#periodic table of elements
Text
And there
154 notes
·
View notes
Photo

#periodic table of elements#palaeolithic#chemisty#elements#physics#prehistory#wrong hands#john atkinson#webcomic#science#stone age
571 notes
·
View notes
Photo
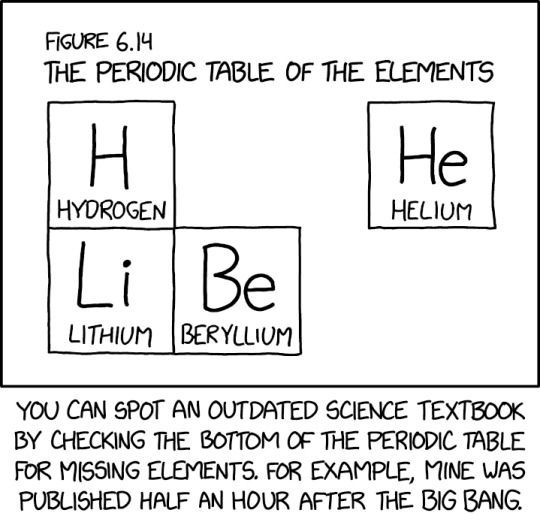
Researchers claim to have synthesized six additional elements in the second row, temporarily named 'pentium' through 'unnilium'.
Outdated Periodic Table [Explained]
#xkcd#xkcd 2723#outdated periodic table#webcomics#chemistry#astronomy#periodic table#periodic table of elements#big bang
705 notes
·
View notes
Text
Periodic table with particles of these same elements.
208 notes
·
View notes
Text
FINALS









CARBON:
Forms the basis of all life
What diamonds, coal, and pencil lead are made of
Used to make graphene, one of the strongest materials (one atom of thickness is 200x stronger than steel)
SILICON:
Not to be confused with Silicone
Could potentially be used to make life instead of carbon
Used in a lot of electronics, hence the name of silicon valley
ARGON:
Has a distinctive purple glow
used in neon signs
often mistaken as a pirate's favorite element
POTASSIUM:
Found in high quantity in bananas
burning it produces a light purple or red flame
the first metal to be discovered by electrolysis
STRONTIUM:
Used in cancer treatment
Used in toothpaste
The most accurate atomic clock (to one second in the 200 million years) uses strontium atoms
FLOURINE:
the most receptive and most electronegative of all the chemical elements
oxygen, helium, neon, and argon are the only elements fluorine can't react with
the only element that can react with noble gases, specifically xenon, krypton, and radon
BISMUTH:
the most diamagnetic metal (meaning it gets repelled by magnets instead of attracted)
Known for its unique shape and colorful style
PROMETHIUM:
the last lanthanoid to be discovered
PLUTONIUM:
Named after the dwarf planet Pluto
Was once thought to have been discovered by Enrico Fermi along with element 93, but he was actually mistaken. He named it Hesperium.
