#moderna coronavirus vaccine
Text
Moderna booster shot update:
I got my first Moderna booster shot on Wednesday, February 22. Day 2, I have a fever, headace, and nausea. I did take medicine for the side effects. Day 3, I feel better.
2 notes
·
View notes
Text
Great news for uninsured adults in the USA who want a COVID-19 booster! It now appears that ALL CVS locations are now active participants in the Bridge Access Program. The Bridge Access Program gives out free Covid-19 vaccinations to 18+ adults who otherwise can't afford one, so if you have a CVS near you, please go get one! For others who don't have a CVS near them, please go to vaccines.gov, click on "Find Covid-19 vaccines", fill out which vaccines you prefer (you can mix different vaccines if you have to so i reccomend just marking all of them for the age groups you need), and when the next page loads mark the "Bridge Access Program Participant" option to see only locations that are Bridge Access Program participants. Hopefully, other places that aren't CVS will start participating soon, so just check back every so often to see if there are any updates. The CDC Bridge Access Program website also has more details on what locations will be participating, but only CVS is appearing as an active participant on the vaccines.gov location finder at the moment.
#covid19#covid#coronavirus#vaccines#covid vaccine#bridge access program#CDC#signal boost#please share#coronavirus vaccine#covid19 vaccine#covid 19 vaccine#novavax#moderna#pfizer#also interesting side note but i havent been able to find any vaccine other than novavax near me#perhaps this is just a regional thing or maybe novavax is cheaper to make so those are the most common?#anyway thats why i made sure to tell people its okay to mix up because im going to have to bc i got moderna every other time lol#mayyybe other vaccines will become available in the future??? but ive had close family catch covid left and right so im not waiting#also does anyone know why the bridge program only bridges access to 18 or older individuals?#like i knew the gov didnt care about children but god damn lmao
16K notes
·
View notes
Text
Two Idaho lawmakers have introduced a bill to charge those who administer mRNA vaccines with a misdemeanor.
Sen. Tammy Nichols, R-Middleton, and Rep. Judy Boyle, R-Midvale, sponsored HB 154. It was introduced in the House Health & Welfare Committee on Feb. 15 by Nichols. According to the bill text, "A person may not provide or administer a vaccine developed using messenger ribonucleic acid technology for use in an individual or any other mammal in this state."
That person would then be charged with a misdemeanor.
Nichols said during her presentation to the committee, "We have issues this was fast tracked."
Nichols said there is no liability, informed consent or data on mRNA vaccines. She later clarified she was referring to the two COVID-19 vaccines, Pfizer and Moderna.
"I think there is a lot of information that comes out with concerns to blood clots and heart issues," Nichols said.
Rep. Ilana Rubel, D-Boise, questioned Nichols' statement that the vaccines were fast-tracked. She said her understanding was that the vaccines were approved and survived the testing, later approved by the FDA.
Nichols said she is finding it "may not have been done like we thought it should've been done."
"There are other shots we could utilize that don't have mRNA in it," Nichols said.
MRNA is a molecule that assists in making proteins. The COVID-19 vaccines, which are known as mRNA vaccines, help your body make proteins that mimic the COVID virus to help bodies fight off the infection, according to John Hopkins Medicine. MRNA was discovered in the early 1960's, John Hopkins states. Some were used to fight the Ebola virus. Researchers are also currently working to use mRNA to prevent other respiratory viruses.
The bill requires a future vote in the committee to pass onto the House floor for debate.
