#what forms negatively charged ions
Text
Enhanced Protection: Harnesses negative ions to shield against electromagnetic radiation.
Advanced Technology: Utilizes cutting-edge negative ion technology for effective defense.
Easy Application: Convenient sticker for hassle-free attachment on various devices.
Versatile Shield: Guards against EMF radiation emitted by smartphones, laptops, and more.
Health-conscious Solution: Provides a proactive approach to reducing exposure to harmful radiation.
Click Here To More Information For This Product
#EMFDEFENSE™ Negative Ions Sticker#emfdefensetm negative ions sticker emf shield for phone smartphone home radio#emfdefensetm negative ions sticker emf shield for phone and other electronics#what forms negative ions#how do positive ions and negative ions form#how to test for negative ions#emfdefensetm negative ions sticker emf shield for phone smartphone home radio low cancelation rate#what group forms negative ions#what elements form negative ions#what atoms form negative ions#what forms negatively charged ions#what group always forms negative ions#what elements will form negative ions#what atoms typically form negative ions#which groups form negative ions on the periodic table#what tend to form negative ions#what group forms negative 2 ions#what forms when positive and negative ions attract#what forms a negative ion#what produces negative ions#form negative ions by gaining electrons#what releases negative ions#what elements form negatively charged ions#form negative ions called#what tend to form negatively charged ions#what usually forms the negative ion#what are negative charged ions called#what forms positive ions#what is negative ions and positive ions#metals form negative ions
1 note
·
View note
Text
A Meta on Crowley's Miracle Meltdown

gif by phaxxion via Tenor
What exactly is happening in this moment? Why is it happening? Why is it included in this story?
I don't mean "Why does Crowley have a frustration meltdown after a huge argument with his partner about helping their former abuser Jeff Bezos?" We've all been there. But not all of us discharge a huge bolt of red infernal energy when we lose our temper, and more to the point, Crowley doesn't usually do that either. What gives?
And, as ixi of Fuck Yeah Good Omens asks and illustrates, why does some of the infernal energy Crowley discharges travel sideways into Give Me Coffee?
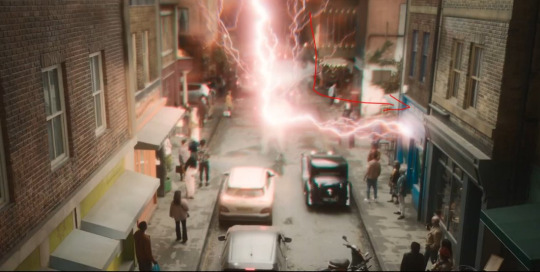
Here's my proposal for answers to these questions.
Insofar as angels and demons were created expressly for the purpose of channeling miracle, maybe to angelic stock refraining from miracles is a bit like holding one's breath is to a human. Human bodies move air in and through and out of themselves as an automatic process. They can refrain from that process, but because breathing is the default, refraining takes a positive sustained effort.
And if you're holding your breath when someone punches you hard in the gut, you don't just lose track of holding your breath, you gasp, you make sounds, you breathe hard. You move a lot of air through your respiratory system for a few seconds until you regain your equilibrium.
Since coming to "a sort of generalised understanding" with Hell, Crowley hasn't been doing many miracles. Aziraphale says he thinks Heaven would notice if he "performed even a minor miracle," and Crowley agrees, "I don't want Hell taking an interest either"; this implies that they've both been trying to keep their miracle usage minimal. (From his continued abuse of London's traffic lights we can infer that Crowley, as usual, has somewhat more latitude in this regard than does Aziraphale.) But he's so freaked out by the appearance of Jeff Bezos Jimbriel and so angry about Aziraphale's line-in-the-sand insistence that the two of them help Jim instead of protecting themselves that Crowley feels approaching a loss of the ability to hold his breath, miraculously speaking.
So before he accidentally curses the bookshop, he goes out into the street away from everyone and tries to get control of himself. If he's not successful, then he's at least away from everyone and able to discharge miracle into the sky where it can dissipate, just like the spiritual smog of evil produced by the M25 dissipates without affecting any one person or thing.
Crowley channels the miracle into the form of lightning, but Crowley's lightning behaves like miracle, not like lightning. Lightning is a huge shock of static electricity, i.e., the sudden mass movement of positively and negatively charged ions toward each other; it does not come up through a person's body from Hell, and it doesn't damage mobile phones.
It takes a direct lightning strike to damage a mobile phone, and when that happens it doesn't just fry the phone, it burns it. An arc of circuit electricity strong enough to fry an unplugged mobile phone would be strong enough to shock and burn the person holding it as well. Nina and Maggie both have their phones on them when the lightning hits Give Me Coffee, but neither woman gets struck by lightning or electrocuted. Their phones get bricked because that is how Crowley thinks lightning works and therefore his lightning works that way.
And it is for this same reason that the "lightning" Crowley puts out hits Give Me Coffee: because it's not lightning, it's miracle. Miracle depends on the miracledoer's familiarity with the thing the miracle is acting upon.
When Crowley has his frustrationgasm, he doesn't have perfect control over where he's aiming miracle in that moment and/or he's concentrating on keeping it away from the bookshop and Aziraphale and the Bentley, so a tongue of miraculous "lightning" (which behaves the way Crowley thinks lightning behaves) slips sideways towards the next most familiar thing on the street to him: Nina's coffee shop. Crowley went there just a few minutes before this scene.
As far as I can tell, this moment serves two functions in the story. Firstly, as many others have pointed out, it establishes the color of Crowley's miracle to be red (continuing the motif of red as the color that represents him) in order to indicate the importance of the Supreme Archangel's purple and the purple of the miracle plume Crowley and Aziraphale's joint miracle produced.
But maybe it's also included because it explicitly establishes a mechanic we see repeated on a smaller scale in 1827: that an angel or a demon's control of the miraculous power they channel isn't always perfect. It can slip if they get really upset.

