#1000 Renal Questions
Text
Do pharmaceuticals pose a threat to aquatic life? Experts weigh in
https://sciencespies.com/nature/do-pharmaceuticals-pose-a-threat-to-aquatic-life-experts-weigh-in/
Do pharmaceuticals pose a threat to aquatic life? Experts weigh in
Pharmaceuticals, the chemicals found in medicines and drugs, are widely used across the world and allow us to treat and prevent disease.
There is a growing concern that some of these pharmaceuticals are leaching into natural waterways like rivers, streams, and lakes.
To find out if this poses a problem, we asked 4 experts in toxicology and environmental sciences “Are current pharmaceuticals levels in natural waterways dangerous?” Here is what we found out.
What is the current level of pharmaceuticals in our natural waterways?
A USA study found measurable amounts of pharmaceuticals in 80 percent of the water samples they collected from streams across 30 different states. The issue of drugs in natural waterways is not just in the USA but across the globe. A 2014 review of over 1000 studies found pharmaceuticals residues in 71 countries worldwide.
The 2014 review found over 600 different pharmaceutical chemicals in lakes, rivers, and groundwater globally. These chemicals included antibiotics, painkillers, beta-blockers, contraceptive hormones, and antidepressants, amongst others.
How do pharmaceuticals get into natural waterways?
There are a variety of ways that medicines and drugs end up in streams, rivers, and lakes.
One of these ways is through sewage. When we take medications, we only absorb a fraction of the chemicals into our bodies, the rest is excreted out. Improperly disposing of medications by flushing them down the toilet also adds to this.
Pharmaceuticals can also leach into natural waterways from agriculture, where drugs, particularly antibiotics and hormones, are used for rearing livestock.
Finally, pharmaceutical chemicals can also be released from industrial plants that make medications. For example, a wastewater treatment plant that is close to many pharmaceutical factories in India was found to have very high levels of some antibiotics.
Although wastewater is cleaned in sewage treatment plants, Dr Bruno Nunes, an expert in toxicology from Aveiro University, says, “Water treatment processes are not effective to prevent [pharmaceutical] release into the aquatic ecosystem.”
What effect does this have on aquatic life?
It is difficult to know the full extent of how these pharmaceuticals affect aquatic life due to lack of data.
However, there are some examples where drug contaminants have had toxic effects on wildlife. For example, the anti-inflammatory medication diclofenac has been found in natural waterways and can damage renal and gastrointestinal tissues in fish, which can kill them.
It is not always possible to predict the effect of pharmaceuticals in the environment based on experiments from the laboratory.
Dr Nunes explains that this is because “some of these drugs may interact with other substances and show some potential to compromise physiological traits of many aquatic species, even in the levels in which these substances occur”.
Pharmaceuticals also act in more indirect ways to damage aquatic life. Dr Mike Grace, an expert in environmental science from Monash University, says that current pharmaceutical levels are dangerous as they act “as a mechanism for inducing sub-lethal effects in stream organisms and essential ecosystem services like photosynthesis and nutrient cycling”.
Dr Grace explains that “pharmaceuticals at concentrations found in most waterways around the world are termed ‘Ecosystem Disruptors'”. Estrogen pharmaceuticals have been shown to be ecosystem disrupters by feminizing male fish of certain species. This disrupts the male to female ratio of the fish and causes collapse of populations.
Is there any risk to humans?
Dr Grace says that current pharmaceutical levels in natural waterways are not dangerous to “humans touching the water” or “drinking a few mouthfuls”. Currently, the level of pharmaceuticals in drinking water is far below the concentration required for any physiological effect.
The takeaway
Current pharmaceutical levels in natural waterways are dangerous for aquatic life but pose no current threat to humans.
Article based on 4 expert answers to this question: Are current pharmaceuticals levels in natural waterways dangerous?
This expert response was published in partnership with independent fact-checking platform Metafact.io. Subscribe to their weekly newsletter here.
#Nature
3 notes
·
View notes
Text
LONG VENT POST: Family issues, part 1 of ?
So, a bit ago I said I’m not as active right now, due to family issues. I didn’t want to fill the thread of that post with all the long, sordid details.
Right now I’m trying to get my mother into an “assisted living and memory care” community (nursing home, basically) and doing everything I can to stop her credit union accounts from hemorrhaging from all her incompetence (she’s been scammed a bunch and generally taken advantage of).
Yesterday’s biggest takeaway was the discovery of numerous scams she fell for over the past few years... plus three months worth of fraudulent Uber and Uber Eats charges... and the fact that she pays about $550/mo on car insurance but the last couple times (at least, maybe more) she got collision repairs done? She didn’t file claims and pay a $500 deductible. No, she paid in full, out of pocket. Out $7k instead of $1k for two repairs in just a few months’ time. How can you pay huge insurance premiums and never notify them when you need collision repairs?!
Found out just recently that about a couple years ago, someone scammed her for an easy $5k. Found out she never deposits the full amount on car payments I send her. She never makes full deposits on the rent a tenant pays her. Instead, she cashes much of them out, and I have no idea what she does with the cash.
She buys stuff in bulk but cannot use most of it before it goes bad, but she refuses to toss out expired foods. Her hoarding tendencies have gotten worse. Even though she tells my sister and I not to send her gifts that would add to the clutter in the house, I found out yesterday she’s been dropping $200-$300 on random stuff from places like TJ Maxx and Tuesday Morning just because they made her “happy”. She hasn’t even unpackaged the hanging glass butterflies or other things. I told her she can take them to the “home”....
She’s wrecked two brand new Priuses (about $30k each, each paid up front/in full with inheritance money from my dad, who died five years ago) within about two years’ time. The first was “totaled”, but I have yet to verify whether she ever opened a claim to get money for it. This one hasn’t been officially declared totaled or repairable. I had to file the claim on it on her behalf. For all I know, the $550/mo premium might be on both cars. I’ll know for sure soon. If she’s been paying insurance on a car that was “totaled” two years ago instead of getting market value of about $22k, I will definitely break down and cry. For at least the 50th time in the past few days. Seriously, if she just found out the previous one couldn’t be fixed and walked away from it without filing a claim and getting the huge payout... and is therefore also still paying for insurance on it? I’ll probably scream, too.
The latest (and last ever) car wreck was last Wednesday. We’ve been telling her for over a year she shouldn’t be driving. Her doctors have told her the same for at least 6mo. My sister and I were planning to visit her and take away her keys, but the wreck happened before we could even finalize our travel plans.
This time, she was trying to get to dialysis (she goes three days a week), and she couldn’t use Uber anymore, so she was determined to drive herself. Just before 5 am, she was driving down her own residential street and blacked out (apparently) and hit three parked (and unoccupied) vehicles. Police showed up and she got out of her car and told them she needed a ride to dialysis. One of the officers took her. She can’t recall hitting three cars. Told me she hit a curb and one car. Later told someone else she only hit a curb. I don’t know anything about the curb, but probably. However, I definitely believe the police report that three parked cars were hit badly and had to be towed away, too. After the police spoke to her tenant, they said they’d make things easier for us and revoke her license. Phew.