#us politics#news#ktvb#2023#vaccines#vaccine bans#misdemeanor#idaho#HB 154#idaho legislature#Idaho House Health & Welfare Committee#Tammy Nichols#Judy Boyle#mrna vaccines#Ilana Rubel#covid vaccine#coronavirus vaccine#moderna#Pfizer#food and drug administration#trust the science#conservatives#republicans#gop#gop platform#gop policy
2K notes
·
View notes
Text
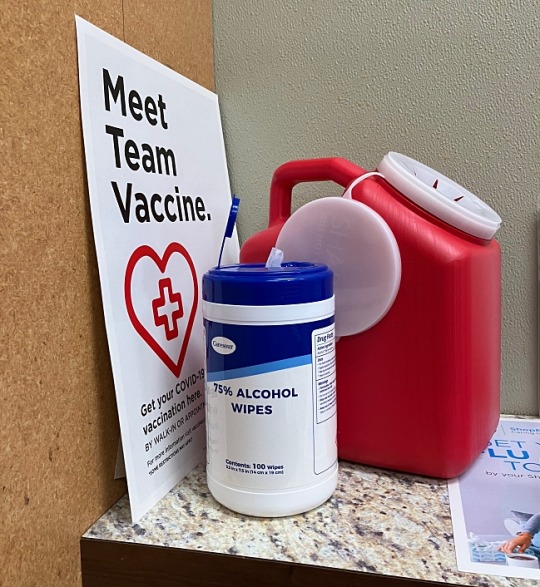
So I got the latest update for the COVID-19 vaccine before the weekend.
The pharmacy section at a large local supermarket was offering it and it was free. They were also giving this year's flu vaccination for free so I got one of those as well.
This was my 5th COVID shot since March of 2021. Contrary to what former President Jair Bolsonaro of Brazil once claimed about COVID vaccines, I did not turn into a crocodile.
One change I noticed is that the Moderna vaccine now has a brand name: SPIKEVAX. But the pharmacists still referred to it as the Moderna type.
The latest subvariant is called JN.1. It now accounts for about a quarter of the cases in the US. It originated in the US, so that is something the "America first" stans might be happy about.
There has been a recent spike in COVID hospitalizations (orange), though the weekly deaths (green) from the virus remain relatively low.

COVID has become a permanent feature of our disease landscape – much like influenza. And as with the flu, vaccinations reduce the possibility of infections and make them less serious if you do get them.
Even if there is not a deadly variant like Omicron (winter 2021-2022) on the horizon, it's worth getting vaccinated just to avoid getting sick at an inconvenient time.
10 notes
·
View notes
Text
FDA proposes annual covid-19 vaccines

View On WordPress
#America#coronavirus#covid#covid-19#FDA#health#healthcare#meme#memes#Moderna#news#pfizer#united states#vaccination#vaccine
20 notes
·
View notes
Text
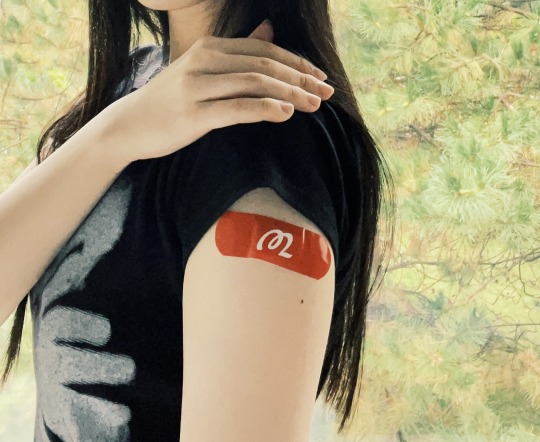
Today I got the newly updated COVID booster targeting XBB.1.5 (the variant driving the current surge*) at Walgreens! It just became available yesterday, and when I double-checked with the pharmacist to make sure I was getting the brand new vaccine, he said that it's the only one they offer now, and that the CDC told them to no longer offer the bivalent one. 👍
*I and a couple pharmacists were the only people wearing masks, and this isn't being widely reported, so for those who don't know: "U.S. COVID infections are hovering near levels of the pandemic’s first peak in 2020 and approaching the Delta peak of late 2021" and "650,000 Americans are becoming infected daily" (via Fortune). Protect each other and yourselves! <3
// (c) Jenny Lam 2023
#moderna#spikevax#health#science#medicine#healthcare#covid#covid19#coronavirus#pandemic#walgreens#photo#photography#selfie#me#trees#vaccine#vaccination#community care#care
6 notes
·
View notes
Text
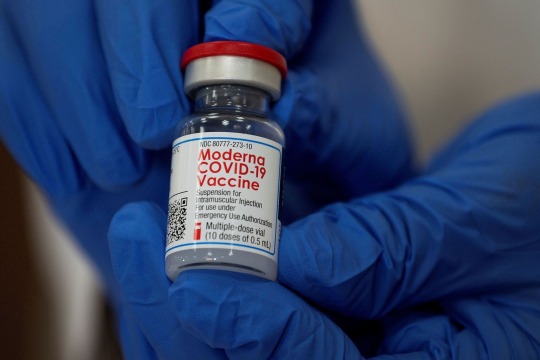
Moderna COVID-19 vaccine, 5ml vial
#uploads#moderna#covid-19#immunizations#vaccines#vaccination#covid shot#moderna covid vaccine#coronavirus#coronavirus vaccine#medcore#medicore#medicalcore#medicine#pharma#pharmacore#pharmacology#pharmcore#pharmacy#immunology#medical aesthetic#pharmacy aesthetic#colorless#vials#covid-19 vaccine#injections#non-controls
20 notes
·
View notes
Link
From the report by Beth Mole, posted 10 Jan 2023:
Moderna is considering raising the price of its COVID-19 vaccine by over 400 percent—from $26 per dose to between $110 and $130 per dose—according to a report by The Wall Street Journal.
