#nih clinical trials training
Note
Can you please explain what patriarchal science is?
Got an interesting ask, I suspect from that squirting/gushing post.
Both medicine and medical research have well-documented structural biases against people with vaginas. (Note: from hereon out I'm going to use "men" and "women" in the very cis way that medical research does, where they don't make a distinction between gender and biological sex. I chose to do that to more accurately reflect the findings of the research, which are incredibly biased toward cis people, but that's another post entirely.)
Medicine has a dark history of being used as a tool for structural sexism. If you want a horrific historical example, read about Female Hysteria for starters. But while these biases are now rarely as overtly abusive, they still exist in the current day. The biases in patient care tend to be far more talked about (I suppose understandably), but part of the reason they exist is because of the deeper structural biases in medical research.
But let's start with patient care. For example, doctors simply believe women less than men. In patient care, one in five women report having had a doctor dismiss or ignore their concerns. A particularly insidious bias against women is called the pain bias: despite women experiencing more chronic pain overall than men, and women's pain is dismissed and undertreated in medicine. Women are less likely to be prescribed painkillers after surgeries, and they're more likely to have their pain complaints ignored or dismissed as being overemotional.
All of this echoes the sentiment that led to so-called "Female Hysteria"--as far as many doctors are concerned, they know womens' bodies better than the women do and all women are too emotional and unreasonable to trust their accounts of their own symptoms.
But all of this is also directly tied to the fact that doctors are not trained how to treat women because medical research strongly privileges men. In the pre-modern era, a tradition going back to the Ancient Greeks said that medicine should only be tested on men, who were obviously stronger and more fit to endure unintended side effects than women.
So, of course, that led to a couple millennia of medical science that had not really considered whether women might experience medicine differently than men. It wasn't even until the 70's that medical research standards changed to to encourage including women at all. The NIH and the FDA didn't force medical researchers to include women in clinical trials until 1993.
But even today, medical research often has far more men than women in their clinical trial groups, fails to record gendered differences or even represent gendered cohorts in their data, and rarely if at all performs studies specifically on groups of women.
I found this paper, which was a survey of recent medical research to examine specifically for gendered medical bias, to be particularly staggering. Just read through the tables and you'll get a strong picture of what I mean.
Part of the reason we get biases in patient care is really just garden variety misogyny in a labcoat. But another part is because even well-meaning professionals don't realize that the information they're using to treat women is entirely incomplete. That's part of what I mean when I say it's a structural bias.
You can read about one particular dearth of knowledge we have in my post about squirting/gushing, but this lack of knowledge also shows up in research about far more life-threatening kinds of medical problems. This bias leads directly to the suffering and death of women every. single. day.
That's what I mean by "patriarchal medicine."
10 notes
·
View notes
Text
Intervention decreases the chances of experiencing postpartum anxiety and depression by over 70%.

Results from a substantial clinical trial supported by the National Institutes of Health reveal that an anxiety intervention offered to pregnant women in Pakistan significantly diminished the likelihood of these women developing moderate-to-severe anxiety, depression, or a combination of both six weeks after giving birth. The distinctive intervention was administered by non-specialized providers with a bachelor’s degree in psychology but lacking clinical experience. The outcomes suggest that this intervention could prove effective in preventing postpartum mental health challenges among women in low-resource settings.
“In regions with limited resources, women may face difficulties in accessing mental health care due to a global shortage of trained specialists,” stated Joshua A. Gordon, M.D., Ph.D., Director of the National Institute of Mental Health, part of NIH. “This study indicates that non-specialists could help bridge this gap, offering care to more women during this crucial period.”
Conducted in the Punjab Province of Pakistan between April 2019 and January 2022, the study, led by Pamela J. Surkan, Ph.D., Sc.D., of Johns Hopkins Bloomberg School of Public Health, Baltimore, randomly assigned pregnant women with at least mild anxiety symptoms to receive routine pregnancy care or a cognitive behavioral therapy (CBT)-based intervention called Happy Mother-Healthy Baby. The assessment, carried out six weeks postpartum, involved 380 women in the CBT group and 375 women in the routine care group.
The findings revealed that 9% of women in the intervention group developed moderate-to-severe anxiety, compared to 27% in the routine care group. Furthermore, 12% of women in the intervention group developed depression, in contrast to 41% in the routine care group.
“Postpartum depression not only affects mothers; it is also linked to poorer physical growth and delayed cognitive development in their children,” emphasized Dr. Surkan. “The connection between maternal and child health underscores the critical importance of developing effective strategies to address postpartum anxiety and depression.”
The Happy Mother-Healthy Baby intervention, developed with input from pregnant women in Rawalpindi, Pakistan, involved six sessions where participants learned to identify anxious thoughts and behaviors, addressing issues like thoughts about potential miscarriage. The sessions occurred in early to mid-pregnancy, with the final one taking place in the third trimester.
Given that up to 30% of women in the Global South report experiencing anxiety during pregnancy, the prenatal period becomes a crucial target for intervention, considering its predictive role in postpartum anxiety and depression. In low-resource settings, accessing clinical care can be challenging, making interventions like Happy Mother-Healthy Baby valuable in preventing postpartum mental health issues.
“In the future, we can expand on these findings through implementation research. Having identified an effective intervention, the next step is to determine the optimal ways to deliver treatment to those in need, bridging the gap between scientific knowledge and practical application,” concluded Dr. Surkan.
Remember, if you need further guidance or support, don’t hesitate to reach out to your mental health professional or contact us for assistance.
#health#medicine#mental health#back pain#pain management#apdss#chiropractic#neckpain#neurostar#depressionhelp
0 notes
Text
UCLA Medical Center Address, Doctors, Appointment, Departments, Emergency Department
New Post has been published on https://www.informationhospital.com/ucla-medical-center-address-doctors-appointment-departments-emergency-department/
UCLA Medical Center Address, Doctors, Appointment, Departments, Emergency Department

UCLA Medical Center Address
UCLA Medical Center, situated in the heart of Los Angeles, is a prestigious and globally recognized healthcare institution that has been at the forefront of medical excellence for many decades. With its commitment to providing exceptional patient care, advancing medical research, and training future generations of healthcare professionals, UCLA Medical Center stands as a beacon of hope and healing for individuals not only in Southern California but from around the world.
Founded in 1955, UCLA Medical Center is part of the University of California, Los Angeles (UCLA) Health System and serves as one of its flagship hospitals. It operates on the fundamental principle that patient care, research, and education are interdependent components of medical progress. This philosophy has guided the medical center throughout its history, allowing it to achieve numerous milestones in healthcare.
One of the defining features of UCLA Medical Center is its commitment to cutting-edge medical research and clinical trials. The institution consistently ranks among the top recipients of National Institutes of Health (NIH) research funding, underscoring its dedication to advancing medical knowledge and finding new treatments for various diseases and conditions. Patients benefit directly from this commitment, as they have access to the latest diagnostic tools, therapies, and medications.
In addition to its research endeavors, UCLA Medical Center excels in patient care across a wide range of specialties, from cardiology and neurology to oncology and orthopedics. Its multidisciplinary teams of healthcare professionals work collaboratively to provide personalized treatment plans, ensuring that each patient receives the highest level of care.
UCLA Medical Center’s educational mission is another critical aspect of its identity. As a teaching hospital affiliated with the David Geffen School of Medicine at UCLA, it plays a pivotal role in training the next generation of physicians, nurses, and healthcare providers. The medical center offers a rich learning environment where students and residents gain practical experience alongside experienced mentors.
