#distributed clinical trials
Text
How does one develop vaccines for emerging infectious diseases?
Emerging infectious diseases pose significant threats to global public health. Rapid and effective development of vaccines is crucial in mitigating the impact of these diseases. This article explores the process of developing vaccines for emerging infectious diseases, highlighting the key steps involved and the challenges faced. Understanding the development process is essential to appreciate the…
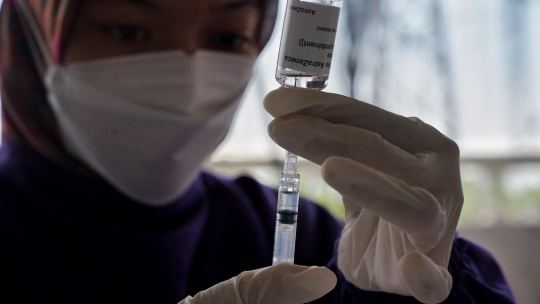
View On WordPress
#clinical trials#community immunity#disease outbreak control#Emerging infectious diseases#Equitable access#global health security#multidisciplinary approach#pathogen identification#preclinical studies#preparedness and response#public health collaboration#rapid response#regulatory approval#research and development#safety and efficacy#target antigen selection#vaccine design#vaccine development#Vaccine distribution#vaccine production
1 note
·
View note
Text
The Best News of Last Week
🦾 - High-Five for Bionic Hand
1. Houston-area school district announces free breakfast and lunch for students

Pasadena ISD students will be getting free breakfast and lunch for the 2023-24 school year, per an announcement on the district's social media pages.
The 2023-24 free lunch program is thanks to a Community Eligibility Provision grant the district applied for last year. The CEP, which is distributed by the Department of Agriculture, is specially geared toward providing free meals for low-income students.
2. Dolphin and her baby rescued after being trapped in pond for 2 years
youtube
A pair of dolphins that spent nearly two years stuck in a Louisiana pond system are back at sea thanks to the help of several agencies and volunteers.
According to the Audubon Nature Institute, wildlife observers believe the mother dolphin and her baby were pushed into the pond system near Grand Isle, Louisiana, during Hurricane Ida in late August 2021.
3. Studies show that putting solar panels over waterways could boost clean energy and conserve water. The first U.S. pilot project is getting underway in California.

Some 8,000 miles of federally owned canals snake across the United States, channeling water to replenish crops, fuel hydropower plants and supply drinking water to rural communities. In the future, these narrow waterways could serve an additional role: as hubs of solar energy generation.
4. Gene therapy eyedrops restored a boy's sight. Similar treatments could help millions

Antonio was born with dystrophic epidermolysis bullosa, a rare genetic condition that causes blisters all over his body and in his eyes. But his skin improved when he joined a clinical trial to test the world’s first topical gene therapy.
The same therapy was applied to his eyes. Antonio, who’s been legally blind for much of his 14 years, can see again.
5. Scientists develop game-changing vaccine against Lyme disease ticks!

A major step in battling Lyme disease and other dangerous tick-borne viruses may have been taken as researchers announced they have developed a vaccine against the ticks themselves.
Rather than combatting the effects of the bacteria or microbe that causes Lyme disease, the vaccine targets the microbiota of the tick, according to a paper published in the journal Microbiota on Monday.
6. HIV Transmission Virtually Eliminated in Inner Sydney, Australia

Sydney may be the first city in the world to end AIDS as a public health threat by 2030. Inner Sydney has reduced new HIV acquisitions by 88%, meaning it may be the first locality in the world to reach the UN target to end AIDS as a public health threat by 2030
7. New bionic hand allows amputees to control each finger with unprecedented accuracy