Ars has reached out to Moderna for comment but has not yet received a response. The plan, if realized, would match the previously announced price hike for Pfizer-BioNTech's rival COVID-19 vaccine.
The Journal spoke with Moderna CEO Stephane Bancel at the JP Morgan Healthcare Conference in San Francisco Monday, who said of the 400 percent price hike: "I would think this type of pricing is consistent with the value.”
Until now, the mRNA-based COVID-19 vaccines from Moderna and Pfizer-BioNTech have been purchased by the government and offered to Americans for free. In the latest federal contract from July, Moderna's updated booster shot cost the government $26 per dose, up from $15–$16 per dose in earlier supply contracts, the Journal notes. Similarly, the government paid a little over $30 per dose for Pfizer-BioNTech's vaccine this past summer, up from $19.50 per dose in contracts from 2020.
But now that the federal government is backing away from distributing the vaccines, their makers are moving to the commercial market—with price adjustments.
Financial analysts had previously anticipated Pfizer would set the commercial price for its vaccine at just $50 per dose but were taken aback in October when Pfizer announced plans of a price between $110 and $130. Analysts then anticipated that Pfizer's price would push Moderna and other vaccine makers to follow suit, which appears to be happening now.
Lawmakers have already lambasted Pfizer for the steep increase. In a letter sent last month to Pfizer CEO Albert Bourla, Senators Elizabeth Warren (D-Mass.) and Peter Welch (D-Vt.) called the price hike "pure and deadly greed" and accused the company of "unseemly profiteering."
"We urge you to back off from your proposed price increases and ensure COVID-19 vaccines are reasonably priced and accessible to people across the United States," they wrote.
The revelation that Moderna may match Pfizer's price increase comes just a day after Moderna announced that its COVID-19 vaccine sales in 2022 totaled approximately $18.4 billion. "We enter 2023 in a great position, with significant momentum across our clinical pipeline, a highly energized team and a strong balance sheet of over $18 billion of cash and cash equivalents," Moderna's Bancel said in a press release Monday. Moderna also noted in the release that it expects to make a minimum of $5 billion in COVID-19 vaccine sales in 2023.

#holy fucking hell#corporate greed#moderna#pfizer#coronavirus#covid vaccine#covid#covid—19#ars technica#science
18 notes
·
View notes
Video
youtube
Why manufacturers are hiking COVID vaccine prices, January 14, 2023
Since COVID vaccines first became available in the U.S., the federal government has been buying them from manufacturers and distributing them for free. But soon, the manufacturers will be distributing them at higher prices. Jen Kates, senior vice president and director of global health at the Kaiser Family Foundation, joins John Yang to discuss what this means for future vaccination costs.
PBS NewsHour
#pbs newshour#covid 19#coronavirus#pandemic#public health#accessibility#vaccination#pfizer#moderna#corporations#capitalism#medicine#kaiser family foundation#Jen Kates#profiteering#health
9 notes
·
View notes
Text
Experts Await Data on Moderna’s Vaccine for Kids; Criticize Lifting of Mask Mandates
The medical community is celebrating drug maker Moderna’s recent announcement that it is seeking FDA approval for its Covid-19 vaccination for younger kids, though experts add they are “anxiously” awaiting further data on its efficacy and safety.