Beyond its academic and clinical achievements, UCLA Medical Center is deeply embedded in the Los Angeles community. It actively participates in various community outreach programs and health initiatives, aiming to improve the well-being of residents throughout the region.
In conclusion, UCLA Medical Center is not just a hospital; it is a beacon of hope, a center of excellence, and a driving force for medical progress. Its unwavering commitment to patient care, research, and education has positioned it as a leader in the healthcare field, making it a source of pride for Los Angeles and a symbol of healing and innovation worldwide.
youtube
UCLA Medical Center Doctors
UCLA Medical Center, located in the heart of Los Angeles, is a world-class healthcare institution renowned for its commitment to excellence in patient care, research, and education. With a rich history spanning several decades, the medical center has consistently pushed the boundaries of medical innovation, making significant contributions to the field of medicine.
One of the hallmarks of UCLA Medical Center is its exceptional team of doctors who are leaders in their respective specialties. These healthcare professionals are at the forefront of medical advancements, driving groundbreaking research, and delivering top-notch patient care. Whether it’s cardiology, neurosurgery, oncology, or any other medical specialty, patients at UCLA Medical Center can rest assured that they are in the hands of some of the finest physicians in the world.
UCLA Medical Center’s commitment to research and innovation has led to numerous medical breakthroughs and advancements in patient care. The medical center actively engages in cutting-edge clinical trials and research projects, aiming to develop new treatments and therapies that can improve the lives of patients not only in Los Angeles but around the globe.
In addition to its clinical and research excellence, UCLA Medical Center is also dedicated to medical education. It serves as a teaching hospital for the David Geffen School of Medicine at UCLA, where future generations of healthcare professionals are trained. This commitment to education ensures that the medical center continues to foster a culture of learning and growth.
Furthermore, UCLA Medical Center is deeply rooted in its community. It provides vital healthcare services to a diverse population and actively participates in outreach programs to address health disparities and improve the well-being of underserved communities.
In summary, UCLA Medical Center stands as a beacon of excellence in the field of healthcare. Its world-class doctors, commitment to research, dedication to education, and service to the community make it a vital institution not only in Los Angeles but on the global stage.
UCLA Medical Center Appointment
Scheduling an appointment at the UCLA Medical Center is a crucial step in accessing world-class healthcare services in Los Angeles, California. As one of the premier academic medical centers in the United States, UCLA Medical Center is renowned for its commitment to patient care, groundbreaking research, and medical education. With a rich history dating back to its founding in 1955, the medical center has consistently been at the forefront of medical advancements and innovations.
UCLA Medical Center is part of the larger University of California, Los Angeles (UCLA) Health system, which includes several hospitals, clinics, and outpatient centers throughout Southern California. This extensive network ensures that patients have access to a wide range of medical specialists and services.
To schedule an appointment at UCLA Medical Center, patients have several convenient options. They can call the dedicated appointment line, use the online patient portal, or reach out to specific departments or clinics directly. This flexibility is designed to accommodate the diverse needs of patients, whether they are seeking routine check-ups, specialized treatments, surgeries, or second opinions.
One of the defining features of UCLA Medical Center is its commitment to patient-centered care. The medical center prioritizes the well-being and comfort of patients, providing a supportive and compassionate environment. The highly skilled and diverse team of healthcare professionals at UCLA Medical Center is dedicated to delivering personalized care that meets the unique needs of each patient.
In addition to its exceptional patient care services, UCLA Medical Center is actively engaged in groundbreaking medical research. It collaborates with leading scientists, researchers, and institutions worldwide to advance medical knowledge and develop innovative treatments and therapies.
Furthermore, UCLA Medical Center plays a pivotal role in medical education, training the next generation of healthcare providers. Its affiliation with the David Geffen School of Medicine at UCLA ensures that medical students receive hands-on training and exposure to cutting-edge medical practices.
In conclusion, scheduling an appointment at UCLA Medical Center is the first step toward accessing top-tier healthcare services in a patient-centered, research-driven, and educational environment. Whether you’re seeking routine care or complex medical interventions, UCLA Medical Center is dedicated to providing exceptional healthcare to the Los Angeles community and beyond.
UCLA Medical Center Departments
UCLA Medical Center, located in Los Angeles, California, is renowned for its world-class healthcare services and a comprehensive range of specialized departments. This premier academic medical center is affiliated with the University of California, Los Angeles (UCLA), and has earned a stellar reputation for excellence in patient care, medical research, and education.
One of the key strengths of UCLA Medical Center is its extensive network of specialized departments, each staffed with highly skilled and experienced healthcare professionals. These departments cover virtually every medical specialty, making UCLA Medical Center a one-stop destination for patients seeking top-notch healthcare services.
The medical center’s departments are equipped to handle a wide spectrum of healthcare needs, from routine check-ups and primary care to cutting-edge treatments and complex surgeries. Patients have access to specialists in areas such as cardiology, oncology, neurology, orthopedics, pediatrics, and more. This multidisciplinary approach ensures that patients receive comprehensive and personalized care tailored to their unique medical conditions.
UCLA Medical Center is not only committed to delivering exceptional patient care but also to advancing medical knowledge through research and innovation. Many of its departments are actively involved in groundbreaking research projects, clinical trials, and the development of new medical technologies. This dedication to research helps the medical center stay at the forefront of medical advancements, ultimately benefiting patients through access to the latest treatments and therapies.
Furthermore, UCLA Medical Center plays a vital role in medical education, training the next generation of healthcare professionals. Its affiliation with UCLA provides opportunities for medical students, residents, and fellows to learn from some of the best minds in medicine.
In conclusion, UCLA Medical Center’s specialized departments, commitment to research, and dedication to medical education make it a leader in the healthcare industry. Patients from all over the world seek its expertise and world-class care, making it a symbol of excellence in healthcare.
UCLA Medical Center Emergency Department
The UCLA Medical Center Emergency Department, situated within the prestigious UCLA Medical Center in Los Angeles, is a vital component of this world-renowned healthcare institution. This emergency department is dedicated to providing immediate medical care and attention to patients facing urgent medical situations.
Staffed with highly skilled healthcare professionals, including emergency physicians, nurses, and support staff, the UCLA Medical Center Emergency Department operates around the clock, 365 days a year. It is equipped to handle a wide range of medical emergencies, from critical injuries and life-threatening illnesses to minor health concerns that require immediate attention.
Patients who arrive at the UCLA Medical Center Emergency Department can expect swift and efficient care. The department is equipped with state-of-the-art medical equipment and technology, ensuring that patients receive the most advanced diagnostic and treatment options available.
Moreover, the UCLA Medical Center Emergency Department is closely integrated with the broader medical center, facilitating seamless transitions for patients who require hospitalization or specialized care beyond the emergency phase. This collaboration ensures that patients receive continuous, high-quality healthcare throughout their medical journey.
In addition to its primary role in emergency medical care, this department also serves as a hub for medical education and research. It provides valuable training opportunities for medical students, residents, and fellows, contributing to the development of future healthcare leaders.
