#clinical trials monitor
Text
Clinical Research Monitoring: A Guide to Clinical Monitoring
Clinical research monitoring is a vital part of clinical trials and it involves various activities to ensure the safety and accuracy of the data collected. It is important that the clinical trial is conducted in a way that meets regulatory standards, protects human studies participants, and minimizes potential risks to their health and well-being. Clinical trial monitoring can include activities such as auditing study sites, evaluating data for accuracy and completeness, review of protocols and amendments, reviewing case report forms (CRFs), identifying any deviations from the standard operating procedures (SOPs) or protocols, managing corrective action plans (CAPs), following up on safety reports, tracking progress against enrollment goals and much more. Apart from evaluating data quality, clinical research monitoring also ensures compliance with all regulatory standards like GCP (Good Clinical Practices) ICH (International Conference on Harmonization), FDA regulations and local laws. In addition to this ongoing monitoring throughout a study's duration, there may be audits conducted by sponsors or regulatory authorities at any time during or after completion of a clinical trial. All these efforts are dedicated towards ensuring that the results obtained from a clinical trial are accurate, reliable and applicable for use in making medical decisions.
Steps to Clinical Monitoring
1. Establish an Effective Monitoring Plan: Ensure that the plan is comprehensive and contains all applicable elements, such as the types of monitoring activities to be conducted, frequency of monitoring visits, data collection methods, and specific criteria for acceptable performance.
2. Develop Appropriate Documentation: Design protocol-specific monitoring tools and forms to document information from site visits including source documents, data collection instruments, case report forms (CRF). In addition, develop a Monitoring Log or Tracking System which will enable better accountability for study activities.
3. Execute Monitors’ Visits: Depending on the complexity of the trial and regulatory requirements, conduct pre-study qualification visits (PSQV), pre-initiation visits (PIV), initiation visits (IVs), periodic monitoring visits (PMV) and close out visits (COV). During each visit, ensure that good clinical practice is followed at all times by reviewing source documents and data collection instruments. Review patient enrollment logs to ensure accuracy and record any discrepancies in the visit report.
4. Report Findings: Generate detailed yet concise reports per each monitor's visit with clear recommendations for corrective actions if required; provide professional feedback to investigators regarding their performance; identify any areas of noncompliance with protocol requirements or applicable regulations; recommend training or educational sessions when appropriate; track all follow up activities related to corrective actions taken in response to findings from monitors' visits; ensure that essential documentation is complete before closing out a particular study site.
5. Quality Assurance: Validate accuracy of tracking systems used by monitors during their visits; assess risk associated with various deficiencies identified during monitoring process; carry out periodic internal audits/assessments to ensure compliance with established SOPs/guidelines related to clinical research monitoring activities; take preventive measures based on audit/assessment results in order to strengthen internal quality system processes.
Types of Clinical Trial Monitoring
1. Types of Clinical Research Monitoring: Clinical research monitoring is the process to assess the quality and integrity of clinical trial data and ensure compliance with applicable regulatory requirements. It can be done through three primary methods: onsite monitoring, centralized or remote monitoring, and risk-based approaches.
2. Onsite Monitoring: Onsite monitoring is considered the "gold standard" for clinical research monitoring, as it requires the presence of a monitor at a study site during the entire duration of a trial. The monitor will typically review source documentation such as patient records, lab results, and investigational product dispensing logs to assess accuracy and conformance with study protocols and good clinical practices (GCP). The monitor also interviews staff members responsible for conducting the trial to verify that procedures are being followed properly.
3. Centralized or Remote Monitoring in Clinical Trials: Centralized or remote monitoring enables sponsors to conduct clinical research monitoring without needing to send someone onsite to each study location. This is accomplished by using technology such as web portals, video conferencing, and virtual meetings that allow monitors to remotely review data from various sites simultaneously and quickly flag any issues that arise. Additionally, centralized/remote monitoring allows sponsors to be more proactive in identifying potential risks associated with a trial prior to sending monitors onsite for an assessment.
4. Risk-Based Approaches: Risk-based approaches use data analytics tools such as descriptive statistics and predictive algorithms to identify potential trends or outliers in clinical trial data that may represent heightened risk of noncompliance with GCPs or other regulations. By leveraging technology, these approaches can help sponsors identify issues earlier in the course of a trial so they can take corrective action before something goes wrong.
5. Benefits of Clinical Research Monitoring: Utilizing effective clinical research monitoring strategies helps ensure that trials are conducted ethically, safely, correctly according to protocol standards, within timelines agreed upon with regulatory authorities, and within budget constraints set out by sponsors/CROs/investigators/other stakeholders involved in a study’s execution.. Clinical research monitors act as an independent third party who are able to provide objective insight into how studies are being conducted across multiple sites which helps minimize errors due to bias from investigators or other personnel who may have vested interests in outcomes associated with their studies.. In addition, effective clinical research monitoring helps ensure patient safety by providing oversight about how drugs or medical devices used in trials are administered as well as ensuring patient confidentiality is maintained throughout the course of a study.. Lastly, robust clinical research monitoring protocols help reduce costs associated with delays caused by errors made during trials which can add up significantly over time if not avoided through proper oversight methods both pre-study start up until closeout occurs after all enrolled patients have completed their participation in a given trial
Clinical Research Monitoring Guide
1. Understand the Basics of Clinical Research Monitoring: Clinical research monitoring is a key part of the clinical research process, ensuring the safety and accuracy of results. It involves periodically assessing study sites to confirm that data is being collected properly, according to ethical and legal requirements, as per Good Clinical Practice (GCP) guidelines.
2. Know What Types of Studies are Monitored: Clinical research monitoring can be used for a variety of studies, including clinical trials, observational studies, epidemiologic studies, and public health surveys. It is important to know what type of study you are monitoring in order to ensure that the appropriate procedures are followed.
3. Understand How to Monitor a Study Site: The primary goal of clinical research monitoring is to confirm that the protocol and informed consent form have been followed properly at each site. This requires a thorough review of all relevant documents such as case report forms (CRFs), source documentation (e.g., physician notes), internal audit reports (audit trails), and external quality assurance reports. Additionally, it involves evaluating compliance with GCP guidelines during study visits or remote reviews, as well as conducting interviews with staff members to assess how they are handling data collection and reporting processes.
4. Become Familiar With Regulatory Requirements: In addition to GCP guidelines, there may be applicable regulations from local governments or other institutions that must be adhered to when conducting clinical research monitoring activities. Understanding these regulations is essential for ensuring compliance with applicable laws and regulations related to clinical research activities.
5. Develop an Effective Monitoring Plan: An effective monitoring plan should include a detailed timeline for visiting sites, information about any specific areas where focused attention is required (e.g., enrolling/randomizing patients or managing adverse events), and plans for auditing/reviewing data generated by the study site(s). Additionally, it should incorporate measures for controlling risk associated with data collection processes so that issues can be identified early on in the study process before they become problematic later on down the line.
Clinical Research Monitor Job
The job of a Clinical Research Monitor is to ensure that clinical trials are conducted ethically, safely and in compliance with established standards. The primary responsibility of the monitor is to protect the rights, safety and well-being of the human subjects enrolled in the trial. Duties typically include developing protocols for clinical studies; coordinating study start up activities; conducting site visits; monitoring data for timeliness, accuracy and completeness; auditing files for regulatory compliance; managing investigator queries/issues; preparing visit reports; reviewing update protocols related to study operations; resolving issues raised through audit reports or other sources; providing technical guidance to sites regarding protocol implementation or study conduct; and escalating complex issues or potential risks as needed.