“We have seen that our youngest patients are getting sick and hospitalized with COVID… even previously healthy kids can develop complications,” said Pediatric Pulmonologist Dr. Manisha Newaskar with Stanford Children’s Health during an April 29 press briefing hosted by Ethnic Media Services.
“The data still needs full review, but we have to educate our families and encourage them to get their young ones vaccinated,” Newaskar added.
The vaccine will require two doses one month apart and is expected to be authorized by the FDA in June. Parents have been hesitant about shots for their kids in part due to the risk of myocarditis—an inflammation of the muscles around the heart—though experts note the risk decreases for younger patients.
“Schools may have a role to play in getting the vaccine out there,” said Dr. Ben Neuman, professor of Biology and chief virologist with the Global Health Research Complex, Texas A&M University.
“There are 500 deaths per year from bacterial meningitis, and that is one of the things that schools would normally require vaccination against,” explained Dr. Neuman. “COVID-19 is around 350 deaths in the same age group in roughly the span of a year. This needs to be one of the vaccines to make school work.”
Moderna previously requested FDA authorization for its vaccine for kids ages 6 to 11 and ages 12 to 17. It expects to submit data on the efficacy of these vaccines by May 9.
The news comes as Moderna also announced progress on a “bivalent” vaccine that would provide immunity to the original Covid-19 strain along with newer variants.
“Each year we update our influenza vaccine and it’s now a quadrivalent vaccine. It has four different antigens in it. So, the thinking among vaccine scientists is to do something quite similar with COVID,” explained Dr. William Schaffner, professor of medicine in the Division of Infectious Diseases at Vanderbilt University School of Medicine.
“Expanding the antigens in the vaccines, we get broader coverage against this array of variants going forward,” Schaffner noted. Dr. William Schaffner, professor of medicine in the Division of Infectious Diseases at Vanderbilt University School of Medicine
The speed at which variants of the Covid-19 virus are appearing, along with their relative transmissibility, calls for the development of multivariant vaccines, speakers said. The BA2 Omicron variant, which emerged in late November in the United States, is almost gone but has been replaced by a subvariant that is even more contagious: the BA.2.12.1
“We need to advocate the FDA to update the vaccines more quickly considering that we’re just tweaking the spike protein a little bit,” said Dr. Eric Feigl-Ding, co-founder of the World Health Network and chief of the COVID Task Force at the New England Complex Systems Institute. “We should be able to do a phase two (of approval), and then allow these brand-new phases to adapt the vaccines pretty quickly…”
Experts also weighed in on the removal of mask mandates on public transportation which several U.S transit systems, including airlines, have already adopted.
“We don’t just stop wearing helmets, unbuckle our seatbelts, and allow drunk driving because hospital beds are not full. I think the CDC is forgetting prevention (strategies) in trying to push for a return to normal,” added Dr. Feigl-Ding, who highlighted the fact that even for people who are boosted, the protection against hospitalization and death is 90-95%, but the protection against infection is only about 45%.
We don’t just stop wearing helmets, unbuckle our seatbelts, and allow drunk driving because hospital beds are not full.
“Adults now are going out to gatherings, parties, bars, restaurants, nightclubs… so the likelihood of transmission is higher,” continued Dr. Feigl-Ding, “and someone may have immunocompromised children at home or family members who could actually have a severe outcome (from infection).”
Dr. Schaffner agreed that the ending of the mask mandate might increase the vulnerability of already fragile people and suggested that people consider their personal circumstances when deciding to leave the mask at home. “Are you older? Do you have an underlying illness, heart disease or lung disease? Are you immune-compromised? Are you a person that’s providing care for someone? Continue to be very careful.”
The speakers emphasized that vaccination is safe and that boosters are essential to strengthen immunity against COVID-19 and its long-term effects such as headaches, fatigue, sleep disturbance, stomach aches, chest tightness, and loss of appetite among others.
Originally published here
Want to read this piece in Spanish? Click here
2 notes
·
View notes
Text
Daily Comic Journal: November 15, 2021: "Branding!"