An unnaturally poor conductor of electricity
Used to make atomic bombs, even more powerful than uranium
Known by some as the "forbidden gummy"
COBALT:
Turns a vibrant blue when heated to extreme temperatures
Named after kobolds, who are "mythical, death-dealing goblins" (not the lizard kobold)
One of only three elements that are ferromagnetic at room temperature
#element#periodic table#periodic table of elements#poll#polls#tournament#elements#carbon#silicon#argon#potassium#strontium#flourine#bismuth#promethium#plutonium#cobalt
164 notes
·
View notes
Text
308 notes
·
View notes
Text
PSE, but make it gallifreyan and queer 😌
If you're missing a particular flag, I'll be glad to add more :)
For now I'mma do these ten Tumblr let's me post in one go





#circular gallifreyan#gallifreyan#doctor who#time lords#doctor who fanart#dw art#dw fanart#my art#pse#periodic table of elements#periodic table#chemistry#queer#trans#transgender#gay#bi#bisexual#ace#acespec#lesbian#aro#asexual#aroace#aromantic#ftm#mtf#Spotify
23 notes
·
View notes
Text


#periodic table poll#periodic table of elements#bismuth#mercury#polls#bracket poll#science#chemistry#semifinals
166 notes
·
View notes
Text
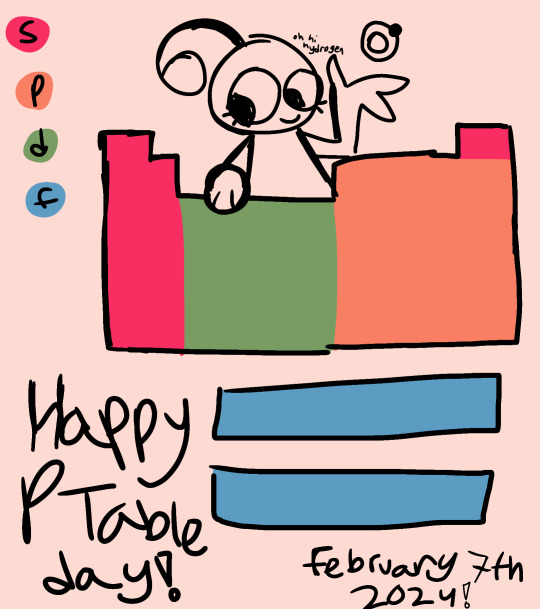
Happy Periodic Table Day! The table is officially 105 years old!
Bonus Doodle Page Under Cut
From left to right and top to bottom: oxygen, hydrogen, chlorine, an electron, actinium, helium, and an alpha particle
tags: @allhailhelium @averagecarbonenjoyer @sweetpumpkinmouse @official-atoms
25 notes
·
View notes
Text
By: Dyani Lewis
Published: May 31, 2023
In India, children under 16 returning to school this month at the start of the school year will no longer be taught about evolution, the periodic table of elements or sources of energy.
The news that evolution would be cut from the curriculum for students aged 15–16 was widely reported last month, when thousands of people signed a petition in protest. But official guidance has revealed that a chapter on the periodic table will be cut, too, along with other foundational topics such as sources of energy and environmental sustainability. Younger learners will no longer be taught certain pollution- and climate-related topics, and there are cuts to biology, chemistry, geography, mathematics and physics subjects for older school students.
Overall, the changes affect some 134 million 11–18-year-olds in India’s schools. The extent of what has changed became clearer last month when the National Council of Educational Research and Training (NCERT) — the public body that develops the Indian school curriculum and textbooks — released textbooks for the new academic year that started in May.
Researchers, including those who study science education, are shocked. “Anybody who’s trying to teach biology without dealing with evolution is not teaching biology as we currently understand it,” says Jonathan Osborne, a science-education researcher at Stanford University in California. “It’s that fundamental to biology.” The periodic table explains how life’s building blocks combine to generate substances with vastly different properties, he adds, and “is one of the great intellectual achievements of chemists”.
Mythili Ramchand, a science-teacher trainer at the Tata Institute of Social Sciences in Mumbai, India, says that “everything related to water, air pollution, resource management has been removed. “I don’t see how conservation of water, and air [pollution], is not relevant for us. It’s all the more so currently,” she adds. A chapter on different sources of energy — from fossil fuels to renewables — has also been removed. “That’s a bit strange, quite honestly, given the relevance in today’s world,” says Osborne.
More than 4,500 scientists, teachers and science communicators have signed an appeal organized by Breakthrough Science Society, a campaign group based in Kolkata, India, to reinstate the axed content on evolution.
NCERT has not responded to the appeal. And although it relied on expert committees to oversee the changes, it has not yet engaged with parents and teachers to explain its rationale for making them. NCERT also did not reply to Nature’s request for comment.
Chapters closed
A chapter on the periodic table of elements has been removed from the syllabus for class-10 students, who are typically 15–16 years old. Whole chapters on sources of energy and the sustainable management of natural resources have also been removed.