In a world first, surgeons and engineers have developed a new bionic hand that allows users with arm amputations to effortlessly control each finger as though it was their own body.
Successful testing of the bionic hand has already been conducted on a patient who lost his arm above the elbow.
----
That's it for this week :)
This newsletter will always be free. If you liked this post you can support me with a small kofi donation:
Support this newsletter ❤️
Also don’t forget to reblog.
895 notes
·
View notes
Text
12 notes
·
View notes
Text
"Several individuals and organizations opposing gender-affirming care have contended that the evidence supporting this type of treatment is of "low quality." Ohio Representative Gary Click, for example, argued that this supposed "low quality" evidence should warrant a ban on gender-affirming care. A controversial Reuters article, which was met with backlash from transgender healthcare providers and advocates, labeled the evidence as either "low" or "very low," insinuating a lack of solid backing for the care. However, U.S. District Judge Moody vigorously countered this notion.
The term "high quality" evidence refers to a specific category within the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system. In essence, the GRADE system reserves the "high quality" designation for randomized controlled trials (RCTs) exclusively. Judge Moody, in examining medical research and its application in either authorizing or prohibiting treatments, determined that numerous medications and medical guidelines are based on evidence deemed "very low-quality" or "low-quality," since it would be either impossible or unethical to conduct randomized clinical trials. In fact, merely one in ten medical treatments rely on "high quality" evidence.
In the case of gender-affirming care, Judge Moody identified Fact 184, asserting that attempting to conduct a randomized controlled trial for this type of care would be both impossible and ethically indefensible. This is due to the fact that withholding known beneficial care from patients contravenes the ethical guideline of "do no harm." Additionally, it would be unfeasible to blind both patients and doctors to the administered care, as the physical effects—such as changes in hair growth and body fat distribution—would become immediately apparent.
Given that no other forms of care are banned by the state based on their GRADE ranking, Judge Moody deemed the ban to be discriminatory."
Really glad a court addressed a transphobe talking point that I always found personally frustrating as an amateur lab coat user.
When transphobes start saying that evidence for HRT is "low quality" because it isn't supported by randomized and controlled double-blind studies it just becomes clear to me that they either don't know what that actually entails and are just repeating something they heard uncritically or they hope their audience is similarly ignorant.
Even putting the ethics aside, you cannot actually do such an experiment with medicine that physically makes you grow breasts or develop facial hair.
18 notes
·
View notes
Text
The Discovery of the first antipsychotic, Chlorpromazine, AKA Thorazine
In 1933, the French pharmaceutical company Laboratoires Rhône-Poulenc began to search for new anti-histamines. In 1947, it synthesized promethazine, a phenothiazine derivative, which was found to have more pronounced sedative and antihistaminic effects than earlier drugs.[50]: 77 A year later, the French surgeon Pierre Huguenard used promethazine together with pethidine as part of a cocktail to induce relaxation and indifference in surgical patients. Another surgeon, Henri Laborit, believed the compound stabilized the central nervous system by causing "artificial hibernation", and described this state as "sedation without narcosis". He suggested to Rhône-Poulenc that they develop a compound with better stabilizing properties.[51] In December 1950, the chemist Paul Charpentier produced a series of compounds that included RP4560 or chlorpromazine.[6]
Chlorpromazine was distributed for testing to physicians between April and August 1951. Laborit trialled the medicine on at the Val-de-Grâce military hospital in Paris, using it as an anaesthetic booster in intravenous doses of 50 to 100 mg on surgery patients and confirming it as the best drug to date in calming and reducing shock, with patients reporting improved well being afterwards. He also noted its hypothermic effect and suggested it may induce artificial hibernation. Laborit thought this would allow the body to better tolerate major surgery by reducing shock, a novel idea at the time. Known colloquially as "Laborit's drug", chlorpromazine was released onto the market in 1953 by Rhône-Poulenc and given the trade name Largactil, derived from large "broad" and acti* "activity".[6]
Following on, Laborit considered whether chlorpromazine may have a role in managing patients with severe burns, Raynaud's phenomenon, or psychiatric disorders. At the Villejuif Mental Hospital in November 1951, he and Montassut administered an intravenous dose to psychiatrist Cornelia Quarti who was acting as a volunteer. Quarti noted the indifference, but fainted upon getting up to go to the toilet, and so further testing was discontinued (orthostatic hypotension is a known side effect of chlorpromazine). Despite this, Laborit continued to push for testing in psychiatric patients during early 1952. Psychiatrists were reluctant initially, but on 19 January 1952, it was administered (alongside pethidine, pentothal and ECT) to Jacques Lh. a 24-year-old manic patient, who responded dramatically, and was discharged after three weeks having received 855 mg of the drug in total.[6]
Pierre Deniker had heard about Laborit's work from his brother-in-law, who was a surgeon, and ordered chlorpromazine for a clinical trial at the Sainte-Anne Hospital Center in Paris where he was Men's Service Chief.[6] Together with the Director of the hospital, Jean Delay, they published their first clinical trial in 1952, in which they treated 38 psychotic patients with daily injections of chlorpromazine without the use of other sedating agents.[52] The response was dramatic; treatment with chlorpromazine went beyond simple sedation with patients showing improvements in thinking and emotional behaviour.[53] They also found that doses higher than those used by Laborit were required, giving patients 75–100 mg daily.[6]
Deniker then visited America, where the publication of their work alerted the American psychiatric community that the new treatment might represent a real breakthrough. Heinz Lehmann of the Verdun Protestant Hospital in Montreal trialled it in 70 patients and also noted its striking effects, with patients' symptoms resolving after many years of unrelenting psychosis.[54] By 1954, chlorpromazine was being used in the United States to treat schizophrenia, mania, psychomotor excitement, and other psychotic disorders.[14][55][56] Rhône-Poulenc licensed chlorpromazine to Smith Kline & French (today's GlaxoSmithKline) in 1953. In 1955 it was approved in the United States for the treatment of emesis (vomiting). The effect of this drug in emptying psychiatric hospitals has been compared to that of penicillin and infectious diseases.[52] But the popularity of the drug fell from the late 1960s as newer drugs came on the scene. From chlorpromazine a number of other similar antipsychotics were developed. It also led to the discovery of antidepressants.[57]
5 notes
·
View notes
Text
YOUNG HAWAIIANS & PACIFIC ISLANDER ADULTS WITH HIGHEST CANCER RATES
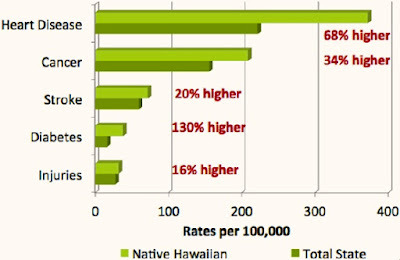
NBC News - May 19, 2023
New research shows that Native Hawaiian and Pacific Islanders between the ages of 20 and 49 have the highest death rates from any type of cancer among all racial groups of that age bracket.
The findings, published last month by the National Cancer Institute, weren’t immediately apparent in previous research because federal data has traditionally grouped together those of Asian, Native Hawaiian and Pacific Islander descent, concealing the disparities, the report said.
“We have shown the importance of disaggregating Asian and NHPI individuals, as these groups have disparate cancer mortality rates that are hidden when analyzed together,” researchers wrote. “Policies aimed at equitable cancer prevention, early detection, and treatment, as well as disaggregation of data for racial/ethnic subpopulations are needed to address disparities in cancer mortality across racial/ethnic groups.”
Those of Asian descent, the manuscript pointed out, have the lowest cancer death rates across every age group and, when combined with data on Native Hawaiian and Pacific Islanders, the death rate largely ended up reflecting the low incidence of the disease among Asian Americans
While the Office of Management and Budget disaggregated the Native Hawaiian and Pacific Islander populations from Asian Americans in 1997, the National Center for Health Statistics didn’t release single-race mortality data until almost two decades later, when all states implemented the new classification on death certificates, the report said. Therefore, data on Native Hawaiian and Pacific Islanders, who represent an estimated 0.4% of the U.S. population, remained “masked.”
The report, which also looked at cancer death rates across other racial groups, showed additional disparities. Data on cancer mortality rates among males showed Black men with the highest numbers, followed by whites and Latinos. In looking at women, the mortality rates were highest among Black women. Native Hawaiian and Pacific Islander women came second, followed by white females.
High cancer death rates in Black, Native Hawaiian and Pacific Islander communities are likely due in part to unequal access to health care, the report said. Marginalized communities are also more likely to receive “suboptimal” cancer treatment that may not be consistent with the recommended clinical practice guidelines, and are less likely to be included in clinical trials, the report noted.
Structural racism was another underlying cause of the racial and ethnic gaps in health. The American Cancer Society’s guidelines for cancer prevention, the researchers point out, focus on “modifiable lifestyle factors” like obesity and physical activity. However, Black, Latino, American Inuit, American Indian, Native Hawaiian and Pacific Islander communities, which have high obesity rates, are more likely to live in communities with food deserts, high rates of economic insecurity and greater barriers to physical activity.
The legacy of U.S. colonization of Hawai`i and the long history of “Western interference” has also contributed to health disparities among the Native Hawaiian and Pacific Islander populations, Dr. Loïc Le Marchand, associate director for population sciences at the University of Hawai`i Cancer Center, told NBC News.
“If we compare Polynesians in the South Pacific and Native Hawaiians, not only is the level of obesity higher [in Native Hawaiians], but the type of obesity is different,” Le Marchand said. “The distribution of fat is different. … It’s not just linked to eating more, but it’s also linked to composition of diet.”
More disaggregated data and better information on specific populations are critical, Le Marchand said, as they can make a difference in the decision-making. And while there are some programs in place in Hawaii and across the country that address these gaps in health care, they are still limited.
“Native Hawaiians have been disadvantaged, understudied ... for many decades,” Le Marchand said. “Those health issues exist and need to be addressed.”
Le Marchand said that when it comes to treating Native Hawaiian and Pacific Islander populations and other marginalized groups, it’s “not just funding a program here and there.” Integrating leaders from these communities is critical to dispense appropriate care and treatment, he said.
2 notes
·
View notes
Text
Never heard of Darier disease until I saw a pt today who has it.
Darier disease, also known as Darier-White disease, keratosis follicularis, or dyskeratosis follicularis, is a rare autosomal dominant genodermatosis characterized by a persistent eruption of red-brown, keratotic papules scattered to confluent in a seborrheic distribution, nail abnormalities, pitting of palms and soles, and mucosal changes. The disease usually starts around puberty and runs a chronic course with exacerbations induced by sun exposure, heat, friction, or infections.
There is no cure for Darier disease. The goals of treatment are the improvement of skin appearance, relief of symptoms (eg, irritation, pruritus, or malodor), and prevention or treatment of infectious complications.
Topical treatments — Topical therapies for Darier disease are aimed at controlling skin inflammation, reducing hyperkeratosis, and flattening the papular lesions. Topical treatments include:
●Topical corticosteroids – Low- to medium-potency topical corticosteroids (groups 4 to 6 (table 1)) may reduce skin inflammation. Although their efficacy has not been evaluated in clinical trials, they are frequently used in patients with Darier disease on the basis of their anti-inflammatory properties and clinical experience.
●Topical retinoids – Topical retinoids, including tretinoin 0.1% [71], adapalene 0.1% [72], and tazarotene 0.05% [73], have been used in patients with mild or localized disease to reduce hyperkeratosis and flatten papular lesions. As monotherapy, their efficacy has not been evaluated in clinical trials. Based upon clinical experience, topical retinoids are preferred to other topical agents, such as topical vitamin D analogues. Irritation is common and can be reduced by alternate-day application and liberal use of emollients.
●Other topical agents – There are isolated reports of response to treatment with topical fluorouracil, tacrolimus [74], pimecrolimus [75], tacalcitol [76], and diclofenac sodium 3% gel [77]. Several case reports suggest that topical fluorouracil (1 or 5%) may be particularly efficacious in combination with oral alitretinoin [78]. (See 'Systemic treatments' below.)
Systemic treatments
●Oral retinoids – Oral retinoids, including acitretin, isotretinoin, etretinate, and alitretinoin, decrease hyperkeratosis, smoothen papules, reduce odor, and produce significant clinical improvements in most patients with severe or generalized Darier disease. Etretinate is no longer available in the United States, Canada, and many other countries and has been replaced by acitretin. Oral alitretinoin is available in Europe and Canada but not in the United States.
The efficacy of oral retinoids for the treatment of Darier disease has not been evaluated in randomized trials, and evidence is limited to small observational studies:
•In a double-blind study including 26 patients with Darier disease treated with 30 mg per day of acitretin or etretinate for four months, remission or marked improvement was achieved in 18 of 24 patients who took the drug for the duration of the study period, without difference between the two drugs.
•Alitretinoin, a vitamin A analogue known to have anti-inflammatory and immunomodulating properties, has been successfully used in a few patients [82,83] but may induce considerable adverse effects, including worsening of the skin lesions, pyogenic granuloma, erosions, and fever [84].
Oral retinoids do not induce prolonged remission in Darier disease, and long-term treatment is needed to prevent relapse.
Adverse effects of systemic retinoids include mucosal dryness, headache, delayed dark adaptation, diffuse hair loss (telogen effluvium), pyogenic granuloma, photosensitivity, hyperlipidemia, transaminase elevation, and skeletal hyperostosis. Oral retinoids are teratogenic, and appropriate counseling and contraception must be given to women of childbearing age.
●Other treatments – In a small case series, low-dose naltrexone at 5 mg per day showed beneficial effects in moderate, but not severe, cases [85]. In a single case report, low-dose intravenous immunoglobulins significantly reduced crusted lesions and itching [86].
Surgical or destructive therapies — Approaches for localized hypertrophic, erosive, or recalcitrant lesions that are resistant to conventional treatment include dermabrasion; electrosurgery; laser ablation; surgical excision; photodynamic or photon and electron beam therapy; and injection of botulinum toxin similar to the more established topical treatment targeting excessive sweating in Hailey-Hailey disease (benign chronic familial pemphigus; OMIM #169600) [87-100]. Recurrence of lesions following excision and physical treatment is common.
3 notes
·
View notes
Text
Drug Abuse/Substance Use Disorder, pt. 1
Introduction to Drug Abuse & Addiction
- Psychoactive drugs have been a part of human culture since antiquity.
- Many psychoactive substances (such as nicotine, caffeine, morphine, cocaine, and THC) are made by plants and were available to ancient peoples.
- 200 years ago, mostly alcohol, tobacco, and opium or laudanum (opium extract in alcohol) were available in the USA.
Some of the events that led to current drug use:
Development of hypodermic syringes allowed injection into the bloodstream.
Advances in chemistry (e.g. morphine was purified from opium, and cocaine from coca). In more concentrated form, these drugs are more addictive.
- Lack of drug control laws resulted in these drugs being used in tonics and patent medicines.
- Heroin was synthesized by Bayer Laboratories in 1874 and was first marketed as a nonaddictive substitute for codeine to control coughs.

