#immunology
Text
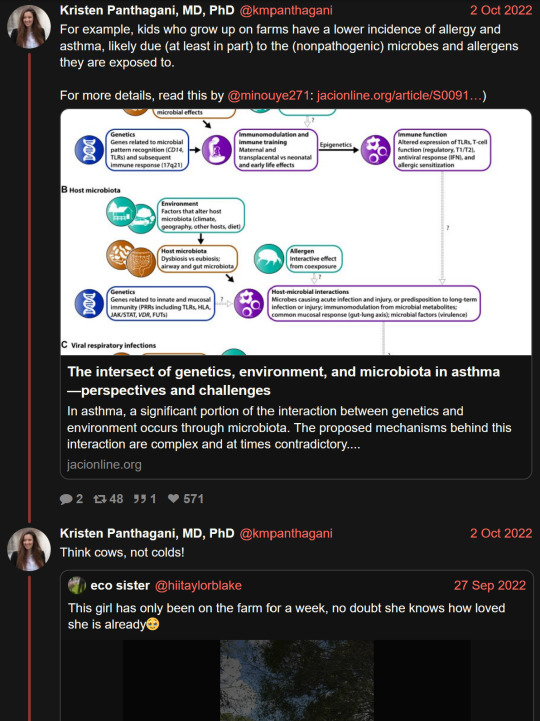
Researchers have revealed the regulatory mechanism of a specific protein that plays a key role in balancing the immune response triggered by viral infections in mammal cells. These findings could help drive the development of antiviral therapies and nucleic acid medicines to treat genetic disorders. The research is published in the journal Nucleic Acids Research.
For cells to protect themselves from viral infections, a series of immune responses typically occur, including programmed cell death called apoptosis and interferon signaling. While apoptosis is a normal process, that occurs with or without the presence of viral molecules, following a cascade of steps to end with the death of a cell—which might not sound advantageous to the host—it can help prevent the reproduction of abnormal cells, including those infected by viruses, and eliminate them from the body.
Continue Reading.
48 notes
·
View notes
Text
"A team of researchers at Washington University in St. Louis has developed a real-time air monitor that can detect any of the SARS-CoV-2 virus variants that are present in a room in about 5 minutes.
The proof-of-concept device was created by researchers from the McKelvey School of Engineering and the School of Medicine at Washington University...
The results are contained in a July 10 publication in Nature Communications that provides details about how the technology works.
The device holds promise as a breakthrough that - when commercially available - could be used in hospitals and health care facilities, schools, congregate living quarters, and other public places to help detect not only the SARS-CoV-2 virus, but other respiratory virus aerosol such as influenza and respiratory syncytial virus (RSV) as well.
“There is nothing at the moment that tells us how safe a room is,” Cirrito said, in the university’s news release. “If you are in a room with 100 people, you don’t want to find out five days later whether you could be sick or not. The idea with this device is that you can know essentially in real time, or every 5 minutes, if there is a live virus in the air.”
How It Works
The team combined expertise in biosensing with knowhow in designing instruments that measure the toxicity of air. The resulting device is an air sampler that operates based on what’s called “wet cyclone technology.” Air is sucked into the sampler at very high speeds and is then mixed centrifugally with a fluid containing a nanobody that recognizes the spike protein from the SARS-CoV-2 virus. That fluid, which lines the walls of the sampler, creates a surface vortex that traps the virus aerosols. The wet cyclone sampler has a pump that collects the fluid and sends it to the biosensor for detection of the virus using electrochemistry.
The success of the instrument is linked to the extremely high velocity it generates - the monitor has a flow rate of about 1,000 liters per minute - allowing it to sample a much larger volume of air over a 5-minute collection period than what is possible with currently available commercial samplers. It’s also compact - about one foot wide and 10 inches tall - and lights up when a virus is detected, alerting users to increase airflow or circulation in the room.
Testing the Monitor
To test the monitor, the team placed it in the apartments of two Covid-positive patients. The real-time air samples from the bedrooms were then compared with air samples collected from a virus-free control room. The device detected the RNA of the virus in the air samples from the bedrooms but did not detect any in the control air samples.
In laboratory experiments that aerosolized SARS-CoV-2 into a room-sized chamber, the wet cyclone and biosensor were able to detect varying levels of airborne virus concentrations after only a few minutes of sampling, according to the study.
“We are starting with SARS-CoV-2, but there are plans to also measure influenza, RSV, rhinovirus and other top pathogens that routinely infect people,” Cirrito said. “In a hospital setting, the monitor could be used to measure for staph or strep, which cause all kinds of complications for patients. This could really have a major impact on people’s health.”
The Washington University team is now working to commercialize the air quality monitor."
-via Forbes, July 11, 2023
-
Holy shit. I know it's still early in the technology and more testing will inevitably be needed but holy shit.
Literally, if it bears out, this could revolutionize medicine. And maybe let immunocompromised people fucking go places again
Also, for those who don't know, Nature Communications is a very prestigious scientific journal that focuses on Pretty Big Deal research. Their review process is incredibly rigorous. This is an absolutely HUGE credibility boost to this research and prototype
#covid#covid 19#pandemic#plague#rsv#influenza#the flu#science and technology#medical research#medical technology#biochemistry#immunology#good news#hope#hope posting
6K notes
·
View notes
Text






