#food microbiology
Text
Clearing Misconceptions About Microbiology In Nigeria::
As a microbiology undergraduate currently in her finals (4th year) at Rivers State University, I have come to understand that a lot of the things we were made to believe about microbiology are actually false. Today I want to clear up some common misconceptions about this field.
Let me start by explaining what microbiology is. Microbiology is the study of tiny organisms like bacteria, viruses, fungi and algae.
Now, here in Nigeria, people have some wrong ideas about microbiology that I think need to be corrected.
Firstly, many Nigerians think all microbes are dangerous and that's not true because there are some bacteria that help us digest food and make tasty fermented drinks. Microbes also keep the soil healthy and fertile. So you see, some microbes are really useful!
Another big misunderstanding is that microbes are way too small to see without a microscope but this is another misconception because some fungi and algae can actually be seen with the naked eye! Only bacteria and viruses need a microscope to view them properly.
A lot of Nigerians also think microbiology is only useful for healthcare stuff. That's not accurate! Farming relies on microbiology too for bio-fertilizers and improving soil health. Food companies use good microbes to make yogurt, bread and more. So microbiology is super important for medicine, agriculture, and other industrial uses.
People often assume microbiology just studies insignificant creatures that don't matter. Well, here's a newsflash - microbes enable great scientific discoveries! Microbiology also leads to new medicines, industrial applications and so much more. Don't underestimate it - this is serious science, not just the study of small, unimportant things.
To wrap this up, here in Nigeria many people wrongly think all microbes are dangerous, invisible, only useful in healthcare and insignificant. We need better awareness campaigns, workshops, media outreach and microbiology education in schools to correct these myths. This will also help Nigerians understand microbiology better and support this exciting field.

2 notes
·
View notes
Text
Chef WK, lead charcuterie specialist in Alberta Canada
Table of contents
1. Control Program Requirements for Fermented Meat Products
2. Facility and Equipment Requirements
3. Starter Culture
4. Chemical Acidification
5. Water Activity Critical Limits
6. Time and Temperature for Fermented Products
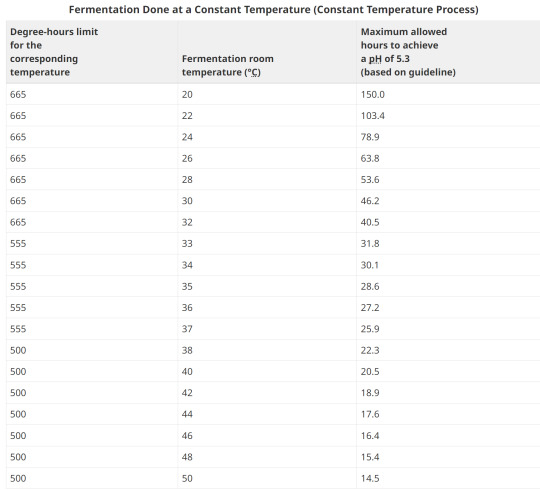
7. Fermentation Done at a Constant Temperature
8. Examples of Degree-hours at constant room temperatures
9. Fermentation Done at Different Temperatures
10. Fermentation done at Different temperatures
11. What happens if fermentation fails to hit critical limit?
12. E. coli and Salmonella Control in Fermented Sausages
13. Options for E. coli validation
14. Option1; Heating
15. Option 2; pH, heating, holding, diameter
16. Safety and consistency
Control Program Requirements for Fermented Meat Products
The producer must have a program in place to assess the incoming product. This program should outline specifications for the incoming ingredients. This may include criteria including receiving temperature, farm/ supplier, lot code or packed on date, species/cut etc.
2. Facility and Equipment Requirements
Equipment used in the fermentation process must be included in the operator's prerequisite control programs. These must include the following elements:
Temperature in the fermentation, drying and smoking chambers must be uniform and controlled to prevent any fluctuation that could impact on the safety of the final product.
Fermentation, drying and smoking chambers must be equipped with a shatter resistant indicating thermometer, (or equivalent), with graduations of 1°C or less. If mercury thermometers are used, their mercury columns must be free from separations. All thermometers must be located such that they can be easily read.
Fermentation and smoking chambers must be equipped with a recording thermometer for determining degree-hours calculations in a reliable manner. Recording thermometers are also preferable in drying and aging rooms but, in these rooms, it may be sufficient to read and record the temperatures 2 times a day.
Drying and aging rooms must be equipped with humidity recorders in order to prevent uncontrolled fluctuations of the relative humidity. The only alternative to an automatic humidity recorder in these rooms would be for the company to manually monitor and record ambient humidity twice a day (morning and afternoon) every day with a properly calibrated portable humidity recorder.
For routine monitoring, accurate measurement electronic pH meters (± 0.05 units) should be employed. It is important that the manufacturer's instructions for use, maintenance and calibration of the instrument as well as recommended sample preparation and testing be followed.
When the aw of a product is a critical limit set out in the HACCP plan for a meat product, accurate measurement devices must be employed. It is important that the manufacturer's instructions for use, maintenance and calibration of the instrument be followed.
3. Starter Culture
The operator must use a CFIA approved starter culture. This includes Freeze-dried commercially available culture as well as back-slopping (use of previously successful fermented meat used to inoculate a new batch). When performing back-slopping, the operator must have a control program in place to prevent the transmission of pathogens from when using the inoculum from a previous batch to initiate the fermentation process of a new batch. These must include:
The storage temperature must be maintained at 4°C or less and a pH of 5.3 or less.
Samples for microbiological analysis must be taken to ensure that the process is in line with the specifications.
The frequency of sampling is to be adjusted according to compliance to specifications.
Any batch of inoculum which has a pH greater than 5.3 must be analysed to detect at least Staphylococcus aureus. Only upon satisfactory results will this inoculum be permitted for use in back slopping.
This can be an expensive and a time exhaustive process and is generally avoided due to food safety concerns. AHS does not allow back-slopping.
[Chef WK was in communication with the U of A to get his method, a starter mix, studied.]
4. Chemical Acidification
If product is chemically acidified by addition of citric acid, glucono-delta-lactone or another chemical agent approved for this purpose, controls must be in place and records kept to ensure that a pH of 5.3 or lower is achieved by the end of the fermentation process. These acids are encapsulated in different coatings that melt at specific temperatures, which then release the powdered acids into the meat batter and directly chemically acidulate the protein.
Summer sausage is a very common chemically acidified product. The flavor profile tends to be monotone and lacking depth.
5. Water Activity Critical Limits
The aw may be reduced by adding solutes (salt, sugar) or removing moisture.
Approximate minimum levels of aw (if considered alone) for the growth of:
molds: 0.61 to 0.96
yeasts: 0.62 to 0.90
bacteria: 0.86 to 0.97
Clostridium botulinum: 0.95 to 0.97
Clostridium perfringens: 0.95
Enterobacteriaceae: 0.94 to 0.97
Pseudomonas fluorescens: 0.97
Salmonella: 0.92 - 0.95
Staphylococcus aureus: 0.86
parasites: Trichinella spiralis will survive at an aw of 0.93 but is destroyed at an aw of 0.85 or less.
The above levels are based on the absence of other inhibitory effects such as nitrite, competitive growth, sub-optimum temperatures, etc., which may be present in meat products. In normal conditions, Staphylococcus aureus enterotoxins are not produced below aw 0.86, although in vacuum packed products this is unlikely below aw 0.89.
6. Time and Temperature for Fermented Products
Certain strains of the bacteria Staphylococcus aureus are capable of producing a highly heat stable toxin that causes illness in humans. Above a critical temperature of 15.6°C, Staphylococcus aureus multiplication and toxin production can take place. Once a pH of 5.3 is reached, Staphylococcus aureus multiplication and toxin production are stopped.
Degree-hours are the product of time as measured in hours at a particular temperature multiplied by the "degrees" measured in excess of 15.6°C (the critical temperature for growth of Staphylococcus aureus). Degree-hours are calculated for each temperature used in the process. The limitation of the number of degree-hours depends upon the highest temperature in the fermentation process prior to the time that a pH of 5.3 or less is attained.