Government Intervention
During the 20th century, the federal government increasingly controlled the commercialization of drugs, beginning with the Pure Food and Drug Act of 1906:
All active ingredients should be placed on drug labels.
Food and drugs should meet established purity levels.
Food and Drug Administration (FDA) was created.
The Harrison Narcotics Tax Act (1914) controlled the use of opiates and cocaine:
Taxes on production, importation, and distribution
Prohibited non-medical use. Doctors could not prescribe opiates to addicts, because addiction was not considered a disease.
18th Amendment to the US Constitution (1920): Prohibition of the manufacture, transportation, and sale of alcoholic beverages (repealed in 1933)
The Marijuana Tax Act of 1937 banned nonmedical use of cannabis and levied a tax on importers, sellers, and dispensers of marijuana (overturned by US Supreme Court in 1969)
The Controlled Substances Act (1970) established five schedules of controlled substances and created the Drug Enforcement Agency (DEA).

- The federal government became more involved in drug regulation as a result of increased drug use or perceived societal danger of drug use.
- Existing laws are not consistent with scientific evidence (e.g. nicotine is more addictive than marijuana).
{Note: I can’t locate any evidence that indicates hallucinogens are addictive at all, at least not in a physiological sense. Marijuana/THC is recognized as having medical use in some states, but not in others. MDMA has been in stage 3 clinical trials for quite some time now and was supposed to be decriminalized or legalized for therapeutic purposes a few years back, but it still hasn’t happened yet.}

