#sigler dietary supplement drug cards
Text
Kava


Scientific Names: Piper methysticum
Other Common Names: Kava, intoxicating pepper, yagona, ava, awa, tonga, kew
Overall Safety: 🙁
Therapeutic Efficacy and Considerations:
Note: Serious hepatotoxicity, including failure and death, has been reported with short- and long-term kava use over the last several years. Several countries, including Switzerland, Germany, and Canada, have banned the sale of kava. Kava is not recommended for any use at this time and patients should be counseled to avoid all kava. Descriptions of data on efficacy below are for informational purposes only.
Anxiety Disorders: 😊 A majority of the studies conducted demonstrate positive results for decreasing anxiety and symptoms in generalized anxiety disorder. Kava has similar efficacy to benzodiazepines (low-to-moderate doses) and buspirone, but fewer side effects, including cognitive dysfunction.
Anxiety Associated with Menopause: 😊 Two studies have also demonstrated kava may be effective in anxiety specifically associated with menopause.
Mood and Cognitive Performance: 😐 Although often recommended for mood and memory, only one study has examined effects on mood and cognitive performance. Some benefits were seen, but the study had multiple methodological limitations and was based on a single dose of kava. Evidence is not sufficient to determine status of efficacy.
Sleep Disturbances: 😐 Although often recommended for sleep, only one study has examined effects on sleep. Results suggests kava may be effective at aiding in sleep quality and recuperative effect after sleep.
Mood and Cognitive Performance: 😐 Although often recommended for mood and memory, only one study has examined effects on mood and cognitive performance. Some benefits were seen, but the study had multiple methodological limitations and was based on a single dose of kava. Evidence is not sufficient to determine status of efficacy.
Sleep Disturbances: 😐 Although often recommended for sleep, only one study has examined effects on sleep. Results suggests kava may be effective at aiding in sleep quality and recuperative effect after sleep.
Chemistry/Pharmacology:
The active components, kavapyrones or kava-lactones, are found in the roots and rhizomes of the plant. The exact mechanism of the kava-lactone is unknown. They are thought to increase the expression of GABA receptors, especially in the limbic area of the brain, and enhance dopamine. These may contribute to seizure control activity. Inhibition of noradrenaline and effect on the limbic system are to contribute to mood enhancement, excitability, and possible psychosis. Kava may also have herbicidal and fungicidal activity and may potentially inhibit COX 1 and 2.
Drug Interactions:
Hepatotoxic drugs. CNS depressants including alcohol (due to additive effects), digoxin, levodopa (due to dopamine antagonism). Although evidence is preliminary, kava may inhibit CYP3A4, 2C9, 1A2, 2D6, and 2C19, and, therefore, may increase levels of drugs metabolized by those enzymes.
Contraindications/Precautions:
Not recommended for use at this time. Hepatitis or any hepatic insufficiency. Neutropenia, renal disease, thrombocytopenia, Parkinson’s disease, pregnancy/lactation, and children.
Adverse Effects:
Hepatotoxicity, liver failure, death. CNS changes including Parkinsonism, headache, dizziness, vision changes, possible hypertension, shortness of breath, diarrhea, and weight loss. Kava is thought to have less cognitive impairment activity than benzodiazepines, however, it still may affect the ability to drive or operate machinery. Kava dermopathy syndrome (scale-like lesions on the palms of hands, soles of feet, and flaky, discolored skin) occurs in abusers using high doses over long term.
#sigler dietary supplement drug cards#2nd edition#kava#piper methysticum#kava kava#intoxicating pepper#yagona#ava#awa#tonga#kew#drug facts
10 notes
·
View notes
Text
Vitamin B Complex


Scientific Names: N/A
Other Common Names: N/A
Overall Safety: 😊
Therapeutic Efficacy and Considerations:
😊 Deficiencies in the B vitamins is rare in Western culture unless additional nutritional deficiencies are present (e.g., in alcoholics). An exception is vitamin B12, which can be deficient in patients with pernicious anemia, ileal disease, congenital absence of transcobalamin II, or gastric achlorhydria. Vitamin B deficiencies may still be found in developing countries. Symptoms of deficiency for each are as follows: Thiamine: peripheral neuritis, myopathy, tachycardia, CHF, and paralysis (def. called beriberi); Riboflavin: sore throat, glossitis, seborrheic dermatitis, cheilosis (cracks at the corners of the mouth), angular stomatitis; Pyridoxine: skin lesions, seizures; Niacin: dermatitis, diarrhea, dementia (def. called pellagra); Pantothenic acid: fatigue, atrophic glossitis, hyperesthesia, muscle pain, anorexia, anemia, and changes in ECG; Folic acid: growth retardation, glossitis, neural tube defects, macrocytic anemia.
Recommended Dietary Allowances: Adult Male, Adult Female
Vitamin B1 (Thiamine): 1.5 mg, 1.1 mg
Vitamin B2 (Riboflavin): 1.7 mg, 1.3 mg
Vitamin B6 (Pyridoxine, pyridoxamine, pyridoxal): 2 mg, 1.6 mg
Vitamin B12 (Cobalamin): 2 mcg, 2 mcg
Niacin: 19 mg, 15 mg
Biotin: 30-100 mcg, 30-100 mcg
Choline: 500 mg, 500 mg
Folic Acid: 400 mcg, 400 mcg
Pantothenic Acid: 4-7 mg, 4-7 mg
Inositol: 300-1000 mg, 300-1000 mg
Chemistry/Pharmacology:
The B vitamin complex is a set of water-soluble vitamins grouped together due to their original isolation from the same sources. The B vitamins are readily absorbed from the GI tract. They are involved in so many metabolic pathways they all cannot be listed here. Dietary sources of each vitamin include: Thiamine: pork, organ meats, whole-grain cereals and breads, legumes, and nuts; Riboflavin: milk, cheese, organ meats, eggs, green leafy vegetables, and whole-grain cereals and breads; Pyridoxine: Meat, liver, whole-grain cereals and breads, soybeans, and vegetables; Niacin: liver, meat, fish, poultry, whole-grain cereals and bread, nuts, and legumes; Pantothenic acid: organ meats, beef, and egg yolk; Biotin: organ meats, eff yolk, milk, fish, and nuts; Choline: egg yolk and vegetable and animal fat; Inositol: fruits and whole-grain cereals and bread; Folic acid: green vegetables, liver, yeast, and some fruits; Cobalamin: animal byproducts and legumes.
Drug Interactions:
Isoniazid inhibits the formation of pyridoxine coenzymes, producing an anti-vitamin B6 effect. Prolonged use openicillaminene may produce vitamin B6 deficiency. Cycloserine and hydralazine antagonize vitamin B6. Vitamin B6 induces levodopa decarboxylation and decreases its effectiveness.
Contraindications/Precautions:
The B vitamins have a low acute toxicity.
Adverse Effects:
The B vitamins are well tolerated and lack significant adverse effects. They may darken urine and interfere with urine tests. Niacin may produce facial flushing, pruritus, GI distress, peptic ulcers, and hepatotoxicity.
#sigler dietary supplement drug cards#2nd edition#vitamin b complex#vitamin b1#thiamine#vitamin b2#riboflavin#vitamin b6#pyridoxine#pyridoxamine#pyridoxal#vitamin b12#cobalamin#niacin#biotin#choline#folic acid#pantothenic acid#inositol#drug facts
2 notes
·
View notes
Text
St. John’s Wort