Clinical Research Monitor Salary
Salaries for this position tend to vary depending on education level, experience and geographical location but can range from $60,000 per year for entry level positions up to around $90,000 per year for more experienced professionals. In addition to salary many employers also offer benefits such as paid vacation days, health insurance plans and retirement packages.
Resources for Clinical Research Monitoring
1. National Institutes of Health (NIH): Clinical Research Monitoring
This link provides information on NIH's guidelines for monitoring clinical research, which include topics such as the roles and responsibilities of the investigator, data safety monitoring boards, and protocols for reporting unanticipated problems and adverse events.
2. National Institutes of Health (NIH): Guide to Clinical Research Monitoring
This comprehensive guide walks readers through all aspects of clinical research monitoring, including topics such as study design, randomization strategies, regulatory compliance requirements, data management, monitoring plans and reports, quality improvement initiatives, and safety assessments.
3. US Food and Drug Administration (FDA): Guidelines for Clinical Trials Monitoring
This resource from the FDA outlines the importance of effective monitoring in clinical trials and provides an overview of the different roles within a clinical trial as well as details about essential elements for implementation of an effective monitoring strategy such as risk assessments and adverse event tracking.
4. International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH)
ICH has developed standards that provide a set of harmonized technical requirements for clinical trials conducted across countries in the European Union (EU), Japan, and US with an emphasis on quality assurance and safety monitoring during trials.
5. Association of Clinical Research Professionals (ACRP)
ACRP's guidelines provide best practice recommendations for conducting clinical research studies in accordance with applicable regulations and standards to ensure patient safety monitoring during studies as well as data integrity throughout the process from start to finish.
6. Pharmaceutical Research & Manufacturers of America (PhRMA)
The PhRMA guidelines provide an overview of expectations around clinical research activities with respect to ethics, data integrity, safety reporting, resource allocation and more. It defines roles and responsibilities of all those involved in overseeing a clinical trial such as a Clinical Research Monitor or CRA who has primary responsibility for ensuring that the protocol is implemented correctly throughout a study’s duration
Clinical Research Monitoring Review
1. What is the main purpose of clinical research monitoring?
A) To ensure that a research study is conducted in accordance with applicable regulations and ethical standards
B) To ensure that data collected during a research study is accurate and reliable
C) To evaluate the safety of participants enrolled in a research trial
D) To oversee the financial management of a research project
Answer: A) To ensure that a research study is conducted in accordance with applicable regulations and ethical standards. Clinical Research Monitors are responsible for ensuring compliance with Good Clinical Practice guidelines, protecting participant privacy, verifying data accuracy, and evaluating protocol deviations. In addition, they may also be involved in reviewing participant eligibility requirements, conducting site assessments, providing training to investigators and staff on proper study procedures, as well as monitoring progress towards completion of all requirements of the study.
2. What type of individuals typically serve as clinical research monitors?
A) Physicians
B) Nurses
C) Regulatory specialists
D) All of the above
Answer: D) All of the above. Clinical Research Monitors can come from various backgrounds such as medical doctors (MDs), nurses (RNs), pharmacists (RPhs), regulatory specialists (e.g., Regulatory Affairs Professionals or Paralegals), or biostatisticians/data analysts who have experience in clinical trials and understand local regulations related to human subject protection. Each monitor has specific job duties depending on their education and experience, such as assessing compliance with regulatory guidance or analyzing data sets for accuracy, completeness, integrity, or validity.
3. What kind of activities do clinical research monitors need to perform?
A) Protocol reviews or verifications
B) Ensuring appropriate documentation completion
C) Site visits to observe investigator conduct
D )All of the above
Answer: D )All of the above. Clinical Research Monitors need to perform several activities including protocol reviews or verifications; ensuring appropriate documentation completion; site visits to observe investigator conduct; liaising between sponsors and sites; assisting with resolving issues associated with adverse events; reviewing case report forms for completeness, accuracy, consistency and correctness; evaluating subject safety throughout enrollment process;and writing reports detailing their findings at each visit.
4. What is one benefit gained from having an effective Clinical Research Monitor on-site? A) Reduced risk for legal liability stemming from negligence
B) Improved protocol adherence by investigators
C) Increased patient engagement during trial period
D )All of the above
Answer: D) All of the above . An effective Clinical Research Monitor encompasses several benefits such as reduced risk for legal liability stemming from negligence due to thorough oversight and accurate record keeping; improved protocol adherence by investigators through continued communication between sponsor representatives and researchers on-site regarding best practices; increased patient engagement during trial period due to more detailed explanations about potential risks/benefits offered by having monitor on-site ; and improved efficiency when dealing with complex protocols that require multiple levelsof oversight due to familiarity with protocol specifics which decreases time spent troubleshooting errors or unclear instructions..
5. How often should Clinical Research Monitors visit a particular site?
A) Weekly B) Biweekly C) Monthly D) Quarterly
Answer: C) Monthly . It is recommended that Clinical Research Monitors visit sites at least once per month in order to maintain active surveillance over ongoing studies at each location while also providing timely feedback regarding any issues discovered while on-site visits are taking place within a shorter timeframe if needed based upon changes made midstream or other unanticipated circumstances which might require immediate attention by sponsor personnel.
#clinical monitoring#clinical trial monitoring#remote monitoring clinical trials#clinical research monitor#clinical research monitor salary#risk based monitoring in clinical trials#clinical trial monitor#clinical trial monitor salary#monitoring of clinical trials by industry sponsors#centralized monitoring clinical trials#clinical research monitoring#clinical site monitoring#clinical trial monitoring services#clinical trial remote monitoring#clinical trials monitor#medical monitor clinical trial#risk based monitoring clinical trials#clinical monitor#clinical research monitor jobs#clinical trials monitoring#clinically validated blood pressure monitors for home use#monitoring in clinical trials#clinical monitoring services#clinical trial monitor jobs#journal of clinical monitoring and computing#monitoring clinical trials#remote monitoring in clinical trials#central monitoring clinical trials#clinical data monitoring#clinical monitoring jobs
0 notes
Text
Strategic Insights into the Clinical Trial Investigative Site Network Market
The global clinical trial investigative site network market size is expected to reach USD 12.5 billion by 2030. Growing investments in pharmaceutical R&D, increasing demand for new therapies and complications associated with site management of clinical trials are some of the major factors driving the growth of the industry. There has been a consistent rise the clinical trials in the last 5 years. For instance, according to ClinicalTrials.gov, over 262,298 trials were registered in 2018, whereas as of September 2022, over 399,518 trials were registered. The clinical trials are expected to grow even further as the funding for research improves.

Gain deeper insights on the market and receive your free copy with TOC now @: Clinical Trial Investigative Site Network Market Report
This is expected to propel the growth of the industry post-pandemic. There is a growing focus on reducing the cost associated with clinical research. Hiring a clinical trial investigative site network supports the regulatory function, improves the enrollment of participants, assists in data management, and quality assurance. It increases process compliance, reduces process issues with each trial, and helps with faster trial initiations, and shorter trial timelines. These factors are supporting the demand for clinical investigative site networks. The governments are actively trying to improve R&D by providing tax deductions. For instance, in January 2022, the Indian government stated that it is providing a weighted average tax deduction of up to 200% in R&D.
Such initiatives are expected to improve the R&D activities on drugs and thus support industry growth. According to the IQVIA, report on oncology trends, clinical trials for cancer have been increasing for the last 10 years. For instance, in 2011, 1,242 trials were registered for cancer, and as of 2021, 2,335 trials were registered for cancer. The number of clinical trials for cancer is expected to rise even further owing to the growing prevalence of the disease. This is expected to improve the demand for clinical investigative site networks for cancer clinical trials post-pandemic.