#this all seems super obvious now I've typed it out#but maybe if it wasn't obvious to me it isn't obvious to someone else either?#good omens#good omens s2#good omens 2#crowley#crowley's emotional meltdown#good omens meta
62 notes
·
View notes
Text
Monday Musings
Let's talk about macrocrystalline quartz vs microcrystalline quartz. I see sooooo many people argue over these in rockhounding groups that I think it is important to address. First, they are the same chemical formula

Whether it's amethyst or chert this is what it is on an atomic level. So, what makes them different?
Well, a big part is how they are formed. Macrocrystalline quartz grows by adding molecules to the surface, layer by layer. It essentially grows in three different environments:
1.) In silica-rich molten rock during cooling and solidification

2.) in pegmatites, during and following pneumatolytic processes

3.) In hot water solutions of silica under various conditions (usually hydrothermal) The water is between 100 and 450 degrees Celsius and often at high pressures. (underground)



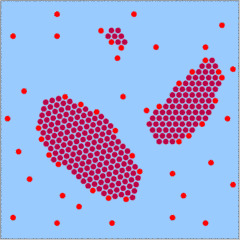

There are no free SiO2 molecules in the solution, instead quartz crystals grow by the addition of dissolved orthosilicic acid (H2SiO4). The four photos above show the process on a very basic level.
In igneous rocks, the formation of quartz is caused by positively and negatively charged ions floating around in the molten rock soup. In fact, these silicate ions cause magmas to become more viscous. SiO4- usually forms long chains in the magma.
When magmas cool rapidly, like at the point of eruption, the chains don't have time to break and new bonds to form so silica-rich magmas often for volcanic glass or pumice.

When magmas cool slowly under the surface, like in granite, crystals of different minerals will in the melt in order of chemical composition. With granite, micas form first, the feldspars, then finally quartz. Because quartz is last, it usually doesn't have great shape since it is filling in all available space.
Quartz in magmas will often have an onion-like internal structure of layers added on top of each other reflecting a gradual change in the residual liquid parts of the solidifying magma.
Okay, now for the microcrystalline quartz: chert and chalcedony (and the bazillion names given to different colored varieties of that).

Chert can form under two different processes: biochemical and and replacement through solution in water.
Biochemical chert is formed when siliceous skeletons of marine plankton are dissolved during diagenesis with silica precipitating out from the resulting solution.

Replacement chert forms when other material is replaced by silica usually by water such as petrified wood.

Chert has the same properties as macrocrystalline quartz i.e. same hardness, fracture, etc.
Chalcedony also forms by precipitation of dissolved silica in water but it is usually formed from watery silica gels which give it the botryoidal look it is well-known for.
It is often deposited in cavities and fractures, spaces too small to form proper quartz crystals, by the release of silica from weathering of other rocks (often volcanic in origin).

Isn't quartz wild?
#geology#mineralogy#quartz#chert#chalcedony#science#too many names#formation#igneous rock#silica solution
28 notes
·
View notes
Photo


Exotic water ice contributes to understanding of magnetic anomalies on Neptune and Uranus Ordinary everyday ice, like the ice produced by a fridge, is known to scientists as hexagonal ice (ice Ih), and is not the only crystalline phase of water. More than 20 different phases are possible. One of them, called “superionic ice” or “ice XVIII”, is of particular interest, among other reasons, because it is thought to make up a large part of Neptune and Uranus, planets frequently referred to as “ice giants”. In the superionic crystalline phase, water loses its molecular identity (H2O): negative oxygen ions (O2-) crystallize into an extensive lattice, and protons in the form of positive hydrogen ions (H+) form a liquid that floats around freely within the oxygen lattice. “The situation can be compared to a metal conductor such as copper, with the big difference that positive ions form the crystal lattice in the metal, and electrons bearing a negative charge are free to wander around the lattice,” said Maurice de Koning, a professor at the State University of Campinas’s Gleb Wataghin Physics Institute (IFGW-UNICAMP) in São Paulo state, Brazil. De Koning led the study that resulted in an article published in Proceedings of the National Academy of Sciences of the United States of America (PNAS) and featured on the cover of its November 8, 2022 issue. Superionic ice forms at extremely high temperatures in the range of 5,000 kelvins (4,700 °C) and pressure of around 340 gigapascals, or over 3.3 million times Earth’s standard atmospheric pressure, he explained. It is therefore impossible for stable superionic ice to exist on our planet. It can exist on Neptune and Uranus, however. In fact, scientists are confident that large amounts of ice XVIII lurk deep in their mantles, thanks to the pressure resulting from these giants’ huge gravitational fields, as confirmed by seismographic readings. “The electricity conducted by the protons through the oxygen lattice relates closely to the question of why the axis of the magnetic field doesn’t coincide with the rotation axis in these planets. They’re significantly misaligned, in fact,” De Koning said. Measurements made by the space probe Voyager 2, which flew by these distant planets on its journey to the edge of the Solar System and beyond, show that the axes of Neptune’s and Uranus’s magnetic fields form angles of 47 degrees and 59 degrees with their respective rotation axes. Experiments and simulations On Earth, an experiment reported in Nature in 2019 succeeded in producing a tiny amount of ice XVIII for 1 nanosecond (a billionth of a second), after which the material disintegrated. The researchers used laser-driven shock waves to compress and heat liquid water. According to the paper in Nature, six high-power laser beams were fired in a temporally tailored sequence to compress a thin water layer encapsulated between two diamond surfaces. The shock waves reverberated between the two stiff diamonds to achieve a homogeneous compression of the water layer resulting in the superionic crystalline phase for an extremely short time. “In this latest study, we didn’t perform a real physical experiment but used computer simulations to investigate the mechanical properties of ice XVIII and find out how its deformations influence the phenomena seen to occur on Neptune and Uranus,” De Koning said. A key aspect of the study was the deployment of density functional theory (DFT), a method derived from quantum mechanics and used in solid-state physics to resolve complex crystalline structures. “First of all, we investigated the mechanical behavior of a flawless phase, which doesn’t exist in the real world. We then added defects to see what kinds of macroscopic deformations resulted,” he explained. Crystal defects are typically point defects characterized by ion vacancies or intrusion of ions from other materials into the crystal lattice. Not so in this case. De Koning was referring to linear defects known as “dislocations”, which are due to angular differences between adjacent layers resulting in puckering somewhat like a rumpled rug. “In crystal physics, dislocation was postulated in 1934 but first observed experimentally in 1956. It’s a type of defect that explains a great many phenomena. We say dislocation is to metallurgy what DNA is to genetics,” De Koning said. In the case of superionic ice, the sum of dislocations produces shear, a macroscopic deformation familiar to mineralogists, metallurgists and engineers. “In our study, we calculated, among other things, how much it’s necessary to force the crystal for it to break up owing to shear,” De Konig said. To this end, the researchers had to consider a relatively large cell of the material with about 80,000 molecules. The calculations entailed extremely heavy and sophisticated computational techniques, including neural networks, machine learning, and the composition of various configurations based on DFT. “This was a most interesting aspect of the study, integrating knowledge in metallurgy, planetology, quantum mechanics and high-performance computing,” he said.
43 notes
·
View notes
Text
Meet the Chemiballs: The Metagaming Metalloids!
So you know metals? They’re metallic, conductive, and usually form positively charged ions? And you know nonmetals? They’re not metallic, insulators, and make negatively charged ions. So yeah, what do you call stuff that’s like, not one of those two things? Y’know, semiconductors and shit.
Metals are only metallic because they’re bad at holding on to their valence electrons, and elements tend to get worse at that the closer they are to the bottom left of the periodic table. Because physics.
But there’s a stair-shaped boundary line between the two where it’s really hard to decide if they’re one or the other so we sort of just gave up and called them metalloids.







