#nitrocellulose membrane
Text
The blog "The Concept, Processes, and Applications of Western Blotting" published on PraxiLabs outlines the Western blot (or protein immunoblot) technique, highlighting its indispensability in modern biomedical research and various laboratories. This analytical method, rooted in molecular biology and immunogenetics, employs antibodies to specifically detect antigens, allowing for the identification of a particular protein within a sample alongside information on protein size and abundance.
Originating from the collaborative work of Harry Towbin, Julian Gordon, and Theo Staehelin in 1979, the development of protein blotting techniques marked a significant advancement in the field. Their pioneering work facilitated the electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets, thereby setting a foundational technique for numerous scientific investigations.
Western blotting operates on the principle of separating proteins within a sample based on molecular weight through gel electrophoresis. These proteins are then transferred (blotted) onto a more durable surface, such as a nitrocellulose or PVDF membrane. The detection of the protein of interest is facilitated by the use of specific antibodies.
Let’s explore the Western Blot method step-by-step.
Sample preparation: Involves preparing and loading the sample onto a gel for electrophoresis.
Electrophoretic separation: Proteins are separated by size using gel electrophoresis.
Protein transfer: The separated proteins are transferred from the gel to a membrane.
Blocking: Nonspecific binding sites on the membrane are blocked.
Antibody probing: The membrane is incubated with primary and secondary antibodies to detect the protein of interest.
Detection: Various methods, including chemiluminescence and fluorescence, are used to visualize the protein-antibody complexes.
Imaging and analysis: The final steps involve capturing images of the membrane and analyzing the detected signals to estimate protein size and quantity.
The article highlights the benefits and applications of Western Blotting.
One of the key advantages of Western Blotting lies in its sensitivity and specificity, making it highly effective in analyzing complex protein structures. This is crucial not only in research for obtaining molecular insights but also in clinical settings for making accurate diagnoses.
Sensitivity: demonstrates the ability to identify minuscule protein quantities within samples, as slight as 0.1 nanograms, which is an invaluable tool for early diagnostics in detecting minimal immune reactions triggered by viruses or bacteria in patient samples.
Specificity: originates from two principal factors attributing to the western blot technique. Firstly, the application of gel electrophoresis allows the classification of proteins based on their size, charge, and structure. This initial sorting is instrumental in the identification process, as the resulting patterns on the gel can provide preliminary insights regarding the protein or peptide of interest.
What about the applications of Western Blotting?!
The Western blot technique has a wide range of uses in scientific and clinical approaches, such as:
Biological sciences: It enables researchers to ascertain the presence, size, and amount of protein expression related to various tissues or cell types. This technique is pivotal for understanding protein expression levels, monitoring protein purification processes, and studying the effects of diseases or treatments on protein behavior. Its application extends to comparing protein expressions across different tissues or observing how proteins respond to specific treatments or diseases.
Medical diagnosis: Western blotting has found significant applications in the medical diagnostic field, especially as a confirmatory test for various diseases. For instance, it is widely used in the diagnosis of HIV, where it confirms the presence of HIV antibodies in blood samples following a preliminary ELISA test. In addition, Western blotting assists in diagnosing other conditions, such as Lyme disease, by detecting antibodies against specific pathogens, offering a layer of confirmation that enhances the accuracy of initial screening tests.
If you are interested in exploring more details about the Western Blotting Technique, check our PraxiLabs blog about “How to Analyze Western Blot Data”
0 notes
Text
Molecules, Vol. 28, Pages 8135: An #aptamer-Based Lateral Flow Biosensor for Low-Cost, Rapid and Instrument-Free Detection of Ochratoxin A in Food Samples
In this work, a simple and cost-efficient aptasensor strip is developed for the rapid detection of OTA in food samples. The biosensor is based on the lateral flow assay concept using an OTA-specific #aptamer for biorecognition of the target analyte. The strip consists of a sample pad, a conjugate pad, a nitrocellulose membrane (NC) and an absorbent pad. The conjugate pad is loaded with the OTA-specific #aptamer conjugated with gold nanoparticles (AuNPs). The test line of the NC membrane is loaded with a specific OTA-#aptamer probe and the control line is loaded with a control probe. The assay is based on a competitive format, where the OTA present in the sample combines with the OTA #aptamer-AuNP conjugate and prevents the interaction between the specific probe immobilized on the test line and the OTA #aptamer-AuNP conjugates; therefore, the color intensity of the test line decreases as the concentration of OTA in the sample increases. Qualitative detection of OTA is performed visually, while quantification is performed by reflectance colorimetry using a commercial scanner and image analysis. All the parameters of the assay are investigated in detail and the analytical features are established. The visual limit of detection (LOD) of the strip is 0.05 ng mL−1, while the LOD for semi-quantitative detection using reflectance colorimetry is 0.02 ng mL−1. The lateral flow strip aptasensor is applied to the detection of OTA in wine, beer, apple juice and milk samples with recoveries in the range from 91 to 114%. The assay exhibits a satisfactory selectivity for OTA with respect to other mycotoxins and lasts 20 min. Therefore, the lateral flow strip aptasensor could be useful for the rapid, low-cost and fit-for-purpose on-site detection of OTA in food samples. https://www.mdpi.com/1420-3049/28/24/8135?utm_source=dlvr.it&utm_medium=tumblr
0 notes
Text
Lateral Flow Assay Uncut Sheets
Lateral Flow Assay Uncut Sheets uncut sheets are specialized materials.