The operator is encouraged to measure temperatures at the surface of the product. Where this is not possible, the operator should utilize fermentation room temperatures. The degree hour calculations are based on fermentation room temperatures. Temperature and humidity should be uniform throughout the fermentation room.
A process can be judged as acceptable provided the product consistently reaches a pH of 5.3 using:
fewer than 665 degree-hours when the highest fermentation temperature is less than 33°C;
fewer than 555 degree-hours when the highest fermentation temperature is between 33° and 37°C; and
fewer than 500 degree-hours when the highest fermentation temperature is greater than 37°C.
This means that as the temperature increases, the amount of time that you have available to reach 5.3 or under is shorter. The warmer the temperature, the sharper the log growth phase of bacteria, which equates to more overshoot in lactic acid production, faster.
8. Examples of Degree-hours at constant room temperatures
Example 1:
Fermentation room temperature is a constant 26°C. It takes 55 hours for the pH to reach 5.3.
Degrees above 15.6°C: 26°C - 15.6°C = 10.4°C
Hours to reach pH of 5.3: 55
Degree-hours calculation: (10.4°C) x (55) = 572 degree-hours
The corresponding degree-hours limit (less than 33°C) is 665 degree-hours.
Conclusion: Example 1 meets the guideline because its degree-hours are less than the limit.
Example 2:
Fermentation room temperature is a constant 35°C. It takes 40 hours for the pH to reach 5.3.
Degrees above 15.6°C: 35°C - 15.6°C = 19.4°C
Hours to reach pH of 5.3: 40
Degree-hours calculation: (19.4°C) x (40) = 776 degree-hours
The corresponding degree-hours limit (between 33 and 37°C) is 555 degree-hours.
Conclusion: Example 2 does not meet the guideline because its degree-hours exceed the limit
9. Fermentation Done at Different Temperatures
When the fermentation takes place at various temperatures, each temperature step in the process is analyzed for the number of degree-hours it contributes. The degree-hours limit for the entire fermentation process is based on the highest temperature reached during fermentation.
Example 1:
It takes 35 hours for product to reach a pH of 5.3 or less. Fermentation room temperature is 24°C for the first 10 hours, 30°C for second 10 hours and 35°C for the final 15 hours.
Step 1
Degrees above 15.6°C: 24°C - 15.6°C = 8.4°C
Hours to reach pH of 5.3: 10
Degree-hours calculation: (8.4°C) x (10) = 84 degree-hours
Step 2
Degrees above 15.6°C: 30°C - 15.6°C = 14.4°C
Hours to reach pH of 5.3: 10
Degree-hours calculation: (14.4°C) x (10) = 144 degree-hours
Step 3
Degrees above 15.6°C: 35°C - 15.6°C = 19.4°C
Hours to reach pH of 5.3: 15
Degree-hours calculation: (19.4°C) x (15) = 291 degree-hours
Degree-hours calculation for the entire fermentation process = 84 + 144 + 291 = 519
The highest temperature reached = 35°C
The corresponding degree-hour limit = 555 (between 33°C and 37°C)Conclusion: Example 1 meets the guideline because its degree-hours are less than the limit.
10. Fermentation done at Different temperatures
Example 2:
It takes 38 hours for product to reach a pH of 5.3 or less. Fermentation room temperature is 24°C for the first 10 hours, 30°C for the second 10 hours and 37°C for the final 18 hours.
Step 1
Degrees above 15.6°C: 24°C - 15.6°C = 8.4°C
Hours to reach pH of 5.3: 10
Degree-hours calculation: (8.4°C) x (10) = 84 degree-hours
Step 2
Degrees above 15.6°C: 30°C - 15.6°C = 14.4°C
Hours to reach pH of 5.3: 10
Degree-hours calculation: (14.4°C) x (10) = 144 degree-hours
Step 3
Degrees above 15.6°C: 37°C - 15.6°C = 21.4°C
Hours to reach pH of 5.3: 18
Degree-hours calculation: (21.4°C) x (18) = 385.2 degree-hours
Degree-hours calculation for the entire fermentation process = 84 + 144 + 385.2 = 613.2
The highest temperature reached = 37°C
The corresponding degree-hour limit = 555 (between 33°C and 37°C)
Conclusion: Example 2 does not meet the guidelines because its degree-hours exceed the limit.
11. What happens if fermentation fails to hit critical limit?
What happens if the batch takes longer than degree-hours allows? For restaurant level production, it's always safer to discard the product. The toxin that Staph. Aureus produces is heat stable and cannot be cooked to deactivate. In large facilities that produce substantial batches, the operator must notify the CFIA of each case where degree-hours limits have been exceeded. Such lots must be held and samples of product submitted for microbiological laboratory examination after the drying period has been completed. Analyses should be done for Staphylococcus aureus and its enterotoxin, and for principal pathogens, such as E. coli O157:H7, Salmonella, and Clostridium botulinum and Listeria monocytogenes.
If the bacteriological evaluation proves that there are fewer than 104 Staphylococcus aureus per gram and that no enterotoxin or other pathogens are detected, then the product may be sold provided that it is labelled as requiring refrigeration.
In the case of a Staphylococcus aureus level higher than 104 per gram with no enterotoxin present the product may be used in the production of a cooked product but only if the heating process achieves full lethality applicable to the meat product.
In the case where Staphylococcus aureus enterotoxin is detected in the product the product must be destroyed.
12. E. coli and Salmonella Control in Fermented Sausages
Business' that manufacture fermented sausages are required to control for verotoxinogenic E. coli including E. coli O157:H7 and Salmonella when they make this type of product. This includes:
establishments which use beef as an ingredient in a dry or semi-dry fermented meat sausage;
establishments which store or handle uncooked beef on site;
Establishments which do not use beef and do not obtain meat ingredients from establishments which handle beef are not currently required to use one of the five options for the control of E. coli O157:H7 in dry/semi-dry fermented sausages.
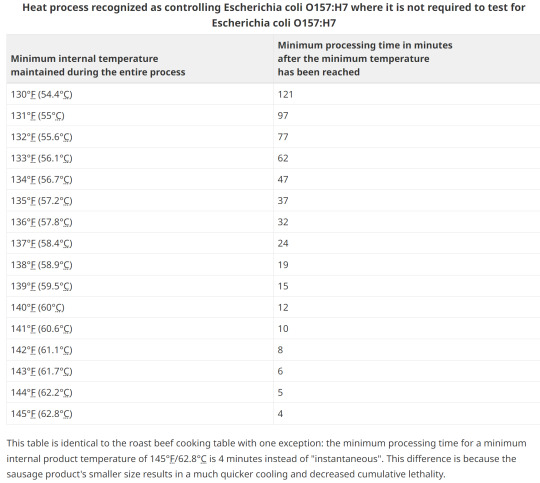
Any processed RTE product containing beef or processed in a facility that also processed beef, must be subjected to a heat treatment step to control E. coli O157:H7. Heating to an internal temperature of 71°C for 15 seconds or other treatment to achieve a 5D reduction is necessary. This is a CFIA requirement and is not negotiable.
Uncooked air dried products produced as RTE, must meet shelf stable requirements as detailed for Fermented-Dry products.
13. Options for E. coli validation
Without lab testing, the two main methods of validation are with heat treating by either low temp and a long duration, or various hotter processing temperatures for a shorter timeframe.
A challenge study to validate a process can take 1 year and over $100,000!
14. Option1; Heating
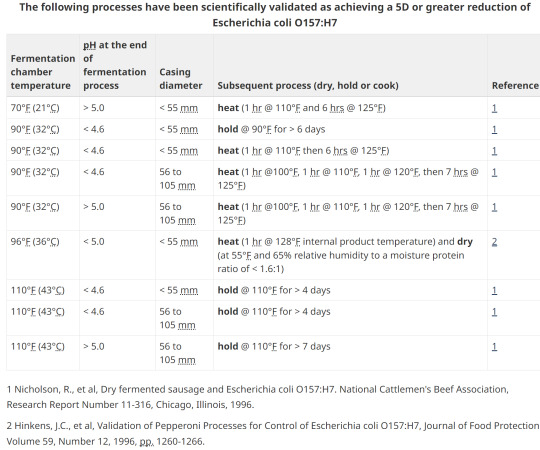
15. Option 2; pH, heating, holding, diameter
16. Safety and consistency
The aw and pH values are critical in the control of pathogens as well as to ensure shelf-stability in all semi-dry and dry fermented meat products. Each batch must be tested for aw and/or pH in order to verify that the critical limits are met.