READ MY OLD BLOG IT HAS JOKES AND FUN FACTS ABOUT ELEMENTS AAAAHHH
#chemiballs#science#Boronball#Siliconball#Germaniumball#Arsenicball#Antimonyball#Telluriumball#Poloniumball
5 notes
·
View notes
Note
From my understanding of the sales pitch the accessories are made of materials that naturally radiate negative ions that serve as a form of low grade negative ion exposure therapy. My cursory reading without a deep dive shows that there's been some success with exposure therapy to negative ions to treat SAD and anxiety nothing on whether accessories do anything or are a neo science grift even if they do actually do anything achievable I don't doubt they upcharge to hell and back the starter wrist band which was just plastic somehow rigged to be negatively charged was like 20 bucks. Actually I think this may be why my cousin has been buying up silver chains and jewelry now that I think of it.
That's interesting about using ion therapy to treat depression. I wouldn't be surprised if walking outside barefoot on natural surfaces would achieve similar results, although it would be difficult to control for that given the known positive benefits of physical activity and sunlight exposure.
What seems less grifty to me is the concept of grounding, which involves connecting oneself to the earth via conductive metal. There are grounded sheets, which are woven with silver and typically have a stake on a line which you can lead through a window and stick into the ground. The theory is that we used to sleep on the ground and thus have ion exchange with the earth throughout the night. People report sleeping more soundly and feeling less physical pain when they use grounded sheets.
Is this real or placebo? I don't know. It seems completely plausible from a scientific perspective, but again I have not sought out any literature on it. Still, it's pretty interesting to think about!
17 notes
·
View notes
Text
What Is an Industrial Water Treatment System and How Does It Work?
Water is the lifeblood of countless industrial processes. But unlike the kind that comes out of your tap, industrial water often needs a little extra TLC before it's ready for action. That's where industrial water treatment systems come in. At PureBact, we understand the importance of clean, reliable water for your facility's operations.
Here's a breakdown of what industrial water treatment systems are and how they work:
What is an Industrial Water Treatment System?
An industrial water treatment system is a customized set of processes designed to remove impurities and adjust the properties of water to make it suitable for a specific industrial application. This could involve anything from making boiler feed water ultra-pure to treating wastewater before it's released back into the environment.
Why is Industrial Water Treatment Important?
Untreated water can wreak havoc on your industrial processes. Minerals can cause scaling and corrosion in pipes and equipment, while contaminants can affect product quality. Proper water treatment helps to:
Protect equipment: By removing impurities that can cause corrosion and scaling, you can extend the lifespan of your valuable machinery.
Ensure product quality: Consistent water quality is essential for producing consistent, high-quality products.
Minimize environmental impact: Industrial wastewater treatment helps to remove pollutants before they are released back into the environment.
Reduce operating costs: By preventing equipment damage and ensuring efficient operation, proper water treatment can save you money in the long run.
How Does Industrial Water Treatment Work?
The specific treatment processes used will vary depending on the source water and the desired end product. However, some common methods include:
Pre-treatment: This stage removes large particles such as sand, debris, and organic matter through filtration or sedimentation.
Clarification: Coagulation and flocculation techniques help suspended solids clump together and settle out of the water.
Filtration: Various filtration technologies, like sand filters or membrane filters, remove finer particles and contaminants.
Ion exchange: This process removes unwanted ions by exchanging them for harmless ones.
Deionization: A specialized form of ion exchange that removes both positively and negatively charged ions to create high-purity water.
Disinfection: Chemicals like chlorine or ultraviolet light can be used to kill bacteria and other microorganisms.
PureBact: Your Partner in Industrial Water Treatment
At PureBact, we offer a comprehensive range of industrial water treatment solutions. Our team of experts will work with you to assess your specific needs and design a custom system that ensures optimal water quality for your operations.
Contact PureBact today to learn more about how we can help your business thrive with clean, reliable industrial water.
2 notes
·
View notes
Note
Any fun facts about sodium? (Can sodium be fun???) Also, what got you interested in chemistry?
Oh you shouldn’t have asked me about sodium. I’m fucking FERAL about sodium.
Sodium is a member of the Alkali Metals on the periodic table, and a common theme with these metals is that they fucking explode when in contact with water. One of my first experiences in a chemistry class was watching a piece of sodium spinning rapidly in water, catching fire, and detonating before my gleeful gaze.
This is because sodium has a single electron in its outer shell, and it does not give a single shit about holding onto this electron. It will get rid of it by giving that thing to literally whatever might maaayyyybe want it, so it’s going to violently react with anything.
“But teet,” I hear you asking, “Why is table salt so chill then?” And that’s because that is not neutral sodium. Sodium chloride (aka table salt) is what occurs when sodium was already able to give away it’s electron to something that wanted it (the chloride). Held together by electrostatic forces, the newly stable sodium ions forms a lattice with the chloride ions to create salt crystals. It’s safe to consume and pretty inert for the most part.
