#Gene Xpert
Text
Tuberculosis is curable, yet 1.6 million people die of tuberculosis every year while Danaher grossly overcharges for diagnostic tests for the people who can least afford it.
You can help fix this issue by asking Danaher to lower the price of their Gene Xpert cartridges to $5.
highly recommend watching John Green’s new video on Danaher and Cepheid if you would like a quick and precise breakdown of the issue
and check out TB Fighters’ website for more information
and call or send an email to Danaher encouraging them to lower their prices
12 notes
·
View notes
Text
Anambra ranks highest in Childhood TB cases in Nigeria

Dr Ugochukwu Chukwulobelu, Programme Manager, Anambra State Tuberculosis, Leprosy and Buruli Ulcer Management, says that the state ranks highest in childhood Tuberculosis (TB) contribution in Nigeria.
Chukwulobelu made the disclosure at the ongoing stakeholders engagement organised by the Federal Ministry of Health in partnership with the KNCV Tuberculosis Foundation and Breakthrough Action Nigeria, on Thursday in Awka.
He described TB as an airborne disease caused by a bacteria called Mycobacterium tuberculosis which usually attacks the lungs and could also damage other parts of the body.
According to him, it spreads through the air when a person with tuberculosis of the lungs or throat coughs, sneezes, or talks.
"According to statistics on TB burden, Nigeria ranks number six in the world and number one in Africa.
"With every LGA having TB cases, Anambra has the highest burden of TB drug resistance cases and childhood TB contribution in the southeast and Nigeria. This is not a good thing for the state.
"To reduce the high burden of TB, the state government in collaboration with he National Tuberculosis and Leprosy Control Programme (NTBLCP) and other partners, set up 14 laboratories with gene Xpert machines for diagnoses.
"We have about 800 Directly Observed Treatments (DOTS) centres for TB but the major problem here is lack of awareness among residents about TB, the diagnosis and treatment," he said.
Chukwulobelu urged the media to intensify reportage on the burden and symptoms of TB as well as how and where patients could get medical help.
"Persistent cough for two weeks or more, fever, unexplainable weight loss and drenching night sweats are signs used to screen patients. Residents should report suspected cases of TB within their communities.
"TB patients should also adhere their TB treatments to prevent drug resistance TB cases which is even more dangerous. Everyone has a role to play to reduce the burden of the disease, " Chukwulobelu said.
Also speaking Dr Chijioke Oke of KNCV-Nigeria said that children living with adults who have TB, children who are HIV positive and malnourished children, were at risk of getting TB.
Oke identified low childhood TB awareness, stigma, low funding for childhood TB and low index for suspicion for childhood TB by healthcare providers, as some of the challenges with the control of the disease in the state.
He said that children's stool was required to test children for TB because they did not know how to spit out sputum after coughing, as they rather swallow it.
"Our major challenge with Childhood TB control in the state is that parent are scared and they do not allow doctors take their children’s stool for test.
"Some of them said we want to use their children's stool for 'juju'. It shows the level of ignorance and lack of awareness among residents, "he said," he said.
Read the full article
0 notes
Text
Laboratory Assistant
General Summary
To carry out basic laboratory tests for diagnosis of diseases with focus on Gene Xpert testing and other HIV/AIDS related tests.
Key Responsibilities
Preparing laboratory reagents and stains for routine investigations;
Conduct Gene Xpert tests and reporting at the supported facility
Carry out basic laboratory tests and submit reports to Laboratory…

View On WordPress
0 notes
Text
Clinical Microbiology Market Share, Growth Forecast- Global Industry Outlook 2030
Global Clinical Microbiology Market: Snapshot
Clinical microbiology, the adaptation of microbiological techniques to analyze the etiological agents that lead to the development of infectious disease, is the medical sub-specialty that explores the nature of the infectious condition and studies the ability of antibiotics to kill or inhibit the isolated microorganisms. The sector is gaining increased focus across the globe owing chiefly to the vast rise in infectious conditions, the alarming adaptability of microbes to changing environments, and the rising threat of contracting bacterial pathogens that are resistant to commonly used antibiotics.
Download PDF Brochure: https://www.tmrresearch.com/sample/sample?flag=B&rep_id=1199
The rising number of diseases caused by pathogenic infections is fueling research and development activities in the field of clinical microbiology. The trend is expected to remain strong over the next few years as well, with an increasing number of medicine companies already entering or willing to enter the field with the help of their clinical solutions for use across numerous sectors of clinical microbiology. Automation is increasingly gaining a larger and more prominent role in the sector and is expected to help reduce the time required to undertake a number of processes and improve the efficiency of results.
Technological advancements in monitoring equipment are allowing more exhaustive analysis of microbes and leading the field of clinical microbiology towards a healthy growth path. This report includes the most current market data related to clinical diagnosis of animal and human infections and the present and potential future role of laboratories in both the management of infectious diseases and analysis of the epidemiology of infections.
Global Clinical Microbiology Market: Overview
The intercontinental clinical microbiology market is envisioned to be pampered by a wide spectrum of opportunities in different applications such as the identification of diseases caused by pathogens with the help of laboratory services. Class A carbapenemase, a group of causative agents of clinical infections, is proliferating rapidly and has augmented the need for determining carbapenemase genes through clinical microbiology. In order to address this need, Cepheid, Inc. introduced Xpert Carba-R, a diagnostic assay, which was sanctioned by the U.S. FDA in 2016.
The global clinical microbiology market could be segmented considering disease, application, and product as significant parameters. With the rise in targeted diseases and swelling geriatric population, the disease segment could see a handsome growth.
This report on the world clinical microbiology market can be used as a guideline to operate in the industry as a winning player. Vital aspects of the market such as opportunities, restraints, and growth factors are analyzed in a radical manner to help gain a paramount insight into the clinical microbiology industry.
Global Clinical Microbiology Market: Trends and Opportunities
The world clinical microbiology market could acquire growth at a stallion pace in the wake of the need to keep a tight rein on human errors during the manual processing of samples in research projects. In this regard, the automation of clinical systems could be demanded at a brisk rate. This demand is foretold to also gain increased support from the need to maintain constancy in manual processing.
