#what are negative charged ions called
Text
The Chemistry of Mineral Pigments

Nowadays, most artists need to give little thought to what goes into making their colors. Indeed, even if they did, most modern pigments are synthetically made, designed and mass produced in a laboratory. However, it was not too long ago that painters needed to be intimately familiar with the chemistry of their pigments, which came primarily from ground minerals mixed with a binding agent.
Many well known compounds that are used in paint making come from the transition metal elements (these ones:)
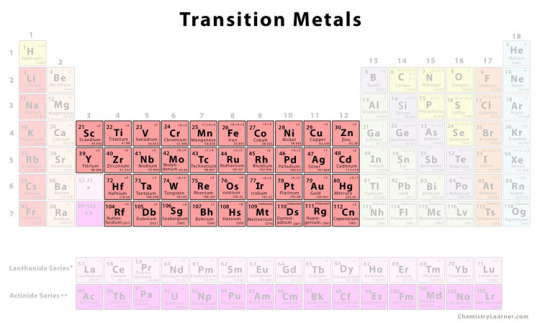
Some of these are instantly recognizable even to those who know little about chemistry- most artists know that cobalt is blue, iron is red, and titanium is white. Chromium can produce an entire rainbow of colors in different compounds. But how does this work?
When they exist in the crystalline structures used in pigments, transition metals are ions- they have a positive charge which is balanced out by the negative charge of their neighboring atoms, the oxygens, sulfurs, and the like. The electrons in a transition metal exist in specific levels called orbitals. Electrons can jump up to a higher orbital if they absorb energy of the exact wavelength as the gap between orbital energies.

In transition metals, these energy wavelengths often fall into the visible light spectrum. For example, oxidized copper's electrons absorb the red and orange light that hits them, so the color that gets reflected back into our eyes turns out to be a bluish green (the opposite color on the color wheel).

However, these gaps in energy levels can change based on the surrounding elements, the oxidation state of the transition metal (how much of a positive charge it has) and even how the different nonmetal ligands are arranged around the central transition metal element. For some elements, this doesn't make too much of a difference, but others like chromium can produce a wide variety of colored compounds depending on its structure. Chromium impurities are responsible for the color in many gemstones, such as the red in ruby, the green in emerald, and the blue in sapphire.

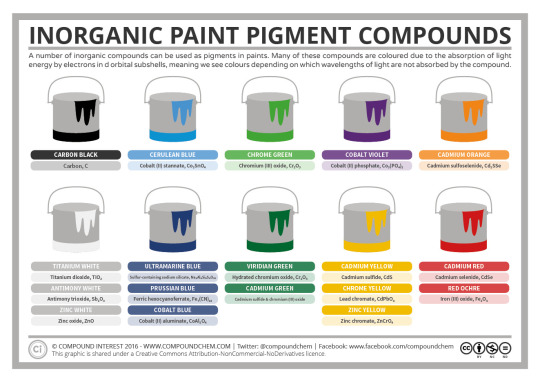
Here is a reference for the chemical compounds that go into creating some common colored pigments (note that the formula for lead chromate should actually be PbCrO4).
70 notes
·
View notes
Photo


Exotic water ice contributes to understanding of magnetic anomalies on Neptune and Uranus Ordinary everyday ice, like the ice produced by a fridge, is known to scientists as hexagonal ice (ice Ih), and is not the only crystalline phase of water. More than 20 different phases are possible. One of them, called “superionic ice” or “ice XVIII”, is of particular interest, among other reasons, because it is thought to make up a large part of Neptune and Uranus, planets frequently referred to as “ice giants”. In the superionic crystalline phase, water loses its molecular identity (H2O): negative oxygen ions (O2-) crystallize into an extensive lattice, and protons in the form of positive hydrogen ions (H+) form a liquid that floats around freely within the oxygen lattice. “The situation can be compared to a metal conductor such as copper, with the big difference that positive ions form the crystal lattice in the metal, and electrons bearing a negative charge are free to wander around the lattice,” said Maurice de Koning, a professor at the State University of Campinas’s Gleb Wataghin Physics Institute (IFGW-UNICAMP) in São Paulo state, Brazil. De Koning led the study that resulted in an article published in Proceedings of the National Academy of Sciences of the United States of America (PNAS) and featured on the cover of its November 8, 2022 issue. Superionic ice forms at extremely high temperatures in the range of 5,000 kelvins (4,700 °C) and pressure of around 340 gigapascals, or over 3.3 million times Earth’s standard atmospheric pressure, he explained. It is therefore impossible for stable superionic ice to exist on our planet. It can exist on Neptune and Uranus, however. In fact, scientists are confident that large amounts of ice XVIII lurk deep in their mantles, thanks to the pressure resulting from these giants’ huge gravitational fields, as confirmed by seismographic readings. “The electricity conducted by the protons through the oxygen lattice relates closely to the question of why the axis of the magnetic field doesn’t coincide with the rotation axis in these planets. They’re significantly misaligned, in fact,” De Koning said. Measurements made by the space probe Voyager 2, which flew by these distant planets on its journey to the edge of the Solar System and beyond, show that the axes of Neptune’s and Uranus’s magnetic fields form angles of 47 degrees and 59 degrees with their respective rotation axes. Experiments and simulations On Earth, an experiment reported in Nature in 2019 succeeded in producing a tiny amount of ice XVIII for 1 nanosecond (a billionth of a second), after which the material disintegrated. The researchers used laser-driven shock waves to compress and heat liquid water. According to the paper in Nature, six high-power laser beams were fired in a temporally tailored sequence to compress a thin water layer encapsulated between two diamond surfaces. The shock waves reverberated between the two stiff diamonds to achieve a homogeneous compression of the water layer resulting in the superionic crystalline phase for an extremely short time. “In this latest study, we didn’t perform a real physical experiment but used computer simulations to investigate the mechanical properties of ice XVIII and find out how its deformations influence the phenomena seen to occur on Neptune and Uranus,” De Koning said. A key aspect of the study was the deployment of density functional theory (DFT), a method derived from quantum mechanics and used in solid-state physics to resolve complex crystalline structures. “First of all, we investigated the mechanical behavior of a flawless phase, which doesn’t exist in the real world. We then added defects to see what kinds of macroscopic deformations resulted,” he explained. Crystal defects are typically point defects characterized by ion vacancies or intrusion of ions from other materials into the crystal lattice. Not so in this case. De Koning was referring to linear defects known as “dislocations”, which are due to angular differences between adjacent layers resulting in puckering somewhat like a rumpled rug. “In crystal physics, dislocation was postulated in 1934 but first observed experimentally in 1956. It’s a type of defect that explains a great many phenomena. We say dislocation is to metallurgy what DNA is to genetics,” De Koning said. In the case of superionic ice, the sum of dislocations produces shear, a macroscopic deformation familiar to mineralogists, metallurgists and engineers. “In our study, we calculated, among other things, how much it’s necessary to force the crystal for it to break up owing to shear,” De Konig said. To this end, the researchers had to consider a relatively large cell of the material with about 80,000 molecules. The calculations entailed extremely heavy and sophisticated computational techniques, including neural networks, machine learning, and the composition of various configurations based on DFT. “This was a most interesting aspect of the study, integrating knowledge in metallurgy, planetology, quantum mechanics and high-performance computing,” he said.
43 notes
·
View notes
Text
Meet the Chemiballs: The Metagaming Metalloids!
So you know metals? They’re metallic, conductive, and usually form positively charged ions? And you know nonmetals? They’re not metallic, insulators, and make negatively charged ions. So yeah, what do you call stuff that’s like, not one of those two things? Y’know, semiconductors and shit.
Metals are only metallic because they’re bad at holding on to their valence electrons, and elements tend to get worse at that the closer they are to the bottom left of the periodic table. Because physics.