Because sodium loves giving away it’s outer electron, it’s really good at creating strong bases, as whatever it attaches to is gonna be negatively charged and isn’t gonna be super hindered by the sodium if it’s in something like water. You’ve probably used some of these in your life, like maybe sodium hypochlorite (bleach) or sodium bicarbonate (baking soda). It also can be used for organic chemistry as another reducing agent, sodium borohydride (NaBH4), which is very nice to use :).
However, if we want to have some fun, let’s get into using sodium’s bases for making polymers. Polymers are long chains or networks of repeating patterns of simple molecules, called monomers. Your clothes, water bottles, and even your cells contain polymer ingredients, so these are definitely important. One way to make polymers is to use a base like, say, sodium amide (NaNH2). The negative NH2- ion is going to be reactive enough so that it can immediately start building chains with monomers, and if you’re particularly careful, the negatively charged chain can be prevented from terminating, so that any time you want you can just add more monomer and make it grow even longer. These are called “living” polymers and they’re incredibly cool!
So those are a few applications of sodium that I just think are really neat, but with regards to your last question, I think it goes back to when I was a kid and I just thought it was cool seeing what chemicals could do (especially the explosions lol). Back then I told everyone I wanted to be a mad scientist, but what I meant was a chemist. That dream still lives on, but I’ve found that my talents lie especially in mathematics and physics, so I’ve been focusing a lot more on the physical chemistry side of things lately.
3 notes
·
View notes
Text
Stabilizing the Element
or Even our atoms need each other
When atoms are far apart, they attract each other. This attraction is stronger for some kinds of atoms than others. At the same time, the heat, or kinetic energy, of atoms makes them always move. If the attraction is strong enough, relative to the amount of heat, atoms will form a solid. If the attraction is weaker, they will form a liquid, and if it is even weaker, they will form a gas. (Wikipedia, 6/26/2023)
It's the hot Central Valley summer of the second long distance, the second hand ticking away uncountable minutes, the seconding of everyone in my orbit that I am not quite interesting enough to really bond with. No one said it first, but I am pitifully made aware that I cannot be created nor destroyed, only transmuted transfixed and transformed, the ever unstable element on the table for discussion.
Chemical bonds are the strongest kinds of attraction between atoms. The movement of electrons explains all chemical bonds. Atoms usually bond with each other in a way that fills or empties their outer electron shell. The most reactive elements have an almost full or almost empty outer shell. Atoms with a full outer shell, called noble gases, do not usually form bonds. (Wikipedia, 6/26/2023)
I do not understand my parents, well off and independent of one another. How do you breathe the same oxygen every night and remain unmoved? I cannot help but react and I reach, desperate to fill myself, desperate to fill others, to give and receive. But in this hot Central Valley summer I grasp only air.
There are three main kinds of bonds: ionic bonds, covalent bonds, and metallic bonds.
In an ionic bond, one atom gives electrons to another atom. Each atom becomes an ion: an atom or group of atoms with a positive or negative charge. The positive ion (which has lost electrons) is called a cation; it is usually a metal. The negative ion (which has gained electrons) is called an anion; it is usually a nonmetal. Ionic bonding usually results in a regular network, or crystal, of ions held together. (Wikipedia, 6/26/2023)
I had forgotten what it was like to live on the affluent side of town, to not see people starving, to not have people all around who are unstable, reactive. Knowing they are still here, still reacting, only kept out of this sterile lab environment through aggressive sanitizing of unwanted elements to avoid cross contamination, I am not comforted by comforts. I am lonely.
In a covalent bond, two atoms share electrons. This usually happens when both atoms are nonmetals. Covalent bonds often form molecules, ranging in size from two atoms to many more. They can also form large networks, such as glass or graphite. The number of bonds that an atom makes (its valency) is usually the number of electrons needed to fill its outer electron shell. (Wikipedia, 6/26/2023)
Board games and conversations on the street corner spread conversations far and wide, and everything belongs to everyone on that street corner for the day. I meet a man who asks me if I want a cigarette while beating me thoroughly at chess, a woman who has a little dog wearing a sweater in better condition than her own, a guy who watches each tournament and pulls at his sleeves, someone is writing resources on the community board.
In a metallic bond, electrons travel freely between many metal atoms. Any number of atoms can bond this way. Metals conduct electric current because electric charge can easily flow through them. Atoms in metals can move past each other, so it is easy to bend, stretch, and change the shape of metals. (Wikipedia, 6/26/2023)
Remember the weeks of watching for news of Seattle's community? Of parsing through hyperbole and lies for glimmers of a people committed to radical restructuring? Remember what it felt like to wonder if this was the beginning of something significant? I remember changing and stretching into something new, something I thought was stable but could not have been more reactive. Maybe that isn't a bad thing.
#science#chemistry#english literature#poetry#free verse#original poem#poetic#writing#creative writing#elements#electrons#leftist#love poem#actually bipolar#bipolardisorder#wrote in my biology lecture
4 notes
·
View notes
Text
The Importance of the Lightsaber
What follows is a series of excerpts from the novel I, Jedi by Michael A. Stackpole:

