#Human coronavirus OC43
Text
COVID-19 Infection Has More Than 50 Long-Term Effects
COVID-19 Infection Has More Than 50 Long-Term Effects
MADRID, Spain — Clinical experiences in approaching COVID-19 from different perspectives, results obtained by various therapeutic options and, above all, the challenges posed by a new healthcare reality — long COVID — were all the focus of a recent discussion at the 7th International Congress of the Spanish Society of Precision Health.
In this forum, titled Precision Health: A COVID-19…

View On WordPress
#2019 Novel Coronavirus#2019-nCoV#Coronavirus#dyspnea#exercise#HCoV-229E#HCoV-OC43#Human coronavirus 229E#Human coronavirus HKU1#Human coronavirus OC43#idiopathic pulmonary fibrosis#mon#physical activity#pneumonia#Post-acute sequelae of SARS-CoV-2 infection; post-acute sequelae of SARS-CoV-2 infection (PASC); PASC - post-acute sequelae of SARS-CoV-2 in#Wuhan coronavirus
0 notes
Text
Moringa and Spirulina: Mini Review on their use against COVID-19

Abstract
The world woke up in 2020 with a new virus called coronavirus 2019 (COVID-19). The virus spread easily from Wuhan, a western province in China to the whole world and caused a pandemic situation. Some preventive measures such as wearing of face masks and the use of alcohol-based sanitizers have been prescribed by the World Health Organization (WHO). All these measures could not effectively handle the virus, and the world started to search for a new solution by using herbal medicines. This mini-review discusses the use of Moringa and spirulina to combat COVID-19.
Keywords: Moringa; Spirulina; covid-19; Herbal medicines
Introduction
Discovered in 1965 by Tyrrell and Bynoe, human coronaviruses are group of viruses that display crown like spikes on their surface [1]. There are several types of coronaviruses (four sub groups) in the world; namely alpha, beta, gamma and delta. Six of them including alpha coronaviruses (229E and NL63) [2,3], two types of beta coronaviruses (OC43 and HKU1) [3,4] were explored before 2019. These alpha-coronaviruses and the two types of beta-coronaviruses are common, but they do not cause severe biological damage in humans. Some of the coronaviruses can cause middle east respiratory syndrome called MERS-Cov [5, 6]. Another beta coronavirus was able to induce severe acute respiratory syndrome (SARS) and was labelled as SARS-Cov [5,6]. In 2019, a new form of SARS-Cov came out with a severe pandemic impact. The name SARS-Cov 2 or COVID-19 was given to this new virus [7]. The COVID-19 identified in Wuhan (a Western China province) had spread quickly to other parts of the world. This situation resulted in locking down the world and its economy, akin to the 2008 economic crunch. Some prevention methods like the use of alcoholic or gel-based sanitizers were firstly prescribed, and the use of face masks to prevent the spread of viruses in the air. These two methods could help protect against the viruses; however, they do not get rid of the spread completely. In the middle of 2020, the idea of developing a vaccine had emerged. Some pharmaceutical research industries investigated and came out with some vaccines, which have already been given out in many countries All these precautions do not rapidly eliminate the spread of the virus completely. Moreover, the virus keeps on mutating, and new variants have been identified, which are more dangerous than the earlier types (delta). In middle of this, the population in many parts of the world went back to explore nature by using herbal medicines that could potentially control the virus. Among these herbal medicines are Moringa oleifera and Spirulina, which have been used by some herbal practitioners to treat COVID-19. In this mini review we seek to discuss how this could happen for these two old nutraceutical medicines to help combat the virus amidst the pandemic.
Spirulina and Covid-19
Spirulina, a microalgae used as a diet worldwide is rich in protein, vitamin B1, Vitamin B2, vitamin B3, copper and iron [8]. It is an aquatic organism which needs the presence of salt and fresh water to grow by photosynthesis. It is well-known that spirulina is the best candidate for nutritional supplement for different disease such as cancer and HIV [9-13]. A lot of researchers have proven that spirulina can be used as an antioxidant and anti-inflammatory agent; it helps to lower bad cholesterol and triglyceride levels, thereby controlling type 2 diabetes [14,15]. Spirulina has been explored to reduce blood pressure and has been confirmed to be efficacious against anemia. With the presence of protein and vitamins, spirulina has been consumed to strengthen muscles and improve performance by endurance. Since the appearance of covid-19, there have been 7 research publications that have reported the consumption of spirulina for the control of this virus. Carbone et al., showed that by their antiviral activities, microalgae such as spirulina could help to boost immunity [16]. By the strength of their immunity, the person affected or a healthy person can control the virus, however, the authors did not define the quantity of spirulina to take. The mechanism by which spirulina can act as an antiviral agent was not well-established. In fact, all these research papers suggested that, spirulina may down regulate anti-inflammatory signal by the presence of phycocyanobilin in its components [17-20]. The stimulation of the immune system by increasing phagocytic activity of macrophage which are recruited to fight against the virus have also been reported by Ferreira et al., and Ratha et al., [21,22]. Tzachor et al. [23] had published original work which showed the different effects of spirulina against covid-19 is dependent on the type of spirulina. As was mentioned in the beginning, the growth of spirulina nutriment is photosynthesis-dependent. They compared the effect of solar and light-emitting diode (LED) on the photosynthesis of spirulina. They found that LED spirulina had more anti-inflammatory effect than solar spirulina due the presence of bioactive components like sorbitol, adenosine derivates and C-phycocyanin (CPC) [20]. After extraction, the authors stated that the amount of all these components were significantly increased by using LED as compared to solar [23]. Covid-19 has an acute inflammatory part, and as such by using spirulina, especially LED spirulina extract, it could interfere with tumor necrosis factor (TNFα), which might help to control the inflammatory aspect of covid-19 disease [23]. All these researches demonstrated the case and the importance of spirulina in the apprehension of covid-19 disease.
Moringa and Covid-19
Moringa oleifera, a plant widely used as a supplement nutrition is rich in vitamin C, potassium, calcium, protein, iron, and amino acid [24,25]. These nutrients are responsible for building of muscles after the consumption of M. oleifera. A lot of research reported that M. oleifera could act as antioxidant, immune system booster, lower blood pressure and reduce fat in blood and body [26]. Some research emphasized its use on rheumatoid arthritis, diabetes, cancer, and memory loss [27]. Hamza et al., by using molecular peptide docking proved the effect of M. oleifera on covid-19. They found the presence of flavonoid which may interact with 15 peptides of SARS Cov 2 and reduce the activity of the virus. Their findings revealed that the antiviral activity of M. oleifera is due to the presence of those flavonoids [28]. Moreover, Mathpal et al. [29] used computational approaches to screen the potential of the compounds in M. oleifera on SARS-Cov 2. Among 294 phytochemicals compounds of M. oleifera they found that two of them (Kaempfenol-3-o-rutinoside and vitexin) showed good stability and high binding to the SARS-CoV-2 receptors [29]. These compounds are also flavonoids, and further confirmed the findings by Hamza et al. [28]. Sen et al [30]., demonstrated that the antiviral activity of M. oleifera was dependent on the presence of three flavonoids in the plant. These three flavonoids are isorhamnetin, kaempferol and apigenin. They displayed good binding by virtual screening and dynamic simulations [30]. In addition, Muhammad et al. [31], confirmed the antiviral activity of M. oleifera on covid-19 by performing in silico molecular docking and dynamic studies. According to these authors, the presence of ellagic acid and apigenin is responsible for the antiviral activity of M. oleifera. They evaluated the pharmacokinetics and toxicology profiles of these compounds and revealed the safety of the plant. The molecular docking of these compounds showed clearly their druggability [31].