But there’s a stair-shaped boundary line between the two where it’s really hard to decide if they’re one or the other so we sort of just gave up and called them metalloids.







READ MY OLD BLOG IT HAS JOKES AND FUN FACTS ABOUT ELEMENTS AAAAHHH
#chemiballs#science#Boronball#Siliconball#Germaniumball#Arsenicball#Antimonyball#Telluriumball#Poloniumball
5 notes
·
View notes
Text
past and present
Title: past and present
Author: Shiro (TeitoxAkashi [AO3]/ seijuurouxryuu [tumblr])
Rating: Gen
Pairing: Giotto & Tsuna
Event: @khrrarepairweek
Prompts: Monster Hunter AU | Ghosts
Tags/Warnings: No Archive Warnings Apply
Day 1: Storm Day
The thunder was loud, booming and deafening in the background. It was calming, it was unnerving. Because it was on these days that Tsuna could see the shadows of the past, the shadows of the dead.
AO3
The thunder was loud, booming and deafening in the background. It was calming, it was unnerving. Because it was on these days that Tsuna could see the shadows of the past, the shadows of the dead.
“Giotto-san…” He frowned at his ancestor, blurry frame solidifying with the lightning’s shine. Akin to charged light bulb. Something something about how the positive and negative ions become disarray during a thunderstorm.
The dead was hovering by the window, staring out silently. At Tsuna’s call, he looked back and blinked. In a way that reminded Tsuna of a lizard. Again, unnerving.
“Yes, child?”
The almost thirty-four-year-old man twitched at the ‘endearment’. He decided to ignore it seeing how ancient Giotto was and instead asked, “Are you alright?”
Giotto blinked again.
“Why wouldn’t I be?”
“Well, you… You look,” Tsuna paused, thinking of a word to describe the ex-mafia boss. “Burnout.” Tired. Lost. Dead. Figuratively and literally.
Giotto smiled. “I am, aren’t I?” He murmured and look out of the window again. It was a foreign yet familiar sight to see—it was after all the town he vowed to protect forever, the town he grew up and bleed for. It was the island that chained his very soul to, to be forgotten by all but his descendants and the dark side they chose to join.
He was persistent, he was filled with fire and will to fight for them. He was young, then, like the only child who could see him and hear him. He was more willful too. But he was older soon and the fire slowly was doused with tears and blood, burning slower and quieter. His bones grew colder at the harsh reality he had faced and soon, he could no longer burn the will.
Ricardo—his brother who rage brighter than he burned with hope—took his continued dimming as the ending of legality and brought them into the world of darkness. He then reigned Vongola with growing glory, burning brighter and stronger than Giotto, bringing Vongola its peak.
And the part the fully douse Giotto’s flame was how Ricardo protected their home better than Giotto despite drowning the walls with more blood of their enemies.
Giotto decided to leave the limelight and take Alaude and Asari’s hands to move to the land of rising sun to warm his bones and blood once more.
Sawada Ieyasu was the name Asari gave—peaceful family. A representative—a reminder—of Giotto’s vows. A reassurance that Giotto had brought peace to his family—them.
And it was a name that he never really believed that it suited him despite what they said.
“How are you doing?” He asked instead, returning Tsuna the question. Tsuna shrugged as he walked closer, leaning against the wall as he weakly laughed at himself.
“As well as I can ever be.” He sighed. “I’m just tired of dealing with all the chaos that Xanxus and Reborn caused. I just want to sleep…”
Giotto smiled, understanding the feeling because he had his fair share of continuously trying to reign in Ricardo and Lampo from whatever fights or chaos they were up to. It wasn’t fun, but he wouldn’t have them any other way. Even if it almost always ended up with him bedridden in exhaustion. Even then, it was probably the most fun he had.
Now that he thought about it, he had the time of his life whenever he was with his family, be it good or bad. And even if he had gone back to that time, he wouldn’t want to change anything except for the incidents involving Cozarto and Elena.
Ahh, how he missed the carefree times he was with his family.
Looking at how Tsuna was, he was sure that the young boy felt the same. Only, he would burn brighter. More so than how Giotto once did. He would fight for his family, and more importantly, he would fight for himself. He would survive better than Giotto.
And Giotto want that to happen.
He smiled and leaned back; His frame more relaxed. “Could you tell me about them? Your family.”
Tsuna blinked and grinned. “Yes. In return, tell me about yours!”
-----------
Notes:
Honestly I churned this out in an hour or so time, and separately in another day for the ending. I had three drafts for the ending but none seemed to fit so,,, I could only do like this. The plunny for this died halfway and I can't remember what I planned so rip.
But still!!! First day of RPW!! I'm late but yay!! :DD
Just them bonding honestly. I think that Giotto would linger around Vongola mansion, hovering here and there to reminisce his past by himself. The rest of the primo gen are probably somewhere chillin but Giotto was haunted by his own past i think. Daemon too. Both of them are just. Suffering. I didn't write Daemon because I think whatever he does, he wants it private while Giotto? Giotto doesn't care who sees him. He just stuck in the abyss [vtuber joke]
Anywaysssssss,,,, I'm late by so many days so im gonna go ahead and post the other days one first CIAO
[I apologize for any grammar, spellings, etc. etc. mistakes]
14 notes
·
View notes
Text

Alfred Nobel stipulated that his annual prizes be awarded to those who “have conferred the greatest benefit to humankind”. Few scientific advances have had a greater impact on our lives than that made by the American materials chemist John Goodenough, a chemistry Nobel laureate in 2019 for his role in inventing the rechargeable lithium battery.
If you are reading this on a handheld device, it will almost certainly have a lithium battery inside. These power packs have been instrumental to the advent of electric cars, and their ability to store power such as that generated by ephemeral renewable sources could aid the transition away from a fossil-fuel energy economy.
For year after year Goodenough, who has died aged 100, featured in the list of Nobel predictions. Only his remarkable longevity saved the Swedish committee from an embarrassing injustice – he is the oldest person to have been awarded a Nobel. He seemed phlegmatic about being repeatedly overlooked, even though he did not enjoy any financial reward for his breakthrough either: in the 1980s he was not encouraged to take out a patent on the battery breakthrough he made at Oxford University. He was glad enough still to be able to do research, which he sustained almost until the very end of his life.
He left Oxford in 1986 for the University of Texas at Austin to escape compulsory retirement at 65, convinced – rightly – that he had a lot more still to offer. “Why would anyone retire and simply wait to die?” he asked. His vitality and enjoyment in the lab well into his 90s, punctuated by his loud and high-pitched laugh, was a constant cause of amazement.
One would hardly have guessed from that demeanour how unhappy his childhood had been, as the second of three children of extremely distant parents in what he called “a disaster” of a marriage. He was born in the city of Jena, Germany, to Helen (nee Lewis) and Erwin Goodenough.
They were both Americans who were living in Oxford – Erwin was studying for a DPhil at the university and, according to his son, “enjoyed the culture of the Weimar Republic; he spent much of his long summer vacations in Germany as well as in Rome”.
John was taken as a baby to the US, where his father became a professor of religious history at Yale University. John grew up mostly in a boarding school in Massachusetts, from where, despite being an undiagnosed dyslexic, he won a place to study mathematics at Yale. After wartime military service as a meteorologist, he gained a doctorate in physics at the University of Chicago and in 1952 began research on magnetic materials for information storage at the Massachusetts Institute of Technology.
That work qualified him to switch to inorganic materials chemistry when in 1976 he moved to Oxford. At that time, interest was growing in electric vehicles, which were being held back by the lack of suitable batteries.
The potential benefits of electric cars as quieter and less polluting than those using the petrol-fired internal combustion engine had been recognised since their inception. But the lead-acid batteries used as starter batteries and the power source for vehicle electronics were utterly unequal to the task of supplying the motive power: they were too heavy and offered too little power.