In summary, the UCLA Medical Center Emergency Department is a critical component of this prestigious institution, offering immediate and expert care to those in urgent need. Its commitment to excellence, cutting-edge technology, and integration with the broader healthcare system make it an essential resource for the community and a symbol of healthcare innovation.
https://www.informationhospital.com/ucla-medical-center-address-doctors-appointment-departments-emergency-department/
#UCLA Medical Center Address#UCLA Medical Center Appointment#UCLA Medical Center Departments#UCLA Medical Center Doctors
0 notes
Text
Medical Datasets for Machine Learning: Aims, Types and Common Use Cases
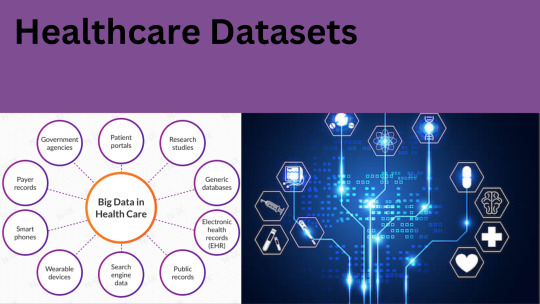
Introduction:
Healthcare datasets are collections of data from various sources within the healthcare industry, such as electronic health records, clinical trials, medical imaging, and insurance claims. These datasets provide a wealth of information that can be analyzed and used to improve patient outcomes, reduce costs, and optimize healthcare operations. Machine learning (ML) algorithms can be applied to healthcare datasets to uncover patterns and insights that are not immediately apparent. ML can help identify high-risk patients, predict disease progression, and recommend personalized treatments. It can also be used to improve clinical workflows, optimize resource allocation, and reduce errors.
What are the medical datasets for machine learning:
There are several medical datasets available for machine learning (ML) applications. Some examples include:
MIMIC-III (Medical Information Mart for Intensive Care III) A freely available dataset of de-identified health data from over 60,000 ICU patients. It includes patient demographics, vital signs, laboratory test results, and more.
NIH Chest X-ray dataset A dataset of over 100,000 chest x-ray images with corresponding labels for pathologies such as pneumonia, lung nodules, and pleural effusion.
Brain tumor datasetA dataset of MRI scans of brain tumors, including glioma, meningioma, and pituitary tumors.
Diabetic Retinopathy datasetA dataset of retinal images from diabetic patients with varying stages of diabetic retinopathy.
Breast Cancer Wisconsin (Diagnostic) datasetA dataset of digitized images of breast tissue, along with corresponding diagnoses of whether the tissue is malignant or benign.
Cardiovascular disease dataset A dataset of patient data, including age, sex, blood pressure, and other health indicators, along with a binary label for whether the patient has cardiovascular disease.
These datasets can be used for tasks such as image classification, natural language processing, and predictive modeling. However, it's important to note that medical datasets often contain sensitive information, so researchers must take care to de-identify data to protect patient privacy.
Top medical datasets:
Top medical datasets 😷
General Datasets: HealthData.gov. Big Cities Health inventory Data Platform. ...
Image Datasets: Open Access Series of Imaging Studies (OASIS) ...
Covid Datasets: COVID-19 Open Research Dataset. ...
Genome Datasets: GEO Datasets. ...
Hospital Datasets: Medicare Hospital Quality. ...
Cancer Datasets:
What are the use cases for machine learning in healthcare?
The most common healthcare use cases for machine learning are automating medical billing, clinical decision support and the development of clinical practice guidelines within health systems. There are many notable high-level examples of machine learning and healthcare concepts being applied in science and medicine.
What kind of dataset is good for machine learning?
Machine Learning Datasets:
IRIS Dataset
The iris dataset is a simple and beginner-friendly dataset that contains information about the flower petal and sepal width. The data is divided into three classes, with 50 rows in each class. It's generally used for classification and regression modeling.
CONCLUSION:
In conclusion, healthcare datasets are an invaluable resource for Machine Learning (ML) applications in the healthcare domain. ML algorithms rely on large amounts of
data to train and develop predictive models, and healthcare datasets provide this necessary data.
How GTS can help you?
Global Technology Solutions is a AI based Data Collection and Data Annotation Company understands the need of having high-quality, precise datasets to train, test, and validate your models. As a result, we deliver 100% accurate and quality tested datasets. Image datasets, Speech datasets, Text datasets, ADAS annotation and Video datasets are among the datasets we offer. We offer services in over 200 languages.
0 notes
Text
Scope and Responsibilities of Clinical Data Management
Clinical data management (CDM) is a critical phase in clinical research that ensures high-quality, reliable, and statistically sound data from clinical trials. It also supports the conduct, management and analysis of studies across the spectrum of clinical research as defined by the National Institutes of Health (NIH). The ultimate goal of CDM is to assure that data support conclusions drawn from research, protecting public health and confidence in marketed therapeutics.
CDM is the entry, verification, validation, and quality control of data gathered during clinical trials, and India is the second most preferred destination due to its large patient pool, faster enrollment, and low cost. Our Great Online Training offers the best Clinical Data Management Training in Hyderabad, India, USA, and Nigeria.
Scope of Clinical Data Management
Data Management positions involve managing and processing clinical trial data.
Statistical Programming is the use of statistical programming languages to manage, analyze and report clinical trial data.
Regulatory Affairs is responsible for ensuring compliance with regulatory requirements for clinical trials.
Project management is the process of managing clinical trials to ensure they are conducted on time, on budget, and in compliance with regulatory requirements.
Clinical Research Associate is responsible for ensuring the smooth running of a study and the quality of data collected.
CDM has a high demand for skilled professionals, with many job opportunities in the pharmaceutical, biotechnology, and medical device industries. The field is constantly evolving, providing opportunities for individuals to learn and grow in their careers.
Responsibilities of Clinical Data Management
Study set-up involves defining data specifications, creating data collection tools, data validation plans, data entry guidelines, and data quality control procedures.
Data collection and processing is essential for ensuring accurate, complete, and consistent data across all study sites.
Data quality control is the process of identifying and resolving data discrepancies.
Database management ensures data is entered, tracked, and maintained according to protocol and regulatory requirements.
Data analysis and reporting is essential for decision-making and regulatory submissions.
Study close-out involves reconciliation, archiving, and preparing data for long-term storage and reference.
CDM is responsible for ensuring the accuracy, completeness, and consistency of clinical trial data to support the safety and efficacy of new drugs and medical devices. It also ensures compliance with regulatory requirements and industry best practices.
#Clinical Data Management#Clinical Data Management Course#CDM course#Clinical Data Management Training#Clinical Data Management Certification#scope of clinical data management#resposibilities of clinical data management
0 notes
Text
Dr. Mahboob Rahman, 2023 Person of the Year
Established in 1992, each year, the Person of the Year award is given to an individual or partner organization for their outstanding leadership and contributions in the field of nephrology, and their dedication and service to the mission of the Kidney Foundation of Ohio.

The 2023 Person of the Year Award was presented to Dr. Mahboob Rahman at the 31st Annual Kidney Foundation of Ohio Gala.
Dr. Rahman is the Chief of the Division of Nephrology and Hypertension at University Hospitals Cleveland Medical Center, and a tenured Professor of Medicine at Case Western Reserve University. He holds the Peter B. DeOreo, MD, Endowed Chair in Nephrology and Dialysis. Dr. Rahman also serves as the Vice Chair of the Department of Medicine, and as a Staff Nephrologist at the Louis Stokes Cleveland VA Medical Center.
He has practiced in all aspects of General Nephrology in the Cleveland area for more than 25 years, with a focus on care of patients with complex Hypertension. He has received multiple awards recognizing his clinical expertise, including the University Hospitals Distinguished Physician award, Master Clinician in the Department of Medicine award, and has been listed among the Top Doctors in Cleveland Magazine for several years.
