#monitoring of clinical trials by industry sponsors
Text
Clinical Research Monitoring: A Guide to Clinical Monitoring
Clinical research monitoring is a vital part of clinical trials and it involves various activities to ensure the safety and accuracy of the data collected. It is important that the clinical trial is conducted in a way that meets regulatory standards, protects human studies participants, and minimizes potential risks to their health and well-being. Clinical trial monitoring can include activities such as auditing study sites, evaluating data for accuracy and completeness, review of protocols and amendments, reviewing case report forms (CRFs), identifying any deviations from the standard operating procedures (SOPs) or protocols, managing corrective action plans (CAPs), following up on safety reports, tracking progress against enrollment goals and much more. Apart from evaluating data quality, clinical research monitoring also ensures compliance with all regulatory standards like GCP (Good Clinical Practices) ICH (International Conference on Harmonization), FDA regulations and local laws. In addition to this ongoing monitoring throughout a study's duration, there may be audits conducted by sponsors or regulatory authorities at any time during or after completion of a clinical trial. All these efforts are dedicated towards ensuring that the results obtained from a clinical trial are accurate, reliable and applicable for use in making medical decisions.
Steps to Clinical Monitoring
1. Establish an Effective Monitoring Plan: Ensure that the plan is comprehensive and contains all applicable elements, such as the types of monitoring activities to be conducted, frequency of monitoring visits, data collection methods, and specific criteria for acceptable performance.
2. Develop Appropriate Documentation: Design protocol-specific monitoring tools and forms to document information from site visits including source documents, data collection instruments, case report forms (CRF). In addition, develop a Monitoring Log or Tracking System which will enable better accountability for study activities.
3. Execute Monitors’ Visits: Depending on the complexity of the trial and regulatory requirements, conduct pre-study qualification visits (PSQV), pre-initiation visits (PIV), initiation visits (IVs), periodic monitoring visits (PMV) and close out visits (COV). During each visit, ensure that good clinical practice is followed at all times by reviewing source documents and data collection instruments. Review patient enrollment logs to ensure accuracy and record any discrepancies in the visit report.
4. Report Findings: Generate detailed yet concise reports per each monitor's visit with clear recommendations for corrective actions if required; provide professional feedback to investigators regarding their performance; identify any areas of noncompliance with protocol requirements or applicable regulations; recommend training or educational sessions when appropriate; track all follow up activities related to corrective actions taken in response to findings from monitors' visits; ensure that essential documentation is complete before closing out a particular study site.
5. Quality Assurance: Validate accuracy of tracking systems used by monitors during their visits; assess risk associated with various deficiencies identified during monitoring process; carry out periodic internal audits/assessments to ensure compliance with established SOPs/guidelines related to clinical research monitoring activities; take preventive measures based on audit/assessment results in order to strengthen internal quality system processes.
Types of Clinical Trial Monitoring
1. Types of Clinical Research Monitoring: Clinical research monitoring is the process to assess the quality and integrity of clinical trial data and ensure compliance with applicable regulatory requirements. It can be done through three primary methods: onsite monitoring, centralized or remote monitoring, and risk-based approaches.
2. Onsite Monitoring: Onsite monitoring is considered the "gold standard" for clinical research monitoring, as it requires the presence of a monitor at a study site during the entire duration of a trial. The monitor will typically review source documentation such as patient records, lab results, and investigational product dispensing logs to assess accuracy and conformance with study protocols and good clinical practices (GCP). The monitor also interviews staff members responsible for conducting the trial to verify that procedures are being followed properly.
3. Centralized or Remote Monitoring in Clinical Trials: Centralized or remote monitoring enables sponsors to conduct clinical research monitoring without needing to send someone onsite to each study location. This is accomplished by using technology such as web portals, video conferencing, and virtual meetings that allow monitors to remotely review data from various sites simultaneously and quickly flag any issues that arise. Additionally, centralized/remote monitoring allows sponsors to be more proactive in identifying potential risks associated with a trial prior to sending monitors onsite for an assessment.
4. Risk-Based Approaches: Risk-based approaches use data analytics tools such as descriptive statistics and predictive algorithms to identify potential trends or outliers in clinical trial data that may represent heightened risk of noncompliance with GCPs or other regulations. By leveraging technology, these approaches can help sponsors identify issues earlier in the course of a trial so they can take corrective action before something goes wrong.
5. Benefits of Clinical Research Monitoring: Utilizing effective clinical research monitoring strategies helps ensure that trials are conducted ethically, safely, correctly according to protocol standards, within timelines agreed upon with regulatory authorities, and within budget constraints set out by sponsors/CROs/investigators/other stakeholders involved in a study’s execution.. Clinical research monitors act as an independent third party who are able to provide objective insight into how studies are being conducted across multiple sites which helps minimize errors due to bias from investigators or other personnel who may have vested interests in outcomes associated with their studies.. In addition, effective clinical research monitoring helps ensure patient safety by providing oversight about how drugs or medical devices used in trials are administered as well as ensuring patient confidentiality is maintained throughout the course of a study.. Lastly, robust clinical research monitoring protocols help reduce costs associated with delays caused by errors made during trials which can add up significantly over time if not avoided through proper oversight methods both pre-study start up until closeout occurs after all enrolled patients have completed their participation in a given trial
Clinical Research Monitoring Guide
1. Understand the Basics of Clinical Research Monitoring: Clinical research monitoring is a key part of the clinical research process, ensuring the safety and accuracy of results. It involves periodically assessing study sites to confirm that data is being collected properly, according to ethical and legal requirements, as per Good Clinical Practice (GCP) guidelines.
2. Know What Types of Studies are Monitored: Clinical research monitoring can be used for a variety of studies, including clinical trials, observational studies, epidemiologic studies, and public health surveys. It is important to know what type of study you are monitoring in order to ensure that the appropriate procedures are followed.
3. Understand How to Monitor a Study Site: The primary goal of clinical research monitoring is to confirm that the protocol and informed consent form have been followed properly at each site. This requires a thorough review of all relevant documents such as case report forms (CRFs), source documentation (e.g., physician notes), internal audit reports (audit trails), and external quality assurance reports. Additionally, it involves evaluating compliance with GCP guidelines during study visits or remote reviews, as well as conducting interviews with staff members to assess how they are handling data collection and reporting processes.
4. Become Familiar With Regulatory Requirements: In addition to GCP guidelines, there may be applicable regulations from local governments or other institutions that must be adhered to when conducting clinical research monitoring activities. Understanding these regulations is essential for ensuring compliance with applicable laws and regulations related to clinical research activities.
5. Develop an Effective Monitoring Plan: An effective monitoring plan should include a detailed timeline for visiting sites, information about any specific areas where focused attention is required (e.g., enrolling/randomizing patients or managing adverse events), and plans for auditing/reviewing data generated by the study site(s). Additionally, it should incorporate measures for controlling risk associated with data collection processes so that issues can be identified early on in the study process before they become problematic later on down the line.
Clinical Research Monitor Job
The job of a Clinical Research Monitor is to ensure that clinical trials are conducted ethically, safely and in compliance with established standards. The primary responsibility of the monitor is to protect the rights, safety and well-being of the human subjects enrolled in the trial. Duties typically include developing protocols for clinical studies; coordinating study start up activities; conducting site visits; monitoring data for timeliness, accuracy and completeness; auditing files for regulatory compliance; managing investigator queries/issues; preparing visit reports; reviewing update protocols related to study operations; resolving issues raised through audit reports or other sources; providing technical guidance to sites regarding protocol implementation or study conduct; and escalating complex issues or potential risks as needed.
Clinical Research Monitor Salary
Salaries for this position tend to vary depending on education level, experience and geographical location but can range from $60,000 per year for entry level positions up to around $90,000 per year for more experienced professionals. In addition to salary many employers also offer benefits such as paid vacation days, health insurance plans and retirement packages.
Resources for Clinical Research Monitoring
1. National Institutes of Health (NIH): Clinical Research Monitoring
This link provides information on NIH's guidelines for monitoring clinical research, which include topics such as the roles and responsibilities of the investigator, data safety monitoring boards, and protocols for reporting unanticipated problems and adverse events.