The dream of battery-powered cars was resurrected in the 60s, but it was only a decade later, with the Opec oil crisis in full swing, that the industry took them seriously.
The key was to find the right materials for the battery electrodes. Lithium metal looked attractive because it is lightweight and capable of delivering high voltages. The idea was that lithium at the positive electrode would provide electrically charged ions that travel to the negative electrode, where they could be trapped between the layers of atoms in materials called intercalators.
The British chemist Stanley Whittingham, one of Goodenough’s co-laureates, working at the Exxon laboratories in New Jersey, found a suitable intercalator called titanium disulfide in 1976. Four years later, Goodenough in Oxford identified the material – a form of cobalt oxide – that became the industry standard, offering a higher voltage and greater power density.
Early lithium batteries had a tendency to catch fire because of the high chemical reactivity of pure lithium. But the third 2019 laureate, the Japanese researcher Akira Yoshino, of the Asahi Kasei Corporation in Tokyo, replaced lithium electrodes with graphite-like carbon made from petroleum coke, which also intercalates lithium so that the ions merely shuttle back and forth between the two sets of layers, making them easily rechargeable.
The lithium-ion battery was commercialised in 1991 by the Sony Corporation, and now commands an estimated $92bn market. Without it there could have been none of today’s handheld electronics – laptops, smartphones, tablets. Elon Musk’s Tesla electric cars depend on them.
There is still room for improvement and Goodenough never stopped seeking it. In the past decade he was working, among other things, on making batteries that operate at low temperatures, suitable for powering cars in the winter.
He was also seeking a new, safer way to reinstate pure lithium electrodes, which could give lithium batteries more energy capacity. At the same time, he expressed concerns about the international tensions that might arise over the limited global supplies of lithium.
Goodenough maintained a strong Christian belief throughout his life, seeing no conflict with his scientific work. “The scientist is trying to do something for society and for his fellow man,” he said. “In that sense why should there be a conflict?” During his 90s he cared for his wife, Irene (nee Wiseman), who had Alzheimer’s disease. They had married in 1951; she died in 2016.
“I’d like to get all the gas emissions off the highways of the world”, Goodenough said in 2018. “I’m hoping to see it before I die.” It was always an ambitious aspiration, even for someone with his staying power. But if it happens one day, Goodenough will have played a central part in that.
🔔 John Bannister Goodenough, materials scientist, born 25 July 1922; died 25 June 2023
Daily inspiration. Discover more photos at http://justforbooks.tumblr.com
17 notes
·
View notes
Note
Any fun facts about sodium? (Can sodium be fun???) Also, what got you interested in chemistry?
Oh you shouldn’t have asked me about sodium. I’m fucking FERAL about sodium.
Sodium is a member of the Alkali Metals on the periodic table, and a common theme with these metals is that they fucking explode when in contact with water. One of my first experiences in a chemistry class was watching a piece of sodium spinning rapidly in water, catching fire, and detonating before my gleeful gaze.
This is because sodium has a single electron in its outer shell, and it does not give a single shit about holding onto this electron. It will get rid of it by giving that thing to literally whatever might maaayyyybe want it, so it’s going to violently react with anything.
“But teet,” I hear you asking, “Why is table salt so chill then?” And that’s because that is not neutral sodium. Sodium chloride (aka table salt) is what occurs when sodium was already able to give away it’s electron to something that wanted it (the chloride). Held together by electrostatic forces, the newly stable sodium ions forms a lattice with the chloride ions to create salt crystals. It’s safe to consume and pretty inert for the most part.
Because sodium loves giving away it’s outer electron, it’s really good at creating strong bases, as whatever it attaches to is gonna be negatively charged and isn’t gonna be super hindered by the sodium if it’s in something like water. You’ve probably used some of these in your life, like maybe sodium hypochlorite (bleach) or sodium bicarbonate (baking soda). It also can be used for organic chemistry as another reducing agent, sodium borohydride (NaBH4), which is very nice to use :).
However, if we want to have some fun, let’s get into using sodium’s bases for making polymers. Polymers are long chains or networks of repeating patterns of simple molecules, called monomers. Your clothes, water bottles, and even your cells contain polymer ingredients, so these are definitely important. One way to make polymers is to use a base like, say, sodium amide (NaNH2). The negative NH2- ion is going to be reactive enough so that it can immediately start building chains with monomers, and if you’re particularly careful, the negatively charged chain can be prevented from terminating, so that any time you want you can just add more monomer and make it grow even longer. These are called “living” polymers and they’re incredibly cool!
So those are a few applications of sodium that I just think are really neat, but with regards to your last question, I think it goes back to when I was a kid and I just thought it was cool seeing what chemicals could do (especially the explosions lol). Back then I told everyone I wanted to be a mad scientist, but what I meant was a chemist. That dream still lives on, but I’ve found that my talents lie especially in mathematics and physics, so I’ve been focusing a lot more on the physical chemistry side of things lately.
3 notes
·
View notes
Text
Stabilizing the Element
or Even our atoms need each other
When atoms are far apart, they attract each other. This attraction is stronger for some kinds of atoms than others. At the same time, the heat, or kinetic energy, of atoms makes them always move. If the attraction is strong enough, relative to the amount of heat, atoms will form a solid. If the attraction is weaker, they will form a liquid, and if it is even weaker, they will form a gas. (Wikipedia, 6/26/2023)
It's the hot Central Valley summer of the second long distance, the second hand ticking away uncountable minutes, the seconding of everyone in my orbit that I am not quite interesting enough to really bond with. No one said it first, but I am pitifully made aware that I cannot be created nor destroyed, only transmuted transfixed and transformed, the ever unstable element on the table for discussion.
Chemical bonds are the strongest kinds of attraction between atoms. The movement of electrons explains all chemical bonds. Atoms usually bond with each other in a way that fills or empties their outer electron shell. The most reactive elements have an almost full or almost empty outer shell. Atoms with a full outer shell, called noble gases, do not usually form bonds. (Wikipedia, 6/26/2023)
I do not understand my parents, well off and independent of one another. How do you breathe the same oxygen every night and remain unmoved? I cannot help but react and I reach, desperate to fill myself, desperate to fill others, to give and receive. But in this hot Central Valley summer I grasp only air.
There are three main kinds of bonds: ionic bonds, covalent bonds, and metallic bonds.
In an ionic bond, one atom gives electrons to another atom. Each atom becomes an ion: an atom or group of atoms with a positive or negative charge. The positive ion (which has lost electrons) is called a cation; it is usually a metal. The negative ion (which has gained electrons) is called an anion; it is usually a nonmetal. Ionic bonding usually results in a regular network, or crystal, of ions held together. (Wikipedia, 6/26/2023)
I had forgotten what it was like to live on the affluent side of town, to not see people starving, to not have people all around who are unstable, reactive. Knowing they are still here, still reacting, only kept out of this sterile lab environment through aggressive sanitizing of unwanted elements to avoid cross contamination, I am not comforted by comforts. I am lonely.
In a covalent bond, two atoms share electrons. This usually happens when both atoms are nonmetals. Covalent bonds often form molecules, ranging in size from two atoms to many more. They can also form large networks, such as glass or graphite. The number of bonds that an atom makes (its valency) is usually the number of electrons needed to fill its outer electron shell. (Wikipedia, 6/26/2023)
Board games and conversations on the street corner spread conversations far and wide, and everything belongs to everyone on that street corner for the day. I meet a man who asks me if I want a cigarette while beating me thoroughly at chess, a woman who has a little dog wearing a sweater in better condition than her own, a guy who watches each tournament and pulls at his sleeves, someone is writing resources on the community board.