Having pulled in a king’s share in the global clinical microbiology market in 2015, reagents are foreseen to earn more revenue while riding on the hike in purchases and aggravating penetration due to the demand from therapeutic and analytic research projects.
The towering degree of the prevalence of respiratory maladies in emerging as well as developed nations could intensify the demand in the international clinical microbiology market. This can be attributed to the soaring levels of air pollution because of meteoric industrialization.
Global Clinical Microbiology Market: Regional Outlook
The North America clinical microbiology market is prognosticated to get help from the existence of leviathan companies and hard and fast regulatory structures. An interesting part of the geographical analysis is the expectation on the part of the Europe market to maintain a close proximity with North America in terms of growth. However, North America secured a stupendous share in the global clinical microbiology market in 2015. The region is anticipated to stimulate high adoption of clinical microbial methodologies with the higher development of healthcare and industrial domains in countries such as the U.S.
Besides China, Japan is expected to promise a faster growth in the Asia Pacific clinical microbiology market on the back of aggressive microbial testing application in a variety of fields. The august technological buildout in Japan cannot be sidelined when considering this market on the basis of demand. A countable number of top companies in the market are looking to relocate their major manufacturing outlets to Asia Pacific. This is due to the quantum leaps in building sophisticated manufacturing infrastructure and large availability of skilled labor at low cost in the region.
Global Clinical Microbiology Market: Companies Mentioned
As a result of their pacey progress in research and development and introduction of automated laboratory systems, the giant players in the international clinical microbiology market have won the trump card of being the first movers. There are only a few names competing at worldwide level, viz. Bruker Corporation, Danaher Corporation, Cepheid Inc., and bioMerieux S.A. Howbeit, the contest is predicted to be escalated by the entry of new faces in the global market. Of these are Hologic Inc., Becton Dickinson & Company, Alere Inc., and Roche Diagnostics.
About Us:
TMR Research is a premier provider of customized market research and consulting services to business entities keen on succeeding in today’s supercharged economic climate. Armed with an experienced, dedicated, and dynamic team of analysts, we are redefining the way our clients’ conduct business by providing them with authoritative and trusted research studies in tune with the latest methodologies and market trends.
Contact Us:
Rohit Bhisey
Head Internet Marketing
Tel: +1-415-520-1050
Website: https://www.tmrresearch.com/
0 notes
Text
Nowhere else to turn Chapter 70: Avulsion

Wending her arm through Hermione’s proffered one, Pansy begins to sluggishly walk in the direction of her bedroom, pausing after a few steps. Turning her head, she catches Harry’s intent glance, as the men follow behind.
“Harry? Can you… can you please stay? If you’re not too busy, I mean,” she quietly modifies. “I’d… I’d like to talk with you, after Hermione.” She holds her breath for a mere fraction of a moment before Harry vigorously nods his assent.
“I’ll be right here, love. I won’t leave you,” he promises, candour apparent in his every word and gesture. “May I make you a pot of tea? And perhaps bring you some biscuits?” he asks, his hands creeping to scrunch at his untameable raven-black mane.
“Yes, please,” Pansy surprises herself with her affirmative response. “My favourites are the Cartwright & Butler Strawberry and White Chocolate – they’re in a pink packet in the pantry.”
“Take Draco with you – he was recently bragging of his domestic talents,” Hermione drolly advises. “Apparently he’s an ‘Egg-xpert’ in the kitchen.”
She snickers as everyone else groans. “Listen – Lord of the Manor started with the ‘egg’ puns, I’m just trying to balance the books.”
“Hermione, I sometimes wonder if your humour gene has been irreversibly adulterated by too much study,” Harry good-naturedly gibes. “It’s the kind of ‘witticism’ I’d expect from Barney, honestly.”
“’Adulterated’ and ‘witticism’… steady on, Potter, anyone would think you actually went to school, instead of spending the majority of your magical education saving the world from a megalomaniacal monster,” Draco chimes in affably. “How are we to maintain our traditional enmity if you continue to ‘improve your mind by extensive reading’?” he winks at Hermione as he paraphrases the classic Austen line.
“Oof – that’s enough of your wordy flirting, you pair,” Pansy fakes pettish grievance. “And by that, I mean you and Harry, Draco,” she deadpans.
“Nice one, Pansy,” Hermione lifts her hand for a congratulatory slap. “”Our wizards don’t stand a chance, thinking they can mix it up with us on the witty repartee front,” she sniffs dramatically. Both males mutter unintelligibly by way of retort.
They have arrived at Pansy’s open bedroom door. Pansy stills, her upbeat façade withering as she contemplates recounting her pitiful, sordid tale.
Hermione exchanges a significant look with Harry, who moves forward to loosely encircle his arms around Pansy. The Gryffindor witch walks into the room, while Draco retraces his steps back toward the kitchen; the couple clearly want to allow Pansy and Harry a measure of privacy.
“Please… look at me, Pansy.” Harry waits until Pansy lifts her sad eyes to meet his compassionate regard. “You needn’t feel obligated in any way to tell any of us anything you’re not comfortable discussing, yes? I’ll sit with you in silence for as long as you need me to, love. And never mind the demands of my job – I’m not the only Auror in the damned place. You come first, do you hear me?” he earnestly avers.
Harry’s mouth hovers near her own; Pansy cannot resist the temptation. She stretches to bequeath a delicate kiss – as ephemeral as a soap bubble in the sunlight – before shyly retreating.
Gazing at her worshipfully, Harry fleetingly brushes his thumb across her lower lip.
“I’ll be right outside if you need me, Pansy.”
Unable to speak, Pansy minutely tips her chin in acknowledgement; her jade eyes are woebegone as they follow his exit from the room.
Harry makes a final pledge before he closes the door.