2. National Institutes of Health (NIH): Guide to Clinical Research Monitoring
This comprehensive guide walks readers through all aspects of clinical research monitoring, including topics such as study design, randomization strategies, regulatory compliance requirements, data management, monitoring plans and reports, quality improvement initiatives, and safety assessments.
3. US Food and Drug Administration (FDA): Guidelines for Clinical Trials Monitoring
This resource from the FDA outlines the importance of effective monitoring in clinical trials and provides an overview of the different roles within a clinical trial as well as details about essential elements for implementation of an effective monitoring strategy such as risk assessments and adverse event tracking.
4. International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH)
ICH has developed standards that provide a set of harmonized technical requirements for clinical trials conducted across countries in the European Union (EU), Japan, and US with an emphasis on quality assurance and safety monitoring during trials.
5. Association of Clinical Research Professionals (ACRP)
ACRP's guidelines provide best practice recommendations for conducting clinical research studies in accordance with applicable regulations and standards to ensure patient safety monitoring during studies as well as data integrity throughout the process from start to finish.
6. Pharmaceutical Research & Manufacturers of America (PhRMA)
The PhRMA guidelines provide an overview of expectations around clinical research activities with respect to ethics, data integrity, safety reporting, resource allocation and more. It defines roles and responsibilities of all those involved in overseeing a clinical trial such as a Clinical Research Monitor or CRA who has primary responsibility for ensuring that the protocol is implemented correctly throughout a study’s duration
Clinical Research Monitoring Review
1. What is the main purpose of clinical research monitoring?
A) To ensure that a research study is conducted in accordance with applicable regulations and ethical standards
B) To ensure that data collected during a research study is accurate and reliable
C) To evaluate the safety of participants enrolled in a research trial
D) To oversee the financial management of a research project
Answer: A) To ensure that a research study is conducted in accordance with applicable regulations and ethical standards. Clinical Research Monitors are responsible for ensuring compliance with Good Clinical Practice guidelines, protecting participant privacy, verifying data accuracy, and evaluating protocol deviations. In addition, they may also be involved in reviewing participant eligibility requirements, conducting site assessments, providing training to investigators and staff on proper study procedures, as well as monitoring progress towards completion of all requirements of the study.
2. What type of individuals typically serve as clinical research monitors?
A) Physicians
B) Nurses
C) Regulatory specialists
D) All of the above
Answer: D) All of the above. Clinical Research Monitors can come from various backgrounds such as medical doctors (MDs), nurses (RNs), pharmacists (RPhs), regulatory specialists (e.g., Regulatory Affairs Professionals or Paralegals), or biostatisticians/data analysts who have experience in clinical trials and understand local regulations related to human subject protection. Each monitor has specific job duties depending on their education and experience, such as assessing compliance with regulatory guidance or analyzing data sets for accuracy, completeness, integrity, or validity.
3. What kind of activities do clinical research monitors need to perform?
A) Protocol reviews or verifications
B) Ensuring appropriate documentation completion
C) Site visits to observe investigator conduct
D )All of the above
Answer: D )All of the above. Clinical Research Monitors need to perform several activities including protocol reviews or verifications; ensuring appropriate documentation completion; site visits to observe investigator conduct; liaising between sponsors and sites; assisting with resolving issues associated with adverse events; reviewing case report forms for completeness, accuracy, consistency and correctness; evaluating subject safety throughout enrollment process;and writing reports detailing their findings at each visit.
4. What is one benefit gained from having an effective Clinical Research Monitor on-site? A) Reduced risk for legal liability stemming from negligence
B) Improved protocol adherence by investigators
C) Increased patient engagement during trial period
D )All of the above
Answer: D) All of the above . An effective Clinical Research Monitor encompasses several benefits such as reduced risk for legal liability stemming from negligence due to thorough oversight and accurate record keeping; improved protocol adherence by investigators through continued communication between sponsor representatives and researchers on-site regarding best practices; increased patient engagement during trial period due to more detailed explanations about potential risks/benefits offered by having monitor on-site ; and improved efficiency when dealing with complex protocols that require multiple levelsof oversight due to familiarity with protocol specifics which decreases time spent troubleshooting errors or unclear instructions..
5. How often should Clinical Research Monitors visit a particular site?
A) Weekly B) Biweekly C) Monthly D) Quarterly
Answer: C) Monthly . It is recommended that Clinical Research Monitors visit sites at least once per month in order to maintain active surveillance over ongoing studies at each location while also providing timely feedback regarding any issues discovered while on-site visits are taking place within a shorter timeframe if needed based upon changes made midstream or other unanticipated circumstances which might require immediate attention by sponsor personnel.
#clinical monitoring#clinical trial monitoring#remote monitoring clinical trials#clinical research monitor#clinical research monitor salary#risk based monitoring in clinical trials#clinical trial monitor#clinical trial monitor salary#monitoring of clinical trials by industry sponsors#centralized monitoring clinical trials#clinical research monitoring#clinical site monitoring#clinical trial monitoring services#clinical trial remote monitoring#clinical trials monitor#medical monitor clinical trial#risk based monitoring clinical trials#clinical monitor#clinical research monitor jobs#clinical trials monitoring#clinically validated blood pressure monitors for home use#monitoring in clinical trials#clinical monitoring services#clinical trial monitor jobs#journal of clinical monitoring and computing#monitoring clinical trials#remote monitoring in clinical trials#central monitoring clinical trials#clinical data monitoring#clinical monitoring jobs
0 notes
Text
Introduction to the world of CRO (Contract Research Organisation)
Greetings! We, at Team Auxillium, are here to be your encyclopaedia on everything that is about Contract Research! Contract Research Organisation, also called Clinical Research Organization (CRO) is a service organization that provides support to the pharmaceutical and biotechnology industries in the form of outsourced pharmaceutical research services (for both drugs and medical devices). CROs range from large, international full service organizations to small, niche specialty groups and can offer their clients the experience of moving a new drug or device from its conception to FDA marketing approval without the drug sponsor having to maintain a staff for these services. Contract Research Organizations (CROs) have existed since the 1930s, are of various sizes, offer a variety of services, and are present worldwide. Business strategies of CROs vary and it is important for the sponsor to select a CRO with a philosophy that fits their style. A variety of resources are available to identify CROs. There are key points to consider in the selection of any CRO. Work can be placed at CROs in China, India, etc., if proper precautions are taken. Unexpected events may occur during work and the management of these ‘mistakes’ is critical to the success of any study.
What does employing a CRO cover?
A Contract Research Organization (CRO) is a company that provides clinical trial services for the pharmaceutical, biotechnology, and medical device industries. There are different types of CROs, but typical CRO services in the medical device industry include regulatory affairs, clinical trial planning, site selection and initiation, recruitment support, clinical monitoring, data management, trial logistics, biostatistics, medical writing, and project management. CROs are hired by sponsors who want to run a clinical trial. This eliminates the need to hire full time staff to complete the project and provides an opportunity to work with the CRO on a project-by-project basis. The CRO is hired to plan, coordinate, execute, and manage the lifecycle of the clinical trial, safely and efficiently. Serving as the main contact between the sponsor and other stakeholders throughout the trial, the CRO communicates with ethics and compliance committees, regulatory personnel, vendors, physicians, and research coordinators. They do clinical trial planning, clinical data management, clinical project management, and clinical trial monitoring.
What does a CRO mean in clinical trials?
CROs have the knowledge, capabilities, processes and procedures that are needed to develop and run a successful clinical trial, while ensuring trial quality and compliance with national and international standards. Working with a contract research organization can offer innovative tools that can increase efficiencies, leading to decreased timelines and cost. Choosing the right CRO to run your clinical trial is crucial to the trial success. In addition to consideration of their own project needs, requirements, and budget when selecting a CRO, Sponsors should evaluate the qualification, experience, and quality system processes of the CRO.
2 notes
·
View notes
Text
Transforming the Trial: How Digital Innovation is Reshaping Clinical Research

Clinical trials are the backbone of medical progress, but they've traditionally been slow, expensive, and geographically limited. Thankfully, the winds of change are blowing through the industry, driven by a powerful force: digital transformation.
The Rise of the Digital Patient
Today's patients are tech-savvy and expect convenience. Digital platforms are making clinical trials more accessible than ever before. Imagine enrolling in a study from the comfort of your couch, using a mobile app to report symptoms, or wearing a wearable device that transmits health data remotely. This is the reality of decentralized trials, and it's attracting a wider pool of participants, leading to more diverse and generalizable results.