In a metallic bond, electrons travel freely between many metal atoms. Any number of atoms can bond this way. Metals conduct electric current because electric charge can easily flow through them. Atoms in metals can move past each other, so it is easy to bend, stretch, and change the shape of metals. (Wikipedia, 6/26/2023)
Remember the weeks of watching for news of Seattle's community? Of parsing through hyperbole and lies for glimmers of a people committed to radical restructuring? Remember what it felt like to wonder if this was the beginning of something significant? I remember changing and stretching into something new, something I thought was stable but could not have been more reactive. Maybe that isn't a bad thing.
#science#chemistry#english literature#poetry#free verse#original poem#poetic#writing#creative writing#elements#electrons#leftist#love poem#actually bipolar#bipolardisorder#wrote in my biology lecture
4 notes
·
View notes
Text
What is Electricity?
[The following is a rough script for a student-led presentation to my HVAC classmates, who are currently building and testing basic AC circuits with no formal introduction to the fundamental concepts of electricity and energy. Like, line voltage. I wish I was joking.]
Electricity is all around us. It charges our phones, amplifies our voices, heats our homes, and allows us to communicate from vast distances. It is humanity's greatest discovery. Greater than fire, greater than the internal combustion engine. Greater than nuclear fission. Nothing in this room would exist without it.
For almost two weeks, now, we have been building circuits for electricity to pass through and do work for us. But do you know what this invisible force even is? Today, we are going to change that.
We will start small, at the atomic level. This is the basic atomic model you may have seen before. We have a nucleus, made of neutron and proton particles, and orbitals around the nucleus occupied by electron particles. The neutron has a neutral charge, the proton has a positive charge, and the electron has a negative charge.
This is the atomic model for copper, the most common conductor used in electrical circuits. It is a kind of metal. We use copper over other conductive metals because it is inexpensive and is good enough for general use. Copper conducts electricity so well because of this guy. The valence electron. The valence shell is the outermost orbital of any given atom, and in copper, there is just one.
The fewer valence electrons there are, the more conductive an element is [simplification, necessary]. There can be up to eight electrons in an atom's valence shell [simplification].
This is a copper wire. There are two kinds: solid core and stranded core. When the wire isn't connected to anything, those valence electrons are idle, remaining with their respective atoms [simplification]. But when we apply a voltage across the wire, those electrons are moved from negative to positive along the wire simultaneously. This movement of electrons is what we call current, and is measured in amperage, or amps, symbolized by "I".
Let's step back for a moment. I mentioned voltage, but what exactly is it? Voltage is the difference in electrostatic potential energy between two points [simplification]. Voltage can be produced by chemicals and plates in a battery, or can be produced by a generator. I will go into detail on generators later. For now, let us examine a battery.
The standard battery in your car is a 12v lead-acid battery. The voltage between the positive and negative terminals is 12.6v in a healthy battery. Inside the battery are lead plates connected like so, and immersed in an acidic liquid we call electrolyte. There are 6 cells in this battery, and each has an individual voltage of 2.1v. Together in series, they produce 12.6v across the terminals. When we charge the battery, all we are doing is moving electrons from one side of the battery to the other with an external circuit. When the battery is charged, we will have a collection of negatively charged atoms on the negative plates, and positively charged plates on the positive plates.
[Sidebar: When an atom looses an electron, it becomes a positive ion, because the total charge balance between the protons and electrons has been skewed so that there are more protons than there are electrons. The opposite is true when an atom takes on an electron; it becomes a negative ion.]
The electrons of the negative ions are compelled to move over to fill the vacancy of the positive ions. This compulsion is our voltage. It's a potency of opposing ions in this battery.
When we complete a circuit, such as this one, we provide a path from those electrons to move and equalize the potential difference. When they recombine with the positive ions on the positive side, the battery loses voltage; the potential is reduced and the battery will be depleted. This is why your phones and your cars need to be charged regularly; to reset this potential.
What I just described is a DC, or direct-current, circuit. The electrons moved in only one direction. But we have been building AC circuits. What is an AC circuit? AC stands for alternating-current. AC will reverse the direction of electron flow periodically, and this rate of this change in flow direction is called frequency. In the US, our outlets output 60hz (hz, or Hertz, is the unit of frequency), or 60 direction changes per second. This is how we represent AC on a graph. This is the wave model. If we measure a wave like this, we can calculate its frequency by the time between the two nearest positive zero-crossings. It could be any point on the wave, in truth, so long as they are the same.
If we could see the electrons moving in the wire, we would see them move in one direction before slowing down and then moving in the other direction, like a train. [simplification, we really don't need to get into transmission line theory or wave theory].
How do we produce AC? We don't get it from a battery, but it's available at any given outlet in this room. To answer this, we will have to explore the fascinating realm of electromagnetism.
A magnet, like this one, is what we call a permanent magnet. It is iron that has been polarized through exposure to an magnetic field. If I place it on this steel frame, we see that it sticks to it when it gets close enough. This is possible because iron, cobalt, and nickel are elements that are magnetically permeable. Aluminum is not, for example, and cannot be influenced by magnetism. This property-based magnetism is what we call ferromagnetism. It's not important to know that term, though [perhaps cut this part out].
So, we can magnetize iron, but how was that accomplished? Through electromagnetism. This is a wire, just like I showed you before. When we apply a voltage across it and current flows, something else happens: a small magnetic field is generated perpendicular to the flow of the electrons in the wire. The direction of this field depends on the direction of current flow. The direction doesn't matter for this demonstration, but know that it can change.
This small field is useless to us in its current state, but if we coil the wire around in close proximity, such at this, those small fields combine to create a larger, more powerful magnetic field. What we've just created is an electromagnet. We have turned electrostatic potential energy into magnetic potential energy. This field can do many different things for us, and we will explore them in the next lecture about circuit components.
There is a reverse side of electromagnetism, though. If we pass a magnetic field, either from a permanent magnet or an electromagnet coil, over a wire, a current is induced in the wire. The passing of the magnetic field over the wire causes electrons to move in the wire. Again, in this current configuration, the effect is relatively weak. If we want to extract more current from this effect, we need to coil the wire. Now, we have more of the wire being exposed to the field at once, and this induces a stronger voltage and current in the wire [simplification].
"magnetism is seen whenever electrically charged particles are in motion—for example, from movement of electrons in an electric current, or in certain cases from the orbital motion of electrons around an atom's nucleus."
This means that electricity and magnetism are always present, together. We cannot have current flow with magnetic field generation, and vice versa. To dig any deeper into it is the realm of physics. But magnetism is not magic, it is an understood property of particles in motion.
So, stepping back, how do we get AC electricity? This is the job of powerplants. Have any of you heard the phrase "energy cannot be created nor destroyed"? Its meaning is literal. I cannot summon energy from nothing. Energy exists in many states and is always being converted between them. There is kinetic energy, which is the movement of atoms; this includes temperature--heat--and my arm moving to throw this marker.
There is gravitational potential energy (GPE), which results from the force of gravity. If I stand on a beach, I have a certain GPE. If I stand on the top of a cliff over the beach, I have more GPE than if I were down on the beach. Just like EsPE, I am the electron on the negatively charged battery plate, and the beach down below is the positive plate. The circuit is the air and all the trees and rocks I hit on the long fall down.
There is chemical potential energy (CPE), this is the energy that is stored in the bonds in, and between, elements. Calories is the unit of CPE in food, for example. It is the amount of energy stored in the food we eat.
There is elastic potential energy (ElPE), which is the energy stored in a spring or your tendons, for example, when stretched or compressed.
There is magnetic potential energy (MPE), which is the energy stored is magnetic fields.
There is electrostatic potential energy (EsPE), which we just covered.
And lastly, there is nuclear potential energy (NPE) which is the energy in the bonds between the particles in the nucleus of the atom.
All this energy potential can be used to perform work; it can be converted to kinetic energy through machinery. In Puget Sound, we get most of out energy from natural gas powerplants and hydroelectic dams. To the east, there are large wind and solar farms, and to the south, there is a single coal powerplant.