Even though there’s this new strain of the Covid virus, what a difference a year makes! Last November people were staying at home, masked when they did leave their homes and everyone was getting prepared for a lonely Thanksgiving. Now, thanks to the vaccines it doesn’t feel as dire and scary. Yes, even with the Delta Force bursting through!
Still would like a booster shot though.

View On WordPress
0 notes
Text
Moderna will keep its COVID vaccine on the market at no cost to consumers, even after the federal government stops paying for it, the company announced Wednesday.
"Everyone in the United States will have access to Moderna's COVID-19 vaccine regardless of their ability to pay," the company said in a statement.
Last month, the vaccine maker was slammed for reportedly considering a dramatic price increase for the shot, which it had developed with the help of the federal government.
The proposal was also bad timing: The Biden administration was moving toward ending its designation of a public health emergency on May 11, which meant that federal funding for vaccines would soon dry up and uninsured Americans would have to pay out of pocket for their boosters.
Among the critics of Moderna's reported consideration of a price increase -- from about $26 a shot to as much as $130 -- was Sen. Bernie Sanders, who has long advocated for government-funded health care and alleged the move would result in deaths.
"How many of these Americans will die from COVID 19 as a result of limited access to these lifesaving vaccines?" Sanders, I-Vt., wrote in a January letter to Moderna.
"While nobody can predict the exact figure, the number could well be in the thousands. In the midst of a deadly pandemic, restricting access to this much needed vaccine is unconscionable," he added.
Now, Moderna will be the sole manufacturer of COVID vaccines offering its shot for free to the uninsured. Under federal regulation, insurance companies are already required to foot the bill for COVID vaccines.
"Moderna remains committed to ensuring that people in the United States will have access to our COVID-19 vaccines regardless of ability to pay," the company wrote in its statement.
"Moderna's COVID-19 vaccines will continue to be available at no cost for insured people whether they receive them at their doctors' offices or local pharmacies. For uninsured or underinsured people, Moderna's patient assistance program will provide COVID-19 vaccines at no cost" after the public health emergency expires.
To date, the federal government paid for all COVID vaccines for Americans, whether they were insured or not using emergency money passed by Congress. But President Joe Biden says he plans to let the nationwide public health emergency expire May 11.
Once that happens, federal support ends for many of the programs put in place to help uninsured Americans, including expanded Medicaid, testing and treatments.
Last month, the World Health Organization said COVID-19 remains a public health emergency worldwide, but that the pandemic was at a "transition point."
WHO Director-General Dr. Tedros Adhanom Ghebreyesus said the "global response remains hobbled because in too many countries, these powerful, life-saving tools are still not getting to the populations that need them most – especially older people and health workers."
#us politics#news#abc news#2023#moderna#covidー19#coronavirus#coronavirus pandemic#covid vaccine#coronavirus vaccine#get the vaccine#get the shot#sen. bernie sanders#us healthcare#us health insurance#us health system#biden administration#World Health Organization#Tedros Adhanom Ghebreyesus
81 notes
·
View notes
Text
FDA authorizes Moderna, Pfizer-BioNTech bivalent COVID-19 vaccines for use as a booster dose
- By U.S. Food and Drug Administration (FDA) -
Today, the U.S. Food and Drug Administration amended the emergency use authorizations (EUAs) of the Moderna COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine to authorize bivalent formulations of the vaccines for use as a single booster dose at least two months following primary or booster vaccination.
The bivalent vaccines, which we will also refer to as “updated boosters,” contain two messenger RNA (mRNA) components of SARS-CoV-2 virus, one of the original strain of SARS-CoV-2 and the other one in common between the BA.4 and BA.5 lineages of the omicron variant of SARS-CoV-2.
The Moderna COVID-19 Vaccine, Bivalent, is authorized for use as a single booster dose in individuals 18 years of age and older. The Pfizer-BioNTech COVID-19 Vaccine, Bivalent, is authorized for use as a single booster dose in individuals 12 years of age and older.
The monovalent COVID-19 vaccines that are authorized or approved by the FDA and have been administered to millions of people in the United States since December 2020 contain a component from the original strain of SARS-CoV-2.