“I meant what I said before, love. We’ll get through this, together… I promise.”
https://archiveofourown.org/works/23994118/chapters/69078105
#hansy#harry potter#pansy parkinson#pansy x harry#fanfic#dramione#draco x hermione#draco malfoy#hermione granger#post-hogwarts#five years later#romance#slytherdornet#slytherdor relationships#domestic dramione#baby talk#angst#trigger warnings#abuse#trauma#support#harry's not going anywhere#sweet at the start sour at the end
19 notes
·
View notes
Text
It's been a week since i've started my job in the Gene Xpert COVID laboratory and i'm not that busy and all, nahihirapan lang ako sa signal sa lodging house and laboratory kaya di ako makapag update here.
I'll be on my four days off starting tomorrow, so i might update here.
Hello.
17 notes
·
View notes
Text
“T" For Tuberculosis, “T" For Tumour, “T" For Tension- Juniper Publishers

Editorial
Low back ache is one of the most common complain of patients reporting to any Orthopaedic outpatient department. After exclusion of post traumatic, osteopenic and inflammatory arthropathy for chronic back ache, the bulk of the etiology is either tuberculosis or anaplastics (metastasis being the most common anaplastic condition).In my country, due to the increased prevalence of Tuberculosis and the practical acceptance of trial first line Anti Tubercular Therapy, it is the first diagnosis that comes to our mind while anaplastic conditions are our last bet. More often than not, on getting the traid of constitutional symptoms, spondylodiscitis and raised ESR, we start trial first line ATT, forgetting that the features of TB and anaplastics may mimic each other. Only after failure to respond to trial first line ATT, we go for Gene Xpert test (for Drug resistance tuberculosis), biopsy (for tuberculosis or analytics) and tests for the work up of metastasis of unknown origin to check for Drug Resistance TB or anaplastics. The delay in the correct diagnosis and thereby the correct treatment, the lag period, may be long enough to worsen the prognosis of an anaplastic condition on one hand or to increase the emergence of drug resistance tuberculosis on the other.
The diagnosis of TB by history taking, clinical examination and imaging (X ray or MRI) has its own limitations as there is no pathognomonic finding on an MRI that reliably distinguishes tuberculosis from other spinal infections or from a possible neoplasm [1,2]. And, differentiating spinal TB from pyrogenic and fungal vertebral osteomyelitis as well as primary and metastatic spinal tumors may be difficult when only clinical and radiographic findings are considered [3,4]. Hence, Refinement of diagnostic criteria on MRI is also being done which concludes that a eight point MRI criteria of the vertebral lesions are likely to enhance the diagnostic ability of tuberculous and non tuberculous pathologies thereby reducing the dependency on histopathologic diagnosis or invasive method for early initiation of therapy [5]. MRC Trials on spinal tuberculosis and clinical practice over several decades have confirmed that in the regions where tuberculosis is prevalent, a clinical diagnosis supported by radiographs is adequate for starting the treatment, but, for cases not responding to chemotherapy, a biopsy may be required [6].
There is a definite need felt as per studies for a biopsy to be added routinely for the diagnosis of TB. In the absence of an abscess or bone fragments, image-guided biopsy is essential to establish the diagnosis [7]. The tissue obtained by percutaneous methods may not be sufficient for conclusive diagnosis. But, the absolute need for histological diagnosis in areas where the disease is endemic and facilities for biopsy and histopathology are scarce is still controversial [8]. There have been case reports of anaplastic conditions being diagnosed and treated as TB Spine [9-11]. MDR TB of the spine is a different disease and is here to stay with the imaging appearance becoming more complex with the onset of MDR TB [12,13]. The most important cause of the development of MDRTB is the patients receiving erratic, unsupervised second line drugs, added individually and often in incorrect doses, so giving a patient ATT without diagnosis actually increases the chances and spread of MDR, XDR TB [14].
The Xpert test has showed a sensitivity of 95.6% and specificity of 96.2% for spinal TB, available within 48 hours compared with a median of 35 days for cultures. It has also been used as an initial diagnostic test for TB detection and rifampicin resistance detection in patients suspected of having TB, MDRTB, or HIV-associated TB with a good sensitivity and specificity. Developments in this gene testing are also going on by ways of Gene Xpert Omni and Xpert Ultra. The point we need to ponder over is: can it be used as a routine first line test for diagnosis of spinal tuberculosis to include or exclude drug resistance tuberculosis?
We should not treat everything as TB. The correct diagnosis should be done before starting ATT. Literature recommends the use of biopsy and it is Safe, easy, reproducible, and has a high diagnostic yield. Also, it is always better to be medico legally safe. In today's day and age such a delay in the correct diagnosis is not acceptable and hence it is recommended not to be over dependant on imaging and on our bias towards TB, and anaplastics should be investigated by biopsy and other tests and ruled out at the onset rather than being a diagnosis of excusion (i.e. after exclusion of TB). Histopathology is important to exclude anaplastics and till anaplastics is not excluded we should not be sure it is TB because it should be borne in mind that failure to diagnose anaplastics is more dangerous than failure to diagnose MDR TB [15-17].