A small section on Michael Faraday’s contributions to the understanding of electricity and magnetism in the nineteenth century has also been stripped from the class-10 syllabus. In non-science content, chapters on democracy and diversity; political parties; and challenges to democracy have been scrapped. And a chapter on the industrial revolution has been removed for older students.
In explaining its changes, NCERT states on its website that it considered whether content overlapped with similar content covered elsewhere, the difficulty of the content, and whether the content was irrelevant. It also aims to provide opportunities for experiential learning and creativity.
NCERT announced the cuts last year, saying that they would ease pressures on students studying online during the COVID-19 pandemic. Amitabh Joshi, an evolutionary biologist at Jawaharlal Nehru Centre for Advanced Scientific Research in Bengaluru, India, says that science teachers and researchers expected that the content would be reinstated once students returned to classrooms. Instead, the NCERT shocked everyone by printing textbooks for the new academic year with a statement that the changes will remain for the next two academic years, in line with India’s revised education policy approved by government in July 2020.
“The idea [behind the new policy] is that you make students ask questions,” says Anindita Bhadra, an evolutionary biologist at the Indian Institute of Science Education and Research in Kolkata. But she says that removing fundamental concepts is likely to stifle curiosity, rather than encourage it. “The way this is being done, by saying ‘drop content and teach less’”, she says, “that’s not the way you do it”.
Evolution axed
Science educators are particularly concerned about the removal of evolution. A chapter on diversity in living organisms and one called ‘Why do we fall ill’ has been removed from the syllabus for class-9 students, who are typically 14–15 years old. Darwin’s contributions to evolution, how fossils form and human evolution have all been removed from the chapter on heredity and evolution for class-10 pupils. That chapter is now called just ‘Heredity’. Evolution, says Joshi, is essential to understanding human diversity and “our place in the world”.
In India, class 10 is the last year in which science is taught to every student. Only students who elect to study biology in the final two years of education (before university) will learn about the topic.
Joshi says that the curriculum revision process has lacked transparency. But in the case of evolution, “more religious groups in India are beginning to take anti-evolution stances”, he says. Some members of the public also think that evolution lacks relevance outside academic institutions.
Aditya Mukherjee, a historian at Jawaharlal Nehru University in New Dehli, says that changes to the curriculum are being driven by Rashtriya Swayamsevak Sangh (RSS), a mass-membership volunteer organization that has close ties to India’s governing Bharatiya Janata Party. The RSS feels that Hinduism is under threat from India’s other religions and cultures.
“There is a movement away from rational thinking, against the enlightenment and Western ideas” in India, adds Sucheta Mahajan, a historian at Jawaharlal Nehru University who collaborates with Mukherjee on studies of RSS influence on school texts. Evolution conflicts with creation stories, adds Mukherjee. History is the main target, but “science is one of the victims”, she adds.
==
Well, at least it'll put them on par with the anti-science and biology-denial of US classrooms. China no longer has anything to worry about.
#India#hinduism#islam#science denial#evolution#evolution denial#science#anti science#biology denial#math#mathematics#physics#religion#religion is ignorance#religious stupidity#periodic table#periodic table of elements#religion is a mental illness
114 notes
·
View notes
Text
🧑🏻🔬THE PERIODIC TABLE OF CHEMICAL ELEMENTS👩🏿🔬
(Note: I am not a chemist, just someone who enjoys science, if I made any errors please let me know, thank you and I hope you enjoy reading this)

Ever wonder about this bad boy? While here's a quick crash course on it and some of it groups!
What is it?
The Periodic Table is an arrangement of rows and columns used to display and predict what elements exist and their properties based upon where they fall.
The farther right and down you go, the "heavier" the elements get as they have more Protons, going from 1 Proton for Hydrogen to as of now 118 Protons for Oganesson. Each Collum, excluding the Transition Metals and Rare Earth Series, have similar chemical properties.