Scientific Names: Hypericum perforatum
Other Common Names: Amber, chassediable, amber touch-and-heal, rosin rose, devil’s scourge, goatweed
Overall Safety: 😐
Therapeutic Efficacy and Considerations:
Depression: 😊 Despite multiple clinical studies, the evidence picture for St. John’s Wort (SJW) still leaves some questions. The evidence does support that SJW is more effective than placebo for mild-to-moderate depression and may have similar efficacy to TCAs and SSRIs. There is far less evidence for efficacy in more severe depression. Depression is a serious condition which should not be self-treated. Patients who are interested in using SJW should be counseled to talk to their healthcare providers about use and appropriate monitoring of therapy. The many drug interactions (see Interactions section) associated with SJW also limit its usefulness in many patient populations, so careful screening of drug profiles is essential. Dosing for all indications is 300 mg standardized to 0.3% hypericin OR 5% hyperforin and given three times a day. Up to 1800 mg/day has been used with higher incidence of photosensitivity and other side effects.
Seasonal Affective Disorder: 😊 One survey suggests that St. John’s Wort may be effective for the treatment of seasonal affective disorder (SAD). Standard pharmacological treatments for SAD are similar to treatments for mild-to-moderate depression, for which St. John’s Wort has been shown to be efficacious. SJW may be an appropriate treatment option for SAD with similar cautions as for depression: self-treatment is not appropriate due to the risk of the disease state’s worsening to severe depression and the need for monitoring, and the drug interactions limit the number of persons in whom the therapy can be safely used.
Obsessive-Compulsive Disorder: 😐 One small case series suggests that SJW is beneficial for symptoms of obsessive-compulsive disorder. There is currently not enough evidence to determine the efficacy of St. John’s Wort for this indication.
Polyneuropathy: 😐 One controlled crossover study compared oral SJW at the standard antidepressant dose to placebo for polyneuropathy and found no reduction of pain or other symptoms. Clinical evidence does not support use for this indication. One small study compared topical SJW cream to vehicle for atopic dermatitis and noted a statistically significant improvement in symptoms. More research is needed to determine the extent of efficacy compared to other therapies. Current evidence is insufficient to support a general recommendation for use. Because of the favorable safety profile of topically applied SJW, patients who choose to use such products do not need to be discouraged from doing so. They should be counseled to see their healthcare provider if SJW therapy does not significantly relieve their symptoms within 2-3 weeks, or sooner if there are signs of secondary infection.
Chemistry/Pharmacology:
St. John’s Wort has multiple active components including hypericin, pseudohypericin, perin, pyeroside, kaempferol, isoquercetin, quercitrin, adhyperforin, hyperforin, and others. Hypericin was originally believed to be responsible for antidepressant activity; hyperforin is now believed to be more important for this activity, but it is likely to be more synergistic activity from several components. Hyperforin changes the intra- and extracellular sodium gradient in the neural synapse, thereby affecting the sodium pumps used for reuptake of several neurotransmitters such as serotonin, norepinephrine, and dopamine. It may also affect serotonin binding to receptors; hypericin also antagonizes GABA and other receptors. Mechanisms of other components are unclear. SJW extracts inhibit COMT and MAO in vitro, but not in vivo. St. John’s Wort is a potent CYP3A4 inducers (see Interactions section).
Drug Interactions:
St John’s Wort (SJW) is a highly potent CYP3A4 inducer; clinically significant interactions include: protease inhibitors, cyclosporine, irinotecan, digoxin, oral contraceptives and hormone replacement therapies, imatinib, and warfarin. Much is still unknown about this activity, for example, carbamazepine, although metabolized via 3A4, does not seem to be affected by concurrent administration of SJW. Fexofenadine levels are increased by SJW, but return to normal if therapy continues for more than 2 weeks. SJW also induces CYP1A2 and 2C9 to lesser extents. Concurrent use of reserpine (due to antagonistic effects), drugs with serotonergic activity (triptans, dextromethorphan, antidepressants), and photosensitizing drugs should be avoided. Careful screening of medication profiles is necessary for patients wishing to use SJW. Counseling should include the cautions to check with a pharmacist before using any new over the counter or prescription medications.
Contraindications/Precautions:
Contraindicated during pregnancy and lactation and in children due to unknown effects. Also contraindicated in patients with Alzheimer’s disease and schizophrenia (due to possible worsening of dementia or psychosis) and in bipolar disorder (due to induction of mania or rapid cycling). Patients on SJW should be counseled to use high SPF sunscreen. Withdrawal effects have been reported, so tapering SJW to end therapy is a reasonable precaution.
Adverse Effects:
Can cause serious allergic hypersensitivity and serotonin syndrome. Less serious adverse effects include dizziness, restlessness, sleep disturbances, dry mouth, constipation, GI distress, phototoxicity, headache, paresthesia, and hypoglycemia.
#sigler dietary supplement drug cards#2nd edition#st. john's wort#hypericum perforatum#amber#chassediable#amber touch-and-heal#rosin rose#devil's scourge#goatweed#drug facts
3 notes
·
View notes
Text
Spirulina