Shaji et al. [32] investigated the binding properties between covid-19 (the main protease (Mpro)) and several compounds of M. oleifera by performing protein-ligand docking. 12 compounds (morphine, kaempferol, quercetin, pterygospermin, benzoic acid, gallic acid, benzyl isothiocyanate, niazirin, niaziminin, niazinin, o-ethyl-carbamothionate and niazirinin) found in M. oleifera were evaluated. After docking, the result demonstrated that only niaziminin bound strongly to the Mpro, probably by it OH groups. Niaziminin could form hydrogen bonds with the sequences Glu 166 and Phe 140 of the Mpro of covid-19 [32]. This was confirmed by Ullah and Ullah, who also evaluated the binding of natural and synthetic inhibitors to Mpro as promising vaccine strategies against covid-19 [33]. Sundhari et al. [34] have also encapsulated M. oleifera in electrospum nanofiber and evaluated its effect on covid-19. The nanofibers were able to control the viruses’ particles and they developed a new face mask to protect safe and sick people [34]. Usually, researchers assume that old medicines cannot work on new diseases. These two well used natural drugs showed promising efficacy against covid-19 [35,36]. This still confirmed that natural product work as combination strategies to enhance the activity of each component. This review encourages the use of these two products (spirulina and M. oleifera) to boost immunity of healthy people and re-boost the immunity of sick people under this covid-19 pandemic. Further research may help to investigate the combination of spirulina and M. oleifera on covid-19.
Conclusion
The promotion of the consumption of these two herbal medicines is welcome to combat the Covid-19. By interacting with the receptor of this virus, Moringa and spirulina helps to control the inflammation part of the covid-19. These two products can boost the immunity of healthy and sick patients, thereby giving a protection against covid-19. These products might be used by the world population in this pandemic situation. Further research needs to be conducted to evaluate the impact of this combination.
To Know More About Nutrition and Food Science International Journal
Please click on: https://juniperpublishers.com/nfsij/index.php
For more Open Access Journals in Juniper Publishers
please click on: https://juniperpublishers.com/index.php
#Food biotechnology#Mass spectrometry in food technology#Advanced food processing technologies#Food process engineering#Juniper Publishers#open acess journals
0 notes
Text
Origin of SARS-CoV-2: Two Schools of Thought

Origin of SARS-CoV-2: Two Schools of Thought in Biomedical Journal of Scientific & Technical Research
https://biomedres.us/fulltexts/BJSTR.MS.ID.005989.php
The causative pathogen, Severe Acute Respiratory Syndrome Coronavirus 2(SARS-CoV-2) also referred to as SC2 or H CoV-19 is the seventh coronavirus known to infect humans; SARS-CoV, MERSCoV and SARS-CoV-2 can cause severe disease, whereas HKU1, NL63, OC43 and 229E are associated with mild symptoms. In the COVID-19 pandemic, SARS-CoV-2 is the agent. The infectiousness (ability to infect), pathogenicity (ability to cause disease) and the number of virus particles needed to get infected determine the disease pattern. SARS-CoV-2 is a respiratory virus, which spreads mainly through droplets that enter the nose and mouth. It can bind itself to a receptor, typically in our lungs. Some new strains of the virus have also emerged over the last few months. Every individual is a host for SARS-CoV-2. Health condition, risk of exposure and medical history determine an individual’s response to the agent.
For more articles in Journals on Biomedical Sciences click here bjstr
Follow on Twitter : https://twitter.com/Biomedres01
Follow on Blogger : https://biomedres01.blogspot.com/
Like Our Pins On : https://www.pinterest.com/biomedres/
#journals on biomedical imaging#journals on emergency medicine#preventive medicine#journal of biomedical research and reviews impact factor#biomedical research articles
0 notes
Text
Immunopeptidome profiling of human coronavirus OC43-infected cells identifies CD4 T cell epitopes specific to seasonal coronaviruses or cross-reactive with SARS-CoV-2
Preliminary report; Seasonal "common-cold" human coronaviruses are widely spread throughout the world and are mainly associated with mild upper respiratory tract infections. The emergence of highly pathogenic coronaviruses MERS-CoV, SARS-CoV, and most recently SARS-CoV-2 has prompted increased attention to coronavirus biology and immunopathology, but identification and characterization of the T cell response to seasonal human coronaviruses remain largely uncharacterized. Here we report the repertoire of viral peptides that are naturally processed and presented upon infection of a model cell line with seasonal human coronavirus OC43. We identified MHC-I and MHC-II bound peptides derived from the viral spike, nucleocapsid, hemagglutinin-esterase, 3C-like proteinase, and envelope proteins. Only three MHC-I bound OC43-derived peptides were observed, possibly due to the potent MHC-I downregulation induced by OC43 infection. By contrast, 80 MHC-II bound peptides corresponding to 14 distinct OC43-derived epitopes were identified, including many at very high abundance within the overall MHC-II peptidome. These peptides elicited low-abundance recall T cell responses in most donors tested. In vitro assays confirmed that the peptides were recognized by CD4+ T cells and identified the presenting HLA alleles. T cell responses cross-reactive between OC43, SARS-CoV-2, and the other seasonal coronaviruses were confirmed in samples of peripheral blood and peptide-expanded T cell lines. Among the validated epitopes, S903-917 presented by DPA1*01:03/DPB1*04:01 and S1085-1099 presented by DRB1*15:01 shared substantial homology to other human coronaviruses, including SARS-CoV-2, and were targeted by cross-reactive CD4 T cells. N54-68 and HE128-142 presented by DRB1*15:01 and HE259-273 presented by DPA1*01:03/DPB1*04:01 are immunodominant epitopes with low coronavirus homology that are not cross-reactive with SARS-CoV-2. Overall, the set of naturally processed and presented OC43 epitopes comprise both OC43-specific and human coronavirus cross-reactive epitopes, which can be used to follow T cell cross-reactivity after infection or vaccination and could aid in the selection of epitopes for inclusion in pan-coronavirus vaccines. https://www.biorxiv.org/content/10.1101/2022.12.01.518643v1?rss=1%22&utm_source=dlvr.it&utm_medium=tumblr Read more ↓
0 notes
Text
Specific antibodies may be effective against multiple coronavirus types
Specific antibodies may be effective against multiple coronavirus types
Patients who have been exposed to a coronavirus may produce a versatile, cross-reactive coronavirus antibody; this may be useful for the eventual development of a broad-acting vaccine.
There are seven human coronavirus types, of which, four cause the common cold, named OC43, HKU1, 229E, and NL63. Most people become infected with at least one of these four coronaviruses at some point in their…

View On WordPress
0 notes
Text
Analysis of the seasonality of common human coronaviruses
Analysis of the seasonality of common human coronaviruses
Mild upper respiratory illness is known to be caused by the four common human coronaviruses (HCoVs), which include 2 beta (HCoV-HKU1 and HCoV-OC43) and 2 alpha (HCoV-NL63 and HCoV-229E) coronaviruses. These HCoVs, unlike betacoronaviruses (SARS-CoV, SARS-CoV-2, and Middle East respiratory syndrome coronavirus), have been observed to be endemic among humans, as indicated by their continuous and…

View On WordPress
0 notes
Text
Improve Immune System of Your Kids - Dr Ajay Prakaash, Pediatrician

Being a new dad means you have to adopt new ways of thinking and doing things. One aspect that comes with fatherhood is the immunisation of your young son or daughter. Learning how amazing our immune system is is one of the best ways of actually appreciating it. Read the advice of our tirunelveli pediatrician Dr Ajay Prakaash to boost your child's immunity.
