Text
hey guys! so, things to do today 🌺
finish the historical context for that MUN guide
past papers in chemistry
revise math (quadratic functions)
#study motivation#chaotic academia#academia#studyspo#ib#student#studyblr#high school#study tips#study#ib studyblr#studying#study blog#study aesthetic#school#college#teachers#university#middle school#motivation#mindset#habits
8 notes
·
View notes
Text
Sharing my schedule for next week with you! I have to study this much because I have 6 exams when I come back (4 on chemistry...) so wish me luck!
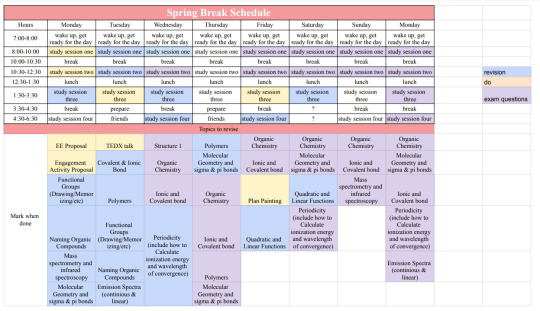
#academia#study#study tips#ib#student#studyspo#chaotic academia#study motivation#high school#studyblr#study routine#study hard#studying#study blog#studyspiration#study movitation#study aesthetic#student life#ib studyblr#chemistry#stem#organic chemistry#middle school#school#college#university
32 notes
·
View notes
Text


Spring Break Study Plan
March 24th
Watch the glopol documentaries
Finish copying the Spanish analysis
March 25th
Finish planning the English Paper 2
Continue the guide for my friend's MUN (introduction to topic, and historical context)
March 26th
Continue the guide for my friend (the rest of things)
Begin the engagement activity
March 27th
Finish the MUN guide
#study motivation#academia#study#chaotic academia#studyspo#studyblr#student#ib#study tips#high school#studying#study blog#study aesthetic#study movitation#studyspiration#student life#school#middle school#university#college#light academia#pink academia#dark academia#academia aesthetic#stem#women in stem#stem student#stem academia#phd#chemistry
91 notes
·
View notes
Text
I’m so mad that a t4 bacteriophage actually looks like that and that it’s appearance isn’t made up
118K notes
·
View notes
Text
this week's schedule 🐚💨

wednesday 🥝
review for global politics
review for biology
upload my Spanish works for the folder ⭕️
finish English IO
thursday 🍓
review for biology
review English IO
friday 🥭
write tok essay
#academia#study#study tips#studyspo#chaotic academia#high school#studyblr#study motivation#ib#student#study movitation#studymaterial#schedule#study aesthetic#studying#studies#studyspiration#study blog#desk#study with me#study hard#ibdp student#international baccalaureate#ibd problems#ibdp#ib diploma#ib studyblr#school#college#academics
82 notes
·
View notes
Text
things on my mind
study organic chemistry
biology, viruses
presentation global politics
IO english
#academia#chaotic academia#study tips#study#study motivation#student#ib#studyblr#high school#studyspo#motivation#studies#studying#study aesthetic#study movitation#studyspiration#study blog#university#desk#desksetup#desk aesthetic#desktop#study desk
28 notes
·
View notes
Text
things on my mind
write a script for a video of my MUN (done)
write my TOK essay (done)
print my notes for the open notebook glopol exam
finish my imath (done)
assign delegates for the MUN (done)
Spanish presentation (done)
#study motivation#academia#study#ib#study tips#chaotic academia#student#studyblr#studyspo#high school#studying#study aesthetic#studyspiration#study movitation#study blog#student life#ib student#college#university#pink academia#dark academia#light academia#school#academics#middle school
22 notes
·
View notes
Text

Monday february 19
so today im feeling so bad that im going to rant. I did a mock test for the national exam and i was the only one that never received the result. Today my chem teacher aka my father came and asked me if I'm okay, if my house if stable etc and i was like yeah why. And he told me that I FAILED THE EXAM. I was like wtf, and asked to see the results and i got 11% on bio and 33% on chem. Such results are absolutely incoherent because i have 7 in both classes which i take in HL.
I went and asked my bio teacher what the hell was going on and she told me that she didn't put the grade up (because these results are the 15% of final grades) because there was no way that I failed everything when I got a 7 in all my classes. So now, I had to write an email asking for a re-evaluation because if this is not a mistake, then i'm failing ALL MY CLASSES for this trimester.
I'm overwhelmed, I want to disappear and yet i can't. I just need this to be a mistake.
🎧 - not strong enough by boygenius
THINGS I NEED TO DO FOR THIS WEEK
monday
-TOK essay
-chem internal
-study for bio
TUESDAY
- chem internal
- finish spanish l drive folder
- meeting at 6.30
-study for bio
WEDNESDAY
-prepare glopol exam
THURSDAY
-prepare for MUN
#chaotic academia#studyspo#ib#study tips#academia#studyblr#student#study#high school#study motivation#studyabroad#studyspiration#study blog#studying#study aesthetic#study movitation#student life#study hard#stem#stemblr#stem academia#women in stem#academics#scientific research#stem student#phd#ibdp#iupac#ibd problems#school
52 notes
·
View notes
Text
he's so me

