#clinical research and trials
Text

Story from the Washington Post here, non-paywall version here.
Washington Post stop blocking linksharing and shit challenge.
"The young woman was catatonic, stuck at the nurses’ station — unmoving, unblinking and unknowing of where or who she was.
Her name was April Burrell.
Before she became a patient, April had been an outgoing, straight-A student majoring in accounting at the University of Maryland Eastern Shore. But after a traumatic event when she was 21, April suddenly developed psychosis and became lost in a constant state of visual and auditory hallucinations. The former high school valedictorian could no longer communicate, bathe or take care of herself.
April was diagnosed with a severe form of schizophrenia, an often devastating mental illness that affects approximately 1 percent of the global population and can drastically impair how patients behave and perceive reality.
“She was the first person I ever saw as a patient,” said Sander Markx, director of precision psychiatry at Columbia University, who was still a medical student in 2000 when he first encountered April. “She is, to this day, the sickest patient I’ve ever seen.” ...
It would be nearly two decades before their paths crossed again. But in 2018, another chance encounter led to several medical discoveries...
Markx and his colleagues discovered that although April’s illness was clinically indistinguishable from schizophrenia, she also had lupus, an underlying and treatable autoimmune condition that was attacking her brain.
After months of targeted treatments [for lupus] — and more than two decades trapped in her mind — April woke up.
The awakening of April — and the successful treatment of other people with similar conditions — now stand to transform care for some of psychiatry’s sickest patients, many of whom are languishing in mental institutions.
Researchers working with the New York state mental health-care system have identified about 200 patients with autoimmune diseases, some institutionalized for years, who may be helped by the discovery.
And scientists around the world, including Germany and Britain, are conducting similar research, finding that underlying autoimmune and inflammatory processes may be more common in patients with a variety of psychiatric syndromes than previously believed.
Although the current research probably will help only a small subset of patients, the impact of the work is already beginning to reshape the practice of psychiatry and the way many cases of mental illness are diagnosed and treated.
“These are the forgotten souls,” said Markx. “We’re not just improving the lives of these people, but we’re bringing them back from a place that I didn’t think they could come back from.” ...
Waking up after two decades
The medical team set to work counteracting April’s rampaging immune system and started April on an intensive immunotherapy treatment for neuropsychiatric lupus...
The regimen is grueling, requiring a month-long break between each of the six rounds to allow the immune system to recover. But April started showing signs of improvement almost immediately...
A joyful reunion
“I’ve always wanted my sister to get back to who she was,” Guy Burrell said.
In 2020, April was deemed mentally competent to discharge herself from the psychiatric hospital where she had lived for nearly two decades, and she moved to a rehabilitation center...
Because of visiting restrictions related to covid, the family’s face-to-face reunion with April was delayed until last year. April’s brother, sister-in-law and their kids were finally able to visit her at a rehabilitation center, and the occasion was tearful and joyous.
“When she came in there, you would’ve thought she was a brand-new person,” Guy Burrell said. “She knew all of us, remembered different stuff from back when she was a child.” ...
The family felt as if they’d witnessed a miracle.
“She was hugging me, she was holding my hand,” Guy Burrell said. “You might as well have thrown a parade because we were so happy, because we hadn’t seen her like that in, like, forever.”
“It was like she came home,” Markx said. “We never thought that was possible.”
...After April’s unexpected recovery, the medical team put out an alert to the hospital system to identify any patients with antibody markers for autoimmune disease. A few months later, Anca Askanase, a rheumatologist and director of the Columbia Lupus Center,who had been on April’s treatment team, approached Markx. “I think we found our girl,” she said.
Bringing back Devine
When Devine Cruz was 9, she began to hear voices. At first, the voices fought with one another. But as she grew older, the voices would talk about her, [and over the years, things got worse].
For more than a decade, the young woman moved in and out of hospitals for treatment. Her symptoms included visual and auditory hallucinations, as well as delusions that prevented her from living a normal life.
Devine was eventually diagnosed with schizoaffective disorder, which can result in symptoms of both schizophrenia and bipolar disorder. She also was diagnosed with intellectual disability.
She was on a laundry list of drugs — two antipsychotic medications, lithium, clonazepam, Ativan and benztropine — that came with a litany of side effects but didn’t resolve all her symptoms...
She also had lupus, which she had been diagnosed with when she was about 14, although doctors had never made a connection between the disease and her mental health...
Last August, the medical team prescribed monthly immunosuppressive infusions of corticosteroids and chemotherapy drugs, a regime similar to what April had been given a few years prior. By October, there were already dramatic signs of improvement.
“She was like ‘Yeah, I gotta go,’” Markx said. “‘Like, I’ve been missing out.’”