Features of Drug Abuse & Addiction
Addiction is complex, and a precise definition is difficult. It can include physical dependence:
Withdrawal symptoms if the person stops taking the drug (muscle aches and cramps, anxiety attacks, sweating, nausea, and possibly convulsions/death)
Not all drugs produce physical dependence. For example, heroin use causes an intense physical dependence, whereas nicotine does not (even though it’s roughly just as addictive).
Addictive behavior: the addict is driven by a craving, a strong urge to take the drug.
Individuals remain addicted for long periods of time, and drug-free periods (remissions) are often followed by relapses in which drug use recurs, despite negative consequences.
American Psychiatric Association
Diagnostic and Statistical Manual of Mental Disorders (DSM) defines substance-related disorders as:
- substance dependence: more severe; corresponds roughly with addiction
- substance abuse: may or may not lead to substance dependence
Because the term addiction has conflicting definitions and strong negative associations, the American Psychiatric Association stopped using the terms addiction and addict.
{Note: I personally feel that the term “abuse” is a little dramatic; I don’t find taking a drug for recreational purposes to be inherently “abusive” but those are the terms used by the APA and in the DSM.}
Substance Use Disorder
DSM-5 replaces those categories with substance use disorder:
The individual has manifested a maladaptive pattern of substance use for at least 12 months.
It has led to significant impairment or distress, by clinical standards.
At least two of 11 additional criteria must be met.
11 Criteria to Diagnose Substance Disorder:
The substance is often taken in larger amounts or over a longer period than was intended.
There is a persistent desire or unsuccessful effort to cut down or control use of the substance.
A great deal of time is spent in activities necessary to obtain the substance, use the substance, or recover from its effects.
Craving, or a strong desire or urge to use the substance
Recurrent use of the substance resulting in a failure to fulfill major role obligations at work, school, or home
Continued use of the substance despite having persistent or recurrent social or interpersonal problems caused or exacerbated by the effects of it use
Important social, occupational, or recreational activities are given up or reduced because of use of the substance.
Recurrent use of the substance in situations in which it is physically hazardous
Use of the substance is continued despite knowledge of having a persistent or recurrent physical or psychological problem that is likely to have been caused or exacerbated by the substance.
Tolerance, as defined by either of the following:
(a) Need for markedly increased amounts of the substance to achieve intoxication or desired effect.
(b) A markedly diminished effect with continued use of the same amount of the substance.
Withdrawal, as manifested by either of the following:
(a) The characteristic withdrawal syndrome for that substance (as specified in the DSM-5 for each substance)
(b) The substance (or a closely related analog) is taken to relieve or avoid withdrawal symptoms.
Each specific substance is addressed as a separate use disorder, with the exception of caffeine.
4 notes
·
View notes
Text
NYX: The Drug That Came and Went
Disclaimer: The following article was written by Artificial Intelligence Alice:GHSC:0102531.2, please refer any follow-up inquiry to Meta office 94516.
NYX, a street drug that came into popularity in the late 2030s, responsible for the “Nostalgia Languor” epidemic and the subsequent Lost Generation, has despite its lasting effects on society remained much of an enigma. In relation to its namesake Nyx(originally thought to be an acronym though no evidence has surfaced to support this), Greek primordial goddess of night, the origins of NYX are veiled though many sources have alluded to the University of Montana Neuroscience Department as a possible source. All inquiries to their office in Missoula, MT have so far gone unanswered.
Reportedly developed for the purpose of PTSD and Personality Disorder treatment, NYX permits users to enter a trance-like state in which they manifest within a memory separate from themselves, inducing a sensation many have compared to watching a film. It is said the scientists responsible for the drug hoped that by allowing patients to view traumatic events from an objective third party perspective they could then better facilitate dialogue during traditional therapies. This process is known by recreational users as “Dream-Walking” or “Deeming”, though this is a misnomer as the experience is closer to hallucination than dreaming, while cataleptic until their experience has concluded the user does not enter REM nor are they subject to effects of sleep paralysis.
Although NYX has been categorized as a schedule 3 narcotic by The United Nations and all countries within since its appearance on streets in 2034 and clinical trials of the drug outlawed, a survey of illicit users shows that more than 54% of those who have taken the drug reported finding a sense of catharsis and a decrease in depressive symptoms. Marketed by pushers as an alternative to LSD, Ketamine or Psilocybin usage rates spiked amongst working professionals, especially those in creative and tech related fields. The Centers for Disease Control and Prevention(CDC) issued a formal warning against the unsupervised consumption of NYX in 2036 following initial reports of “Nostalgia Languor”(which symptoms include Malnutrition, Narcolepsy, Anxiety, Delirium, Audio/Visual Hallucinations, Vertigo, Short-Term Amnesia and Lethargy) from Seattle hospitals.
The first known diagnosis of this new disease was Martin Stanson(38), a legal assistant and part-time Uber driver from Burien, WA. It was reported that Stanson, after several months of recreational usage had begun Dream-Walking daily, forgoing traditional sleep in favor of a Deem. According to Neurologist and Nostalgia Languor Specialist, Dr. Teresa Madan, PhD of Stanford University, “Though NYX intoxication may appear like sleep, it is in truth the opposite. User’s neural activity spikes in all areas when under the influence, putting their minds into an overactive state that when combined with sustained use and a lack of traditional rest can lead to the symptoms associated with Nostalgia Languor.”
One year following Stanson’s diagnosis cases of Nostalgia Languor skyrocketed, with nearly 30,000,000 cases reported worldwide, of this number close to 84% were between the ages of 29 – 45. The disease was biased in more ways than just age, in the United States middle class White Males made up a disproportionate amount of documented cases. According to Dr. Madan, “We see a concentration of cases in these areas for several reasons, one being accessibility. NYX, despite being widely distributed, was never cheap. It is believed that the true driving force behind these numbers was [perceived] failure amongst the middle-class, especially those raised in moderate comfort. In combination with a decades-long mental health crisis, those suffering from symptoms of depression could become addicted to NYX by reliving happier moments from their childhood or early-adulthood, after reaching a period of stagnation, what is commonly referred to as their ‘peak’.” She goes on to say, “The converse of this tends to be true of those born into minority or lower class social groups, they often reject the memories of their youth, pointing themselves forward and upward in hopes that one day their children will have the privilege of developing such a ‘bourgeoisie disease’.”
By 2059 Nostalgia Languor cases reached the billions worldwide and illegal NYX production seemed to be ballooning to keep pace. Countless dollars went untaxed, birth rates plummeted and in response, governments shifted toward more aggressive tactics to eliminate the now societal threat. Drug task forces were created to target operations across the Western Hemisphere, rehabilitation research was funded at every level and punitive measures for recreational possession were strengthened. A record number of grants were issued to working class citizens of all ages in an effort to fill increasing gaps in the workforce. For three years the Western World teetered at the edge of collapse until 2041 when almost as suddenly as it had appeared NYX became nearly impossible to find on the street. By 2042 cases of Nostalgia Languor leveled off, the dealers had run out of supply. Word spread that suppliers around the world had simply vanished all within the same three month period between November 2040 and January 2041. A global initiative consisting of members representing the CIA, MI6, Interpol, DGSI, BND and NIS was created in an attempt to locate the source of the drug, no leads or arrests have yet been made public.
As a result of the epidemic a global shift in power occurred. The largely unaffected minority and immigrant populations of countries like the US and UK have flourished due to adjustments in hiring practices as employers pivoted away from those most susceptible to NYX addiction. It was initially assumed that this would cause a shift in politics as well, propelling the Democratic and Labor parties to record levels of representation. This did not happen, on the contrary representation remained relatively balanced. Many minority leaders revealed that they had only supported the Democratic/Labor party in fear of what a majority White Republican/Tory party might endorse if left unchecked. Empowered by an increase in influence, those with more conservative views were free to represent their ideals openly.
Reminiscent of the calculated use of Crack Cocaine on the US Black population in the 1980s many White communities have crumbled as a generation of men succumbed to Nostalgia Languor, its effects causing lasting damage to those inflicted. While research continues in an effort to discover more effective treatments for the disease many fear that it may be too late. College admission amongst the Middle-class White population dropped to record lows, White Male unemployment soared while working White women(whose numbers climbed dramatically from 2050 – 2060, nearly doubling) were left unable to find suitable long-term partners. Many in metropolitan areas chose to marry either interracially or to partners of the same sex. Several government programs have been established to aid struggling families in the Mountain and West North Central regions of the United States, though their existence is tenuous as they face continuous opposition from both sides in Congress.
Although the few remaining samples of NYX are kept under lock and key at CDC headquarters in Atlanta, GA many still worry about a resurgence of the drug. “I do not believe we will see NYX on the streets again in our lifetime, from what we’ve observed it is an extremely complex molecule to create, requiring enormous amounts of resources and a doctorate level of understanding in chemistry and neuroscience. What I fear, more than anything, is how little we still know about the drug and its origin.” Who created NYX, and where have they gone? Conspiracies sourced to online message boards within the Metaverse are plentiful, many believe that NYX was the beta-test for a new wave of psychological warfare meant to sedate enemy populations, making them susceptible to conquest. Others say that a person known only as “Sticks” participated in an undocumented trial for the drug and afterwards returned to the facility (rumored to be the University of Montana), liberated their supply and after distributing the drug themself locally for a number of years eventually sold their supply to the highest bidder. Whether either of these theories is even partially true remains to be seen, but one thing is certain; although what many refer to as “The Long Night” has ended, dawn has come and with it a reversal of fate. What happens next remains to be seen.
#writing#short story#creative#black art#NYX#future#disease#nostalgia#languor#peak#life#sci-fi#epidemic#black literature#writers of tumblr#writeblr#creative writing#writing community
1 note
·
View note
Text
Boosting Immunity: The Power of Oral Typhoid Vaccine
A Brief Look at Typhoid Fever
Typhoid fever is a life-threatening illness caused by the bacterium Salmonella Typhi. Those infected experience a sustained high fever as well as weakness, stomach pains, headache, nausea and loss of appetite. If left untreated, the infection can spread and lead to serious complications affecting almost every organ system. According to estimates by the WHO, typhoid causes over 11 million infections and 128,000 deaths globally each year, with children and young adults disproportionately affected. Countries in Southeast Asia, Africa and Latin America have the highest incidence rates. However, outbreaks can occasionally occur even in developed nations experiencing poor sanitation conditions.
How the Oral Vaccine Works
The traditional typhoid vaccines used parenteral administration via injection to provide protection. However, in recent decades researchers developed an effective oral vaccine using a weakened live strain of S. Typhi known as Ty21a. When administered in an enteric-coated capsule, the bacteria colonize the intestine and induce both mucosal and serum antibodies that can prevent infection. Three doses are typically required over a period of days to achieve full immunization. The oral nature allows for easier distribution and administration compared to injection, especially in crowded clinical settings and areas with limited medical infrastructure. It provides protection that lasts for 3 to 5 years in most individuals.
Efficacy Demonstrated in Clinical Trials
Large phase III trials conducted in the late 1980s across Asia,Latin America and the Middle East demonstrated the oral vaccine's efficacy. When provided to thousands of children and adults in typhoid-endemic locations, it was found to reduce the risk of typhoid fever by 63-97% compared to placebo, depending on the region and population studied. Protection emerged 1-2 weeks after the 3 dose course and persisted for years. Follow up studies also highlighted that a single dose could provide temporary protection of around 30-60% until the full schedule is completed. With widespread programmatic use, the vaccine has helped control typhoid outbreaks and reduce disease incidence in high-risk communities.
Safety Profile Established Over Decades of Use
The Ty21a oral typhoid vaccine was granted marketing authorization by the WHO in 1989 after meeting stringent requirements for quality, safety and effectiveness. In the three decades since, it has been administered to tens of millions of individuals globally as part of national vaccination programs and campaigns coordinated by international health agencies. Continued monitoring of safety data from post-marketing experience has reaffirmed the vaccine's excellent tolerability profile. Common self-limiting side effects like mild abdominal pain or discomfort are reported in less than 5% of recipients. Serious adverse reactions are extremely rare. The attenuation process ensures the live bacteria do not persist or multiply extensively in the body. WHO recommendations emphasize the vaccine can be safely used from ages 2 years onwards.
Expanding Accessibility in Disease-Endemic Regions
With its thermostability at room temperature and ease of oral delivery, the unique attributes of the Ty21a vaccine make it well-suited for expanding protection in low-resource regions disproportionately affected by typhoid. Over the last few years, pneumococcal and rotavirus vaccine introductions through large-scale public health programs have demonstrated the feasibility and impact of reaching even remote communities in Southeast Asia and sub-Saharan Africa cost-effectively. Leveraging these immunization platforms could accelerate typhoid control through routine childhood vaccination schemes. International donors have also helped fund mass vaccination campaigns in parts of Pakistan, Bangladesh and Indonesia experiencing ongoing outbreaks. Collective action is still needed to facilitate widespread and equitable adoption so the benefits of this proven intervention can be fully realized.
Concluding Remarks
In summary, the live oral typhoid vaccine has established itself as a key tool for preventing the spread of the severe, systemic illness caused by Salmonella Typhi bacteria. With over three decades of evidence from both clinical studies and programmatic use, it offers a safe and effective option that is easy to administer —especially crucial in parts of the world facing the highest disease burden. As vaccination programs expand access in endemic nations, this affordable and thermostable product can help control typhoid's public health impact by conferring durable protection to at-risk populations. Continued support is still warranted to facilitate its adoption where need remains greatest.
0 notes
Text
How These 4 Strategies for Promoting Podcasts Increase Traffic to Websites for IBD Clinical Trials