#Clinical Trials#Investigative Sites#Clinical Research#Clinical Trial Network#Medical Research#Clinical Site Management#Trial Coordination#Research Networks#Patient Recruitment#Healthcare Innovation#Data Integrity#Patient Centricity#Trial Management#Research Collaboration#Protocol Compliance#Site Monitoring#Clinical Operations#Healthcare Quality#Patient Engagement#Clinical Data Management
0 notes
Text
Enhancing Clinical Studies with GCP Audit and Monitoring
Understanding GCP Audit and Monitoring
GCP guidelines set forth by the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) provide a framework to ensure the ethical conduct, safety, and quality of clinical trials. Audit and monitoring are essential components of GCP compliance and play a vital role in the success of clinical studies.
These processes involve the systematic review and verification of trial data, procedures, and processes to ensure compliance with GCP guidelines. It helps identify and rectify any deviations or discrepancies, guaranteeing the reliability and validity of trial results.

The Importance of GCP Audit and Monitoring
Data Integrity:
GCP audit and monitoring verify the accuracy and reliability of trial data, reducing the risk of errors or fraudulent practices. By maintaining high data integrity, researchers and regulatory bodies can have confidence in the trial results.
Participant Safety:
Ensuring GCP compliance helps safeguard the well-being and safety of trial participants. With thorough monitoring, potential risks and adverse events can be identified early, enabling prompt intervention
Early Detection of Issues:
Regular monitoring and audits help identify potential issues early on, allowing researchers to take corrective actions promptly. This proactive approach can prevent larger problems that may arise later in the trial.
Regulatory Compliance:
Trials that adhere to GCP guidelines are more likely to gain regulatory approval and acceptance, expediting the path to market for potential life-saving treatments.
Enhanced Trial Efficiency:
Regular monitoring and audits improve the efficiency of clinical trials by identifying and addressing issues promptly. This minimizes costly delays and accelerates the overall trial timeline.
Zenovel's Contribution:
Zenovel offers a comprehensive GCP audit service that thoroughly examines all aspects of your clinical trial. Their team of experts meticulously assesses protocol adherence, data accuracy, informed consent processes, and investigator compliance.
By partnering with Zenovel, your trial gains an extra layer of assurance, knowing that any potential issues will be proactively addressed. Here are some key ways Zenovel has contributed to the advancement of clinical trials:
Experienced and Trained Professionals:
Zenovel boasts a team of experienced and highly trained professionals with expertise in GCP guidelines and clinical trial monitoring. Their auditors and monitors possess a keen eye for detail, ensuring no aspect of the trial goes unnoticed.
Tailored Monitoring Strategies:
Recognizing that each clinical trial is unique, Zenovel devises customized monitoring strategies to suit the specific needs and complexity of each study. This approach optimizes resource utilization while maintaining the highest standards of quality
Real-time Oversight and Data Monitoring
GCP monitoring involves ongoing oversight of the trial’s progress and data collection to ensure accurate and reliable results. Monitoring activities identify and resolve data discrepancies, verify source data, and verify that the trial is being conducted in line with the approved protocol.
Early Identification of Risks and Mitigation Strategies
GCP audit and monitoring enable the early detection of potential risks and challenges during the trial. Identifying these issues promptly allows the trial sponsor and investigators to implement mitigation strategies, ensuring the study’s success and participant safety.
Enhanced Compliance with Regulatory Authorities
Adherence to GCP guidelines is crucial for obtaining regulatory approvals and ensuring acceptance of trial data by regulatory authorities. Non-compliance with GCP can lead to data rejection and delays in bringing life-saving treatments to patients.
Conclusion
In conclusion, GCP audit and monitoring significantly enhance the efficacy and reliability of clinical trials. By ensuring compliance with ethical standards, maintaining data accuracy, identifying risks early, and streamlining interactions with regulatory authorities, GCP audit and monitoring contribute immensely to the success of your clinical study.
Zenovel’s invaluable contribution to enhancing clinical studies lies in their comprehensive GCP audit and monitoring services. Their expertise and commitment to quality and compliance provide trial stakeholders with the confidence and tools needed to conduct safe and successful clinical trials.
When it comes to your clinical trial, don’t compromise on quality and compliance. Partner with Zenovel to unlock the full potential of your research and contribute to advancing medical science for the betterment of patients worldwide.
Adherence to GCP guidelines is crucial for obtaining regulatory approvals and ensuring acceptance of trial data by regulatory authorities. Non-compliance with GCP can lead to data rejection and delays in bringing life-saving treatments to patients.
0 notes
Text
Inato obtains $20M funding to make clinical trials more accessible

- By InnoNurse Staff -
Paris, France-based Inato, a platform that makes clinical trials more accessible, has secured $20 million in Series A2 funding round.
Read more at Tech Funding News
///
Other recent news and insights
Diabetes: Bluedrop Medical, based in Ireland, has raised €10.5 million in funding (Tech.eu)
#inato#clinical trials#france#medtech#health tech#digital health#accessibility#diversity#inclusion#bluedrop medical#diabetes#remote patient monitoring
0 notes
Text
The Approval Process for New Drugs in Turkey
The approval process for new drugs in Turkey is a rigorous and multi-step process that involves preclinical testing, clinical trials, evaluation and approval by the Turkish Medicines and Medical Devices Agency (TMMDA), and post-market monitoring.

0 notes
Text
Clinical trial Technology trends : Digital Patient Engagement tools & Risk-based monitoring for Clinical Trials
Clinical trials play a crucial role in the development and approval of new medical treatments and drugs. As technology continues to advance, Clinical Trial Technology is becoming a more important factor in shaping the way trials are conducted. In 2023, we can expect to see several technology trends that are set to further revolutionize the design, conduct, and analysis of trials. Some of these trends include decentralized trials, wearable devices, machine learning, and Risk-Based Quality Management (RBQM).
https://www.clinion.com/insight/clinical-trial-technology-trends/

0 notes
Text
Importance of setting up Power Of Attorney
Betsy Wurzel’s guest is Ryan McEniff the CEO of Minute Women Home Care located in Lexington, MA. Ryan McEniff is also Co-Owner of Well Aware Care which is a software fall detector monitoring service that is available nationwide and Ryan is the Host of “The Caregiver’s Toolbox” Podcast which is available wherever you hear your favorite podcasts.
Ryan McEniff discusses how he became involved …

View On WordPress
#Alzheimers#Betsy Wurzel#Caregivers#Dementia#Depression#elderly falls#Finding Clinical Trials#Medication Therapy#Power of Attorney#Ryan McEniff#software fall detector monitoring service
0 notes
Text
Clinical Research Services for Local and Global Clinical Trials - Contract Research Organisation (CRO)
CliniExperts Research Services is your one stop expert solution to conducting clinical trials in India, stress-free.
Clinical trials have several phases and must follow strict protocols all throughout, until the very end. Clear objective of the clinical trial, design, methodology, statistical considerations to the organization of the project, etc must be well-designed and equally well-executed. Errors at any step can lead to complications in the application process.
To enable a glitch-free clinical trial, we provide the following services:
Clinical Trial Management
Clinical trials are complex undertakings and effective trial management is crucial. Our experienced project and site managers along with experts in vendor and data management ensure that each phase of the trial is executed meticulously and timely.
Clinical Project Management
Our excellent team promises to deliver the project as per pre-decided timelines with all the components of the project well-aligned. We ensure this by building a strongly structured trial and executing all it just as efficiently.