After several treatments, Devine began developing awareness that the voices in her head were different from real voices, a sign that she was reconnecting with reality. She finished her sixth and final round of infusions in January.
In March, she was well enough to meet with a reporter. “I feel like I’m already better,” Devine said during a conversation in Markx’s office at the New York State Psychiatric Institute, where she was treated. “I feel myself being a person that I was supposed to be my whole entire life.” ...
Her recovery is remarkable for several reasons, her doctors said. The voices and visions have stopped. And she no longer meets the diagnostic criteria for either schizoaffective disorder or intellectual disability, Markx said...
Today, Devine lives with her mother and is leading a more active and engaged life. She helps her mother cook, goes to the grocery store and navigates public transportation to keep her appointments. She is even babysitting her siblings’ young children — listening to music, taking them to the park or watching “Frozen 2” — responsibilities her family never would have entrusted her with before her recovery.
Expanding the search for more patients
While it is likely that only a subset of people diagnosed with schizophrenia and psychotic disorders have an underlying autoimmune condition, Markx and other doctors believe there are probably many more patients whose psychiatric conditions are caused or exacerbated by autoimmune issues...
The cases of April and Devine also helped inspire the development of the SNF Center for Precision Psychiatry and Mental Health at Columbia, which was named for the Stavros Niarchos Foundation, which awarded it a $75 million grant in April. The goal of the center is to develop new treatments based on specific genetic and autoimmune causes of psychiatric illness, said Joseph Gogos, co-director of the SNF Center.
Markx said he has begun care and treatment on about 40 patients since the SNF Center opened. The SNF Center is working with the New York State Office of Mental Health, which oversees one of the largest public mental health systems in America, to conduct whole genome sequencing and autoimmunity screening on inpatients at long-term facilities.
For “the most disabled, the sickest of the sick, even if we can help just a small fraction of them, by doing these detailed analyses, that’s worth something,�� said Thomas Smith, chief medical officer for the New York State Office of Mental Health. “You’re helping save someone’s life, get them out of the hospital, have them live in the community, go home.”
Discussions are underway to extend the search to the 20,000 outpatients in the New York state system as well. Serious psychiatric disorders, like schizophrenia, are more likely to be undertreated in underprivileged groups. And autoimmune disorders like lupus disproportionately affect women and people of color with more severity.
Changing psychiatric care
How many people ultimately will be helped by the research remains a subject of debate in the scientific community. But the research has spurred excitement about the potential to better understand what is going on in the brain during serious mental illness...
Emerging research has implicated inflammation and immunological dysfunction as potential players in a variety of neuropsychiatric conditions, including schizophrenia, depression and autism.
“It opens new treatment possibilities to patients that used to be treated very differently,” said Ludger Tebartz van Elst, a professor of psychiatry and psychotherapy at University Medical Clinic Freiburg in Germany.
In one study, published last year in Molecular Psychiatry, Tebartz van Elst and his colleagues identified 91 psychiatric patients with suspected autoimmune diseases, and reported that immunotherapies benefited the majority of them.
Belinda Lennox, head of the psychiatry department at the University of Oxford, is enrolling patients in clinical trials to test the effectiveness of immunotherapy for autoimmune psychosis patients.
As a result of the research, screenings for immunological markers in psychotic patients are already routine in Germany, where psychiatrists regularly collect samples from cerebrospinal fluid.
Markx is also doing similar screening with his patients. He believes highly sensitive and inexpensive blood tests to detect different antibodies should become part of the standard screening protocol for psychosis.
Also on the horizon: more targeted immunotherapy rather than current “sledgehammer approaches” that suppress the immune system on a broad level, said George Yancopoulos, the co-founder and president of the pharmaceutical company Regeneron.
“I think we’re at the dawn of a new era. This is just the beginning,” said Yancopoulos."
-via The Washington Post, June 1, 2023
#mental illness#schizophrenia#schizoaffective#psychotic disorders#psychology#neurology#autoimmune#autoimmine disease#neuroscience#medical news#medical research#catatonia#immunotherapy#immune system#clinical trials#good news#hope
6K notes
·
View notes
Text
Most Common Clinical Trial Therapy Areas
2023 Top Clinical Trial Areas
Clinical trials are a fundamental part of the medical research process. They help determine the safety and effectiveness of new treatment approaches, contributing to advancements in the medical field. With an ever-growing number of clinical trials conducted worldwide each year, it's essential to identify the most impactful and relevant therapy areas being researched. This article will discuss the top five clinical trial areas that have taken center stage in 2023. We will cover the prevalence of these clinical trials, funding, and expected outcomes for each therapy area, as well as the future of clinical trials in 2023.