"Do not let anyone convince you that you need to get sick to be healthy."
#kristen panthagani#immunology#sars cov 2#long post#i s2g if i see one more bullshit article/comment about IMmUNiTY deBT........................#for the millionth billionth fucking time: IMMUNITY DEBT ISN'T REAL. there is NO SUCH THING as immunity debt caused by avoiding illness#and ANY dr/immunologist worth their salt in the year 2023 will tell you that#“but you have to train your immune system for it to get stronger!” nope doesn't work like that. the immune system is not a muscle#you might as well tell everyone that the reason they don't have skin like armoured steel is that they haven't been stabbed enough#tell all those drs treating tuberculosis patients that they're weakening their immunity by wearing respirators and NOT catching tb#let's all stop filtering the water while we're at it. have everyone get cholera - that'll fortify ppl's immune systems for sure#this world is a joke.#ppl buy into this shit and then wonder why the anti-vaxx/anti-science movement keeps growing
2K notes
·
View notes
Text

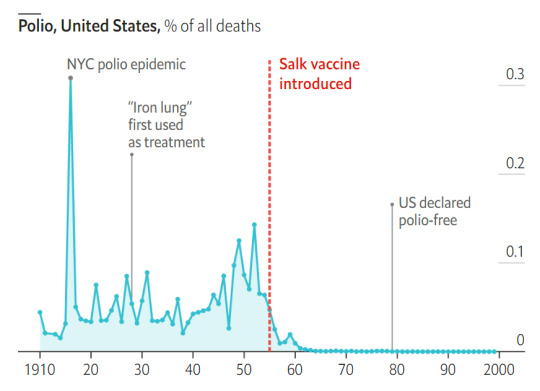



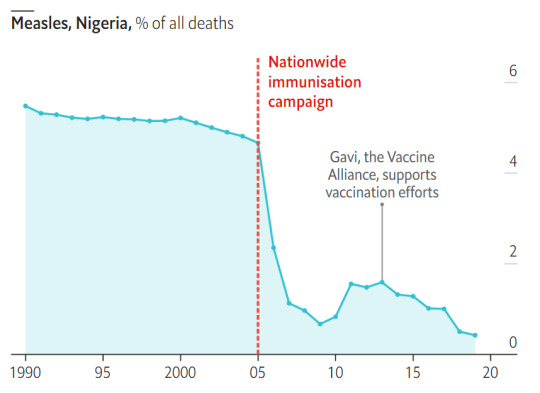

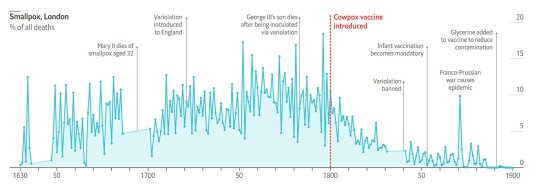
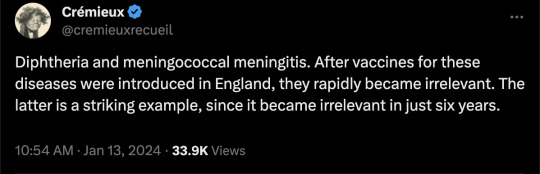
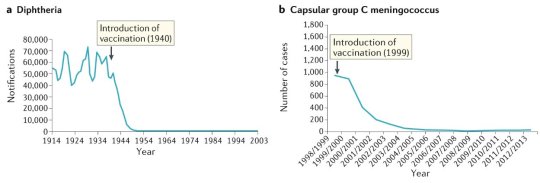

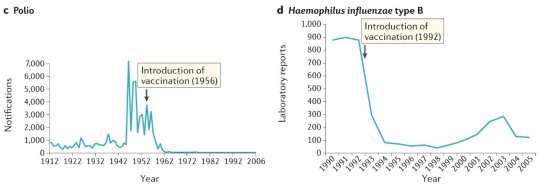

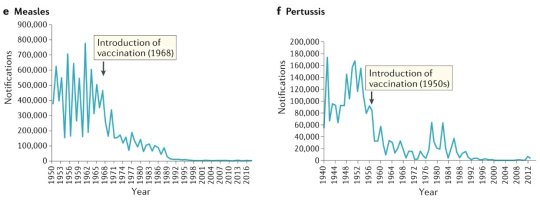

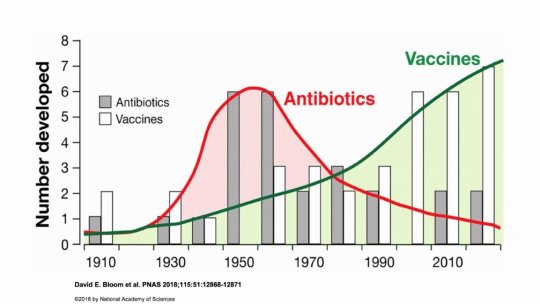


#vaccines#polio#measles#smallpox#diphtheria#meningococcal meningitis#haemophilus influenzae type B#pertussis#vaccine hesitancy#anti vaxxers#anti vax movement#antivaxers#COVID vaccine#anti vaccination#anti vaccine#science#medicine#religion is a mental illness#immunology
250 notes
·
View notes
Text