Vladimir Nabokov, Letters to Véra
3K notes
·
View notes
Text
it pisses me how i will always be inferior to that guy in the eyes of my chem professor (I LITERALLY HAVE BETTER GRADES THAN HIM)
#study motivation#academia#study tips#study#student#studyblr#studyspo#ib#chaotic academia#high school#ibdp#ibdp student#ibd problems#international baccalaureate#ib student#biology#ib diploma#studyabroad#studying#studyspiration#study aesthetic#study with me#study movitation#study blog
13 notes
·
View notes
Text
Sorry for breaking under the pressure of studies and crying. That wasn't very overachieving-model-student of me.
2K notes
·
View notes
Text
Notes for Metallic Bonding
METALLIC BONDING AND STRUCTURE
Delocalised electrons - electrons that are not associated with one specific atom and are free to move within the molecule structure
Metallic bond - the electrostatic attraction between a lattice of cataions and a sea of delocalised electrons.
In metals, state which electrons are the delocalised electrons present between positive ions in the lattice = valence electrons
Mg(s) has metallic bonding in the interaction between positive metal ions and delocalised valence electrons in a three-dimesional lattice structure. The metal itself is neutral and is made up of many, many atoms.
Identify the ways in which solid metals are similar to solid ionic and covalent network substances:
I. Lattice structures II. Non-directional bonding III. Electrostatic attractions between positive and negative species
Solid metals, ionic compounds and network covalent solids form three-dimensional lattices. In all three types of bonding there is an electrostatic attraction between positively and negatively charged species. Metallic – between cations and delocalised electrons, ionic – between cations and anions, covalent – between positive nuclei and shared electron pair.
PHYSICAL PROPERTIES AND APPLICATION OF METALS
Lustre (shiny appearance)
Delocalised electrons in a metal lattice interact with visible light. When visible light hits the surface of a metal, the electrons absorb some of that energy and vibrate. This vibration generates a second wave of light, which radiates from the surface.
Sonority (sound when struck)
When a metal surface is struck, the free electrons in the metallic lattice can move easily, propagating the incoming sound energy easily throughout the material.
Malleability (can be reshaped on compression) & Ductility (can be drawn out into a wire)
When stress is applied (for example, by bending, hitting with a hard object or pulling), layers within the lattice shift in response to that stress. As these layers shift, the cations in the lattice remain surrounded by delocalised valence electrons, meaning the metallic bonding also remains unaffected.
Electrical conductivity
The delocalised valence electrons can move throughout the metallic lattice. When a potential energy difference is applied to the metal, the delocalised electrons are repelled by the negative terminal and attracted to the positive terminal. This is why metals can conduct electricity in their solid state and why metals are used for electrical wires and cables.
Thermal conductivity
Thermal conductivity in metals is a result of the free electrons in the lattice.
STRENGTH OF THE METALLIC BOND
Strength of the metallic bond
The smaller the radius of the metal ion, the stronger the metallic bond. This is because of the shorter distance between the positive nucleus of the cation and the surrounding delocalised electrons. Dictionary
Charge of the metal ion
The higher the ionic charge, the stronger the metallic bond. This is because:
greater charge on the metal ion
greater number of delocalised valence electrons
The greater the ionic charge and the smaller the ionic radius, the stronger the metallic bond. The stronger the metallic bond, the higher the melting point.
TRANSITION METALS
As there are a large number of valence electrons from both the s and d orbitals, this results in a greater electron density within the metallic lattice. This increased electron density in turn increases the strength of the metallic bond.
Hardness
valence electrons (delocalised) increase attraction and increase metallic bond which results in greater hardness
Electrical Conductivity
Transition elements are electrically conductive. These metals form their metallic bonds through the delocalisation of electrons in unfilled d orbitals. The electrostatic attraction between metal ions in the lattice and delocalised electrons increases with an increasing number of electrons in d orbitals.
In comparison to s block metals, the melting point and electrical conductivity of transition metals are HIGHER and HIGHER
#academia#study#study tips#ib#student#study motivation#high school#studyblr#chaotic academia#studyspo#chemistry#notes#study notes#chem#stem#stemblr#school#college#metallic bond#organic chemistry#stem academia#stem student#stem studyblr
11 notes
·
View notes
Text
I realized that people who aspire to be successful academically will never have a sizeable group of friends. I mean, at least most of us, or at least when we are young. Now, that we live i a society that has normalized self-destruction and ephemeral living, it would be much to ask for the people who would rather get drunk and high to hang out with the future scholars that would rather do translations of Latin texts or analyze the deep ocean. That loneliness is part of the academic life, and it’s a pain we must endure until we meet other people with the same interests and such.
55 notes
·
View notes
Text
I gave a 30min Ted talk on why it made me feel uncomfortable AND HES GOING WTF
Quick poll my loves,
Would you be fine if your bf, ex-fuck-party-boy, would want to go back to parties with his fuck boy friends?
I'm not and i wanna know if im crazy
14 notes
·
View notes
Text
My cat is the only living organism that I can say that has seen me cry because of all of my academic crisis
#study motivation#academia#chaotic academia#ib#student#study tips#studyspo#studyblr#high school#study#study hard#studying#study blog#study movitation#study aesthetic#studyspiration#pink academia#academics#light academia#dark academia#academia aesthetic#stem academia#stem#stemblr#women in stem#stem student#science education#chemistry#cat#paula rants
46 notes
·
View notes