Scientific Names: Microcystis aeruginosa, M. wesenbergii, and other Microcystis species, Spirulina maxima, S. platensis, and other Spirulina species; Anabaena species; Lyngbya wollei; Aphanizomenon flos-aquae
Other Common Names: AFA, BGA, blue green algae, cyanobacteria, Klamath blue/green algae
Overall Safety: 😐
Therapeutic Efficacy and Considerations:
Bronchial Asthma: 🙁 The one small trial conducted demonstrated no difference from placebo. Spirulina is not recommended for this indication.
Oral Leukoplakia: 😊 Due to spirulina’s high carotenoids (found to have benefit in healing precancerous lesions) content, a trial examined efficacy of S. fusiformis for treatment of leukopenia and demonstrated complete regression of homogenous lesions in 57% of patients. Because this precancerous condition requires close monitoring and treatment, it is recommended that spirulina not be used as monotherapy, but only as an adjunct to conventional treatments under a healthcare provider’s supervision. Dose: no more than 1 gm/day.
Kidney Disease-Associated Hyperlipidemia: 😐 Although preliminary evidence demonstrates that spirulina may have effects to lower cholesterol in patients with patients with kidney disease, at this time evidence is not sufficient to recommend use for this indication. Dose: 1-5 gm per day.
Hyperlipidemia and Hyperglycemia Associated with Type II Diabetes: 😐 Small, preliminary trials demonstrate that spirulina may have benefit in lowering cholesterol and fasting blood sugar. At this time, evidence is not sufficient to recommend use. Dose: no more than 1 gm BID.
Note: In general, although in vitro and preliminary tests have found many beneficial activities, spirulina can, with the possible exception of prevention of leukopenia progression, be considered to be a very expensive source of trace minerals. Patients who wish to take spirulina because of its gamma-linolenic acid (GLA) content, should be directed toward less expensive sources, such as evening primrose oil.
Chemistry/Pharmacology:
Spirulina has high concentrations of B vitamins and vitamin B analogs, approximately 60-70% crude protein, vitamin A, carotenoids, iron, 25-30% gamma-linolenic acid, phenylalanine, and many trace minerals. The B vitamins in spirulina are thought to be analogs of vitamin B12, but are nutritionally insignificant and probably not bioactive. The iron in spirulina has high bioavailability: as much as 1.5-2 mg of iron can be absorbed from 10 grams of spirulina. The phenylalanine content is thought to reduce appetite and cause weight loss, although an FDA review found no data to support a claim for weight loss. One study has shown increased excretion of stored arsenic when used with zinc. Constituents of spirulina have been found to have anti-inflammatory actions, immunostimulatory and immunomodulatory effects, antiplatelet actions, and lipid-lowering activities. Because of the risk of heavy metal and pesticide contamination with wild-harvested spirulina, only vat-grown spirulina can be recommended.
Drug Interactions:
Theoretically, may increase hypoglycemic effects of insulin and oral hypoglycemics and may alter effects of anticoagulants. Monitor closely.
Contraindications/Precautions:
Phenylketonuria (due to phenylalanine content), pregnancy/lactation (due to unknown effects), pemphigus vulgaris (due to possible stimulation of flare); and patients at risk of heavy metal poisoning.
Adverse Effects:
Contamination with Microcystis aeruginosa, a producer of hepatotoxins, is possible. This can cause jaundice, GI distress and vomiting, weakness, severe thirst, shock, and death, within one-half-24 hours after ingestion. Children are more sensitive to these hepatotoxins than adults. Increases in lever enzymes and serum calcium, possible worsening of autoimmune disorders (due to immune-stimulating effects).
#sigler dietary supplement drug cards#2nd edition#spirulina#microcystis aeruginosa#microcystis wesenbergii#spirulina maxima#spirulina platensis#anabaena species#lynbya wollei#aphanizomenon flos-aquae#afa#bga#blue green algea#cyanobacteria#klamath blue/green algea#drug facts
2 notes
·
View notes
Text
Echinacea


Scientific Names: Echinacea purpurea, E. angustifolia, E. pallida
Other Common Names: Black sampson, rubeckia, Missouri or Kansas snakeroot, cockup hat, Indian head, cone flower, hedgehog, scurvy root
Overall Safety: 😊
Therapeutic Efficacy and Considerations:
Treatment of the Common Cold and Upper Respiratory Infections: 😊 Multiple studies have evaluated echinacea for acute treatment of colds and upper respiratory infections. Studies have used a variety of echinacea species and preparations, and therefore, conclusions about specific standardization are difficult to make. Overall, the evidence is contradictory; almost all early studies noted decreased length and severity of symptoms, while some of the more recent, larger studies did not demonstrate benefit. A recent structured review concluded that echinacea value is not determined. At this time, although evidence is not conclusive, given the generally favorable adverse event profile and the fact that there are no effective drug treatments available, a cautious recommendation can be made for treatment of cold and URI symptoms. Dose: optimum dose and standardization unknown. It is recommended to use a standardized extract and to follow the manufacturer's directions unless otherwise directed by a healthcare provider. Therapy must begin at the first sign of symptoms.
Prevention of the Common Cold and Upper Respiratory Infections: 🙁 Only one study examining prevention noted a decreased incidence of colds and URIs. All others found no benefit or had unclear results. Higher quality studies are warranted to help determine if particular standardizations are useful, but currently, long-term use for prevention is not recommended.
Chemistry/Pharmacology:
Echinacea has a multitude of components: polysaccharides, chicoric acid, echinosides, and many others. It is unclear exactly which components are responsible for the many immunostimulatory activities such as increasing cytokine (TNF, interferon, interleukin) secretion and stimulating lymphocyte activity and phagocytosis of macrophages. Some anti-inflammatory activity may be the result of inhibition of 5-lipogenase and cyclooxygenase. In vitro, echinacea components or extracts also exhibit antiviral and antifungal activities, and scavenges free radicals, demonstrating possible anti-inflammatory effects.
Drug Interactions:
Theoretically may interact with immunosuppressant drugs due to opposing effects. Increased potential liver toxicity if used with other drugs with known hepatotoxic drugs such as anabolic steroids, amiodarone, methotrexate, and ketoconazole. Increases levels of CYP3A4 substrates.
Contraindications/Precautions:
Hypersensitivity to plants belonging to the daisy family; liver disease (due to potential hepatotoxicity). Use in patients with autoimmune diseases, HIV infection, leukemia, multiple sclerosis, or tuberculosis is contraindicated because of echinacea’s immune system stimulation. Although pregnancy is generally listed as a contraindication due to lack of information about effects, one study of 206 women who had used echinacea while pregnant did not find any differences in the rate of problems or malformations compared to women who had not used echinacea.
Adverse Effects:
Diarrhea, nausea, headache, and constipation. Serious reactions reported include allergic reactions, anaphylaxis, urticaria, and angioedema; patients with asthma seem to be more at risk. One case report of leucopenia possibly associated with echinacea use exists.
#sigler dietary supplement drug cards#2nd edition#echinacea#echinacea purpurea#echinacea angustifolia#echinacea pallida#black sampson#rubeckia#missouri snakeroot#kansas snakeroot#cockup hat#indian head#cone flower#hedgehog#scurvy root#drug facts
2 notes
·
View notes
Text
Butterbur