Along with this, excluding the Transition Metals, as you go left to right the number of Valence Electrons increases by 1 up to 8 before flipping around back to 1. Valence Electrons can be thought of as the Hands of Atoms, being how they interact and react with each other; a free valence electron spot being a place another atom can bond to like an open hand, while an occupied valence electron spot is like a full hand slapping away other potential electrons.
Each valence group, along with the Transition Metals, builds up the base of all of Chemistry and the universe. So lets have a look at a few of them, shall we?
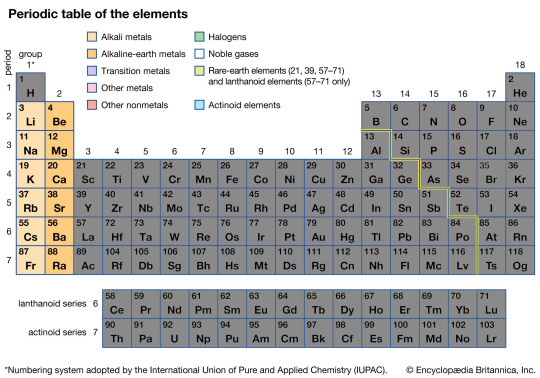
The Alkaline-Earth Metals
The Alkaline-Earth Metals are the two first columns of the Periodic table, and are often sub-divided into the Alkaline Metals and Alkaline Earth Metals. Both groups however do share some properties, often being softer metals that are extremely reactive.
Most famously, they react with water to produce Hydrogen and some heat amount of heat. On the more reactive side such as Lithium and Sodium, this means upon contact with water they will explode, sending red hot metal flying with a violent blast. On the less extreme end such as Magnesium or Calcium, they can be used as ways to produce Hydrogen in small quantities.
Not only are they reactive with water, but also air, oxidizing rapidly in the air and in the case of Magnesium having an extremely violent and energetic reaction when lit on fire.
They also all like to bond with chlorine to produce salts such as Sodium chloride, or more commonly known as humble Table Salt.
After the neat uniformity of the Alkaline-Earth metals, we reach,

The Transition Metals
The Transition Metals are not transgender metals, but rather a large collection of various metals that don't as neatly fit into rows or columns as the other Elemental groupings. Most notably, they have no Valence Electron Shell patterns, meaning you often have to search out the specific Transition Metal you are working with to know how many spots it has still open. They are by far the largest single group of elements within the Periodic Table, and chances are if you think of a metal it will be a transition metal.
It includes some famous stars such as Iron, Gold, Copper, Titanium, Lead, Zinc, Osmium, Tungsten, and Silver. As you can tell from the diverse cast of elements, all of these have wildly varying properties; take the strength and hardness of Iron and Tungsten compared Gold and Zinc as an example.
However they are not totally dissimilar as they are all still metals, meaning they share the properties of Metals. These include high thermal and electrical conductivity, liking to gobble up ions, are highly ductile (the ability to be pulled and bent into wire without breaking), form Cations (positively charged ions), and other such Metal traits.
Truly a party of metals, followed by,

The Other Metals
As the name would suggest, these are some of the other metals and can be thought of as an extension of the Transition Metals.
However their proximity to the other groups gives a few of these metals such as Aluminum more unique chemical behaviors compared to the standard transition metals, which is why sometimes you'll see some disagreement and debate about which ones, if any, should be placed into the next group,

The Metalloids
The Metalloids border between the Metals and the Non Metals, and as such are unique in having properties of both Metals and Non Metals.
Most famous of the Metalloids is arguably Silicon, which makes of the base of the modern world through its semiconductor properties in circuit boards and chips like the one bring used to allow you to read these very words.
Another famous, or perhaps infamous, Metalloid is Arsenic, used in as many poisoning murders for its toxic lethality as paintings and dyes for its beautiful vibrant green hue when turned into a pigment.