Don't stress your child
Embrace the benefits of emotional stress. Emotional stress is a good thing, because it is the body's way of preparing for action. It can be exhausting, but it's also nice to have something to do.
Emotions are signals from the body that tell us what we need to do, and how we should do it. When we feel stressed and tired, our bodies know we need to rest. When we feel happy and energetic, our bodies know that we need to run around outside playing with friends or going on adventures in nature.
Encourage to eat healthy food
Children's diets should consist of a variety of foods from all food groups. This is because children develop their immune system during their first two years. Therefore, they need to be fed with healthy and nutritious food that is rich in vitamins, minerals and other nutrients.
The best way to feed your child is through breast milk or formula as these are the best alternatives for infants when it comes to feeding babies. However, if you are not able to do so or if you are breastfeeding your child, then read on some tips on how to feed your child a nutrient-rich diet:
1. Feed your child with breast milk or formula every day
2. Feed them fruits, vegetables and whole grains for breakfast
3. Make sure that you give them fruits for snacks and lunch time
4. Limit the intake of processed foods in their diet
Vaccines is important to Immunization
Immunization is important to protect against vaccine-preventable diseases. The immune system is a complex network of cells, proteins and chemicals that work together to protect us from germs. Vaccines are made from germs or weakened versions of germs to stimulate the creation of antibodies that fight off disease.
Immunizations help prevent illness, but they also build up resistance to infection because vaccines contain weakened versions of the diseases we want to avoid. For this reason, it’s important for everyone ages 6 months through 18 years old to get vaccinated against diphtheria, tetanus and pertussis (whooping cough); measles, mumps and rubella (MMR); hepatitis A and B; polio; rotavirus; varicella (chickenpox); seasonal influenza; human papillomavirus (HPV) types 6 and 11; invasive meningococcal disease; HPV types 16 and 18; human coronavirus OC43; yellow fever; Japanese encephalitis virus
Smoking is bad for kids health
Smoking around your child is bad for their health. It can cause problems in their lungs, heart, brain and more.
Children are more sensitive than adults to the effects of harmful substances such as second-hand smoke. This means their bodies react to smoking differently to an adult's body. A child's body has not yet developed resistance to the harmful effects of tobacco smoke and nicotine, so they are more likely to experience health problems after being exposed to someone who smokes regularly in the same way that an adult would.
Sleep Boosting the immune power
Sleep is important for the immune system so make sure your child gets enough rest.
A lack of sleep can impact your child's ability to fight off infections, says Lisa Shives, PhD, director of the Centre for Sleep and Circadian Neurobiology at Wake Forest Baptist Medical Centre in Winston-Salem, N.C. In fact, a study published in Science found that children who missed more than five hours of sleep per night were more likely to develop ear infection or sinusitis.
Sleep helps reset your body's internal clock, Dr. Shives says. "If you don't get enough sleep on a regular basis, it's going to affect how well you're able to regulate your body's circadian rhythm."
0 notes
Text
RBD and ACE2 Embedded Chitosan Nanoparticles as a Prevention Approach for SARS-COV 2

RBD and ACE2 Embedded Chitosan Nanoparticles as a
Prevention Approach for SARS-COV 2 in Biomedical Journal of Scientific & Technical Research
https://biomedres.us/fulltexts/BJSTR.MS.ID.005960.php
A new type of coronavirus-associated persistent pneumonia outbreak called SARS-CoV-2, which causes severe acute respiratory syndrome, was reported in Wuhan, China in Hubei Province in December 2019. In the following weeks, infections spread rapidly to China and other countries around the world [1]. Coronaviruses (CoVs) are known to cause enteric and respiratory diseases in animals and humans, which are positive stranded RNA viruses that are not segmented into large envelopes. Most human CoVs such as hCoV - 229E, OC43, NL63 and HKU1 cause mild respiratory diseases, but two previously unknown CoVs, severe acute respiratory syndrome CoV (SARS - CoV) and Middle East respiratory syndrome CoV (MERS - The worldwide spread of CoV) has drawn global attention to the deadly potential of human CoVs. Genomic analysis shows that SARS - CoV - 2 belongs to the same betacoronavirus family as MERS - CoV and SARS - CoV and shares a sequence that shows a high homology with SARS - CoV. A cellular receptor angiotensin-converting enzyme 2 (ACE2) is mainly mediated by the introduction of SARS - CoV into human host cells. It is expressed in the human respiratory epithelium, lung parenchyma, vascular endothelium, kidney cells and small intestine cells [2].
For more articles in Journals on Biomedical Sciences click here bjstr
Follow on Twitter : https://twitter.com/Biomedres01
Follow on Blogger : https://biomedres01.blogspot.com/
Like Our Pins On : https://www.pinterest.com/biomedres/
#medical and medicinal journal#journals on cancer medicine#Journals on Biomedical Intervention#journal of biomedical sciences research review#biomedical open access journals
0 notes
Text
SARS-CoV-2 mRNA vaccination elicits broad and potent Fc effector functions to VOCs in vulnerable populations
Preliminary report; SARS-CoV-2 variants have continuously emerged even as highly effective vaccines have been widely deployed. Reduced neutralization observed against variants of concern (VOC) raises the question as to whether other antiviral antibody activities are similarly compromised, or if they might compensate for lost neutralization activity. In this study, the breadth and potency of antibody recognition and effector function was surveyed in both healthy individuals as well as immunologically vulnerable subjects following either natural infection or receipt of an mRNA vaccine. Considering pregnant women as a model cohort with higher risk of severe illness and death, we observed similar binding and functional breadth for healthy and immunologically vulnerable populations. In contrast, considerably greater functional antibody breadth and potency across VOC was associated with vaccination than prior infection. However, greater antibody functional activity targeting the endemic coronavirus OC43 was noted among convalescent individuals, illustrating a dichotomy in recognition between close and distant human coronavirus strains that was associated with exposure history. Probing the full-length spike and receptor binding domain (RBD) revealed that antibody-mediated Fc effector functions were better maintained against full-length spike as compared to RBD. This analysis of antibody functions in healthy and vulnerable populations across a panel of SARS-CoV-2 VOC and extending through endemic alphacoronavirus strains suggests the differential potential for antibody effector functions to contribute to protecting vaccinated and convalescent subjects as the pandemic progresses and novel variants continue to evolve. https://www.medrxiv.org/content/10.1101/2022.09.15.22280000v1?rss=1%22&utm_source=dlvr.it&utm_medium=tumblr Read more ↓
0 notes
Text
Disparities Seen in COVID-19–Related Avoidance of Care
Disparities Seen in COVID-19–Related Avoidance of Care
[ad_1]
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
In the early weeks and months of the COVID-19 pandemic, many people were trying to avoid the coronavirus by staying away from emergency rooms and medical offices. But how many people is “many”?

Turns out almost 41% of Americans delayed or avoided some form of medical care…
View On WordPress
#2019 Novel Coronavirus#2019-nCoV#Avoidance#Care#caregiver#Coronavirus#COVID19Related#Disparities#HCoV-229E#HCoV-OC43#health insurance#Human coronavirus 229E#Human coronavirus HKU1#Human coronavirus OC43#Wuhan coronavirus
0 notes
Text
The virucidal effects of tea tree oil against FCoVII and HCoV-OC43
The virucidal effects of tea tree oil against FCoVII and HCoV-OC43
In a recent study published in Molecules, researchers assessed the probable efficacy of tea tree oil (TTO) as a natural disinfectant against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) using the human coronavirus OC43 (HCoV-OC43) and feline coronavirus (FCoVII) as surrogate models.