But these are just the names for the buildings. What's happening inside them that gives us this power? Let us use the hydroelectric powerplant as our first example. A dam is a structure that obstructs the flow of water in a river to create a reservoir. The reservoir water has a higher GPE than the outflowing water, and is compelled to fall to reach a lower energy state. To convert that GPE to EsPE, we need some kind of machine. This is the job of the generator and turbine. The water is piped down into a turbine, which has dozens or hundreds of blades that the falling water turns. The blades rotate a shaft, which is attached to a magnet. This magnet spins around coils of wire arranged like so. This is why you see power lines in sets of three; they are the output of a generator like this.
After the water passes through the turbine, it is then exhausted to the river below, having provided as much energy conversion as we could extract. So, we took GPE in the water, converted it to kinetic energy with the turbine, and then turned the kinetic energy to EsPE with the spinning magnet. Isn't that fascinating?
Let's look at a natural gas plant. We take methane, CPE, burn it, which converts the CPE to kinetic energy, so that it boils water to create steam. This is still kinetic energy. Latent heat. Then, we pipe that steam through a turbine to do the exact same thing as the hydropower plant: to spin a magnet over coils of wire. The coal plant works exactly the same. The high pressure generated from the boiling of water is compelled to flow to a low-pressure area; the exhaust to the outside, somewhere. And on its way, we have it spin a turbine.
In fact, a nuclear powerplant does the same thing, as well. Through fission reaction, NPE is converted to kinetic energy as heat to boil water, rinse and repeat.
Okay, let's look at solar. Solar power introduces some nuance into our energy model because sunlight is an electomagentic wave. It's a compound form of energy that is both electrostatic and magnetic. Specifically, we are harnessing the EMPE in a particle called the photon. The photon strikes the solar panel, which is made up of photovoltaic cells, and its energy is given up to the circuit inside. The insides of a solar panel are too complex for this lecture, but that photon's energy gets converted to EsPE. But unlike the turbine generators previously described, the current is DC, not AC. To turn DC to AC, we must first pass it through an inverter. Not going to describe how it works here, but know that it converts DC to AC for use on the grid.
Lastly, let us look at wind. Kinetic energy in the air as wind is used to rotate the turbine which are the windmill blades. The blades are connected to a shaft which is connected to a magnet which rotates around three packs of coiled wire (there is a gearbox in there as well, but that's not necessary to describe). Boom! Three-phase AC power from the sky.
As you can see, there are many ways to produce AC. Through other electrical infrastructure, such as transmission towers and substations, the AC power makes it wherever it is needed, including this classroom. All by harnessing energy in its various forms.
This concludes our introduction to electricity. I hope you will join me later for the introduction to schematics and circuit components; the devices that allow us to harness electricity to perform work.
Any questions?
4 notes
·
View notes
Text
The Hunt to solve the chemical mysteries
Imagine one morning you wake up and find yourself in a cold freezing and snowy environment and it seems like your in one of the movies of Harry Potter. The next moment a woman comes up to you and asks “ciamar a tha thu?” before you recover from your amazement a boy appears out of a snowman and goes like “Madainn Mhath”.
Well before this article turns into a language guide let me tell you what language was being spoken and where you were. Ok, so you were in Britan and you just heard Gaelic Scottish.
You might have been perplexed for a moment, well that’s ok .Fun fact ,Chemistry i.e. a branch of science will leave you in the same state of confusion if you aren’t well-versed with ‘Chemstrian’.
Yes ,you read that right ,Chemstrian is what people call the unique language of Chemistry that is a whole system of speaking and writing that’s unique to the study of chemicals. An idiosyncratic way of translating numbers and chemical formula into words, that can be understood and spoken.
Well, Chemstrian might seem difficult but by the time you finish this exciting journey of this article, I assure you that you’ll start loving chemistry and be one of its native speakers.
This article is a guide which will be the map for the hunt of a special treasure whose clues are hidden in the Periodic table which is our phrase book.By the end of this article I wouldn’t expect you to walk out and go around spouting off ‘three-keto-two-carboxy- arabinitol' but I would surely help you set off in the right way.
Ay-Ay…let’s begin our hunt!
Our stepping stone to the unique treasure of chemistrian is that the rules for describing an element changes with its place in the Periodic table.
The guiding star for this treasure is that these rules not only apply to their formulas that is chemical symbols we use in the equation but also to their names.
For example…Ions become positively or negatively charged on the basis of gaining or losing electrons
Cations are positively charged ions and anions are negatively charged ions .Which are going to be the special kings of whom we’ll be learning today and by praising them we will be able to find our treasure. Cause we might not want to face opposition from these powerfully charged kingdoms(Ions).
REMINDER: DO NOT GET BORED YOU’RE HALF THE WAY FOR THE TREASURE
Here's a clue ,
When it is a cation we add the suffix ION For example- sodium Ion (Na±)
When it is an anion we add the sufffix IDE For example- Cl– = chloride
To make this trip more exciting..let me get it to your notice that we say the cation first then the anion for eg, Sodium chloride (sodium- cation & Chloride- anion) Isn’t that amazing? For sure the anions must be jealous of cations right? What do you think?
Ok, so here’s our final key for opening the treasure, that is to know which element is what.Here comes the periodic table to the rescue!
The first two columns on the left are your Alkali and Alkaline earth metals..well that was pretty much of fancy names right? Ok back to topic, so the Alkali and Alkaline earth metals form ions readily when they battle against(react) the non metals and when they have the war they lose their soldiers that is electrons and form the cations, and on the other side of the table we'll mostly find anion forming elements. That’s How the kingdom of Cation and Anion was formed . Well, all that praising work paid off.
Here you go! You’ve just mastered the basic concepts of chemistry! You’ve also earned yourself a great treasure. Welcome aboard !to this amazing group of chemistrian native speakers, in order to enjoy future trips with our fellow passengers,
Keep reading chemistry and solve the life’s mystery!
Happy reading!
Aishwarya Upadhye☺
Manchi,Udupi
3 notes
·
View notes
Text
Big Think: How plants can perform feats of quantum mechanics
It is spring now in the Northern Hemisphere, and the world has greened around us. Outside my window, trees are filled with leaves that act as miniature factories, collecting sunlight and converting it into food. We know this basic transaction takes place, but how does photosynthesis really happen?
During photosynthesis, plants utilize quantum mechanical processes. In an attempt to understand how plants do this, scientists at the University of Chicago recently modeled the workings of leaves at the molecular level. They were blown away by what they saw. It turns out that plants act like a strange, fifth state of matter known as a Bose-Einstein condensate. Even stranger is that these condensates are typically found at temperatures near absolute zero. The fact that they are all around us on a normal, temperate spring day is a real surprise.
States of low energy
The three most common states of matter are solid, liquid, and gas. When either pressure or heat is added or removed, a material can shift between these states. We often hear that plasma is the fourth state of matter. In a plasma, atoms break down into a soup of positively charged ions and negatively charged electrons. This typically occurs when a material is super-heated. The Sun, for example, is mostly a big ball of super-hot plasma.
If matter can be superheated, it can also be supercooled, causing particles to fall into very low energy states. Understanding what happens next requires some knowledge of particle physics.
There are two main types of particles, bosons, and fermions, and what differentiates them is a property called spin — a weird, quantum-mechanical characteristic that relates to the particle’s angular momentum. Bosons are particles with integer spin (0, 1, 2, etc), while fermions have a half-integer spin (1/2, 3/2, etc). This property is described by the spin-statistics theorem, and it means that if you swap two bosons, you will retain the same wave function. You cannot do the same for fermions.
In a Bose-Einstein condensate, the bosons within a material have such low energy that they all occupy the same state, acting as a single particle. This allows quantum properties to be seen on a macroscopic scale. A Bose-Einstein condensate was created in a lab for the first time in 1995, at a temperature of a mere 170 nanokelvin.
Quantum Photosynthesis
Now, let’s look at what happens in a typical leaf during photosynthesis.