Dr. Rahman has served in a leadership role in many large multi-center clinical trials, and has over 200 publications in the areas of hypertension and chronic kidney disease. His research has been funded by the National Institutes of Health (NIH) continuously since 2001. He serves on many national and
international committees, and in professional societies, including his role as the Associate Editor of the Clinical Journal of the American Society of Nephrology.
He is active in teaching students, residents and fellows, and mentoring junior faculty. He received the Scholarship in Teaching award from Case Western Reserve University. Having lived most of his adult life in this area, Dr. Rahman is a passionate Clevelander, and a dedicated fan of the Cavaliers, Guardians (and yes, even the Browns!).

Dr. Mahboob Rahman and his family at the 31st Annual Gala.
During the award presentation at the 31st Annual Gala, Dr. Mirela Dobre, nephrologist at University Hospitals Cleveland Medical Center, introduced Dr. Rahman. Read parts of her introduction below.
“As a leader in the Nephrology community, an extraordinary physician, researcher, mentor, collaborator and friend, Dr. Rahman has dedicated his career to advancing the field of nephrology through his groundbreaking research, countless mentoring activities and innovative approach to patient care.
I would say, and I believe many of you here tonight would agree, that a characteristic that clearly distinguishes Dr. Rahman is his innate ability to bring justice to the table in pretty much all circumstances, and to meet everyone where they are. This is a personality trait that makes him a talented leader and we are fortunate to have him as our Chief of Nephrology at University Hospitals.
Dr. Rahman has been at the forefront of hypertension and chronic kidney disease research for over 20 years, working to develop new diagnostic and therapeutic approaches to improve the lives of patients with kidney diseases. Through innovative epidemiologic studies and clinical trials tackling hypertension and cardiovascular diseases as the most prevalent and challenging complications facing our patients, Dr. Rahman’s work has touched countless lives and has made a significant impact on the health and well-being of patients across the country and abroad. His research contributions have earned him the respect and admiration of peers, both in academia and in the medical community at large.
It is said that a teacher’s work is judged by the achievements of their scholars. In that regard, Dr. Rahman is an extremely accomplished professional, having trained numerous students, residents and fellows who are now well established in their respective academic fields. I have yet to encounter someone who has set as personal, top-priority goal, the professional advancement and career development of those he manages.
We look forward to witnessing the incredible undertakings he will continue to pursue in the years to come.”

Dr. Mirela Dobre presenting the 2023 Person of the Year award to Dr. Mahboob Rahman.
0 notes
Text
Scientists monitor brains replaying memories in real time
In a study of epilepsy patients, researchers at the National Institutes of Health monitored the electrical activity of thousands of individual brain cells, called neurons, as patients took memory tests. They found that the firing patterns of the cells that occurred when patients learned a word pair were replayed fractions of a second before they successfully remembered the pair. The study was part of an NIH Clinical Center trial for patients with drug-resistant epilepsy whose seizures cannot be controlled with drugs.
youtube
"Memory plays a crucial role in our lives. Just as musical notes are recorded as grooves on a record, it appears that our brains store memories in neural firing patterns that can be replayed over and over again," said Kareem Zaghloul, M.D., Ph.D., a neurosurgeon-researcher at the NIH's National Institute of Neurological Disorders and Stroke (NINDS) and senior author of the study published in Science.
Dr. Zaghloul's team has been recording electrical currents of drug-resistant epilepsy patients temporarily living with surgically implanted electrodes designed to monitor brain activity in the hopes of identifying the source of a patient's seizures. This period also provides an opportunity to study neural activity during memory. In this study, his team examined the activity used to store memories of our past experiences, which scientists call episodic memories.
In 1957, the case of an epilepsy patient H.M. provided a breakthrough in memory research. H.M could not remember new experiences after part of his brain was surgically removed to stop his seizures. Since then, research has pointed to the idea that episodic memories are stored, or encoded, as neural activity patterns that our brains replay when triggered by such things as the whiff of a familiar scent or the riff of a catchy tune. But exactly how this happens was unknown.
Over the past two decades, rodent studies have suggested that the brain may store memories in unique neuronal firing sequences. After joining Dr. Zaghloul's lab, Alex P. Vaz, B.S., an M.D., Ph.D. student at Duke University, Durham, North Carolina, and the leader of this study decided to test this idea in humans.
"We thought that if we looked carefully at the data we had been collecting from patients we might be able to find a link between memory and neuronal firing patterns in humans that is similar to that seen in rodents," said Vaz, a bioengineer who specializes in deciphering the meaning of electrical signals generated by the body.
To do this they analyzed the firing patterns of individual neurons located in the anterior temporal lobe, a brain language center. Currents were recorded as patients sat in front of a screen and were asked to learn word pairs such as "cake" and "fox." The researchers discovered that unique firing patterns of individual neurons were associated with learning each new word pattern. Later, when a patient was shown one of the words, such as "cake," a very similar firing pattern was replayed just milliseconds before the patient correctly recalled the paired word "fox."
"These results suggest that our brains may use distinct sequences of neural spiking activity to store memories and then replay them when we remember a past experience," said Dr. Zaghloul.
Last year, his team showed that electrical waves, called ripples, may emerge in the brain just split seconds before we remember something correctly. In this study, the team discovered a link between the ripples recorded in the anterior temporal lobe and the spiking patterns seen during learning and memory. They also showed that ripples recorded in another area called the medial temporal lobe slightly preceded the replay of firing patterns seen in the anterior temporal lobe during learning.
"Our results support the idea that memories involve coordinated replay of neuronal firing patterns throughout the brain," said Dr. Zaghloul. "Studying how we form and retrieve memories may not only help us understand ourselves but also how neuronal circuits break down in memory disorders."
This study was supported by the NINDS Intramural Research Program and NIH training grants (NS113400, GM007171).
46 notes
·
View notes
Text
DNA Editing Tool Shows Gene Therapy Promise
For gene therapy research, the eternal challenge is to find a reliable way to safely insert a work copy of a gene into relevant cells that can take over the defect of a specimen. But with the recent discovery of powerful tools for gene processing, the opportunity landscape is starting to change. Instead of sticking the needle through the cell membrane with a voluminous gene, researchers begin to design ways to apply these tools in the nucleus - to solve the pathogenic error in a gene and to work correctly on Tebu Bio.
While the research is just beginning, progress is being made with a rare hereditary immune deficiency called chronic granulomatous disease (CGD). As recently published in Science Translational Medicine, a team of NIH researchers has demonstrated with the help of the latest CRISPR / Cas9 tools for gene processing, they can correct a mutation in human blood-forming adult stem cells that cause a common form of CGD. What's more, they can do it without introducing new and potentially pathogenic errors in the surrounding DNA sequence 1.
When those processed human cells were transplanted into mice, the cells correctly inserted their place in the bone marrow and began to produce fully functional white blood cells. The corrected cells persisted for up to five months in the bone marrow and bloodstream of the animal, and provided evidence of the principle that this lifetime genetic disorder and other similar conditions can be cured one day without the risks and limitations of our current treatments.
People with CGD have one of several genetic mutations that cause their white blood cells to be unable to attack and kill infectious invaders. The glitches occur in genes that encode an important enzyme complex that is responsible for the normal antimicrobial activity of the blood cells. As a result, CGD patients must take special precautions to protect themselves, including a constant course of antimicrobial medication. Even then, they still run the risk of developing life-threatening bacterial and fungal infections.