Study: Potential Use of Tea Tree Oil as a Disinfectant Agent against Coronaviruses: A Combined…

View On WordPress
0 notes
Text
In May 1889, people living in Bukhara, a city that was then part of the Russian Empire, began sickening and dying. The respiratory virus that killed them became known as the Russian flu. It swept the world, overwhelming hospitals and killing the old with special ferocity.
Schools and factories were forced to close because so many students and workers were sick. Some of the infected described an odd symptom: a loss of smell and taste. And some of those who recovered reported a lingering exhaustion.
The Russian flu finally ended a few years later, after at least three waves of infection.Its patterns of infection and symptoms have led some virologists and historians of medicine to now wonder: Might the Russian flu actually have been a pandemic driven by a coronavirus? And could its course give us clues about how our pandemic will play out and wind down?
If a coronavirus caused the Russian flu, some believe that pathogen may still be around, its descendants circulating worldwide as one of the four coronaviruses that cause the common cold. If so, it would be different from flu pandemics whose viruses stick around for a while only to be replaced by new variants years later that cause a new pandemic.
If that is what happened to the Russian flu, it might bode well for the future. But there is another scenario. If today’s coronavirus behaves more like the flu, immunity against respiratory viruses is fleeting. That might mean a future of yearly Covid shots.
But, some historians voice caution about the Russian flu hypothesis.
“There is very little, almost no hard data” on the Russia flu pandemic, said Frank Snowden at Yale.
There is, though, a way to solve the mystery of the Russian flu. Molecular biologists now have the tools to pull shards of old virus from preserved lung tissue from Russian flu victims and figure out what sort of virus it was.
Some researchers are now on the hunt for such preserved tissue in museums and medical schools that might have old jars of specimens floating in preservative fluid that still contain fragments of lung.
The Russian Flu
Tom Ewing of Virginia Tech, one of the few historians who has studied the Russian flu, can’t help noticing striking parallels with today’s coronavirus pandemic: Institutions and workplaces shut down because too many people were ill; physicians overwhelmed with patients; and waves of infection.
“I would say, maybe,” Dr. Ewing said when asked if the Russian flu was a coronavirus.Dr. Scott Podolsky, a professor of global health and social medicine at Harvard Medical School, called the idea “plausible.”And Dr. Arnold Monto, professor of public health, epidemiology and global health at the University of Michigan, considered it “a very interesting speculation.”
“We have long wondered where coronaviruses came from,” Dr. Monto said. “Has there ever been a coronavirus pandemic in the past?”
Harald Bruessow, a retired Swiss microbiologist and editor of the journal Microbial Biotechnology, points to a paper published in 2005 concluding that another coronavirus circulating today, known as OC43, which causes severe colds, may have jumped from cows to humans in 1890.
Three other less virulent coronaviruses circulate, too. Perhaps one of those viruses, or OC43, is a variant left over from the Russian flu pandemic.
Dr. Bruessow, while acknowledging the uncertainties, would bet that the Russian flu was caused by a coronavirus. His work, which involved delving into old newspaper and journal articles, and public health reports on the Russian flu, uncovered that some patients had complained about conditions like a loss of taste and smell and long Covid-like symptoms.
Some historians speculated that the 19th century’s fin de siècle might actually have been lassitude caused by sequelae of the Russian flu.
Such symptoms are not typical of flu pandemics.Like Covid, Dr. Bruessow reports, the Russian flu seems to have preferentially killed older people but not children. Dr. Ewing, examining 1890 records from the State Board of Health in Connecticut, found a similar pattern. If true, that would make the 1890 virus unlike influenza viruses which kill the very young as well as the very old.But historical records cannot readily answer the question of whether a coronavirus caused the Russian flu.And Dr. Snowden of Yale cautioned that any lessons he could draw from that pandemic that could apply to a world in which the novel coronavirus has shaken societies would be “fantasy.”
At this point, the idea that the Russian flu might have been caused by a coronavirus remains speculative, said Peter Palese, a flu researcher and professor of medicine at the Icahn School of Medicine at Mount Sinai in New York. There is nothing, he said, that clearly ties the Russian flu pandemic to a coronavirus and excludes influenza.
But for those seeking hints to how the current coronavirus pandemic might end, some think those past two pandemics could offer a clue.
As the Russian flu pandemic waned, said J. Alexander Navarro, a historian at the University of Michigan, “people rather quickly went on with their lives.” It was the same with the 1918 flu pandemic. Newspaper stories about it dwindled. And, he said, “grieving was almost entirely a private affair.”
“I highly suspect that the same will occur today,” Dr. Navarro said.
“In fact, in many ways, I think it already has.”
When Pandemics Burn Out
Quite a few pandemics — at least in the past 100 years when their causes can be known — have been caused by respiratory viruses. Recent exceptions are Zika and chikungunya — old mosquito-borne viruses — and H.I.V., which is spread by sexual intercourse and sharing needles.
Great plagues terrorized humanity in ancient and pre-modern times, most notably the bubonic plague. It was mostly spread by rat fleas, and it ushered in a horrendous period, killing multitudes among the European population from 1347 to 1352. So many died that they were buried in pits, in piles.
The bubonic plague kept returning to Europe for centuries after it first emerged. But how that plague ended offers few relevant lessons for today’s pandemic.
Researchers have also been unable to find answers in animal studies. They have tried for decades to find general laws to predict how pandemics progress by infecting hundreds of thousands of mice with various viruses and bacteria, said Dr. George Davey Smith, professor of clinical epidemiology at the Bristol Medical School in England. The experiments went on year after year in England, Germany, the United States and Australia. All looked for ways to predict when and how an epidemic could end.
None were found.
“They couldn’t predict what was going to happen,” Dr. Davey Smith said.
So researchers trying to understand how respiratory pandemics conclude can only study the flu and the current coronavirus pandemic.
Only the flu pandemics have ended. That, said Dr. David Morens, a flu researcher and senior adviser to the director of the National Institute of Allergy and Infectious Diseases, is a real limitation in trying to understand the natural history of respiratory disease pandemics.
“We have only 104 years and four different pandemics to make predictions from,” he said.
Flu pandemics are also baffling.The first of the four flu pandemics for which the virus is known began in 1918. The pandemic waned after three waves of infections and that virus, H1N1, remained in circulation, in a less virulent form until 1957, when it disappeared.
“As far as we could tell, in 1957, that virus was gone forever,” Dr. Morens said.
Then H2N2 emerged. It was substantially different from H1N1 and caused a pandemic. That pattern repeated itself with H3N2 emerging in 1968.
But in 1977, something strange happened. H1N1 came back after being gone for two decades. It and another virus, H3N2, have been circulating ever since.
“Until 1977, we never had two subtypes circulating at the same time,” Dr. Monto said. “We don’t understand why one subtype pushed out the other and why it didn’t happen in 1977.”
And in 2009, the H1N1 that had re-entered the human population in 1977 was displaced by a genetically distinct version that came from pigs, causing another pandemic.
But why would a new variant make the previous one go away?
That, Dr. Morens said, “is another mystery.”
At least there are vaccines which are useful against the flu. But they have to be administered every year because of waning immunity. In a study in England with common cold coronaviruses, researchers found that immunity from infections with these viruses also diminishes within a year.
“Would we need a Covid vaccine every year?” asked Dr. Jeffery Taubenberger, chief of the viral pathogenesis and evolution section at the National Institute of Allergy and Infectious Diseases. “That’s the direction we’re heading.”
Then there is the question of why the Russian flu, and now the Covid pandemic produced waves of escalating and declining mortality.“We are pretty clueless, and this extends to the waves we are seeing over the past two years with Covid,”
Dr. Morens said. The evolution of viruses is not the full answer, he added.
“There are no good explanations I know of.”