Data Sharing on Steroids
Clinical trials generate a mountain of data. But siloed systems and paper-based processes often hinder its efficient collection and analysis. Secure digital platforms are changing this game. By facilitating seamless data sharing between researchers, sponsors, and CDMOs, these platforms accelerate trial progress and provide real-time insights.
The CDMO Advantage in a Digital Age
Contract Development and Manufacturing Organizations (CDMOs) are at a crossroads. Those that embrace digital transformation will be the frontrunners in this evolving landscape. By integrating cutting-edge technologies like remote monitoring, data analytics, and telehealth into their services, CDMOs can offer pharma companies a significant edge:
Faster Trial Completion: Streamlined data collection and analysis shave valuable time off trial timelines.
Reduced Costs: Decentralized trials and remote monitoring minimize travel expenses for patients and sites.
Enhanced Patient Engagement: Digital tools empower patients to be active participants in their own healthcare journey.
Improved Data Quality: Real-time data capture and standardized platforms minimize errors and ensure data integrity.
The Future is Now
The digital transformation of clinical trials is not a futuristic vision; it's happening now. CDMOs that invest in these technologies are positioning themselves as invaluable partners in the race to bring life-saving treatments to patients faster and more efficiently. This digital revolution promises a brighter future for medical research, one that benefits patients, researchers, and the healthcare industry as a whole.
0 notes
Text
Clinical Trial Management Systems Market Set to Reach US$ 4.6 Billion Surge in Demand by 2032
By 2022, the clinical trial management systems market is expected to be valued at approximately US$ 1.3 billion.
Clinical trial management system deployment is expected to grow at a rapid rate of 13.6% CAGR to reach US$ 4.6 billion by 2032. It is anticipated that the market for web-based clinical trial management systems will grow at a 13.9% compound annual growth rate.
Download a Sample Copy Of Report:
In the realm of healthcare and pharmaceuticals, the efficient management of clinical trials stands as a cornerstone for innovation and progress. With the advent of advanced technologies, the landscape of clinical trial management is undergoing a profound transformation, propelled by the emergence of Clinical Trial Management Systems (CTMS). This article delves into the pivotal role of CTMS in shaping the future of clinical research and the burgeoning market surrounding it.
Competitive Landscape:
The majority of businesses are concentrated on brand development, delivery, portfolio expansion, investments, and acquisitions. Important firms also enjoy a dominant position in the worldwide market and a sizable consumer base, giving them a competitive advantage over rivals.
In addition, developers fully engage in marketing activities to increase their exposure in the industry. Large companies are shifting their attention to smart service platforms that let patients use their products from a distance.
Kyoto University Hospital and Parexel, a preeminent worldwide clinical research organization, formed a strategic partnership in October 2021 with the goal of improving clinical research prospects and developing effective means of supporting clinical investigations.
Revolutionizing Clinical Research
Clinical Trial Management Systems (CTMS) serve as comprehensive software solutions designed to streamline and optimize the entire lifecycle of clinical trials. From study planning and participant recruitment to data collection and analysis, CTMS platforms provide researchers, sponsors, and regulatory authorities with the tools and capabilities necessary to conduct trials efficiently, accurately, and compliantly.
Enhancing Efficiency and Compliance
One of the primary drivers behind the adoption of CTMS is the pressing need for increased efficiency and compliance in clinical research. By centralizing study protocols, documents, and data within a unified platform, CTMS eliminates redundancies, minimizes errors, and accelerates trial timelines. Moreover, CTMS facilitates compliance with regulatory requirements and industry standards, ensuring that trials adhere to ethical guidelines and data integrity principles.
Optimizing Resource Management
CTMS platforms enable researchers to manage critical resources such as personnel, equipment, and funding more effectively. By automating workflows, tracking study progress, and monitoring resource utilization in real-time, CTMS enhances resource allocation and budget management, leading to cost savings and improved operational efficiency. Additionally, CTMS provides stakeholders with actionable insights and analytics, enabling data-driven decision-making and strategic planning.
Driving Market Growth
The Clinical Trial Management Systems market is experiencing rapid growth and expansion, driven by several key factors. The increasing complexity and scale of clinical trials, coupled with the growing demand for personalized medicine and innovative therapies, underscore the need for robust CTMS solutions. Furthermore, advancements in technology, such as artificial intelligence, machine learning, and cloud computing, are fueling innovation within the CTMS market, driving the development of more sophisticated and user-friendly platforms.
Read More: https://www.factmr.com/report/832/clinical-trial-management-systems-market
Future Outlook
Looking ahead, the future of the Clinical Trial Management Systems market appears exceptionally promising. Analysts project sustained growth and continued innovation as the demand for CTMS solutions continues to rise. With the global clinical trials market expected to expand further in response to emerging healthcare challenges and therapeutic advancements, CTMS will play an increasingly vital role in facilitating efficient, compliant, and successful clinical research endeavors.
Key Segments Covered in Clinical Trial Management System Industry Survey:
By Mode of Deployment :
Cloud-based Clinical Trial Management Systems
Web-based
On-premise
By Component :
Hardware
Services
Software
By Product Type :
Enterprise-based
Site-based
By End User :
Pharmaceuticals
Clinical Research Organizations (CROs)
Healthcare Providers
In conclusion, the Clinical Trial Management Systems market stands as a beacon of innovation and progress in the realm of healthcare and pharmaceuticals. By providing researchers, sponsors, and regulatory authorities with advanced tools and capabilities for managing clinical trials, CTMS is revolutionizing the way research is conducted, accelerating the pace of discovery, and ultimately improving patient outcomes. As the healthcare landscape continues to evolve, CTMS will remain at the forefront of clinical research, guiding stakeholders towards a future marked by innovation, efficiency, and excellence.
0 notes
Text
Introduction:
Embark on an exciting career journey with KV Clinical Research Pvt Ltd, a renowned name in the field of clinical research. We are currently seeking passionate individuals to join us as Clinical Research Coordinators (CRC) for oncology trials at our Raipur, C.G. location. If you possess a background in B & M Pharmacy, Pharma D, or hold a diploma in clinical research, this could be the opportunity you've been looking for. Join us in our mission to contribute to groundbreaking research and make a difference in the field of healthcare.
About the Company (KV Clinical Research Pvt Ltd):
KV Clinical Research Pvt Ltd is a leading player in the realm of clinical research, committed to conducting ethical and high-quality trials that pave the way for medical advancements. Founded with a vision to transform healthcare through innovation and excellence, we specialize in oncology trials and strive to bring novel therapies to patients in need. Our team comprises dedicated professionals driven by a shared passion for improving patient outcomes and advancing medical science.
Company Vacancies List:
Explore the available position at KV Clinical Research Pvt Ltd:
Clinical Research Coordinator (CRC)
Job Description:
Role: Clinical Research Coordinator
Industry Type: Clinical Research
Department: Oncology Trials
Employment Type: Full-time
Role Category: Research & Development
Location: Raipur, C.G.
Qualifications and Requirements:
Educational Background: B & M Pharmacy, Pharma D, or Diploma in Clinical Research
Experience: Experience in oncology trials preferred
Proficiency in English, Hindi, and regional languages
Strong organizational and communication skills
Ability to work effectively in a team and manage multiple tasks simultaneously
About the Department & Responsibilities:
As a Clinical Research Coordinator, you will play a pivotal role in the successful execution of oncology trials. Your responsibilities will include:
Coordinating and overseeing all aspects of clinical research studies, ensuring compliance with protocols and regulatory requirements
Recruiting and screening eligible participants for clinical trials
Collecting and maintaining accurate and complete study documentation
Collaborating with investigators, sponsors, and other stakeholders to facilitate study activities
Monitoring participant safety and adherence to study protocols
Contributing to data analysis and reporting of study results
How to Apply:
Ready to take the next step in your career? Send your CV to [email protected] and be part of our mission to drive innovation in clinical research. To learn more about us and apply online, visit www.kvclinicalresearch.com.