Although aw measurement is mandatory only for shelf stable products, it is strongly recommended that the producer determine the aw values achieved for each product type they manufacture and for each product. Once this has been established, frequent regular checks should be made to ensure consistency. In the U.S., they rely on moisture to protein ratio and have set targets. This lab-tested value is a direct correlation of the % water to % meat protein and not aw. This gives more consistency to common names. For example, to legally call a product "jerky" it must have a MPR of 0.75:1 or lower.
Remember your ABCs:
Always be compliant.
-AND-
Documentation or it didn't happen.
(tags)
Charcuterie,Fermented Meat,Food Safety,Starter Culture,Chemical Acidification,Water Activity,Fermentation Process,Degree-Hours Method,Foodborne Pathogens,Meat Processing Guidelines,Chef WK Alberta Canada,Food Industry Standards,pH Critical Limits,Thermal Processing,Food Preservation,Food Microbiology,Sausage Fermentation,Charcuterie Expertise,Fermented Meats ,Food Safety Standards,Food Processing Guidelines,Starter Cultures,Chemical Acidification,Water Activity (a_w),Critical Limits,Degree-Hours Method,Foodborne Pathogens,Meat Processing Equipment,Processing Facility Requirements,Hazard Analysis and Critical Control Points (HACCP),Food Preservation Techniques,Temperature Control,Pathogen Reduction,Food Industry Compliance,Documentation Practices,Heat Treatment,pH Control,Food Stability,Consistency in Production,Microbial Testing,Real-time Monitoring,Process Validation,Regulatory Requirements,Verotoxigenic E. coli,Lethality Standards,Product Labelling,Spoilage Prevention,Enterotoxin Detection,Shelf-Stable Products,Moisture to Protein Ratio (MPR)
#Charcuterie#Fermented Meat#Food Safety#Starter Culture#Chemical Acidification#Water Activity#Fermentation Process#Degree-Hours#Meat Processing Guidelines#Thermal Processing#Food Preservation#Food Microbiology#Sausage Fermentation#Starter Cultures#Critical Limits#Meat Processing#Food Preservation Techniques#Temperature Control#Pathogen Reduction#Food Industry#Heat Treatment#pH Control#Food Stability#Microbial Testing#Real-time Monitoring#Process Validation#Spoilage Prevention#Enterotoxin Detection#Shelf-Stable Products#Moisture to Protein Ratio (MPR)
1 note
·
View note
Text
Understand the Spiderman of the Microverse: XLD Agar
Learning XLD’s X, Y, and Z can be challenging, but not with us. In the previous article, we discussed the various needs and applications of XLD (click on the link if you haven’t read it). In this, we’ll talk more about its composition, principle, observations, and modifications.
What is the composition of XLD Agar?
It was developed by Welton Taylor in 1965. XLD stands for Xylose Lysine Deoxycholate Agar and is a bright pink or red-colored solid opalescent gel medium. Knowing its key contents will help you better understand its principles and differentiating abilities.
Ingredients g/l
Yeast Extract 3 g
L-lysine 5 g
Xylose 3.75 g
Lactose 7.5 g
Sucrose 7.5 g
Sodium Deoxycholate 1 g
Sodium Chloride 5 g
Sodium Thiosulfate 6.8 g
Ferric Ammonium Citrate 0.8 g
Phenol red 0.08 g
Agar 12.5 g
Distilled water 1 Litre
Yeast extract provides the medium with nutrients, vitamins, peptides, and other essential growth factors. Xylose, lactose, and sucrose are rich sources of fermentable carbohydrates. For the detection of fermentation of these carbs in the medium, a pH indicator, phenol red, is added. The addition of xylose to the medium accounts for the differentiation of various fermenting enteric bacteria from species of Shigella that do not ferment it. Most enteric pathogens, including Salmonella,can ferment xylose, which results in the formation of acidic byproducts and a change in the color of the medium from pink to yellow, whereas Shigella colonies remain red. In some cases, Salmonella imitates Shigella colonies and gives red colonies, which are then differentiated by the production of Hydrogen sulfide gas by the metabolism of thiosulfate and give black centers. Enterobacteria such as E. coli can ferment lactose in the medium.
XLD Agar’s composition can be adjusted according to one’s needs and choices and is hence available in many different variations, but the basic principle remains the same.
Observation and Inferences on XLD Agar:
Observation Inferences
Red colonies, some with black centers Salmonella spp.
Red colonies Shigella spp.
Yellow to orange colonies Coliforms
Pink, flat, and rough colonies Pseudomonas aeruginosa

While learning about XLD Agar, a question that must have run through your thoughts would’ve been, “Where is XLD Agar available?” Let’s look into it.
Where is XLD Agar available?
XLD Agar is available on the market as Dehydrated Culture Media in a number of variations according to one’s requirements, including plant-based and animal-based options.
These Dehydrated Culture Media can be dissolved in distilled water according to the concentration mentioned on the package, autoclaved, poured, and used.
TM Media, the microbiology division of Titan Biotech Ltd., offers a wide range of XLD Agar as Dehydrated Culture Media in a number of variations suitable for all your needs, operations, and specific requirements.

The product range includes the following:
XLD AGAR MODIFIED (as per ISO) TM 1621: XLD Agar, modified, is a selective and differential medium for the isolation of gram-negative enteric pathogens from clinical specimens or food products. It is a modification of the original formulation of Taylor that allows selective isolation of Salmonella typhi, E. coli, Salmonella enteritidis, Salmonella typhimurium, and Shigella flexneri. It is recommended by the ISO committee, and the composition and performance criteria of this medium are as per the specifications laid down in ISO 6579-1:2017.
XLD AGAR (VEG.) TMV 492: Xylose Lysine Deoxycholate Agar (Veg) is prepared by replacing Sodium Deoxycholate with synthetic detergent No.III, which makes the medium free of BSE/TSE risks. Xylose Lysine Deoxycholate Agar (Veg) is suitable for the isolation and identification of enteric pathogens from stool samples.
XLD AGAR (as per USP/EP/JP/BP) TMH 112: This medium is employed for pharmaceutical testing and non-sterile product testing for the detection of Salmonella after enrichment in Rappaport VassialidiasSalmonella Enrichment Broth in accordance with the harmonized method of USP/EP/BP/JP/IP.
XLD AGAR TM 1448: It is used for pharmaceutical testing and nonsterile product testing for the detection (or absence) of Salmonella after enrichment in Rappaport Vassialidias Salmonella Enrichment Broth in accordance with IP.
XLD AGAR TM 492: XLD Agar exhibits increased selectivity and sensitivity as compared to other plating media, e.g., SS Agar, EMB Agar, and Bismuth Sulphite Agar. The media formulation does not allow the overgrowth of other organisms over Salmonella and Shigella. Samples suspected of containing enteric pathogens, along with other mixed flora, are initially enriched in Modified Semisolid RV Medium Base.
Why choose TM Media’s XLD Agar?
TM Media’s superior-quality and diverse Microbiological Culture Media are certified by ISO, CE, GMP, FSSAI, and FSSC.
TM Media provides unrivaled convenience, dependability, and efficacy, as well as versatility and flexibility, by manufacturing a contamination-free medium with a longer shelf life and reproducible outcomes.
Conclusion:
XLD Agar is a selective and differential agar medium primarily used for the isolation and differentiation of different enteropathogenic organisms that are well-known to cause diseases like Food poisoning, Gastroenteritis, and other Digestive illnesses.
By choosing TM Media, consumers invest in precision, quality, and excellence. TM Media's product portfolio includes over 2000 Dehydrated Culture Media, along with Ready-to-Use Culture Media, Biological Media Bases, Media Supplements, Lab Chemicals, and many more.