1. Oncology: Leading the Charge in Clinical Trials
Cancer is among the leading causes of death globally, and the need for innovative therapies has never been higher. In 2023, the oncology domain is still the most common area in clinical trial research, representing nearly 50% of clinical trials worldwide. The United States bears the majority of these trials, boasting over 25,000 active studies in oncology alone. Europe is a close second, with multiple countries working together to fund and conduct innovative cancer research.
Government agencies, pharmaceutical companies, and non-profit organizations have invested billions of dollars into cancer research. In 2023, the National Cancer Institute (NCI) in the United States received more than $6.5 billion in funding for cancer research. Many prominent clinical trials this year target hard-to-treat cancers, such as lung, breast, and pancreatic cancer, with a focus on immunotherapy, targeted therapies, and cellular therapies. The future of oncology research is bright, as advancements in technology and global collaboration continue to push the boundaries of cancer treatment.
2. Neurology: Addressing the Growing Burden of Neurological Disorders
Neurological disorders, including Alzheimer's disease, Parkinson's disease, and multiple sclerosis, affect millions of people worldwide. With the growing prevalence of these disorders, neurology has emerged as a leading area in clinical trial research. The United States leads the world in neurological clinical trials, followed closely by Europe and Asia.
Global funding for neurological clinical trials reached new heights in 2023, with a focus on addressing neurodegenerative conditions like Alzheimer's and Parkinson's. Public and private sectors invested heavily in this research, with organizations like the National Institutes of Health (NIH) allocating over $3 billion to neurological clinical trials in 2023. The outcomes of these trials aim to slow down, prevent, or cure neurological diseases, and hope to improve overall quality of life for affected individuals.
3. Infectious Diseases: Tackling Emerging and Reemerging Pathogens
The COVID-19 pandemic has underscored the importance of research and preparedness in combating infectious diseases. In response to this global challenge, the field has seen significant growth in clinical trials focused on infectious diseases. Research funding for these trials has boasted increased support worldwide, with both public and private sectors contributing to the development of novel vaccines and treatments.
In 2023, clinical trials in infectious diseases tackled novel pathogens, as well as reemerged diseases like tuberculosis and malaria. The World Health Organization (WHO) played a significant role in funding and initiating these trials, collaborating with governments and pharmaceutical companies to ensure rapid response and treatment development. The outcomes of these trials will contribute to global health security and preparedness for future pandemics and disease outbreaks.
4. Cardiology: Addressing the Global Burden of Cardiovascular Disease
Cardiovascular disease (CVD) is a leading cause of death worldwide, with almost 18 million annual fatalities. In 2023, cardiology clinical trials aimed to improve prevention, diagnosis, and treatment of CVD, encompassing areas such as heart failure, coronary artery disease, and hypertension.
Globally, funding for cardiology research came from government agencies, non-profit organizations, and pharmaceutical companies. The United States, Europe, and several Asian countries allocated substantial resources to support these clinical trials. The anticipated outcomes of these trials will emphasize personalized and precision medicine approaches in cardiovascular healthcare and ultimately reduce the burden of CVD around the world.
5. Rare Diseases: Advancing Treatment for Orphan Disorders
In recent years, the focus on rare diseases has grown significantly, resulting in more clinical trials aimed at developing treatments for orphan disorders affecting less than 200,000 individuals in the United States. Developing therapies for rare diseases is often financially challenging due to the small patient populations. However, regulatory incentives and growing public awareness have resulted in an increase in funding and clinical trials in this area.
Rare disease clinical trials are prevalent in both the United States and Europe, with a focus on gene therapy, enzyme replacement therapy, and targeted treatments. Public health agencies
Oncology: One of the most common clinical trials by therapy area is oncology, which involves testing medications and treatments with the goal of helping to improve patient outcomes when dealing with various types of cancer. Examples of these clinical trials include those that seek to determine the efficacy of new drugs in treating particular forms of cancer, or researching novel therapeutic approaches such as immunotherapy.
Cardiovascular Disease: Clinical trials related to cardiovascular disease are also quite common. These tests may involve assessing the effectiveness of new medications that can help lower blood pressure or improve cardiac function, as well as examining lifestyle interventions such as diet and exercise for their potential to reduce risk factors associated with heart disease.
Diabetes: Clinical trials related to diabetes are also a frequent occurrence due to its prevalence in many parts of the world. These studies often aim to understand how better management strategies for diabetes can improve quality of life for patients and reduce long-term complications associated with this condition.
Neurology: Clinical trials pertaining to neurology are commonplace in research settings because there is still much unknown about how the brain and nervous system work, as well as treatment effectiveness for conditions like epilepsy, Parkinson’s disease, multiple sclerosis, and stroke recovery.