They consist of a large sheet or roll of the various components that make up a lateral flow , including the nitrocellulose membrane, sample pad, conjugate pad, absorbent pad, and backing card.
The uncut sheets serve as a precursor to the individual lateral flows test strips that are commonly seen in in vitro diagnostic kits.Firstly Manufacturers typically purchase these uncut sheets and then customize them according to their specific test requirements, including the target analyte, detection method, and desired performance characteristics. Secondly The uncut sheets allow for flexibility in the manufacturing process, as they can be customized to produce lateral flow test strips with different configurations and specifications.
Thirdly Manufacturers can cut the sheets into smaller strips of specific dimensions, apply additional coatings or reagents, and assemble them into the final test format.

0 notes
Text
Ningbo Mangomore Trade Co., Ltd.
https://www.bikudo.com/company/ningbo-mangomore-trade-co-ltd-101698.html
#nitrocellulose #membrane #test #tube
0 notes
Text
METHODS FOR PURIFYING AND ANALYSING PROTEINS VIA WESTERN BLOTTING
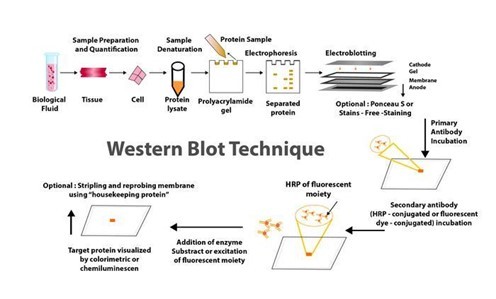
For many years, western blotting was a primary method in molecular biology and techniques such as proteomics (the large-scale study of proteomes: a set of proteins produced in an organism, system, or biological context). Given that western blot is a multistep process that frequently necessitates specialised interpretation, potential errors and variations at any step can jeopardise the reliability and reproducibility of its results.
Given this situation, many advanced and sophisticated approaches to improving the western blot protocol have been developed over the last two decades. These enhancements are more automated and sensitive, with high potential for reducing potential issues with the traditional western blot method. Western blotting's improved sensitivity and innovative equipment advancements have the potential to broaden the field of clinical applications for this fundamental method.
Helvetica Health Care explains the western blot technique as a method of purifying and analysing proteins, as well as new developments in western blotting methodology, in this article.
WHAT EXACTLY IS THE WESTERN BLOT TECHNIQUE?
Traditionally, the western blotting method involves seven steps, as below.
Sample preparation from cells or tissue lysates
Separation of proteins by gel electrophoresis
Protein transfer in a nitrocellulose or polyvinylidene fluoride (PVDF) membrane,
Blocking of non-specific proteins on membrane
Primary Antibody incubation
Secondary Antibody incubation
Protein detection & analysis
WHAT ARE THE NEW DEVELOPMENTS IN THE WESTERN BLOT DOMAIN AND WHAT CHARACTERISTICS DO THEY HAVE?
Below are some innovations that have enhanced the western blot protocol and addressed the potential problems in its application in protein purification and analysis.
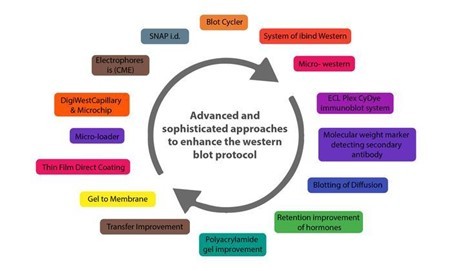
CAPILLARY AND MICROCHIP ELECTROPHORESIS (MCE)
MCE has higher sensitivity and resolution than traditional techniques, allowing for the detection of multiple target proteins from a single cell lysate sample. The method not only allows for parallel multiplexed tests of a set of proteins using a small sample amount, but it also eliminates blocking stages and has faster analysis times (8 min for electrophoretic resolution). MCE, while still in development, has the potential to be significantly improved with additional work.
AUTOMATED MICROFLUIDIC PROTEIN IMMUNOBLOTTING
Automated protein immunoblotting is a programmable and controllable technology that combines PAGE (polyacrylamide gel electrophoresis) and blotting in a single device. It saves time, avoids multiple test stages, and reduces the amount of equipment and reagents required. Because it can detect free prostate-specific antigens in a sample of human seminal fluid in less than 5 minutes, this technology is both cost-effective and reagent-efficient. The method is constantly being improved to improve sensitivity and enable protein quantification.
SINGLE CELL-RESOLUTION WESTERN BLOTTING
This sensitive method has also been used to investigate protein variations in stem cell signalling and differentiation. It can detect specific cell-to-cell changes in protein expression. MiloTM (ProteinSimple), the world's first Single-Cell Western platform, can measure protein expression (up to 4 proteins per cell simultaneously) in 1000 single cells in about 4 hours.