I convinced dialysis staff (actually, they completely agreed without question) to send her to hospital afterwards instead of letting yet another friend take her home. Good thing, too, because before dialysis was even up that morning, she was in a lot of pain; she had told them earlier she didn’t need to be looked at. Well... no broken bones, no major injuries, and her labs were ok except slightly low potassium. However, a brain scan showed something I already knew just by dealing with her: it showed ischemic changes associated with dementia. Monday of that week, I had called her renal doctor to tell him I worried about her mental health and wondered if it had anything to do with the renal failure. He said he didn’t think so; it’s got to be something else causing the mental decline we are seeing. When I told him she’s still driving sometimes, he became furious and said he’d refer her to get a full dementia evaluation. Well, before he could even get the referral to her, she’d wrecked again. He’s seen her now, but I haven’t heard any updates from him. Mom says she hasn’t done the evaluation (that she knows of), and she heard someone at the hospital mention “dementia”, but she doesn’t recall what they said about it. 😔
I didn’t take photos when I finally saw her car, but I’m going back up to Dallas tomorrow and staying in a Motel 6 overnight (with my dog) to take care of as much financial matters for her as I can in these next two days. I’ll get another chance to see the car (to clean out items), so I’ll take pics then. I might not get back to Austin until sometime Wednesday. Not sure about Wednesday yet, but I already requested Monday and Tuesday off from work by email and left a vm with coworkers. I’m about to run out of annual leave because of this. I know I’ll be making many weekend trips coming up until my sister and I have gotten her moved into the nursing facility. And for a while afterwards, too, since we have to clean out the house, put some of her stuff into a storage unit, and sell the house ASAP. Plus, we need to visit often, at least at first, to make sure she’s settled in, isn’t hating it too much, and is being taken care of properly.
It’s a good thing my sister is paying for my hotel charges and has also offered gas money (though I haven’t asked for gas money... yet). This is still way cheaper for her than booking herself flights back and forth between Olympia, WA and Dallas, TX. The more leg work I do on this, the happier my sister is to help with my travel costs. Honestly, she really doesn’t want to come down here until it’s time to move our mom, clean the house, and put it up for sale.
And, since we have so little time to get her affairs in order, we are placing her in the only community my mom and I have toured, so far. I told her if it turns out to not be a good place (at all) once she moves in, we can keep looking at others (while she still lives at that one) and move her again. But, honestly, this place does seem nice enough, and none of these places are perfect. Plus, it’s right next to the hospital where she always goes... the one where her doctors are associates. I joked that if they needed to send her to the hospital, they could put her on a gurney and wheel her down the street. She laughed at that and said the location is perfect.
Sigh. She’s being compliant and has even said thanks for us (her two daughters) stepping up to help her and get things taken care of. We were afraid she would refuse to leave her house of almost 40years. She’s not even batting an eye at us deciding to sell the house to make sure she can afford the rent and services (the suites at the community are rented out like apartments, but with three meals a day and unlimited snacks, weekly cleaning service, weekly laundry service, landline phone, cable, and internet included. We will have to pay more for “memory care” and probably for medical transport they provide (unless that’s included, too), plus whatever else. She might take her cat with her, or she might leave him with a friend of the family. But it’s a one-time, non-refundable fee of $500 if she keeps him. I kind of hope she gives him up, and they just bring him along on visits to her. She would have trouble taking care of him.
She’s never shown me her finances before. We had no idea how bad (completely uncontrolled) her spending was. It was probably bad enough before our dad died, but afterwards, she started going downhill fast. Now she’s in renal failure and requires dialysis three days a week. She’s recently lost an unhealthy amount of weight in a very short time, apparently because she can’t remember to eat and sometimes she’s too disoriented to get up. She can’t cook anymore, and she’s hardly done cleaning chores since she had kids (that’s what us kids were for: housework). So she’s a money-wasting hoarder in a house full of dirty dishes, dirty clothes, clutter everywhere, and $100’s — maybe $1000 worth — of groceries she can’t get through but won’t throw out when they go bad. And she won’t let anyone else touch them while she’s still living there.
I have so much to do the next couple days, I had to write a list of each thing I need to look into and take care of before I return home. I still have some stuff to get ready for the trip, so this is the end of the first vent post.
I hope I get more sleep tonight than I have the past week....
#off topic#PLEASE DO NOT REBLOG#will reblog to off topic#might delete later#venting#family problems#dementia#assisted living#nursing home#memory care#long post#personal post
17 notes
·
View notes
Text

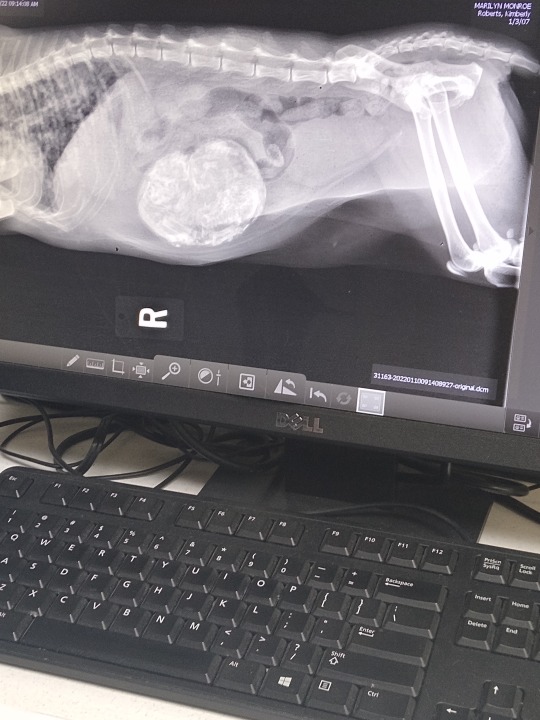
TL;DR: Marilyn Monroe, my 14 year old cat, has a mass in her stomach. I need help paying the other $500. I am paying $100 every two weeks, but would love to pay it all up front.
There's a lot more that goes with the story, but this is the TL;DR version
Donations can be made at: paypal.me/bronzecoffee
Reblogs are MORE than welcomed.
Marilyn Monroe has been with me for 13-14 years now. She's at least 14 because she was already a fully grown cat when I got her. She's never had a heat and has only been around neutered males as long as I've owned her.
A couple of week ago my sister, that I live with, said she thought Marilyn lost weight, and she was right. She did look really slender. Marilyn's always been round. To the point people asked me a lot if she was pregnant, and I always said no.
About a week ago she stopped eating all her dry treats. Then she didn't eat any of them. I was concerned, but also thought maybe she got tired of the flavor. It's been the same one for a while now.