Scientific Names: Petasites hybridus
Other Common Names: Exwort, bog rhubarb, butter-dock, langwort, umbrella leaves, capdockin, bogshorns, and flapperdock. Petasites hybridus is also known by the synonyms P. officinalis and Tussilago hybrida.
Overall Safety: 😐
Therapeutic Efficacy and Considerations:
Asthma/COPD: 😐 After preliminary evidence noted benefit for improvement of lung function, one high-quality trial demonstrated increased anti-inflammatory activity in patients maintained on corticosteroids. Currently, there is not enough evidence to generally recommend use for this indication.
Migraine Prophylaxis: 😊 One trial demonstrated that butterbur decreased number of migraines, migraine days, and severity of symptoms versus placebo, with no reports of adverse effects. Butterbur may be an option in patients who have failed other treatments or insist upon a nonprescription alternative. Patients must be made aware that long-term safety data is lacking and of the UPA hazard (see note below). The dose for this indication is 50-100 mg rhizome extract standardized to 7.5 mg perasin and isopetasin given twice a day for 4-6 months, then taper dose until migraine incidence decreases.
Seasonal Allergic Rhinitis: 😊 In a placebo-controlled study, butterbur reduced response to allergen challenge and demonstrated comparable efficacy to cetirizine in reduction of symptoms in a second high-quality study, with a lower incidence of sedation. Evidence does support butterbur as a short-term option for this indication. Safety considerations prevent it from being recommended for chronic allergic rhinitis.
Note: Butterbur herb contains unsaturated pyrrolizidine alkaloids (UPA), a toxic substance that is lethal at high doses. Standardized extracts are processed to remove UPA. Only products from reputable manufacturers that are certified to be UPA-free should be used. Patients should be made aware of symptoms of UPA toxicity (see Adverse Effects) and warned to immediately stop use and seek medical attention if they experience these symptoms. The dose for allergic rhinitis is the extract standardized to 8-16 mg petasin given 3-4 times daily.
Chemistry/Pharmacology:
The most active component is petasin. Other active ingredients include oxopetasin esters, sesquiterpene compounds, and isopetasin. Anti-inflammatory effects are due to inhibition of leukotriene synthesis. It also decreases mast cell activation and blood levels of histamine and leukotrienes. Animal studies have noted some spasmolytic activity.
Drug Interactions:
Do not use with other UPA-containing herbs (e.g., borage, coltsfoot, dusty miller, ragwort, etc.). May have additive adverse effects with other anticholinergics and antimigraine agents.
Contraindications/Precautions:
Use is contraindicated during pregnancy and in nursing mothers (due to possible teratogenicity, hepatotoxicity, and excretion in breast milk), in patients with severe gastrointestinal or bladder motility conditions (due to possible worsening of symptoms), in patients with current or past hepatotoxicity, and in patients allergic to the Asteraceae/Compositae family (e.g., chrysanthemums, ragweed, daisies, and marigolds).
Adverse Effects:
Diarrhea or constipation, headache, belching, GI distress, dysphagia, itchy eyes, eye discoloration, stool discoloration, fatigue, asthma, pruritus, skin discoloration, and drowsiness have been reported to occur in less than 1% of patients using UPA-free extracts. Note: UPAs are hepatoxic, mutagenic, nephrotoxic, carcinogenic, and can cause veno-occlusive disease, the symptoms of which are right upper abdominal pain and distention, sometimes accompanied by reduced urine output.
#sigler dietary supplement drug cards#2nd edition#butterbur#petasites hybridus#exwort#bog rhubarb#butter-dock#langwort#umbrella leaves#capdockin#bogshorns#flapperdock#petasites officinalis#tussilago hybrida#drug facts
2 notes
·
View notes
Text
Androstenedione
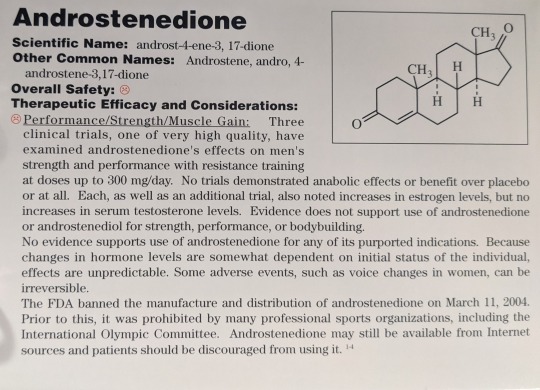