#XLD Agar#dehydrated culture media#food microbiology#clinical microbiology#Microbiology#Xylose Lysine Deoxycholate Agar
0 notes
Text
Prebiotics Market by Ingredient (Inulin, Fructo Oligosaccharides, Galacto Oligosaccharides, Mannan Oligosaccharides), Application (Food & Beverages {Dairy Products, Beverages, Infant Food Products}, Dietary Supplements), and Geography - Global Forecast to 2029
#Prebiotics Market#prebiotics#microbiology#food microbiology#nutrition#nutraceuticals#food#food and nutrition#food and beverages#food industry
0 notes
Text
Obesity in Obstetrics

Mini review
The people in industrialized countries have experienced a dramatic increase in obesity in recent times. Prevalence of obesity has doubled in the last 25 years. In the United States, 17-th on the list of most obese places in the world - average BMI 28.8 Kg/m2, more than 60% of reproductive-age women are overweight and 35% are obese, representing a 70% increase in pre-pregnancy obesity. In Romania, 75th on the list- average BMI is 22.2 Kg/m2, the lowest average BMI in the European Union (9.4% obesity in 2016). [1] One of three Romanians is overweight, and one of four is obese. There are over 3.5 million obese in Romania. The highest obesity rate is recorded in Moldova, where the percentage is 23.8%. Only 10% of them see a doctor. Only one percent are included in a national obesity education program [2].
Not all ethnic groups are at equal risk. Of particular concern is the rapid increase in adolescent overweight and obesity. Concordantly, pregnancy obesity rates are also increasing. Obesity is associated with increased morbidity and 6- to 12-fold increase in mortality. Obesity is highly complex in terms of etiology and prevalence. Genetic predisposition, race, socioeconomic status, built environment (e.g., the presence of sidewalks or community design), accessibility of healthy and affordable foods, sleep habits, and geographic region all play a role. Lifestyle changes, which include consuming foods and beverages with a high glycemic index, increased food portion sizes, decreased structured physical activity, and increased screen-based sedentary behavior, have influenced the prevalence of obesity.
Antenatal Monitoring
An evaluation of dietary intake and exercise habits can provide insight into women at risk. All pregnant women without contraindications should participate in regular exercise. During prenatal visits women should be questioned and advised about their diet and exercise habits. Where available, nutritional counselling can be a helpful adjunct for women not meeting the weight gain recommendations.
The sonographer’s ability to evaluate fetal structures is largely dependent on maternal size. Approximately 15% of normally visible structures will be sub optimally seen in women with a BMI above the 90th percentile. In women with a BMI above the 97.5th percentile, only 63% of structures are well visualized. Obstetric care providers should take BMI into consideration when arranging for fetal anatomic assessment in the second trimester. Anatomic assessment at 20 to 22 weeks may be a better choice for the obese pregnant patient.
Use all available technical tools improving image quality in obesity: lower transducer emission frequencies; harmonic imaging; compound imaging; speckle reduction filters. Consider approaching the fetus through the four major abdominal areas with least subcutaneous fat: periumbilical area, suprapubic area, right and left iliac fossae. Consider using the transvaginal approach for the assessment of the central nervous system (CNS) in fetuses in vertex presentation.
Gently inform the patient and her partner that obesity will reduce the diagnostic accuracy of the scan. Consider including the BMI value among the demographic data in the report to document the presence or absence of maternal obesity. Report other cofactors of limited acoustic window, such as previous cesarean section (for the scar), twinning and myomata.
Pregnancy Complications
The risk of spontaneous abortion is increased in obese women. Lashen et al. identified an odds ratio for spontaneous abortion of 1.2 (95% CI 1.01 to 1.46) for obese women (BMI > 30 kg/m2). The authors also identified an increased risk of recurrent early miscarriages (more than 3 successive miscarriages < 12 weeks’ gestation) in the obese population, odds ratio 3.5 (95% CI 1.03to 12.01).[8] Similar risks have been identified in obese women undergoing in vitro fertilization treatment [3].
Pre-gestational diabetes is more prevalent in obese women. Therefore, testing during early in pregnancy for women with risk factors is recommended. Obese women are also at increased risk of developing gestational diabetes (GDM). Not surprisingly, obese women are also at increased risk of having a macrosomic child. Physical activity is inexpensive and can significantly reduce the risk of gestational diabetes. More relevant to the obese population, they also reported a 34% reduction in the development of gestational diabetes in women who did not participate in vigorous exercise but who did participate in brisk walking compared with those who participated in easy pace walking. Women with GDM have a 30% chance of developing type 2 diabetes later in life [4].
Intrapartum Complications and Management
Macrosomia and shoulder dystocia
The use of antenatal ultrasound to detect fetal macrosomia is associated with such obstetric interventions as labor induction and cesarean section. The rate of cesarean section is affected. Higher cesarean section is more frequent when ultrasound examination indicates a macrosomic fetus.
Fetal monitoring
The obese abdominal wall may make monitoring more difficult than in other cases, and of course, the positive predictive value of antenatal testing (e.g. cardiotocography, nonstress testing, biophysical profile assessment) is limited. There is no evidence to support the routine use of internal fetal monitoring in this population, but it may be more effective in some women. Monitoring contractions and ensuring adequate labor in obese women poses a special challenge. Obese women require more oxytocin in labor. Consider allowing longer first stage of labor before performing a cesarean for labor arrest. Although most obstetric care providers rely on manual palpation and/or external tocometry, the use of an intrauterine pressure catheter may be advantageous in some cases.
Cesarean section
The risk of cesarean section is increased in the obese parturient. The increase in cesarean section rate may be partly due to the fact that overweight and obese nulliparous women have a slower progression of the first stage of labor. When faced with lack of descent in the second stage of labor, some practitioners may opt for cesarean section rather than operative vaginal delivery because of concerns about fetal macrosomia and shoulder dystocia. This may explain the low rate of operative vaginal delivery in some series [5]. Obese women undergoing caesarean section experience more complications, including blood loss > 1000 mL, increased operative time, increased postoperative wound infection and endometritis, and need for vertical skin incision. The obese diabetic women who undergo cesarean section have an odds ratio for postoperative wound infection of 9.3 (95% CI 4.5 to 19.2), and those who require a vertical skin incision have a 12% rate of wound complication serious enough to require opening the incision [6].
For morbidly obese patients, two standard 50-cm-width operating tables secured together may be necessary. Specially constructed wider operating tables would be ideal. Weighing scales suited for obese patients are necessary not only to measure weight and evaluate weight gain during pregnancy, but also for calculating medication dosages. A wider delivery bed that is easy to move around and that may be used at all stages of delivery, including cesarean section, without the need to move the patient into another bed is most useful. Nursing care of obese patients requires ergonomic adaptation and knowledge about the special risks involved in caring for these patients. More trained nurses are necessary to care for morbidly obese patients.
The decision-to-delivery interval may be longer when an emergent or urgent cesarean section is required in obese parturient. Causes for this delay may include patient transport and bed transfer, the time to establish adequate anesthesia, and the operative time from incision to delivery. The 30-minute rule of emergency cesarean section is an arbitrary threshold rather than an evidence-based standard.
Vaginal birth after cesarean section
In the absence of contraindications, women who have had their first child by cesarean section are asked to consider vaginal birth in subsequent pregnancies. The success of vaginal birth after cesarean section is commonly quoted at 80% [7]. Obese women are less likely than their lean peers to be successful in delivering vaginally after previous cesarean section (VBAC). In women with a BMI > 29 kg/m2 the success rate is 54% to 68% [8]. The success rate is further reduced in even heavier women. Chauhan et al. found a 13% VBAC success rate in women >300 lbs (136 kg) [9].
Thromboembolism
The risk of thromboembolism is high in obese parturients. Edwards et al. reported 683 obese women (BMI > 29 kg/m2) who were matched to 660 normal weight women (BMI 19.8 to 26.0 kg/m2). The incidence of thromboembolism was 2.5% in the obese women, and only 0.6% in the controls.[29] BMI >30 plus one additional risk factor qualify for seven days of postpartum Clexane; BMI >30 plus two additional risk factors require Clexane antenatally and for 6 weeks postpartum; BMI>40 should be regarded as already having two risk factors. Clexane dosage should be calculated by weight:
Early mobilization and T.E.D. anti-embolism stockings are clinically proven to reduce the incidence of deep vein thrombosis by up to 50% and to promote increased blood flow velocity in the legs 138% of baseline by compression of the deep venous system.