And if Arsenic is a bit too deadly for your liking, Gallium is always an option, used in old vacuum tube era computing and lighting. Along with its vintage past, it also has a melting point of 85.58°F or 29.76°C, just shy of room temperature; meaning if you hold a piece of Gallium in your hand, it will melt into a liquid - and unlike Mercury, it is non toxic making it far safer to play with, although it does stick to glass and stain objects so be careful if you do play with it to not let it touch something you don't want getting stained.
And as hinted just before, let us introduce the one and only,
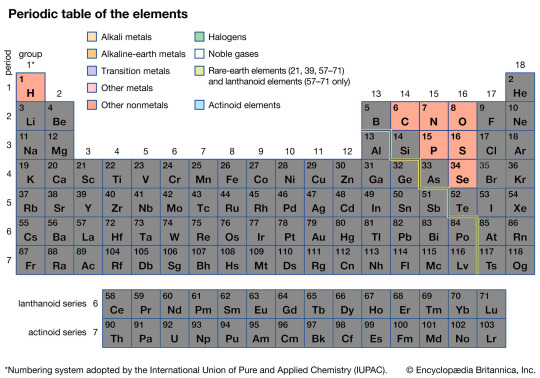
The Non Metals
The Non Metals are a small but extremely unique and important group. All of these elements as the name would suggest sharing the properties of being Non Metals, meaning they are all for the most part poor thermal and electrical conduct, have poor ductility, and form Anions (negatively charged ions).
If you are biologist of any kind, you may also notice this group contains many elements crucial for organic life; the three most important of which without doubt being Carbon, Oxygen, and Hydrogen.
Along with Nitrogen and a few other elements, these elements firm the basis of all life on Earth. From the air you breathe being a Nitrogen-Oxygen mix, to your cells being based on Carbon, to the very DNA that created you. All of it based on Carbon, Oxygen, Nitrogen, and Hydrogen.
And while Oxygen may have taken the honor of the name of Oxidation, it has nothing on the next group,
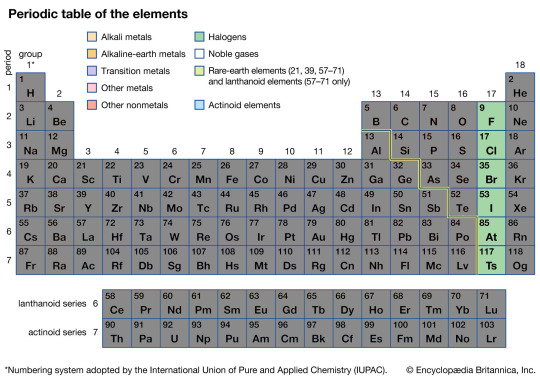
The Halogens
The Halogens, due to missing just one election to be totally stable, are some of the most violently reactive elements on the entire Periodic Table, with Fluorine even known to be able to eat through glass if given enough time.
It might be surprising to hear then that the Halogens also produce some of the most chemically stable compounds known on Earth, such as Polytetrafluoroethylene (PTFE), A.K.A., Teflon. This is because when these elements create compounds they become stable, meaning to break them apart you need an equal amount of energy to do so, and the energy requirement for Halogen compounds is often massive, making them chemically hardy.
Outside of Teflon and eating glass, the less reactive of the Halogens also are used in medicine as a way to sterilize an area. Most commonly and famously used would be Iodine, which not only has a beautiful purple hue, but is also used in surgeries to sterilize equipment and areas before operations, saving countless lives from infections every day.