Clinical Investigation
Clinical investigation refers to studies performed on live subjects. These could be studies concerning diagnosis or treatment of certain diseases or clinical drug development and methodology. We ensure that the investigative trials are carried out smoothly right from patient selection, to sample collection, testing and analysis.
Clinical Performance Evaluation
Performance evaluation of a clinical trial is all about the authenticity and accuracy of the results and the scientific validity of the results. Trial safety must be ensured at each step. Foreseeing, preventing and navigating through possible pitfalls is what ensures a well-executed Clinical performance evaluation.
#Clinical Research Services#clinical trial services#global clinical trials#local clinical trials#clinical trial solutions#Clinical Trial Management Services#Clinical Trial Registry India#Ethics Committee Registration#Contract Research Organisation#CRO#Medical Writing#Medical Monitoring
0 notes
Text

"Guys, I cannot believe my college talked me into signing up for unlimited clinical trials, like it was some noble thing I'd earn extra credit doing while supporting a good cause like medical research. This is totally out of control.... I was almost an A-Cup only eight months ago. I start college and immediately I'm sent into these trials. I'm seated next to a girl so pregnant she can hardly get out of her chair, taking selfies, giggling about bursting. And a trans girl with the biggest cock I've ever seen, inflated to the point that it couldn't possibly be used to penetrate anyone. She was sitting on her testicles like cushions, whispering to me that her cock produced three gallons of cum a day..... but the researchers overlooking her trial wanted to double that.
I got so nervous. I was sat in a room with a team of researchers, they said they were going to monitor the unimpeded growth of various cysts and tumors as a form of 'natural' breast growth, an alternative to implants and pharmaceuticals. The sample cysts and cancerous growths were surgically inserted into my breasts. They shot my breasts with radiation to help them grow and mutate. My team is very happy with my progress and want to increase my radiation dose. Look at these things! They're completely out of control already.....
I can actually feel the growths inside of them and I'm told not to worry about them. That everything is 'safe' despite them admitting this is a new breast expansion technique. But they're assuring me I'm extremely lucky since I get to test the technology and benefit from such immense levels of fast, 'safe' growth. I ask them how big my breasts will have to get before the test is over and they tell me each breast should weigh close to 200lbs by graduation. 200lbs!? Of pure cysts and tumors, growing and spreading, my breasts basically incapable of not getting bigger, so either I'll have to get them removed or endure breasts that never stop growing, despite the fact that I'll be bedbound long before I graduate... Going to class in a wheelchair probably, no clothes big enough to cover my boobs, so they'll be exposed for everyone to see. My poor boobs! I guess I have no choice but to watch helplessly as they each get so big they weigh twice as much as me a piece! God.... and I think I have back trouble now...."
620 notes
·
View notes
Note
What is phage therapy? I heard the word somewhere and now it’s stuck in my head but I don’t know what it is. Thanks 🙌
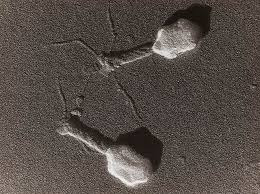
Phage therapy is the therapeutic use of viruses to kill bacteria.
Viruses are semi-living things that use other cells to reproduce. They do this by injecting DNA or RNA into the cell, letting the cell make copies of the genetic material, manufacture proteins, assemble new viruses, and then those new viruses burst out of the cell. In the process of reproducing, the cell is killed.
We typically think of viruses that replicate in human tissue, like influenza or SARS-CoV-2. But as it turns out, where there is a cell, including a plant cell or a bacterial cell, there is a virus that wants to kill it to make baby viruses.
Theoretically, then, if someone has a bacterial infection, we can find the extremely specific virus that kills only the bacteria that are causing the infection, infect the person with that virus, let that virus replicate and kill a bunch of the bacterial cells without harming the human cells, and voila, no more infection. Once the virus runs out of bacterial cells it can't replicate any more and it dies off.
And when I say "theoretically" I mean we have absolutely, definitely done this. Like a lot. And while phage therapy is extremely difficult to do good clinical trials with, as far as we can tell it's been pretty effective, and has few side effects that we know about.
So why don't we use phage therapy all the time?
Well, probably because of how specific the phages are. The phage that kills one kind of staph probably won't work on another kind of staph. So you need giant libraries of phages in order for them to really be useful to a large number of people.
Also, there's politics:
See, depending on what you consider the start of the antibiotic age, phage therapy and antibiotic therapy kind of came into being around the same time. By the start of WWII we had a couple of each worldwide.
Then the war happened and the Iron Curtain came into being. On the Western side resources were funneled into the mass production of a new antibiotic called penicillin, and on the Eastern side, resources were funneled into further developing phage therapy.
Throughout the Cold War this pattern would continue, with the Soviet Union eventually using both antibiotics and phages, and the West using only antibiotics (honestly, it's probably capitalism's fault- making money from phages is extremely difficult because they can't be mass produced like antibiotics can). When the Soviet Union fell apart, the research on phage therapy largely disappeared with it.
The West, now saddled with the burden of antibiotic resistance after decades of overprescription and use in agriculture, is trying to rebuild some of the knowledge that was lost with the fall of the Soviet Union.
Unfortunately, there have only been a handful of people who have been treated with phage therapy in the West. This is because the way phages work makes them extremely difficult to do high quality studies on, which makes them impossible to get FDA approval for in the US. Another factor standing in the way of approval is that they tend to change over time as the bacteria they replicate in evolve. So there are potential approval problems if we approve one type of phage but not the type it becomes in a few years.
So if something needs to change itself to work, how do you monitor to make sure that the changes aren't something dangerous? Do you have to repeatedly apply for approval? It just has all kinds of legal and policy issues.
If you want more info, there is a book called The Perfect Predator by Steffanie Strathdee. The author ended up saving her husband's life using phage therapy after he ended up with a life-threatening multidrug resistant infection.
If you want something shorter than a book, I highly recommend this video by Patrick Kelly.
43 notes
·
View notes
Text
Introduction to Clinical Trial Training
The field of clinical research has seen a significant rise in demand over recent years, with the evolving landscape of the pharmaceutical and biotechnology industries playing a significant role. A clinical research associate (CRA) is a crucial part of the clinical trial process, as they are responsible for overseeing the well-being of subjects and ensuring that the study complies with regulatory requirements. With the clinical research market set to experience growth, it's essential to have a thorough understanding of clinical research training, encompassing its components, course options, and benefits.
I. Clinical Research Training: Background and Importance
Before diving into the specifics of clinical research training, it's necessary to understand what clinical research is and its importance in the field of medicine. Clinical research refers to the systematic study of potential new drugs, medical devices, and techniques to establish their safety and efficacy before they can be approved for use by regulatory authorities. In simple terms, clinical research aims to ensure that new treatments and therapies are both safe and effective for human use, protecting the public from potentially harmful side effects or complications.
Clinical research, thus, plays a key role in the process of bringing novel medical treatments to market. It is a multifaceted process that requires a range of skill sets, from data analysis to ethics and compliance. Clinical research associates (CRAs) are responsible for managing clinical trials and ensuring that they adhere to relevant laws and ethical guidelines. As such, clinical research training equips potential CRAs with the necessary skills to excel in their roles and contribute to the safe development of new therapies and medical products.
II. Components of Clinical Research Training
Clinical research training typically comprises several essential components, each designed to provide a comprehensive understanding of the clinical research process. Some of the critical elements of clinical research training include the following:
1. Basic Principles of Clinical Research: An overview of the fundamentals of clinical research, including the phases of clinical trials and the importance of randomization, blinding, and placebo controls.