The lightsaber, while an elegant and deadly weapon, was not that complex. Getting the parts to put one together was not difficult at all. To serve as the hilt, for example, I salvaged the throttle assembly and handlebar tube from a junked speeder bike.
(...) I got the dimetris circuitry for the activation loop from an old capital-ship-grade ion cannon fire initiation controller (...) The recharger port and wiring came from a comlink. A milled down Tri-fighter laser flashback suppressor became the parabolic, high-energy flux aperture to stabilize the blade and I pulled the dynoric laser feed line from the same broken laser cannon to act as the superconductor for energy transference from the power cell to the blade.

Buttons and switches were easy to find, and dear old Admiral Tavira, with her gift of the brandy decanter and snifters, provided me all the jewels I needed to make half a dozen lightsabers.
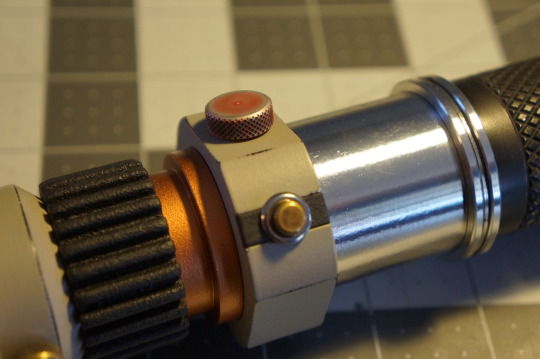
(...) Before I could figure out how to put Tavira off for another month, Elegos decoded an annotation to the instructions for constructing lightsabers. It turned out that during the Clone Wars, Jedi Masters developed a way to create a lightsaber in two days. Nejaa included this method, noting it was to be used only in times of pressing need, but not in haste. I read it over and felt a certain peace settle upon me. I knew the words had not been written for me, but they sank deep into my core. Urgency without panic, action without thoughtlessness.
(...) I sat in the middle of the floor, with the parts for the blade laid out in a semicircle around me. I studied each one and used the Force to enfold it and take a sense of it into myself. My hands would fit the pieces together, but I wanted the parts to mesh as if they had been grown together. The lightsaber would be more than just a jumble of hardware, and to make it I had to see the parts as belonging together.
I fitted the activation button into its place on the handlebar shaft and snapped the connectors into the right spots on the dimetris circuit board.

I worked that into the shaft itself, then inserted a strip of shielding to protect it from even the slightest leakage from the superconductor. Next I snapped into place the gemstones I was using to focus and define the blade. At the center, to work as my continuous energy lens, I used the Durindfire. That same stone gave my grandfather’s blade its distinctive silver sheen.



I used a diamond and an emerald in the other two slots. I wasn’t certain what I would get in the way of color tints from the emerald, and with the diamond I hoped for a coruscation effect.
Onto the end of the hilt where the blade would appear I screwed the high-energy flux aperture. It would carry a negative charge which would stabilize the positively charged blade and provide it a solid base without allowing it to eat its way back through to my hands.
(...) I clipped the discharged energy cell in place, then connected the leads to the recharging socket.


I screwed the recharging socket into the bottom of the hilt, but didn’t fasten on the handlebar’s original butt cap that would protect it because I needed to charge the power cell for the very first time.
In what amounts to just a handful of pages in a singular book, Stackpole so vividly describes not only the physical process of constructing a lightsaber, but the spiritual essence of what it means to be a Jedi, in its purest form:
(...) With my finger poised on the transformer button that would start the energy flowing, I drew in a deep breath and lowered myself into a trance. I knew that manipulating matter sufficiently to meld the part and forge the weapon would have been all but impossible for anyone but a Jedi Master like Yoda, but doing just that as part of the construction of a lightsaber had been studied and ritualized so even a student could manage it. It was very much a lost art, a link to a past that had been all but wiped out, and by performing it I completed my inheritance of my Jedi legacy.
I hit the button, allowing the slow trickle of energy to fill the battery. I opened myself to the Force and with the hand I had touching the lightsaber’s hilt, I bathed the lightsaber with the Force. As I did so subtle transformations took place in the weapon. Elemental bonds shifted allowing more and more energy to flow into the cell and throughout the weapon. I was not certain how the changes were being made, but I knew that at the same time as they were being made in the lightsaber, they were being made in me as well.
In becoming a conduit for the Force for this purpose, the final integration of the people I’d been occurred. The fusion became the person I would be forever after. I was still a pilot: a little bit arrogant, with a healthy ego and a willingness to tackle difficult missions. I was still CorSec: an investigator and a buffer between the innocents in the galaxy and the slime that would consume them.
And I was Jedi. I was heir to a tradition that extended back tens of thousands of years. Jedi had been the foundation of stability in the galaxy. They had always opposed those who reveled in evil and sought power for the sake of power. People like Exar Kun and Palpatine, Darth Vader and Thrawn, Isard and Tavira; these were the plagues on society that the Jedi cured. In the absence of Jedi, evil thrived.
In the presence of just one Jedi, evil evaporated.
Just as with the lightsaber, the changes being made in me were not without cost. What the Force allowed me to do also conferred upon me great burdens. To act without forethought and due deliberation was no longer possible. I had to be very certain of what I was doing, for a single misstep could be a disaster. While I knew I would make mistakes, I had to do everything I could to minimize their impact. It was not enough to do the greatest good for the greatest number, I had to do the best for everyone.
There was no walking away from the new responsibility I accepted. Like my grandfather I might well choose when and where to reveal who and what I was, but there was no forgetting, no leaving that responsibility and the office. My commitment to others had to be total and complete. I was an agent of life every day, every hour, every second; for as long as I lived, and then some.
(...) I nodded and brandished the lightsaber. I punched the button under my thumb, giving birth to the silver blade 133 centimeters in length.