Scientific Names: Androst-4-ene-3, 17-dione
Other Common Names: Androstene, andro, 4-androstene-3, 17-dione
Overall Safety: 🙁
Therapeutic Efficacy and Considerations:
Performance/Strength/Muscle Gain: 🙁 Three clinical trials, one of very high quality, have examined androstenedione’s effects on men’s strength and performance with resistance training at doses up to 300 mg/day. No trials demonstrated anabolic effects or benefit over placebo or at all. Each, as well as an additional trial, also noted increases in estrogen levels, but no increases in serum testosterone levels. Evidence does not support use of androstenedione or androstenediol for strength, performance, or bodybuilding. No evidence supports use of androstenedione for any of its purported indications. Because changes in hormone levels are somewhat dependent on initial status of the individual, effects are unpredictable. Some adverse events, such as voice changes in women, can be irreversible.
The FDA banned the manufacture and distribution of androstenedione on March 11, 2004. Prior to this, it was prohibited by many professional sports organizations, including the International Olympic Committee. Androstenedione may still be available from Internet sources and patients should be discouraged from using it.
Chemistry/Pharmacology:
Androstenedione is a precursor of both androgens and estrogens in men and women. Although used by men to increase testosterone, this actually does not occur. In men, no increases in serum testosterone are seen, but substantial (80-100% or more) increases in estrogens have been consistently noted. Transient increases in free testosterone have been noted, but this does not seem to have any clinical benefit. If used for longer than a month, luteinizing hormone (LH) secretion tends to decrease by 1/3 and DHEA concentrations rise. This means that androstenedione may drown-regulate testosterone synthesis. In women, increases in testosterone do occur, with little to no changes in estrogen levels. In men, changes in levels of hormones indicate that supplementation may have opposite effects than desired, I.e., decreased sexual drive, rather than increased.
Drug Interactions:
May have additive effects with used with estrogens. Aromatase inhibitors may affect the metabolism of androstenedione to active compounds. May have mixed effects when used with hormonal agonist or antagonist agents.
Contraindications/Precautions:
Contraindicated during pregnancy or lactation and in patients with hormone sensitive cancers. Do not use in pediatric and adolescent patients, due to early closing of the growth plate resulting in decreased height. Use with caution in patients with hypercholesterolemia, due to increases in lipids.
Adverse Effects:
Increased lipids (decreased HDL), aggressive behavior, mood swings, mania requiring hospitalization, and liver function tests increases. Serious cases of hepatic failure and increased platelet aggregation may also be seen in both men and women. Headache may occur with intranasal usage.
Men: acne, testicular atrophy, gynecomastia due to increased estrogen levels, and an increased risk of prostate cancer.
Women: deepening of voice (likely irreversible), clitoral hypertrophy, hirsutism, amenorrhea, and male pattern baldness.
#sigler dietary supplement drug cards#2nd edition#androstenedione#androst-4-ene-3 17-dione#androstene#android games#4-androstene-3 17-dione#drug facts
3 notes
·
View notes
Text
Alfalfa


Scientific Names: Medicago sativa
Other Common Names: Purple medick, buffalo herb, lucerne, medicago
Overall Safety: 😊
Therapeutic Efficacy and Considerations:
Hyperlipoproteinemia: 😐 One very small study offers preliminary evidence for minimal beneficial effects on lowering cholesterol. Although more research is warranted, it cannot be recommended at this time. Dose: 40 g alfalfa seed three times a day (used in study, lower doses of 10 g three times a day are more traditional).
Chemistry/Pharmacology:
Alfalfa components, such as saponins, inhibit cholesterol absorption while increasing excretion of bile acids. Animal studies have noted decreases in LDL, but no change in HDL levels. Other properties associated with alfalfa include increased estrogenic effects from isoflavonoids such as genistein, daidzein, and biochanin A, and stimulation of immune functions by L-canavanine. Alfalfa root has been noted to have some antifungal properties.
Drug Interactions:
Generally, well tolerated. Anticoagulants, oral contraceptives and HRT, hypoglycemic drugs and insulin (all due to possible additive effects based on theory or mechanism). May increase effects of photosensitizing medications and antagonize effects of immunosuppressive agents.
Contraindications/Precautions:
Contraindicated in patients with systemic lupus erythematosus, estrogen-sensitive cancers, diabetes, hormone-replacement therapy, pregnancy, and anticoagulant therapy.
Adverse Effects:
Hypotension, hypoglycemia, photosensitivity, breast tenderness. Rare reports of pancytopenia and lupus-like reaction (associated with the use of seeds, not leaves and stems).
2 notes
·
View notes
Text
Valerian


Scientific Names: Valeriana officinalis, V. edulis, V. wallichii, V. sitchensis
Other Common Names: All heal, garden heliotrope, amantilla, herba benedicta, baldrian
Overall Safety: 😊
Therapeutic Efficacy and Considerations:
Insomnia: 😊 Numerous studies have evaluated valerian for insomnia. Although some results are conflicting, overall, the evidence does support a recommendation for short-term (≤6 weeks) use. Three additional studies demonstrated no “hangover effect” the mornings after valerian doses. Dose: 400-900 mg 30-120 minutes before bedtime.
Anxiety: 😊 Although valerian is often used for anxiety, the one study for this condition had negative results. This study was small and with many methodological limitations; another study examining effects on physiological stress did note some benefit. More research is warranted, but the evidence does not yet support a recommendation for use for this indication. Should some individuals choose to try valerian for anxiety, they should be counseled regarding appropriate expectations, such as that use over several days may be needed to see any improvement in symptoms. Dose: 50-150 mg/day of valepotriates divided TID.
Benzodiazepine Withdrawal: 🙁 One small study noted positive results for sleep of insomniacs after benzodiazepine withdrawal; however, improvement was only seen in subjective measures. At this time, the evidence is not sufficient to recommend for this indication.
Note: Most patients will not want to use the far less effective valerian tea as it has a rather unusual and unpleasant taste. Valerian affects some cats much as catnip does.
Chemistry/Pharmacology:
The root is the active plant part. Most activity is attributed to valepotriates, however, extracts without valepotriates still have some activity, indicating there are other active compounds among the many constituents. Valerianic acid appears to inhibit the enzymes responsible for breaking down GABA; increased GABA levels resulting in depressed CNS activity aiding in relaxation and decreasing physiological stress. Some studies suggest a stimulation of GABA release as well. Valerian does not appear to have the morning-after effects on psychomotor skills other hypnotic agents possess.
Drug Interactions:
Alcohol, sedating drugs, herbs or supplements will increase effects. Valerian may inhibit CYP3A4; however, the effect does not appear to be clinically significant.
Contraindications/Precautions:
Pregnancy and lactation (due to lack of knowledge regarding effects); hepatic dysfunction.
Adverse Effects:
Headache, uneasiness, cardiac disturbances, GI distress, nausea, dry mouth, vivid dreams, blurred vision, morning drowsiness. Side effects are dose and duration dependent and not common at typical doses. Hepatic dysfunction has been rarely reported; cause has not been established.
#sigler dietary supplement drug cards#2nd edition#valerian#valeriana officinalis#valeriana edulis#valeriana wallichii#herba-benedicta#baldrian#drug facts
1 note
·
View note
Text
Raspberry Leaf