Mental Health: Mental health-related clinical trials are becoming increasingly more common as researchers continue to investigate and develop better treatments for depression, anxiety disorders, bipolar disorder, schizophrenia, PTSD, addiction and other issues related to mental health and wellbeing.
Respiratory Disease: Clinical trials involving respiratory diseases, such as asthma or chronic obstructive pulmonary disease (COPD), have become more commonplace in recent years due to their rising prevalence throughout the world; they typically involve testing new medications or therapies that can help manage symptoms and reduce exacerbations associated with these conditions.
Immunology: Immunology-focused clinical research has become more popular over recent years due to its potential implications for developing treatments for autoimmune diseases like rheumatoid arthritis or lupus; these clinical trials often involve testing existing medications or creating new ones from scratch in order to achieve desired results regarding immune system regulation within individuals living with autoimmune conditions .
Gastroenterology: Gastroenterological clinical research is commonplace due primarily to its relevance within digestive disorders such as Crohn’s Disease or Irritable Bowel Syndrome (IBS). Research conducted in this area generally seeks to gain an understanding into how certain dietary changes or drug treatments might be effective at managing symptoms associated with gastrointestinal problems while reducing side effects associated with traditional pharmacological approaches .
Endocrinology: Endocrinological clinical research is yet another form of study found in medical circles due primarily via its relevance within hormone-related issues such as diabetes mellitus type 1 & 2; this type of study typically involves testing ways in which different hormones might interact differently between individuals who have similar conditions but don't respond positively/negatively the same way when it comes to traditional forms of treatment .
Ophthalmology: Last but not least is ophthalmology which looks at vision disorders like glaucoma and age related macular degeneration (AMD); here researchers test existing medications/treatments looking for improvement when it comes both short term relief from eye pain/blurring but also long term protection against further loss/damage occurring over time via regular monitoring sessions
Learn more about clinical trials and become involved in management of clinical trials through further training with CCRPS.
#Keyword#recent clinical trials#clinical trials news#latest clinical trials#trials update#trilas#available clinical trials#clinical research news#clinical research trial#clinical trial news#clinical trials today#new clinical trials#new medical trials#ongoing clinical trials#trial website#us clinical trials database#available trials#cancer clinical trials database#clinical gov trials#clinical research and trials#clinical research newsletter#clinical search#clinical studies gov#clinical study gov#clinical study search#clinical trial data#clinical trial database#clinical trial finder#clinical trial search#clinical trial support
0 notes
Link
During a clinical trial, participants receive specific interventions, based on which researchers determine if those interventions are safe and effective. Interventions studied in clinical trials could be new cancer drugs or new combinations of drugs, new medical procedures, new surgical techniques or devices, new ways to use existing treatments, and even lifestyle or behavior changes.
0 notes
Text


More information about Stanford research goals and the RECOVER Program/Initiative:
#covid-19#covid 19#sars cov 2#covid#long covid#long haul covid#Stanford#Clinical Trials#Paxlovid#Antivirals#Research#RECOVER#RECOVER Program#National Institute of Health#NIH#Institutonal Review Board#IRB
91 notes
·
View notes
Text
my mother has found the cause of my sleeplessness guys its bc i was watching howls moving castle the other night :0
#“its cause of those scary cartoon faces you watch”#apparently howl as a one legged bird is why i couldnt fall asleep till 4 in the morning the other night#based on research and clinical trials (involving implementation + controls + placebo) and results btw uwu
2 notes
·
View notes
Text
yeah this is going as well as anticipated -_-
#scribbling notes: clinical... trials... indicate... live... execution... not... effective... anger... management... treatment.....#more... research... funding... needed
4 notes
·
View notes
Text
Despite Challenges, Clinical Research Must Include Women of Reproductive Age
HealthyWomen hosted a congressional briefing, “Women in Clinical Trials: The Challenge of Research During the Reproductive Years,” on June 1, 2023.Clinical trials have long been focused on white men, leaving women woefully underrepresented. Lack of diversity in clinical trials means that healthcare providers (HCPs) often don’t have enough data about how certain conditions affect women or what…

View On WordPress
#access#Age#babie blue#Challenges#Clinical#Clinical Trials#health policy#Include#policy#Reproductive#research#Women
2 notes
·
View notes
Text
See, the thing about the Zones is that they are bigger than people give them credit for. Sure, most killjoys live out East and taking a pod down to Wolfblood Beach is only going to take you about two and a half hours after leaving the Lobby, but that doesn't mean there's nothing else out there.