This past Thursday, Jan. 6, we took her to the vet for a senior screening, which is blood work, her vaccines, and rabies shot. I tried to warn the vet we took her two that she doesn't like being handled. I mentioned they could use a muzzle and gloves, even a blanket. They didn't, and it took two of the techs to hold her down while scruffed. They stressed her so badly she urinated all over herself, bit the clippers while scruffed, and it was a mess.
The stress elevated her kidney levels for the exam enough for them to call me and tell me she had 'elevated levels'. I didn't really understand what she was talking about, it was so fast and over the phone. Didn't really give me time for questions. She mentioned the words 'renal' and KD diet, which I had to Google just meant a kidney diet.
Took her home and she was mad but seemed OK. She didn't eat Thursday, Friday, or Saturday. I called the emergency vet in the area and asked if that was normal, and they said no. I should probably bring her in. So I did that.
They sedated her after I told them what happened at the first vet. They retook her blood work and ran a urinalysis. Her kidney levels were well within normal, but her blood looked like she was fighting an infection/inflammation. They found a hard mass her in abdomen. They also gave her under the skin fluids, a pain relief shot that would last a few days, because it is squishing up her organs, and a nausea medication because she was throwing up prior to when she stopped eating. (Also had her claws clipped so she stopped taking out the techs.)
Told me to go back to the vet for an xray because it would be cheaper than at their hospital. The first vet that stressed her out so much was the only one that could take her in today.
So I did that. They said it went a lot better than normal.
Today they found what looks like a couple of calcified/mummified kittens in her abdomen/uterus. They're not 100% sure that's what it is.
She's having surgery tomorrow, Jan. 11, 2022. It needed to be done quickly since it was clearly impacting her eating and drinking.
I paid $480 on a $1000 bill today. They let me set up a payment plan, but I can only do $100 every two weeks. I don't get paid much. Any and all help is appreciated, from reblogs to donations.
1 note
·
View note
Text
Abstract algebra an introduction 3rd edition solutions pdf
ABSTRACT ALGEBRA AN INTRODUCTION 3RD EDITION SOLUTIONS PDF >> DOWNLOAD LINK
vk.cc/c7jKeU
ABSTRACT ALGEBRA AN INTRODUCTION 3RD EDITION SOLUTIONS PDF >> READ ONLINE
bit.do/fSmfG
abstract algebra an introduction hungerford solutions pdf
slader abstract algebra hungerford
abstract algebra: an introduction 3rd edition pdf
9781111569624 pdf
hungerford solutions chapter 2slader abstract algebra 3rd edition
Access Abstract Algebra 3rd Edition solutions now. Our solutions are written by Chegg experts so you can be assured of the highest quality! Download Free Abstract Algebra An Introduction 3rd Edition eBook by Thomas W. Hungerford in PDF Format, found under Abstract Algebra Books MathSchoolinternational.com provides 1000+ free mathematics eBooks, worksheets, shortcuts, formulas and question with solution.Microeconomics 4th Edition by Paul Krugman Description Type: E-Textbook This is a digital products , eBook ( PDF ) NO ONLINE ACCESS CARD/CODE INCLUDED. NO 8 hours ago Unlike static PDF Abstract Algebra 3rd Edition solution manuals or printed answer keys, our experts show you how to solve each problem step-by-step. 11 downloads 325 Views 21MB Size. Report. DOWNLOAD .PDF Abstract Algebra Solutions Abstract Algebra (Herstein 3rd Ed). Full description Access all of the textbook solutions and explanations for Hungerford's Abstract Algebra: An Introduction (3rd Edition). dms to .pdf or .zip or .rar. If you encounter any problems or need samples, please contact us. What is Solution Manual ? The solution Free abstract algebra an introduction 3rd edition solutions PDF Book Download Link from FreePDFBook.com, in Books.
https://somumubuqida.tumblr.com/post/666799880274132992/2018-fll-mission-model-building-instructions, https://poxejorixo.tumblr.com/post/666727981087129600/houzetek-s657-manual, https://comecoviwisa.tumblr.com/post/666839191553982464/fpdf-codeigniter, https://vilipupiru.tumblr.com/post/666807057355276288/nutrition-care-manual-renal-diet, https://powipevok.tumblr.com/post/666833402652180480/physical-chemistry-4th-edition-solutions-manual.
0 notes
Text
300+ TOP MEDICAL SCIENCE Objective Questions and Answers
MEDICAL SCIENCE Multiple Choice Questions :-
1) Regarding serratus anterior muscle which is incorrect?
A. Multipinnate muscle
B. Lifts arm above the shoulder
C. Supplied by long thoracic nerve
D. Originates from lower eight ribs
Ans: D
2) The treatment of choice for atticoantral variety of chronic suppurative otitis media is:
A. Mastoidectomy
B. Medical management
C. Underlay myringoplasty
D. Insertion of ventilation tube
Ans:A
3) All of the following are the complications in the new born of a diabetic mother except?
A. Hyperbilirubinemia
B. Hyperglycemia
C. Hypocalcemia
D. Hypomagnesemia
Ans:B
4) The correct line of management in child who has swallowed a coin is?
A. Fiber optic endoscopy
B. Rigid endoscopy
C. Laparotomy
D. Wait and Watch
Ans: D
5)Helper cells belong to?
A. T cells
B. Macrophages
C. B cells
D. Monocytes
Ans:A
6)Mac Ewen's triangle can be felt through the?
A. Superior conchae
B. Cymba conchae
C. Middle conchae
D. Posterior part of auricle
Ans:B
7)Most common strain of E.coil giving rise to traveller's diarrhoea is?
A. Entero-invasive E.coil
B. Entero-pathogenic E.coil
C. Entero-toxigenic E.coil
D. Entero-aggregative E.coil
Ans:C
8)With urine turning green on ferric chloride test, the diagnosis is:
A. Phenylketonuria
B. Alkaptonuria
C. Multiple carboxylase deficiency
D. Glutaric aciduria
Ans:A
9)For assessing the ability of protein utilisation the best index is?
A. Urea
B. Uric acid
C. Blood ammonia
D. Urinary nitrogen content
Ans:D
10)Screening test is not useful when?
A. Incidence of the disease
B. Incidence is low in the community
C. Early detection leads to favorable outcome
D. The disease has a lead time
Ans:B

MEDICAL SCIENCE MCQs
11)Nerve not related to humerus is?
A. Radial
B. Median
C. Axillary
D. Musculocutaneous
Ans:D
12)What is the percentage of chances of hydatidiform mole to develop choriocarcinoma?
A. 5%
B. 15%
C. 50%
D. 80%
Ans:B
13)Vasomotor reversal of Dale is because of?
A. Block of alpha receptors
B. Block of alpha & beta receptors
C. Agonistic action on alpha receptors
D. Adrenaline only
Ans:A
14)Primary visual field is situated around the ______ sulcus?
A. Central
B. Calcarine
C. Superior temporal
D. Inferior occipital
Ans:B
15)Medical treatment for BPH includes?