#shitpost#immunology#immunology shitposting#meme#mhc#b cell receptor#bcr crosslinking#this meme brought to you by my mom and I making fun of a question on a quiz I had for literature class#where one of the questions was ‘which of these is NOT a feature of the Greek underworld’#and I guess the professor just picked a random Greek sounding word but the specific word they picked was leukocyte#so we were pretending that leukocytes were in different literary traditions#and that morphed into talking about ‘immuno-christian values’ and I got inspired to throw this nonsense together
77 notes
·
View notes
Text
eeeee my mom got her paper on long covid published in Nature Medicine!
it supports a body of evidence she’s been gathering over decades that indicates that many more people might be at risk of serious health complications from infections than our available data gathering methods can detect - and that dysfunction in the immune system, pre-existing or not, might play some role in post-acute infectious syndromes.
#not horses#covid#long covid#covid 19#coronavirus#immunology#immunity#science#also the gut is a mass of neuronal and immune cells. so it fits multiple categories
3K notes
·
View notes
Link
A research team led by Prof. Nir Hacohen from the Broad Institute of MIT and Harvard, Cambridge, designed the study Dictionary of Immune Responses to Cytokines at single-cell resolution. This dictionary is a compilation of single-cell transcriptomic profiles of over 17 immune cell types in response to each of 86 cytokines (>1,400 cytokine–cell type pairings). Immune Dictionary expands our understanding of the activation states of all immune cell types, generates new hypotheses for cytokine functions, highlights the pleiotropic effects of cytokines, and offers a framework for determining the roles of particular cytokines and cell-cell communication networks in any immune response.
A large class of tiny, secreted proteins known as cytokines attach to specific receptors on target cells to initiate local or systemic action. This initiates downstream signaling and coordinates immune system cell types’ actions. Numerous illnesses, such as cancer and autoimmune diseases, are treated with cytokine-based medicines and cytokine antagonists. A large number of studies have been conducted depicting the central role of cytokines in immune function. However, a generalized global view of the cellular responses of each immune cell type to each cytokine was lacking. To fix this breach, the Immune Dictionary was introduced.