“A lightsaber and robes. Looks like a little justice has arrived on Courkrus, and it’s about time.”
This is what almost everybody gets wrong about the Jedi - never mind the Prequels, the Sequels, or the vast majority of EU novels - the Jedi are an absolute good. They are life. They bring order to chaos. Every moment of their lives is spent, their spirits grappling against the disorder of a universe torn between Dark and Light.
If you haven’t read I, Jedi, you might consider picking it up.
As I’m sure many of you have already deduced, the accompanying images are of my own personal Corran Horn lightsaber. I’ve just recently finished a complete overhaul and rebuild of it, and I just had to show it off. I’ll be posting more info & pics about it soon, but I want to address one key aspect of saber building here, as it pertains directly to my own personal journey and growth.
This portion of the book holds incredible significance for me; it allows me to imagine, however briefly, that I’m undertaking a similar spiritual ritual, imbuing my own sabers with the same energy that Stackpole so flawlessly describes here. It’s so rare to feel so seen and be so moved by a piece of fiction. For the discerning Sabersmith, it’s very much like Corran says:
I knew the words had not been written for me, but they sank deep into my core.
Thank you for this book, Michael. 🥂
#star wars#lightsaber#lightsabers#sabersmith#ijedi#new jedi order#michael a stackpole#goat#expanded universe#eu#legends#corran horn#dual-phase lightsaber#mirax terrik horn#elegos a'kla#leonia tavira#admiral tavira#nejaa halcyon#nikkos tyris#jensaarai#saarai-kaar#halcyon days of star wars
7 notes
·
View notes
Text
The Hunt to solve the chemical mysteries
Imagine one morning you wake up and find yourself in a cold freezing and snowy environment and it seems like your in one of the movies of Harry Potter. The next moment a woman comes up to you and asks “ciamar a tha thu?” before you recover from your amazement a boy appears out of a snowman and goes like “Madainn Mhath”.
Well before this article turns into a language guide let me tell you what language was being spoken and where you were. Ok, so you were in Britan and you just heard Gaelic Scottish.
You might have been perplexed for a moment, well that’s ok .Fun fact ,Chemistry i.e. a branch of science will leave you in the same state of confusion if you aren’t well-versed with ‘Chemstrian’.
Yes ,you read that right ,Chemstrian is what people call the unique language of Chemistry that is a whole system of speaking and writing that’s unique to the study of chemicals. An idiosyncratic way of translating numbers and chemical formula into words, that can be understood and spoken.
Well, Chemstrian might seem difficult but by the time you finish this exciting journey of this article, I assure you that you’ll start loving chemistry and be one of its native speakers.
This article is a guide which will be the map for the hunt of a special treasure whose clues are hidden in the Periodic table which is our phrase book.By the end of this article I wouldn’t expect you to walk out and go around spouting off ‘three-keto-two-carboxy- arabinitol' but I would surely help you set off in the right way.
Ay-Ay…let’s begin our hunt!
Our stepping stone to the unique treasure of chemistrian is that the rules for describing an element changes with its place in the Periodic table.
The guiding star for this treasure is that these rules not only apply to their formulas that is chemical symbols we use in the equation but also to their names.
For example…Ions become positively or negatively charged on the basis of gaining or losing electrons
Cations are positively charged ions and anions are negatively charged ions .Which are going to be the special kings of whom we’ll be learning today and by praising them we will be able to find our treasure. Cause we might not want to face opposition from these powerfully charged kingdoms(Ions).
REMINDER: DO NOT GET BORED YOU’RE HALF THE WAY FOR THE TREASURE
Here's a clue ,
When it is a cation we add the suffix ION For example- sodium Ion (Na±)
When it is an anion we add the sufffix IDE For example- Cl– = chloride
To make this trip more exciting..let me get it to your notice that we say the cation first then the anion for eg, Sodium chloride (sodium- cation & Chloride- anion) Isn’t that amazing? For sure the anions must be jealous of cations right? What do you think?
Ok, so here’s our final key for opening the treasure, that is to know which element is what.Here comes the periodic table to the rescue!
The first two columns on the left are your Alkali and Alkaline earth metals..well that was pretty much of fancy names right? Ok back to topic, so the Alkali and Alkaline earth metals form ions readily when they battle against(react) the non metals and when they have the war they lose their soldiers that is electrons and form the cations, and on the other side of the table we'll mostly find anion forming elements. That’s How the kingdom of Cation and Anion was formed . Well, all that praising work paid off.
Here you go! You’ve just mastered the basic concepts of chemistry! You’ve also earned yourself a great treasure. Welcome aboard !to this amazing group of chemistrian native speakers, in order to enjoy future trips with our fellow passengers,
Keep reading chemistry and solve the life’s mystery!
Happy reading!
Aishwarya Upadhye☺
Manchi,Udupi
3 notes
·
View notes
Text
Big Think: How plants can perform feats of quantum mechanics
It is spring now in the Northern Hemisphere, and the world has greened around us. Outside my window, trees are filled with leaves that act as miniature factories, collecting sunlight and converting it into food. We know this basic transaction takes place, but how does photosynthesis really happen?
During photosynthesis, plants utilize quantum mechanical processes. In an attempt to understand how plants do this, scientists at the University of Chicago recently modeled the workings of leaves at the molecular level. They were blown away by what they saw. It turns out that plants act like a strange, fifth state of matter known as a Bose-Einstein condensate. Even stranger is that these condensates are typically found at temperatures near absolute zero. The fact that they are all around us on a normal, temperate spring day is a real surprise.
States of low energy
The three most common states of matter are solid, liquid, and gas. When either pressure or heat is added or removed, a material can shift between these states. We often hear that plasma is the fourth state of matter. In a plasma, atoms break down into a soup of positively charged ions and negatively charged electrons. This typically occurs when a material is super-heated. The Sun, for example, is mostly a big ball of super-hot plasma.
If matter can be superheated, it can also be supercooled, causing particles to fall into very low energy states. Understanding what happens next requires some knowledge of particle physics.
There are two main types of particles, bosons, and fermions, and what differentiates them is a property called spin — a weird, quantum-mechanical characteristic that relates to the particle’s angular momentum. Bosons are particles with integer spin (0, 1, 2, etc), while fermions have a half-integer spin (1/2, 3/2, etc). This property is described by the spin-statistics theorem, and it means that if you swap two bosons, you will retain the same wave function. You cannot do the same for fermions.
In a Bose-Einstein condensate, the bosons within a material have such low energy that they all occupy the same state, acting as a single particle. This allows quantum properties to be seen on a macroscopic scale. A Bose-Einstein condensate was created in a lab for the first time in 1995, at a temperature of a mere 170 nanokelvin.
Quantum Photosynthesis
Now, let’s look at what happens in a typical leaf during photosynthesis.
Plants need three basic ingredients to make their own food — carbon dioxide, water, and light. A pigment called chlorophyll absorbs energy from light at red and blue wavelengths. It reflects light at other wavelengths, which makes the plant look green.
At a molecular level, things get even more interesting. Absorbed light excites an electron within a chromophore, the part of a molecule that determines its reflection or absorption of light. This kicks off a series of chain reactions that end up producing sugars for the plant. Using computer modeling, the researchers at the University of Chicago examined what occurs in green sulfur bacteria, a photosynthetic microbe.
Light excites an electron. Now the electron and the empty space it left behind, called a hole, act together as a boson. This electron-hole pair is called an exciton. The exciton travels to deliver energy to another location, where sugars are created for the organism.
“Chromophores … can pass energy between them in the form of excitons to a reaction center where energy can be used, kind of like a group of people passing a ball to a goal,” Anna Schouten, the study’s lead author, explained to Big Think. ��
The scientists discovered that the paths of the excitons within localized areas resembled those seen within an exciton condensate — a Bose-Einstein condensate made of excitons. The challenge with exciton condensates is that the electrons and ions tend to recombine quickly. Once this happens the exciton vanishes, often before a condensate can form.
These condensates are remarkably difficult to create in the lab, yet here they were, right in front of the scientists’ eyes, in a messy organism at room temperature. By forming a condensate, the excitons formed one single quantum state. In essence, they were acting like a single particle. This forms a superfluid — a fluid with zero viscosity and zero friction — allowing energy to flow freely between chromophores.
Their results were published in PRX Energy.
Messy Conditions
Excitons normally decay quickly, and when they do, they can no longer transfer energy. To give them a longer lifetime, they typically need to be very cold. In fact, exciton condensates have never been seen above temperatures of 100 Kelvin, which is a frosty negative-173 degrees Celsius. This is why it is so surprising to see this behavior in a messy, real-world system at normal temperatures.
So what’s going on here? Just another way that nature is constantly surprising us.
“Photosynthesis works at normal temperatures because nature has to work at normal temperatures in order to survive, so the process evolved to do that,” says Schouten.
In the future, room-temperature Bose-Einstein condensates may have practical applications. Since they act as a single atom, Bose-Einstein condensates may give us insight into quantum properties that would be difficult to observe at the atomic level. They also have applications for gyroscopes, atom lasers, high-precision sensors of time, gravity, or magnetism, and higher levels of energy efficiency and transfer.
2 notes
·
View notes
Text
Understanding the Chemistry Behind Lead-Acid Batteries