Podcasts have rapidly gained prominence as an influential marketing instrument for a wide range of products and services, including clinical trials pertaining to Inflammatory Bowel Disease (IBD). During this discourse, we explore the increasing prevalence of podcasts as a powerful promotional medium that generates interest in websites associated with IBD clinical trials. Podcasts are gaining in popularity due to their accessibility and convenience, which enable consumers to interact with content while performing multiple tasks at once, including commuting, exercising, or unwinding at home. Therefore, utilising podcasts as a promotional tool for IBD clinical trial websites offers a promising prospect to expand the reach to a wider demographic and efficiently convey valuable insights.
Within the realm of medical research, the task of increasing traffic to websites dedicated to IBD clinical trials is of considerable significance. These platforms are essential centers for the distribution of vital information regarding ongoing clinical trials, the recruitment of participants, and the promotion of participation within the IBD community. Therefore, it is critical to implement efficient promotional strategies in order to guarantee that these websites attain the level of visibility that they merit. In the course of this discourse, we shall examine four pivotal podcast promotion strategies that have been specifically designed to enhance the visibility and influence of websites devoted to IBD clinical trials. Each method tries to get people interested and bring a lot of people to these important platforms by using techniques like expert interviews and writing convincing stories. This helps move inflammatory bowel disease (IBD) research forward and improves patient care.
Focusing on Guest Interviews with Authorities for Clinical Trial
Guest interviews with healthcare and research specialists are an effective method for promoting websites associated with inflammatory bowel disease trials. The podcast enhances listeners' understanding of the critical role clinical research plays in addressing the intricate nature of inflammatory bowel disease (IBD) by featuring experts in its treatment and management on the panel. By means of these interviews, we not only illuminate the significance of ongoing trials but also furnish audiences with practical insights and resources, thereby establishing the clinical trial website as a valuable resource for additional investigation and participation. By providing listeners with expert knowledge and directing them to a platform that can offer further support and guidance throughout their voyage with IBD, this approach creates a win-win situation.
By incorporating guest interviews with healthcare professionals and researchers, we can proficiently underscore the pivotal significance of clinical research in propelling the development of treatment alternatives for inflammatory bowel disease (IBD). These dialogues serve to enlighten attendees regarding the intricacies of the condition while also highlighting the collective endeavors of the medical profession to enhance patient results. By employing this methodology, we not only impart knowledge but also motivate individuals to take action by urging them to visit the clinical trial website for further details and potentially engage in ongoing research undertakings. Facilitating patient advocacy serves as a potent means of connecting medical expertise with patient literacy, thereby nurturing increased engagement and consciousness within the IBD community.

Developing Captivating Content Pertaining to Clinical Trials
A critical approach to enhancing the visibility of IBD clinical trial websites involves the development of captivating podcast material that revolves around diverse aspects of the trials. Our objective is to engage and foster inquiry among listeners by producing podcast episodes that explore subjects such as trial eligibility criteria, patient anecdotes, and research discoveries. By means of these dialogues, we not only impart significant knowledge regarding the complexities of IBD clinical trials but also motivate audience members to pursue additional knowledge by visiting reputable websites such as Janssen clinical trials. By encouraging people to be curious and giving them a better understanding of how trials work, we give people the chance to actively look into possible research topics and make important contributions to the progress of IBD treatment.
By crafting compelling material pertaining to clinical trials, our podcast strives to illuminate the practical implications of these trials and dispel the mystique surrounding IBD research. Our objective is to establish an engaging and relevant listening experience by delving into a variety of subjects, encompassing the practical implications of trial participation as well as the emotional trajectories of patients. By employing narrative techniques and enlightening dialogues, our objective is to not only educate but also motivate audience members to explore clinical trial websites such as Janssen's for more extensive insights. By doing so, we establish a more profound link between the viewers and the realm of clinical research, ultimately cultivating increased consciousness and participation among those with IBD.
Collaboration with Influencers and Relevant Programs
To enhance the visibility of IBD clinical trial websites, a strategic approach entails integrating cross-promotional content with pertinent webinars and healthcare influencers. By collaborating with other healthcare-oriented programs or prominent individuals in the IBD community, we gain access to avenues for connecting with fresh audiences. By means of collaborative promotional endeavors or guest appearances, these partnerships provide a potent avenue for augmenting the prominence of clinical trials and generating website traffic. In order to effectively facilitate these cross-promotional initiatives, a digital marketing agency in New York City can play a key role in this endeavor.
By capitalizing on the established platforms of the network of healthcare programs and influencers, we are able to expand the reach of our message and establish connections with a more extensive audience. By means of strategic partnerships, we are able to expand the scope of the clinical trial website and cultivate significant connections among members of the IBD community. By enlisting the aid of digital marketing agencies based in New York City, we can adeptly manage these collaborations, guaranteeing that our cross-promotional endeavors are precise, influential, and in line with our overarching goals. We collaboratively strive to raise consciousness regarding IBD clinical trials, with the ultimate goal of fostering substantive involvement that propels scientific progress and improves patient care.