Plants need three basic ingredients to make their own food — carbon dioxide, water, and light. A pigment called chlorophyll absorbs energy from light at red and blue wavelengths. It reflects light at other wavelengths, which makes the plant look green.
At a molecular level, things get even more interesting. Absorbed light excites an electron within a chromophore, the part of a molecule that determines its reflection or absorption of light. This kicks off a series of chain reactions that end up producing sugars for the plant. Using computer modeling, the researchers at the University of Chicago examined what occurs in green sulfur bacteria, a photosynthetic microbe.
Light excites an electron. Now the electron and the empty space it left behind, called a hole, act together as a boson. This electron-hole pair is called an exciton. The exciton travels to deliver energy to another location, where sugars are created for the organism.
“Chromophores … can pass energy between them in the form of excitons to a reaction center where energy can be used, kind of like a group of people passing a ball to a goal,” Anna Schouten, the study’s lead author, explained to Big Think.
The scientists discovered that the paths of the excitons within localized areas resembled those seen within an exciton condensate — a Bose-Einstein condensate made of excitons. The challenge with exciton condensates is that the electrons and ions tend to recombine quickly. Once this happens the exciton vanishes, often before a condensate can form.
These condensates are remarkably difficult to create in the lab, yet here they were, right in front of the scientists’ eyes, in a messy organism at room temperature. By forming a condensate, the excitons formed one single quantum state. In essence, they were acting like a single particle. This forms a superfluid — a fluid with zero viscosity and zero friction — allowing energy to flow freely between chromophores.
Their results were published in PRX Energy.
Messy Conditions
Excitons normally decay quickly, and when they do, they can no longer transfer energy. To give them a longer lifetime, they typically need to be very cold. In fact, exciton condensates have never been seen above temperatures of 100 Kelvin, which is a frosty negative-173 degrees Celsius. This is why it is so surprising to see this behavior in a messy, real-world system at normal temperatures.
So what’s going on here? Just another way that nature is constantly surprising us.
“Photosynthesis works at normal temperatures because nature has to work at normal temperatures in order to survive, so the process evolved to do that,” says Schouten.
In the future, room-temperature Bose-Einstein condensates may have practical applications. Since they act as a single atom, Bose-Einstein condensates may give us insight into quantum properties that would be difficult to observe at the atomic level. They also have applications for gyroscopes, atom lasers, high-precision sensors of time, gravity, or magnetism, and higher levels of energy efficiency and transfer.
2 notes
·
View notes
Note
I saw your posts and to be honest, you’re spreading misinformation about the body neutrality movement because you do NOT understand it fully.
Body neutrality isn’t “fat people exist”. Body neutrality is nothing but the core belief that our bodies are just bodies and that’s alright. There is no type of body that’s superior. No hierarchy. Looks shouldn’t play a role in how we experience the world. Body positivity, on the other hand, is still all about validation of different body types. It’s still about fitting in this harmful mindset driven by capitalism that you have to be considered pretty by society to deserve respect.
Body neutrality is about seeing people for their character and appreciating their bodies for keeping them alive. As a physical disabled person who saw their self and their community getting harmed by that (how body positivity reinforces the need to be considered attractive), it’s quite sad to see you talking down on body neutrality without even understanding it.
Wrong. Thanks for playing!!!!
Body positivity is not driven by capitalism, it was co-opted by capitalism and you let capitalism create the narrative. You let capitalism steal a body movement from fat people and decided that the problem was the movement, not the way capitalism reframed it. Like its meaning, its true meaning belongs with the bullshit capitalism ascribed to it and not with the fat people who pioneered it for decades prior. Good job!!! You know what I call that????? Fatphobic.
Body positivity is not about being pretty, it's about respect and the right to exist free of shame. I'll say it again slower this time but...
You cannot fight negativity with neutrality.
Let's take your definition of body neutrality that "bodies are just bodies and that's alright."
"Your body is too fat!!!"
"My body is a body and that's alright"
Does the body neutral statement contradict the fatphobic statement? No? Huh, that's weird. Since you brought up disability (by the way... I'm also multiply physically disabled!), let's try that!
"Your body is supposed to walk!!"
"My body is a body and that's alright"
Does the body neutral statement contradict the ableist statement? No? Huh, that's weird. Let's try body positivity for shits and giggles.
"Your body is too fat!!!"
"Fat is not a bad thing deserving of disrespect"
Well, that kinda sounds better... Let's try disability....
"Your body is supposed to walk!!"
"Disability is not a bad thing deserving of disrespect"
Wow. If we define body positivity as fat people did from the 1970's-the 2010's, it becomes the only actual helpful solution here!!!
See, body neutrality doesn't fight harmful societal narratives. It helps individuals get good at ignoring the harmful societal narratives. It's like an ion... An ion with a lot of negatively charged particles (electrons) and neutrally charged particles (neutrons) is still going to hold a negative charge. If you add positively charged particles (protons), you have a neutral charge.
If the message society sends is "bodies should be xyz way to receive respect" and your counter is "bodies are bodies and that's alright," you don't counter that. You have to fight for the right to receive respect. "Bodies deserve respect because there is no wrong way to have a body, so all bodies are correct" takes a positive stance.
I have seen a lot people define their "body neutrality" by this statement, but again this is how fat people defined body positivity for almost 50 years before capitalism twisted it (which btw they could only have done by the fact that thin people had already taken it and twisted its meaning). So if that's how you define "body neutrality," you took something from fat people, mangled it and twisted it, fucked it up, recreated it, and then said that somehow it was better than what fat people did. Fatphobic.
I completely understand body neutrality. I just also understand body positivity, which is a piece that you, unfortunately, are missing.
It’s quite sad to see you talking down on body positivity without even understanding it.
2 notes
·
View notes
Photo

SPORT and petitSat CubeSats to shed light on space weather disturbances Two CubeSats, or small satellites, are on a quest to provide insight on space weather disturbances and the subsequent impact on communication signals. The dynamic duo, the Plasma Enhancements in the Ionosphere-Thermosphere Satellite (petitSat) and Scintillation Prediction Observations Research Task (SPORT), arrived at the International Space Station on Nov. 27, 2022, as part of SpaceX’s 26th commercial resupply mission for NASA. Both CubeSats deployed from the space station on Dec. 29, 2022, at 8:55 a.m. EST. Scientists on both missions are most interested in studying a layer in Earth’s upper atmosphere known as the ionosphere. The ionosphere is where the impacts of space weather on our technology are felt most strongly. It's home to many satellites, including the International Space Station. Radio waves and GPS signals travel through the ionosphere, and variations there can interfere with, or even disrupt, our communication signals. Space weather can also create electric currents that can induce electrical charge in orbiting satellites, and, in extreme cases, cause power outages on the ground. Day in and day out, the ionosphere is cooked by the Sun's radiation into a soup of positively charged ions and negatively charged electrons, called plasma. Fluctuations in the ionosphere cause low-density and high-density regions – bubbles and blobs – to form in the plasma. These bubbles and blobs can scatter radio signals, sometimes sending them crashing into each other in a phenomenon called scintillation. The result is noisy radio signals, which can reduce the reliability of communication and navigation systems, or even disrupt signals completely. “If you put a pencil into a glass of water that’s half full, the pencil appears broken,” said Linda Habash Krause, the project scientist for SPORT at NASA’s Marshall Space Flight Center in Huntsville, Alabama. “What happens when you have bubbles? Similar to the pencil in the water, the signals go through ample bends.” Unfortunately, scientists do not understand exactly how the plasma bubbles and blobs arise. Once petitSat and SPORT are launched from the space station, the two CubeSats will use complementary scientific instruments to investigate the conditions that cause these disruptive features to form. “The idea is that the science teams will work together and cross compare,” said Jeff Klenzing, the principal investigator of petitSat at NASA’s Goddard Space Flight Center in Greenbelt, Maryland. SPORT is equipped with six instruments to make measurements throughout the ionosphere. It will help determine the conditions that exist just before plasma bubbles form and, ultimately, how their evolution impacts ground-based communications signals. SPORT will transmit data back to the Brazilian National Institute for Space Research (INPE), where the data will be distributed to researchers at INPE, NASA, and other U.S. partners. In a complementary fashion, petitSat will work to determine what triggers plasma blobs, when they appear, or even how large a region they occupy. Both petitSat and SPORT will provide improved observations and insights into space weather phenomena which impact communications. These missions will collectively enhance our understanding of our ever-changing space environment and amplify current capabilities of small satellites to directly benefit our society. The more we learn about space weather – and how to predict it – the better we can protect our astronauts, spacecraft, and technology. IMAGE....The ionosphere constantly glows and will be the main focus of study for these two satellites. Here, an aurora is captured as seen from the International Space Station. CREDIT NASA
2 notes
·
View notes
Text
Types and Functions of Diodes - A Comprehensive Guide
Email: [email protected]
WhatsApp & Wechat: +86 18038197291
www.xygledscreen.com
Diodes are a crucial electrical component. They appear in various items, including computers, televisions, radar circuits, power supply systems, and communications systems. Understanding diodes can help one understand why it is such an essential component.