In contrast to the reactive Halogens, we have next,
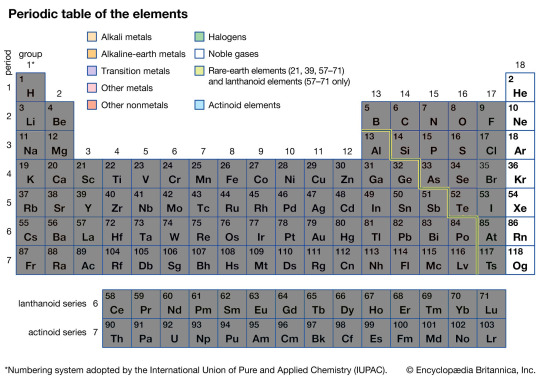
The Nobel Gases
The Nobel Gases like any true Nobels with wealth and status don't like to get involved in the common peasantry's squabbles. In the world of chemicals and elements, this means their full valence electron shells don't stubbornly refuse to chemically react with any other element, and the compounds they do form are often unstable and want to fall apart back into a Nobel Gas and whatever common element it wound up stuck with.
While this lack of reacting does make them rather boring from a reaction perspective, it does make them extremely useful when you do not want things chemically reacting by acting as a buffer between other elements; most easily seen in high end car lights, often filled with Xeon or other Nobel Gases to make sure no matter how hot the light gets it doesn't react chemically and degrade.
Along with protecting lights, they can also be used for lights. Due to their unreactive nature in even high energy environments, it makes them perfect to be used to fill a sealed glass tube that has power shot through it; otherwise known as a Neon Light. However despite the name, not all Neon Lights are filled with Neon, only the orange-red ones are. Other colors such as blues and greens come from the other Nobel Gasses depending on their spectrum emissions.
And on light and degradation, last but not least we have,
The Rare Earth Metals
The Rare Earth Metals can be and often are split into two smaller groups, the upper Lanthanide series and the lower Actinide series.
The upper Lanthanides are all rather chemically similar, meaning they can often be treated somewhat interchangeably in a chemical sense. Their most notable use is without a doubt their ferro magnetic properties, making some of the most powerful magnets on Earth barring electro-magnets. Neodymium magnets are by far the most famous of them, being known to be able to leap across across tables and smash fingers if not handled carefully, while holding up hundreds of pounds of weight.
In contrast, the lower Actinide series is the domain of many of the Radioactive elements and sees the boundary line between the natural and artificial elements. All of them are radioactive to various degrees with decreasing half life lengths.
It is here Thorium and Uranium is found, being the heart of both terrifying bombs and the cleanest sources of energy known to mankind. Beyond Uranium, all the elements are artificial and must be made by humans in breeder reactors.
Despite their association with atom bombs and nuclear reactors, many of these elements also have far more mundane uses. Americium is used in common household Smoke Detectors, saving untold numbers of lives and dollars in property through early warnings of smoke and fire. Other ones are used by NASA and other space agencies in Radioisotope thermoelectric generators or R.T.G.s, used to power spacecraft like Voyager 2 and the Mars Curiosity Rover thousands of miles away from Earth where conventional solar panels would be too heavy, unwieldy, and inefficient so far away from the Sun.
#periodic table of elements#science#elements#chemistry#chemical elements#periodic table#science stuff#science side of tumblr
148 notes
·
View notes
Text








This is gonna take 3 posts
149 notes
·
View notes
Text
me @ my chemistry nerd gf
hey do you think i’m boron
am i a good samarium
do you not appreciate my iron-y
are you not slapping your neon
i would tell you a good joke but all the best ones argon
should i zinc of a new joke
i’m sodium thoughtful aren’t i
you know most of these jokes were xenon television
hey girl are you gold cause you’re pretty au-some
alright these jokes are really boron, sorry for making you sulfur through them
might have to stop soon cause sleep is krypton up on me (but don’t worry, i’ve got my ion you)
#i’m sorry#stella’s misc#i’m not funny#periodic table of elements#chemistry#bad puns#terrible puns#awful puns
20 notes
·
View notes
Text


This is what I do when I am presented with the periodic table of elements in my science textbook.