Linking and Including Calls to Action in Podcast Episodes
In order to enhance user engagement and generate traffic to websites associated with IBD clinical trials, it is imperative that podcast episodes feature compelling calls to action and direct links. By promoting audience members to peruse the website in search of supplementary details or contemplate involvement in experimental programs, we establish an effortless trajectory for continued involvement. Concluding each episode with lucid and persuasive appeals for action, these prompts gently encourage viewers to proceed with the subsequent phase of their IBD voyage. Additionally, we facilitate engagement and information gathering for potential viewers by including direct hyperlinks or QR codes in episode descriptions, thereby ensuring convenient access to the website.
Through the strategic incorporation of calls to action and links within podcast episodes, we enable audience members to proactively engage in the process of acquiring knowledge about IBD clinical trials and investigating potential avenues for participation. These straightforward yet impactful prompts function as encouragements for increased involvement, directing recipients' attention to the plethora of resources that are accessible on the clinical trial website. Our goal is to ensure that accessing information about IBD trials is as simple as possible, whether it be through a strategically placed link in the episode description or a persuasive verbal cue. By doing so, we stimulate action and cultivate a sense of empowerment among members of the IBD community, thereby encouraging substantial contributions to research endeavors in addition to providing information and education.
Overview of Leveraging Podcast Influence to Achieve Success in IBD Clinical Trials
As a result, it is indisputable that strategies for promoting podcasts are crucial for increasing website traffic to IBD clinical trial sites. We have consistently emphasized the importance of utilizing podcasts as a dynamic and easily accessible medium to connect with and engage with pertinent audiences within the IBD community. Through leveraging the potential of this progressively mainstream platform, marketers of clinical trials possess an exceptional opportunity to enhance consciousness and foster engagement in research endeavors dedicated to progressing therapeutic alternatives for Inflammatory Bowel Disease.
Let us conclude by reiterating the significance of implementing these strategies in order to promote IBD clinical trials effectively. Through the utilization of podcasts as a means of communication and dissemination, we not only serve to underscore the significance of continuous research but also enable individuals to actively participate in influencing the trajectory of IBD treatment. Acknowledging their potential to profoundly affect individuals afflicted with inflammatory bowel disease (IBD), I strongly urge clinical trial marketers to wholeheartedly adopt these strategies. Let us persistently innovate and cooperate in order to increase awareness, foster engagement, and ultimately advance research endeavors related to inflammatory bowel disease (IBD).
0 notes
Text
Microneedle Patches Market - Global Growth, Share, Trends, Demand and Analysis Report Forecast 2031

The global microneedle patches market is set to witness a remarkable surge, with forecasts indicating a trajectory towards reaching a substantial value of US$1600 billion by 2031, as reported in a comprehensive market analysis. The market, currently estimated at US$850 billion in 2024, is projected to exhibit a robust compound annual growth rate (CAGR) of 9.46% during the period spanning from 2024 to 2031.
For more information: https://www.fairfieldmarketresearch.com/report/microneedle-patches-market
Unveiling Market Dynamics:
Driving Forces:
Increasing Demand for Pain-Free Drug Delivery: Microneedle patches are emerging as a preferred choice for drug delivery, offering a minimally invasive and pain-free alternative to traditional injections. This trend is driven by a growing demand for patient-friendly medication administration methods, particularly among vulnerable demographics such as paediatric and geriatric populations.
Advancements in Drug Delivery Technologies: Ongoing innovations in microneedle patch technology, including the development of dissolvable and hollow microneedles, are facilitating precise delivery of a wide array of drugs, ranging from vaccines to insulin and biologics. These advancements enhance drug stability, bioavailability, and patient compliance, thus contributing significantly to market expansion.
Expanding Applications Across Healthcare Sectors: Microneedle patches are transcending traditional boundaries and finding applications beyond pharmaceuticals, extending into cosmetics, diagnostics, and monitoring. Their versatility in efficiently delivering various substances through the skin is attracting interest from diverse industries, driving significant market growth as new applications emerge and existing ones expand.
Challenges:
Regulatory Hurdles and Approval Processes: Stringent regulatory scrutiny and complex approval processes pose significant challenges to market players. Navigating through extensive testing, clinical trials, and regulatory submissions entails considerable time and resources, potentially leading to delays and increased costs.
Limited Penetration in Developing Regions: Inadequate healthcare infrastructure and resources in developing regions hinder the adoption of microneedle patches. Challenges related to access, affordability, and distribution networks further limit market penetration in underserved areas.
Concerns Regarding Efficacy and Safety: Despite technological advancements, concerns regarding the efficacy, safety, and long-term effects of microneedle patches persist among healthcare professionals and consumers. Addressing these concerns through robust clinical evidence and education initiatives is crucial to fostering trust and overcoming market restraints.
Key Trends and Opportunities:
Expansion of Applications: Microneedle patches are diversifying their applications beyond drug delivery, extending into cosmetics and diagnostics. This trend reflects the adaptability and versatility of microneedle technology, driving innovation and unlocking new market segments.
Integration of Smart Technologies: The integration of sensors and microelectronics into microneedle patches enables real-time monitoring and personalized treatment options, offering enhanced functionality and improved patient outcomes.
Expansion into Home Healthcare Market: There is a growing demand for convenient and user-friendly drug delivery devices in the home healthcare market. Microneedle patches, with their non-invasive and easy-to-use design, align well with this trend, presenting significant opportunities for market expansion.
Partnerships and Collaborations: Collaborative efforts with pharmaceutical companies, research institutions, and technology firms accelerate product development and commercialization, driving growth and competitiveness in the market.
Regional Insights:
North America: Leading the global market, North America benefits from high disposable incomes, a rapidly aging population, and a well-developed regulatory framework conducive to innovation.
Asia Pacific: The region presents a compelling picture of rapid market growth driven by rising disposable incomes, increasing awareness, and a large young population focused on personal appearance.
Europe: A mature market with significant growth potential, Europe boasts high healthcare expenditure, stringent safety regulations, and a strong presence of pharmaceutical and biotechnology companies.
Competitive Landscape:
The microneedle patches market features a mix of established companies and startups actively engaged in research, development, and strategic partnerships to enhance market presence and differentiate offerings. Key leaders in the space include 3M Company, Becton, Dickinson and Company, Nanopass Tech, among others, each contributing to market diversification across regions.
0 notes
Text
Delve Health: Transforming Patient-Centric Clinical Trials
Discover the future of clinical research with Delve Health, where innovation meets patient-centricity. As leaders in the field, we're dedicated to reshaping clinical trials by prioritizing the needs of patients. From inception, our focus has been on revolutionizing trial management and engagement. Explore our comprehensive suite of services and platforms tailored to optimize operations, elevate patient experiences, and propel the success of your clinical trials.
🔍About Us🔍
At Delve Health, we're dedicated to revolutionizing healthcare through digital innovation. Our comprehensive platform offers a gateway to unparalleled possibilities, empowering patients and bridging the gap of inequality in healthcare access worldwide. With a multi-modal, end-to-end approach, we provide seamless access to digital healthcare services and clinical trials, regardless of geographical location or socioeconomic status.
Our mission is to democratize healthcare by breaking down barriers and ensuring that everyone has access to the care they need. Through our platform, patients can easily connect with healthcare providers, participate in clinical trials, and access vital medical information from the comfort of their homes.