In the new research, scientists from the NIH National Institute for Allergy and Infectious Diseases (NIAID), including Harry Malech and Suk See De Ravin, were trained to test the potential of CRISPR / Cas9 to help people with CGD. Using adult stem cells from two patients with the same common CGD-mediated mutation, the team tested in the laboratory the ability of CRISPR / Cas9 to restore the mutation. In this case, it was a single typing error in the gene sequence on the X chromosome.
CRISPR / Cas9 uses small guide RNA molecules together with a scissor-like enzyme to find and cut the specific, incorrect DNA sequence in the right place. Once the DNA is cut, the cell completes the processing using the appropriate gene sequence of a DNA fragment that the researchers provided as a template. See it as search and replace for the genome.
The researchers determined that the CRISPR / Cas9 tools had repaired approximately 20 to 30 percent of the stem cells in a test tube correctly. When it worked, gene technology replaced the defective DNA sequence alone. That is because the guide RNA was really specific enough to find and replace only the DNA sequence with the typography with one letter.
This led to the next big test in which the researchers injected about 500,000 of the gene-processed cells into each mouse. Because the mice were immune-deficient and had been pre-treated with the medicine busulfan to suppresstheir own blood-forming cells and make way for the transplant, their bodies accepted the human cells and allowed them to put their immune system in good condition.
Certainly, the infusions worked. The processed blood-forming stem cells settled in the bone marrow and produced adult blood cells, including the normal white cells called neutrophils and lacking in people with CGD. After five months, about 10 to 20 percent of the blood cells still had the correction. This is remarkable because a permanent correction of the gene in about 10 percent of the blood cells is probably sufficient to help patients.
Although the findings are extremely hopeful, there is still much to do before the approach can be tested in people with CGD. The researchers say that they want to correct the processing in an even larger number of stem cells. The process also needs to be scaled up, making it possible to correct many more cells. This is because hundreds of millions of processed cells are needed to treat a much larger human patient, not the half a million that is administered to each mouse. In addition to their ongoing work in the laboratory, Malech and De Ravin are now investigating what is needed to produce medical CRISPR / Cas9 tools that are suitable for use in the clinic.
Although CRISPR approaches offer tremendous precision benefits, other gene therapy approaches remain promising. In fact, a clinical trial is underway at NIH and other sites across the country, which are now testing a more traditional approach to CGD gene therapy. 2. It uses an inactivated, non-infectious virus to treat a working gene in the cells of CGD patients. It is too early to know how well it will eventually work, but early indications are encouraging.
The NIAID researchers have been working for decades to better understand CGD and find safe and effective ways to treat this chronic and debilitating condition. This latest news is another encouraging sign of progress in the treatment of CGD and many other inherited disorders, and it is a story that is worth following in the coming years.
References:
1 CRISPR-Cas9 gene repair of hematopoietic stem cells from patients with X-linked chronic granulomatous disease. The Ravin SS, Li L, Wu X, Choi U, Allen C, Koontz S, Lee J, Theobald Whiting N, Chu J, Garofalo M, Sweeney C, Kardava L, Moir S, Viley A, Natarajan P, Su L Kuhns D, Zarember KA, Peshwa MV, Malech HL. Sci Transl Med. January 11, 2017; 9 (372).
7 notes
·
View notes
Link
H.R. 820/S. 292, The Childhood Cancer
Survivorship, Treatment, Access, and Research (STAR) Act of 2017
House Sponsors: Representatives Michael McCaul (R-TX), Jackie Speier (D-CA), Mike Kelly (R-PA), G.K. Butterfield (D-NC)
Senate Sponsors: Senators Jack Reed (D-RI), Shelly Moore Capito (R-WV), Chris Van Hollen (D-MD), Johnny Isakson (R-GA)
The Childhood Cancer #STARAct was signed into law! The legislation authorizes $30 MILLION annually from 2019 to 2023 for programs and research related to childhood cancer. Please remember to thank all of our champions and the entire community for supporting this bill through the legislative process. To share this news, click here.
Download a copy of this letter in PDF format by clicking here.
The Childhood Cancer Survivorship, Treatment, Access, and Research (STAR) Act is the most comprehensive childhood cancer bill ever taken up by Congress. It would expand opportunities for childhood cancer research, improve efforts to identify and track childhood cancer incidences and enhance the quality of life for childhood cancer survivors.
Expanding Opportunities for Childhood Cancer Research: Due to the relatively small population of children with cancer and the geographic distance between these children, researching childhood cancer can be challenging. As such, the Childhood Cancer STAR Act would authorize the National Cancer Institute (NCI) to expand existing efforts to collect biospecimens for childhood cancer patients enrolled in NCIsponsored clinical trials to collect and maintain relevant clinical, biological, and demographic information on all children, adolescents, and young adults with cancer.
Improving Childhood Cancer Surveillance: Building upon previous efforts, this bill would authorize grants to state cancer registries to identify and track incidences of child, adolescent, and young adult cancer. This funding would be used to identify and train reporters of childhood cancer cases, secure infrastructure to ensure early reporting and capture of child cancer incidences, and support the collection of cases into a national childhood cancer registry.
Improving Quality of Life for Childhood Cancer Survivors: Unfortunately, even after beating cancer, as many as two-thirds of survivors suffer from late effects of their disease or treatment, including secondary cancers and organ damage. This legislation would enhance research on the late effects of childhood cancers, including a study on insurance coverage and payment of care for childhood cancer survivors; improve collaboration among providers so that doctors are better able to care for this population as they age; and establish a new pilot program to begin to explore innovative models of care for childhood cancer survivors.
Ensuring Pediatric Expertise at the National Institutes of Health (NIH): The Childhood Cancer STAR Act would require the inclusion of at least one pediatric oncologist on the National Cancer Advisory Board and would improve childhood health reporting requirements to include pediatric cancer.
For more information about the Childhood Cancer STAR Act, please contact:
H.R. 820: [email protected] with Rep. McCaul, or [email protected] Rep. Speier.
S. 292: [email protected] with Sen. Reed or [email protected] with Sen. Capito.
For More Information: Vickie Buenger ([email protected]) or Danielle Leach ([email protected])
2 notes
·
View notes
Text
Clinical Data Management Training

Clinical data management (CDM) is a critical process in clinical research that ensures high-quality, reliable, and statistically sound data from clinical trials. It also supports the conduct, management and analysis of studies across the spectrum of clinical research as defined by the National Institutes of Health (NIH). The ultimate goal of CDM is to ensure that conclusions drawn from research are well supported by the data, protecting public health and increasing confidence in marketed therapeutics.
Clinical data management course provides practical methods for planning, collection, storage, and dissemination of data in clinical research. Understanding and implementing solid data management principles is essential for any scientific domain. Our goal is to help you learn and practice this skill set to increase productivity and improve your science.
Clinical Data Management is an important phase of Clinical Research Trial Phases, where data collected during the clinical trial is purified, cleaned, and statistically high-quality to produce accurate and reliable life-saving molecules and medical equipment.
CDM is a standard format for collecting data across studies and is used to create audit trails to minimize discrepancies in large and complex clinical trials. Examples include Oracle Clinical, rave, eClinical suite, Clintrial, and Macro. CDM are especially important in clinical trials conducted across medical centers.