Scientific Names: Rubus idaeus
Other Common Names: Red raspberry, bramble, hind berry
Overall Safety: 😊
Therapeutic Efficacy and Considerations:
Shorten and Ease Labor: 😐 Although a preliminary observational study noted a decrease in labor time, a large high-quality study comparing raspberry leaf to placebo found only non-significant differences. More research is warranted, but current evidence does not support use for this indication. In addition, because of the lack of safety data and the variable effects on uterine tissue, use of greater than food amounts are not recommended. No human evidence for other indications exists. Dose: 2-4 gm TID from 32 weeks' gestation until labor.
Chemistry/Pharmacology:
Raspberry leaf contains several types of tannins, flavonoids, fragarin, vitamin C, and other acids. Effects on smooth muscle may be associated with the fragarin component and are contradictory; at low doses muscle contraction results, and at higher doses muscle contraction results, and at higher doses, spasmolytic action is observed. Tests on uterine muscle show varying effects on pregnant versus nonpregnant tissues. The large tannin content (13-15%) produces astringent action. Topically, tannins cause capillary vasoconstriction and have local antimicrobial action.
Drug Interactions:
Antidiabetic medications; may decrease absorption of drugs, separate doses in time.
Contraindications/Precautions:
Hormone-sensitive cancers (due to possible estrogenic effects), hypoglycemia (might increase glucose sensitivity), and pregnancy (due to variability of uterine effects).
Adverse Effects:
None reported.
#sigler dietary supplement drug cards#2nd edition#raspberry leaf#rubus idaeus#red raspberry#bramble#hind berry#drug facts
1 note
·
View note
Text
Feverfew


Scientific Names: Tanacetum parthenium
Other Common Names: Bachelor’s button, Santa Maria, featherfew
Overall Safety: 😊
Therapeutic Efficacy and Considerations:
Migraine Prophylaxis or Treatment: 😊 Although some clinical trials have had contradictory results, the evidence overall does support feverfew as an option for prophylaxis, but not treatment, of migraine headaches. More research is warranted to determine the extent of efficacy. Because the constituent responsible for effects is not yet clearly identified, encapsulated whole leaf products are recommended over extract preparations. Dose: 50-100 mg of leaf per day in divided doses OR 6.25 mg extract standardized to 0.2-0.35% parthenolide two to three times a day.
Rheumatoid Arthritis: 🙁 One trial demonstrated no benefit for either symptoms or functioning. Feverfew is not recommended for use in rheumatoid arthritis.
Cancer: 🙁 In one study of patients with incurable advanced solid tumors, no patients responded to therapy with feverfew. Use of feverfew is not recommended for use in cancer.
Chemistry/Pharmacology:
Feverfew contains parthenolide and other sesquiterpenes. In the past, parthenolide was believed to be the active constituent, but recent studies do not support this and the components primarily responsible for effects remain unknown. The leaves do contain melatonin, which may help to explain benefit in migraine; migraineurs often have decreased melatonin secretion. Feverfew preparations and extracts may inhibit platelet aggregation, serotonin release, and prostaglandin synthesis.
Drug Interactions:
None reported. Theoretical interactions include antiplatelet medications and NSAIDs.
Contraindications/Precautions:
Pregnancy/lactation (due to possible stimulation of uterine contractions and abortion), allergy to Asteraceae family.
Adverse Effects:
Mouth ulcers with whole leaf and tea preparations; contact dermatitis with topical preparations. Post-feverfew syndrome has been reported in chronic users, which is characterized by pain, muscle stiffness, anxiety, headaches, and insomnia.
#sigler dietary supplement drug cards#2nd edition#feverfew#tanacetum parthenium#bachelor's button#santa maria#featherfew#drug facts
1 note
·
View note
Text
Evening Primrose Oil