0 notes
Text
Role of Clinical Research Services in Advancing Healthcare
In the dynamic landscape of healthcare, clinical research plays a pivotal role in driving innovation, improving patient outcomes, and advancing medical knowledge. Clinical research services encompass a wide range of activities aimed at evaluating the safety, efficacy, and effectiveness of medical interventions, from pharmaceutical drugs to medical devices and beyond. Through rigorous scientific inquiry and collaboration between researchers, clinicians, and industry partners, clinical research services contribute to the development of new therapies, treatments, and healthcare solutions. Let's delve into the world of clinical research services and explore their profound impact on the future of healthcare.
Comprehensive Study Design and Protocol Development
At the heart of clinical research services lies the meticulous design and development of study protocols that guide the conduct of clinical trials and research studies. Experienced research teams work closely with sponsors, investigators, and regulatory agencies to design studies that adhere to ethical principles, regulatory requirements, and scientific standards. This involves defining study objectives, selecting appropriate study populations, determining study endpoints, and establishing methodologies for data collection and analysis.
By employing robust study design and protocol development methodologies, clinical research services ensure that research studies are conducted with scientific rigor and integrity. This helps to minimize bias, confounding variables, and other sources of error, leading to reliable and credible study results that inform clinical practice and regulatory decision-making.
Patient Recruitment and Enrollment
Patient recruitment and enrollment are critical aspects of clinical research services that involve identifying and enrolling eligible participants in research studies. Research teams employ various strategies to recruit participants, including advertising, outreach efforts, and collaboration with healthcare providers and patient advocacy groups. Additionally, electronic health record systems and patient registries may be utilized to identify potential participants who meet study criteria.
Efficient and timely patient recruitment is essential for the successful completion of clinical trials and research studies. By engaging with diverse patient populations and communities, clinical research services strive to ensure adequate representation and participation in research studies, ultimately enhancing the generalizability and applicability of study findings to real-world clinical settings.
Regulatory Compliance and Ethical Oversight
Ensuring regulatory compliance and ethical oversight is paramount in clinical research services to protect the rights, safety, and well-being of research participants. Research teams work closely with regulatory agencies, institutional review boards (IRBs), and ethics committees to obtain approvals and oversee the conduct of research studies in accordance with applicable regulations and guidelines.
This involves submitting study protocols, informed consent forms, and other study-related documents for review and approval, as well as addressing any regulatory or ethical concerns that may arise during the course of the study. By adhering to rigorous regulatory and ethical standards, clinical research services uphold the highest standards of integrity and accountability in the conduct of clinical research.
Data Collection, Monitoring, and Analysis
Data collection, monitoring, and analysis are essential components of clinical research services that involve gathering, recording, and analyzing data generated during the course of research studies. Research teams utilize electronic data capture systems, case report forms, and other data collection tools to collect study data in a standardized and systematic manner.
Furthermore, clinical research services employ rigorous monitoring and quality assurance measures to ensure the accuracy, completeness, and integrity of study data. This may include on-site monitoring visits, source data verification, and data validation checks to identify and address any discrepancies or deviations from the study protocol.
Once data collection is complete, research teams conduct statistical analysis and interpretation of study findings to assess the safety, efficacy, and effectiveness of the investigational intervention. This involves employing advanced statistical methods and techniques to analyze study endpoints, identify trends, and draw meaningful conclusions from the data.
Dissemination of Study Results and Knowledge Translation
The dissemination of study results and knowledge translation are essential components of clinical research services that involve sharing research findings with the broader scientific community, healthcare providers, policymakers, and the public. Research teams publish study results in peer-reviewed journals, present findings at scientific conferences, and communicate results through various channels to facilitate knowledge dissemination and translation into clinical practice.
Furthermore, clinical research services play a vital role in promoting evidence-based medicine and informing healthcare decision-making by providing stakeholders with timely access to accurate and reliable research evidence. This helps to bridge the gap between research and practice, ultimately improving patient care and outcomes.
Conclusion
In conclusion, clinical research services are essential drivers of innovation and progress in healthcare, facilitating the development and evaluation of new therapies, treatments, and healthcare solutions. Through comprehensive study design, patient recruitment, regulatory compliance, data collection and analysis, and dissemination of study results, clinical research services contribute to advancing medical knowledge, improving patient outcomes, and shaping the future of healthcare. By embracing the principles of scientific inquiry, collaboration, and ethical conduct, clinical research services play a vital role in promoting evidence-based medicine and ultimately enhancing the quality of care for patients worldwide.
0 notes
Text
Biopharmaceutical CMO And CRO Market Is Estimated To Witness High Growth Owing To Increasing Demand For Outsourcing

Biopharmaceutical CMOs and CROs provide services like clinical trial management, data management, medical writing, clinical monitoring to biopharmaceutical and biotechnology companies. They help reduce development costs and improve productivity. With rise in complex drug development projects, outsourcing non-core functions have become essential.
The global biopharmaceutical CMO and CRO market is estimated to be valued at US$ 41.71 Mn in 2024 and is expected to exhibit a CAGR of 5.0% over the forecast period 2024 to 2031, as highlighted in a new report published by Coherent Market Insights.
Market Dynamics:
One of the key drivers for the growth of the biopharmaceutical CMO and CRO market is the increasing demand for outsourcing from biopharmaceutical companies. Due to high costs associated with drug development and pricing pressures, biopharma companies are increasingly outsourcing non-core activities like clinical trials to CROs and manufacturing to CMOs. This allows them to focus on drug discovery and commercialization while reducing fixed costs. Additionally, biopharmaceutical CMOs and CROs have capabilities across diverse therapeutic areas and expertise in handling complex projects involving novel biologics, vaccines, and cell and gene therapies. Their specialized expertise and economies of scale help accelerate development timelines and bring down costs for biopharma sponsors.
SWOT Analysis
Strength: The biopharmaceutical CMO and CRO market has experienced strong growth in recent years due to the increasing demand for biologics. CMOs provide services to drug makers from drug development to commercial manufacturing which helps biopharma companies focus on drug discovery. CROs help biopharma companies by outsourcing various research functions like preclinical and clinical trials which reduces operational costs.
Weakness: CMOs and CROs face significant compliance and regulatory challenges to meet stringent manufacturing standards and quality controls set by regulatory agencies. Any issues with quality can damage reputation and business. Outsourcing clinical research also risks loss of control and intellectual property exposure.
Opportunity: The growth of the biologics market and rising demand for personalized medicines is driving significant opportunity for CMOs and CROs. Emerging biotech companies also lack manufacturing capabilities and rely on CMOs. CROs can tap into the market for clinical trials outsourcing. Asia Pacific region offers lower costs that can boost outsourcing.
Threats: Changing regulations and trade policies can disrupt operations and supply chains. Increasing M&A activity among CMOs and CROs leads to consolidation reducing client choice. Intense competition limits pricing power and pressure to diversify service offerings.
Key Takeaways
The global biopharmaceutical CMO and CRO market size is expected to witness high growth driven by the expanding biologics industry. The market size is projected to reach US$ 41.71 Mn in 2024 from US$ 31.13 Mn in 2021, registering a CAGR of 5.0% during the forecast period.
Regional analysis:
North America currently dominates due to presence of major pharmaceutical players and global centers for drug development. However, Asia Pacific is expected to grow at the fastest pace due to rising scientific talent, lower costs and growing pharma industry in China and India. Countries are attracting CMOs and CROs through industry-friendly policies to boost local manufacturing and clinical research.
Key players:
Key players operating in the biopharmaceutical CMO and CRO market are Albemarle Corporation, Greenchemicals S.r.l., ICL, Jordan Bromine Company, Kingboard Holdings Limited, LANXESS, Novel Chem, Shandong Brother Sci. & Tech. Co., Ltd., Shandong Futong Chemical Co., Ltd. and Tianjin Changlu Hangu Saltern Co., Ltd. The market has flourished due to strategic collaborations between biopharma firms and major global players. Market leaders are investing in expansions using advanced technologies to strengthen capabilities across various stages of drug development. Get more insights on this topic: https://www.newsstatix.com/biopharmaceutical-cmo-and-cro-market-industry-insights-trends-biopharmaceutical-cmo-and-cro-market/
#Biopharmaceutical CMO And CRO#Biopharmaceutical CMO And CRO Market#Biopharmaceutical CMO And CRO Market size#Biopharmaceutical CMO And CRO Market share#Biopharmaceutical CMO And CRO Market demand#Biopharmaceutical CMO And CRO Market analysis
0 notes
Text
Exploring the Excellence of Clinical Research Services in India: A Comprehensive Guide

Introduction: Understanding Clinical Research Services and Their Significance
Clinical Research Services play a pivotal role in advancing medical knowledge and improving patient care by conducting rigorous studies to evaluate the safety and efficacy of healthcare interventions. These services encompass a wide range of activities, including protocol development, patient recruitment, data collection, and statistical analysis. In recent years, India has emerged as a key player in the field of Clinical Research services, offering a cost-effective and quality-driven environment for conducting clinical trials. The significance of Clinical Research services in India lies in its ability to contribute valuable data to global research efforts, accelerating the development of new therapies and medical innovations for the benefit of patients worldwide.