A. Finesteride
B. Methyl testosterone
C. Oestrogens
D. Osmic acid
Ans:A
16)Anterior dislocation of shoulder causes all except?
A. Circumflex artery injury
B. Avascular necrosis head of humerous
C. Brachial plexus injury
D. Chip fracture scapula
Ans:D
17)HIV is a?
A. Retrovirus
B. Flavivirus
C. Oncovirus
D. Arbovirus
Ans:A
18)In shigella dysentery associated hemolytic uremic syndrome, the false statement is?
A. Leucocytosis
B. Neurological abnormalities
C. Hepatic failure
D. Thrombotic angiopathy
Ans:C
19)Dengue hemorrhagic fever is caused by?
A. Type I secrotype
B. Re-infection with the same serotype of dengue virus
C. Re-infection with the different serotype of the dengue virus
D. Re-infection in immunocompromised host
Ans:C
20)When the sample size is less than 30, one of the following modifications is made in the formula of standard deviation?
A. Numerator is increased
B. Denominator is decreased
C. Both numerator and denominator are changed
D. Numerator is decreased
Ans:B
21)Oesophagus receives blood supply from all except?
A. Inferior thyroid artery
B. Inferior phrenic artery
C. Internal mammary artery
D. Bronchial artery
Ans:C
22)All are pencillinase resistant except?
A. Methicillin
B. Nafcillin
C. Penicillin
D. Dicioxacillin
Ans:C
23)Mild hemolyti anaemia is associated with vitamin.. . Deficiency?
A. B6
B. E
C. A
D. C
Ans:B
24)Trimethoprim acts by?
A. Inhibiting DHFR
B. Inhibiting cell metabolism
C. Inhibiting DNA
D. Inhibiting RNA
Ans:A
25)Paradoxically split second heart sound signifies severe?
A. Pulmonary stenosis
B. Mitral stenosis
C. aortic stenosis
D. tricuspid stenosis
Ans:C
26)Riboflavin nutritional status is assessed by?
A. Xanthine oxidase levels in RBC's
B. Glutathione reductase activation in RBC's
C. Urine excretion of Riboflavin
D. Cytochrome- C-reductase levels in kidneys
Ans:B
27)Provision of free medical care to the people at government expense is known as?
A. State medicine
B. Social therapy
C. Social medicine
D. Social insurance programme
Ans:A
28)Shortest sacrocotyloid diameter causing narrowing of pelvis is a feature of which type of maternal pelvis?
A. Android
B. Gynaecoid
C. Platypelloid
D. Anthropoid
Ans:C
29)What is Bennett's fracture?
A. Fracture dislocation of base of first metacarpal
B. Fracture dislocation of base of first metatarsal
C. Fracture of first metatarsal
D. Fracture of first metacarpal
Ans:B
30)All the following techniques are helpful in the diagnosis of haemoglobinopathies, except?
A. Alkali denaturation test
B. Cellulose acetate electrophoric
C. Sickling test
D. Osmotic fragility test
Ans:D
31)Which of the following is not likely in patients taking amiodarone?
A. Pulmonary fibrosis
B. Hypothyroidism
C. Hyperthyroidism
D. Gynaecomastia
Ans:D
32)Which of the following helps in preventing colon cancer?
A. High fiber diet
B. Selenium
C. Antioxidants
D. Fatty food
Ans:A
33) Size of ovary, above which considered to be malignant?
A. 2 cm
B. 5 cm
C. 8 cm
D. 10 cm
34)Cadaveric spasm
A. Instant in onset
B. Confined to small group of muscles
C. Occurs only in voluntary muscles
D. All of the above
Ans:D
35)The radionuclide used for ventriculography is
A. Thallium
B. Technetium
C. Gallium
D. Pottasium
Ans:B
36)"Signet ring cells" are seen in?
A. Carcinoma cervix
B. Carcinoma endometrium
C. Krukkenberg tumour
D. Carcinoma vulva
Ans:C
37)Cimetidine was synthesized by?
A. Black
B. Upjohn
C. Lindsay
D. Lilly
Ans:A
38)Nutritional status of children between 0-4 years in a community can be assesed by all except?
A. Mortality in 0-4 years
B. Birth weight of less than 2.5 gm
C. Maternal Hb D. Height and weight of all preschool children
Ans:C
39)Pulmonary fibrosis in Bronchogenic carcinoma of lung may follow exposure to?
A. Coal dust
B. Silica
C. Asbestos
D. Bagasse
Ans:C
40)Ejection fraction increases with?
A. Decrease end-systolic volume
B. Decrease end-diastolic volume
C. Decreased peripheral resistance
D. Venodilation
Ans: A
41)Which is NOT visualized on posterior rhinoscopy?
A. Eustachian tube
B. Inferior meatus
C. Middle turbinate
D. Posterior border of nasal septum
Ans:B
42)Which one of the following would cause a metabolic acidosis is with a normal anion gap?
A. Renal tubular acidosis
B. Acute renal failure
C. Diabetic ketoacidosis
D. Aspirin overdose
Ans: A
43)All of the following organs contain aneurysm in polyarteritis nodosa except?
A. Liver
B. Lung
C. Kidney
D. Pancreas
Ans: C
44)Diphtheria toxin acts by?
A. Inhibiting Acetyl Choline release
B. Inhibiting glucose transport
C. Increasing levels of Cyclic AMP
D. Inhibiting protein synthesis
Ans:D
45)Smokeless gun powder is composed of?
A. KMno4
B. HCN
C. Nitrocellulose
D. Sulphur
Ans:C
46)Cetuximab (an EGFR antagonist) can be used in?
A. Palliation in head and neck cancer
B. Anal canal carcinoma
C. Gastric cancer
D. Small cell lung carcinoma
Ans:A
47)The formula showing relations of pressure, thickness and radius?
A. Laplace formula
B. Ohm's law
C. Pascal's law
D. Poisseulle's formula
Ans:A
48)Dapsone is useful for treating all except?
A. Leprosy
B. Dermatitis Herpetiformis
C. Madura Foot
D. Lymphoma
Ans:D
49)The most important factor to overcome protein energy malnuntrition in children less than 3yrs is;
A. Suply of subsidized food from ration shop
B. Early supplimentation of solids in infants
C. immunization to the child
D. Treatment of anaemia and pneumonia in infant and toddlers
Ans:B
50)The binding of 2,3 BPG to Hemoglobin is to?
A. Carboxyterminal
B. Amino terminal
C. Sulphydryl groups
D. None of the above
Ans:B
MEDICAL SCIENCE Objective type Questions with Answers
51)Dissociate anaesthesia is described with which of the following?
A. Propofol
B. ketamine
C. Thipental
D. Halothane
Ans:B
52)In colour Doppler the colour depends upon?
A. Strength of returning echo
B. Relation of transducer to blood flow
C. Frequency of Doppler used
D. Type of Doppler machine used
Ans:B
53)The commonest cause of breech presentation is:
A. Prematuarity
B. Hydrocephalus
C. Placenta praevia
D. Polyhydramnios
Ans:A
54)Which of the following pathologic processes in an example of dysplasia?