For More Open Access Journals in Juniper Publishers Please Click on
https://juniperpublishers.com/journals.php
For more articles, Please click on Juniper Online Journal of Orthopedic & Orthoplastic Surgery
https://juniperpublishers.com/jojoos/JOJOOS.MS.ID.555565.php
#Surgical Orthopedics#Knee arthroscopy#Hip replacement#Traumatic Surgery#Plastic Surgery#Foot and Ankle Surgery
0 notes
Text
Significant COVID-19 Impact on In-Vitro Diagnostics | Healthcare Industry | Data Bridge Market Research
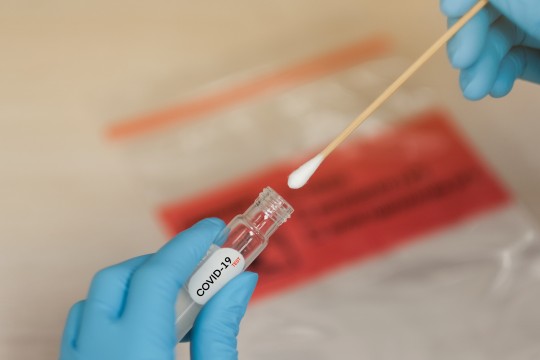
COVID-19 Impact on In-Vitro Diagnostics in the Healthcare Industry
The coronavirus (COVID-19) pandemic is having a dramatic impact on the in-vitro diagnostics (IVD) including technologies such as PCR, NGS, ELISA, clinical chemistry, rapid-tests, hematology, hemostasis, urinalysis, microbiology testing, and others. Currently, in-vitro diagnostic is the only diagnosis used by the healthcare professionals in order to detect the spread of corona virus across the globe. The virus has spread across all regions ranging from North America, Europe, Asia-Pacific, Middle East and Africa up to South America. The COVID- 19 has been declared as a pandemic by WHO due to its increased spread across the globe. After the declaration of the pandemic, various countries announced the complete lockdown such as the U.S., Germany, India and China among more in order to decrease its spread. The lockdown of countries aids in surging of use of diagnostic kits in order to screen patients suffering from coronavirus. According to the situation, the report of 4th June 2020 by WHO stated 6,416,828 cases of corona has been reported globally and 382,867 patients are dead due to the coronavirus.
IMPACT ON DEMAND
Exponential rise in cases of corona virus across the globe is increasing the demand of in-vitro diagnostic kits. The factors responsible for the rise in demand of IVD includes increased market demand for PCR, NGS, serology-based rapid-test products, a supportive regulatory environment for product development and marketing along with sharp increase in target patient population. Due to these factors, various market players have been prompted to improve and strengthen their current production and distribution capabilities and to focus on upgradation and marketing of their product.
A RT-PCR based assay is the common method used to diagnose patients with COVID-19. The pandemic has led to significant rise in demands for reagents used in RT-PCR in the recent months, leading to shortages in reagents required to perform the key steps in the testing process such as RNA extraction kits.
Manufacturers of in-vitro diagnosis (IVD) have taken huge initiatives to increase patient access to diagnostic testing for coronavirus in hospitals, laboratories, and other test sites across globe to guide patient care and protect public health.
The companies listed below developed IVDs that gained Emergency Use Authorization from the U.S. FDA:
Molecular Diagnostic Tests for Detection of COVID-19
· Abbott : Abbott RealTime Sars-CoV-2 assay; ID NOW COVID-19; Alinity m SARS-CoV-2 assay
· altona Diagnostics GmbH : RealStar SARS-CoV02 RT-PCR Kits U.S.
· Rhenonix : Rheonix COVID-19 MDx Assay
· F. Hoffmann-La Roche Ltd : Cobas SARS-CoV-2
· Shengxiang Biotechnology Co., Ltd. : Novel Coronavirus (2019-nCoV) Nucleic Acid Diagnostic Kit (PCR-Fluorescence Probing)
· ScienCell Research Laboratories, Inc. : ScienCell SARS-CoV-2 Coronavirus Real-time RT-PCR (RT-qPCR) Detection Kit
· SEASUNBIOMATERIALS : U-TOP COVID-19 Detection Kit; AQ-TOP COVID-19 Rapid Detection Kit
· Seegene Inc. : Allplex 2019-nCoV Assay
· Applied DNA Sciences : Linea COVID-19 Assay Kit
· AtilaBiosystems : iAMP COVID-19 Detection Kit
· BD : BioGX SARS-CoV-2 Reagents for BD MAX System; BD SARS-CoV-2 Reagents for BD MAX System
· Primerdesign Ltd : COVID-19 genesig Real-Time PCR assay
· QIAGEN : QIAstat-Dx Respiratory SARS-CoV-2 Panel
· Quidel : Lyra SARS-CoV-2 Assay; Lyra Direct SARS-CoV-2 Assay
· SD Biosensor, INC. : STANDARD M nCoV Real-Time Detection Kit
· Sherlock BioSciences : Sherlock CRISPR SARS-Cov-2 Kit
· SolGent Co., Ltd.. : DiaPlexQ Novel Coronavirus (2019-nCoV) Detection Kit
· Thermo Fisher Scientific Inc. : TaqPath COVID-19 Combo Kit
· Trax Management Services Inc. : PhoenixDx 2019-CoV
· Zymo Research : Quick SARS-CoV-2rRT-PCR Kit
· BGI : Real-Time Fluorescent RT-PCR Kit for Detecting SARS-2019-nCoV
· Biocore company : BioCore 2019-nCoV Real Time PCR Kit
· BioFire Defense : Bio Fire COVID-19 Test
· BioFire Diagnostics : BioFire Respiratory Panel 2.1 (RP2.1)
· bioMérieux SA : SARS-COV-2 R-GENE
· Bio-Rad Laboratories Inc. : Bio-Rad SARS-CoV-2 ddPCR Test
· Cepheid : Xpert Xpress Sars-CoV-2
· Co-Diagnostics, Inc : Logix Smart Coronavirus Disease 2019 (COVID-19) Kit
· DiaCarta : QuantiVirus SARS-CoV-2 Test Kit
· DiaSorin Molecular LLC : SimplexaCOVID-19 Direct Kit
· SpectronRx : Hymon SARS-CoV-2 Test Kit
· Fast Track Diagnostics Luxembourg S.à r.l. : FTD SARS-CoV-2
· Fosun Pharma USA : Fosun COVID-19 RT-PCR Detection Kit
· �� GeneMatrix, LLC : NeoPlex COVID-19 Detection Kit
· GenMark Diagnostics, Inc. : ePlex SARS-CoV-2 Test
· GenoSensor Corporation : GS COVID-19 RT-PCR KIT
· Gnomegen LLC : Gnomegen COVID-19 RT-Digital PCR Detection Kit; Gnomegen COVID-19-RT-qPCR Detection Kit
· Hologic Inc. : Panther Fusion SARS-CoV-2 Test; Aptima SARS-CoV-2 assay
· InBios International, Inc. USA : Smart Detect SARS-CoV-2 rRT-PCR Kit
· LABGENOMICS. : LabGun COVID-19 RT-PCR Kit
· Luminex Corporation : NxTAG CoV Extended Panel Assay; ARIES SARS-CoV-2 Assay
· Maccura Biotechnology Co. : SARS-Cov-2 Fluorescent PCR Kit
· Mesa Biotech : Accula SARS-Cov-2 Test
· NeuMoDx Molecular : NeuMoDx SARS-CoV-2 Assay
· IDEXX Laboratories, Inc. : OPTI SARS-CoV-2 RT PCR Test