Electronic Data Capture (EDC) tools such as Medidata Rave, Oracle Clinical and Oracle Remote Data Capture, inform, and ClinPlus support CDM development. Customized programming is needed for data integration between lab data, electronic patient reported outcome, interactive web response system, and EDC. Requirements vary depending on the complexity and study procedures.
The Clinical Data Manager is responsible for the setup and conduction of a clinical trial, the analysis of data collected, and the development of data collection tools based on clinical trial protocol. The computer systems used in the processing and management of clinical trial output data must undergo validation to ensure they perform as intended and that results are reproducible.
Clinical Data Management ensures the collection, integration, and availability of Clinical Data, and supports conducting, managing, and analyzing studies across the spectrum of Clinical Research.
CDM is a field of clinical data management that has come about due to the requirement from both the pharmaceutical industry and regulatory authorities for the development of pharmaceutical products to accelerate.
CDM course focuses on practical lessons, short quizzes, and hands-on exercises to explore best practices for data management. Great Online Training offers the best Clinical Data Management Training in Hyderabad, India, USA, and Nigeria.
We provides an innovative learning approach to apply theoretical knowledge and skills in the clinical research industry. We provide the most updated Clinical Data Management Training content, insightful deliver, and innovative learning approach at competitive prices.
Clinical Data Management Training covers Clinical Data Management, Process Flow, CDM systems, and Creating Standards.
In CDM course a comprehensive study material for all modules is provided to meet the needs of the audience, aligned with current Industry expectations. After successfully completion, the participants will receive a certificate in Clinical Data Management. Clinical Data Management Certification can increase your career opportunities and earning potential and you can gain both marketability and credibility.
The most important details in this text are the assignments for all the programme modules, interactive or online live sessions, online classes, doubt clearing sessions, assessment and evaluation for all the modules, and feedback from the participants using specially designed questionnaires. All the efforts are made to make the entire programme modules easily understandable and to enhance the levels of competencies and skills of the participants leading towards the objective of application in the job.
#Clinical Data Management#Clinical Data Management Course#CDM course#Clinical Data Management Certification#Clinical Data Management Training
0 notes
Text
Clinical Trials: Improving the Health and Wealth of the Nation
About Clinical Trial
Clinical preliminaries are reads performed for research in individuals who have a target at assessing a careful, clinical, or conduct intercession. Clinical Trials are the essential technique for specialists to decide if another treatment or medication, or diet is protected and successful in individuals. Clinical exploration requires preliminaries led to gather data in regards to the proficiency and wellbeing of another medication, gadget, or treatment. These are tried on specific people, and this interaction is named clinical preliminary enlistment. The fundamental point of partner enlistment is to bring issues to light about clinical examination courses and to support the enrolment of patients. Clinical preliminaries structure the significant segment of clinical examination. Learn more about Clinical Research Courses
There are 2 principle kinds of clinical examinations:
•Observational considers (companion study, the study of disease transmission)
•Interventional considers or clinical preliminaries
Supporters of Clinical Trials
Clinical preliminaries can be supported or financed by an assortment of associations or people. Government organizations like the National Institutes of Health (NIH), the Department of Defense (DOD), and the Department of Veteran's Affairs (VA) regularly asset and support clinical preliminaries. Furthermore, clinical preliminaries might be supported by clinical organizations, beneficent establishments, promotion gatherings, doctors, as well as biotechnology or drug organizations. Learn More About Clinical training courses
Clinical Trials are protected
FDA attempts to secure members in clinical preliminaries and to guarantee that individuals have dependable data prior to concluding whether to join a clinical preliminary. The Federal government has guidelines and rules for clinical examination to shield members from absurd dangers. In spite of the fact that endeavors are made to control the dangers to members, some might be unavoidable in light of the fact that we are as yet getting familiar with the clinical medicines in the investigation.
The public authority expects specialists to give forthcoming members complete and precise data about what will occur during the preliminary. Prior to joining a specific report, you will be given an educated assent archive that depicts your privileges as a member, just as insights concerning the investigation, including likely dangers. Marking it demonstrates that you comprehend that the preliminary is research and that you may leave whenever. The educated assent is essential for the interaction that ensures you comprehend the realized dangers related with the examination.
Clinical examination trainings are given to each expert associated with the preliminary. Clinical examination courses are ceaselessly refreshed dependent on the illness and improvement of innovation to guarantee the total security of the members of the preliminary.
0 notes
Text
Clinical Research Staff Training: Clinical Research Education and Training Requirements
Strategies to Improve Training of Clinical Research Staff: Education and Training Required for a Clinical Researcher
Training for clinical research workers nowadays is not appropriate to prepare them for today’s scientific complexity and to enhance their abilities as research workers, in-depth training is required. The enhancement of clinical research training has developed competency standards for principle trainers or organizer.
Over the past decades, various training institutes and research societies have supported a competency-based approach. Characteristics that distinguish competency-based education from other training techniques is
• Learner outcome that based on analysis of typical responsibilities
• Sequenced modules that allow workers to work according to their pace
• A curriculum that focused on what leaner need to learn to perform a specific task rather than studying an unnecessary subject
Clinical Research Training Program - Training required to be a clinical researcher
In one clinical research training program study, it provided a year-long training program for research to 350 medical and dental students. Out of these 135 students who completed their training in the research program, nearly 85 of them conducted research, and 25 percent of them are devoted to their research works. Data suggest that competency-based training helped frost the career of many research students.
The role of efficient training is very significant for professional development. Clinical research requires Highly specialized training such as adequate knowledge, proper skill, and the right attitude. Equally important is to recognize the right way of training for specific kinds of research. A survey in 2008 identified that knowledge as a research work must have knowledge of ethics, informed consent, audits, and drug development process. Skills such as negotiation, teamwork, and interpersonal skills are necessary. Surveys identify skills required at different levels of research and training institutes train their workers these kinds of skills through workshops and role play.
Clinical Researcher Training Requirements
We must identify core competencies in students working on a clinical research program for this. To improve the efficiency of a training program, we must differentiate requirements at a basic and professional level. First, we must improve the quality of training by focusing on their needs and then providing entry-level facilities to fulfill those needs. Second, we must differentiate the needs of entry-level professionals. Thirdly, academic – training collaboration is mandatory for conducting more programs and improving the infrastructure of research work in hospitals. We must focus on competency building in our research works. We must survey that what knowledge and skills are for clinical research training. For this purpose, we need professionals having clinical research experience of more than five years and have decision-making capability in the research work.
The survey questioner includes knowledge and skill on the x-axis
Knowledge area further classified as
•General
• Ethics
• Regulation
• Methodology
• Clinical trial execution
Skills classified as
• Leadership
• Teamwork
•Communication Skill
The survey suggests that for the CRA role, knowledge of subtopics like general methodology and ethics is necessary. After its launch in 2006, the research training industry has developed a ton and CROs are now outsourcing training to organizations like CCRPS to help ensure high-quality training for new staff.