Hunting Russian Flu Samples
The mysteries about the evolution of flu viruses and flu pandemics lead back to the mystery of the Russian flu and the coronavirus hypothesis.
Some, like Dr. Navarro, the historian at the University of Michigan, said that he finds the evidence for the “interesting hypothesis” about the Russian flu “circumstantial at best.”
Dr. Taubenberger predicts better evidence will emerge. He and John Oxford, emeritus professor of virology at the University of London, have been looking for flu or coronavirus in old lung tissue from patients who were ill with a respiratory disease in the years before the 1918 flu. They had hoped to find them embedded in tiny blocks of paraffin no bigger than a pinky fingernail in the Royal London Hospital, a place that has tissue from patients dating back to around 1906.
“We sampled hundreds of tissues,” Dr. Taubenberger said, without finding viruses. “We continue to look,” he said.But, he said, with renewed interest in the 1890 pandemic, he hopes some tissues containing the Russian flu virus — whatever it is — might be found, perhaps lying unnoticed in the basements of museums or medical schools in different corners of the world.Finding the tissue, though, has been challenging.
“The people running institutions in which they might be housed very likely would have no way to easily access records about them,” Dr. Taubenberger said.
“Paradoxically, genetic analysis of these samples would be less difficult than locating them in the first place.”
Dr. Podolsky of Harvard and Dominic W. Hall, the curator of the Warren Anatomical Museum at Harvard, are also looking for tissue archives that might have lung tissue from that era. Mr. Hall has been reaching out to those in charge of collections of tissue samples.
On Thursday, he spoke with Anna Dhody, director of the research institute at the Mütter Museum, a collection of anatomical specimens and items from medical history in Philadelphia. She thinks items in the museum’s climate-controlled storage room may help.
The archive contains jars of tissue from the late 19th century, including a few whole lungs, all floating in jars of pale yellow liquid, the alcohol that was used as a preservative.
With funding and the right technology, she says outside researchers may be able to analyze the specimens.The work, Ms. Dhody said, “is so imperative.”
“It’s life and death information.”
3 notes
·
View notes
Photo


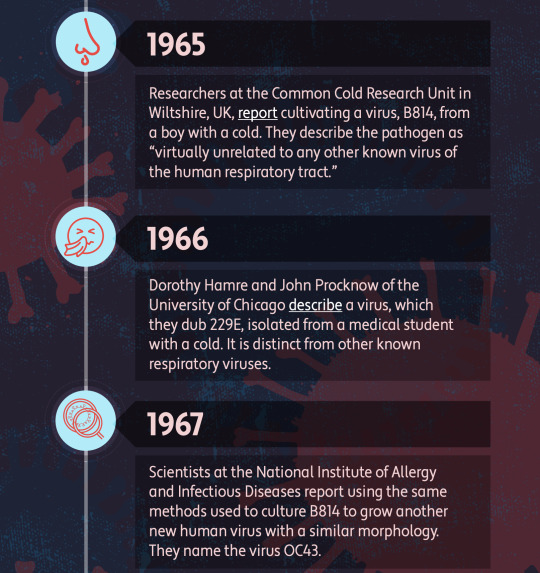



A Brief History of Human Coronaviruses
By Shawna Williams (The-Scientist).
Top Image: MERS virus particles on the surface of an infected cell. FLICKR.COM, NIAID. Infographic by The-Scientist.
Milder, cold-causing members of this pathogenic viral family long remained under the radar, although they aren’t entirely harmless.
On January 9 of this year, Chinese state media reported that a team of researchers led by Xu Jianguo had identified the pathogen behind a mysterious outbreak of pneumonia in Wuhan as a novel coronavirus. Although the virus was soon after named 2019-nCoV, and then renamed SARS-CoV-2, it remains commonly known simply as the coronavirus. While that moniker has been catapulted into the stratosphere of public attention, it’s somewhat misleading: Not only is it one of many coronaviruses out there, but you’ve almost certainly been infected with members of the family long before SARS-CoV-2’s emergence in late 2019.
Coronaviruses take their name from the distinctive spikes with rounded tips that decorate their surface, which reminded virologists of the appearance of the sun’s atmosphere, known as its corona. Various coronaviruses infect numerous species, but the first human coronaviruses weren’t discovered until the mid-1960s. “That was sort of the golden days, if you will, of virology, because at that time the technology became available to grow viruses in the laboratory, and to study viruses in the laboratory,” says University of Texas Southwestern Medical Center pediatrician Jeffrey Kahn, who studies respiratory viruses. But the two coronaviruses that were identified at the time, OC43 and 229E, didn’t elicit much research interest, says Kahn, who wrote a review on coronaviruses a few years after the SARS outbreak of 2003. “I don't believe there was a big effort to make vaccines against these because these were thought to be more of a nuisance than anything else.”
The viruses cause typical cold symptoms such as a sore throat, cough, and stuffy nose, and they seemed to be very common; one early study estimated that 3 percent of respiratory illnesses in a children’s home in Georgia over seven years in the 1960s had been caused by OC43, and a 1986 study of children and adults in northern Italy found that it was rare to come across a subject who did not have antibodies to that virus (an indicator of past infection).
Coronaviruses’ mild-mannered reputation changed with the SARS outbreak. Although related to OC43 and 229E, SARS-CoV was far deadlier, killing about 10 percent of people it infected—a total of 774 worldwide, according to the United Kingdom’s National Health Service. While it’s still unclear exactly where SARS-CoV came from, similar viruses were later found in bats, and some studies suggested the virus could have jumped to humans via an intermediary such as civets.
While SARS was a wake-up call that coronaviruses posed a greater risk to humans than had been thought, it’s unsurprising that a virus that’s newly spilled over to humans would be more deadly than its human-adapted cousins, says Rachel Roper, a virologist and immunologist at East Carolina University. “When a species is co-evolving with its pathogen . . . they tend to come to a detente, where the virus will be surviving in the population, and the population is surviving without that much illness,” she explains. “But when a virus jumps species, then you’ve got a real problem.”
In the wake of the SARS epidemic, some researchers went looking for other human coronaviruses—and found them. In 2004, researchers in the Netherlands reported they’d found a novel coronavirus, related to 229E, in a child with pneumonia and in four other people with respiratory disease. That virus came to be known as NL63, and Kahn and his colleagues found it at around the same time in hospitalized children in New Haven, Connecticut (Kahn worked at Yale University School of Medicine at the time).
Although first identified in people with severe infections, NL63 is, much like 229E and OC43, a widespread virus that causes colds in most people it infects. But there were hints that its effects could extend beyond the respiratory tract. In their 2005 paper on the newly-discovered virus, Kahn and his colleagues reported finding it in 8 of 11 patients they tested who had Kawasaki disease, an inflammation of the blood vessels that occurs primarily in young children. But most follow-up studies failed to find such an association.
“It could be that it was just chance,” Kahn says of his finding, or “there may be a signal there.” His 15-year-old study has acquired new salience in light of reports that COVID-19 is associated with Kawasaki-like symptoms in some children, but he cautions that this inflammatory syndrome has been defined slightly differently by different groups reporting on it, and isn’t identical to Kawasaki disease.
Close on the heels of NL63’s discovery came a report from Hong Kong on yet another common, cold-causing coronavirus, this one called HKU1. As with NL63, researchers in other parts of the world went looking for HKU1, finding it in Australia, France, and the United States, among other places.