DIGIWEST
DigiWest, a simplified version of the standard procedure, improves western blotting throughput by requiring less lysate, target detection, and antibody. However, this method requires biotinylation of the target proteins, requires specialised reagents and equipment, incurs initial startup costs, has issues with translating the digital DigiWest results to western blot mimics, and has specifics about some procedures, such as the complex and difficult elution of proteins from PVDF membranes.
SIMPLE WESTERN
Simple Western is a method developed by ProteinSimple (San Jose, California) that is based on CE-SDS (Capillary electrophoresis sodium dodecyl sulphate), in which separated proteins are linked to the capillary wall by a patented photo- (ultraviolet) induced chemical crosslink. The method can use up to 90 different antibodies at the same time, is relatively quick, and requires small amounts of sample. It can also perform standard curve quantification, similar to high-pressure liquid chromatography (HPLC), and improve reproducibility. However, it necessitates the use of specialised reagents.
MICRO-LOADER
The Micro-loader technology has improved the sensitivity of the western blot assay and PAGE. To load samples, it employs a sample micro-loader device with a funnel-like design. This technique is highly effective for measuring protein expression and phosphorylation in samples that are few, difficult to find, and limited in quantity because it only requires a small number of loading samples for signal detection.
THIN-FILM DIRECT COATING WITH SUCTION-WESTERN BLOTTING (TDCS)
Western blotting has significant limitations due to high antibody consumption and lengthy operating times. TDCS, a capillary-tube-based technique, aims to reduce antibody and time consumption in western blotting. This quick and sensitive detection method allows for the quantitative study of multiple antigen-antibody interactions, as well as multiple protein interactions.
BLOTTING OF DIFFUSION
SDS-PAGE Single-prefabricated gels on plastic support are a quick and easy way to make multiple blots using diffusion blotting. Diffusion blotting allows for the comparison of several proteins on blots from the same gel. Protein transmission rates by diffusion blotting are 10% in 3 minutes, 20% in 20 minutes, and 40% to 60% in 3 hours. When compared to electroblotting, the technique may increase the amount of proteins efficiently delivered to the membrane surface. As a result, when quantitative protein exchange is not required, diffusion blotting is the preferred method.
MICRO WESTERN
Soon, micro western will be a proper western blot technique for confirming the diagnosis of purified HIV p24 and gp120 proteins in blood plasma. The Micro-Western may be vital for detecting infectious diseases and cancer, despite not being widely available.
SYSTEM OF IBIND WESTERN
Thermo Fisher Scientific's iBind Western procedure makes use of a low-cost semi-automated western blotting structure that uses sequential flow technology to distribute chemicals and antibodies to different compartments. Cleaner western blots will result from using the iBind Western Systems and highly specialised primary and secondary antibodies.
BLOT CYCLER
Every step of membrane processing is automated with the BlotCycler (Precision Biosystems, USA), and up to 12 membranes can be processed at once. The BlotCyclerTM is an Automated Western Blot Development system that improves repeatability and sensitivity by completing all blot cleaning, trapping, and incubation steps. It is also simple to programme all of the actions for specific operations.
SYSTEM OF SNAP I.D.
In a low-cost system of SNAP i.d. 2.0, solvents are uniformly distributed via the tissue using a technique based on a vacuum and a flow distribution system (Merck, USA). Its main advantage is that washing, antibody incubation, and blocking all take about 30 minutes. The SNAP i.d.® 2.0 gadget actively forces reagents through the membrane, unlike conventional Western blotting, which depends on diffusion to transfer reagents.
Washes, antibody recollection, and antibody-antigen binding have been improved. The SNAP i.d.® 2.0 delivers new, cutting-edge western blotting characteristics with an immunodetection capability in 30 minutes without using additional reagents (e.g., antigen, antibody, or detection reagents).
RETENTION IMPROVEMENT OF HORMONES OF THE PEPTIDE ON THE MEMBRANE OF BLOTTING
Western blotting is significantly improved by treating the PVDF membrane used in this method with glutaraldehyde (0.2%) for fifteen minutes in a saline solution containing tween-80. In western blotting, the addition of glutaraldehyde to the membrane prevented or reduced the quantity of insulin loss.
Apart from these, two more techniques that have improved the conventional method are
Analysis of western blot utilising molecular weight marker detecting secondary antibody
Total and target protein co-detection by immunoblotting of fluorescent ECL and labelling of Cydye
Many innovations in western blotting techniques to transfer proteins from gel to membrane have also been observed, such as vacuum blotting, centrifuge blotting, multiple tissue blotting, and so on. The Trans-Blot Turbo, the iBlot® dry blotting system, and the PierceTM Power Blot Cassette are all improvements in protein transfer methods from gels to membranes. The transfer time is reduced from an hour or overnight to at most 10 minutes when these new techniques are used.
HHC offers the ZeptoMetrix WESTERN BLOT for in vitro detection of antibodies to SIV in serum or plasma and is available in 10 or 30-strip kit formats. We also provide a bench top, a western blot processor, and the AUTOBLOT 3000. It controls incubation times, dispensing volumes and washing programs. Ten user-defined protocols can be programmed easily. Contact us now to know how we can help you get accurate and high-quality output with our products when performing the western blotting procedure.