What you need to know:
The authorized bivalent COVID-19 vaccines, or updated boosters, include an mRNA component of the original strain to provide an immune response that is broadly protective against COVID-19 and an mRNA component in common between the omicron variant BA.4 and BA.5 lineages to provide better protection against COVID-19 caused by the omicron variant.
The BA.4 and BA.5 lineages of the omicron variant are currently causing most cases of COVID-19 in the U.S. and are predicted to circulate this fall and winter. In June, the agency’s Vaccines and Related Biological Products Advisory Committee voted overwhelmingly to include an omicron component in COVID-19 booster vaccines.
For each bivalent COVID-19 vaccine, the FDA based its decision on the totality of available evidence, including extensive safety and effectiveness data for each of the monovalent mRNA COVID-19 vaccines, safety and immunogenicity data obtained from a clinical study of a bivalent COVID-19 vaccine that contained mRNA from omicron variant BA.1 lineage that is similar to each of the vaccines being authorized, and nonclinical data obtained using a bivalent COVID-19 vaccine that contained mRNA of the original strain and mRNA in common between the BA.4 and BA.5 lineages of the omicron variant.
Based on the data supporting each of these authorizations, the bivalent COVID-19 vaccines are expected to provide increased protection against the currently circulating omicron variant. Individuals who receive a bivalent COVID-19 vaccine may experience side effects commonly reported by individuals who receive authorized or approved monovalent mRNA COVID-19 vaccines.
With today’s authorization, the monovalent mRNA COVID-19 vaccines are not authorized as booster doses for individuals 12 years of age and older.
The agency will work quickly to evaluate future data and submissions to support authorization of bivalent COVID-19 boosters for additional age groups as we receive them.
Who is eligible to receive a single booster dose and when:
Individuals 18 years of age and older are eligible for a single booster dose of the Moderna COVID-19 Vaccine, Bivalent if it has been at least two months since they have completed primary vaccination or have received the most recent booster dose with any authorized or approved monovalent COVID-19 vaccine.
Individuals 12 years of age and older are eligible for a single booster dose of the Pfizer-BioNTech COVID-19 Vaccine, Bivalent if it has been at least two months since they have completed primary vaccination or have received the most recent booster dose with any authorized or approved monovalent COVID-19 vaccine.
“The COVID-19 vaccines, including boosters, continue to save countless lives and prevent the most serious outcomes (hospitalization and death) of COVID-19,” said FDA Commissioner Robert M. Califf, M.D. “As we head into fall and begin to spend more time indoors, we strongly encourage anyone who is eligible to consider receiving a booster dose with a bivalent COVID-19 vaccine to provide better protection against currently circulating variants.”
The Moderna COVID-19 Vaccine, Bivalent and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent contain mRNA from the SARS-CoV-2 virus. The mRNA in these vaccines is a specific piece of genetic material that instructs cells in the body to make the distinctive “spike” protein of the original virus strain and the omicron variant lineages BA.4 and BA.5. The spike proteins of BA.4 and BA.5 are identical.
“The FDA has been planning for the possibility that the composition of the COVID-19 vaccines would need to be modified to address circulating variants. We sought input from our outside experts on the inclusion of an omicron component in COVID-19 boosters to provide better protection against COVID-19. We have worked closely with the vaccine manufacturers to ensure the development of these updated boosters was done safely and efficiently. The FDA has extensive experience with strain changes for annual influenza vaccines. We are confident in the evidence supporting these authorizations,” said Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research. “The public can be assured that a great deal of care has been taken by the FDA to ensure that these bivalent COVID-19 vaccines meet our rigorous safety, effectiveness and manufacturing quality standards for emergency use authorization.”
For each of the bivalent COVID-19 vaccines authorized today, the FDA evaluated immunogenicity and safety data from a clinical study of a booster dose of a bivalent COVID-19 vaccine that contained a component of the original strain of SARS-CoV-2 and a component of omicron lineage BA.1. The FDA considers such data as relevant and supportive of vaccines containing a component of the omicron variant BA.4 and BA.5 lineages. Furthermore, data pertaining to the safety and effectiveness of the current mRNA COVID-19 vaccines, which have been administered to millions of people, including during the omicron waves of COVID-19, contributed to the agency’s evaluation.