Check out this comprehensive guide concerning the function of diodes. It will provide insight into what diodes are, how diodes work, their benefits and drawbacks, their various types, and their applications.
What Is a Diode?
A diode is a one-way switch in a circuit. It allows electrical current to move in a specific direction and prevents it from moving in the opposite direction. This device typically has two terminals. One is the positive terminal, the anode, and the other is the negative terminal, the cathode.
Many diodes consist of semiconductor materials, such as selenium. Semiconductors are substances with conductivity levels lower than conductors but higher than insulators. People often rate diodes by their current capacity, type, and voltage.
How Do Diodes Work?
The most common kind of diode is the semiconductor diode. It has a P-type layer of positively charged particles and an N-type layer of negatively charged particles. When these two layers come together, they create a PN junction.
A PN junction impacts the flow of current. The positively charged particles in the P-type layer of the junction are attracted to the negatively charged particles in the N-type layer. Their attraction creates a barrier.
An electrode attached to the P-type layer is an anode, and one attached to the N-type semiconductor is a cathode. When connected to a power source, the current will flow from the anode to the cathode. It will not flow from the cathode to the anode.
What Are the Advantages of a Diode?
There are several advantages associated with using diodes. First, they prevent electrical circuits from sustaining damage from overcurrents, short circuits, and overvoltages. Second, they can change alternating current (AC) to direct current (DC).
Third, diodes decrease power losses within an electrical circuit. Fourth, diodes can lessen electromagnetic interference (EMI). Lastly, you can execute logic operations with diodes because they can produce logic gates.
What Are the Disadvantages of a Diode?
Though diodes can protect electrical circuits, their efficiency is comparatively low. Their voltage drop is ~0.7V, so they use power even when there is no current flow. The low efficiency makes diodes unideal for electrical circuits that need high efficiency, such as solar cells.
Diodes are susceptible to heat damage. They can experience an overload of current, resulting in damage or failure. A drawback of semiconductor diodes is that they cannot handle high reverse voltage. Also, semiconductor diodes have high noise levels at high frequencies.
What Are the Different Types of Diodes?
Several types of diodes are available on the market, such as PN junction diodes, photodiodes, rectifiers, PIN diodes, and light-emitting diodes (LEDs).
A PN junction diode, also known as a general purpose diode, has two terminals, the anode and cathode. The current in this diode moves in one direction, from the anode to the cathode. This type of diode has a P-type layer with positive ions and an N-type layer with negative electrons. You can find these diodes in automotive, computer, and communication devices.
A photodiode, called a light detector or photo-detector, uses light energy to yield a current. This device has two electrodes and a radiation-sensitive junction. It is an optoelectronic component that supports a reverse current that changes with illumination. Photodiodes usually consist of materials such as germanium and silicon. People often employ them to detect and convert optical power.
A rectifier takes in AC that has, on average, zero volts. It converts AC to DC. The DC the rectifier yields has a net value of more than zero. Rectification is the name of this AC to DC process. The diode in the rectifier has an anode and cathode and sustains a current that flows in a single direction.
A PIN diode features three semiconductor regions. One of the regions is a p-type semiconductor, and the other is an n-type one. The layer that is between the p-type and n-type layers is the intrinsic region. This region is large and undoped. The p-type and n-type regions have impurities to facilitate ohmic contacts.
A light-emitting diode gives off light radiation via electroluminescence. It has a PN junction and serves as an illuminator or visual indicator. LEDs on the market can support infrared, visible, and ultraviolet light. Plenty of industries use LEDs. You can find them in automobiles, aircraft carriers, televisions, and lamps.
What Are the Common Applications of Diodes?
People use diodes in a variety of ways. They appear in devices found in industrial, commercial, and residential settings.
Many use diodes for rectification. Converting AC to DC helps because it stops voltage spikes. Thus, you will find diodes in items such as surge protectors.
Diodes appear in logic gates because they can enact digital logic functions. You will find diodes in digital electronics, such as computer processors.
Diodes work well for radio demodulation, also known as signal demodulation. This process isolates signals from a supply of current. People use diodes to get radio signals from a carrier. Look at a present-day radio circuit. A diode will likely be there.
Those who need to measure or manipulate light frequently employ diodes to achieve their desired results. Photodiodes can measure light intensity, and LEDs can function as a light source because they appear in illumination technology, such as light bulbs.
Voltage multiplication is another process that people use diodes to perform. The diode, plus a capacitor, will use AC with a low voltage value and multiply it, increasing its voltage. Many electric devices, such as power supplies, feature voltage multipliers.
In conclusion, diodes are vital electrical devices with strengths, limitations, and multiple applications. There are many types of diodes, including rectifiers, photodiodes, and LEDs. Some use diodes on occasion for special electrical applications. Others use them daily because they appear in household appliances, computers, and communication devices. Many tools, systems, and processes could not exist without the assistance of diodes.
0 notes
Text
Best Alkaline Water Ionizer Machine | Health Center Network

Looking for the best alkaline water ionizer machine to improve your health and well-being? Look no further! In this article, we will explore the top alkaline water ionizer machines on the market, helping you make an informed purchasing decision.
Our brand voice is informative and reliable, guiding you through the process of finding the perfect alkaline ionizer machine to suit your needs.
With the rise in popularity of alkaline water plant, there are numerous options available, making it challenging to choose the right machine. Our experts have done the research for you, analyzing various features such as water filtration, pH levels, ORP, and ease of use.
Discover the best brands and models that offer exceptional alkaline ph water machine quality, enhanced hydration, and potential health benefits. Get ready to experience the power of alkaline water with the best alkaline water machine!
What is Alkaline Water?
Alkaline water is water that has a higher pH level than regular tap water, typically ranging from 8 to 9.5 on the pH scale. The pH level of water determines its acidity or alkalinity, with a pH of 7 being neutral. Alkaline water is believed to help neutralize acid in the body, promoting better overall health.
Drinking alkaline water has gained popularity due to its potential health benefits. It is claimed to have antioxidant properties, improve hydration, aid digestion, and boost the immune system. While scientific research on the benefits of alkaline water is limited, many individuals swear by its positive effects on their well-being.
Understanding Water Ionizers
Water ionizers are devices that transform regular tap water into alkaline water through a process called electrolysis. They use an electrical charge to separate the water into acidic and alkaline components, allowing you to adjust the pH level of the water you consume.
Water ionizers typically consist of a filtration system, electrodes, and a control panel. The filtration system removes impurities and enhances the taste of the water. The electrodes, usually made of platinum or titanium, are responsible for the ionization process. The control panel allows you to adjust the pH level and other settings according to your preferences.