0 notes
Text
Lzip pgc1a

Membrane Blocking and Antibody Incubations Electrotransfer to nitrocellulose membrane ( #12369).Ĭ.NOTE: Loading of prestained molecular weight markers ( #59329, 10 µl/lane) to verify electrotransfer and biotinylated protein ladder ( #7727, 10 µl/lane) to determine molecular weights are recommended. Load 20 µl onto SDS-PAGE gel (10 cm x 10 cm). Heat a 20 µl sample to 95–100☌ for 5 min cool on ice.Sonicate for 10–15 sec to complete cell lysis and shear DNA (to reduce sample viscosity).Immediately scrape the cells off the plate and transfer the extract to a microcentrifuge tube. Lyse cells by adding 1X SDS sample buffer (100 µl per well of 6-well plate or 500 µl for a 10 cm diameter plate).Aspirate media from cultures wash cells with 1X PBS aspirate.Treat cells by adding fresh media containing regulator for desired time.Protein Blotting A general protocol for sample preparation. Detection Reagent: SignalFire™ ECL Reagent ( #6883).ī.Secondary Antibody Conjugated to HRP: Anti-rabbit IgG, HRP-linked Antibody ( #7074).Pore size 0.2 µm is generally recommended. Blotting Membrane and Paper: ( #12369) This protocol has been optimized for nitrocellulose membranes.Blue Prestained Protein Marker, Broad Range (11-250 kDa): ( #59329).Biotinylated Protein Ladder Detection Pack: ( #7727).Primary Antibody Dilution Buffer: 1X TBST with 5% BSA for 20 ml, add 1.0 g BSA to 20 ml 1X TBST and mix well.Blocking Buffer: 1X TBST with 5% w/v nonfat dry milk for 150 ml, add 7.5 g nonfat dry milk to 150 ml 1X TBST and mix well.10X Tris Buffered Saline with Tween ® 20 (TBST): ( #9997) To prepare 1 L 1X TBST: add 100 ml 10X TBST to 900 ml dH 2O, mix.10X Tris-Glycine Transfer Buffer: ( #12539) To prepare 1 L 1X Transfer Buffer: add 100 ml 10X Transfer Buffer to 200 ml methanol + 700 ml dH 2O, mix.10X Tris-Glycine SDS Running Buffer: ( #4050) To prepare 1 L 1X running buffer: add 100 ml 10X running buffer to 900 ml dH 2O, mix.1X SDS Sample Buffer: Blue Loading Pack ( #7722) or Red Loading Pack ( #7723) Prepare fresh 3X reducing loading buffer by adding 1/10 volume 30X DTT to 1 volume of 3X SDS loading buffer.10X Tris Buffered Saline (TBS): ( #12498) To prepare 1 L 1X TBS: add 100 ml 10X to 900 ml dH 2O, mix.20X Phosphate Buffered Saline (PBS): ( #9808) To prepare 1 L 1X PBS: add 50 ml 20X PBS to 950 ml dH 2O, mix.NOTE: Prepare solutions with reverse osmosis deionized (RODI) or equivalent grade water. Solutions and Reagentsįrom sample preparation to detection, the reagents you need for your Western Blot are now in one convenient kit: #12957 Western Blotting Application Solutions Kit NOTE: Please refer to primary antibody product webpage for recommended antibody dilution. For western blots, incubate membrane with diluted primary antibody in 5% w/v BSA, 1X TBS, 0.1% Tween ® 20 at 4☌ with gentle shaking, overnight.

0 notes
Text
Western blot protocol

Place the membrane in blocking buffer (5X DiluObuffer ) for 1 hour on shaker at room temperature or overnight at 4 o C.Stain with Ponceaus to assess the quality of transfer and to mark the MW markers on membrane.For high MW/large glycosylated proteins, reduced the concentration of methanol form 20% to up to 15% and transfer for longer times (plate electrodes: Limit 250mA at 50 volts for 7 hours) the conditions are different for wire electrodes, overnight transfers will be preferred for HMW proteins at low methanol concentrations). Note: Immobilon membranes must be wetted in 100% methanol before using for transfer.Transfer the gel onto nitrocellulose/ immobilon (PVDF) membrane in Towbins buffer.Load the samples (20-65ug protein in 10-25ul volume) into their respective wells run the gel until dye reaches the bottom.Prepare SDS gel to the desired percentages (8% for antibodies >50kDa 10% for antibodies 30kDa 12% gel for antibodies Note: target proteins that are highly glycosylated/large in size should be avoided freeze thaw cycles or they form oligomers that will not enter in to the gel.The cells extracts were heated to 90 o C for 3 minutes before applying on SDS-PAGE.The samples were mixed in SDS-PAGE Laemmli’s buffer and reduced by adding 2.5% BME.Tissues can be grinded and then homogenized at 10% w/v in SolObuffer with protease inhibitors. Cells (106 cells are homogenized/ polytron /sonicate) in 1ml of SolObuffer with protease inhibitor A and B or with phosphatase inhibitors (depending upon experiment).The membrane exposure time to the film/imager will depend on the abundance of the protein and the detection system.Note: If using a commercial kit, follow the manufacturer’s instructions. Proceed to detection using an enhanced chemiluminescence (ECL) system.Wash the membrane with Washing Buffer for 3 x 10 minutes at room temperature.Incubate the secondary antibody in PBS with 1% BSA and 0.1% Tween-20 for 1 hour at room temperature with gentle agitation.Note: If you are using a secondary antibody conjugated to HRP, do not use solutions containing NaN 3 from this point on. Wash the membrane with Washing Buffer (PBS and 0.1% Tween-20) for 3 x 10 minutes at room temperature.Incubate for 2–3 hours at room temperature or overnight at 4☌ with gentle agitation.Note: If you use a blocking peptide as a negative control, refer to our Peptide Blocking Protocol for WB. Add the primary antibody diluted in PBS 1% BSA, 0.1% Tween-20, and 0.05% NaN 3.Block the membrane with Blocking Solution (PBS with 3% BSA and 0.05% NaN 3) for 2–5 hours at room temperature with gentle agitation.Note: High molecular weight proteins may need a longer transfer time (see Western Blot Protocol for High Molecular Weight (HMW) Proteins ). For dry transfer, follow the manufacturer’s instructions. Transfer to a nitrocellulose membrane at 200 mA for 2.5 hours at 4☌ for wet transfer.Run the gel according to the manufacturer’s instructions.