Out South, for example, there is a graveyard. It isn't quite in the literal sense, but the old houses looming over the horizon will have your hair standing on ends just like the ghosts running cables inside of Battery City. Some are benevolent, some not so much, and neither quite like being disturbed, but not all who rest in a cemetery are dead: there are living people, albeit a little odd and fuzzy around the edges, but people nonetheless. There is, of course, Chimp and Newsie's station, then there is the Finch sisters, and the man who leaves things meant to be found— some killjoys even insist that while peering through grimy shop windows and past "Gone fishing" signs they saw Tommy Chow Mein sitting behind his desk and smoking a cigar.
Much as the location suggests, out North is the polar opposite: a bustling suburbia made up of smaller gated communities that feel almost anachronistic when compared to the field of large satellite dishes they are hidden amongst. It is not a welcoming place, lest you wear black and white uniforms and have the stench of bleach on your breath, yet for all the life sealed away from the dangers of the outside world in the so-called "neutral towns", they are more dead than the ghosts living in the walls of southern homes. It is, after all, hard to run clinical trials when the population believes all ailments and desease to have ben erradicated, and that pain is punishment to have gone against the hand that's simply trying to guide you down the right path. What you don't see can't hurt you and you don't hear might as well never have happened
#they're not doing clinical trials for like. meds btw. they're doing clinical trials for less than ethical stuff and also several#psychiatric research thingiemabobs#words are failing me because letters are either very stubbornly not staying in place or making any sense whatsoever but like. headcanon. do#with it what you will#danger days#the zones#zone headcanons#headcanon#killjoys#ttlotfk
14 notes
·
View notes
Text
just had a cute little panic attack because of all the stuff i have to do. my body is being so dramatic like damn dude just do it. i'm so glad i'm starting therapy again on monday
#i couldnt find a therapist that dealt with lgbtq stuff specifically i could get into so im participating in a research study#to get free therapy instead. love accessing free healthcare by partaking in clinical trials
9 notes
·
View notes
Text
I think my fav recent misinformation post that went around on here was that one post with almost 30k notes abt how “ADHD meds don’t work on your period” and they didn’t know this until recently because “the tests for every ADHD med were only done on AMAB people to control for hormonal difference,” where the post’s one source was a link to an Upworthy article that ADMITTED it couldn’t find sources for the anecdotal claim like. Lol. Lmao
#I was reminded of this bc I was going through my drafts and found a post I never made where I was mad abt it#The cool and fun thing is that you can actually look up clinical trial results for drugs pretty easily#and pretty quickly verify that clinical trials for these meds weren't done only on AMAB people and that the claim is just an outright lie#It's true that there isn't a lot of research on how periods affect ADHD -- but there is SOME research#There's a 2018 study (with a sample size of only 32) that found a connection between hormone levels and reported severity of ADHD symptoms#This is a small study and even they say that further research is needed#But if it's true that would mean that it's not that the medication is working -- it's that the symptoms are just worse around then anyway#So theoretically like. Stopping your meds around that time because 'they don't even work' would set you up for an EVEN WORSE time#Anyway checking sources and doing research is cool and sexy. Please take ur meds#* not that the medication ISN'T working#runner up for funniest is whatever is happening with the soy sauce conspiracy posts#you are not immune to conspiratorial thinking etc etc
5 notes
·
View notes
Link
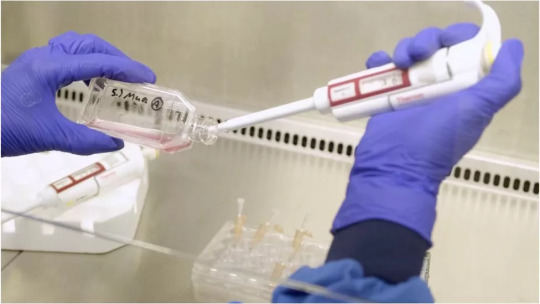
“British scientists have grown human red blood cells in a lab for the first time, and conducted a clinical trial to give it to patients.
The blood is grown by encouraging stem cells found in a blood donor’s sample to become new red blood cells, and opens the door to transfusion treatments for those with ultra-rare blood types.
For the near total majority of blood transfusions, British hospitals will still rely on people rolling up their sleeves and donating. This is, and will remain the case for, A, B, O, and AB blood types.
But what if a patient needs a blood transfusion of the “Bombay” blood group? It’s a tough call, as the British NIH knows of three people in the whole of the UK with this ultra-rare blood type.
Certain diseases, such as sickle-cell anemia, require regular blood transfusions, and if this patient were to also have the Bombay blood type, or “Jka-b-” or “Rh-null” also called “golden blood,” or “SARA” type after the first person it was discovered in, they are in serious danger...