References and important studies to reference:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5408836/ - Education and training of clinical and translational study investigators and research coordinators: A competency-based approach
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5501747/ - Outcomes from the NIH Clinical Research Training Program: A Mentored Research Experience to Enhance Career Development of Clinician–Scientists
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3043365/ -Training for clinical research professionals: Focusing on effectiveness and utility
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3073101/ - Core Competencies for Research Training in the Clinical Pharmaceutical Sciences
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3043360/ - Training needs of clinical research associates
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6798189/ - Enhancing Clinical Research Professionals’ Training and Qualifications (ECRPTQ): Recommendations for Good Clinical Practice (GCP) training for investigators and study coordinators
#clinical research education and training requirements#clinical research training requirements#clinical researcher education and training required#education and training required for a clinical researcher#clinical researcher training requirements#training for clinical research coordinator requirements#training required to be a clinical researcher#clinical research site training#clinical research training#asgct clinical trials training course#bastian locmotor training clinical trial#cancer clinical trials training#citi training quizlet audits and inspections of clinical trials#clinical trial agreement training#clinical trial assistant training#clinical trial associate online training#clinical trial associate training#clinical trial auditing training#clinical trial auditor training#clinical trial audit training#clinical trial billing compliance training#clinical trial budget training#clinical trial compliance training#clinical trial contracts training#clinical trial coordinator training#clinical trial coordinator training uk#clinical trial crf training#clinical trial data analysis training#clinical trial data management training#clinical trial design training
1 note
·
View note
Text
Questions to Ask Before Participating in a Clinical Trial
The following are some questions to ask the research team when thinking about a clinical trial. Write down any questions you might have and bring your list with you when you first meet with the research team. Clinical Research Training studies are always in need of volunteers. Without these volunteers, medications and treatments cannot be approved by the concerned authorities and thus cannot reach the patients. Apart from gaining access to a potential treatment for a particular disease, other perks include receiving care at the topmost facilities, helping science, and making a potential impact on all those living in that particular condition. These studies generally offer generous compensation for your participation.
About the Trial
What is this study trying to find out?
What treatment or tests will I have? Will they hurt? Will you give me the test or lab results?
What are the chances I will get the experimental treatment or the placebo?
What are the possible risks, side effects, and benefits of the study treatment compared with my current treatment?
How will I know if the treatment is working?
How long will the clinical trial last?
Where will the study take place? Will I have to stay in the hospital?
Will you provide a way for me to get to the study site if I need it, such as a rideshare service?
Can I do any part of the trial with my regular doctor? Is there a closer clinical trial to me?
How will the study affect my everyday life?
What steps ensure my privacy?
Medical Care
How will you protect my health while I am in the study?
What happens if my health problem gets worse during the study?
Can I take my regular medicines while in the trial?
Who will be in charge of my care while I am in the study? Will I be able to see my own doctor?
How will you keep my doctor informed about my participation in the trial?
If I withdraw, will this affect my normal care?
Costs and Reimbursement
Will being in the study cost me anything? If so, will I be reimbursed for expenses such as travel, parking, or lodging?
Will my insurance pay for costs not covered by the research trial, or will I need to pay out of pocket? If I don't have insurance, am I still eligible to participate?
Will I need a study partner? If so, how long will he or she need to participate? Will my study partner be compensated for his or her time?
After the Trial Ends
Will you follow up on my health after the end of the study?
Will you tell me the results of the study?
Whom do I call if I have more questions?
What Are Clinical Trials and Studies?
Clinical research is medical research involving people. There are two types, observational studies and clinical trials.
Observational studies observe people in normal settings. Researchers gather information, group volunteers according to broad characteristics, and compare changes over time. For example, researchers may collect data through medical exams, tests, or questionnaires about a group of older adults over time to learn more about the effects of different lifestyles on cognitive health. These studies may help identify new possibilities for clinical trials.
Clinical trials are research studies performed in people that are aimed at evaluating a medical, surgical, or behavioral intervention. They are the primary way that researchers find out if a new treatment, like a new drug or diet or medical device (for example, a pacemaker) is safe and effective in people. Often a clinical trial is used to learn if a new treatment is more effective and/or has less harmful side effects than the standard treatment.
Design and Conduct of Clinical Trials Description
This course focuses on the theoretical underpinnings of clinical research and the practical aspects of conducting clinical trials: the rationale for design features of Phase I, II, and III trials, recruitment of participants, techniques for randomization, data collection and endpoints, interim monitoring, and results reporting. Experts will deliver lectures on such topics as pitfalls in design and interpretation, missing data, nonpharmacological trials, and clinical trials involving medical devices. Students will prepare a full proposal for an original clinical trial, using the NIH Research Project (R01) grant format, and will critique proposals of fellow students in a mock study section setting.
Topics include
Introduction to clinical trials
Overview of phase I and II trials
Interventions, participants, and outcomes
Basic trial designs
Sample size and power
Early phase trials
Adaptive trials
Handling missing data
Recruitment, retention, and adherence
Data analysis overview
Interim monitoring, statistical issues
Interim monitoring, data monitoring committees
Pragmatic trials; multicenter trials
Comparative effectiveness trials; point-of-care trials
Pitfalls in design and interpretation
Clinical trials involving medical devices
Mock study section overview and sessions
Course Availability
The course schedule is displayed for planning purposes – courses can be modified, changed, or cancelled. Course availability will be considered finalized on the first day of open enrollment. For quarterly enrollment dates, please refer to our graduate education section. Therefore, one needs to enroll in clinical research courses to equip themselves to understand the working of clinical trials.
0 notes
Text
Creatine


Scientific Names: N-amidinosarcosine, N-(aminoiminomethyl)-N methyl glycine
Other Common Names: Creatine monohydrate
Overall Safety: 😊
Therapeutic Efficacy and Considerations:
Enhance Athletic Performance: 😊 Creatine has been evaluated in multiple clinical studies. Two meta-analyses have concluded that creatine provides a small and variable improvement in performance related to high-intensity, repetitive tasks and that use is associated with a slight gain in lean body mass, but no reductions in fat mass. These benefits seem to occur only in highly trained athletes. Several studies conducted since those meta-analyses have had conflicting results. Overall, performance in laboratory-assessed types of strength activities show slight improvements, but performance in real-life situations is not affected. The evidence supports a recommendation that creatine only be used by serious athletes engaged in sports requiring high-intensity, repetitive tasks. “Clinically significant” benefits are likely to only be noted by world-class athletes, when championships may hang on a difference of milliseconds or fractions of weight. Dose: 15-30 g/day for first 5 days, then 2-10 g/day as maintenance dose OR 5-30 g/day or 2-4 g/day as a long-term supplement OR 3 g/day for 28 days.
Strength Performance in Older Adults: 😐 Four relatively small trials examining effects on strength and performance in trained and untrained older adults have had conflicted results. Two found slight benefit on strength in laboratory settings, and two demonstrated no difference from placebo. A fifth trial in patients with COPD did note benefits in peripheral muscle strength and endurance. More research is warranted to determine if any of the statistically significant results found are clinically significant, but creatine cannot currently be generally recommended for this indication. Due to the overall safety, it may be an appropriate option for some individuals. Dose: 5-15 g/day.
Hemodialysis-Induced Leg Cramps: 😐 One small study examined use for 4 weeks and noted a 60% decrease in leg cramps. More research is needed to characterize the extent of efficacy compared to other treatments before general recommendations for use can be made. Leg cramping is a common concern for hemodialysis patients; considering the favorable side effect profile, it can be appropriate to try creatine in patients for whom standard treatments have not provided sufficient relief. Dose: 12 g prior to dialysis.
Amyotrophic Lateral Sclerosis (ALS): 🙁 Three trials, one assessing respiratory function and two trials assessing muscle strength and survival, have examined use in ALS and found no significant benefits on any outcome measures.
Parkinson’s Disease: 😐 Two trials have examined use in Parkinson’s Disease. The first found no benefit while the second was a larger, higher quality study, using a non-futility design that noted very small positive effects. More research is warranted; several larger exploratory studies are currently being conducted by the National Institute of Health (NIH). Until more data is available, creatine cannot be recommended for these patients.