*Load 80–100 µg tissue lysate/lane or lysate from 2–5 x 10 5 cells/lane. Heat the samples in Laemmli buffer at 70–100 ☌ for 10 minutes.If you have any problems, please see our extensive troubleshooting guides. An important and widely used tool in biology, western blotting involves separating proteins according to their size via gel electrophoresis and then transferring them to a membrane to detect with specific antibodies. Subscribe – Newsletters and Email UpdatesĪ western blot (WB) lets you detect and evaluate the size of specific proteins in cell or tissue extracts.

0 notes
Text
DNA - FINGERPRINTING
DNA fingerprinting is also know as DNA profiling is a process of determining an individuals DNA characteristics. This is a forensic technique in criminal investigations, comparing criminal suspect's profiles to DNA to identify the like hood of their involvement in the crime. Also can be used for paternity tests. 99 % of human DNA sequences are the same in every person. Remaining sequences are different . It makes every individual unique.

This is used to distinguish and identify the individuals of a same species by using their DNA samples. These biological samples may be blood, hair, saliva, semen, body tissue cells. The DNA samples are isolated first. Then digested by restriction endonucleases. After this electrophoretic separation of fragments are done. These fragments are transferred into nylon or nitrocellulose membrane. This is followed by probing, hybridization and autoradiography. From this banding pattern is analyzed.
youtube
FOR FURTHER INFORMATION VISIT : -
https://readitupwithswathy.blogspot.com/2022/04/dna-fingerprinting.html
#dna#dna fingerprinting#dna profiling#forensic science#crime#suspect#dna sequence#restriction endonucleases#nylon#nitrocellulose membrane#probe#hybridization#autoradiography#banding pattern#genetics#genome
1 note
·
View note
Link
Axiflow offers Nitrocellulose membrane, HPLC Columns, Filter Paper, Centrifuge Tubes and HPLC Vials at best prices. Our technical team works with clients to deliver the best solutions for their application.
2 notes
·
View notes
Text
Transfer Membrane Market | Increasing R&D Spending By Pharmaceutical and Biotechnology Companies

According to the new market research report " Transfer Membrane Market by Type (PVDF, Nitrocellulose, Nylon), Transfer Method (Tank, Semi-dry, Dry), Application (Western, Northern, Southern Blot, Protein Sequencing), End user (Academia, Diagnolab, Pharmaceutical Companies) - Global Forecast to 2023" the transfer membrane market is projected to reach USD 187.9 million by 2023 from USD 174.8 million in 2018, at a CAGR of 1.5% during the forecast period.
Factors such as increasing public and private funding for life science research, the significantly high prevalence of target diseases across the globe, and increasing R&D spending by pharmaceutical and biotechnology companies are expected to drive the growth of market.
Browse 64 market data Tables and 35 Figures spread through 124 Pages
Download Brochure: https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=36975865
The report analyzes the global market by type, transfer method, application, end user, and region.
On the basis of type, the market is divided into nylon membranes, nitrocellulose membranes, and PVDF membranes. In 2018, PVDF Membranes are expected to command for the major share of the market. This can be attributed to the advantages of PVDF membranes over its counterparts, such as better protein retention, strength, chemical compatibility, and wide applications in western blotting.
Based on end user, the market is segmented into academic and research institutes, pharmaceutical and biotechnology companies, diagnostic laboratories, and other end users. Among these, the academic and research institutes segment is expected to account for the largest share of the transfer membrane market in 2018, owing it to the rising financial support from private as well as government bodies for life science research in various nations.
Get Sample Copy of This Report
@https://www.marketsandmarkets.com/requestsampleNew.asp?id=36975865
Regional Growth, Development and Demand Analysis:
North America is expected to account for the largest share of the global transfer membrane market in 2018, followed by Europe. The large share in the North American region is mainly attributed to the presence of leading transfer membrane manufacturers in the region, availability of government and private financial support for life science research, and high target disease prevalence in the region.
