#Chemistry
Text



20 April 2024
It's raining outside and I'm lying in bed sipping coffee and reading after studying for a couple of hours <333
#first time reading a classic in english!#ive read a lot of books in english but never anything written before like#late 20th century#bc i was too intimidated#but apparently my comprehension of english is at that point#where frankenstein isn't too challenging#so that's nice :)#mine#studyblr#chemblr#studyspo#study motivation#chemistry#stemblr#sciblr
187 notes
·
View notes
Text
Getting 3 cats and calling them proton, neutron, and electron that way when they sleep in a big pile they become hydrogen
116 notes
·
View notes
Text
Moments before disaster
You see, when potassium Potato Solution meets water tinted with methyl orange in a supposed titration set-up, the reaction will be so reactive that it will cause an explosion.
Stay in school, everyone. It makes you know not to imitate the above set-up in-person.
107 notes
·
View notes
Text
my liege, a new element just dropped. the ball

#i think this is some sort of /R carbon group#but in all faithfullness it's my first time seing it#chemistry#resin synthesys#?#chemistry memes#organic chemistry#REM resin#saw it in a REM resin synthesys paper. maybe this is a resin chemistry thing#don't want to ofend the#resin chemists#by accident#maybe it's a cultural thing
36 notes
·
View notes
Text
A team of geologists and planetary scientists from the California Institute of Technology, the University of California Santa Cruz, New York University, and NASA Goddard Space Flight Center reports evidence that Io's volcanic activity has been ongoing since the beginning of the solar system. In their study, published in the journal Science, the group studied sulfur isotopes in Io's atmosphere to determine how long the moon has been volcanically active.
Prior research has shown that the solar system is approximately 4.5 billion years old and that Jupiter's moon, Io, is the most volcanically active body in the solar system. But until now, researchers did not know how long the moon has been active. To find out, the research team used data from ALMA to analyze the gases present in Io's atmosphere.
Continue Reading.
46 notes
·
View notes
Text


More fun things I got from that science lab
32 notes
·
View notes
Text
look, I know I've talked about this essay (?) before but like,
If you ever needed a good demonstration of the quote "Any sufficiently advanced technology is indistinguishable from magic", have I got an exercise for you.
Somebody made a small article explaining the basics of atomic theory but it's written in Anglish. Anglish is basically a made-up version of English where they remove any elements (words, prefixes, etc) that were originally borrowed from romance languages like french and latin, as well as greek and other foreign loanwords, keeping only those of germanic origin.
What happens is an english which is for the most part intelligible, but since a lot everyday english, and especially the scientific vocabulary, has has heavy latin and greek influence, they have to make up new words from the existing germanic-english vocabulary. For me it kind of reads super viking-ey.
Anyway when you read this article on atomic theory, in Anglish called Uncleftish Beholding, you get this text which kind of reads like a fantasy novel. Like in my mind it feels like it recontextualizes advanced scientific concepts to explain it to a viking audience from ancient times.
Even though you're familiar with the scientific ideas, because it bypasses the normal language we use for these concepts, you get a chance to examine these ideas as if you were a visitor from another civilization - and guess what, it does feel like it's about magic. It has a mythical quality to it, like it feels like a book about magic written during viking times. For me this has the same vibe as reading deep magic lore from a Robert Jordan book.
#off topic#literature#language#linguistics#science#science history#science fiction#fantasy#physics#atomic theory#anglish#chemistry#robert jordan#the wheel of time#uncleftish beholding
42K notes
·
View notes
Text
Cesium-133, let it be. Cesium-134, let it be even more.
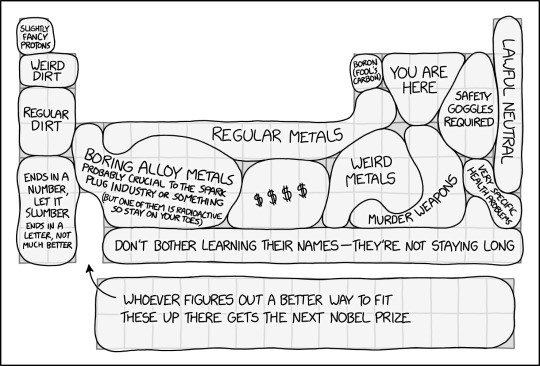
Periodic Table Regions [Explained]
Transcript
[A periodic table with regions labeled.]
[Hydrogen:] Slightly fancy protons
[Lithium and Beryllium:] Weird dirt
[Group 1 & 2 metals, Periods 3-4:] Regular dirt
[Group 1 & 2 metals, Periods 5-7:] Ends in a number, let it slumber ends in a letter, not much better
[Left side of the transition metals group:] Boring alloy metals Probably critical to the spark plug industry or something (but one of them is radioactive so stay on your toes)
[Most of the top row of the transition metals + aluminum:] Regular metals
[Below the rightmost "regular metals" - the "ordinary metals" and some transition metals:] Weird metals
[The platinum group:] $$$$
[Boron:] Boron (fool's carbon)
[Carbon, Nitrogen, Oxygen, and Phosphorus:] You are here
[The Halogens:] Safety goggles required
[Noble Gases:] Lawful neutral
[Iodine and Radon:] Very specific health problems
[Ordinary metals and metalloids - Arsenic, Antimony, Tellurium, Thallium, Lead, Bismuth, Polonium] Murder weapons
[Astatine and Period 7 from Rutherfordium onwards:] Don't bother learning their names - they're not staying long
[Lanthanides and Actinides:] Whoever figures out a better way to fit these up there gets the next Nobel Prize
8K notes
·
View notes
Text
#i have full exam season next week and coming up with the names gave me some extra fun#personally i’m more of a#chemistry#person myself#(though my recent grades are working hard to prove me wrong)#anyways#how does one tag again?#poll#!!#science#stem#science side of tumblr#never mind#nadirants
9K notes
·
View notes
Text