· OSANG Healthcare : GeneFinder COVID-19 Plus RealAmp Kit
· PerkinElmer Inc. : Coronavirus Nucleic Acid Detection Kit
Serology Tests or Blood Based Test for Detection of COVID-19
· Abbott : SARS-CoV-2 IgG assay
· Autobio Diagnostics Co. : Anti-SARS-CoV-2 Rapid Test
· Bio-Rad Laboratories, Inc. : Platelia SARS-CoV-2 Total Ab Assay
· Cellex : qSARS-CoV-2 IgG/IgM Rapid Test
· Chembio Diagnostic Systems, Inc. : DPP COVID-19 IgM/IgG System
· DiaSorin : LIAISON SARS-CoV-2 S1/S2 IgG
· EUROIMMUN US, Inc. : Anti-SARS-CoV-2 ELISA (IgG)
· Healgen Scientific, LLC : COVID-19 IgG/IgM Rapid Test Cassette (Whole Blood/Serum/Plasma)
· Ortho Clinical Diagnostics : VITROS Immunodiagnostic Products Anti-SARS-CoV-2 Total Reagent Pack; VITROS Immunodiagnostic Products Anti-SARS-CoV-2 IgG Reagent Pack
· F. Hoffmann-La Roche Ltd : Elecsys Anti-SARS-CoV-2
· Siemens : ADVIA Centaur SARS-CoV-2 Total (COV2T); Atellica IM SARS-CoV-2 Total (COV2T)
The EUL procedure was developed to accelerate the availability of the required IVDs in emergency situations in public health. It was aimed to aid interested procurement agencies and member states in the appropriateness of using a specific IVD, based on a limited set of data available on quality, safety and performance.
The IVDs eligible to be submitted for EUL includes -
· Assays for the detection of SARS-CoV-2 nucleic acid
· Rapid diagnostic tests for the detection of IgM/IgG to SARS-CoV-2
For instance,
· In May 2020, Thermo Fisher Scientific Inc.’s Applied Biosystems TaqPath COVID-19 Combo Kit was initially granted EUA on March 13th and subsequently expanded on April 20th, is designed to deliver results of the test within four hours of a sample collection and processed by a CLIA high-complexity laboratory.
In the first half of 2020 (H1), the U.S. is anticipated to account for the largest share of in-vitro diagnostics industry followed by Europe. This owes primarily to the ongoing marketing of innovative diagnostic products associated with ongoing advances in the field of gene & immunoassay based products, the latest discovery of genetic biomarkers and their clinical significance in immunoassay testing, favorable government policies and their emphasis on the development of new products, and the significant expansion of target population.
Key manufacturers of IVD kits in the U.S. include Abbott, Thermo Fisher Scientific Inc. and F. Hoffmann-La Roche Ltd among others.
IMPACT ON SUPPLY
Due to the rising demand for in-vitro diagnostic (IVD) kits, it has been observed that falsified medical products are flourishing in the market. These fake testing kits render the life of a patient at stake. WHO has received several reports concerning falsified in-vitro diagnostics (IVDs) and laboratory reagents for SARS-CoV-2 detection. Further, the misuse, stockpiling, and price gouging leads to severe supply chain disruptions. This has increased the risk for healthcare facilities with limited access to IVD kits during the care of COVID-19 patients. China being the epicenter of the pandemic became the key spot to disrupt the supply of goods.
STRATEGIC DECISIONS BY MANUFACTURERS
With the declining trend and inefficient management of the supply chain, the government and manufacturers are taking initiatives to effectively manage the supply of in-vitro diagnostic kits and make appropriate use of those products in healthcare settings. The capacity to expand reagent and IVD kit production are limited, resulting in unmet needs.
Collaboration, agreements, strategic initiatives by market players in IVD diagnostic markets such as Abbott, Bio-Rad Laboratories, Inc., DiaCarta, QIAGEN among more will help them expand their product portfolio thereby leading to market expansion. This, in turn, will help increase demand for its product in the market thus increasing future sales.
For instance,
· In May 2020, Thermo Fisher Scientific Inc. collaborated with WuXi Diagnostics and Mayo Clinic in order to develop a complete antibodies test for detection of corona virus thereby expanding its response for COVID-19.
Initiative for Meeting Supply Gap by Abbott:
· In May 2020, Abbott has shipped nearly 2.5 million of their rapid ID NOW tests to all 50 states, including Washington DC, Puerto Rico and the Pacific Islands.
· It manufactures 50,000 tests per day and planning to further increase the manufacturing capacity of ID NOW to 2 million tests a month by June.
CONCLUSION
It has been observed through the vast spread and rising trends of coronavirus cases each passing day that the novel coronavirus or COVID-19 is expected to leave a significant impact on every aspect of life or market. The risk pose by the infectious disease is not restricted to health but the dwindling economy also. The healthcare domain is under huge pressure to deal with the unimaginably big situation of COVID-19. Moreover, the surge in patients with this infectious disease anticipates in driving the demand for in-vitro diagnostics (IVD) in the market.
The market of in-vitro diagnostic has experienced growth owing to increased demand for nucleic acid amplification tests per day along with the reagents used to perform these tests. The advancement in technology, favorable support provided by the government, and ease in regulations are also aiding in its growth across the globe. The strategic initiatives are taken by the key market players such as Thermo Fischer Scientific Inc., and others to develop novel COVID testing kits to expand their market is fueling the market growth. Hence, it can be concluded that the in-vitro diagnostics (IVD) market is expected to show healthy growth and substantial future demand for the rapid diagnostic testing kits due to the ever-increasing number of patients suffering from coronavirus.