Some of The Major Players In Transfer Membrane Market :
Merck KGaA (US), Thermo Fisher Scientific (US), Bio-Rad Laboratories (US), GE Healthcare (US), PerkinElmer (US), Pall Coporation (US), Advansta (US), GVS (Italy), Santa Cruz Biotechnology (US), Abcam (UK), ATTO Corporation (Japan), Carl Roth (Germany), Macherey-Nagel (Germany), Azure Biosystems (US), and Axiva Sichem Biotech (India)
#Transfer Membrane Market#PVDF Transfer Membranes#Nitrocellulose Transfer Membranes#Nylon Transfer Membranes
1 note
·
View note
Conversation
Tom Wilson: Can someone please explain to me what evaporated milk is? Wouldn’t that just be gas by definition?
TJ Oshie: No no, it’s what's left behind after the milk has been evaporated cause only the water goes, not the other stuff.
Tom: THERE’S WATER IN MILK?!
TJ: WHAT DID YOU THINK THE LIQUID WAS?
Tom: IDK ISN'T MILK ITS OWN LIQUID?
TJ: NO IT’S MILK STUFF MIXED WITH WATER!
Tom: MILK STUFF? DOESN'T IT JUST COME FROM THE COW’S TIT?
TJ: IT'S LIKE TIT JUICE, THERE IS WATER IN JUICE AND THERE IS WATER IN MILK!
Lars Eller: It’s fat droplets suspended in water, with some nutrients and so forth dissolved in it. You know, like ranch dressing. Evaporated milk is just dehydrated milk.
John Carlson: Obsessed with the fact that Tom assumed milk was its own element on the periodic table.
Tom: It’s the forbidden 119th element. Here, I made a chart.
Garnet Hathaway: Tom seems to have classified it as a special case of halfnium, reclassified as a lanthanide. This has fascinating implications for electron orbital geometry. Anyway it’s a rare earth metal apparently.
Tom: Yes I definitely classified it intentionally and knew exactly what I was doing when I put it with the lanthanides because I am never wrong. MILK *IS* A RARE EARTH METAL.
Garnet: I thought so, I took one look at your classification and immediately thought “this is definitely someone with a deep understanding of how the periodic table works”. I’m glad that we have reached a consensus on the expected elemental properties of milk.
Nic Dowd: MILK IS SEVERAL COMPOUNDS PLEASE Y'ALL ARE KILLING ME OVER HERE. We have a container of dry milk because in addition to a little fat and sugars, it contains proteins, which settle into the pores of nitrocellulose membranes, making sure analytical proteins (specific antibodies) don’t get trapped.
Nic: We could just use casein (one of the proteins in milk), but milk is much cheaper and can also be found at Walmart.
Garnet: No milk is a lanthanide, keep up.
Evgeny Kuznetsov: A cube of milk with 3 inches of edge length can blow up the galaxy.
Garnet: Our galaxy is actually the result of such an explosion. That’s why we call it the Milky Way.
#source: tumblr#long post#tom wilson#tj oshie#lars eller#john carlson#garnet hathaway#nic dowd#evgeny kuznetsov#washington capitals#incorrect capitals quotes#incorrect nhl quotes#incorrect hockey quotes
30 notes
·
View notes
Text
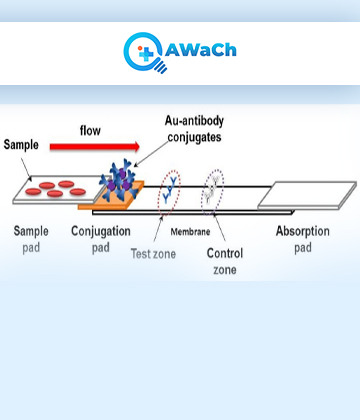
Lateral Flow Assay
A lateral flow assay (LFA), also known as a lateral flow test or rapid diagnostic tests (RDTs), is a simple and rapid diagnostic tool used to detect the presence or absence of a specific substance or analyte in a sample.
LFAs are commonly used in medical diagnostics, food safety testing, environmental monitoring, and other fields. In case of medical diagnostic tests based out of lateral flow assay, a specific biomarker or a protein is biochemically interacted with its counter-molecule like antibodies to help aid detection. The basic principle of a lateral flow assay involves the capillary action of fluids through a strip of nitrocellulose membrane, which contains specific components to facilitate the detection of the target analyte. The test strip typically consists of the major components like, Sample Pad, Conjugate Pad, Nitrocellulose Membrane, Absorbent Pad and a Backing Laminate on which all components of a LFA are assembled. LFA based test can detect a single analyte or could detect more than one analyte thus making a multiplex LFAs.
0 notes
Photo

Nitrocellulose Transfer Membrane Market Size By Technology By Operating Principle By End-Use, Industry Outlook Report, Regional Analysis, Application Potential, Price Trends, Competitive Market Share & Forecast, 2019 – 2024 Nitrocellulose Transfer Membrane Market Report The report offers an overview of the Nitrocellulose Transfer Membrane with the help of application segments and geographical regions (United States, Europe, China, Japan, Southeast Asia, India, Central & South America, ROW) that govern the market currently.