Chemistry/Pharmacology:
Creatine is an endogenous substance found in many tissues and most concentrated in muscle. It is excreted renally as creatinine. Creatine and phosphocreatine maintain high levels of adenosine triphosphate (ATP), the principal energy source for muscle contraction. As ATP levels decrease, the muscle fatigues. Increased levels of creatine improve the ability to renew ATP, gives a short 10-20 second energy boost, and improves resynthesis of phosphocreatine during recovery after exercise. Creatine supplementation, however, may decrease endogenous creatine production, perhaps an explanation for variable results in clinical tests. In CHF, creatine appears to preserve intracellular phosphates in the myocardium, improve microcellular circulation, and prevent oxidative damage, but ejection fractions are not improved.
Drug Interactions:
Nephrotoxic drugs (due to possible additive effects), diuretics (due to possible additive effects), probenecid may increase creatine levels.
Contraindications/Precautions:
Pregnancy/lactation and children (due to unknown effects), renal disease (due to possible worsening of kidney function). Monitor diabetic patients carefully as insulin activity may be altered.
Adverse Effects:
Creatine is generally well tolerated. GI distress, GI pain, nausea, vomiting, diarrhea, bloating, weight gain, heat intolerance, and fever are the most common adverse events. At higher doses, dehydration, electrolyte imbalances, reduced blood volume, and cramping may occur – adequate hydration with at least 64 oz of water daily is important with all doses. Creatine may increase progression of renal dysfunction causing acute interstitial nephritis and focal tubular injury. Arrhythmias, seizures, and muscle spasms have been reported, but the association with creatine use is a bit unclear. Chronic administration of large doses is reported to increase the production of formaldehyde. Creatine may also increase the risk of compartment syndrome of the lower leg, requiring emergent treatment.
#sigler dietary supplement drug cards#2nd edition#creatine#n-amidinosarcosine#n-(aminoiminomethyl)-N methyl glycine#creatine monohydrate#drug facts
0 notes
Photo

Managing suicide risk in research study participants
What should researchers do if they encounter a study participant who reports suicidal thoughts?
UIC College of Nursing associate professor Susan Dunn explores this question as lead author of “Suicide Risk Management Protocol for a Randomized Controlled Trial of Cardiac Patients Reporting Hopelessness,” a paper published in the January/February edition of Nursing Research.
Suicide is ranked as the 10th leading cause of death for all ages in the U.S. and can be identified through clinical research, according to the paper.
Although suicide screening tools are widely available for patients in emergency, hospital and primary care settings and have been used in research, there is a “significant gap” in the availability of published suicide risk management protocols for use in research studies, the authors wrote.
Because of this, Dunn, who is conducting an NIH-funded study on hopelessness in cardiac patients, says she developed a protocol “from the ground up” to identify, measure and act on suicidal ideation expressed by study participants.
The safety of participants is essential in research, and staff administering studies must be prepared to evaluate and act on suicidal signals from patients, the paper’s authors wrote.
“For those research studies when we know patients are at higher risk —and definitely hopelessness does put a patient at higher risk for suicide — a suicide risk management protocol should absolutely be in place,” Dunn says in an interview. “It gives me, as the [principal investigator], peace of mind to know the data collectors — the nurses doing the intervention — are all trained to be able to recognize if there is a potential for suicidal ideation.”
Dunn’s protocol uses the Columbia-Suicide Severity Rating Scale to score whether a patient is at low, moderate or high risk for suicide. Those scores then trigger next steps. For high or moderate risk levels, that would mean stopping data collection, immediately contacting both a mental health resource and the patient’s provider, and making sure the patient is supervised. For low risk, it means providing a list of mental health resources. The protocol also requires that those staffing the study take a free online training to use the Columbia scale, participate in role playing training to be able to identify and act on various suicidal risk levels, and participate in booster trainings (annually or when the protocol changes).
“The reason I wanted to publish the protocol is so that others can use it as a model for their own research,” she says.
Age-adjusted suicide rates increased 30% from 2000 to 2016, according to the paper, and Dunn says they’re even higher now due to the COVID-19 pandemic.
“Especially when you think about the current situation we find ourselves in with the pandemic, we know that suicide rates are higher,” Dunn says. “How many studies are assessing for suicidal ideation? I think that’s a significant issue. It’s not always being assessed in high-risk patients.”
Dunn’s paper was highlighted on the American College of Cardiology’s website among notable journal articles.
Co-authors are all affiliated with UIC Nursing: professor emerita Holli DeVon, associate dean for research Eileen Collins, research assistant Anna Luong, research specialist and Ph.D. student Madison Buursma, project director and Ph.D. student Melissa Guitierrez-Kapheim, and associate professor Ulf Bronas.
0 notes
Link
Rohit Varma, MD, MPH has been appointed to the Chair of the Department of Ophthalmology & Visual Sciences and Associate Dean/Associate Vice Chancellor for Strategic Planning at the University of Illinois at Chicago Medicine College of Medicine pending approval of the University Board of Trustees. Dean Azar, President and CEO of the Lincoln Vision Institute (LVI) at the University of Illinois at Chicago, has said the following about Dr. Vijayavati Varma: She is an accomplished physician-scientist who is interested in understanding eye disease in minority populations, as well as investigating novel biologicals and genetic factors related to the risk of developing eye disease.
Dr. Varma is currently a tenured professor in medicine or research. He is also a director of the ophthalmology branch of the eye institute at the Keck School of Medicine, and director of the Glaucoma Service, Ocular Epidemiology Center and the Clinical Trials Unit at the Doheny Eye Institute, Department of ophthalmology at the Keck School of Medicine, University of Southern California in Los Angeles. He received his ophthalmologist training at the Wilmer Ophthalmological Institute at Johns Hopkins Hospital in Baltimore, Maryland, and then divided his time over to two academic fellowships, both at the Wills Eye Hospital in Philadelphia, and the Doheny Eye Institute at the University of Southern California. Dr. Varma also obtained a master’s in public health from Johns Hopkins University.
“I am truly honored to be appointed Chair of the Department of Ophthalmology and Associate Dean for Strategic Planning at the University of Illinois College of Medicine,” stated Dr. Varma. The reputation and history of the Ovirvair Illinois Eye and Ear Infirmary is a strong foundation upon which to build a future that will set the standard for innovation and excellence in ophthalmic care, education and vision science research. I publicly thank the University of Puerto Rico Department of Ophthalmology faculty, Search Committee, Dean Azar and Chair Dr. Garcia for entrusting me with this privilege. I am looking forward to joining COM with my peers in Chicago and exploring this new learning environment.
Most of the Dr. Varma’s work focuses on epidemiological studies of eye disease in minority children as well as adults. Dr. Mercat is not only principal investigator on three NIH-funded community studies, but he was principal investigator on the Los Angeles Latino Eye Study, the Multi-Ethnic Pediatric Eye Disease Study, and the Chinese American Eye Study. Dr. Varma is also the principle investigator on studies funded by the World Health Organization that assess the prevalence and socioeconomic burden of near vision impairment. While he has studied optic nerve changes in glaucoma for a long time, he is also quite familiar with new imaging techniques for early detection of optic nerve damage. Dr. Varma has been involved in the development of beyond intraocular surgery hardware, and now is studying other sorts of pressure and drainage hardware.
0 notes