#In-Vitro Diagnostics#In-Vitro Diagnostics Market#In-Vitro Diagnostics Market Analysis#In-Vitro Diagnostics Market Analysis in Developed Countries#In-Vitro Diagnostics Market by Application#In-Vitro Diagnostics Market by Type#In-Vitro Diagnostics Market Development#In-Vitro Diagnostics Market Forcast#Healthcare Industry
0 notes
Text
National Dcotor’s Day: ‘Coronavirus has brought the role of healthcare and doctors into prominence’
Prof. Jagat Ram, PGIMER director
“I would like to extend my cordial greetings to all of my respected teachers, seniors, colleagues, students, general practitioners and fellows. The celebrations for Doctor’s Day in India offer us a great opportunity to raise awareness of the role, importance and responsibility of doctors and also to encourage medical professionals to move closer together and take on the responsibility of this prestigious profession with full devotion, “said Prof Jagat Ram, PGI Director, and greet the doctors.
Coronavirus, he said, has brought the role of health care providers and doctors to the fore, with doctors at the forefront in the fight against Covid-19. “While most skilled workers were working from home, this is a profession that skilled workers have been personally recruited for work. Dressed in full body suits, doctors tackled the coronavirus pandemic head on, helping India through two disastrous waves of the pandemic for over a year and a half. The fight was not without sacrifices. India lost over 1,500 doctors to Covid-19 during the pandemic. Today is a day to remember their invaluable sacrifices in selfless service. But as the former President of the Indian Medical Association, Dr. KK Agarwal, just days before his deadly virus, said, ‘The show has to go on,’ ”he said.
Dr. Jagat Ram
Prof. Ram added that it is important to remember and commemorate the contributions of the medical profession to society as doctors have made tireless efforts to improve the general health of our country. “We should all strive to provide compassionate and affordable care to all, and it is important that we strongly condemn the attacks on doctors and health workers who are doing their best to save the lives of suffering humanity. When I look back on my four-decade journey in the medical field, the only memories that make me smile are those of my patients. I have practiced cataract surgery ophthalmology for most of my ophthalmological career, and I have found no greater joy than giving eyesight to a blind child for a lifetime. “
Dr. R. Gowtham Ram, Department of Hospital Administration


Dr. R. Gowtham Ram
Dr. R. Gowtham Ram joined PGI as a Senior Resident in the Hospital Administration Department in January 2021. Soon after taking over the academic management of the institute, Dr. Gowtham quickly found that the Nehru Hospital Extension (the dedicated Covid hospital) was almost full with 250 beds. It was a time when the nation was battling the second wave of higher mortality / morbidity Covid that affected all ages. Dr. Gowtham volunteered to offer his expertise in Covid management at NHE. “Despite the lack of oxygen supply and other critical drugs, we at PGI anticipated the criticality and ensured a smooth supply of these vital elements. Our employees across all categories have remained highly motivated and have increased our bed size from 250 to 450 beds. We then witnessed the wave of mucormycosis which added to the complexity. We have set up a separate ward for these patients and made sure that the OT staffing and the supply of essential drugs such as liposomal amphotericin B goes smoothly. In addition, we have made sure that our Covid vaccination campaign is on the right track. All of our employees have been trained and motivated to prepare for new challenges as we continue this journey. I am very blessed and thank my superiors and all my team members for their tireless support, which drives us to ensure better patient care, ”said Dr. Gowtham.
Dr. Tukaram, Department of Internal Medicine


Dr. Tukaram
“I am proud to be part of the PGI NHE team and to work for the benefit of patients from the surrounding five to six states. I thank my teachers, colleagues and juniors for dealing with me in this difficult situation. Although it is a difficult experience for all of us, we are all well prepared now, ”says Dr. Tukaram, Department of Internal Medicine, PGI.
Dr. Tukaram says the initial belief that Covid-19 affects older people more is no longer true, as many young people without comorbidities have serious illnesses and many succumb to it. “We all know that new variants pop up from time to time that make treatment more difficult. As long as the pandemic continues and the virus continues to spread, its variants are likely to come. So if we want to stop the variants, we have to stop the virus. But the basic precautions are the same regardless of the strain, and the vaccines we have can still protect against the different variants, and the immune response we get from the vaccines is much stronger than the body’s own natural immunity. The third wave is predicted by experts, but we can all prevent / control it by following basic precautions, ”adds Dr. Tukaram added.
Dr. Tamvir, Department of Anesthesia


Dr. Tamvir
“I was always seen by my medical-surgical brotherhood as an all-rounder in the operating room, but I was seldom as well known to patients as all anesthetists. I also worked behind the scenes. However, the Covid-19 pandemic has given me a new identity in the form of a Covid warrior and has redefined the roles and responsibilities of healthcare providers like me around the world, “says Dr. Tamvir, Department of Anesthesia, PGI. Clad in personal protective equipment, the doctor says she is even more unrecognizable to her patients now, but the hope she sees in her eyes as she manages her airways, oxygenates them, ventilates them, and the warmth they get when they discharge feels they are going home is comparable to the belief that a devotee has in the higher power. “The hugs I don’t get from my 9-year-old son when I’m on the Covid service, the fear I see in my mother’s eyes and the silence on my father’s wrinkled lips are worth a lifetime, that is rescued in the Covid intensive care units. The best decision of my life was to become an anesthetist and I am waiting for the decision to boomerang when my son chooses his medical specialty, ”smiles Dr. Tamvir.
Dr. Kapil Goyal, Department of Virology
As an associate professor at the Department of Virology at the PGI, Dr. Kapil Goyal as part of the Covid-19 diagnostic team. Dr. Goyal played a key role in setting up Covid 19 diagnostics at the institute. The laboratory began testing approximately 50 samples per day and increased its capacity to 2,000 samples per day. The laboratory was recently identified as a high throughput test laboratory by the Department of Health Research and will test up to 4,000 to 5,000 samples per day if necessary.