Data Supporting the Moderna COVID-19 Vaccine, Bivalent Authorization
To evaluate the effectiveness of a single booster dose of the Moderna COVID-19 Vaccine, Bivalent for individuals 18 years of age and older, the FDA analyzed immune response data among approximately 600 individuals 18 years of age and older who had previously received a two-dose primary series and one booster dose of monovalent Moderna COVID-19 Vaccine. These participants received a second booster dose of either the monovalent Moderna COVID-19 Vaccine or Moderna’s investigational bivalent COVID-19 vaccine (original and omicron BA.1) at least 3 months after the first booster dose. After 28 days, the immune response against BA.1 of the participants who received the bivalent vaccine was better than the immune response of those who had received the monovalent Moderna COVID-19 Vaccine.
The safety of a single booster dose of the Moderna COVID-19 Vaccine, Bivalent for individuals 18 years of age and older is supported by safety data from a clinical study which evaluated a booster dose of Moderna’s investigational bivalent COVID-19 vaccine (original and omicron BA.1), safety data from clinical trials which evaluated primary and booster vaccination with the monovalent Moderna COVID-19 Vaccine, and postmarketing safety data with the monovalent Moderna COVID-19 Vaccine.
The safety data accrued with the bivalent vaccine (original and omicron BA.1) and with the monovalent Moderna COVID-19 Vaccine are relevant to the Moderna COVID-19 Vaccine, Bivalent because these vaccines are manufactured using the same process.
The clinical study that evaluated the safety of a booster dose of the bivalent vaccine (original and omicron BA.1) included approximately 800 participants 18 years of age and older who had previously received a two dose primary series and one booster dose of the monovalent Moderna COVID-19 Vaccine, and then at least 3 months later, received a second booster dose with either the monovalent Moderna COVID-19 Vaccine or Moderna’s investigational bivalent COVID-19 vaccine (original and omicron BA.1).
Among the study participants who received the bivalent vaccine, the most commonly reported side effects included pain, redness and swelling at the injection site, fatigue, headache, muscle pain, joint pain, chills, swelling of the lymph nodes in the same arm of the injection, nausea/vomiting and fever.
Data Supporting the Pfizer-BioNTech COVID-19 Vaccine, Bivalent Authorization
To evaluate the effectiveness of a single booster dose of the Pfizer-BioNTech COVID-19 Vaccine, Bivalent for individuals 12 years of age and older, the FDA analyzed immune response data among approximately 600 adults greater than 55 years of age who had previously received a 2-dose primary series and one booster dose with the monovalent Pfizer-BioNTech COVID-19 Vaccine. These participants received a second booster dose of either the monovalent Pfizer-BioNTech COVID-19 Vaccine or Pfizer-BioNTech’s investigational bivalent COVID-19 vaccine (original and omicron BA.1) 4.7 to 13.1 months after the first booster dose. After one month, the immune response against BA.1 of the participants who received the bivalent vaccine was better than the immune response of those who had received the monovalent Pfizer-BioNTech COVID-19 Vaccine.
The safety of a single booster dose of the Pfizer-BioNTech COVID-19 Vaccine, Bivalent for individuals 12 years of age and older is based on safety data from a clinical study which evaluated a booster dose of Pfizer-BioNTech’s investigational bivalent COVID-19 vaccine (original and omicron BA.1), safety data from clinical trials which evaluated primary and booster vaccination with the monovalent Pfizer-BioNTech COVID-19 Vaccine, and postmarketing safety data with the monovalent Pfizer-BioNTech COVID-19 Vaccine.
The safety data accrued with the bivalent vaccine (original and omicron BA.1) and with the monovalent Pfizer-BioNTech COVID-19 Vaccine are relevant to Pfizer-BioNTech COVID 19 Vaccine, Bivalent because these vaccines are manufactured using the same process.
The clinical study that evaluated the safety of a booster dose of the bivalent vaccine (original and omicron BA.1) included approximately 600 participants greater than 55 years of age who had previously received a 2-dose primary series, one booster dose of the monovalent Pfizer-BioNTech COVID-19 Vaccine, and then 4.7 to 13.1 months later, received a second booster dose of either the monovalent Pfizer-BioNTech COVID-19 Vaccine or Pfizer-BioNTech’s investigational bivalent COVID-19 vaccine (original and omicron BA.1). Among the study participants who received the bivalent vaccine, the most commonly reported side effects included pain, redness and swelling at the injection site, fatigue, headache, muscle pain, chills, joint pain, and fever.