Perinatal outcomes
Maternal obesity is also an established risk factor for stillbirth. The reported risk of stillbirth is 2-5 times higher in obese compared with normal-weight women. The risk of stillbirth associated with obesity increases with gestational age. Infant mortality rates increase from 2.4/1000 among normal weight women (BMI 18.5-24.9) to 5.8/1000 among women with grade 3 obesity (BMI ≥ 40.0). Maternal overweight and obesity are associated with increased risks of infant mortality due to increased mortality risk in term births and an increased prevalence of preterm births. Maternal obesity may increase the risk for intellectual disability or cognitive deficits in offspring from 1.3- to 3.6-fold. Maternal prepregnancy obesity and high gestational weight gain of > 18 kg was associated with a 3-fold increase in offspring IQ deficit (mean of 6.5 points lower) [10]. The majority of studies that have examined a link between high maternal BMI and childhood diagnosis of autism spectrum disorders have found a significant positive association. This risk may be further augmented by intrauterine growth restriction (IUGR), preterm birth, high gestational weight gain, gestational or pre-gestational diabetes, and preeclampsia [11].
Conclusion
A national information campaign is required to exploit women’s interest in having as healthy a pregnancy as possible by giving them the information they need to become fit and have a normal BMI before they consider pregnancy. Periodic health check-ups and other appointments for gynecologic care prior to pregnancy offer ideal opportunities to raise the issue of weight loss before conception. Women should be encouraged to enter pregnancy with a BMI < 30 kg/m2, and ideally < 25 kg/m2. Although obesity is not an indication for the transfer of routine obstetric care, consultation with or referral to physicians with expertise in obesity may be appropriate if the obstetrician cannot safely and effectively care for the patient because of the lack of the specialized training, experience or institutional resources.
To Know More About Nutrition and Food Science International Journal
Please click on: https://juniperpublishers.com/nfsij/index.php
For more Open Access Journals in Juniper Publishers
please click on: https://juniperpublishers.com/index.php
1 note
·
View note
Text

0 notes
Text
Antibacterial Efficacy of Vernonia Amygdalina Against Bacteria Strains Recovered from Hospital Fomites, Nigeria
Abstract
This study was carried out to evaluate the occurrence of bacteria from hospital fomites and the antibacterial activity extract from Vernonia amygdalina against bacteria isolates. The colonies obtained were subjected to colonial characteristics and conventional biochemical test with reference to Bergey’s Manual of Determinative Bacteriology. The antibiotic susceptibility of the isolates was performed using the Kirby-Bauer’s disc diffusion methods while the antimicrobial activity of the extract was performed by using well diffusion method. Proteus species (18%) were the most prevalent bacteria followed by Staphylococcus spp (16%) while Actinobacter spp and Photobacterium spp have the least of 1%. All the isolates showed high resistant (100%) to various antibiotics tested while they are sensitive to ofloxacin. The bioactive extract of Vernonia amygdalina revealed the presence of some active medicinal constituent. The antibacterial activity of the extract against the organisms produced a zone of inhibition which ranged between 4.5-15mm at 100mg/ml concentration while it ranged between 2.0-12.1mm at 50mg/ml. In conclusion, this study showed that hospital fomites harbour highly pathogenic bacteria which have the potentials of causing epidemics in the nearest future. Therefore, the efficacy of Vernonia amygdalina against clinical resistant isolates could be explored for further pharmaceutical use and should be encouraged in the formulation and production of new antibiotics.
Read more about this article: https://lupinepublishers.com/biotechnology-microbiology/fulltext/antibacterial-efficacy-of-vernonia-amygdalina-against-bacteria-strains-recovered-from-hospital-fomites-nigeria.ID.000131.php
Read more Lupine publishers Goggle Scholar Articles: https://scholar.google.com/citations?view_op=view_citation&hl=en&user=X4tPijcAAAAJ&citation_for_view=X4tPijcAAAAJ:M05iB0D1s5AC
1 note
·
View note
Text
“Why do we have Peyer’s patches, if not for this?”
Before consuming food of dubious origins.
#medicine#med school#university#microbiology#histology#immunology#food poisoning#food safety#gastroenterology#pinned
105 notes
·
View notes
Text
So. Much. Life !!!
(Rotten milk... Again... And these little things swimming everywhere are paramecia)
#life#microscope#biology#microbiology#microscopic life#microorganisms#nature#little animals#paramecium#milk#rotten#food#protists#paramécie#disgusting#organisms#organic#eukaryotes#cells
130 notes
·
View notes
Text
Well, not even antidepressants can help me from being on the verge of tears from hearing an older coworker you actually really like and respect absolutely going to town while blasting you and how much you fucked up to another coworker.
I will never do anything for this lab again.
#i didn't fuck up!#i did exactly what my boss told me!#and what also you told me#they were in the office across from mine talking so so loudly#i hate this job i hate this job#so there are these new guidelines for food microbiology and we have the old ones too#the lab boss told me to add the new ones#i did it on the last worker day of 2023#and last week i was like should i delete the old ones#i asked him multiple times if he was sure#and he said yes#so i deleted them#and now it's my fault#and apparently that also messed up the whole system which it didn't and it couldn't#i hate it here#and she's going basically from person to person saying O fucked up#like she was talking to the head of s completely different lab right now#my lab boss stood up from me yesterday apparently#it just doesn't make me feel any better because he's like the only one
2 notes
·
View notes
Text
Fungal Protein Market By Type (Fusarium Venenatum Extract, Mushrooms, Yeast Extract), Application (Food & Beverage {Beverages, Bakery}, Animal Nutrition {Poultry Feed, Aquafeed}, Pharmaceuticals, and Other Applications), and Geography - Global Forecast to 2029
#Fungal Protein Market#Fungal Protein#mushrooms#yeast extract#plant based#plant based food#nutrition#biotechnology#food microbiology#food#food industry
1 note
·
View note
Text
Pandanus Conoideus Lamk Protects Inflammation by Regulating Reactive Oxygen Species and Nuclear Factor Kappa B in Lps-Induced Murine Macrophages
Abstract
Background: Pandanus conoideus Lamk (Red fruit) is a Papuan traditional food which has been used to treat various diseases. Despite these various effects of Red fruit, little is known about the physiological mechanism. Aims: The aim of this study was to investigate the anti-inflammatory properties of Red fruit oil (RFO) and establish the signal pathway of leading compounds.
Methods: Raw 264.7 murine macrophage cells were used with lipopolysaccharide (LPS). Cell viability and the pro-inflammatory factors were investigated using MTT assay, real time PCR, western blot analysis, and Enzyme linked immuno-sorbent assay (ELISA). The quantification of leading compounds in RFO was performed using high performance liquid chromatography (HPLC).
Results: RFO did not affect cell viability. RFO significantly reduced the production of nitric oxide (NO) and prostaglandin E2 (PGE2), and both the protein level and mRNA level of iNOS in LPS-induced macrophages. RFO also regulated the reactive oxygen species (ROS) in LPS-induced macrophages. RFO attenuated the translocation of NF-κB p65 subunit, phosphorylation of I-κB, extracellular signal-regulated kinase (ERK), and c-Jun N-terminal kinase (JNK) in a dose-dependent manner. HPLC analysis determined that 1 g of RFO had 14.05±0.8 mg of β-cryptoxanthin and 7.4±0.7 mg of β-carotene.
Conclusion: RFO provides an anti-inflammatory effect by regulating ROS and NF-κB through MAPK due to the antioxidant activity.