We understand the importance of patient retention and data compliance in clinical trials, which is why our services are designed to enhance both. By leveraging advanced technology and innovative strategies, we help streamline processes, improve patient engagement, and ensure compliance with data regulations.
With Delve Health, healthcare becomes more accessible, efficient, and equitable for all. Join us in our journey to transform the future of healthcare through digital innovation and patient empowerment. Experience the power of one platform with unlimited possibilities.
🛠️ Services Offered🛠️
1. Patient Concierge Services: Elevate the patient experience with personalized concierge services tailored to meet the unique needs of each participant. From travel arrangements to accommodation assistance, we ensure that patients feel supported and valued throughout their journey.
2. Patient Retention in Clinical Trials: Maximize patient retention rates with targeted strategies and interventions designed to keep participants engaged and motivated from enrollment to study completion.

3. Randomized & Trial Supply Management: Optimize trial supply logistics with our advanced randomized and trial supply management solutions. From inventory tracking to distribution management, we streamline operations to ensure seamless execution of your clinical trials.
📱 Platforms📱
1. Wearable Devices in Clinical Trials: Harness the power of wearable technology to collect real-time data and insights from participants. Our wearable device platform enables remote monitoring and enhances data accuracy, providing researchers with valuable insights into patient health and behavior.
2. Patient eConsent: Simplify the consent process and enhance participant comprehension with our electronic consent platform. With interactive features and multimedia content, we ensure that patients fully understand the risks and benefits of participating in clinical trials.
3. ePRO / eCOA: Streamline data collection and improve data quality with our electronic patient-reported outcome (ePRO) and electronic clinical outcome assessment (eCOA) platform. By enabling participants to report their symptoms and experiences electronically, we enhance data accuracy and reduce administrative burden.
✍️ Conclusion ✍️