2. Good Clinical Practice (GCP): A thorough understanding of GCP guidelines set by regulatory authorities like the International Council for Harmonisation (ICH) and the Food and Drug Administration (FDA) to ensure the safety, integrity, and quality of clinical trials.
3. Protocol Development: Training in the design and development of clinical trial protocols, with an emphasis on creating study objectives, inclusion/exclusion criteria, and the types of assessments required.
4. Ethics in Clinical Research: In-depth exploration of ethical considerations in clinical research, including informed consent, institutional review board (IRB) approval, and data protection.
5. Regulatory Compliance: Gaining a comprehensive understanding of the role of various regulatory authorities in the clinical research process, and ensuring compliance with relevant regulations.
6. Data Management and Biostatistics: Knowledge of essential data management techniques, including data collection and validation, data quality control, and the application of biostatistics in clinical research.
7. Clinical Trial Management: Training on the roles and responsibilities of the clinical trial team and best practices in trial management, including site selection, patient recruitment, and study closeout.
8. Safety Reporting and Pharmacovigilance: An understanding of safety reporting requirements and the importance of pharmacovigilance in maintaining patient safety throughout the clinical trial.
III. Clinical Research Training: Course Options and Certifications
Numerous clinical research training programs are available for those wishing to enter or advance within the clinical research field. These programs typically cater to diverse educational backgrounds and levels of expertise, ensuring that all prospective CRAs have access to the necessary training. Courses generally range from short-duration workshops to comprehensive diploma or degree programs.
One popular and widely recognized accreditation is the Clinical Research Associate (CRA) Certification. Obtaining this certification demonstrates a commitment to excellence and professionalism in clinical research. Several organizations offer clinical research associate certification online, making it an easily accessible option for many individuals.
In conclusion, clinical research training is essential for anyone wishing to pursue a career in clinical research. It equips learners with the skills and knowledge necessary to conduct and manage clinical trials, ensuring public safety and helping to bring life-enhancing treatments to market. With various course options available, including the Clinical Research Associate Certification Online, gaining the required qualifications is more accessible than ever before.
#clinical trials training#clinical trial training#clinical trial manager training#citi clinical trial training#clinical trial administrator training#clinical trial agreement training#clinical trial assistant training#clinical trial associate training#clinical trial audit training#clinical trial budget training#clinical trial coordinator training#clinical trial investigator training#clinical trial management system training#clinical trial management training#clinical trial management training courses#clinical trial project management training#clinical trial protocol training#clinical trial site training#clinical trial training program#clinical trials monitoring training#clinical trials sas programming training#clinical trials training courses#edc training clinical trials#iata training clinical trials#medical device clinical trial training#medical device clinical trials training#nida clinical trials network free gcp training#nida clinical trials network gcp training#nih clinical trials training#oncology clinical trials training
0 notes
Text
It is the first gene therapy approved to treat this debilitating and fatal disease found almost exclusively in boys.
Emma Ciafaloni is a neuromuscular neurologist with the University of Rochester Medical Center (URMC) neurology department and Golisano Children’s Hospital, and director of the UR Medicine Duchenne Muscular Dystrophy Clinic, which treats boys with Duchenne muscular dystrophy (DMD) from across upstate New York.
Ciafaloni has been involved in DMD clinical research for decades and URMC was one of first three sites in the nation to start dosing patients in the phase 3 clinical trial for the new gene therapy. The study, called EMBARK, has since expanded to additional sites in North America, Europe, and Asia. Ciafaloni also served as chair of the independent Data Safety and Monitoring Board for the early phase clinical trials of the therapy. The new drug—delandistrogene moxeparvovec-rokl—is being developed by Sarepta Therapeutics and marketed under the name ELEVIDYS.
Continue Reading
53 notes
·
View notes
Text
I.O.U
(Be There For You Follow Up)

A/N: Same universe as BTFY, different characters, same medicine. You don't need to read BTFY to understand this story tho they're kinda their own thing
Pairing: Qian Kun X Reader
Genre: Be There For You AU, Smut, Doctor Kun, Good Ending, (as always, i know nothing about medicine lol)
Warnings: Mind Break, kun is kinda mean
Word Count: 2.3k
“I mean, every college kid does a clinical trial to make a quick buck at least once, right? That’s just like, part of the freshman experience!” You were on the phone with your friend as you stood outside the clinic, ready to get tied up to an IV for 10 hours or have your sleep monitored.
“Whatever. Don’t let them do anything stupid to you, alright?” Your friend replied, dryly.
“They’re doctors! They wouldn’t intentionally hurt me, right? Anyways, I’m gonna go in!” Way too optimistic, considering you were willing to go through Hell for $1000.
You walked in and went to the front desk.
“Hi, can I help you?” The worker, wearing a name tag that said “Jeno” asked you. He seemed… like your average nerd. Chunky, black rimmed glasses in a rectangle shape.
“I’m actually wondering if you have any studies that I could participate in.” You smiled up at him, biting your lip, as to avoid asking him if his bright pink hair was up to code.
“Hm…” He grabbed a paper. “How about you fill this out to let us get a better grasp of who you are, then I’ll have a doctor chat with you, alright?” He quickly smiled up at you, but his eyes were cold, probably wishing you’d leave him alone already.
You nodded. “Thanks!”
After about 30 minutes and 100 questions later, you were called into an exam room, and you sat on the bed in there.
A young man with wire rimmed glasses and light blue hair entered and applied hand sanitizer. “So, my name is Kun Qian. So I’ve checked your questionnaire and your medical history, and I’ve compiled a list of things you are able to participate in.”
He handed you a stack of papers, and on top, there it was.
Project E 2.0
This medication is being tested to treat PCOS and other hormone irregularities.
Side effects include a heightened libido, sensitivity during sex, as well as more intense and stronger orgasms.
You glanced up at Kun, who was clicking his pen. “I’ll just do this one, it seems simple enough.”
Kun sighed, rolling his neck back to crack his neck. “The E one? It’s important for you to know that the side effects of this medication are… strong, to say the least.”
“What, am I going to go feral? It’s fine, just give me the jab.” You laughed, but Kun didn’t respond.
“Yes. The woman who received the 1.0 version was unable to sleep, eat… she couldn’t live a normal life for several weeks because of it. All she was able to do was have sex and be force-fed by her doctor… Well, boyfriend now, I guess.” He pulled off his glasses and looked you in the eye. “I promise, I will be right here, ready to assist you, or anything you may need, but I need to know that you can deal with the pain.”
“Well, when you put it like that… I guess I should learn a bit more first.”
“Do you have a husband or wife? Any significant other that might be able to help?” Kun started to write stuff down on his clipboard.
“Er, I have a roommate?” You fidgeted with the hem of your skirt, unsure of what Kun meant.
Kun exhaled loudly, leaning back in his chair, his annoyance with you obvious. “I meant someone you could have sex with, madam.”
“I have a vibrator?” You laughed, the hairs on your arms standing due to your nerves.
Kun bit his lip. “I think it’s in your best interest to choose another option.”
“Um, but I think this would be the easiest for me… All I have to do is track possible side effects, right? And I can go home?” You flipped through the other studies, ranging from a hypothermia study to a sociology study.
“Well, you’d have to stay here for a week, only so we can see how you’d react… but yes, you can.” Kun clicked his pen against the clipboard. “I’ll give it to you. Let me lead you to your room.
~
It actually wasn’t that bad, considering this was an independent clinic. You had your own bedroom and bathroom, and your roommate sent you clothes for your week stay.
Kun sat on the chair next to your bed, letting you settle in before giving you the shot.