The Advantages of Choosing Clinical Research Services in India:
Following are the advantages of choosing Clinical Research Services in India
Cost-effectiveness: Clinical research services in India often offer cost-effective solutions compared to many Western countries. The lower cost of conducting trials in India can be attributed to factors such as lower labor costs, infrastructure expenses, and regulatory fees.
Diverse Patient Population: India has a diverse population, offering a wide range of genetic and demographic profiles. This diversity is advantageous in clinical research, as it allows for a more comprehensive understanding of how different populations may respond to treatments. This diversity can enhance the generalizability of study findings.
Experienced Investigator Pool: India has a growing pool of skilled and experienced clinical investigators and researchers. Many healthcare professionals in India have received international training and certifications, contributing to the quality of clinical trials conducted in the country.
Accelerated Recruitment and Enrollment: The large and diverse population, coupled with a robust healthcare infrastructure, often facilitates quicker patient recruitment and enrollment in clinical trials. This can lead to faster completion of studies and quicker access to valuable research outcomes.
Regulatory Framework: Over the years, India has strengthened its regulatory framework for clinical research. The regulatory bodies, such as the Central Drugs Standard Control Organization (CDSCO), have implemented measures to ensure the safety and ethical conduct of clinical trials. A well-defined regulatory environment provides assurance to sponsors and researchers regarding the integrity of the research process.
The Role of Contract Research Organizations (CROs) in Clinical Research Services
Contract Research Organizations (CROs) play a pivotal role in the landscape of clinical research services by offering specialized support to pharmaceutical, biotechnology, and medical device companies. These organizations serve as strategic partners, assisting in the planning, execution, and management of clinical trials, thereby accelerating the drug development process. CROs provide a range of services, including protocol development, patient recruitment, data management, regulatory compliance, and monitoring of clinical trials. Their expertise and infrastructure enable sponsors to navigate the complexities of the research process more efficiently and cost-effectively, ultimately contributing to the timely and successful completion of clinical studies. As outsourcing to CROs continues to grow, their role in facilitating innovation and advancing healthcare solutions remains integral to the progress of the pharmaceutical and life sciences industries.
Regulatory Framework for Clinical Trials and Ethical Considerations in India
In India, the regulatory framework governing clinical trials is primarily overseen by the Central Drugs Standard Control Organization (CDSCO) under the auspices of the Drugs Controller General of India (DCGI). The regulatory process involves obtaining approval from the Institutional Ethics Committee (IEC) and the DCGI before initiating clinical trials. The Drugs and Cosmetics Act, along with the Schedule Y of the Drugs and Cosmetics Rules, provides the legal framework for conducting clinical trials in the country. Ethical considerations are paramount, and trials must adhere to the principles laid out in the Declaration of Helsinki and the Indian Council of Medical Research (ICMR) guidelines. These guidelines emphasize the importance of obtaining informed consent from participants, ensuring patient confidentiality, and maintaining high standards of safety and efficacy throughout the trial process. The evolving regulatory landscape reflects India's commitment to upholding ethical standards and ensuring the safety of participants in clinical research.
The Future of Clinical Research Services in India: Trends and Innovations to Watch Out For
The future of clinical research services in India is poised for significant advancements, marked by emerging trends and innovative approaches. With a growing emphasis on technological integration, virtual trials, and real-world evidence, the landscape is evolving to enhance efficiency and accelerate drug development. India's robust healthcare infrastructure, coupled with a vast and diverse patient population, positions the country as a key player in the global clinical research arena. Collaborations between industry stakeholders and research organizations, along with a focus on data analytics and precision medicine, are expected to shape the future of clinical research services in India, offering new opportunities for stakeholders to contribute to cutting-edge advancements in medical science.
Conclusion: Leveraging the Benefits of Clinical Research Services in India for Successful Outcomes
In conclusion, harnessing the advantages offered by clinical research services in India is pivotal for achieving successful outcomes in the field of healthcare. The country's robust infrastructure, diverse patient population, and cost-effective yet high-quality research facilities make it an ideal hub for clinical trials. Leveraging these resources not only accelerates the pace of research but also ensures the generation of valuable data for advancing medical knowledge. With a collaborative approach between global pharmaceutical companies and Indian research institutions, the potential for groundbreaking discoveries and improved patient care is substantial, affirming India's position as a key player in the realm of clinical research.
#Clinical Research Services in India#Clinical Trials services in India#Clinical Research Organization in India#Clinical Research Company in India
0 notes
Text
Why is a Clinical Trial Management System Essential for Success?
Clinical trials are fundamental in advancing healthcare and bringing new treatments to patients. Effective management of these trials is crucial for success. Clinical Trial Management streamlines this process, enhancing efficiency and aiding in better decision-making.
Understanding the Clinical Trial Management System (CTMS)
Clinical Trial Management System, often abbreviated as CTMS, is a centralized software solution designed to streamline and manage every aspect of clinical trials. From planning and tracking to reporting and analyzing, a CTMS system is a comprehensive tool used by researchers, sponsors, and stakeholders to ensure the smooth execution of clinical trials.
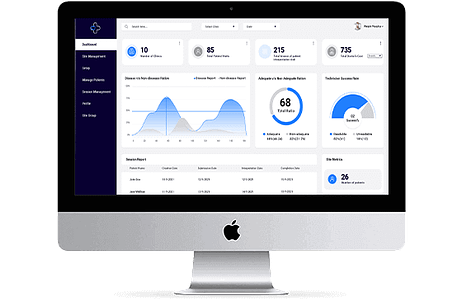
The Key Components of a CTMS System
Trial Planning and Design: Creating a blueprint for the trial, including protocols, endpoints, and participant criteria.
Subject Management: Managing participant information, recruitment, and enrollment into the trials.
Document Management: Organizing and storing trial-related documents and essential paperwork.
Data Collection and Analysis: Collecting, organizing, and analyzing trial data to derive meaningful insights.
Budget and Financial Management: Monitoring and controlling trial-related expenses and budgets.
Advantages of Implementing a Clinical Trial Management System
Efficient Data Management: Streamlining data collection, reducing errors, and enabling real-time access to data for all stakeholders.
Enhanced Compliance and Regulatory Adherence: Assisting in maintaining compliance with regulatory standards and guidelines throughout the trial.
Improved Communication and Collaboration: Facilitating seamless communication among trial stakeholders, promoting collaboration and efficiency.
Cost and Time Efficiency: Optimizing clinical trial software solutions processes, resulting in cost savings and faster development of treatments.
The Evolving Landscape of Clinical Trial Management
Clinical trial management has come a long way from paper-based systems to advanced CTMS solutions. These systems now integrate features like Artificial Intelligence (AI) and Machine Learning (ML) to predict outcomes, optimize resource allocation, and enhance decision-making.
Emerging Trends in Clinical Trial Management
Decentralized Clinical Trials: Leveraging technology for remote monitoring and data collection, minimizing the need for physical site visits.
Patient-Centric Approach: Placing more emphasis on patient experience and involvement in the trial process.
Real-world Evidence Integration: Incorporating real-world data to complement traditional clinical trial data, providing a more comprehensive understanding of treatment outcomes.
How To Choose the Right Clinical Trial Management Software?
When selecting clinical trial software, organizations should consider factors such as ease of use, scalability, integration capabilities, compliance with industry standards, and the specific needs of their trials.