#marvel#mcu#bucky barnes#periodic table#periodic table of elements#winter soldier#bucky#james buchanan barnes#science
23 notes
·
View notes
Note
PLEASE RAMBLE ABT THE PERIODIC TABLE
OMFG I WOULD LOVE THAT
*casually scrolls through my inbox*
*spits my coffee*
Chemistry isn't my favourite science field. In fact, I don't really like it, but it is important for other scientific fields as well, so I do have a compact knowledge about it. And since you all asked so nicely...
⚛️The periodic table of chemical elements:⚛️
Often called just the periodic table, organises all discovered chemical elements in rows (called periods) & columns (called groups) according to increasing atomic number.
An element's period number is the highest unexcited energy level for an electron of that element.
Columns help to distinguish groups in the periodic table. Elements within a group share several common properties & often have the same outer electron arrangement.
It is used to quickly refer to information about an element, like atomic mass & chemical symbol.
The periodic table’s arrangement also allows to discern trends in element properties, including electronegativity, ionization energy & atomic radius.
Dmitri Mendeleev published his first version of the periodic table in 1869, so he is often credited as the inventor.
Over 150 years of scientific development & understanding in chemistry & physics, the number of known elements as significantly risen: there are 118 known elements.
98 elements can be found in nature. The rest were artificially created.
There is a limit, though: the periodic table does not allow to have more than 137 elements since the atoms electrons would circle the nucleus faster than the speed of light (Richard Feynman predicted this).
The periodic table is considered one of the most significant achievements in the history of science.
Some interesting facts to cheer you up:
Carbon is unique in that it is known to form up to 10 million different compounds. Carbon is important to the existence of life!
Francium is the rarest element on earth. There are probably no more than a few ounces of it on earth at any given time.
Helium is the second most abundant element in the universe. Hydrogen & Helium, together make over 99% of the observable universe.
Thorium is named after the Nordic god of thunder - Thor!
Iridium is the least corrosive element: that means it is least bothered about reaction with water, acid, air or other chemicals.
The heaviest naturally occurring element is Uranium with atomic number 92 & atomic weight 238.0289.
There are only two liquid elements in the Periodic Table: Mercury & Bromine. They remain in liquid form at room temperature.
The only letter not in the periodic table is the letter J.
The country Argentina is named after the element silver (symbol Ag), which is argentum in Latin.
Although there is helium on Earth, it was first discovered by observing the sun.
The first ever discovered artificial element is called Technetium. It can be artificially produced in nuclear power stations. It is the lightest element on the table that only holds unstable radioactive isotopes.
There are around 90 metals in the periodic table. The six groups of metals are alkali metals, basic metals, alkaline earth, lanthanides, transition metals & actinides.
I thank you kindly for the sweet inbox messages about asking me to infodump. 💜🎵
I enjoy these A LOT. I get so lost in research & I do learn new things while doing so... it's refreshing!

I might have forgotten to drink & eat....
#donnie speaks#donnie answers#donatello infodumps#donnies exceptional mind#turtle net#rise of the teenage mutant ninja turtles#rottmnt#rottmnt donnie#rottmnt donatello#rise autistic donnie#rottmnt autistic donnie#autistic donatello#periodic table#periodic table of elements#chemistry#infodumping#infodump#autistic coded character
51 notes
·
View notes
Text
FINAL RESULTS
And so concludes the periodic table tournament. Which only leaves one winner:

BISMUTH
Known for its rainbow of colors and its strangely cubic structure, Bismuth has many uses and unique properties. It is used in pepto bismol, its liquid state is denser than its solid state, it's the most diamagnetic metal, and has the lowest thermal conductivity of any metal (aside from mercury).
Apparently, someone has done this kind of tournament before, and bismuth won there, too! That shows that bismuth truly is Tumblr's favorite element!
As for the other finalists, the other placements from 2nd to 10th were:
2nd:Carbon
3rd: Cobalt
4th: tied for Plutonium and Potassium
6th: Argon
7th: Promethium
8th: Silicon
9th: Flourine
10th: Strontium
And thus concludes the periodic table tournament! I hope everybody had a good time voting for their favorite elements!
71 notes
·
View notes