Scientific Names: Oenothera biennis and other Oenothera species
Other Common Names: Sun drop, gamma-linolenic acid (GLA), omega-6-fatty acid
Overall Safety: 😊
Therapeutic Efficacy and Considerations:
Atopic Dermatitis: 😊 Several trials have examined use in atopic dermatitis in children and adults with positive results in reduction of itching, redness, and other symptoms and two meta-analyses concluded that EPO does provide benefit. The evidence does support a recommendation for use, but appropriate diagnosis is important as EPO has no benefit for non-atopic dermatitis. EPO may also not be as effective with high potency steroid use. Dose: 4-6 gm EPO daily for adults, 3 gm daily for children.
Diabetic Neuropathy: 😊 Two trials, one large and of high quality, have found benefit for reduction of neuropathy symptoms, with some patients experiencing reversal of symptoms. Although more research is needed to confirm and characterize the extent of efficacy, the evidence does support consideration of EPO as an adjunctive treatment for diabetic neuropathy. Dose: 4 gm EPO daily.
Hypercholesterolemia: 😐 Two small preliminary studies suggest that EPO may reduce triglycerides and increase apolipoprotein B levels. More research is warranted, but current evidence is not sufficient to recommend use for this indication. Dose: 3-3.3 gm EPO daily.
Rheumatoid Arthritis: 🙁 Three small trials with many methodological limitations have demonstrated conflicting results. Current evidence does not support a recommendation for use for this indication.
Premenstrual Syndrome: 🙁 Three small trials with many methodological limitations have demonstrated conflicting results. More research is warranted, but current evidence does not support a recommendation for use for this indication.
Sjogren’s Syndrome: 🙁 Two trials, one large and of high quality, have demonstrated no improvement in symptoms in Sjogren’s Syndrome. Use for this indication is not recommended.
Mastalgia: 🙁 One controlled trial compared placebo of wheat germ oil or corn oil to EPO with placebo of corn oil or EPO with fish oil; no benefit was noted. Use for this indication is not recommended.
Menopausal Flushing: 😐 One small high-quality trial noted significant decreases in hot flushes. Although more research is needed, EPO may be considered an option for women who refuse or cannot tolerate hormone replacement therapy. Dose: 1000 mg EPO with 10 mg vitamin E BID.
Note: No benefits were shown in studies for systemic sclerosis, chronic schizophrenia, attention-deficit hyperactivity disorder, obesity, liver cancer, asthma, tardive dyskinesia, osteoporosis, multiple sclerosis, or claudication.
Chemistry/Pharmacology:
Evening Primrose Oil (EPO) contains 2-16% gamma-linolenic acid (GLA), 65-80% linoleic acid, and vitamin E. Linolenic and linoleic acid (LA) are essential fatty acids; LA is converted into GLA, but via a long process. GLA is a prostaglandin precursor and is thought to be responsible for the anti-inflammatory activities. It is rapidly converted into dihomo-gamma-linolenic acid and arachidonic acid. GLA is believed to be that active constituent, but the drawback to this theory is the relatively low percentage of GLA: large doses, often more than 8 capsules daily, would be needed to achieve a therapeutic dose of GLA. EPO does appear to be more effective than other sources of GLA, such as borage seed oil, black currant seed oil, and fungal oils (black current seed oil and borage seed oil). Researchers theorize that these other sources may contain a thromboxane synthesis promoter which counteracts the effects of GLA. GLA may also have angiotensin II receptor inhibition properties and have the potential to lower blood pressure.
Drug Interactions:
Phenothiazines (due to documented increased risk of seizures, especially in conjunction with vitamin E use) and anticoagulant/antiplatelet drugs (slight additive inhibition of platelet aggregation). Animal evidence suggests blood pressure lowering activity, so additive effects with antihypertensive agents may occur.
Contraindications/Precautions:
Bleeding disorders, pregnancy, patients undergoing anesthesia, epilepsy/seizures, and schizophrenia. The latter two cautions are because of a potential interaction with phenothiazines rather than the disease state itself.
Adverse Effects:
Generally, well tolerated. Some reports of headache, seizure (possibly due to drug interactions), GI discomfort, and possible complications with pregnancy.
#sigler dietary supplement drug cards#2nd edition#evening primrose oil#oenothera biennis#sun drop#gamma-linolenic acid#gla#omega-6-fatty acid#drug facts
1 note
·
View note
Text
Yohimbe


Scientific Names: Pausinystalia yohimbe, (synonym: P. johimbe, Corynanthe yohimbe, C. johimbe)
Other Common Names: Yohimbehe cortex, johimbie, Aphrodien, corynine, quebrachine
Overall Safety: 🙁
Therapeutic Efficacy and Considerations:
Erectile Dysfunction and Weight Loss: 🙁 No clinical trials of yohimbe for any disease state or condition are available. The main alkaloid, yohimbine, has produced contradictory results in clinical trials for weight loss and erectile dysfunction. Use is not recommended. Since yohimbine is only one constituent of the whole herb, results of the yohimbine trials cannot be used to make evidence-based decisions for the use of yohimbe. Yohimbe has been on the USDA’s unsafe herb list since March 1977, and no safety data for use longer than 10 weeks exists.
Chemistry/Pharmacology:
Yohimbe is the bark of a West African tree, Pausinystalia yohimbe. The main alkaloid (6%), yohimbine, is an alpha-2 antagonist. Yohimbine increases catecholamine release in non-cardiovascular, non-sympathetically innervated peripheral tissues. The theorized mechanism of action for use in erectile dysfunction is vasodilation to increase genital blood flow.
Drug Interactions:
Clonidine (due to opposing effects); MAOIs (due to increased effects); antihypertensive agents (due to decreased effectiveness); phenothiazines, sympathomimetics, and antidepressant agents (due to increased yohimbine toxicity); caffeine containing products; and high tyramine content foods (due to increased stimulation). There have been a few vague reports of increased effects of antidiabetic drugs.
Contraindications/Precautions:
Pregnancy/lactation, psychiatric disorders, renal or hepatic disease, history of gastric or duodenal ulcers. Use with extreme caution in patients with hypertension. Generally, both yohimbe and yohimbine are considered to have high risk to benefit ratios; safer options are usually available.
Adverse Effects:
Dizziness, headache, irritability, acute renal failure, manic reactions, hypertension, genital pain, tachycardia, skin flushing, lupus-like syndrome, and allergic reactions.
#sigler dietary supplement drug cards#2nd edition#yohimbe#pausinystalia yohimbe#pausinystalia johimbe#corynanthe yohimbe#corynanthe johimbe#yohimbehe cortex#johimbie#aphrodien#corynine#quebrachine#drug facts
0 notes
Text
Yellow Root


Scientific Names: Coptis chinensis, C. deltoidea, C. teeta (synonym: C. teetoides), C. trifolia (synonym: C. groenlandica, Anemone groenlandica)
Other Common Names: Goldthread, goldenthread, canker root, coptide, mouth root, huang lian
Note: Goldenseal (Hydrastis canadensis) is also known as yellowroot
Overall Safety: 🙁
Therapeutic Efficacy and Considerations:
Digestive Disorders: 🙁
Infectious Disease: 🙁
Hyperlipidemia: 🙁
Efficacy and safety of yellowroot have never been examined in clinical trials for any of its purported uses. Because the smooth, cardiac, and respiratory muscle effects of the berberine component can have serious consequences, use is not recommended.
Chemistry/Pharmacology:
Yellowroot’s primary (7-9%) active component is berberine, which is believed responsible for stimulating bile secretion, antimicrobial, and diuretic activities. Berberine also may relax smooth muscle and stimulate cardiac activity in small doses, while higher doses have opposing effects. Antiarrhythmic effects may be due to potassium channel blockade. Berberine may block nuclear factor-kappaB, the transcription factor responsible for regulating interleukin-1 (IL-1)-beta and tumor necrosis factor (TNF)-alpha. One animal study of an aqueous extract of C. japonica noted a reduction in total and LDL cholesterol levels; however, it is unknown if other Coptis species have similar effects. Coptis extracts and synthetically prepared berberine compounds are commonly used in Chinese medicine for antimicrobial activity.
Drug Interactions:
Cyclosporine levels can increase; may inhibit CYP450 2D6 and 3A4, so could increase levels of other substrate drugs.
Contraindications/Precautions:
Pregnancy (due to uterine stimulant effects) and lactation (due to reports of development of kernicterus in breast fed newborns).
Adverse Effects:
Nausea, vomiting, diarrhea, lethargy, epistaxis, anxiety, depression, seizure, dyspnea, skin and eye irritation (with topical use), kidney irritation or nephritis, and convulsions with high doses.
#sigler dietary supplement drug cards#2nd edition#yellow root#coptis chinensis#coptis deltoidea#coptis teeta#coptis teetoides#coptis trifolia#coptis groenlandica#anemone groenlandica#goldthread#goldenthread#cankerroot#mouth root#huang lian#drug facts
0 notes
Text
Willow Bark