Keywords: Pandanus conoideus Lamk; Macrophages; Anti-inflammation; ROS; NF-κB; β-cryptoxanthin
Abbreviations: RFO: Red fruit (Pandanus conoideus Lamk ) oil; LPS: Lipopolysaccharide; NO: Nitric oxide; iNOS: Inducible NO synthase; IL: Interleukin; ROS: Reactive oxygen species; ELISA: Enzyme linked immuno-sorbent assay; HPLC: High performance liquid chromatography; COX-2: Cyclooxygenase-2; PGE2: Prostaglandin E2; ERK: Extracellular signal-regulated kinase; JNK: c-Jun N-terminal kinase; MAPK: Mitogen-activated protein kinase; DMEM: Dulbecco’s modified Eagle medium; FBS: Fetal bovine serum; DCFH-DA: 2’7’-dichlorofluorescein diacetate; MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; RT- PCR: Real time polymerase chain reaction
Introduction
The inflammation process is tightly regulated by both initiation and maintenance signals and considered to be a major risk factor in the pathogenesis of chronic diseases where the macrophages are important immune cells which regulate inflammation producing expression of inflammatory proteins and pro-inflammatory chemokines, cytokines, and nitric oxide (NO) [1,2]. Macrophages are highly sensitive to initiators of inflammation as lipopolysaccharide (LPS) which respond by the release of mediators not only interleukins (ILs) and cytokines, but also inducible NO synthase (iNOS) and reactive oxygen species (ROS), which inducing the inflammatory gene expression where each is associated somehow with the pathophysiological of the inflammation [3-5]. Because macrophages produce a wide range of biologically active molecules participated in both beneficial and detrimental outcomes in inflammation, modulation of macrophage activation is a good strategy to prevent this diseases. Red fruit (Pandanus conoideus Lamk) is Papuan traditional food which has been used to treat various diseases such as cancer [6] preeclampsia [7], hepatitis [8], liver cirrhosis [9], diabetes mellitus [10], and sinusitis [11]. This bioavailability of red fruit has been due to unsaturated fatty acids such as palmitoleic acid, oleic acid, linoleic acid, linolenic acid and some carotenoids [10,12]. Despite these many biological effects, few researches were reported on the mechanism of red fruit oil (RFO). β-cryptoxanthin is a typical carotenoid found abundantly in persimmon, papaya, paprika, and carrot. β-cryptoxanthin has been reported to possess several beneficial functions, such as antioxidant, cancer-preventive effects, and anti-metabolic syndrome effects [13-16]. In present study, we hypothesized that the cause of this anti-chronic inflammation and anti-cancer effect is due to antioxidant function of RFO, and evaluated the anti-inflammatory effect of RFO on LPSstimulated RAW 264.7 macrophage cells. We also investigated the mechanism of inflammatory effect of reduced ROS by RFO in LPS-stimulated macrophages and investigated the component of β-cryptoxanthin in RFO.
Materials and Methods
Chemicals and reagents
RFO (APOTEK®) was supplied from Smile international Co., Ltd (Seoul, Korea). Dulbecco’s modified Eagle medium (DMEM), fetal bovine serum (FBS), and penicillin–streptomycin was purchased from Corning (Oneonta, NY, USA). 2’7’-dichlorofluorescein diacetate (DCFH-DA) and anti-iNOS antibody were purchased from BD (San Jose, CA, USA). Peroxidase-conjugated secondary antibodies and TriZol were purchased from Life technologies (Grand island, NY, USA). Phosphor-JNK, phosphor-ERK, phosphor-p38, phosphor-IκB and NF-κB antibodies were purchased from Cell Signaling Technology Inc. (Beverly, MA, USA). The enzyme immunoassay kit used for prostagladin E2 (PGE2) was obtained from R&D Systems (Minneapolis, MN, USA). The ECL detection reagents were purchased from GE Healthcare (Buckinghamshire, UK). LPS (Escherichia coli 0111: B5) was purchased from Creative Biolabs (Shirley, NY, USA). β-actin, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and other chemicals were purchased from Sigma–Aldrich (St. Louis, MO, USA).
Cell culture
RAW 264.7, the murine macrophage cell line was purchased from American Type Culture Collection and maintained in DMEM supplement with 1 mg/mL glucose, 10% FBS, 100 mg/mL penicillin-streptomycin at 37 °C with 5% CO2
Cell viability assay
The cytotoxic effect of RFO against RAW264.7 cell lines was evaluated by MTT assay. Briefly, cells were seeded at a density of 5 × 103 cells/well in a 96-well plate for 24 h. Then, the cells were treated with at various concentrations of fractions with or without 1 μg/mL LPS. After 24 h, 2 mg/mL MTT was added onto each well, then incubated until formazan was constituted for 3h. The formazan was dissolved in dimethyl sulfoxide (DMSO) and the absorbance at 550 nm was measured using microplate reader (Molecular Devices, Sunnyvale, CA). Cell viability was calculated as a percentage of viable cells in drugs treated group versus untreated control. Each experiment was repeated three times.
Nitrite assay
Cells were treated with various concentrations of RFO for 30 min and incubated with 1 μg/mL LPS for 24 h. Because NO production is reflected in the accumulation of nitrite in the cell culture medium, 50 μL of supernatants were removed and mixed with the same volume of Greiss reagent (Promega, Madison, WI). After incubation for 10 min, the absorbance of mixture at 450 nm was measured using a spectrophotometer (TECAN, Austria). The nitrite levels were estimated as the percentage of absorbance of the sample to the respective controls.
Cyclooxygenase2 (COX-2) assay
Cells were treated with various concentrations of RFO for 30 min and incubated with 1 μg/mL LPS for 24 h. After incubation, the supernatants were removed and followed COX-2 measurement. The COX-2 concentrations were evaluated using a specific enzyme immunoassay (EIA) kit (Cayman, Ann Arbor, MI) according to the manufacturer’s instructions.
Prostaglandin E2 assay
Cells were treated with various concentrations of RFO for 30 min and incubated with 1 μg/mL LPS for 24 h. After incubation, the supernatants were removed and followed PGE2 measurement. The PGE2 concentrations were evaluated using a specific enzyme immunoassay (EIA) kit (Cayman, Ann Arbor, MI) according to the manufacturer’s instructions.
iNOS gene measurement by real-time PCR
The cells from the supernatants had been removed were subjected to RNA isolation. RNA isolation was performed using TRIzol reagent according to the manufacturer’s instructions. cDNA was synthesized using hyperscript RT master mix (GeneAll, Daejeon, Korea). The primers were described as; iNOS forward: 5′-ATGTCCGAAGCAAACATCAC-3′, reverse: 5′-TAATGTCCAGGAAGTAGGTG-3′, and GAPDH forward: 5′-TGTGATGGTGGGAATGGGTCAG-3′, reverse: 5′-TTTGATGTCAC GCACGATTTCC-3′. The PCR was amplified using ABI 7500 and Taqman gene expression master mix (Applied Biosystems, Waltham, MA, USA). The quantitative analysis was performed to compare the Δ Δ Ct after the normalization by GAPDH as an internal control. After analysis, PCR products were electrophoresed on 3% agrose gel and images were taken by cybergreen detection using Kodak imagestation FX® (Kodak, Rochester, NY, USA)
Analysis of ROS by flowcytometry
Cells were treated with various concentrations of RFO for 30 min and incubated with 1 μg/mL LPS for 24 h. Cells were followed by the addition of 10 mg/mL DCFH-DA). The suspensions were washed with PBS after incubation for 20 min. The suspensions were then assayed with a flowcytometer (C6 Accuri, BD, Bedford, MA, USA) according to Rhee et al. [4].