“I’m ready.” You sat on the bed, shuffling over to where Kun was sitting.
“There.” Kun injected it. “I’ll stay with you for the first hour, if you don’t mind.”
“Not at all. Do you wanna get in bed with me?” You scooched over, letting him in.
“Um…” Kun stood up, sitting down next to you, taking his shoes off before moving to sit next to you.
“So, Doctor, how’s your day going?” You turned on the TV in front of you, switching the channel to some cheesy reality Lifetime show.
“You’re the first person I’ve seen today, so I guess… Good.” Kun inspected your body, from the palms of your hands down to your chest, then your thighs and feet. “So… what made you want to do this?”
“Money?” You glanced at him, noticing how plump his lips were after he bit his bottom lip. “Why does anyone let themselves do something like this?”
“Well, you’re not wrong, I guess.” The palm of his hand pressed against your forehead, trying to feel for your temperature. “Are you feeling okay?”
“It’s only been like… 5 minutes, so I guess I feel the same.” You looked up at Kun, who was now standing up and sliding his shoes back on.
A minute turned to two minutes, two turned to twenty, twenty turned to sixty…
“I want to check something.” Kun grabbed his stethoscope, putting it in his ears and against your heart. “I’ll be back in a minute…”
You just sat there, scrolling through your phone.
And it started. Your back hit the bed’s back board, the heat getting to you. You knew you were going to get horny, but you didn’t know it was going to be this bad…
The heavy wooden door opened again. “Okay, so I wanted to see if-”
“Doctor…” Your voice was so whiny, you hardly knew you could make that kind of tone. “Need you.” You sat on your knees, reaching out for him, lips pouting, drawling out every word. Blood coursed through your veins, your heart beating out of your chest. And you felt all the blood rush to your clit at the sight of Kun.
“Fuck.” Kun pressed the stethoscope back against your chest, and after about 10 seconds he ripped it out of his ears, falling down towards your body, one of his hands on your shoulder, the other on your back. “Come on sweetie, let’s get you in the shower… or something, I…” Kun frantically looked around, his concern for you resulting in a furrowed brow. “Did your roommate bring you anything, a dildo, or…”
“You…” You finally got out a word. “Please, fuck me.” Tears began to well up in your eyes. It truly was a feeling you never felt, it was like you had been edging for days without release.
A million thoughts rushed into Kun's mind all at once. The loudest one being the word pathetic. You were a pathetic, whining mess, begging him for his cock, needing to fuck him. And it made him so hard. Any man would get hard in his situation, he figured. But he knew he had to stay professional, even if it was for a few more minutes. “I… I don’t think I can, I mean, I’m your doctor!” Kun pushed you off him, then slipped your jacket off you, trying to get you to cool down.
You had a ball of the fabric of Kun’s lab coat in your fist. “Please, I need… anyone.”
“Fuck, fuck… I thought Jaemin fixed it… I knew I shouldn’t have fucking trusted him.” Kun bit his lip, half wanting to search the suitcase and dressers in your room, but also knowing he needed to comfort you right now.
“Cum… Cum inside me…” You whined, your head hitting Kun’s chest. You breathed in the thick scent of his cologne. Minty, musky and woody. He smelled so good, and that just made things worse for some reason. It truly felt like you were going feral, like you were turning back to your primal instincts, needing him inside you, his warm release, to be filled up. “Need… you inside me…”
“Come on baby… work with me.” Kun helped you to your feet, leading you into the bathroom. The lock on the door automatically locked behind the two of you. Kun started undressing you as quickly as possible then pushed you into the bathtub. “Tell me if the water’s too cold.” He turned on the water, the cold helping lower your temperature.
“Kun…” You cooed, wrapping an arm around his neck. “Please, fuck me.”
You caught Kun looking at your body, up at your breasts and down to your thighs…
“I’ll, um…” Kun’s hand entered the water, and began to rub your clit, making you immediately reach your climax.
“Doctor!” You cried out. “More, more please!”
“I don’t know, I don’t know how to help you anymore…” Kun mumbled, thinking hard.
“Cock, please…” You whined.
“Fuck…” Kun helped you out of the bathtub and wrapped you in a towel, quickly drying you off then leading you to the bed, bending you over the mattress.
“Cum inside, please, please, please…” You really felt like you were actually going brain dead, the only thing on your mind being Kun’s cock.
And he finally was inside you. You let Kun do whatever he wanted to with your body. You figured he must’ve been pent up, too. The way his cock slammed into your womb, hitting your cervix with ease, letting you know how sore you were going to be tomorrow. His hands made their way into your mouth, both middle and ring fingers hooking into your cheeks, stretching your lips horizontally, making you drool, tongue sticking out on its own.
He was rough, nothing like the shy boy he was two seconds ago, who didn’t even know if he should be fucking you. It was like a flip switched in his brain, like he was just pretending to be kind so his cock would feel even better when it went inside you…
“Doctor, please be gentler!” You felt like you were melting on his dick, so hot and so hard…
“I don’t want to.” His hands moved to your neck, wrapping around your neck, choking you until you were unable to breathe.
“Cumming!” You screamed out, hitting your second climax.
You felt your womb filling up with Kun’s cum before he pulled out.
He picked you up and laid you back down. “Do you feel better?”
You nodded, sitting up, covering yourself with the blanket.
“Good. I’ll be back with your dinner.” Kun slid his pants back up then left.
~
And after about 30 minutes you felt like a bitch in heat all over again.
“Dinner.” Kun entered again after 45 minutes, catching you with your fingers inside yourself. “Jesus Christ…” He sighed, slamming the tray onto the nearest table.
“Doctor…” You whimpered, hoping he would fuck your again.
“You’re so… pathetic. And whiny.” Kun ran his hands through your hair, then grabbed your hand, pulling your fingers out of yourself and licking your juices off. He stood at the foot of the bed, grabbing your foot to pull you down and began to lick your clit.
And again, you practically immediately reached your high…
Kun wiped his face, your cum on his nose and lips. “Do you feel better?”
You shook your head. “Cum…” You whined. “Please?”
Kun facepalmed, sighing. “Ride me.” He sat on the couch in your room.
You slid off your bed and sat on Kun’s lap, facing away from him, lining his cock into you.
“You know, it’ll hurt more if you take it slow. Put it in all at once.” Kun his hand on your waist, stabilizing you.
“I know!” You whined, shoving it in. “Fuck!”
“Hm? Is that it?” Kun’s tone became more mocking than annoyed. “Do I have to do everything for you?” His arm went under your knees, fucking you under you.
“You’re in too deep, Doctor!” You cried out. You felt like Kun was in your womb. “You’re not gonna be able to pull out!”
“Don’t think.” Kun instructed.
And you did as told, letting your brain melt into nothingness other than the feeling of Kun’s cock inside you. You probably wouldn’t be able to recall your own name…
That was, until Kun’s cum flowed inside you again, bringing you into your own orgasm.
Kun dropped you back onto his lap, your arms wrapping around his neck and your head fell onto his chest.
~
You woke up still on Kun’s lap.
“How long-”
Kun cut you off. “45 minutes. It’s fine.” But really, the entire left side of Kun’s body was numb.
You nodded.
“So, what did you think?” Kun asked you as you climbed off his lap and back onto your own bed.
“It was… good. Amazing, actually, I’ve never felt so good…” You started dreaming about getting stuffed full of Kun’s cum all over again…
“I was talking about the medicine, but thanks for rating my dick.” Kun scoffed, getting up to give you your dinner.
“Oh. Not as fun.” You watched Kun.