“This world-leading research lays the groundwork for the manufacture of red blood cells that can safely be used to transfuse people with disorders like sickle cell,” said Dr. Farrukh Shah, the medical director of transfusion at NHS Blood and Transplant.” -via Good News Network, 11/16/22
#blood#medical research#medical science#blood type#sickle cell disease#good news#hope#clinical trials
30 notes
·
View notes
Text
Dealing with challenges in Quality Evidence Generation with a Real-Time Analytical Framework that makes Clinical Sense for Innovators
Evidence linking interventions with health outcomes is vital for healthcare decision-making. Making sound choices about healthcare requires the best possible and quality evidence from clinical research. However, some of the decisions currently made during the drug development process are not supported by high-quality evidence. As such, making informed decisions for allocating adequate resources to guide clinical Research development becomes challenging. At mid-stage clinical development, the challenge entails in determining the specific indication, if there are multiple potential indications. Moreover, evidence that is complete for some individuals or groups may be incomplete for others, leading to inefficiencies in decision-making.
Evidence generation strategies are especially important at Phase III and Phase IV trials to allow for effective navigation through competitive and regulatory hurdles, while it may be difficult to effectively communicate potentially attractive product attributes to the stakeholders, especially when it has no clear advantage over comparators. Stakeholders also lack the evidence needed to make real-world decisions on approval, coverage and use of treatments as most current processes used in evidence generation focus narrowly on the safety and efficacy of treatment.
Datasets to inform real-time decision making
The traditional demarcation between pre- and post-approval phases does not fit many medical products, as regulatory decisions could be informed by the same evidence that informs the use and coverage decisions, though the criteria for decisions should not be the same for both cases. Validated tools, based on large datasets to help inform real-time decision making are invaluable, yet they are currently limited. When new treatments are approved, healthcare payers and those who participate in shared savings base coverage determination on their value which is calculated by the evidence of benefit and net costs. The incorporation of real-world data (RWD) and patient-reported outcomes (PRO) into the evidence generation process could assist in making coverage determinations by rendering clinical evidence and research more immediately translatable to the beneficiary population.

Real-world data (RWD) and real-world evidence (RWE)
Additionally, large differences usually exist between the evidence required for initial adopters, such as surveys and studies, and that required for most prospective randomized control trials (RCTs). While the healthcare community uses RWD and RWE to develop decision support tools for use in clinical practices, medical product developers use these data to support clinical trial designs and observational studies to generate innovative treatment approaches. FDA uses RWE and RWD to monitor adverse events, post-market safety of the drug, and to make regulatory decisions. While RWD can be collected from various sources such as electronic health records (EHRs) and product and disease registries, RWE can be generated by different study designs including observational studies and randomized trials.
Aligning stakeholders for evidence generation
Aligning stakeholders is another big challenge of evidence generation as different stakeholders will have their own perspectives on uncertainties throughout the drug development lifecycle. Regulators may have different views as to what is acceptable to that of the patient. As such, it remains an industry-wide challenge to provide credible evidence for clinical research to innovators and investigators. Challenges exist for healthcare innovators to keep up to date with compliance and regulations about evidence generation as regulatory space evolves fast.
Because pharmaceutical companies tend to delegate evidence generation to individual departments that are often siloes, the process occurs sequentially, resulting in delays in crucial milestones such as getting regulatory approval before initiating an outcomes-based study.
https://www.futuremedicine.com/doi/10.2217/cer-2017-0073

An analytical framework model that makes clinical sense
There is a pressing need for high-quality evidence generation as regulators and payers seek more long-term data on product safety and effectiveness. As such, more efficient methodologies for generating evidence are required for decision-making, and to enhance clinical evidence collection and interpretation. An analytical framework model makes clinical sense as an evidentiary pathway, however, the challenge for investigators in evidence gathering is to fill out the framework. If the study design is weak, then the link in the evidence chain is also weak. Studies need to be carefully and prospectively designed, and opportunities exist to add well-designed studies into current practices. Study teams and researchers should consider how to most effectively translate diagnostic tests into practice needs within clinical settings.
Quality clinical evidence of safety and efficacy
The Jeeva™ eClinical Cloud platform provides clinical decision-makers with a modular and integrated approach to evidence planning and generation. From a single dashboard, study leaders can monitor data in real time to track safety and efficacy in representative patient populations across vast distances. The Jeeva™ eClinical Cloud is designed for efficient, remote long-term follow-up, natural history and other observational studies as well as interventional clinical trials regardless of therapeutic area. Jeeva™ enables quality clinical evidence generation to evaluate treatment safety and efficacy and tracks patients’ adherence to medications, in compliance with regulatory agencies such as Institutional Review Boards, EMA, FDA, and GDPR.