How do Alkaline Water Ionizer Machines Work?
Alkaline water ionizer machines work by utilizing a process called electrolysis. When water enters the machine, it passes through a series of filters to remove impurities and improve the water’s quality. The filtered water then flows over the electrodes, which are charged with electricity.
The electrode plates in the ionizer machine are divided into two sections: the cathode and the anode. The cathode produces alkaline water, while the anode produces acidic water. The electrical charge causes the water molecules to split into positive and negative ions. The positive ions combine with minerals in the water, such as calcium and magnesium, creating alkaline water.
By adjusting the settings on the control panel, you can customize the pH level and the strength of the ionization process. This allows you to create alkaline water with various pH levels, depending on your preferences and needs.
Factors to Consider When Choosing an Alkaline Water Ionizer Machine
When choosing an alkaline water ionizer machine, there are several factors to consider to ensure you make the right decision. These factors include:
Water Filtration: Look for machines with effective filtration systems that remove contaminants and improve water quality.
pH Range: Consider the pH range the machine offers, ensuring it provides the desired level of alkalinity for your needs.
ORP (Oxidation Reduction Potential): ORP measures the antioxidant properties of water. Look for machines with a negative ORP, as it indicates a higher antioxidant capacity.
Ease of Use: Choose a machine with a user-friendly interface and intuitive controls for easy operation.
Durability and Warranty: Consider the build quality of the machine and the warranty provided by the manufacturer.
Size and Installation: Determine the size of the machine and whether it fits your available space. Additionally, check the installation requirements to ensure compatibility with your plumbing system.
By considering these factors, you can select an alkaline water ionizer machine that meets your specific requirements and delivers the desired results.
Top Features to Look for in an Alkaline Water Ionizer Machine
When researching alkaline water ionizer machines, it’s essential to pay attention to the features they offer. Here are some top features to look for:
Multiple pH Levels: Choose a machine that provides a wide range of pH levels, allowing you to customize your alkaline water according to your preferences.
Advanced Filtration System: Look for machines with multi-stage filtration systems that effectively remove impurities, chlorine, and heavy metals.
Self-Cleaning Function: Opt for a machine with a self-cleaning function to prevent the buildup of mineral deposits and ensure optimal performance.
Adjustable ORP Levels: Consider machines that allow you to adjust the ORP levels, providing you with antioxidant-rich water.
Flow Rate: Check the flow rate of the machine to ensure it can meet your household’s water consumption needs.
Filter Life and Replacement: Evaluate the lifespan of the filters and the cost of replacement to determine the long-term maintenance requirements.
By prioritizing these features, you can find an alkaline water ionizer machine that suits your preferences and provides the desired health benefits.
Maintaining and Cleaning Your Alkaline Water Ionizer Machine
To ensure the longevity and optimal performance of your alkaline water ionizer machine, proper maintenance and cleaning are crucial. Here are some tips to keep in mind:
Follow the manufacturer’s instructions: Read the user manual carefully and adhere to the maintenance guidelines provided by the manufacturer.
Regularly clean the machine: Clean the machine periodically using a mild cleaning solution recommended by the manufacturer. This helps remove any mineral deposits or impurities that may accumulate over time.
Replace filters as recommended: Replace the filters according to the manufacturer’s instructions to maintain water quality and prevent clogging.
Keep the machine dry: After each use, make sure to dry the machine properly to prevent the growth of bacteria or mold.
By following these maintenance practices, you can ensure that your alkaline water ionizer machine continues to function optimally and provide you with high-quality alkaline water.
Conclusion: Choosing the Best Alkaline Water Ionizer Machine for Your Needs
In summary, selecting the best alkaline water ionizer machine requires careful consideration of various factors, including water filtration, pH levels, ORP, and ease of use. By understanding the benefits of drinking alkaline water and how water ionizer machines work, you can make an informed decision.
Remember to properly maintain and clean your alkaline water ionizer machine to ensure its longevity and optimal performance. With the best alkaline water ionizer machine by your side, you can enjoy the benefits of alkaline water and take a step towards better health and well-being.
#alkaline water benefits#alkaline water machine#best alkaline water#alkaline water ionizer#alkaline water purifier#alkalinewater#alkaline#healthcenternetwork
0 notes
Text
The Hair Straightener: What to look for
Curls and waves on a person are beautiful. However, if you wish to smooth them out, these hot tools, irons, brushes, and combs can work wonders. Certain flat irons offer us crispy ends and cramped hands, while the others, like the ones listed here, offer us sleek hair. There are multiple options around, but hopefully, our favorite hair straightener for curls can help narrow down your search.
What to Look for in a Good Hair Tool?
A straightener can either be a flat iron, and a flat iron is a straightener, however not all straighteners are flat irons. These come in other forms too, including brushes and combs. No matter which you go with what you call it, or what your budget is, here are a few things you should bear in mind.
It should be easy to hold and manage: If a flat iron requires all your strength to keep the plates closed, you will end up in pain by the end of the straightening session.
It should feature a range of easy-to-read temperatures: It consists of dials with no indication of what temperature you are using that are confusing, and this way you can end up burning your hair and skin.
It should not snag the hair: This is a basic problem among flat irons, as hair can get caught in cheap plates and pulled out. Hence watch out for simple designs, that can help prevent this.
Flat irons should not be used on wet hair. It is advised to style only wet hair if the tool is manufactured for that, like a blow-dry brush.
What are Ions, Explained
Most experts agree that hair is generally positively charged because of its water content, and the negative ions these flat irons generate usually help dissipate that water. “You get controlled application of the heat you are applying, and you can use only as much heat as you need to smooth and straighten your hair, which can prevent damage,” they say.
However, the Ionic hair dryers are quite the same, the experts say “If you have finer hair and you want as much body and volume as possible, the ionic dryer may not be the best,” they add. "So generally it's best to get a dryer with an ionic option that can be turned on and off.”
CONCLUSION
So, there you have it friends, these are some of the facts about hair straighteners, to help you ‘buy better’.
0 notes
Text
About Action Potentials
Alright, listen up broskis. I’m explaining how brain cells (AKA neurons) activate, the technical terms being how they create an action potential.
We start at the resting potential of the membrane at around -70 millivolts, this number is just a comparison of the inside of the neuron to the outside. Other neurons give the membrane signals to become more positive or more negative. Making it more positive increases the likelihood of the neuron actually firing because the membrane has to reach -55 millivolts to activate, anything below this number and nothing will happen (the creatively named ‘All or Nothing’ Principle). From there it will fire up to +40 millivolts, called depolarization. It gets so positive because sodium ions, which are positive, enter the membrane and make it more positive.
As they say, what comes up must also come down, and so we enter repolarization, the nosedive back into the negative millivolts. This part of neural firing involves potassium ions, again positive, to leave the membrane and lower the charge of the membrane to make it more negative. The next part of this journey leads the neuron membrane to become even more negative than it was at the start of this fun process, and professionals call this the refractory period. This period just gives the neuron a bit of a break and makes sure it can’t fire for a small time after an action potential. Towards the end of this time, potassium ions stop leaving the membrane and the membrane voltage returns to the resting potential.
I can explain this using a more familiar process that people might be able to understand better: a laundromat. When you go to use a washer in a laundromat, it’s at a resting potential, that being having no clothes in it. Going to start it, it’ll only start when you pay all the money it requires, not some of the money. This represents that ‘All or Nothing’ Principle. Next the depolarization happens: it washes your clothes with detergent. The repolarization phase is the rinsing and wind down at the end of a cycle. Your clothes being in it and needing taken out is the refractory period because the washer can’t wash another load with the current load still in it. The washer enters its resting potential when you take your clothes out to move them to a dryer.
And that, my friends, is how action potential works. I hope you appreciated my real-life example because I had to work for it.
0 notes