Western blot analysis
Cells were treated as described previously, then total lysates were prepared with lysis buffer (50 mM Tris (pH 7.4), 300 mM NaCl, 5 mM EDTA (pH 8.0), 0.5 % Triton X-100, 1 mM aprotinin, 1 mM leupeptin, 1mM pepstatin, 10mM iodoacetamide, and 2 mM phenylmethylsulfonyl fluoride (PMSF). Meanwhile, each nucleus extracts and cytosol extracts were isolated using a NE-PER nuclear and cytoplasmic extraction reagent kit (Pierce, Rockford, IL). Briefly, cells were washed with PBS, and were prepared with ice-cold extraction buffers sequentially. After centrifugation at 16,000xg, the cytoplasmic protein and nuclear extract were separated. Total lysates and nuclear fractions were estimated with Bio-Rad dye reagent concentrate (Bio-Rad Laboratories, Hercules, CA), then resolved on a 10% SDS-PAGE. After electrophoresis, the proteins were electro transferred to a PVDF membrane, blocked with 1% BSA, and probed with anti-iNOS (1:1,000), phospho- JNK (1:1,000), phospho-ERK (1:1,000), phospho-p38 (1:1,000), phospho-IκB (1:1,000), and NF-κB (1:500) antibodies at 4 °C overnight. The blot was washed, exposed to HRP-conjugated secondary antibodies for 2 h, and finally developed through enhanced chemiluminescence. For ß-actin detection, previously used membranes were soaked in stripping buffer (62.5 mM Tris- HCl, pH 6.8, 150 mM NaCl, 2% SDS, 100 mM ß-mercaptoethanol) at 65 ℃ for 30 min and hybridized with anti-ß-actin. The relative protein expression was densitometerically quantified using the BioRad GS-670 densitometer (BioRad, Hercules, CA) and normalized to β-actin.
High performance liquid chromatography (HPLC)
To determine the content of β-cryptoxanthin in RFO, we performed HPLC analysis according to previous studies [17]. HPLC analysis was performed using Agilent 1100 model with a pump (G1311C), auto sampler (G1329B), column, and diode array detector purchased from Agilent (Santa Clara, CA, USA). The analysis conditions are described in Table 1.
Statistical analysis
All results are presented as mean ± S.D. and are representing three or more independent experiments. Data were compared using the one-way ANOVA using Prism® (GraphPad, La Jolla, CA, USA) with p-values less than 0.05 considered statistically significant.
Results
RFO did not affect cell viability
Figure 1A showed the effect of RFO on viability of RAW 264.7 with or without LPS. Cell viability was not affected against 10- 1,000 μg/mL of RFO with or without LPS.
RFO reduced NO in LPS-induced macrophages
To assess the effects of RFO on NO production in LPSinduced RAW 264.7 macrophages, cells were treated with various concentrations of RFO for 30 min, then incubated with 1 μg/mL LPS for 24 h. NO release was elevated 224 ± 19.24% (p < 0.001) following LPS treatment, which was reduced 224 ± 19.24% at 10 μg/mL (p < 0.05), 161.38 ± 21.81% at 25 μg/mL (p < 0.001), and 136.16 ± 30.56% at 50 μg/mL (p < 0.001) with RFO combination (Figure 1B).
RFO decreased COX-2 production in LPS-induced macrophages
COX-2 production was significantly increased from 33.17 ± 5.23 ng/mL to 86.25 ± 1.88 ng/mL (p < 0.001) following LPS treatment. However, it was reduced 60.52 ± 12.49 ng/mL at 10 μg/mL (p < 0.05), 32.16 ± 8.85 pg/mL at 25 μg/mL (p < 0.001), and 13.27 ± 1.67 ng/mL at 50 μg/mL (p < 0.001) with RFO combination (Figure 1C).
RFO also decreased PGE2 production in LPS-induced macrophages
Meanwhile, PGE2 production was significantly increased 440.6 ± 35.36 pg/mL (p < 0.001) following LPS treatment, which was reduced 227.5 ± 13.6 pg/mL at 10 μg/mL (p < 0.001), 180.77 ± 48.95 pg/mL at 25 μg/mL (p < 0.001), and 103.27 ± 51.67 pg/ mL at 50 μg/mL (p < 0.001) with RFO combination (Figure 1D).
RFO suppressed both mRNA and protein levels of iNOS in LPS-induced macrophages
To determine the inhibitory effects of RFO on proinflammatory mediator NO, COX-2, and PGE2 production, the biosynthesis of transcriptional levels of iNOS was performed with semi-quantitative reverse-transcription PCR and western blot analysis on LPS-induced RAW 264.7 macrophages. Figure 1D indicates that both mRNA level and protein level of iNOS were significantly decreased by treatment of RFO (p < 0.001). Consistent with the findings shown in Figure 1E, RFO had a significant concentration-dependent inhibitory effect on the inflammation through pro-inflammatory mediator NO in LPSinduced RAW 264.7 macrophages.
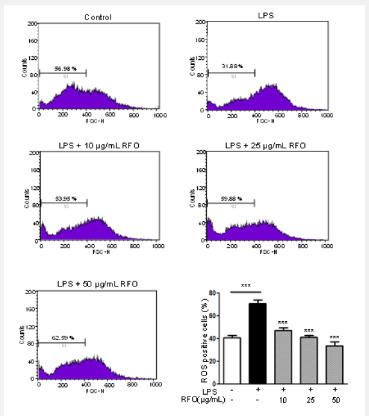
RFO attenuated ROS in LPS-induced macrophages
The excess ROS is known to be injured intracellular proteins, lipids and nucleic acids and induce inflammation [18]. Thus, we investigated the ROS production in response to LPS using flowcytometry. DCFH-DA binds ROS produced cells. Figure 2 showed the DCFH-DA positive cells were increased following LPS treatment from 40.71 ± 2.11% to 70.87 ± 3.09%. However, ROS production was also significantly inhibited by RFO with a dose dependent manner; 47.08 ± 2.45% at 10 μg/mL (p < 0.001),41.34 ± 1.41% at 25 μg/mL (p < 0.001), and 33.76 ± 3.56% at 50 μg/mL (p < 0.001).
RFO suppressed nuclear translocation of the NF-κB p65 subunit via MAPKinase
Since p65 is a major component of NF-κB activated by LPS in macrophages, we evaluated the levels of p65 in nuclear extracts by western blotting analysis. Phosphorylation of IκB results in degradation and release of NF-κB, which then translocates to the nucleus. Therefore, we examined whether RFO could prevent phosphorylation of IκB induced by LPS treatment. Figure 3A shows that IκB phosphorylation was increased by treatment with LPS alone in cytosol level, but that such phosphorylation was significantly inhibited in the presence of RFO, similar to results for the nuclear translocation of p65. Taken together, these data suggest that the inhibitory effect of RFO on the LPS-induced translocation of p65 might be involved in the suppression of IκB phosphorylation. To further investigate whether the inhibition of pro-inflammatory mediators by RFO is modulated through the MAPK pathway, we evaluated the effects of RFO on the LPSinduced phosphorylation of p38, ERK, and JNK (Figure 3B). RFO suppressed LPS-induced phosphorylation of p38, ERK and JNK. These results suggest that RFO blocks MAPK pathways to suppress the inflammatory response in LPS-induced RAW 264.7 macrophages.
HPLC analysis of RFO
Table 2 showed the HPLC analysis of RFO. HPLC revealed that 1 g of RFO had 14.05±0.8 mg of β-cryptoxanthin and 7.4 ± 0.7 mg of β-carotene.
Discussion
Inflammation is an immune response that protects our body against host response to infection and injury [19,20]. All inflammatory responses act through mononuclear cells, macrophages, and lymphocytes. Macrophages play on important innate immune effectors and increase pro-inflammatory factors including nitric oxide (NO), prostaglandin E2 (PGE2) cytokines.