The cold-causing coronaviruses each carry a similar array of symptoms, says Kahn, and it’s not clear how long any of them have been hosted by humans, or where they came from originally. As ubiquitous as they are, other basic questions about these viruses also remain open, such as whether infection with one of them confers lasting immunity. Studies reported in two recent preprints found that it’s not uncommon for a person to be infected with the same coronavirus twice. But Roper suspects that our bodies do learn to fend off coronaviruses they’ve encountered. “There are literally thousands of respiratory viruses, so probably every time you get a cold, you’re getting a new virus that you’ve never seen before,” she says. “Plus, they’re always mutating.”
Until the emergence of SARS-CoV-2, human coronaviruses that have made the news have been both far more deadly and far more contained than their cold-causing counterparts. No cases of SARS have been detected since 2004, and Middle East respiratory syndrome coronavirus (MERS-CoV), first found in a patient in Saudi Arabia in 2012, has caused fewer than 2,500 confirmed cases worldwide, according to the World Health Organization—but it kills about 35 percent of people with confirmed diagnoses. SARS-CoV-2, which, as its name suggests, is closely related to SARS-CoV, appears to be far more transmissible, and its mortality rate has so far proven difficult to nail down.
104 notes
·
View notes
Link
Neutralizing Dr. Bhakdi's attempt at rebuttal
Originale message: https://www.geertvandenbossche.org/post/response-to-dr-bhakdi
Dr. Bhakdi's response (marked in grey below): https://doctors4covidethics.org/rebuttal-to-geert-vanden-bossches-response-to-dr-bhakdi/
Rebuttal to Geert vanden Bossche’s “Response to Dr. Bhakdi”
Michael Palmer, MD and Sucharit Bhakdi, MD
doctors4covidethics.org
Some time ago, Dr. Bhakdi published a video presentation [1] which explained that cross-immunity to SARS-CoV-2 is widespread in the population, and that general vaccination is therefore not necessary or appropriate. This was disputed by Dr. Geert vanden Bossche [2]. Here, we rebut Dr. vanden Bossche’s assertions.
In the following, quotes from van den Bossche’s piece are typeset in italics, and our replies are printed in upright font shape.
I have inserted my comments underneath those of these medical doctors. As I am not sure about the role of Dr. Palmer here, I’ve addressed my comments to Dr. Bhakdi only. I have no interest in criticizing people who, like me and others, argue against mass vaccination. It is important, however, that we use the correct scientific justification as anything else will soon or later backfire and be used against us to invalidate our opposition against mass vaccination campaigns. That’s the only reason why I am spending time on countering the alleged rebuttal of these doctors. I’ll start with my summary:
I maintain that Bhakdi’s interpretation of protective population-level immunity is not built on sound scientific arguments. There is no evidence whatsoever that herd immunity, or what he now calls: ‘widespread protective immunity’, relies on cross-protective memory cells elicited by heterologous Coronavirus (CoV) strains (e.g., common cold CoVs). This probably explains why he’s continuously evading my critical comments on his false assumption that cross-immunity equals cross-protection and why his statements attempting to rebut my comments related to this issue are not backed by any relevant reference from the literature. Indeed, none of the allegedly protective mechanisms he’s alluding to are supported by any kind of evidence of acquired, cross-protective effector memory cells. As previously mentioned, if cross-immunity among different CoVs would be protective, we would not be witnessing fulminant outbreaks of the delta variant in several countries. Bhakdi is simplifying the science down to the level of phantasy. He prefers to ignore some basic immunologic science in an attempt to simplify things and construe interpretations that he can understand himself without having to tap into the different mechanisms that underlie the population’s immune response to the virus. Most strikingly, Bhakdi completely ignores the broadly and universally protective capacity of innate B1 cell-derived oligospecific antibodies (Abs). I can only recommend him to do (a lot!) of literature on these innate, polyreactive Abs. I offer him to start his education on my website where I’ve gathered a number of relevant references from the literature on this very topic.
1. Cross-immunity to SARS-CoV-2 exists and is cross-protective
Dr. Bhakdi is confusing all along cross-reactivity (which basically means that antibodies (Abs) or T cells induced by one CoV can BIND similar [conserved] epitopes on some other CoVs) and cross-protection. None of the publications Dr. Bhakdi is referring to has analyzed or claims cross-PROTECTION elicited by other CoVs.
Cross-protection is supported by the available evidence:
A correlation between cross-protection and cross-reactive antibodies has been explicitly confirmed in several other studies [3–6].
It probably suffices to say that correlations are typically not implying a causal relationship
COVID-19 is more severe in younger children than in older children and adolescents [7]. This is consistent with greater protection of the latter by cross-immunity conferred by past infection with other coronaviruses.
Indeed, exceptional genetic deficiencies in innate immunity typically become clinically manifest in young children. As such deficiencies become manifest at an early stage in life, older children and adolescents who stayed healthy at this stage continue to be well protected by their innate Abs. This has nothing to do with cross-immunity, let alone with cross-protection.
The low disease severity and very low case fatality rates of COVID in the general population further support protection by cross-immunity.
When you take the population segment that is typically featured by poor functionality of their innate Abs (elderly, people with certain co-morbidities), disease severity and case fatality rates are relatively high whereas the opposite applies to the bulk of the population consisting of healthy people and younger age groups (< 65 years). Again, this observation has nothing to do with cross-immunity, let alone with cross-protection.
2. Neutralizing activity of SARS-CoV-2 antibodies
Again, Abs that bind to Sars-CoV-2 do not necessarily neutralize the virus and prevent it from entering the cell.
Apart from the fact that Dr. vanden Bossche erringly equates virus neutralization in laboratory experiments with protection from infection,
On the contrary (!): I am pointing out that cross-reactivity of Abs doesn’t even mean that these Abs can prevent viral entry, let alone they would provide protection!
his above statement applies to all viruses. Each natural virus infection will produce a multitude of clonal antibodies (“idiotypes”). At least some idiotypes will be non-neutralizing; however, other idiotypes typically will neutralize. Moreover, this is true regardless of whether these antibodies are cross-reactive or not. The argument is irrelevant.
Bhakdi doesn’t seem to understand that I am alluding to the affinity of Abs. Cross-reactivity to heterologous antigen (Ag) is based on low(er) affinity interactions of Abs towards immunodominant epitopes. He seems to be considering a natural Ab response a mixture of monoclonal Abs, some of which are neutralizing and some others that are non-neutralizing. This is, of course, an erroneous interpretation as the neutralizing effect of a humoral/ Ab response is determined by the OVERALL effect of the combination of ALL Abs together. Clearly, in case of variants, the overall effect resulting from all Ag-Ab interactions will be dominated by Abs that recognize immunodominant domains. It’s precisely because (CoV) variants, and even more so heterologous (CoV) strains, exhibit changes in immunodominant domains that they become less susceptible, or even not susceptible at all, to cross-reactive Ab immunity!
3. Not all respiratory coronavirus induce cross-reactive antibodies effectively
In addition, Sars-CoV-2-induced Abs were only found to cross-react with 2 out of the 4 common cold CoVs (i.e., only for beta coronavirus HKU1 and OC43) and the cross-reactivity was ‘much lower than that observed for the remaining CoV epitopes.’
This is correct. However, whether it comes from some or from all coronaviruses is immaterial to the existence of cross-immunity as such. The study by Nielsen et al. [8] unambiguously demonstrated cross-immunity in almost all test persons who had been infected with SARS-CoV-2.
How can Bhakdi be so sure that the majority of the population has been exposed to the ‘right’ coronaviruses (i.e., beta coronavirus HKU1 and OC43) rather than to the other common cold viruses? But regardless, researchers have only been demonstrating cross-reactivity of anti-CoV Abs without proving relevant functionality of these Abs. Once again, we’re back to the argument that cross-reactivity doesn’t equal cross-protection!