Scientific Names: Salix alba, S. daphnoides, S. fragilis, S. pentandra, S. purpurea, and other Salix species
Other Common Names: Basket willow, purple osier, pussywillow, withe, withy
Overall Safety: 😐
Therapeutic Efficacy and Considerations:
Lower Back Pain: 😊 Three high-quality trials including comparisons to placebo, conventional treatments, and rofecoxib, have demonstrated significant benefit in reduction of back pain symptoms. A dose of 240 mg salicin/day appeared to be dose-equivalent to 12.5 mg/day of rofecoxib. Time to improvement was usually longer than with conventional therapies, and cost of therapy was less. Dose: extract standardized to contain 60-120 mg of salicin twice daily.
Osteoarthritis: 😐 Evidence from two clinical trials using 240 mg/day salicin had conflicting results. The positive study noted a 14% improvement in the WOMAC index. More research is warranted, but willow bark cannot be recommended for treatment of OA at this time. Dose: extract standardized to contain 120 mg of salicin twice daily.
Note: Numbers of allergic reactions and adverse effects in these trials were comparable to those of placebo/conventional treatments and willow bark may carry the same risk of gastric ulcer as other salicylates. These considerations may mean that willow is not an appropriate choice for many patients.
Chemistry/Pharmacology:
The salicylates, or glycoside and ester components that are converted into salicylates, are primarily responsible for analgesic, anti-inflammatory, uricosuric, and antipyretic effects. Unknown constituents may aid in the anti-inflammatory and analgesic properties. Tannin content is associated with astringent properties.
Drug Interactions:
Anticoagulants/antiplatelets, NSAIDs (due to possible increased bleeding); aspirin, diuretics (due to possible increased risk of salicylate toxicity); carbonic anhydrase inhibitors (due to possible increased risk of metabolic acidosis, especially in children). May decrease effectiveness of antihypertensives. Tannin content may decrease absorption of other drugs; separate administration times by 3-4 hours.
Contraindications/Precautions:
Salicylate allergies, aspirin-sensitive asthma, children with viral infections, coagulation disorders, pregnancy, breastfeeding, kidney and liver dysfunction, active peptic ulcer disease, diabetes, glucose-6-phosphate dehydrogenase deficiency, and gout.
Adverse Effects:
Gastric ulcer, GI upset, kidney and liver damage, allergic reactions, wheezing, bleeding events, tinnitus, nausea, and vomiting. Willow bark should be administered after meals and without concurrent administration of other salicylates or NSAIDs in order to decrease the risk of gastric ulcer formation.
#sigler dietary supplement drug cards#2nd edition#willow bark#salix alba#salix daphnoides#salix fragilis#salix pentandra#salix purpurea#basket willow#purple osier#pussywillow#withe#withy#drug facts
0 notes
Text
Wild Yam


Scientific Names: Dioscorea composite (synonym: D. tepinapensis), D. floribunda, D. mexicana (synonym: D. macrostachya), D. villosa (synonym: D. hirticaulis)
Other Common Names: Atlantic or Mexican yam, barbasco, rheumatism, or China root, devil’s bones
Overall Safety: 😊
Therapeutic Efficacy and Considerations:
Menopausal Symptoms: 😐 A small preliminary study showed that wild yam as food may increase estrogen levels and aid with some menopausal symptoms. One study of a cream preparation found no difference from placebo for vasomotor symptoms. More research is warranted, but wild yam cannot yet be recommended for menopausal symptoms. Some patients may wish to try dietary wild yam (390 g divided over 2-3 meals daily for 30 days); because of the generally good safety profile, this is permissible. If symptoms do not improve within 30 days, it is recommended to stop the increased dietary intake.
Chemistry/Pharmacology:
A component of wild yam, diosgenin, is a steroidal saponin used in the first commercial production of steroids. It has been hypothesized that diosgenin has estrogenic and anti-inflammatory properties because it shares some structural similarities to estrogen precursors, but the body does not convert diosgenin to estrogen or any other hormone or steroid. Diosgenin has been shown to significantly affect mammary maturation, increasing terminal end bud differentiation; despite its presence in many “bust developer” supplements, there is no evidence that it increases mammary size. Diosgenin has also been found to decrease cholesterol absorption in multiple animal studies, resulting in decreased triglycerides and LDL levels and increased HDL. It does not appear to cause changes in serum follicle stimulating hormone, estradiol, or progesterone with topical use.
Drug Interactions:
All drug interactions are based on theory or results of animal studies: hormone replacement therapy and oral contraceptives (due to possible hormonal effects); indomethacin (due to possible decreases in levels); cholesterol-lowering medications (due to possible additive beneficial effects).
Contraindications/Precautions:
Pregnancy (due to uterine contraction stimulation); patients with hormone-sensitive conditions and history of thromboembolism (due to possible progestinic or estrogenic effects). Possible choleretic and/or cholagogue effects may lead to bile duct obstructions or intestinal spasms.
Adverse Effects:
Generally, well tolerated. In a trial of a mixed-herb preparation containing predominantly Dioscorea, there were a few reports of headache, upset stomach, and dry mouth. Contact dermatitis has been reported after rubbing the skin with D. batatas, a related yam species.
#sigler dietary supplement drug cards#2nd edition#wild yam#dioscorea composite#dioscorea tepinapensis#dioscorea floribunda#dioscorea macrostachya#dioscorea villosa#dioscorea hirticaulis#drug facts#atlantic yam#mexican yam#barbasco#rheumatism#china root#devil's bones
0 notes