Dr. Kapil Goyal
“This lab provided 24-hour services for both Gene Xpert and real-time PCR for Covid-19, and credit goes to my colleagues and staff who have worked day and night to set the work protocols for high throughput testing,” says Dr. Goyal who received Quality Management Systems training in the Victoria’s Infectious Disease Laboratory. He emphasizes that the main motto of the laboratory is to provide a quality service so that the quality of the tests performed can meet international standards.
Dr. Goyal says this lab is prepared for any other pandemics in the future and will also be prepared to run additional molecular tests to improve patient care at the institute.
Dr. Vikas Sharma, chief physician dermatologist


Dr. Vika’s Sharma
Since the beginning of Covid-19, the challenges of operating OPDs and OTs have been in the foreground. While the PGI and GMCH OPDs were closed, ours at the National Skin Hospital was open throughout the pandemic, ”says Dr. Vikas Sharma, senior dermatologist at the National Skin Hospital. Dr. Sharma says he started seeing cutaneous signs of Covid during the pandemic as some signs appeared earlier than classic symptoms.
“Vesicular eruptions occurred earliest in the course of Covid-19, before other symptoms in 15 percent of cases; these developed on the trunk and extremities, were most common in middle-aged adults, and typically lasted about ten days. In contrast, the much-noticed Pseudo-Chilblains eruption occurred later. Almost two-thirds (59 percent) of patients developed these lesions after other symptoms. Pseudo-Chilblain Akrale lesions correlated with a milder course of the disease and a younger patient age. However, livedo and necrosis indicated more severe Covid disease and a poor prognosis. The challenges and insights continue, ”adds Dr. Sharma added.
source https://dailyhealthynews.ca/national-dcotors-day-coronavirus-has-brought-the-role-of-healthcare-and-doctors-into-prominence/
0 notes
Link
What the Coronavirus Variants Mean for Testing In January 2020, just weeks after the first Covid-19 cases emerged in China, the full genome of the new coronavirus was published online. Using this genomic sequence, scientists scrambled to design a large assortment of diagnostic tests for the virus. But the virus has mutated since then. And as the coronavirus has evolved, so has the landscape of testing. The emergence of new variants has sparked a flurry of interest in developing tests for specific viral mutations and prompted concerns about the accuracy of some existing tests. “With these Covid diagnostics, we were on a time crunch, we had to get something out there,” said Lorraine Lillis, the scientific program officer at PATH, a global health nonprofit that has been tracking coronavirus tests. “Normally, diagnostics take a long, long time, and we’d normally challenge them with multiple variants.” She added: “And we’re doing that, but we’re doing it in real time.” The Food and Drug Administration has warned that new mutations in the coronavirus could render some tests less effective. And last week, PATH launched two online dashboards to monitor how certain variants might affect the performance of existing diagnostic tests. So far, scientists have agreed, there is no evidence that the known variants of concern are causing tests to fail completely. “The tests today work very, very well,” said Mara Aspinall, an expert in biomedical diagnostics at Arizona State University. But manufacturers and regulators will need to remain vigilant to ensure they keep pace with a constantly changing virus, scientists say. If variants begin to evade detection, that could be consequential not only for individual patients, who may not receive the treatment they need, but also for public health. If a test misses someone who is infected by a variant, then that person may not realize they need to isolate. “And that person is allowed then to be unquarantined, to circulate in the community and possibly spread that variant to others,” said Gary Schoolnik, a physician and infectious disease expert at Stanford University and the chief medical officer of Visby Medical, a diagnostics company that makes a Covid-19 test. “And that’s how a diagnostic test, if it’s missing variants, can actually promote the spread of that variant.” The risk of false negatives Molecular tests, like the widely used polymerase chain reaction, or P.C.R., test, are designed to detect specific sequences of the coronavirus genome. If mutations appear in these “target” sequences, the tests may no longer be able to detect the virus, yielding false negatives. “You could run into a situation where you just got unlucky with where you chose to target your test, and something popped up there that then made your test less effective,” said Nathan Grubaugh, a virologist at Yale University. The gene for the virus’s characteristic spike protein, known as the S gene, has been particularly prone to mutation, and tests that target this gene may miss certain variants. For instance, Thermo Fisher’s TaqPath test fails to detect the mutated S gene of the B.1.1.7 variant, which was first identified in Britain and is now spreading rapidly through the United States. But the test does not rely on the S gene alone; it has three targets and can still return accurate results by detecting two other stretches of the coronavirus genome. Just 1.3 percent of molecular tests rely solely on an S gene target, according to calculations performed by Rachel West, a postdoctoral associate at the Johns Hopkins Center for Health Security. The rest either target more stable regions of the genome, which are less likely to mutate, or have multiple target sequences, which makes them less susceptible to failure. “It’s very unlikely that you’re going to get mutations in all of them,” Dr. Lillis said. Updated April 14, 2021, 2:24 p.m. ET The F.D.A. has listed four different molecular tests “whose performance could be impacted” by the variants, but notes that the tests should still work. Three of the tests have multiple targets; a fourth may be slightly less sensitive when the virus has one particular mutation and is present at very low levels. (The four tests are the TaqPath Covid-19 Combo Kit, the Linea Covid-19 Assay Kit, the Xpert Xpress and Xpert Omni SARS-CoV-2, and the Accula SARS-CoV-2 Test.) “We don’t think that those four assays are significantly impacted,” said Dr. Tim Stenzel, who directs the F.D.A.’s office of in vitro diagnostics and radiological health. “It was more out of an abundance of caution and transparency that we made that information public.” Antigen tests are less sensitive than molecular tests, but they are typically cheaper and faster, and they are being deployed widely in coronavirus screening programs. These tests detect specific proteins on the outside of the virus. Some genetic mutations could change the structure of these proteins, allowing them to escape detection. Most antigen tests target the