Conclusion:
The Evolving Landscape of Clinical Trial Management
Clinical trial management has come a long way from paper-based systems to advanced CTMS solutions. These systems now integrate features like Artificial Intelligence (AI) and Machine Learning (ML) to predict outcomes, optimize resource allocation, and enhance decision-making.
Emerging Trends in Clinical Trial Management
Decentralized Clinical Trials: Leveraging technology for remote monitoring and data collection, minimizing the need for physical site visits.
Patient-Centric Approach: Placing more emphasis on patient experience and involvement in the trial process.
Real-world Evidence Integration: Incorporating real-world data to complement traditional clinical trial data, providing a more comprehensive understanding of treatment outcomes.
How To Choose the Right Clinical Trial Management Software?
When selecting clinical trial software, organizations should consider factors such as ease of use, scalability, integration capabilities, compliance with industry standards, and the specific needs of their trials.
Conclusion:
Clinical Trial Management Systems are transforming the landscape of healthcare trials. By streamlining processes, enhancing collaboration, and leveraging cutting-edge technologies, clinical trial management software plays a pivotal role in expediting the development and approval of life-changing treatments.
0 notes
Text
Mobile healthcare dominates as most commonly used component in decentralized clinical trials, reveals GlobalData

Mobile healthcare dominates as most commonly used component in decentralized clinical trials, according to GlobalData.
The COVID-19 pandemic catalyzed the adoption of decentralized clinical trials (DCTs) even though they have been in use for decades. DCTs offer the advantage of enabling patients to take part in clinical studies from the convenience of their homes, eliminating the need for them to travel to clinical sites. This not only reduces the burden on patients but also leads to higher participation rates. Against this backdrop, out of the virtual components used in clinical trials, mobile healthcare is the most commonly used component, with 47% of DCTs using this element, reveals GlobalData, a leading data and analytics company.
GlobalData’s latest report “Thematic Intelligence: Digital Transformation and Emerging Technologies in the Healthcare Industry,” reveals that web-based technology is the second most commonly used virtual component, with 23% of DCTs using it for activities such as electronic data collection (eCOA, eConsent, eDiary, ePRO, and questionnaire). Mobile healthcare consists of activities such as remote patient monitoring, remote drug delivery, telemedicine, and home nursing.
“As technologies continue to improve, it has become easier to collect, transfer, and store data electronically. After the COVID-19 pandemic, there has been a drastic increase in patients becoming more comfortable with using gadgets. As people were forced to adapt to social distancing measures and lockdowns, many became more comfortable with using technologies for various purposes, including healthcare.”
Shiva Narayana, Associate Project Manager, Pharma at GlobalData, comments:
Progress in network technologies, connected gadgets, medical wearables, sensors, data analytics algorithms, and software is reshaping the healthcare and clinical trial environments. These breakthroughs are facilitating the more effective gathering of data and the provision of advanced healthcare through a variety of means.
With more patients using technologies such as wearable devices, smartphone apps, or other remote monitoring tools to collect and transmit data such as vital signs, medication adherence, and symptoms, clinicians have an opportunity to access real-world data and gain timely insights.
“By introducing virtual components, the study sponsors or clinical research organizations (CROs) can drive more cost-effective and efficient clinical trials. With fewer geographical constraints and increased patient engagement, decentralized trials can potentially be completed more quickly. However, there are also challenges and considerations in implementing decentralization in clinical trials, such as ensuring data privacy and security, addressing the digital divide, and maintaining the integrity of the study.”
Shiva Narayana concludes
Read the full article
0 notes
Text
Biometrics: Revolutionizing Clinical Development with Clinfinite Solutions

Understanding the Basics of Biometrics
Before delving into the specifics of Clinfinite Development Solutions, let's start with the fundamentals of biometrics.
What Are Biometrics?
Biometrics refers to the measurement and statistical analysis of people's unique physical and behavioral characteristics. These characteristics include fingerprints, facial patterns, voice recognition, and even iris scans. The primary objective of biometrics is to establish a person's identity with an exceptionally high level of accuracy.
The Significance of Biometrics in Clinical Development
Biometrics has found its place in clinical development due to its ability to address various challenges faced by the industry.
Enhanced Security and Data Integrity
In clinical trials, data security and integrity are paramount. Biometric authentication ensures that only authorized personnel can access sensitive information, minimizing the risk of data breaches and fraud.
Streamlined Patient Identification
Biometrics simplifies patient identification, reducing the chances of errors and ensuring that the right patients are enrolled in clinical trials. This efficiency is crucial in maintaining trial integrity and data accuracy.
Clinfinite Development Solutions: A Game Changer in Clinical Development
Now that we have a grasp of the importance of biometrics, let's dive into how Clinfinite Development Solutions leverages this technology to transform the clinical development landscape.
Introduction to Clinfinite
Clinfinite is a leading player in the clinical development sector, known for its innovative approach to streamlining processes. With a strong focus on technology, they have pioneered the integration of biometrics into clinical trials.
Biometrics in Clinical Trials
Patient Enrollment and Identification
Clinfinite's use of biometrics in patient enrollment ensures that the right individuals are part of the trials. This minimizes complications and errors during the study, ultimately saving time and resources.
Data Security
Clinfinite takes data security seriously. Through biometric authentication, they provide a secure environment for data storage and access, reassuring sponsors and participants alike.
Monitoring and Compliance
Monitoring the progress of clinical trials and ensuring compliance with protocols are vital aspects of clinical development. Clinfinite's biometric solutions make these tasks more efficient and accurate.
The Future of Clinical Development: Biometrics as the Norm
As biometrics continue to prove their worth in clinical development, it's clear that they are here to stay.
Advancements on the Horizon
The field of biometrics is dynamic, with ongoing research and development. We can expect even more sophisticated methods and technologies to emerge, further improving the clinical development process.
Conclusion
In conclusion, biometrics are revolutionizing clinical development, and companies like Clinfinite Development Solutions are at the forefront of this transformation. With enhanced security, streamlined processes, and improved data accuracy, the future of clinical trials looks promising.
#biometric service provider near me#project management service provider#best clinical development agency in india#Clinical Development Services in Hyderabad
1 note
·
View note
Text
Top Clinical Site Management Services In Hyderabad
Introduction
In the rapidly evolving landscape of clinical trials, ensuring efficient, accurate, and compliant study conduct is of paramount importance. Enter Clinfinite Solutions, a pioneer in Clinical Site Management Services that have redefined the way clinical trials are conducted. With a steadfast commitment to excellence and a proven track record, Clinfinite Solutions provides a suite of services designed to streamline operations, enhance participant experiences, and ensure the successful execution of clinical trials.

Clinical Site Management Services- Clinfinite Solutions: An Overview
Clinical Site Management Services provided by Clinfinite Solutions encompass a wide range of solutions that empower sponsors and sites alike. From protocol development to patient recruitment, data management, and regulatory compliance, Clinfinite Solutions offers a comprehensive suite of services that optimize every aspect of the clinical trial journey.
The Importance of Effective Clinical Site Management
The success of clinical trials hinges on effective site management. Clinfinite Solutions recognizes that sites are the backbone of trials, and their experienced team ensures that sites operate seamlessly. By implementing robust processes and leveraging cutting-edge technology, Clinfinite Solutions maximizes site potential, minimizing errors, and accelerating trial timelines.
Enhancing Patient Recruitment Strategies
Recruiting the right participants is often a challenge in clinical trials. Clinfinite Solutions employs innovative approaches to patient recruitment, leveraging data-driven strategies and digital outreach to connect with potential participants. This targeted approach not only expedites recruitment but also ensures diverse and representative participant populations.
Data Management and Quality Assurance
Accurate and reliable data are non-negotiable in clinical trials. Clinfinite Solutions employs state-of-the-art data management systems to collect, process, and analyze trial data. Their quality assurance protocols identify discrepancies early on, ensuring that data integrity is maintained throughout the trial duration.
Regulatory Compliance Made Easy
Navigating the complex landscape of regulatory compliance is simplified with Clinfinite Solutions. Their experts stay abreast of changing regulations, ensuring that trials are conducted ethically and in full compliance with applicable guidelines. This meticulous approach minimizes the risk of setbacks and ensures the validity of trial outcomes.