A. Actinic keratosis
B. Chronic cystitis
C. Chronic bronchitis
D. Ulcerative colitis
Ans:A
55)True regarding tubercular meningitis:
A. Generally occurs as dissemination of a miliary tuberculosis
B. The cranial nerves frequently are involved
C. The most common affected leptomeninges are at the base of the brain
D. Communicating and obstructive hydrocephalus cortical abscesses, and empyemas are very uncommon complications
Ans:D
56)Haemostasis means?
A. Coagulation
B. Maintenance of electrolyte balance
C. Sufficient hydration
D. Arrest of bleeding
Ans:D
57)The average coronary blood flow in human being at rest is _ % of cardiac output ?
A. 4.5%
B. 5-10%
C. 10-15%
D. 15-20%
Ans:A
58)Rigor mortis first starts in?
A. Upper eyelids
B. Lower eyelids
C. Lower limbs
D. Fingers
Ans:A
59)Madura foot is caused by?
A. Blastomycosis
B. Nocardia
C. Candida albicans
D. Tinea versicolor
Ans:B
60)Population of 10000, birth rate 36 per 1000, 5 maternal deaths, the MMR is?
A. 14.5
B. 13.8
C. 20
D. 5
Ans:B
61)Which of the following is not in WHO surveillance?
A. Rabies
B. Influenza
C. Malaraia
D. Varicella
Ans:D
62)The cause of breech presentation are all except?
A. Previous caesarean section
B. Placenta previa
C. Contracted pelvis
D. Oligohydramnios
Ans:A
63)True about minoxidil is?
A. Increases hair growth
B. Antihypertensive
C. Both
D. None
Ans:C
64)Cold agglutinins are seen in?
A. Mycoplasma pneumonia
B. Psittacosis
C. Legionella pneumonia
D. TB
Ans:A
65)No. of negative stools mandatory to release a case from isolation in typhoid?
A. 3 samples same day
B. 2 samples on first day and 1 sample on the second day
C. 1 sample of first day and 2 samples on the second day
D. 3 samples on 3 separate days
Ans:D
66)The most specific feature of death due to hanging is?
A. Tardieu spots
B. Ligature mark
C. Fracture of thyroid cartilage
D. Dribbling of saliva
Ans:D
67)Cauliflower ear is due to?
A. Haematoma
B. Carcinoma
C. Fungal infection
D. Herpes
Ans:A
68)Following agents have effects on the NMJ, EXCEPT
A. Curare
B. Decamethonium
C. Succinylcholine
D. Hexamethonium
Ans:D
69)Entamoeba, which is not found in gut?
A. E.coli
B. E.histolytica
C. E.gingivalis
D. E.nana
Ans:C
70)most deaths involving placenta previa result from?
A. Infection
B. Toxemia
C. Hemorrhage
D. Thrombophlebitis
Ans:C
71)Tympanic plexus is formed by?
A. Tympanic branch of glossopharyngeal nerve
B. Vagus nerve
C. Facial nerve
D. mandibular nerve
Ans:A
72)Prolactin?
A. Facilitates
B. Prevents ovulation in lactating women
C. In responsible for formation of corpus luteum
D. Is responsible for progesterone secretion
Ans:B
73)Herpes zoster involves?
A. Otic ganglion
B. Gasserian ganlion
C. Geniculate
D. Celiac ganglion
Ans:B
74)In wolf- parkinson white syndrome, there exist a connection between atria and?
A. Bundle of His
B. Ventricles
C. A-V node
D. Purknje fibres
Ans:B
75)Polyhydramnios is seen in all the following except:
A. Diabetes
B. Renal agenesis
C. Esophageal atresia
D. Hydronephrosis
Ans:B
76)Following is the adjuvant for the treatment of nephrotic syndrome?
A. levamisole as immunomodulant
B. B.complex
C. cyclosporin
D. steroid
Ans:D
77)Subarachnoid Haemorrhage is diagnosed by?
A. Lumbar puncture
B. CT scan
C. MRI
D. X-ray skull
Ans:A
78)Disturbances of affect include all except?
A. Panic
B. Apathy
C. Phobia
D. Obsession
Ans:D
79)Deep transverse arrest is seen in?
A. Occipito-posterior position
B. Occipito-anterior position
C. Breech delivery
D. Face presentation
Ans:A
80)Kallu, a 25 yr male pt.presented with a red eye and complains of pain, photophobia ,watering and blurred vision. He gives a history of trauma to his eye with a vegetable matter. Corneal examination shows a dendritic ulcer. A corneal scraping was taken and
A. Herpes simplex
B. Acanthambea
C. Candida
D. Aeno virus
Ans:B
81)Glass vessels and syringes are best sterilised by
A. Hot air oven
B. Autoclaving
C. Irradiation
D. Ethylene oxide
Ans: A
82)Management of extradural hemorrhage is:
A. Immediate evacuation
B. Evacuation after 24 hrs
C. Antibiotics
D. Observation
Ans:A
83)A Post- Thyroidectomy patient develops signs and symptoms of Tetany. The management is?
A. I.v.Calcium gluconate
B. Bicarbonate
C. Calcitonin
D. Vitamin D
Ans:A
84)The most effective treatment in the early stages of trachoma is?
A. Penicillin locally
B. Choromycetin systemically
C. Sulphonamides systemically
D. Soframycin locally
Ans:C
85)Ideal treatment of Tinosporidiosis is:
A. Rifamipicin
B. Excision with cautery at base
C. Dapsone
D. Laser
Ans:B
86)Epithelium of cornea is?
A. Pseudostratified
B. Transitional
C. Stratified squamous keratinized
D. Stratified squamous non-keratinised
Ans:D
87)Bagasosis can be prevented by spraying Bagasse with?
A. 10% acetic acid
B. 5% acetic acid
C. 1% propionic acid
D. 2% propionic acid
Ans:D
88)Mc Ardles disease is due to the deficiency of?
A. Glu 1 phosphatase
B. Gluc1, 6 diphosphatase
C. Gluc 6 phosphatase
D. Myophosphorylase
Ans:D
89) Satiety center in hypothalamus is regulated by?
A. Gastric dilatation
B. Blood glucose levels
C. Blood insulin levels
D. All of the above
Ans:B
90)Coagulative necrosis is seen in all except?
A. Myocardial infarction
B. Burns
C. Tuberculosis
D. Zenker's degeneration
Ans:D
91)The relationship between incidence and prevalence can be expressed as the?
A. Product of incidence and mean duration of disease
B. Divident of incidence and mean duration of disease
C. Sum of incidence and mean duration of disease
D. Difference of incidence and mean duration of disease
Ans:A
92)In twin pregnancy, treatment of choice when first baby is in transverse lie is:
A. Home delivery
B. Cesarean section
C. High forceps
D. Low forceps after external rotation
Ans:B
93)Coose among the following the most important lab finding in nephrotic syndrome?