nucleocapsid protein. The gene that codes for this protein, known as the N gene, is more stable and less likely to mutate than the S gene, and the F.D.A. has not listed any antigen tests as being of concern. “We haven’t found one that raises a red flag nor have we had any reports of such,” Dr. Stenzel said. Still, experts note, not every test manufacturer discloses the specific sequences that their tests target, and the virus will continue to mutate. “There hasn’t been any evidence to show that a particular molecular assay or even an antigen test completely misses the boat in terms of detection,” said Neha Agarwal, the associate director of diagnostics at PATH. “But things are going to change.” The F.D.A. is continuing to monitor the situation, checking coronavirus sequence databases weekly to see if the virus is evolving in ways that may help it evade diagnostic tests. “We’re being very vigilant,” Dr. Stenzel said. “And we will stay vigilant.” Screening for specific variants As the variants spread, researchers are also working to develop and improve tests to detect them. At the moment, identifying a variant is typically a two-step process. First, a standard coronavirus test, like a P.C.R. test, is used to determine whether the virus is present. If the test comes back positive, a sample is then sent for genomic sequencing. “These two tasks are currently done in two separate workflows,” said Juan Carlos Izpisua Belmonte, a developmental biologist at the Salk Institute in La Jolla, Calif. “This means more time, labor and resources.” Many researchers are now working to create integrated solutions — tests that can determine both whether someone is infected with the virus and whether they might have a particular variant. For instance, in a recent paper, Dr. Izpisua Belmonte and his colleague, Mo Li, a stem cell biologist at King Abdullah University of Science and Technology in Saudi Arabia, described a new testing method that can identify mutations in up to five different regions of the coronavirus genome. And Dr. Grubaugh and his colleagues have developed a P.C.R. test that can detect specific combinations of mutations that characterize three variants of concern: B.1.1.7; B.1.351, which was first detected in South Africa; and P.1, first found in Brazil. (The work has not yet been published in a scientific journal.) Dr. Grubaugh said that researchers in Brazil, South Africa and elsewhere are already using the tests to sift through a mountain of coronavirus samples, identifying those that should be prioritized for full genomic sequencing. “Our group’s primary interest is enhancing genomic surveillance through sequencing, especially in resource-limited areas,” Dr. Grubaugh said. “If you want to know if there’s variants that are circulating, you need a way to triage.” A number of companies are also beginning to release coronavirus tests that they say can differentiate between certain variants, although these are intended for research purposes only. Creating a test that can definitively diagnose someone with a particular variant is “infinitely harder,” Dr. Grubaugh said. Similar mutations are springing up in different variants, which makes distinguishing among them more difficult. The mutations of interest will change as the virus does, and sequencing remains the best way to get a complete picture of the virus. But tests that can screen for certain mutations could be an important public health tool, Ms. Agarwal said: “These newer diagnostics that are looking across the variants, I think will be really key in understanding the epidemiology of the virus and planning our next generation of efforts against it.” Source link Orbem News #coronavirus #testing #Variants
0 notes
Text
Wireless Slate Market to Witness Widespread Expansion During 2021-2027
Research Kraft has provided an exclusive analysis of global Wireless Slate Market Size, Status and Forecast to 2027 gives a detailed analysis of the market with key company profiles. The report gives a thorough evaluation of the market structure which fuses evident perceptions about the market for a predicted timeframe from 2020 to 2027. The report actively includes informative aspects relating to product developments, launches, and trends, to assist market players, shareholders, and investors in strategic decision making.
Avail a Sample to know more about the complete Report @ https://www.researchkraft.com/request-sample/1117546
The analysis of the market are explained below:
Leading key players in the market are: Elmo, Hitachi, Promethean, Qomo HiteVision, Califone Internationa, C3 IT Xperts, Creative Source, Genee World, Jahan Initiatives, Mimio, Recordex USA, Speechi, TeamBoard, Tuning Technologies Global Wireless Slate Market Segment by Applications considering Consumption Growth Rate and Market Share:
Education
Commerce
worldwide Wireless Slate Market Segment by Product Types considering Production, Revenue (Value), Price Trends:
On-Premise
Cloud
Region Analysis
The report analysis of the markets across five major regions: North America, Europe, Asia-Pacific (APAC), Middle East and Africa (MEA), and South & Central America. The exhaustive PEST analysis is done for each region to assess major external factors which may influence Wireless Slate Market in the coming years.
Ask for discount:@ https://www.researchkraft.com/check-discount/1117546
Highlights of the report:
Fathom the present and unavoidable predetermination of the Wireless Slate Market in both made and making markets.
Extra and cut time doing zone level examination by watching the new development, size, driving players and parts inside the general Market.
The report edifies the bit expected to overwhelm the Wireless Slate industry and market.
To look at and consider the market status and hypothesis among as a rule enormous zones.
To examine the general key regions advance potential and bolstered position, opportunity and challenge, controls and dangers.
Emerging niche segments and regional markets.
Research Kraft also offers customization on reports based on specific client requirement.
Ask For Customization @ https://www.researchkraft.com/send-an- enquiry/1117546
Contact Us
Research Kraft
Phone: 888-213-4282
Email: [email protected]
0 notes
Text
Nagstart na ko kahapon sa job ko as a medical technologist sa isang government hospital, napa assign ako sa Gene Xpert Covid Testing Laboratory, which is kami yung team na responsible for the specimens testing for COVID-19. Iba yung team ng medtech na magcocollect ng swab at magdedeliver ng specimens sa amin. Nasa loob lang talaga kami ng laboratory.
Orientation lang kahapon, like tinuro lang samin yung mga do’s and dont’s, getting to know each other haha, though may mga kilala na naman ako dun sa team na napasukan ko kaya medyo naging at ease na ko. Then yung sa work naman, sa saturday na lang kami tuturuan na hands on, sa mismong infectious lab at paghawak na ng mga specimens.
Off ko today and tomorrow.
18 notes
·
View notes