Astronomy I-
#physics#science#stem#mathematics#maths#math meme#science meme#physics meme#chemistry#geology#psychology#meme#funny#don’t lick the science#engineer#astronomy#space
73K notes
·
View notes
Text
Paleontologist: I became a paleontologist because dinosaurs are cool
Astronomer: I became an astronomer because space is cool
Chemist: I became a chemist because explosions are cool
Archeologist: I became an archeologist because Indiana Jones is cool
Mycologist: I. Fucking. LOVE. Mushrooms.
Paleontologist: Uh…
Mycologist: IWillLiterallyMurderYouJustSoICanWatchFungiBreakDownYourDecayingRemainsDon’tTestMeBoneBoy
#Science#Mycology#Chemistry#biology#Paleontology#scientists sitcom#scientists specializing in their obscure special interest is my favorite gender#I am one of those#Astronomy#Archeology
4K notes
·
View notes
Text
The Chemical Structure of Redstone
So I was curious about what the chemical structure of Redstone looks like, and Minecraft Education Edition, albeit unintentionally, gives us a canon look into what Redstone is made of:

In Minecraft Education Edition, putting a Redstone Block into a Material Reducer shows that it's composed of 31 Carbon, 31 Uranium, and 38 Unobtanium, which we can assume to be measured in grams
Dividing the Redstone Block into Redstone Dust, each Redstone Dust is then composed of approximately 3.4 Carbon, 3.4 Uranium, and 4.2 Unobtanium
Again assuming that's measured in grams, that's 0.17 cm³ of Uranium, 1.496 cm³ of Carbon, and ???³ of Unobtanium per Redstone Dust
So what does this tell us about the chemical structure of Redstone? Basing this on Redstone Dust's composition, we can estimate that each Redstone molecule is composed of 3 Carbon atoms, 3 Uranium atoms, 4 Unobtanium atoms, a little under half of the time it binds to an extra Uranium and/or Carbon, and 20% of the time it binds to an extra Unobtanium
This also has some horrifying implications for how Redstone works:
Redstone would be extremely volatile as the radioactive decay from Unobtanium and Uranium would occasionally release Helium ions through alpha radiation, sometimes breaking apart Carbon into two Beryllium atoms (as it absorbs the extra proton and neutron from the Uranium) or merging into Oxygen
So Redstone should, in theory, be extremely flammable and potentially explosive, which implies that cave static, or the player mining Redstone with an Iron Pickaxe, could lead to a spark that causes an explosive cave-in
As Unobtanium is just a placeholder for unobtainable elements (hence the name), I'm going to estimate Unobtanium in this case as Unbinilium, the placeholder name for element 120
Why?
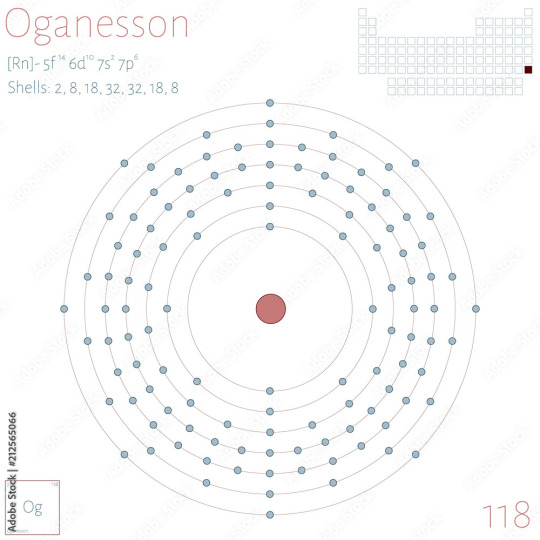
I'm estimating the Unobtanium as Redstone as being larger than the largest man-made element, Oganesson, which holds an impressive 118 protons
Each valence electron shell, from innermost to outermost, can bind with 2, 8, 18, 32, 32, 18, and 8 shells respectively, so I'd like Unobtanium to be an element we haven't discovered yet, and consequently I'd like to jump up to the next shell
While I could estimate with element 119's placeholder, Ununennium, it would have one electron in the next shell, so Unbinilium allows for easier chemical binding
So what does this molecule look like then? Well, horrifyingly...
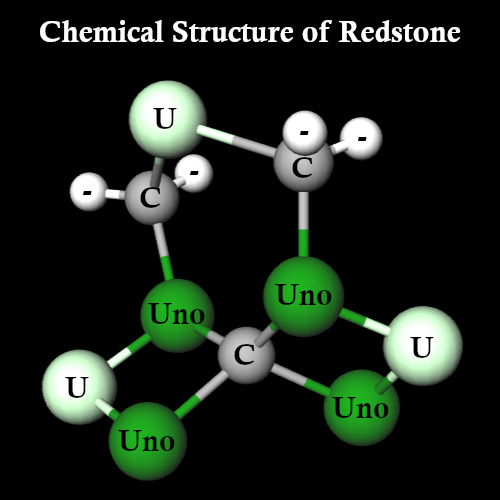
It looks like this. As Redstone forms in crystal lattices, and only two Carbon atoms are free to bind, I can absolutely see why it's so brittle that it breaks into powder.
This makes the structure of Redstone:
C3U3Uno4 (55% of molecules)
C4U3Uno4 (13% of molecules)
C3U4Uno4 (13% of molecules)