The fact sheets for both bivalent COVID-19 vaccines for recipients and caregivers and for healthcare providers include information about the potential side effects, as well as the risks of myocarditis and pericarditis.
With today’s authorization, the FDA has also revised the EUA of the Moderna COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine to remove the use of the monovalent Moderna and Pfizer-BioNTech COVID-19 vaccines for booster administration for individuals 18 years of age and older and 12 years of age and older, respectively. These monovalent vaccines continue to be authorized for use for administration of a primary series for individuals 6 months of age and older as described in the letters of authorization. At this time, the Pfizer-BioNTech COVID-19 Vaccine remains authorized for administration of a single booster dose for individuals 5 through 11 years of age at least five months after completing a primary series of the Pfizer-BioNTech COVID-19 Vaccine.
The amendments to the EUAs were issued to Moderna TX Inc. and Pfizer Inc.
Related Information
Moderna COVID-19 Vaccine
Pfizer-BioNTech COVID-19 Vaccine
COVID-19 Bivalent Vaccine Boosters
COVID-19 Vaccines
Emergency Use Authorization for Vaccines Explained
The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products.
--
Source: U.S. Food and Drug Administration (FDA)
Read Also
Omicron XE is spreading in the UK – a virologist explains what we know about this hybrid variant
#covid19#coronavirus#pandemic#covid variant#omicron#usa#fda#vaccine#vaccines#pfizer#moderna#health#medecine#public health
0 notes
Text
मॉडर्ना ने फाइजर और उसकी पार्टनर कंपनी के खिलाफ मुकदमा किया दर्ज, ये है पूरा मामला
मॉडर्ना ने फाइजर और उसकी पार्टनर कंपनी के खिलाफ मुकदमा किया दर्ज, ये है पूरा मामला
Moderna Sues Pfizer-BioNTech: अमेरिकी फार्मास्युटिकल और बायोटेक्नोलॉजी कंपनी मॉडर्ना (Moderna) ने फाइजर (Pfizer) और उसके जर्मन पार्टनर बायोएनटेक (BioNTech) के खिलाफ यूनाइटेड स्टेट्स डिस्ट्रिक्ट कोर्ट में मुकदमा दायर किया है. मॉडर्ना ने कोविड-19 वैक्सीन (Covid-19 Vaccine) के विकास में पेटेंट उल्लंघन (Patent Infringement) के लिए इन कंपनियों से नुकसान की मांग की है.
बता दें कि मॉडर्ना ने फाइजर और…
View On WordPress
#coronavirus update#coronavirus vaccine latest news#COVID-19#Moderna#moderna sues pfizer#moderna vaccine latest news#moderna vaccine sues pfizer and biontech#moderna versus pfizer#Pfizer#कोरोनावायरस अपडेट#कोरोनावायरस वैक्सीन#मॉडर्न फाइजर मुकदमा#मॉडर्ना बनाम फाइजर#मॉडर्ना वैक्सीन#मॉडर्ना वैक्सीन फाइजर और बायोनटेक मुकदमा
0 notes
Text
FDA authorizes emergency use for Novavax Covid-19 vaccine for ages 12 to 17
FDA authorizes emergency use for Novavax Covid-19 vaccine for ages 12 to 17
A box of the Novavax Covid-19 vaccine arranged at a pharmacy in Schwenksville, Pennsylvania, US, on Monday, Aug. 1, 2022.
Bloomberg | Bloomberg | Getty Images
Biotechnology company Novavax announced on Friday that its Covid-19 vaccine has been authorized for emergency use by the U.S Food and Drug Administration for adolescents between the ages of 12 and 17.
In July, Novavax’s two-dose Covid-19…
View On WordPress
#ages#authorizes#Biotech and Pharmaceuticals#business#business news#Coronavirus#Coronavirus: Prevention#COVID19#emergency#FDA#Health care industry#Moderna Inc#Novavax#Novavax Inc#Pfizer Inc#Science#vaccine
0 notes