A. B-J protine
B. hyperkalemia
C. hypoalbuminemia
D. hypertension
Ans:C
94)Which statement is not true regarding Cryptic military Tuberculosis?
A. X-ray diagnsis is possible
B. It is seen in PEM Children
C. Mntoux test is negative
D. Leucocytosis is seen
Ans:B
95)Commonest histology of carcinoma of endometrium is?
A. Squamous cell
B. Clear cell
C. Adeno carcinoma
D. Anaplastic carcinoma
Ans:C
96)True regarding Felty's syndrome is all EXCEPT
A. Splenomegaly
B. Rheumatoid arthritis
C. Neutropenia
D. Nephropathy
Ans:D
97)The true statement about Zenker's diverticulum is;
A. It is outpouching of ant.pharyngeal wall above the cricopharyngeus muscle
B. Barium swallow lateral view for diagnosis is the best investigation
C. it is a true diverticulum
D. it is congenital
Ans: A
98)Which of the following statements about aging is true?
A. Zoo animals have shorter lifespans than animals in their normal habitat
B. All animal species have approximately the same lifespan
C. Men are programmed to live longer than women.
D. Identical twins have a natural lifespan of approximately similar duration
Ans:D
99)Characteristic features of kwashiorkor include following EXEPT?
A. Anorexia
B. Flaky paint dematosis
C. Hepatomegaly
D. Splenomegaly
Ans:D
100)Most common site of obstruction in gallstone ileus is?
A. Colon
B. Illeum
C. Jejunum
D. Duodenum
Ans: B
MEDICAL SCIENCE Questions and Answers pdf Download
Read the full article
0 notes
Text
Racial & Ethnic Differences in Pregnancy Rates Among Women on Dialysis
MedicalResearch.com Interview with:

Dr. Silvi Shah
Silvi Shah, MD, MS, FACP, FASN| Assistant Professor
Division of Nephrology, University of Cincinnati
Cincinnati, OH-45267
MedicalResearch.com: What is the background for this study?
Response: Our study uses data from the largest retrospective cohort of dialysis patients in the United States from the United States Renal Data System to determine pregnancy rates and factors associated with pregnancy in 47,555 women aged 15-44 years on dialysis. We identified 2,352 pregnancies with a rate of 17.8 pregnancies per 1000 person-years (PTPY) with the highest rate in women aged 20-24 years (40.9 PTPY).
MedicalResearch.com: What are the main findings?
Response: The present study shows racial/ethnic differences in the occurrence of pregnancy among women on dialysis. Native American, black and Hispanic women had a higher likelihood of pregnancy as compared to white women. Women with end-stage kidney disease (ESKD) due to glomerulonephritis, malignancy and hypertension had a higher likelihood of pregnancy than women with end-stage kidney disease due to diabetes, while women on peritoneal dialysis had a lower likelihood of pregnancy than women on hemodialysis.
MedicalResearch.com: What should readers take away from your report?
Response: Our study highlights higher pregnancy rates in women with end-stage kidney disease in the United States than the prior reports. Pregnancy therefore is not uncommon among women undergoing dialysis. Our report uses data from a national registry and did not have the limitation of the reporting bias from the surveys or voluntary registries. The study improves our understanding of various factors associated with pregnancy like race/ethnicity, ESKD cause and type of dialysis modality. This information is particularly important during pre-pregnancy counseling and shared decision making.
MedicalResearch.com: What recommendations do you have for future research as a result of this work?
Response: The real reasons for racial and ethnic differences in pregnancy rates among women on dialysis remain unknown and further study future research is therefore needed to better understand and reduce these differences. We were also not able to determine the differences in unintentional and intentional pregnancies and whether level of education was associated with the likelihood of pregnancy.
MedicalResearch.com: Is there anything else you would like to add?
Response: I would like to thank my entire team at the University of Cincinnati who participated in the project. The research was supported by intramural funds of Division of Nephrology, Kidney C.A.R.E (Clinical Advancement, Research and Education) Program, University of Cincinnati. The authors have no disclosures.
Citation: Racial Differences and Factors Associated with Pregnancy in End Stage Kidney Disease Patients on Dialysis in the United States
Silvi Shah, Annette L. Christianson, Karthikeyan Meganathan, Anthony C. Leonard, Daniel P. Schauer and Charuhas V. Thakar
JASN September 2019, ASN.2019030234; DOI: https://doi.org/10.1681/ASN.2019030234
The information on MedicalResearch.com is provided for educational purposes only, and is in no way intended to diagnose, cure, or treat any medical or other condition. Always seek the advice of your physician or other qualified health and ask your doctor any questions you may have regarding a medical condition. In addition to all other limitations and disclaimers in this agreement, service provider and its third party providers disclaim any liability or loss in connection with the content provided on this website.
Read the full article
0 notes
Photo

Kaplan Step 1 2018 Videos Free Download
DOWNLOAD LINK
https://smarthelpers.blogspot.com/2019/01/kaplan-videos.html
Kaplan is one of the world’s largest and most diverse education providers. Throughout our more than 80-year history, Kaplan has been a beacon for expanding educational access and a leader in instructional innovation.
We help students achieve their educational aspirations. We prep high school students for the SAT, so they can enter college and become teachers or engineers or whatever they dream. We help doctors and nurses, lawyers and financial advisors pass their licensing tests. We help adult learners return to college to earn a degree. We enable global educational experiences through language study and study abroad programs. We provide professional training to improve employees’ productivity and opportunities for career advancement.
Kaplan has a long history and deep experience providing educational services to colleges and universities across the globe. These take the form of university pathway programs, international student recruitment, university hosting, residential design and an array of student support services.
For businesses, Kaplan provides expert exam preparation for professional licensure and certification as well as corporate training, leadership and professional development and educational consultation services.
The company has been a leader in adapting a learning engineering approach to course design and instructional delivery. Kaplan’s pioneering and leadership role in education is well documented. We created the test prep business and were an early leader in online education. We have similarly been a leader in the New Economy Skills Training, which includes immersive computer coding “boot-camps” for people seeking to become web developers. The company has also been a leader in adapting a learning engineering approach to course design and instructional delivery.
We continually strive to make the learning experience for our students the best we can with a rigorous focus on educational performance and results. Kaplan operates in over 30 countries and maintains relationships and partnerships with more than 1,000 school districts, colleges and universities, and over 10,000 corporations and businesses. Our vast breadth and scope in terms of both capabilities and assets sets us apart from our competitors.
For a complete overview of Kaplan’s offerings, please see “Transforming Education Transforming Lives,“ our company brochure.
Mission and Values For 80 years, we have had one mission.
Kaplan helps individuals
achieve
their educational and career goals. We build futures one success story at a time.
Our core values define our company culture and provide the framework for what we deliver to our customers and employees each day:
Integrity: We hold ourselves to the highest ethical standards in everything we do.