0 notes
Text
ANALYSIS OF WESTERN BLOT PROCEDURES
Proteins play many vital roles in our bodies. Essentially proteins or polypeptides are complex compounds of amino acids that are needed by our bodies to regulate, function and construct organs and tissues in the body.
Proteins are integral to organisms, helping almost every cell process and are also critical to metabolism. For these reasons, detecting the presence or absence of protein, their size or molecular weight is vital. Protein analysis is crucial in
investigating a disease,
detecting the presence of allergenic protein in food samples and
evaluating whether genetic manipulation experiments were successful or not
There are different types of proteins in terms of their size and the arrangement of amino acids, having diverse molecular structures, nutritional characteristics, and chemical properties. Given the diverse nature of this vital biological element, there are three traditional techniques that researchers and scientists use for protein analysis:
protein separation,
western blotting, and
protein identification.
In this blog, our team at HHC will help you explore the western blot technique to understand the procedure and its uses for protein analysis
WHAT IS WESTERN BLOTTING?
Developed by Harry Towbin and his colleagues in 1979, the western blot procedure is a typical cell and molecular biology procedure widely used in the analysis of proteins. Often known as protein immunoblotting, the method allows researchers to determine the presence, size and quantity of particular proteins in a given sample. Three elements comprise the western blotting method:
1) separation by size,
2) transfer to a solid support or membrane, and
3) marking target protein using a proper primary and secondary antibody to visualise.
Western blotting is very useful for identifying individual proteins from a complicated mixture of proteins isolated from cells that may have similar characteristics or sizes.
THE WESTERN BLOT PRINCIPLE
The Western blotting technique is based on the use of polypropylene gel electrophoresis and antibodies. In this technique, researchers can separate and identify proteins based on their molecular weight and type from a gel-like sample using electrophoresis, which acts as a molecular sieve. Next, the separated proteins are transferred to a membrane that produces a band for each protein. The membrane is then incubated with labelled antibodies specific to the protein of interest to create a coloured band. These antibodies can be identified, and the size and abundance of the bound proteins can be analysed in relation to established standards or controls.
HOW IS THE WESTERN BLOT PROCEDURE PERFORMED?
The western blotting technique is performed in 7 steps. They are
1) Sample preparation
2) Gel electrophoresis
3) Proteins transfer
4) Blocking
5) Primary Antibody incubation
6) Secondary Antibody incubation
7) Protein detection & analysis
1) Sample preparation
The first step in the western blotting technique involves the extraction of proteins from different samples such as cells or tissues. The extracted samples need to be broken down by a homogeniser or sonication. Phosphatase inhibitors and protease are used to avoid the digestion of the sample at cold temperatures. Once the protein is extracted, the quantity of proteins needs to be determined.
2) Gel electrophoresis
This step involves the separation of the proteins based on their charge and molecular weight. This is done using polypropylene gel electrophoresis like sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) or native PAGE. Usually, separating gels come in 5%, 8%, 10%, 12% or 15%. The size of the prey protein is taken into consideration when choosing the right percentage of separating gel. The relation is inverse. Higher percentage gels are suitable for proteins that weigh less.
3) Proteins transfer or blotting
Once the proteins are separated using gel electrophoresis, they are transferred to a solid support membrane such as nitrocellulose (NC) or polyvinylidene difluoride (PVDF) membrane to facilitate antibody probing. Protein transfers can be performed using various techniques: diffusion transfer, capillary transfer, and vacuum blotting. But the most used is the electroblotting technique, wherein an electric current is applied to the protein gel sandwiched between the membrane and the filter papers. An electric field is aimed perpendicular to the gel’s surface that extracts the protein from the gel and transfers them onto the membrane in a tight manner.
Protein gel transfer through electroblotting can be performed in different ways, such as wet, semi-dry and dry transfer. Wet or semi-dry transfer strategies usually provide reliable results as the buffering is more flexible. However, these methods are time-consuming. On the other hand, the dry transfer method is efficient and quick but offers less buffering flexibility.
4) Blocking
The next and crucial step in the western blot procedure is blocking. Because blotting membranes have a high affinity for proteins, blocking any residue binding sites after the transfer is critical to avoid further non-specific binding of the assay detection antibodies. This is accomplished by exposing the membrane to a liquid that contains protein, such as milk or serum. BSA and non-fat dry milk are the most used typical blockers.
When submerging the membrane in the diluted protein solution, the proteins in the solution bind to all the areas of the membrane that are not bound by the prey protein. The process reduces the “noise” in the western blot’s result to provide more precise findings.
After blocking, antibody probing is performed. There are two strategies for this:
direct detection using a single or primary antibody and
indirect detection using a secondary antibody
For the western blot technique, the indirect method is used most often.
5) Primary Antibody incubation
At this stage, the primary antibody is used to probe the membrane and binds to the prey protein. Depending on the antigen to be determined, the primary antibody is selected.
In the direct detection method, the membrane is then incubated with the secondary antibody to bind to the primary antibody and is detectable.