“I, um… was thinking that you could live with me and help me test the rest of the iterations of the medication.” Kun hooked you back up to the blood pressure monitor.
“Maybe.” You took a bite of the dinner. “But I’ll say yes if it means I can keep having sex with you.
“You can keep having sex with me even when you stop being my patient.”
95 notes
·
View notes
Text

“The NHS is shutting down its gender identity clinic for children after a damning review found that it failed vulnerable under-18s.
The gender identity service at Tavistock & Portman NHS Foundation Trust has been ordered to close by spring 2023.
It will be replaced by regional centres at existing children’s hospitals offering more “holistic care” with “strong links to mental health services”.
Tavistock’s Gender Identity Development Service (GIDS) clinic has been accused of rushing children into life-altering treatment on puberty blockers.
The paediatrician Dr Hilary Cass, who is leading a review of the service, has today issued a series of recommendations for a radical overhaul of how the NHS treats young people who are questioning their gender identity.
She found that the Tavistock clinic was “not a safe or viable long-term option” and that other mental health issues were “overshadowed” when gender was raised by children referred to the clinic.
Cass, former president of the Royal College of Paediatrics and Child Health, said the current model of a sole provider for gender services should be scrapped as it failed to meet the holistic needs of distressed and vulnerable teenagers.
She said Tavistock should be replaced by regional centres with an “appropriate multi-professional workforce to enable them to provide an integrated model of care that manages the holistic needs of this population”.
Amid concerns that the clinic fails to take into account wider health problems before putting children on puberty blockers, Cass added: “Staff should maintain a broad clinical perspective in order to embed the care of children and young people with gender uncertainty within a broader child and adolescent health context.”
NHS England, which commissioned Cass to review the service in September 2020, say they will implement her recommendations in full and decommission the Tavistock clinic.
They have announced they will launch two new clinics for children with gender dysphoria by spring 2023, which will bring together multiple doctors from a broad range of specialities.
The first, in London, will be based at Great Ormond Street Hospital and receive specialist mental health support from the South London and Maudsley NHS Foundation Trust.
The second, in the northwest, will be led by a partnership between Alder Hey Children’s NHS Foundation Trust and the Royal Manchester Children’s Hospital.
Cass said these clinics must have “established academic and education functions” to monitor evidence on children who are put on hormone therapy. The Tavistock clinic failed to collect sufficient data on the impact of puberty blockers in under-16s.
She said there was currently “insufficient evidence” for her to make any firm recommendations around their routine use.
Cass told the NHS to “enrol young people being considered for hormone treatment into a formal research protocol with adequate follow up into adulthood, with a more immediate focus on the questions regarding puberty blockers”.
The NHS said it would launch clinical trials in partnership with the National Institute for Health and Care Research to follow children on puberty blockers into adulthood.
A spokesman said: “This will ensure that there is greater transparency for children and their parents/carers around the uncertain clinical benefits and longer-term health impacts surrounding their use.”
The clinic has been overwhelmed by a sudden increase in referrals, particularly among young girls and children on the autism spectrum. Last year it received more than 5,000 referrals, compared to 250 a decade ago.
Cass’s final report will be published next year. Her interim review published in March found that services had developed without clear rules and that there was a “clinician lottery”, with widely varying approaches to treatment.
She found there was “a lack of agreement, and in many instances a lack of open discussion” about whether unhappiness with gender in adolescence was permanent or temporary. However, last year the Court of Appeal overturned a controversial ruling made by the High Court that children under 16 were unlikely to be able to give informed consent to receiving puberty blockers.
The case was brought against the Tavistock and Portman trust by Keira Bell, 24, who began taking puberty blockers when she was 16 to transition to male before later “detransitioning”.”
Link | Archived Link
249 notes
·
View notes
Text
The NHS is shutting down its gender identity clinic for children after a review found that it failed vulnerable under-18s.
The gender identity service at Tavistock & Portman NHS Foundation Trust has been ordered to close by next spring.
It will be replaced by regional centres at existing children’s hospitals offering more “holistic care” with “strong links to mental health services”.
Tavistock’s Gender Identity Development Service (GIDS) clinic has been accused of rushing children into life-altering treatment on puberty blockers.
The paediatrician Dr Hilary Cass, who is leading a review of the service, issued a series of recommendations today for a radical overhaul of how the NHS treats young people who are questioning their gender identity.
She found that the Tavistock clinic was “not a safe or viable long-term option” and that other mental health issues were “overshadowed” when gender was raised by children referred to the clinic.
Cass, former president of the Royal College of Paediatrics and Child Health, said the existing model of a sole provider for gender services should be scrapped as it failed to meet the holistic needs of distressed and vulnerable teenagers.
She said Tavistock should be replaced by regional centres with an “appropriate multi-professional workforce to enable them to provide an integrated model of care that manages the holistic needs of this population”.
Amid concerns that the clinic fails to take into account wider health problems before putting children on puberty blockers, Cass added: “Staff should maintain a broad clinical perspective in order to embed the care of children and young people with gender uncertainty within a broader child and adolescent health context.”
NHS England, which commissioned Cass to review the service in September 2020, says it will implement her recommendations in full and decommission the Tavistock clinic.
It has announced the launch of two clinics for children with gender dysphoria by next spring, which will bring together multiple doctors from a broad range of specialities.
The first, in London, will be based at Great Ormond Street Hospital and receive specialist mental health support from the South London and Maudsley NHS Foundation Trust.
The second, in the northwest, will be led by a partnership between Alder Hey Children’s NHS Foundation Trust in Liverpool and the Royal Manchester Children’s Hospital.
Cass said these clinics must have “established academic and education functions” to monitor evidence on children who are put on hormone therapy. The Tavistock clinic failed to collect sufficient data on the impact of puberty blockers in under-16s.
She said there was “insufficient evidence” for her to make any firm recommendations around their routine use.
Cass told the NHS to “enrol young people being considered for hormone treatment into a formal research protocol with adequate follow-up into adulthood, with a more immediate focus on the questions regarding puberty blockers”.
The NHS said it would launch clinical trials in partnership with the National Institute for Health and Care Research to follow children on puberty blockers into adulthood. “This will ensure that there is greater transparency for children and their parents/carers around the uncertain clinical benefits and longer-term health impacts surrounding their use,” a spokesman said.
The clinic has been overwhelmed by a sudden increase in referrals, particularly among young girls and children on the autism spectrum. Last year it received more than 5,000 referrals, compared with 250 a decade ago.
Cass’s final report will be published next year. Her interim review published in March found that services had developed without clear rules and that there was a “clinician lottery”, with widely varying approaches to treatment.
She found there was “a lack of agreement, and in many instances a lack of open discussion” about whether unhappiness with gender in adolescence was permanent or temporary. However, last year the Court of Appeal overturned a ruling made by the High Court that children under 16 were unlikely to be able to give informed consent to receiving puberty blockers.
The case was brought against the Tavistock and Portman trust by Keira Bell, 24, who began taking puberty blockers when she was 16 to transition to male before later “detransitioning”.