In order to create a large-scale perturbational single-cell RNA sequencing (scRNA-seq) dataset of the immune system, the researchers methodically profiled single-cell transcriptomic (single-cell RNA sequencing, or scRNA-seq) responses to 86 cytokines across more than 17 immune cell types in mouse lymph nodes in vivo. This allowed them to obtain a comprehensive view of cellular responses to cytokines. The 86 cytokines comprise representative molecules from other families with cytokine functions as well as the majority of members of its major families, such as interleukin-1 (IL-1), common γ-chain/IL-13/thymic stromal lymphopoietin (TSLP), common β-chain, IL-6/IL-12, IL-10, IL-17, interferon (types I, II, and III), complement, and growth factor families.
Continue Reading
66 notes
·
View notes
Text
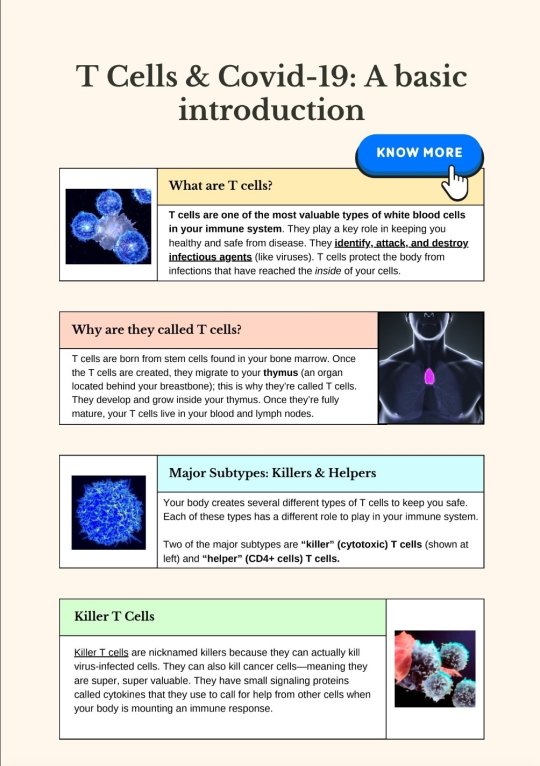
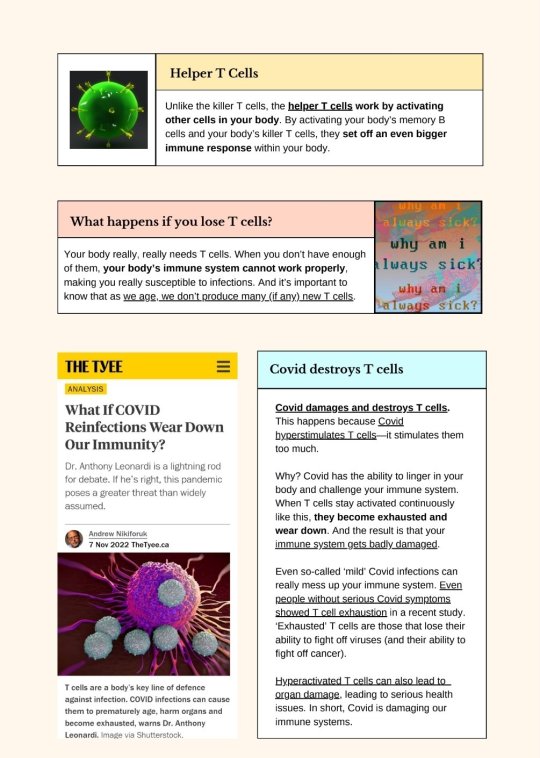
Source.
[ID: A two-page infographic titled "T Cells & Covid-19: A basic introduction."
A box labeled "What are T cells?" Text reads: "T cells are one of the most valuable types of white blood cells in your immune system. They play a key role in keeping you healthy and safe from disease. They identify, attack, and destroy infectious agents (like viruses). T cells protect the body from infections that have reached the inside of your cells." To the left of the box is an image of cells.
A box labeled "Why are they called T cells?" Text reads: "T cells are born from stem cells found in your bone marrow. Once the T cells are created, they migrate to your thymus (an organ located behind your breastbone); this is why they're called T cells. They develop and grow inside your thymus. Once they're fully mature, your T cells live in your blood and lymph nodes." To the right of the box is an image of a simplified human figure. The thymus' location is shown in bright pink in the center of the chest, roughly in the middle of the collarbone.
A box labeled "Major Subtypes: Killers & Helpers." Text reads: "Your body creates several different types of T cells to keep you safe. Each of these types has a different role to play in your immune system. Two of the major subtypes are 'killer' (cytotoxic) T cells (shown at left) and 'helper' (CD4+ cells) T cells." To the left of the box is a depiction of a blue cell.
A box labeled "Killer T Cells." Text reads: "Killer T cells are nicknamed killers because they can actually kill virus-infected cells. They can also kill cancer cells--meaning they are super, super valuable. They have small signaling proteins called cytokines that they use to call for help from other cells when your body is mounting an immune response." To the right of the box is an illustration of what appears to be killer T cells fighting infected or malign cells.
A box labeled "Helper T Cells." Text reads: "Unlike the killer T cells, the helper T cells work by activating other cells in your body. By activating your body's memory B cells and your body's killer T cells, they set off an even bigger immune response within your body." To the left of the box is a depiction of a green cell.