6) Secondary Antibody incubation
When using indirect assay detection, it is vital to wash the membrane using an antibody-buffer solution to reduce background and remove excess or unbound antibodies before the secondary antibody is used to incubate the membrane. After the wash, the specific enzyme-conjugated secondary antibody incubates the membrane. When the second antibody incubation is performed, the labelled secondary antibody binds to the primary antibody that has interacted with the prey protein and is detectable. The selection of the suitable secondary antibody is based on the variety or the species of the primary antibody.
7) Protein detection & analysis
At this final stage, protein detection is achieved when the conjugated enzyme interacts with a substrate introduced to the membrane, causing a chemical change in the form of a coloured substance allowing the researcher to mark the location and the densitometry of the prey protein. Horseradish peroxidase (HRP) and alkaline phosphatase (AP) are the most used enzymes. Due to its stability, suitability for most conjugations, and affordability, HRP is frequently preferred.
Protein detection and the subsequent visualisation can be performed using chemiluminescent, colourimetric, radioactive and fluorescent detection techniques, the most common being chemiluminescent detection.
When using the chemiluminescent detection method, it is essential to note that the duration of the signal or the chemical change lasts only until the substrate reacts with the conjugated enzyme (usually between 1 and 24 hours), making it a very sensitive technique. Creating a permanent record of the signal is possible by using an X-ray film or using digital imaging.
WHAT ARE THE USES OF THE WESTERN BLOTTING TECHNIQUE?
In brief, the western blotting procedure determines the presence or absence, size, and abundance of target proteins in a sample which is beneficial for various scientific reasons across many fields of study. In this regard, the technique proves beneficial to evaluate the following:
Protein DNA interactions
Protein-protein interactions
Post-translational modifications (PTMs)
Protein isoform detection
Antibody characterisation
Epitope mapping
Subcellular protein localisation
Among all the applications of the Western blotting method, the most common are as below.
Diagnosis of infectious diseases
Although time-consuming when compared to other alternatives like the PCR technique, Western Blot proves highly useful in testing and confirming cases of HIV (human immunodeficiency virus), BSE (bovine spongiform encephalopathy), Lyme disease and aspergillosis.
Biomarking non-infectious diseases
As Western Blot is an antibody-based method, the technique often supports and produces reliable results for detecting non-infectious diseases using HTS (High throughput screening). For example, when determining cancers, incongruous isoforms of proteins can become potential markers of the pathology of the disease. Autoantibodies may also indicate an autoimmune disease.
Identifying the differences in protein levels between groups or over time
Western Blot is very efficient in determining changes in protein levels or even different cycle phases for samples studied in a temporal experiment. Additionally, contrasting samples from various disease or treatment groups can draw attention to protein-level changes that could point to a disease’s underlying aetiology or impact a treatment’s effectiveness.
Determine if a protein is expressed
When performing experimental genomic manipulations, Western Blot can help compare the changes made at the genomic level with those that occur at protein levels. (for example, is a novel protein expressed in the expected tissue or location? Or is its expression truncated or lost compared to a wild-type strain?).
Tagged protein detection
Tags can be engineered and assigned to proteins during genomic manipulations facilitating the identification of proteins attached to them from samples of a system under study or of a species. This method enables researchers to localise the protein and understand its course. The process can prove particularly useful for checking recombinant proteins produced through experiments.
Compared to methods like PCR (polymerase chain reaction), western blotting is not usually considered a quantitative procedure. However, the direct identification of proteins it delivers is efficient and has proven significantly advantageous for confirmatory testing for disease diagnosis.
The key to achieving reliable results from a successful blot procedure is the selection of the correct antibody and identifying the optimal concentration. The use of the right equipment is equally essential to get optimised results.
HHC provides in the ZeptoMetrix WESTERN BLOT for in vitro detection of antibodies to SIV (Simian Immunodeficiency Virus, the lentivirus most closely related to Human HIV) in serum or plasma. It is available in 10 or 30 strip kit formats. Additionally, we offer a bench top, western blot processor: the AUTOBLOT 3000, which controls incubation times, dispensing volumes and washing programs. The AUTOBLOT 3000 can programme ten user-defined protocols.
If you want to add the most updated lab equipment to enhance the protein detection services of your lab, don’t hesitate to get in touch with Helvetica Health Care Website now!
Originally Posted On HHC
0 notes
Link
The global market size of PVDF is $XX million in 2018 with XX CAGR from 2014 to 2018, and it is expected to reach $XX million by the end of 2024 with a CAGR of XX% from 2019 to 2024. There are 3 key segments covered in this report: geography segment, end use/application segment, and competitor segment. For geography segment, regional supply, application-wise, and type-wise demand, major players, price is presented from 2013 to 2023. This report covers following regions: * North America * South America * Asia & Pacific * Europe * MEA (Middle East and Africa) The key countries in each region are taken into consideration as well, such as United States, China, Japan, India, Korea, ASEAN, Germany, France, UK, Italy, Spain, CIS, and Brazil, etc. For end use/application segment, this report focuses on the status and outlook for key applications.
End users sre also listed. * Application I * Application II * Application III For competitor segment, the report includes global key players of PVDF as well as some small players. The information for each competitor includes
#pvdf injection molding#solvay pvdf 6010#schedule 80 pvdf pipe dimensions#why is pvdf membrane activated by methanol#when to use pvdf or nitrocellulose
0 notes