Digital-first approach to evidence generation
Study teams, innovators, drug developers, biopharmaceutical sponsors, clinical researchers, hospital sites and contract research organizations (CROs) face challenges to overcome the “no evidence, no implementation—no implementation, no evidence” paradox. Jeeva™ provides a new, digital-first, patient-centric approach to evidence generation that considers patients as partners for clinical trials, not merely subjects.
The Jeeva™ eClinical Cloud is user-designed software-as-a-service (SaaS) platform that allows volunteers to conveniently complete clinical trials wherever they are. The flexible and modular bring-your-own-device (BYOD) solution works on any browser-enabled mobile device and cuts out 70% of logistical burdens for study teams and patients. The modular and flexible Software as a Service (SaaS) subscription-based model is enriched with many features such as automated enrollment workflows, electronic patient-reported outcomes, 2-way email and SMS communication, uploading of lab reports, and more that are designed to encourage innovators to undertake research activities, rather than be intimidated by the complexity, logistical burdens, duration and costs of the traditional evidence generation approaches.
Quickly setup clinical studies of any scale or duration
Jeeva™ applies an innovative approach to remote screening, eConsent, patient registries and natural history studies can enable the generation of higher-quality, low-cost and more timely evidence generation for clinical trials. Jeeva™ offers a cost-effective solution to quickly set up and conduct clinical studies, of any scale or duration, with or without patient travel involved (e.g. hybrid or fully decentralized clinical trial protocols). Jeeva™ provides a more effective clinical trial design in terms of evidence generation, accelerating patient recruitment, site feasibility and endpoints that bring unmatched efficiencies in terms of the quality of evidence, time, and costs.
#clinical trial protocol software#clinical trials recruitment and retention#patient retention in clinical trials#software used in clinical trials#clinical solutions research platforms#clinical research software#software for clinical trials#software used in clinical research#clinical trials software#clinical study software#decentralized clinical trials software#clinical trial recruitment challenges#mobile eclinical#clinical trial cloud software#eclinical platform#eclinical cloud
2 notes
·
View notes
Text
Today at work I shadowed my first patient visit and got to run the labs on the blood and it was so cool
2 notes
·
View notes
Text
>be me
>be enrolled in clinical trial for COVID-19
me: [stands up and sits down 15 times in 30 seconds without getting breathless]
researchers:

me: [internally] I am going to get such a good grade in clinical trial, which is both normal to want and possible to achieve
[one week later]
me: oh no I forgot to do the online questionnaire
me: I am going to get a bad grade in clinical trial, which is both normal to fear and possible to achieve
#yip speaks#yip contributes#clinical trials#covid 19#if you're curious#i did send this to the lead research person because i thought it would make them chuckle#as a screenshot#not showing them my tumblr lmao
3 notes
·
View notes
Link
fundamentally, Phases of studies in research clinic there are 0 to 4 periods of clinical preliminary and in this, there are just three principal progressively eases in clinical examination. Stage 1 preliminaries are the earliest stage preliminaries and stage 3 are Late Phase Clinical Trials. A few preliminaries are randomized. lambda has an answer for clinical exploration. lambda has over 20 years of administration to the biopharmaceutical and conventional industry. Late Phase Clinical Trials.
2 notes
·
View notes
Text
Can Access to Published Research Help Local Science and Innovation?

Low-cost access to information can drive research and clinical trials in developing economies and contribute to SDGs. But different regions are affected in different ways. So how can low-performing institutions catch up?
So far, the public debate on access to medicine, neglected diseases, and patent-protected technology has underplayed the potential of access to information for economic development. Similarly, earlier research has revealed a startling gap between lower- and higher-income countries in terms of access to knowledge, with over half of medical institutions having had no subscriptions to academic literature in lower-income countries.

Several UN agencies and major academic publishers launched the Research
For Life (R4L) initiative to fill this gap. The World Health Organization (WHO) runs Health InterNetwork Access to Research Initiative (Hinari), one of fiveprograms under the R4L umbrella. It provides free or low-cost access to academic literature to at least 270,000 researchers in over 100 developing economies. This is for this WHO-led program alone. The entire initiative includes more than 21,000 peer-reviewed journals, 69,000 e-books and 115 data and other sources. Focusing on Hinari, a new WIPO research paper carried out empirical analysis of millions of data points to understand the strengths and weaknesses of the program. It is the first study to link access to scientific publications in developing countries to welfare along the science-to innovation pipeline. The report shows a local increase in health science publications of up to 75% after joining Hinari. Likewise, involvement in international clinical trials grew
by over 20%, suggesting that research and innovation in local institutions improved. Screening over 36 million scientific papers in PubMed, a repository of health science, the study found more than 167,000 papers coauthored by local researchers in developing economies, which cited clinical trials conducted worldwide over 30 years.