A box labeled "What happens if you lose T cells?" Text reads: "Your body really, really needs T cells. When you don't have enough of them, your body's immune system cannot work properly, making you really susceptible to infections. And it's important to know that as we age, we don't produce many (if any) new T cells." To the right of the box is a graphic where the words "why am i always sick?" appear multiple times in different colours.
A screenshot of an article from the Tyee, titled "What If COVID Reinfections Wear Down Our Immunity?" by Andrew Nikiforuk, dating from 7 November 2022. What text is visible reads: "Dr. Anthony Leonardi is a lightning rod for debate. If he’s right, this pandemic poses a greater threat than widely assumed", followed by an image of cells. Under the image, text reads: "T cells are a body’s key line of defence against infection. COVID infections can cause them to prematurely age, harm organs and become exhausted, warns Dr. Anthony Leonardi. Image via Shutterstock."
A box labeled "Covid destroys T cells." Text reads: "Covid damages and destroys T-cells. This happens because Covid hyperstimulates T cells--it stimulates them too much. Why? Covid has the ability to linger in your body and challenge your immune system. When T cells stay activated continuously like this, they become exhausted and wear down. And the result is that your immune system gets badly damaged. Even so-called 'mild' Covid infections can really mess up your immune system. Even people without serious Covid symptoms showed T cell exhaustion in a recent study. 'Exhausted' T cells are those that lose their ability to fight off viruses (and their ability to fight off cancer.) Hyperactivated T cells can also lead to organ damage, leading to serious health issues. In short, Covid is damaging our immune systems."
/end ID]
To read more on this topic:
How the Coronavirus Short-Circuits the Immune System (26 Jun, 2020)
Dendritic cell deficiencies persist seven months after SARS-CoV-2 infection (21 Jul, 2021)
SARS-CoV-2 Actively Infects And Kills Lymphoid Cells (14 Apr, 2022)
In Cleveland and beyond researchers begin to unravel the mystery of long COVID-19 (22 Oct, 2022)
What if COVID Reinfections Wear Down Our Immunity? (7 Nov, 2022)
Single-cell multiomics revealed the dynamics of antigen presentation, immune response and T cell activation in the COVID-19 positive and recovered individuals (2 Dec, 2022)
SARS-CoV-2 infection weakens immune-cell response to vaccination: NIH-funded study suggests need to boost CD8+ T cell response after infection. (20 Mar, 2023)
Lymphocytopenia: Merck Manual (Revised Apr 2023)
Long COVID manifests with T cell dysregulation, inflammation and an uncoordinated adaptive immune response to SARS-CoV-2 (11 Jan, 2024)
87 notes
·
View notes
Text