226 notes
·
View notes
Text
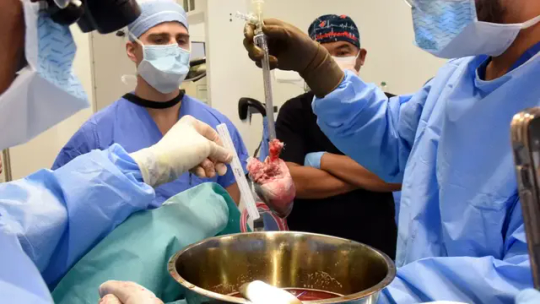
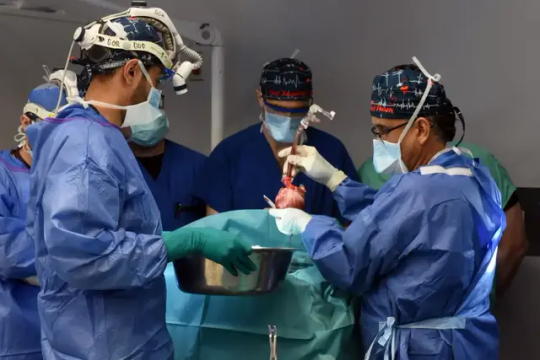
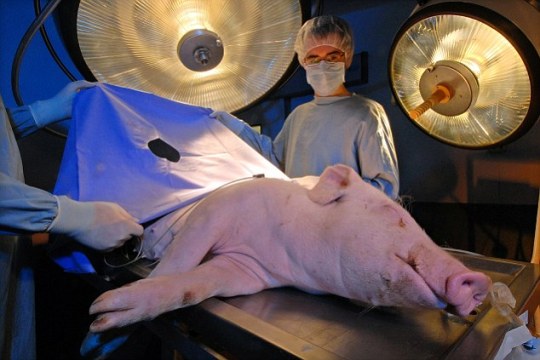
Groundbreaking Pig Heart Transplant is Performed for the Second Time
For the second time ever, a pig heart has been transplanted into a living human recipient, the University of Maryland Medical Center announced on Friday.
The groundbreaking surgery was done on September 20 at UMMC by the same transplant team that preformed the first such experimental surgery in 2022.
In a news release, the hospital said the recipient, 58-year-old Lawrence Faucette, “is currently breathing on his own, and his heart is functioning well without any assistance from supportive devices.”
Faucette had end-stage heart disease. He had pre-existing peripheral vascular disease and complications with internal bleeding making him ineligible for a traditional heart transplant, the hospital said in the release. He was admitted to UMMC on September 14 after experiencing symptoms of heart failure.
“My only real hope left is to go with the pig heart, the xenotransplant,” Faucette told the hospital in an internal interview several days before the surgery.
The experimental xenotransplant surgery was green lit under the US Food and Drug Administration’s “compassionate use” program. According to the FDA, the program is “a potential pathway for a patient with a serious or immediately life-threatening disease or condition to gain access to an investigational medical product (drug, biologic, or medical device) for treatment outside of clinical trials when no comparable or satisfactory alternative therapy options are available.”
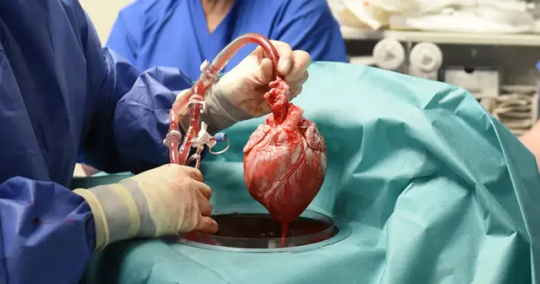
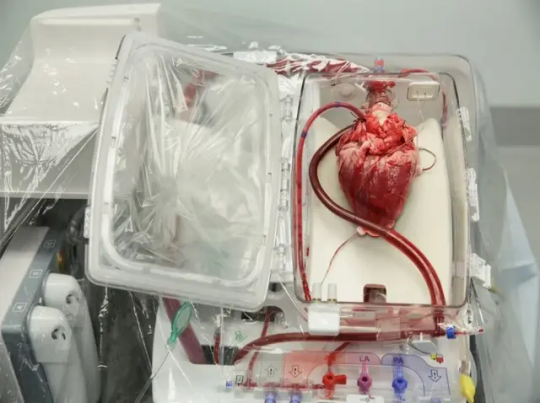
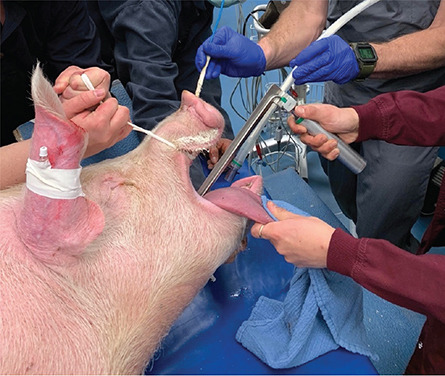
The pig heart used came from a genetically modified pig from Revivcor, a subsidiary the United Therapeutics Corporation. The pig had 10 genes edited, including three genes “knocked out” or inactivated to eliminate the alpha gal sugar in the pig’s blood cells, which can trigger a severe reaction in the human immune system, causing organ rejection. An additional pig gene was modified to control for the growth of the pig’s heart while 6 human genes were added into the pig’s genome to increase acceptance by the immune system. The FDA first approved the gene edited pigs in 2020 for potential therapeutic use and consumption.
Doctors are also treating Faucette with an experimental antibody treatment to further suppress the immune system and prevent rejection. He will be closely monitored for any signs of rejection or any development of pig related viruses. The donor pig was also closely screened for any signs of virus or pathogens.
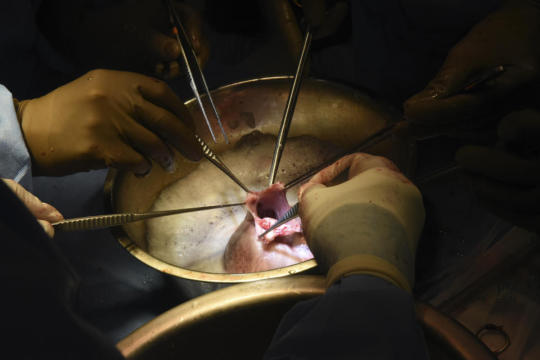
“We are once again offering a dying patient a shot at a longer life, and we are incredibly grateful to Mr. Faucette for his bravery and willingness to help advance our knowledge of this field,” said Dr. Bartley Griffith, in the release. Griffith is the surgeon who performed the transplant and is a professor of surgery at the University of Maryland School of Medicine.
The hospital said Faucette fully consented to the experimental treatment and was informed of all the risks. In addition, he underwent a full psychiatric evaluation and discussed his case with a medical ethicist.
According to the hospital’s news release, Faucette is a married father of two from Frederick, Maryland and a 20-year Navy veteran who had most recently worked as a lab technician at the National Institutes of Health before retiring.
“We have no expectations other than hoping for more time together,” said his wife Ann Faucette, in the release. “That could be as simple as sitting on the front porch and having coffee together.” There are currently no clinical trials that utilize pig organs for transplants in living human beings. The University of Maryland performed the first such experimental surgery on 57-year-old David Bennett in January 2022. Bennett died two months following the surgery.
While there were no signs of rejection in the initial weeks following the transplant, an autopsy concluded that Bennett ultimately died of heart failure from “a complex array of factors,” including Bennett’s condition prior to the surgery. Bennet had already been hospitalized and kept on a heart lung bypass machine for 6 weeks prior to the transplant. However, a case study by the doctors published in the Lancet also noted there was evidence of pig virus that had not been identified previously.
According to the federal government, there are more than 113,000 people on the organ transplant list, including 3,354 people in need of a heart. The group Donate Life America says that 17 people die each day waiting for a donor organ.
By Nadia Kounang,


#Groundbreaking Pig Heart Transplant is Performed for the Second Time#The University of Maryland Medical Center#Lawrence Faucette#heart#pig heart#heart transplant#organ transplant#xenotransplant#animals#strange#stranger things#science#science news
20 notes
·
View notes