Lead-acid batteries have long been the backbone of automotive power solutions, providing reliable and cost-effective energy storage for vehicles of all types. As one of the leading car battery suppliers, Vacuna is dedicated to unraveling the chemistry that powers these essential components. Let’s delve into the intricate chemistry of lead-acid batteries, their working principle, and diverse applications in the automotive industry.
What is a Lead-Acid Battery?
A lead-acid battery is a type of rechargeable battery that utilizes lead plates immersed in an electrolyte solution of sulfuric acid to store and release electrical energy. These batteries are commonly used in vehicles, uninterruptible power supplies (UPS), and other applications requiring reliable energy storage.
Working Principle of Lead-Acid Battery
The working principle of a lead-acid battery involves electrochemical reactions that occur within its cells during charging and discharging cycles. When the battery is charged, electrical energy is converted into chemical energy, causing lead dioxide (PbO2) to form on the positive plate and lead (Pb) to form on the negative plate. This process reverses during discharge, with lead dioxide converting back to lead sulfate (PbSO4) and releasing electrical energy.
Chemistry of Lead-Acid Battery
The chemistry of a lead-acid battery revolves around the following key reactions:
1. Charging Reaction (Positive Plate):
PbO2 + H2SO4 + 2H+ + 2e– → PbSO4 + 2H2O
Lead dioxide, sulfuric acid, and hydrogen ions combine to form lead sulfate and water during charging.
2. Discharging Reaction (Positive Plate):
PbSO4 + 2H2O → PbO2 + H2SO4 + 2H+ + 2e–
Lead sulfate reacts with water to regenerate lead dioxide, sulfuric acid, and release hydrogen ions and electrons during discharge.
3. Charging Reaction (Negative Plate):
Pb + HSO4– → PbSO4 + H+ + 2e–
Lead reacts with bisulfate ions to form lead sulfate, releasing hydrogen ions and electrons.
4. Discharging Reaction (Negative Plate):
PbSO4 + H+ + 2e– → Pb + HSO4–
Lead sulfate is reduced back to lead and bisulfate ions during discharge.
Application of Lead-Acid Battery
Lead-acid batteries find widespread application in the automotive industry, powering vehicles ranging from cars and trucks to motorcycles and recreational vehicles. Lead-acid batteries are engineered to meet the stringent power requirements of modern vehicles, including start-stop systems, advanced electronics, and energy-intensive accessories. They also serve as reliable backup power sources for critical automotive systems, ensuring uninterrupted performance in various driving conditions.
Advantages of Lead-Acid Battery
Cost-Effective: Lead-acid batteries are relatively affordable compared to other types of batteries, making them a cost-effective choice for a wide range of applications, including automotive use.
Proven Technology: Lead-acid batteries have been in use for decades and have a well-established track record of reliability and performance, instilling confidence in their use for critical applications.
High Energy Density: Lead-acid batteries offer a high energy density, providing ample power storage in a compact and efficient package, making them suitable for vehicles with limited space.
Low Self-Discharge Rate: Lead-acid batteries have a low self-discharge rate, meaning they can retain their charge for extended periods, making them ideal for backup power applications.
Recyclable: Lead-acid batteries are highly recyclable, with a significant portion of the materials used in their construction being recoverable and reusable, contributing to environmental sustainability.
In conclusion, Vacuna’s expertise as a car battery supplier extends to understanding the intricate chemistry of lead-acid batteries and harnessing this knowledge to deliver high-quality power solutions. With their proven performance, durability, and cost-effectiveness, lead-acid batteries continue to play a crucial role in powering vehicles and supporting automotive operations worldwide. Trust Vacuna as your reliable partner for automotive power solutions.
0 notes
Photo