Allergies
#science#food#biology#immunology#memes#meme#funny#insidesjoke#student memes#reddit memes#twitter memes#relatable
69 notes
·
View notes
Text
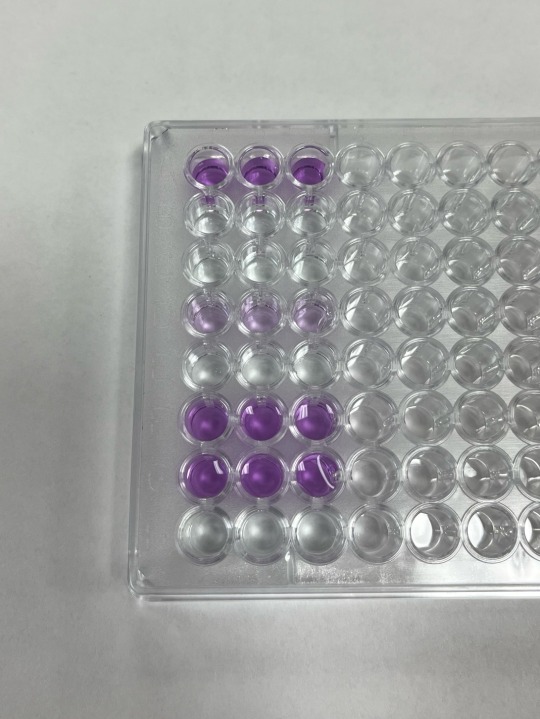
some ELISA results from immunology lab today <3
ELISA stands for enzyme linked immunosorbent assay, and it’s used to detect antibodies or antigens in a sample
the dark purple color indicates a positive result, and the light purple indicates a mild positive result. the clear indicates a negative result.
#college#biology#science#university#student#microbiology#studyblr#laboratory#enzymes#immunology#immunoassay
102 notes
·
View notes
Text
The unbearable itch that accompanies the chronic inflammatory skin condition eczema has a new culprit.
Scientists have discovered that a familiar bacteria, Staphylococcus aureus, ignites persistent itches by directly triggering sensory neurons in the skin, a finding that could help researchers devise new treatments.
Eczema, which is also known as atopic dermatitis, is common in children and teenagers but also affects one in ten adults.
Up until now, immune cells and the inflammatory molecules they secrete were thought to be the main drivers of the insufferable itch that occurs with eczema. That itch so often drives a vicious urge to scratch which only damages the skin further, leaving it red, raw, swollen, and cracked.
Years ago, researchers figured out that people lacking a skin protein called filaggrin were more likely to develop eczema; but what specifically caused the itch that enflames eczema still bugged them.
Continue Reading.
343 notes
·
View notes
Text