Support: We give you the tools you need to succeed.
Knowledge: We offer expert resources to help you achieve your academic and career best.
Opportunity: We open doors and broaden access to education.
Results: We’re dedicated to helping you achieve your goals – we succeed when you succeed.
Fast Facts 1M+ Students Worldwide
13K+ Employees
30+ Countries
10000+ Business Clients
1000+ Educational Partners
80+ Years Transforming Students’ Lives
Graham Holdings Company
Kaplan, Inc. has been part of Graham Holdings, formerly The Washington Post Company, for more than 30 years and has become its largest subsidiary. Based in Arlington, VA, Graham Holdings Company (NYSE:GHC), formerly The Washington Post Company, is a diversified education and media company whose principal operations include educational services, television broadcasting, and online, print, and local TV news, home health and hospice care, and custom manufacturing.
kaplan videos step 1
kaplan videos quora
kaplan videos google drive
kaplan videos online
kaplan videos medicine
kaplan videos review
kaplan videos biochemistry
kaplan videos pharmacology
kaplan videos step 1 kickass
kaplan videos anatomy
kaplan videos amazon
kaplan videos and notes
kaplan anatomy videos download
kaplan anatomy videos free download
kaplan acca videos
kaplan anatomy videos 2014
kaplan anesthesia videos
kaplan app videos
kaplan gross anatomy videos
kaplan videos buy
kaplan videos behavioral sciences
kaplan biostatistics videos
kaplan biochemistry videos 2014
kaplan biochemistry videos 2010
kaplan biochemistry videos lectures free download
kaplan biochem videos download
kaplan biochemistry videos review
kaplan biochem videos step 1
kaplan videos cost
kaplan videos cardiology
kaplan videos cfa
kaplan videos cfa level 2
kaplan videos ck
kaplan videos conrad fischer
kaplan cs videos
kaplan content videos
kaplan ck videos download
robert d kaplan videos
kaplan videos endocrinology
kaplan videos ent
kaplan epidemiology videos
are kaplan videos enough
kaplan clinical examination videos
kaplan physical examination videos
kaplan step 2 ck videos ebay
kaplan step 2 epidemiology videos
kaplan usmle step 1 videos ebay
kaplan videos for step 1
kaplan videos for pharmacology
kaplan videos free download
kaplan videos free
kaplan videos for step 2 ck
kaplan videos for usmle
kaplan videos for step 3
kaplan videos for surgery
kaplan videos genetics
kaplan gre videos
kaplan ged videos
kaplan gmat videos
kaplan gmat videos download
kaplan gre videos free download
kaplan gi videos
kaplan 2015 videos google drive
kaplan histology videos
kaplan hematology videos
kaplan high yield videos
kaplan videos immunology
kaplan immunology videos download
kaplan international videos
kaplan 2010 immunology videos
kaplan usmle immunology videos
kaplan pharmacology videos kickass
kaplan usmle step 1 videos kickass
kaplan usmle step 2 videos kickass
kaplan step 2 ck videos kickass
kaplan videos mega
kaplan mcat videos download
kaplan medical videos step 1
kaplan medical videos step 2
kaplan math videos
kaplan videos neuroanatomy
kaplan videos not working
kaplan videos neurology
kaplan videos nclex
kaplan videos nursing
kaplan neurology videos download
kaplan neuroscience videos
kaplan neuro videos
kaplan neuroanatomy videos free download
kaplan videos obstetrics
kaplan videos offline
kaplan videos on pharmacology
kaplan obstetrics videos download
kaplan obstetrics videos 2010
kaplan ophthalmology videos
kaplan orthopedics videos
kaplan videos pathology
kaplan videos physiology
kaplan videos psychiatry
kaplan videos price
kaplan pharm videos
kaplan videos renal physiology
kaplan videos respiratory
kaplan rheumatology videos
kaplan radiology videos
kaplan renal videos
kaplan pharmacology videos raymon
kaplan videos step 1 2014
kaplan videos surgery
kaplan videos usmle step 2 ck gynecology
kaplan videos usmle step 1 free download
kaplan videos usmle step 2
kaplan videos usmle step 1 download
kaplan videos usmle step 3
kaplan videos usmle 1
kaplan videos usmle forum
kaplan usmle step 1 videos
kaplan usmle videos download
kaplan videos vs dit
kaplan videos vk
kaplan virology videos
kaplan videos 2010 vs 2014
kaplan videos youtube
kaplan high yield videos step 1
kaplan high yield videos step 2
kaplan high yield videos download
kaplan ck high yield videos
kaplan usmle high yield videos 2013.rar
kaplan videos step 1 free download
kaplan videos step 1 2017
kaplan videos step 1 2016
kaplan videos step 1 2017 free download
kaplan videos step 1 2016 free download
kaplan videos step 1 2014 free download
kaplan videos step 1 2018
step 1 kaplan videos
step 1 kaplan videos download
step 1 kaplan videos free download
usmle step 1 kaplan videos 2015
cfa level 1 kaplan videos
usmle step 1 kaplan videos 2014
usmle step 1 kaplan videos 2017
usmle step 1 kaplan videos free
kaplan videos 2014 google drive
kaplan videos 2017
kaplan videos 2018
kaplan videos 2014 free download
kaplan videos 2016
kaplan videos 2010 download
step 2 kaplan videos
cfa level 2 kaplan videos
nbde part 2 kaplan videos
step 2 cs kaplan videos
step 2 ck kaplan videos free
kaplan videos step 3
kaplan step 3 videos download
kaplan cfa level 3 videos
step 3 kaplan videos
step 3 kaplan videos free download
kaplan series 6 videos
kaplan series 63 videos
kaplan series 65 videos
kaplan series 7 videos
kaplan videos google drivekaplan physiology videoskaplan usmle step 1 videos kickasskaplan step 1 lecture videos 2010buy kaplan videos step 1kaplan step 1 high yield videos downloadkaplan medicalkaplan usmle step 1 on demand reviewkaplan usmle step 1 videos 2015 google driveusmle google drivekaplan 2015 videos google drivekaplan usmle step 1 videos google drivekaplan usmle step 1 videos 2015usmle physiology videoskaplan surgery videoskaplan 2010 videos google driveusmle videoskaplan ophthalmology videoskaplan usmle step 1 videos amazonkaplan full length step 1kaplan usmle step 1 bookskaplan medical videoskaplan medical bookskaplan medical loginkaplan medical centerkaplan medical chicagokaplan usmle step 2kaplan usmle step 1kaplan medical new yorkkaplan usmle step 1 qbankusmle step 1 coachingkaplan houston usmleusmle step 1 question bankkaplan usmle step 1 2018kaplan videos
kaplan usmle step 1 videos
kaplan videos step 1
kaplan videos for step 2 ck
kaplan videos step 3
kaplan videos for usmle
kaplan videos usmle
kaplan videos step 1 free download
kaplan step 3 videos download
kaplan videos biochemistry