4. Those ghastly variants
Furthermore, his statement that people who have built immunity against CoV are automatically protected against all Sars-CoV-2 variants is not true either for exactly the same reasons (i.e., Abs that bind to variants do not necessarily neutralize them and could even be at risk of causing Ab-dependent enhancement of disease; ADE).
Dr. vanden Bossche contends that immunity to CoV rests on the presence of neutralizing antibodies that will fail their duty when variants arise. This assumption lacks a scientific basis. It is common knowledge that protection against severe respiratory viral disease derives primarily from cellular immunity.
Where is the evidence for the alleged ‘common knowledge’? And again: where is the immunology? There is no evidence whatsoever that naturally induced, cross-reactive T memory cells are broadly and universally capable of eliminating CoV-infected cells! They can, therefore, not provide population-level protection as Bhakdi tries to make people believe.
Reactive lymphocytes limit viral multiplication at sites of infection.
Limitation of viral multiplication is not sufficient to protect from disease, let alone infection. It could at most mitigate disease, but even bystander T cells could do so! (this is why studies have shown that even immunization with BCG can have a beneficial effect). This is thought to be due to Tc-mediated secretion of immune inflammatory molecules (e.g., cytokines). There is increasing evidence, though, that Sars-CoV-2 is now also developing resistance against a number of these innate immune mediators (see articles on my website).
Antibodies merely play supportive roles in preventing viral spread in the bloodstream.
Where is the evidence for this statement? Is Bhakdi serious in stating that Abs (in general!) have no role to play in fighting the infection at the portal of entry or respiratory tract?
“Variants” (mutant strains) will arise due to “antigenic drift” with any RNA virus that propagates within the human population, and SARS-CoV-2 is no exception. Antigenic drift occurs in minuscule steps and, as is well known, the genetic and antigenic differences between the original (Wuhan) strain of SARS-CoV-2 and any of its variants are vanishingly small relative to the difference between any SARS-CoV-2 strain and the respiratory coronavirus that confer cross-immunity. That such minuscule changes should substantially affect immunity and even promote ADE is not credible.
It seems like Bhakdi missed my point as I stated that Abs that bind with low affinity to Sars-CoV-2 (e.g., such induced by his cross-reactive CoVs) might be suspicious of inducing ADE, precisely because of their weak binding.
Cross-reactive T-cell immunity, which is a pillar of immunological competence against respiratory viruses, has been documented with respect to SARS-CoV-2 since 2020 [9,10]. Antibody response and T cell response are correlated; thus, fixating on the properties of antibody idiotypes only when trying to gauge overall immunity misses the mark.
The mark Bhakdi is continuously missing is that he doesn’t understand that even cross-reactive T cell immunity does not imply cross-PROTECTION!
5. Are the asymptomatically infected doomed?
Vanden Bossche insists that the study by Nielsen et al. [8] “does not truly provide information on asymptomatically infected individuals” but that the people referred to in the study must instead have had mild symptoms, and further that “asymptomatically infected individuals do not develop long-lived or mature anti-S [anti-spike] Abs and have not been reported to develop memory B cells or CYTOTOXIC memory T cells. Consequently, previously asymptomatically infected people (i.e., the majority of the population) cannot rely on ACQUIRED immunity for protection against infection or disease.”
Unlike vanden Bossche, we are not concerned about the difference between mild symptoms and none at all; quite obviously, both groups of patients were protected from severe disease. More interesting than this quibble is his parenthetical assertion that the majority of the population has already been “asymptomatically infected” with COVID.
This is anything but quibble. Qualifying this as ‘quibble’ merely illustrates that Bhakdi doesn’t understand the immune pathogenesis of Sars-CoV-2 infection. From an immunological viewpoint, asymptomatic infection and the immune effector cells involved are completely different from those coming to play in case of symptomatic infection. The immunology here is very different but Bhakdi doesn’t care as he’s exclusively focused on the common clinical denominator, i.e., protection against severe disease. So, my previous comment related to understanding the immunology that underlies asymptomatic infection, not to gauging clinical symptoms.
He further assumes that such individuals do not develop any immunological memory. Several questions then arise:
1. How was it established that the majority of the population had already experienced asymptomatic infection? Positive PCR tests alone cannot be accepted as proof of this assertion.
Does Bhakdi really think that after the virus circulated in the population for over 1.5 years (including several highly infectious variants), the majority of people who didn’t develop symptoms simply didn’t get exposed to the virus yet?
2. If any individuals were indeed asymptomatically infected, then what, if not cross-immunity, prevented their infection from becoming clinically manifest?
My answer: Innate, polyreactive Abs capable of recognizing CoV-derived patterns that are shared among all CoVs (presumably even including some other viruses). See reference from the lit. on my website.
3. If these individuals were protected from manifest disease during their first infection, why should we assume that they will fare any worse when infected again? We hope that vanden Bossche will resist the temptation to trot out the fearsome Delta variant in response.
That is exactly the question Bhakdi ought to be asking himself. If folks got acquired immunity thanks to previous exposure to heterologous CoVs, why would they all of a sudden become sick whereas they were well protected during previous waves of this pandemic? He doesn’t seem to have the answer. I have explained on multiple occasions how high infectious pressure can temporarily suppress innate immune defense in previously asymptomatically protected people. My website is full of explanations on how this works and how this renders previously protected people (e.g., younger age groups) susceptible to C-19 disease. More can be found in the articles I published on TrialSiteNews. Sometimes it’s good to read what other people write before attempting to rebut their arguments.
6. Of people and of test tubes
In the Danish study [Nielsen et al.] at least nine individuals were unable to fully neutralize viral infection
Neutralization is a function of antibodies or serum samples, not of individuals.
Well, if one considers that full-fledged neutralizing Abs were not found in a number of individuals and if those happened to be the people who were truly asymptomatic, then this becomes highly relevant in that it would clearly prove that protection in asymptomatically infected people does not rely on neutralizing Abs
Clinical immunity may exist even without neutralizing antibodies.
Precisely! The bulk of clinical immunity is provided by innate Abs.
Moreover, neutralization was determined using a recombinant pseudo-virus that expressed the SARS-CoV-2 spike protein, but none of the other SARS-CoV-2 proteins. It cannot simply be assumed that none of these could contribute to neutralization.
Where is Bhakdi’s evidence supporting that Abs to proteins other than spike protein have virus-neutralizing capacity?
In addition, it is stated in the discussion [again Nielsen et al.] that ‘some rare individuals have no detectable immunological memory to Sars-CoV-2.’
These “rare individuals” were most likely those with low cross-immunity and false-positive PCR tests. The fact that a lack of immunological memory is rare supports rather than contradicts the prevalence of cross-immunity.
How would that work? It is much more plausible that the lack of immunological memory pertains to those who were truly asymptomatically infected as there is no evidence whatsoever that asymptomatic infection induces B cell memory.
To my knowledge, there is no evidence that Sars-CoV-2-induced CD8+ T cells provide cross-protection from common cold viruses or vice versa and this wasn’t even part of this investigation.
Cross-protection from common cold viruses by immunity to SARS-CoV-2 is not the point. We are only discussing cross-immunity in the opposite direction, which has indeed been detected [9,10] and most likely contributes to the clinically manifest cross-protection.
Bhakdi obviously missed the words ‘or vice versa’. Again: cross-immunity does not mean cross-protection!
7. On herd immunity
So, with all my respect for Dr. Bhakdi, the conclusion that herd immunity would already be established and would simply need to be recalled upon exposure to SARS-CoV-2 is not correct. By the way, if this were true, we would not currently be witnessing a fulminant propagation of the delta variant in several countries.
Herd immunity in the strict sense—namely, a high prevalence of sterilizing immunity that prevents a virus from effectively propagating within the population—is problematic with any respiratory virus.