science doodles done at the lab during reunions
tertiary lymphoid structures
flow cytometry
leucocytes (back of the sketchbook)
neuroendocrine carcinoma
psoriasis
cellular leiomyoma
#sketch#science#medecine#biology#histology#flow cytometry#pathology#psoriasis#immunology#oncology#leucocyte
43 notes
·
View notes
Text

Vitiligo
#vitiligo#wikipedia#wikipedia pictures#skin#dermatology#melanin#immunology#autoimmune disorder#autoimmune disease#medicine
80 notes
·
View notes
Text
Researchers have just uncovered a whole new part of the brain
Researchers have just uncovered a whole new part of the brain.
A new study has discovered a previously unknown structure that surrounds the brain.
Thoughts health innovators?
A new study has discovered a previously unknown structure that surrounds the brain.
A newfound anatomical structure has been discovered in the brain, which appears to play an essential role in the brain’s waste disposal and immune systems, acting as a protective barrier and harboring immune cells that watch for toxic proteins.
Playing a multi-faceted role in the brain’s immunity, the team…

View On WordPress
#anatomy#biology#healthinnovations#immune#immunity#immunology#neuroinnovations#neurology#neuroscience#privileged#science#system
125 notes
·
View notes
Text
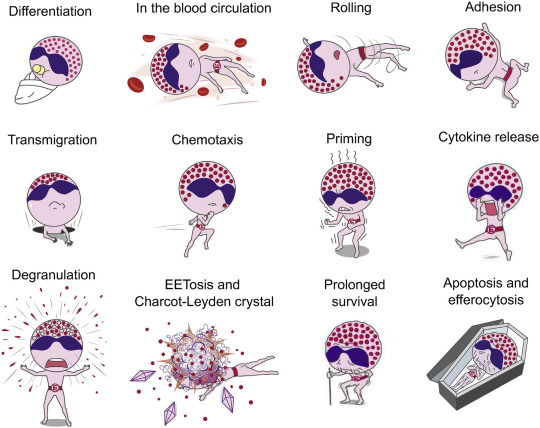
Tag yourself: which fate of eosinophils are you feeling today
51 notes
·
View notes
Text
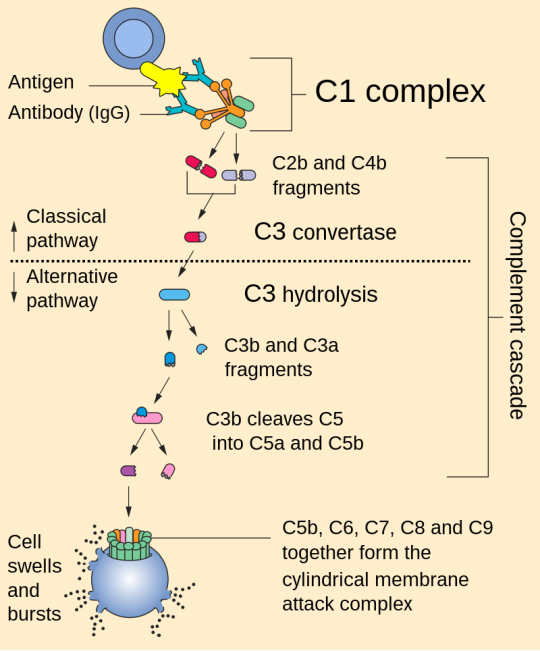
Complement system
1. Activation: The complement system can be activated through three main pathways: the classical pathway, the alternative pathway, and the lectin pathway. Each pathway involves different initiating events but converges on a common cascade of reactions.
2. Cascade of Reactions: Once activated, the complement system triggers a cascade of enzymatic reactions that result in the cleavage of complement proteins. This cascade ultimately leads to the formation of several key components, including C3b, C4b, and C5b.
3. Opsonization: C3b and C4b are opsonins, which means they can bind to pathogens and label them for phagocytosis by immune cells like macrophages and neutrophils. This enhances the removal of pathogens from the body.
4. Inflammation: Complement activation also results in the release of small peptides called anaphylatoxins, such as C3a and C5a. These peptides promote inflammation by increasing blood vessel permeability and attracting immune cells to the site of infection.
5. Membrane Attack Complex (MAC): The final step of complement activation involves the assembly of the membrane attack complex (MAC). C5b, C6, C7, C8, and multiple C9 molecules come together to form the MAC, which can create pores in the membranes of target cells, leading to cell lysis and destruction of pathogens.
References:
1. Walport, M. J. (2001). Complement. First of two parts. New England Journal of Medicine, 344(14), 1058-1066.
2. Ricklin, D., Hajishengallis, G., Yang, K., & Lambris, J. D. (2010). Complement: a key system for immune surveillance and homeostasis. Nature Immunology, 11(9), 785-797.
3. Merle, N. S., Church, S. E., Fremeaux-Bacchi, V., & Roumenina, L. T. (2015). Complement system part I – molecular mechanisms of activation and regulation. Frontiers in Immunology, 6, 262.
Please note that for the most current and detailed medical information on the complement system, I recommend consulting recent textbooks or academic journals in immunology and microbiology.
#science#biology#college#education#school#student#medicine#doctors#health#healthcare#immunology#immune system#complement system
48 notes
·
View notes