The excessive amounts of NO and PGE2 produced by activation of iNOS and COX-2 in response to LPS play an important role in inflammation [21,22]. The overproduction of iNOS-derived NO is involved in the pathology of various inflammatory disorders and tissue damage conditions. A change in the NO level through the inhibition of iNOS enzyme activity or iNOS induction provides a means of assessing the effect of these agents on the inflammatory process. iNOS is implicated in the synthesis of prostaglandin H2 starting of arachidonic acid, which is a precursor of PGE2, in activated macrophages with LPS [23]. In addition, iNOS leads to overproduction of NO, PGE2, and COX-2 which results in the production of inflammatory diseases. Thus, modulation of iNOS and NO expressions could be one of the strategies to reduce inflammatory diseases. The production of inflammatory cytokines is a crucial part of regulating inflammation and tumor progression. The key signaling pathway mediating the inflammatory response, the NF-κB transcription factor, has been well-established in various inflammatory diseases and cancers [24,25]. It is also well known that NF-κB is a significant role factor regulating the expression of inflammation-associated enzymes and cytokine genes, such as iNOS, COX-2, TNF-α and IL-1β, which contain NF-κB binding motifs within their respective promoters [1,26]. Therefore, this signaling pathway is a good target for anti-cancer and antiinflammatory drug development. Many of the upstream kinases and downstream substrates are the same for the each of the major cascades. Our results revealed that anti-inflammatory activities of RFO are mediated through the inhibition of IκB phosphorylation and nuclear translocation of the NF-κB p65 subunit. Besides, these results also indicate that the inhibitory effects of RFO on MAPK and NF-κB signaling are related to a decrease in ROS. It is well known that oxidative stress stimulates ROS production in RAW 264.7 cell line [11,27]. Our data showed the pretreatment with RFO significantly decreased ROS production in LPS-induced RAW264.7 cells using DCFH-DA staining which demonstrated that RFO had a potent to reduce the oxidative stress. We also suggested that RFO regulated MAPK and NF-κB signaling of inflammation operate through oxidative stress. These results demonstrated that RFO could act as scavenging agents or acting on redox state of the cell and other acting as scavenging agents. In previous study, we already demonstrated that RFO regulated the cellular senescence through ROS modulation in H2O2-induced endothelial cells [5].
Carotenoids such as β-cryptoxanthin, β-carotene are one of the antioxidants which are not produced in the human body that must be ingested from outside. Many studies indicated that healthy people had the higher level of β-cryptoxanthin in blood [28-31]. β-cryptoxanthin is the only provitamin A component of carotenoid-based xanthophylls [14,32]. Carotenoids are lipid soluble components that must be ingested with fat to absorb completely in the body. Carotenoids affect the inflammation levels in blood as strong antioxidants and helps purify the blood. Park et al. showed that the daily oral administration of β-cryptoxanthin prevented the progression of osteoarthritis and inhibited proinflammatory cytokines in mice [33]. Therefore, we examined the effects of RFO on the production of several inflammatory mediators and on the expression levels of iNOS in LPS-induced RAW 264.7 macrophage cells. Our results demonstrated that RFO inhibited the expression of iNOS as well as the production of NO and PGE2 and the mechanisms underlying the suppression of the inflammatory response of the NF-κB and ROS. According to the US USDA database, β-carotene content of RFO was significantly higher at 335 times of blackberry, 119 times of broccoli, 13.9 times of pumpkin, and 5.2 times for carrot [34,36]. In addition, β-cryptoxanthin content of RFO was significantly higher at 76 times of orange and 15 times of papaya [30,37]. These findings suggested that RFO might be a beneficial therapeutic agent in the treatment of a variety of inflammatory diseases.
Conclusion
RFO is Papuan traditional food and had been used to treat various disease for long time. In this study, we suggested RFO had an anti-inflammatory effect through regulating inflammatory mediators such as iNOS, COX-2, PGE2, and excessive ROS for the first time. These physiological benefits of RFO may be attributed by regulation of NF-κB transcription. HPLC indicated that large number of carotenoids such as β-cryptoxanthin, β-carotene. This finding may be a synergistic adjuvant therapy for inflammatory diseases by acting as a radical scavenger, ROS inhibitor.
To Know More About Nutrition and Food Science International Journal
Please click on: https://juniperpublishers.com/nfsij/index.php
For more Open Access Journals in Juniper Publishers
please click on: https://juniperpublishers.com/index.php
#food safety#food microbiology#food preservation#Food Engineering#Juniper publishers USA#open access journals
0 notes
Text
so the biggest thing in this whole fructose malabsorption thing I've been diagnosed with and trying to figure out is the fructose to glucose ratio in foods. apparently, when glucose is consumed at the same time as fructose, it increases fructose absorption. so total fructose content, while important, isn't as important as making sure more glucose is consumed than fructose.
which means.
that fucking BRUSSELS sprouts, the vegetable notorious for being bitter and gross, are on the list of things I have to be careful with, bc while they have negligible sugar, that sugar very slightly is more fructose than glucose.
#why does glucose aid in fructose absorption? fuck if I know#I don't know about nutrition really#I got a C in biochem#my biology degree focused on environmental biology and microbiology#I don't know shit my guy#I'm operating off the organic chem courses I took years ago and they aren't as much help as I'd like#anyways now that I've recovered from being sick I'm back to food diary stuff#bc the general gist of everything I've been told is#''well there's broad rules but everyone is different so you're on your own to find out what you can do good luck''#uuuuuuuuuuugggggggggggghhhhhhhh#speechie sucks at health#speecher speaks
2 notes
·
View notes
Text
By now you might have started to suspect: Cheese is fundamentally about decomposition. Like microbes on a rotten log in the woods, the bacteria and fungi in cheese break down their environment — in this case, the milk fats and proteins. This makes cheeses creamy and gives them flavor.
Mother Noella Marcellino, a longtime Benedictine cheesemaker at the Abbey of Regina Laudis, put it this way in a 2021 interview with Slow Food: “Cheese shows us what goodness can come from decay. Humans don’t want to look at death, because it means separation and the end of a cycle. But it’s also the start of something new. Decomposition creates this wonderful aroma and taste of cheese while evoking a promise of life beyond death.”
Exactly how the microbes build flavor is still being investigated. “It’s much less understood,” says Mayo. But a few things already stand out. Lactic acid bacteria, for example, produce volatile compounds called acetoin and diacetyl that can also be found in butter and accordingly give cheeses a rich, buttery taste. A yeast called Geotrichum candidum brings forth a blend of alcohols, fatty acids and other compounds that impart the moldy yet fruity aroma characteristic of cheeses such as Brie or Camembert. Then there’s butyric acid, which smells rancid on its own but enriches the aroma of Parmesan, and volatile sulfur compounds whose cooked-cabbage smell blends into the flavor profile of many mold-ripened cheeses like Camembert. “Different strains of microbe can produce different taste components,” says Cotter.
All a cheesemaker does is set the right conditions for the “rot” of the milk. “Different bacteria and fungi thrive at different temperatures and different humidity levels, so every step along the way introduces variety and nuance,” says Julia Pringle, a microbiologist at the artisan Vermont cheesemaker Jasper Hill Farm. If a cheesemaker heats the milk to over 120 degrees Fahrenheit, for example, only heat-loving bacteria like Streptococcus thermophilus will survive — perfect for making cheeses like mozzarella.
Cutting the curd into large chunks means that it will retain a fair amount of moisture, which will lead to a softer cheese like Camembert. On the other hand, small cubes of curd drain better, resulting in a drier curd — something you want for, say, a cheddar.
Storing the young cheese at warmer or cooler temperatures will again encourage some microbes and inhibit others, as does the amount of salt that is added. So when cheesemakers wash their ripening rounds with brine, it not only imparts seasoning but also promotes colonies of salt-loving bacteria like B. linens that promptly create a specific kind of rind: “orangey, a bit sticky, and kind of funky,” says Pringle.
Even the tiniest changes in how a cheese is handled can alter its microbiome, and thus the cheese itself, cheesemakers say. Switch on the air exchanger in the ripening room by mistake so that more oxygen flows around the cheese and suddenly molds will sprout that haven’t been there before.
— The Science Behind Your Cheese
#ute eberle#the science behind your cheese#food and drink#science#microbiology#chemistry#genetics#bacteriology#mycology#cheese#curd#geotrichum candidum#butyric acid#streptococcus thermophilus#brevibacterium linens#brie#camembert#parmesan#mozzarella#cheddar cheese
6 notes
·
View notes
Text
First textbook I've ever been so interested in that. I want to read the whole thing before we've even had a class! 🤭
Book: Essential Microbiology and Hygiene for Food Professinals by Sibel Roller

#studyblr#studyspo#college#on my desk#school#public health#microbiology#food hygiene#food safety#college life#university
2 notes
·
View notes
Text
I think I accidentally ingested spoiled brussel sprouts because my stomach is doing the worst kinds of backflips and the nausea is unbearable
#Me? Food poisoning myself on the day of my microbiology colloq? It's more likely than you think#The amount of times I've indirectly incapacitated myself during uni life is kinda funny ngl#Argh#Captains log
3 notes
·
View notes