Bhakdi should revisit the definition of herd immunity as herd immunity does not relate to a high prevalence of ‘sterilizing’ immunity. Herd immunity primarily relates to the immune capacity of the population to dramatically reduce viral transmission.
Dr. Bhakdi was using the term loosely, referring widespread immunity that is sufficient to protect from severe disease.
Herd immunity has NOTHING to do with protection from disease. I am not even arguing about Bhakdi’s definition of herd immunity versus “widespread protective immunity” (WPI) but rather about his false hypothesis that this WPI would rely on previous exposure to other CoVs. That is completely wrong, at least as far as durable protection is concerned!
The pervasiveness of such immunological protection is indeed apparent from the very low rates of fatality or severe disease in the general population, and particularly among those without co-morbidities. However, we agree with vanden Bossche that it would have been better to use the term “widespread protective immunity” rather than “herd immunity” in this case.
8. Summary
With the exception of the above single point of terminology, we see no merit in Dr. vanden Bossche’s response. We maintain that Dr. Bhakdi’s interpretation of the studies he highlighted in his video presentation are fully supported by the available scientific literature.
See my summary at the top
References
1. Bhakdi, S. (2021) Proof that puts an end to the SARS-CoV-2 narrative.
2. Vanden Bossche, G. (2021) Response to Dr. Bhakdi.
3. Dugas, M. et al. (2021) Less severe course of COVID-19 is associated with elevated levels of antibodies against seasonal human coronaviruses OC43 and HKU1 (HCoV OC43, HCoV HKU1). Int J Infect Dis 105:304-306
4. Dugas, M. et al. (2021) Lack of antibodies against seasonal coronavirus OC43 nucleocapsid protein identifies patients at risk of critical COVID-19. J Clin Virol 139:104847
5. Yamaguchi, T. et al. (2021) Immunity against seasonal human coronavirus OC43 mitigates fatal deterioration of COVID-19. Int J Infect Dis (preprint)
6. Yaqinuddin, A. (2020) Cross-immunity between respiratory coronaviruses may limit COVID-19 fatalities. Med. Hypotheses 144:110049
7. Tsabouri, S. et al. (2021) Risk Factors for Severity in Children with Coronavirus Disease 2019: A Comprehensive Literature Review. Pediatric clinics of North America 68:321-338
8. Nielsen, S.S. et al. (2021) SARS-CoV-2 elicits robust adaptive immune responses regardless of disease severity. EBioMedicine 68:103410
9. Le Bert, N. et al. (2020) SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 584:457-462
10. Grifoni, A. et al. (2020) Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 181:1489-1501.e15
#Geert Vanden Bossche#Sucharit Bhakdi#virology#viral escape#herd immunity#immunology#fav#family medicine#debate#selection pressure#Natural antibodies#sars-cov-2#covid-19#covid-19 vaccine
3 notes
·
View notes
Photo
Know Your Coronaviruses
March 30, 2020 — While you probably hadn’t heard too much about them before the current pandemic, Coronaviruses are actually quite common — chances are you’ve actually been infected by one. Coronaviruses, aptly named because of the crown-like spikes that stick out of their surface, are a large family of viruses with several members that can infect humans and a variety of animal species. These viruses use RNA as their genetic material, which is similar to the DNA we and other organisms use to store the recipe of who we are.
There are four different groups in this family of viruses: alpha, beta, delta and gamma. So far, only alpha and beta Coronaviruses are known to infect humans. The most common human Coronaviruses are called 229E (alpha), NL63 (alpha), OC43 (beta), and HKU1 (beta). Many of us have been infected with these before and they usually cause symptoms very similar to the common cold and are typically pretty easy to recover from. For decades, these were the only types of infections we thought Coronaviruses could cause.
However, in 2003 with the Severe Acute Respiratory Syndrome (SARS or SARS-CoV) outbreak, we learned that this family is actually capable of much more. SARS infections led to an epidemic that affected 26 countries (mostly in China) with over 8,000 getting infected and almost 800 dying.
In 2012, another Coronavirus — Middle East Respiratory Syndrome (MERS) — led to another human outbreak, this time mostly in the Arabian Peninsula. MERS was responsible for 3,000 infections and over 800 deaths, making it more deadly than SARS. Both SARS and MERS are beta-Coronaviruses, transmitted from person-person through respiratory droplets and cause symptoms such as fever, dry cough and shortness of breath.
So what about this new Coronavirus responsible for the COVID-19 disease that was officially named January of this year?
This beta-Coronavirus is called SARS-CoV-2 because it is highly similar to the SARS virus responsible for the 2003 epidemic. While these viruses are very similar, there are some key, subtle differences that allow the new SARS-CoV-2 to level-up to pandemic status. The way in which SARS-CoV-2 is constructed allows it to more effectively infect its host — Ed Yong’s recent article does a great job at explaining these differences in detail.
Like other Coronaviruses, SARS-CoV-2 is also a respiratory virus, but it can infect both the upper and lower respiratory tracts, making it more difficult to treat once infection has progressed. Like SARS and MERS, the main symptoms of a SARS-CoV-2 infection are fever, dry cough and shortness of breath and it’s transmitted through respiratory secretions.
One commonality between all of these viruses, including SARS-CoV-2, is that their outer bubble-like shell is made up of fats and proteins, so washing your hands with soap and water or rubbing them with a solution containing at least 60 percent alcohol, will inactivate the virus. See UC San Diego Health’s recent video about this and keep those hands washed!
— Christa Trexler, PhD
Photo: Illustration of a Coronavirus provided by the Centers for Disease Control (CDC).
#coronavirus#covid-19#covid 19#infectious disease#faqs#tmyk#sars-cov-2#public health#sars#mers#science#medicine#hand washing#hand sanitizer#academic medicine#ucsd#uc san diego
84 notes
·
View notes
Text
Nitazoxanide is a potent inhibitor of human seasonal coronaviruses acting at postentry level: effect on viral spike glycoprotein
Preliminary report; Coronaviridae is recognized as one of the most rapidly evolving virus family as a consequence of the high genomic nucleotide substitution rates and recombination. The family comprises a large number of enveloped, positive-sense single-stranded RNA viruses, causing an array of diseases of varying severity in animals and humans. To date, seven human coronaviruses (HCoV) have been identified, namely HCoV-229E, HCoV-NL63, HCoV-OC43 and HCoV-HKU1, which are globally circulating in the human population (seasonal HCoV, sHCoV), and the highly pathogenic SARS-CoV, MERS-CoV and SARS-CoV-2. Seasonal HCoV are estimated to contribute to 15-30% of common cold cases in humans; although diseases are generally self-limiting, sHCoV can sometimes cause severe lower respiratory infections, as well as enteric and neurological diseases. No specific treatment is presently available for sHCoV infections. Herein we show that the anti-infective drug nitazoxanide has a potent antiviral activity against three human endemic coronaviruses, the Alpha-coronaviruses HCoV-229E and HCoV-NL63, and the Beta-coronavirus HCoV-OC43 in cell culture with IC50 ranging between 0.05 and 0.15 g/ml, and high selectivity indexes. We found that nitazoxanide does not affect HCoV adsorption, entry or uncoating, but acts at postentry level and interferes with the spike glycoprotein maturation, hampering its terminal glycosylation at an endoglycosidase H-sensitive stage. Altogether the results indicate that nitazoxanide, due to its broad-spectrum anti-coronavirus activity, may represent a readily available useful tool in the treatment of seasonal coronavirus infections. https://www.biorxiv.org/content/10.1101/2022.07.13.499346v1?rss=1%22&utm_source=dlvr.it&utm_medium=tumblr Read more ↓
0 notes