However, this uptick in science publishing and clinical trials only partially translated into global patents and inventions. The study attributes this to developing countries often lacking infrastructure and funding to transfer new findings into patented technologies. This gap reveals the remaining challenges in developing innovation and IP systems. Moreover, the study also finds that local context matters. Institutions in specific regions and those that already had a high research performance benefited most from the Hinari program. This also means that it is harder for others to catch up, despite better access to information.
Access to global knowledge counts on the ground
Empowering local researchers by providing access to information is
essential to their work. Researchers tend to target diseases that affect the local population and may be overlooked by researchers abroad. Enabling such access may help innovation in neglected diseases, mainly by connecting local teams to the global knowledge base. Aside from increased scientific activity, R4L also reports direct effects from Hinari regarding medical practice and patient care. The initiative quotes Dr. Nguyen Duc Chinh from Viet Duc Hospital, Hanoi, Viet Nam: “Good research, in short, leads to better patient care.” The doctor relied heavily on Hinari for his PhD on intestinal TB and surgical treatment. TB is prevalent in Viet Nam, but there is a relative lack of information on intestinal TB. “With the information and knowledge we obtain,” he says, “we feel more confident in practicing and implementing respected medical expertise from around the world.” Dr. Sami Hyacinthe Kambire at Kamboinsé Research Station, Ouagadougou, Burkina Faso, also found his research progressing faster and wrote grantwinning funding proposals thanks to Hinari. Before his institution adopted R4L, Dr. Kambire often devoted considerable time to research already

performed elsewhere. The initiative helped reduce these duplicative
research efforts in global health sciences and increase the quality of local teaching and education.
Access to information affects institutions differently
Despite the impacts, the study also found that the program effects differed for different parts of the world. Research institutions in the Carribean, Central Asia, Europe and Latin America benefited the most in generating new scientific knowledge. On average, their academic paper output increased by 80–100%. Regarding clinical trials, program participation is most impactful for East Asia, the Pacific, the Middle East and North Africa. Trial activity rose by up to 35% at institutions in these regions. That does not mean other regions did not gain from the program, but the impact has been less pronounced.

However, there are also institutional differences. Notably, the study
authors wanted to avoid comparing apples to oranges, because high- and low-performing research institutions differ. The high performers might be more likely to adopt the Hinari program in the first place. Seeing more publications might also be an outcome of the institutionsʼ selection into the program rather than an outcome of the program and better access to knowledge on the ground. To reveal the causal effects rather than mere correlations, the study compares different fields. This means health sciences supported by the program are matched against other research fields not supported by Hinari but conducted at the same institution.
How to make the most of access to information
Having ruled out the factors described above, the report suggests that program management could improve in two ways. First, it shows that already productive institutions benefit more from Hinari. For example, research institutions that have previously published academic papers see an average 60–70% increase in their publications after joining. This increase is only around 40% for institutions that rarely published scientific works previously. This suggests that Hinari preserves the gap between the most and least productive institutions for scientific publications and clinical trials. Under these conditions, the least productive institutions are, all else being equal, less likely to catch up. Still, the study ultimately supports the view that the Hinari program and the R4L initiative contribute to achieving the SDGs. They help boost research
and innovation capacity in developing economies and improve health
services (SDG 3) and education quality (SDG 4) at local institutions. They also aim to build industry, innovation and infrastructure, thus encouraging decent economic growth (SDGs 8 and 9).
The R4L initiative is also an excellent example of how private–public
initiatives can make a difference. It joins private sector stakeholders from
the global publishing industry and research institutions in the UN member states in a win–win situation. For research institutions, the initiative provides a practical solution. Their libraries and labs often need to be better resourced, and R4L improves access to information for students and researchers. It is also a smart way for industry stakeholders to show their corporate social responsibility and enhance their social impact in developing economies. It could also help grow local demand and the customer base in the long term.
Moreover, easing access to published research through initiatives like Hinari and WIPOʼs Access to Research for Development and Innovation (ARDI) program can significantly affect research output and contribute to desired social and economic outcomes laid out in the SDGs. UN agencies like the WHO and WIPO have been vital matchmakers. However, addressing existing gaps through schemes such as WIPOʼs Technology and Innovation Support Centers (TISCs) may help build local infrastructure and contribute to a vibrant IP and innovation system. In conclusion, the reportʼs findings on success and remaining challenges may inform stakeholdersʼ decisions to renew or change their commitment to R4L beyond 2025.

#patent-protected technology#Published Research#Local Science and Innovation#research and clinical trials#patented technologies#world i.p. day#26 april#R4L initiative#sdg3#sdg4#sdg8#sdg9#Technology and Innovation Support Centers (TISCs)#Access to Research for Development and Innovation (ARDI) program#ip and the sdgs
0 notes