Streamlined Monitoring and Reporting
Monitoring trial progress is crucial for making informed decisions. Clinfinite Solutions leverages advanced monitoring tools to track site performance and patient enrollment. This real-time monitoring allows for proactive interventions, minimizing bottlenecks and optimizing trial timelines.
Collaboration and Communication
Effective communication and collaboration between all stakeholders are key to successful trial execution. Clinfinite Solutions facilitates seamless communication channels, ensuring that sponsors, sites, investigators, and participants are all on the same page. This synergy fosters a conducive environment for the exchange of ideas and insights.
The Innovative Edge: Technology Integration
Clinfinite Solutions stands out for its integration of innovative technologies. From e-consent solutions to telemedicine capabilities, technology is harnessed to enhance participant engagement, facilitate remote monitoring, and ensure the smooth conduct of trials even in challenging circumstances.
Expertise and Industry Know-How
With years of experience in the clinical research field, Clinfinite Solutions brings unparalleled expertise to the table. Their team comprises seasoned professionals who understand the nuances of clinical trials and are equipped to navigate complexities effectively.
Our Services:-
Clinical Development Solutions
Biometrics Solutions
Project And Site Solutions
To Know More Visit Here:- Clinfinite Solutions
0 notes
Text
Ecoa Esource and Clinical Trials Market Size and Growth Analysis with Trends, Key players & Outlook to 2030
The latest market report published by Credence Research, Inc. “Global Ecoa Esource and Clinical Trials Market: Growth, Future Prospects, and Competitive Analysis, 2016 – 2028. The global ECoa eSource and clinical trials market has grown steadily in recent years and is predicted to increase at an 8.30% CAGR between 2023 and 2030. In 2022, the market was valued at USD 45.7 billion, and it is predicted to grow to USD 79.8 billion by 2030.
ECoa eSource and clinical trials market has emerged as a crucial segment, driving advancements in medical research and patient-centric approaches. This article provides an in-depth analysis of the ECoa eSource and clinical trials market, its driving forces, major challenges, and potential opportunities for growth. We delve into the key factors that are propelling the market forward, such as the growing adoption of mobile health technology and eClinical technologies, as well as the hurdles posed by regulatory difficulties and data security issues. Moreover, we explore the potential opportunities for personalized medicine expansion and patient monitoring via the internet.
Ecoa Esource and Clinical Trials Market Dynamics refer to the intricate interplay between various factors that shape the landscape of electronic clinical outcome assessment (eCOA) solutions within the realm of clinical trials. As technology continues to advance, eCOA has emerged as a critical tool for collecting patient-reported outcomes and enabling remote data capture in research studies. The market dynamics surrounding Ecoa Esource and Clinical Trials are heavily influenced by several key elements. Firstly, regulatory bodies play a crucial role in shaping this industry, setting guidelines and standards to ensure data integrity and patient privacy. Additionally, advancements in mobile devices, wearables, and telehealth technologies have revolutionized the way researchers collect data from study participants remotely. This has not only increased convenience but also reduced site visits for patients while ensuring real-time access to accurate information for sponsors.
Driving Forces in the Global ECoa eSource and Clinical Trials Market
1. Growing Adoption of Mobile Health Technology
Mobile health technology, encompassing smartphones, wearables, and other connected devices, is rapidly gaining traction in clinical trials. The advantages of real-time data capturing, increased patient interaction, and reduced administrative burden offered by these technologies are driving their adoption. Additionally, the ability to remotely collect data from patients contributes to improved diversity in clinical trial participants, a critical factor in generating robust and representative results.
2. Increased Use of eClinical Technologies in Clinical Trials
While the clinical trials industry had been cautious in embracing digital technologies, there has been a significant shift towards eClinical technologies. Adoption of eCOA eSource technologies for direct data collection from patients has reduced human data entry and enhanced data accuracy. Furthermore, the integration of eClinical technologies can lead to cost and time savings, which are crucial as the demand for innovative medical treatments grows.
3. Potential Opportunities for Personalized Medicine Expansion
Personalized medicine, which tailors treatments to patients based on their genetic information, is gaining prominence. The adoption of eCOA eSource systems and eClinical technologies streamlines the collection and analysis of vast volumes of data necessary for personalized medicine. Consequently, we can expect increased investment in eCOA eSource and other eClinical technologies to support this burgeoning field.
4. Patient Monitoring via the Internet
Remote patient monitoring enables patients to be observed outside clinical settings, improving outcomes and reducing healthcare costs. eCOA eSource systems and other eClinical technologies can facilitate remote patient monitoring by enabling patients to report symptoms and provide data from home. This trend is likely to drive investment in eCOA eSource and other eClinical technologies, presenting growth opportunities in the market.
Browse 220 pages report Ecoa Esource and Clinical Trials Market By Product Type (Educational and research institutes, Pharmaceutical and biotechnology companies, Contract research organizations, Hospitals) By Product Types (ECoa, eSource, Clinical Trails Solutions)- Growth, Future Prospects & Competitive Analysis, 2016 – 2030)- https://www.credenceresearch.com/report/ecoa-esource-and-clinical-trials-market
Outranking the Competition
As a prominent player in the ECoa eSource and clinical trials market, our commitment to innovation and patient-centric approaches has driven our success. We have leveraged the potential of mobile health technology and eClinical technologies to enhance data collection, accuracy, and patient interaction. Our robust data security measures and compliance with regulatory standards ensure the protection of patient data.
Future Outlook
The future of the ECoa eSource and clinical trials market appears promising, with increasing interest in eClinical technologies, growing acceptance of personalized medicine, and rising demand for remote patient monitoring. Investments in healthcare IT and cloud-based eClinical solutions are expected to further bolster market growth. The continuous focus on patient-centric clinical trials will drive the development of innovative solutions tailored to patients' needs.
Why to Buy This Report-
The report provides a qualitative as well as quantitative analysis of the global Ecoa Esource and Clinical Trials Market by segments, current trends, drivers, restraints, opportunities, challenges, and market dynamics with the historical period from 2016-2020, the base year- 2021, and the projection period 2022-2028.
The report includes information on the competitive landscape, such as how the market's top competitors operate at the global, regional, and country levels.
Major nations in each region with their import/export statistics
The global Ecoa Esource and Clinical Trials Market report also includes the analysis of the market at a global, regional, and country-level along with key market trends, major players analysis, market growth strategies, and key application areas.
Browse Full Report: https://www.credenceresearch.com/report/ecoa-esource-and-clinical-trials-market
Visit: https://www.credenceresearch.com/
Related Report: https://www.credenceresearch.com/report/blood-pressure-monitoring-devices-and-accessories-market
Related Report: https://www.credenceresearch.com/report/host-cell-contaminant-testing-market
Browse Our Blog: https://www.linkedin.com/pulse/ecoa-esource-clinical-trials-market-analysis-size-revenue-singh
Browse Our Blog: https://tealfeed.com/ecoa-esource-clinical-trials-market-size-br2pw
About Us -
Credence Research is a viable intelligence and market research platform that provides quantitative B2B research to more than 10,000 clients worldwide and is built on the Give principle. The company is a market research and consulting firm serving governments, non-legislative associations, non-profit organizations, and various organizations worldwide. We help our clients improve their execution in a lasting way and understand their most imperative objectives. For nearly a century, we’ve built a company well-prepared for this task.
Contact Us:
Office No 3 Second Floor, Abhilasha Bhawan, Pinto Park, Gwalior [M.P] 474005 India
0 notes
Text
Clinical Trials Market Insights, New Innovations, Research and Growth Factor till 2033
By the end of 2023, the global Clinical Trials market is anticipated to be worth US$ 115.4 billion, and it will grow at a CAGR of 4.41% to reach US$ 177.7 billion by the year 2033. Industry sponsors are in the lead with a predicted market share of about 54.7% in 2023, according to a recent study by Future Market Insights.
A new study of Future Market Insights (FMI) estimates the global spending on clinical trials in 2019 at ~US$ 90 Bn, recording a ~4% Y-o-Y over 2018. The healthcare sector is closing in on a new era of clinical trials, which are more engaged with patients, streamlined and connected. With development costs reaching record highs and patents approaching cliffs, companies are strengthening their R&D efforts to keep pace with change. Medical device and pharma giants are already eyeing better approaches for clinical trials, implementing a combination of their current systems and better technologies to ebb challenges in critical areas – orphan drugs and rare diseases.