SPORT and petitSat CubeSats to shed light on space weather disturbances Two CubeSats, or small satellites, are on a quest to provide insight on space weather disturbances and the subsequent impact on communication signals. The dynamic duo, the Plasma Enhancements in the Ionosphere-Thermosphere Satellite (petitSat) and Scintillation Prediction Observations Research Task (SPORT), arrived at the International Space Station on Nov. 27, 2022, as part of SpaceX’s 26th commercial resupply mission for NASA. Both CubeSats deployed from the space station on Dec. 29, 2022, at 8:55 a.m. EST. Scientists on both missions are most interested in studying a layer in Earth’s upper atmosphere known as the ionosphere. The ionosphere is where the impacts of space weather on our technology are felt most strongly. It's home to many satellites, including the International Space Station. Radio waves and GPS signals travel through the ionosphere, and variations there can interfere with, or even disrupt, our communication signals. Space weather can also create electric currents that can induce electrical charge in orbiting satellites, and, in extreme cases, cause power outages on the ground. Day in and day out, the ionosphere is cooked by the Sun's radiation into a soup of positively charged ions and negatively charged electrons, called plasma. Fluctuations in the ionosphere cause low-density and high-density regions – bubbles and blobs – to form in the plasma. These bubbles and blobs can scatter radio signals, sometimes sending them crashing into each other in a phenomenon called scintillation. The result is noisy radio signals, which can reduce the reliability of communication and navigation systems, or even disrupt signals completely. “If you put a pencil into a glass of water that’s half full, the pencil appears broken,” said Linda Habash Krause, the project scientist for SPORT at NASA’s Marshall Space Flight Center in Huntsville, Alabama. “What happens when you have bubbles? Similar to the pencil in the water, the signals go through ample bends.” Unfortunately, scientists do not understand exactly how the plasma bubbles and blobs arise. Once petitSat and SPORT are launched from the space station, the two CubeSats will use complementary scientific instruments to investigate the conditions that cause these disruptive features to form. “The idea is that the science teams will work together and cross compare,” said Jeff Klenzing, the principal investigator of petitSat at NASA’s Goddard Space Flight Center in Greenbelt, Maryland. SPORT is equipped with six instruments to make measurements throughout the ionosphere. It will help determine the conditions that exist just before plasma bubbles form and, ultimately, how their evolution impacts ground-based communications signals. SPORT will transmit data back to the Brazilian National Institute for Space Research (INPE), where the data will be distributed to researchers at INPE, NASA, and other U.S. partners. In a complementary fashion, petitSat will work to determine what triggers plasma blobs, when they appear, or even how large a region they occupy. Both petitSat and SPORT will provide improved observations and insights into space weather phenomena which impact communications. These missions will collectively enhance our understanding of our ever-changing space environment and amplify current capabilities of small satellites to directly benefit our society. The more we learn about space weather – and how to predict it – the better we can protect our astronauts, spacecraft, and technology. IMAGE....The ionosphere constantly glows and will be the main focus of study for these two satellites. Here, an aurora is captured as seen from the International Space Station. CREDIT NASA
2 notes
·
View notes
Text
The AIDS/HIV virus which aids also bonds with covid-so to speak. Was what killed off half the population
And hence the purple virus cure is the opposite of purple. Which is red energy.
artificially thermoelectrically charged ions
which is a + positive charged gravity wavelength forcefield.
which makes – negative a inverted electrically AC-like U shape charge. Wave form for actual electricity is U
if you run it through a gold wire
while a silver wire would be S shape
that explains the fuse in…
View On WordPress
0 notes
Text
How Alkaline Water Ionizers Work: A Comprehensive Guide
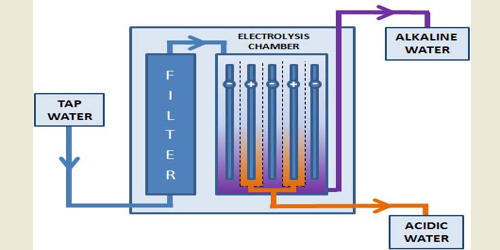
Do you know there’s a great home appliance out there available on the market that can improve your overall health? This miracle home gadget is an alkaline water ionizer’ or simple ‘Water Ionizer’. An alkaline water ionizer is a home appliance that raises the pH of regular drinking water by using electrolysis to separate the incoming water stream into acidic and alkaline components. Let us explore the world of alkaline water ionizers and how they function and turn regular water into the claimed ‘miracle water’.
What is alkaline water?
Normal drinking water has a pH level of 7; On the other hand, Alkaline Water has a higher pH (more basic) than normal tap water, often falling between 7.5 and 9. It is also known as high-pH water or ionized water.
Introduction to alkaline water ionizer
An alkaline water ionizer is a device that can make regular water into alkaline water. This alkaline water is then purified and filtered. Drinking alkaline water is considered best to maintain the pH levels of your body. These alkaline water ionizers are easy to use and are not very expensive, which makes them a great investment.
A guide to how an alkaline water ionizer works.
1. Source Of Water: The water ionizer is connected to a tap water source, which ensures the supply of water for the process of ionization.
2. Filtration Process: This tap water now passes through various stages of filtration, which includes activated sediment filters and carbon filters. These filters then remove the impurities from the tap water such as chlorine, sediment, heavy metals, and other contaminants, making the water clean and safe to drink.
3. Electrolysis Chamber: The filtered water then enters the electrolysis chamber, which contains special electrodes made of materials like platinum or titanium.
4. Electrolysis Process: When the water flows over the electrodes, an electrical current is applied. Electricity is passed through the electrodes, splitting water into alkaline and acidic components. These electrodes are typically divided into two sections: positively charged anodes and negatively charged cathodes.
5. Alkaline Water Production: Alkaline ions accumulate near the cathode to form alkaline water. The pH level of this water is usually higher than that of the original tap water, often from pH 8 to pH 10 or higher, making it more alkaline.
6. Acidic Water Production: The formation of acidic water is caused by the accumulation of positively charged hydrogen ions near the anode. Acidic water is typically less acidic than tap water and is usually obtained by draining or collecting the alkaline water separately.
7. Adjustable Settings: The alkaline water produced by ionizers can be adjusted with the help of user-configured settings, such as pH and strength. These settings may vary by model and manufacturer.
8. Dispensing: The alkaline water is dispensed for immediate consumption by the water ionizer. Certain models may come equipped with storage tanks that can hold alkaline water for convenient usage at a later time.
9. Maintenance: Routine maintenance is essential for the effective functioning of the ionizer. This includes cleaning the electrodes to prevent buildup and changing the filters according to the manufacturer’s recommendations.
Get alkaline water at home
You can make your ionized alkaline water at home by simply installing an Alkaline Water Ionizer at your home. Set up an Alkaline Water Ionizer today with Alkonic. You can also book a free demo from https://alkonic.com.
Source: https://alkonic.com/how-alkaline-water-ionizers-work-a-comprehensive-guide/
0 notes