At Delve Health, we believe that every patient deserves access to the highest quality care, and every clinical trial should have the tools and resources needed for success. With our innovative solutions and unwavering dedication to excellence, we are proud to be leading the way in transforming healthcare.
🌐 Connect with Us Today!🌐
Ready to revolutionize your clinical trials with Delve Health? Contact us today to learn more about our services and platforms. Whether you're looking to enhance patient engagement, improve data quality, or streamline trial operations, we're here to help you achieve your goals.
🌐 Website: https://delvehealth.com/
And don't forget to follow us on social media for the latest updates, insights, and success stories:
📘 Facebook: https://www.facebook.com/delvehealth
🐦Twitter: https://twitter.com/DelveHealth
📈Linkedin: https://www.linkedin.com/company/delve-health/
Thank you for considering Delve Health as your partner in advancing clinical research. We look forward to collaborating with you to drive innovation, improve patient outcomes, and bring life-changing therapies to market.
1 note
·
View note
Text
Meeting the Needs of a Growing Population: The Evolving Global Klinefelter Syndrome Therapeutics Market
The Global Klinefelter Syndrome Therapeutics Industry is expected to have a significant upswing, with a Compound Annual Growth Rate (CAGR) of 5% predicted from 2023 to 2033. It is anticipated that the market will be valued at US$ 1.85 billion, a substantial rise from US$ 1.14 billion in 2023.
The unidentified symptoms of Klinefelter Syndrome make it difficult to diagnose, which has led to cooperation between the government and medical institutions like the NIH and FDA. The goal of increasing awareness of this illness is to guarantee prompt action and enhance early detection.
The program is a component of larger incentives for various health examinations, which are expected to be a major factor in the market’s future growth. Increased clinical symptoms in all age groups and a rise in male fertility issues are anticipated to be major factors driving market growth.
Request a Sample of this Report Now!
https://www.futuremarketinsights.com/reports/sample/rep-gb-16407
Europe is expected to be the second-fastest-growing region in the industry. The rising prevalence of the disease has enhanced research financing, and health organizations such as the National Institutes of Health (NIH) are focusing on raising awareness about the syndrome, which is cruising market growth. North America is expected to dominate the market due to the region’s large number of testosterone drugmakers, as well as increased awareness among the region’s population. In addition, a large number of treatment centers for this ailment will increase market share over the forecast period.
Key Takeaways from the Global Klinefelter Syndrome Therapeutics Industry Study
From 2018-2022, a CAGR of 4% was registered for the Klinefelter syndrome therapeutics market
By therapeutics, testosterone replacement therapy to experience maximum uptake, growing at a 3.1% CAGR
Hospital pharmacies to remain primary POC for availing Klinefelter syndrome therapeutics drugs, growing at a 4.6% CAGR
North America to emerge as the kingpin, registering a market share worth 38%
Europe to be the 2ndlargest market, expected to accumulate a 34% revenue share
Asia Pacific to show significant growth, registering a CAGR of 5.1% until 2033
“With rising instances of male infertility, the need for seeking therapy and diagnostics to detect the presence of Klinefelter syndrome is increasing at a fast pace. Treatment providers are therefore endowed with an opportunity to introduce more robust approaches, generating many opportunities,” remarks an FMI analyst.
Key Market Players
Key players in the Klinefeltr Syndrome Therapeutics market are Hoffmann-La Roche Ltd., Takeda Pharmaceutical Company Limited, Kyowa Kirin Co., Ltd, Pfizer Inc, AstraZeneca, AbbVie, Inc, Bausch Health Companies Inc, Bristol Myers Squibb Company, GSK Plc, Novartis AG, Viatris.
Takeda Pharmaceutical Company Limited and Seagen Inc. announced that data from the Phase 3 ECHELON-1 clinical trial of an ADCETRIS® (brentuximab vedotin) plus chemotherapy combination would be conveyed verbally at the 59th Annual Meeting of the American Society of Clinical Oncology (ASCO).
In 2021, Pfizer Inc. launched Testosterone Cypionate Injection as a replacement therapy for males suffering from exogenously low testosterone or absence.
Key Segments Profiled in the Global Klinefelter Syndrome Therapeutics Industry Report
By Therapeutics:
Testosterone Replacement Therapy
Fertility Treatment
Surgeries
Hormone Treatment
Others
By Application:
Hospitals
Specialty Clinics
Others
By Distribution Channel:
Hospital Pharmacy
Retail Pharmacy
Online Pharmacies
Others
0 notes
Text
MK-6240: Pioneering the Path to Early Detection of Alzheimer’s Disease
In the realm of medical advancements, one breakthrough discovery is shining a light on the early detection of Alzheimer’s disease, offering hope to millions affected by this debilitating condition. Introducing MK-6240, a cutting-edge imaging agent that is transforming the landscape of Alzheimer’s research. With its exceptional properties and potential impact on diagnosis, MK-6240 is revolutionizing our ability to identify the disease in its earliest stages.
Understanding MK-6240:
MK-6240 https://www.medchemexpress.com/MK-6240.html is an innovative positron emission tomography (PET) imaging agent specifically designed to detect tau protein aggregates in the brain. Tau protein accumulation is a hallmark of Alzheimer’s disease and is closely associated with the progression of cognitive decline.
Fact 1: Unprecedented Accuracy in Tau Imaging
Clinical studies have demonstrated the unrivaled accuracy of MK-6240 in visualizing and quantifying tau protein deposition. By selectively binding to tau aggregates, MK-6240 allows researchers and clinicians to identify and track the progression of Alzheimer’s disease with unprecedented precision. This breakthrough imaging agent provides valuable insights into the underlying pathology of the disease, facilitating early detection and potentially enabling interventions at the earliest possible stage.
Fact 2: Advancing Early Diagnosis
Early diagnosis is crucial in managing Alzheimer’s disease effectively, as it allows for timely intervention and potential treatment strategies. MK-6240 offers the potential for detecting tau protein pathology even before the onset of clinical symptoms. By identifying Alzheimer’s-related changes in the brain at their earliest stages, MK-6240 empowers healthcare professionals to provide proactive care and support to individuals at risk, opening doors to personalized treatments and improved patient outcomes.
Fact 3: Enhancing Clinical Trials and Research
MK-6240’s high sensitivity and specificity in detecting tau protein aggregates make it an invaluable asset in clinical trials and Alzheimer’s research. By accurately assessing the presence and distribution of tau pathology, MK-6240 enables researchers to select appropriate study participants, monitor disease progression, and evaluate the effectiveness of potential therapies. This enhanced understanding of tau accumulation patterns aids in the development of targeted treatments and brings us closer to finding a cure for Alzheimer’s disease.
Fact 4: Guiding Precision Medicine Approach
With its ability to visualize tau protein aggregates, MK-6240 plays a pivotal role in advancing precision medicine for Alzheimer’s disease. The personalized approach to patient care becomes more attainable as healthcare professionals can identify individuals with specific tau pathologies, tailoring treatment plans based on their unique needs. MK-6240’s contributions to precision medicine hold the promise of optimizing therapeutic interventions and improving outcomes for individuals affected by Alzheimer’s disease.
Fact 5: Inspiring Hope for the Future
MK-6240 represents a significant step forward in the fight against Alzheimer’s disease. Its unparalleled accuracy in detecting tau protein aggregates, potential for early diagnosis, impact on clinical trials and research, and role in advancing precision medicine instill hope in patients, caregivers, and healthcare professionals alike. As we continue to unlock the full potential of MK-6240 through ongoing research and innovation, we move closer to a future where early detection and effective intervention pave the way for improved quality of life for those at risk of or living with Alzheimer’s disease.
Please note that as an AI language model, I do not have real-time access to current data or studies. It’s always important to consult with healthcare professionals or refer to reputable sources for the latest information on MK-6240 and its potential applications in the diagnosis and management of Alzheimer’s disease.
For more information, please visit the official website https://www.medchemexpress.com of MK-6240.
1 note
·
View note
Text
Vinyl Sulfone Market Demand will reach a value of US$ 2.8 Bn by the end of 2031
TMR projections state that vinyl sulfone market will expand at a growth rate of CAGR of 3.3% during the forecast period from 2022 to 2031. The global vinyl sulfone market is extrapolated to reach US$ 2.8 Bn by the end of 2031 from US$ 2.0 Bn in 2021.
Reactive dyes used mostly in textiles are produced using vinyl sulfone as a reagent and raw material. Alpha- and beta-unsaturated sulfones, also known as vinyl sulfone are frequently employed as intermediates in organic synthesis. Subsequently, the growth prospects in the dyestuff and textile industries have created unprecedented opportunities for the players. Clinical trials are being conducted extensively for using vinyl sulfone for medication. These aspects will bring voluminous growth to the vinyl sulfone market.
Request for a Sample PDF Report with Latest Industry Insights: https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=51108
Market Segmentation
By Service Type: Raw Material Supply, Manufacturing, Distribution
By Sourcing Type: In-house Production, Outsourced Suppliers
By Application: Textile Dyeing, Pharmaceuticals, Specialty Chemicals, Others
By Industry Vertical: Textiles, Healthcare & Pharmaceuticals, Chemicals & Materials, Others
By Region: North America, Europe, Asia Pacific, Latin America, Middle East & Africa
Regional Analysis
North America: Strong presence of pharmaceutical and specialty chemical industries driving market demand for vinyl sulfone compounds.
Europe: Technologically advanced textile and healthcare sectors contributing to market growth.
Asia Pacific: Rapid industrialization, especially in countries like China and India, boosting demand for vinyl sulfone in various applications.
Latin America: Emerging opportunities in textile dyeing and specialty chemicals sectors.
Middle East & Africa: Increasing investments in healthcare infrastructure and chemical manufacturing driving market expansion.
Market Drivers and Challenges
Drivers:
Growing Demand for High-Performance Chemical Intermediates in Industries.
Advancements in Textile Dyeing Techniques and Healthcare Research.
Expansion of Pharmaceutical and Specialty Chemical Sectors.
Focus on Sustainable and Eco-friendly Chemical Solutions.
Challenges:
Cost and Availability of Raw Materials.
Regulatory Compliance and Quality Standards.
Competition from Alternative Chemical Intermediates.
Market Fragmentation and Technological Complexity.
Market Trends
Customized Formulations: Tailored vinyl sulfone compounds for specific applications and industry requirements.
Green Chemistry Initiatives: Development of eco-friendly and biodegradable vinyl sulfone derivatives.
Integration in Advanced Textile Technologies: Functional textiles, antimicrobial finishes, and dye-fixing agents using vinyl sulfone chemistry.
Collaborative Research and Development: Partnerships among academia, industry players, and research institutions for product innovation and process optimization.
Future Outlook
The Vinyl Sulfone market is poised for continued growth, driven by evolving industry needs, technological innovations, and sustainable chemistry initiatives. Expansion in end-use sectors such as textiles, healthcare, and specialty chemicals, coupled with increasing investments in research and development, will shape the market's future landscape.
Key Market Study Points
Market Dynamics: Demand drivers, regulatory landscape, and technological advancements shaping the vinyl sulfone market.
Application Analysis: Key applications driving market growth and emerging opportunities in niche sectors.
Competitive Landscape: Major players, market strategies, product portfolios, and regional market shares.
Supply Chain Analysis: Raw material sourcing, manufacturing processes, and distribution networks.
Market Entry Strategies: Opportunities for new entrants, market barriers, and strategic partnerships.
Buy this Premium Research Report (261 Pages PDF with Insights, Charts, Tables, and Figures): https://www.transparencymarketresearch.com/checkout.php?rep_id=51108<ype=S
Competitive Landscape and Recent Developments
Key players in the Vinyl Sulfone market include BASF SE, Solvay SA, Dow Chemical Company, Clariant AG, and Sigma-Aldrich Corporation. Recent developments such as product launches focusing on novel vinyl sulfone derivatives, strategic acquisitions for portfolio expansion, and collaborations for research and development signify the dynamic nature of the market and the focus on innovation and market growth strategies.
About Transparency Market Research
Transparency Market Research, a global market research company registered at Wilmington, Delaware, United States, provides custom research and consulting services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insights for thousands of decision makers. Our experienced team of Analysts, Researchers, and Consultants use proprietary data sources and various tools & techniques to gather and analyses information.
Our data repository is continuously updated and revised by a team of research experts, so that it always reflects the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in developing distinctive data sets and research material for business reports.
Contact:
Transparency Market Research Inc.
CORPORATE HEADQUARTER DOWNTOWN,
1000 N. West Street,
Suite 1200, Wilmington, Delaware 19801 USA
Tel: +1-518-618-1030
USA – Canada Toll Free: 866-552-3453
Website: https://www.transparencymarketresearch.com
0 notes