To remain ‘ahead’ of your competitors, request for a sample – https://www.futuremarketinsights.com/reports/sample/rep-gb-384
“The ubiquity digital technologies is evidently growing, as these (including but not limited to ingestible devices, sensors, and wearable health monitors) hold the potential to disrupt the aspects of clinical trials. Digital technologies are revolutionizing the antiquated process of new drug development, even as optimizing the way health data is collected, measured, and assessed”.
According to the study, the clinical trials landscape is witnessing a paradigm shift toward patient centricity, in a bid to incorporate perspective of patients during the clinical trial design. Clinical trial sponsors have aligned their methods with the voice of patients, by launching communication channels during the study’s execution. Key enterprises are adopting unconventional approaches by implementing site-centric method, using operational support systems and technology for bringing studies to the trial-naïve physicians.
Get a Customized Scope to Match Your Need Ask an Expert- https://www.futuremarketinsights.com/ask-question/rep-gb-384
Industry Sponsors Account for Majority Clinical Trial Spending
The most remarkable advances in medicine are realized from sponsorships of companies for clinical trials, in the anticipation of turning a profit. A notable percentage of clinical trials being conducted to support approval of vaccines, devices, or drugs, and track their safety, have been industry-sponsored in recent years.
The study estimates industry-sponsored clinical trials to account for ~60% of overall spending in 2019, and in the foreseeable future. Recent studies state that industry-sponsored trials yield positive results compared to academic-run trials, which also account for a significant share of clinical trial spending.
0 notes
Text
In-the-cloud Lims A Tco Analysis
The dashboard lets you manage knowledge, create new tasks, and share lab protocols along with your peers by way of real-time updates. Because the Genemod dashboard is user-friendly and customizable, it eliminates the necessity in your group to rummage via outdated software purposes for individual samples. GetApp offers free software discovery and choice laboratory information management systems sources for professionals such as you. A Laboratory Information Management System (LIMS) that may streamline all of the processes in a clinical research lab can improve the lab’s credibility within the trade. A customized configured LIMS can scale back the incidence of human error and improve the quality of your lab’s data.
Emory's Office of Information Technology (OIT) Laboratory Solutions group helps the Emory Research Natuilus Laboratory Information Management System (LIMS) software. Never miss out on the newest news and updates from Cerebrum and the AP trade by signing up for our communication record. Download a detailed list of LABdivus capabilities for every workflow step under. Nevertheless, the COVID-19 pandemic pressured stakeholders from developed markets, corresponding to the U.S. and Europe, to reduce back their heavy dependence on Asian international locations and shift again to in-house operations.
Faced with rising knowledge volumes and pattern throughput along with frequent changes in expertise, labs should modernize their method to managing, tracking, and centralizing genomics information. ClinLab, a Rennova Health company, offers ClinLab LIS, a laboratory information system supporting physician's workplace labs, personal lab inventory management system reference labs, and student health centers. At the center of it, a LIMS supplies a system to track, standardize, manage, and centralize all the info, processes, and tasks that happen in a lab. Clients who combine their LIMS with MasterControl solutions are solely hosted in our cloud network.
You can filter results by consumer evaluations, pricing, options, platform, area, assist options, integrations, and more. Qualis LIMS has been designed to digitally rework Laboratories to carry out their duties in an automatic and paper-less method, and more importantly allow them to fulfil regulatory compliance requirements and cling to industry requirements. Utilize Clinical Lab Software to trace instrument calibration, monitor high quality control information lab inventory management software, and generate reports for efficient error detection. A LIMS might must satisfy good manufacturing follow (GMP) and meet the reporting and audit wants of the regulatory our bodies and research scientists in many alternative industries. A LIS, nevertheless, should satisfy the reporting and auditing wants of health service agencies e.g. the hospital accreditation company, HIPAA in the US, or different clinical medical practitioners.
The carried out LIMS has many distinctive features offering many user-friendly features. First, the implemented system classifies COVID-19 non-clinical trial subjects into six sub-themes (investigational product, kinds of investigational product, sponsor, experimental animals, research objective, and examine site). It also applies entry control and visualization by classifying the research amassed within the system.
Electronic Trial Master File (eTMF) is designed for the maintenance and storage of the essential paperwork for clinical trials as maintained by the sponsor. PennVault eTMF is designed to perform for any kind of trial, be it single website or multiple sites over a number of international locations. The eTMF may be used for the storage of documents as nicely as the writer, evaluate, transmission of paperwork for e-signature and approval. Automation of such actions as contract management, bill approvals, and billing can reduce administrative workloads and contribute to controlling costs.
New laboratory systems, using the most cutting-edge expertise, are being developed to fulfill the present demand. New strategies for organizing laboratory information are required to keep up with the demands of the healthcare system. An elegant way to overcome the pitfalls of dispersed knowledge clinical research lims software management would be a software package that does not need sophisticated interface solutions and thus grants straightforward information accessibility throughout departments, even including the management and provide chain.
0 notes
Text
"Digitizing Clinical Trials: Exploring the Electronic Trial Master File (eTMF)"
Introduction: Clinical trials are complex endeavors that require meticulous management of various documents and records. Traditionally, Trial Master Files (TMFs) have been maintained in paper format, leading to challenges in organization, accessibility, and compliance. The advent of Electronic Trial Master Files (eTMFs) has revolutionized clinical trial documentation management, offering a digital solution that streamlines processes, enhances collaboration, and ensures regulatory compliance. In this blog post, we explore the concept of eTMF, its benefits, and its impact on the clinical research industry.
Understanding eTMF: An Electronic Trial Master File (eTMF) is a digital system that centralizes and manages the documentation and records related to a clinical trial. It serves as a comprehensive repository for all essential trial documents, including protocols, informed consent forms, regulatory submissions, investigator brochures, and monitoring reports. eTMFs are designed to provide efficient storage, organization, and retrieval of documents, while maintaining data integrity and ensuring compliance with regulatory requirements.
Benefits of eTMF:
Enhanced Document Organization and Accessibility: eTMFs offer a structured and organized approach to document management. Documents can be classified, indexed, and stored in a standardized manner, making it easier to locate and retrieve specific files. With electronic search functionalities, users can quickly find relevant documents based on keywords, dates, or specific criteria, improving efficiency and reducing the time spent searching for information.
Real-time Collaboration and Communication: eTMFs facilitate real-time collaboration and communication among trial stakeholders, including sponsors, investigators, and regulatory authorities. Multiple users can access and review documents simultaneously, allowing for efficient collaboration and streamlined communication. This promotes faster decision-making, ensures timely updates, and reduces the delays associated with physical document exchange.
Improved Regulatory Compliance: Compliance with regulatory guidelines is of utmost importance in clinical trials. eTMFs provide mechanisms for enforcing compliance by maintaining an audit trail of document activities, capturing electronic signatures, and implementing version control. These features help ensure data integrity, traceability, and accountability, which are crucial for regulatory inspections and audits.
Cost and Time Savings: Adopting eTMFs can result in significant cost and time savings. The elimination of physical document storage and manual document handling reduces administrative overheads, such as printing, shipping, and storage costs. eTMFs also facilitate faster document review and approval processes, reducing the overall time required for trial execution and regulatory submissions.
Impact on the Clinical Research Industry: The introduction of eTMFs has had a profound impact on the clinical research industry. It has led to increased efficiency, improved data quality, and enhanced collaboration among trial stakeholders. Regulatory authorities are increasingly accepting eTMFs as a standard practice, further promoting their adoption. As the industry continues to embrace digitization, eTMFs are becoming an integral part of clinical trial management systems, paving the way for a more streamlined, efficient, and compliant approach to documentation management.
Conclusion: Electronic Trial Master Files (eTMFs) have transformed the way clinical trial documentation is managed, offering a digital solution that enhances organization, accessibility, and compliance. With improved document organization, real-time collaboration, and enhanced regulatory compliance, eTMFs provide significant benefits to the clinical research industry. As the industry evolves and embraces digital transformation, eTMFs will continue to play a pivotal role in optimizing document management processes and ensuring the success of clinical trials.
Read more @ https://techinforite.blogspot.com/2023/05/the-future-of-trial-documentation.html
0 notes