C4U4Uno4 (7% of molecules)
C3U3Uno5 (5% of molecules)
C4U3Uno5 (3% of molecules)
C3U4Uno5 (3% of molecules)
C4U4Uno5 (1% of molecules)
An extremely radioactive, flammable, and explosive compound.
#redstone#minecraft#chemistry#physics#education#i would love if someone could submit what a 2D representation of these molecules would look like#i don't actually know how to make them
3K notes
·
View notes
Text
A spectrum of neurodegenerative diseases, including frontotemporal dementia (FTD), progressive supranuclear palsy (PSP), and corticobasal degeneration (CBD) are due to the accumulation of abnormal, misfolded tau proteins in the brain.
A team of researchers has found potential ways to interrupt this process by targeting “sticky” sites along the long form of mutated tau, preventing the misfolding and spreading of the neurofibrillary tangles.
Continue Reading.
49 notes
·
View notes
Text
OKAY THIS ARTICLE IS SO COOL
I'm going to try to explain this in a comprehensible way, because honestly it's wild to wrap your head around even for me, who has a degree in chemistry. But bear with me.
Okay, so. Solids, right? They are rigid enough to hold their shape, but aside from that they are quite variable. Some solids are hard, others are soft, some are brittle or rubbery or malleable. So what determines these qualities? And what creates the rigid structure that makes a solid a solid? Most people would tell you that it depends on the atoms that make up the solid, and the bonds between those atoms. Rubber is flexible because of the polymers it's made of, steel is strong because of the metallic bonds between its atoms. And this applies to all solids. Or so everybody thought.
A paper published in the journal Nature has discovered that biological materials such as wood, fungi, cotton, hair, and anything else that can respond to the humidity in the environment may be composed of a new class of matter dubbed "hydration solids". That's because the rigidity and solidness of the materials doesn't actually come from the atoms and bonds, but from the water molecules hanging out in between.
So basically, try to imagine a hydration solid as a bunch of balloons taped together to form a giant cube, with the actual balloon part representing the atoms and bonds of the material, and the air filling the balloons as the water in the pores of the solid. What makes this "solid" cube shaped? It's not because of the rubber at all, but the air inside. If you took out all the air from inside the balloons, the structure wouldn't be able to hold its shape.
Ozger Sahin, one of the paper's authors, said
"When we take a walk in the woods, we think of the trees and plants around us as typical solids. This research shows that we should really think of those trees and plants as towers of water holding sugars and proteins in place. It's really water's world."
And the great thing about this discovery (and one of the reasons to support its validity) is that thinking about hydration solids this way makes the math so so so much easier. Before this, if you wanted to calculate how water interacts with organic matter, you would need advanced computer simulations. Now, there are simple equations that you can do in your head. Being able to calculate a material's properties using basic physics principles is a really big deal, because so far we have only been able to do that with gasses (PV=nRT anyone?). Expanding that to a group that encompasses 50-90% of the biological world around us is huge.
#science#stem#science side of tumblr#stemblr#biology#chemistry#scientists#biochemistry#studyblr#physics#nature
6K notes
·
View notes