kaplan videos step 1 2017
kaplan usmle step 1 videos kickass
kaplan videos pharmacology
kaplan mcat videos download
kaplan videos physiology
kaplan videos usmle step 2
kaplan videos free
kaplan videos usmle step 1 free download
kaplan videos step 1 2015
kaplan videos step 1 2018
kaplan videos mcat
kaplan high yield videos step 1
kaplan videos for step 1
kaplan cs videos
kaplan videos step 1 2016
kaplan neuroscience videos
kaplan videos internal medicine
kaplan gre videos
kaplan gmat videos
kaplan medical videos step 1
kaplan ob gyn videos
kaplan ophthalmology videos
kaplan videos anatomy
kaplan usmle videos download
kaplan videos google drive
kaplan biostatistics videos
kaplan high yield videos
kaplan pharmacology videos free download
kaplan videos step 1 2016 free download
kaplan videos step 1 kickass
kaplan videos 2010
kaplan 2015 videos google drive
kaplan anatomy videos download
kaplan anatomy videos free download
kaplan hematology videos
kaplan high yield videos download
kaplan high yield videos step 2
kaplan immunology videos download
kaplan medical videos free download
kaplan microbiology videos free download
kaplan neurology videos download
kaplan obstetrics videos 2010
kaplan obstetrics videos download
kaplan orthopedics videos
kaplan pediatrics videos free download
kaplan pharm videos
kaplan physiology videos free download
kaplan rheumatology videos
kaplan videos 2014
kaplan videos 2015
kaplan videos free download
kaplan videos medicine
kaplan videos online
kaplan videos pediatrics
kaplan videos step 1 2014
kaplan videos step 1 2014 free download
kaplan videos step 1 2017 free download
kaplan videos youtube
kaplan pharmacology videos kickassusmle videos step 1
usmle videos google drive
usmle videos step 2
usmle videos kaplan
usmle videos free download
usmle videos download
usmle videos 2017
usmle videos telegram
usmle videos free
usmle anatomy videos
usmle algorithm videos
kaplan usmle videos amazon
usmle audio lectures
usmle anatomy lectures
usmle first aid videos
usmle success academy videos download
usmle success academy videos
kaplan usmle step 1 videos amazon
usmle biochem videos
usmle biochemistry lectures
kaplan usmle biochemistry videos
usmle step 1 biochemistry videos
kaplan usmle step 1 videos biochemistry
kaplan usmle step 1 videos buy
usmle step 1 best videos
becker usmle videos
b&b videos usmle
usmle cse videos download
usmle cs videos free download
usmle cse videos
usmle ck videos
usmle cardiology videos
usmle world cs videos
usmle step cs videos
usmle express videos review
usmle embryology videos
usmle express videos free
usmle examination videos
usmle ent lectures
usmle rx express videos 2015 download
usmle rx express videos 2017
usmle lectures free download
usmle lectures free
usmle step 1 videos free download
usmle cse videos free download
usmle gynaecology lectures
usmle step 2 gynecology videos
usmle immunology videos
usmle immunology lectures
kaplan usmle immunology videos
usmle kaplan videos free download
usmle kaplan videos 2017
usmle kaplan videos 2014
usmle kaplan videos 2015
usmle kaplan lectures
usmle step 1 kaplan videos free download
usmle step 2 videos kaplan
usmle step 1 kaplan videos 2015
usmle step 1 kaplan videos 2014
usmle lecture videos
usmle video lectures
kaplan usmle lecture videos
usmle step 1 lecture videos
kaplan usmle lecture videos free download
free usmle lecture videos
usmle anatomy lecture videos
usmle step 1 latest videos
best usmle lecture videos
download usmle lecture videos
usmle microbiology videos
usmle motivational videos
usmle medicine videos
usmle medicine lectures
medical videos usmle
kaplan usmle step 1 videos microbiology
usmle internal medicine videos
kaplan usmle microbiology videos
best usmle microbiology videos
medtube usmle videos
usmle neuroanatomy videos
usmle nephrology lectures
kaplan usmle neurology videos
kaplan usmle step 1 neuroscience videos
usmle videos online
usmle ophthalmology videos
usmle lectures online
usmle step 1 videos online
kaplan usmle step 1 videos online
kaplan usmle obstetrics videos
usmle step one videos
kaplan usmle step 1 videos on dvds
usmle step 2 obstetrics videos
usmle prep videos
usmle pathoma videos
usmle preparation videos
usmle psychiatry videos
usmle pathology videos
usmle lectures ppt
usmle pharmacology lectures
usmle pediatrics lectures
usmle review videos
usmle step 1 review videos
best usmle review videos
rx usmle videos
usmle step 2 review videos
usmle study videos
usmle surgery videos
usmle step 3 videos
usmle step videos
usmle step 1 videos download
kaplan usmle videos step 2
usmle youtube videos
usmle lectures youtube
usmle step 1 videos youtube
usmle 1 videos
kaplan usmle 1 videos
usmle step 1 videos kaplan
usmle step 1 videos free
usmle step 1 videos 2016
usmle step 1 videos
usmle 2014 videos
usmle 2 videos
usmle 2018 videos
kaplan usmle videos 2017
usmle express videos 2018
kaplan usmle videos 2015
usmle step 2 ck videos free download
usmle step 2 ck videos kaplan 2015
usmle step 2 ck videos kaplan 2014
usmle 2 cs videos
becker usmle step 2 ck videos
usmle step 3 videos download
step 3 usmle videos
step 3 usmle videos downloadusmle videos
usmle step 1 videos kaplan
usmle videos step 1
usmle videos kaplan
usmle step 3 videos
usmle first aid videos
usmle cs videos
usmle videos step 2
usmle lecture videos
usmle video lectures
usmle express videos
usmle kaplan lectures
usmle step 1 videos 2018
usmle step 2 cs videos youtube
kaplan usmle 1 videos
usmle step 1 lecture videos
usmle lectures online
usmle step 1 videos free download
usmle review videos
usmle step 2 videos kaplan
kaplan usmle lecture videos
kaplan usmle step 1 videos amazon
usmle anatomy videos
usmle step 3 videos free download
usmle lectures free download
usmle videos free download
usmle ck videos
usmle prep videos
usmle step 1 kaplan videos free download
usmle audio lectures
usmle physiology videos
usmle step 1 biochemistry videos
usmle success academy videos download
usmle success academy videos
usmle videos free
becker usmle videos download
usmle pharmacology videos
usmle cse videos
kaplan usmle biochemistry videos
becker usmle videos
usmle express videos download
kaplan usmle step 1 videos biochemistry
kaplan usmle videos 2015
kaplan usmle videos 2017
usmle 1 videos
usmle algorithm videos
usmle anatomy lectures
usmle biochemistry videos
usmle cs videos free download
usmle ent lectures
usmle kaplan videos free download
usmle pathology videos
usmle pediatrics lectures
usmle pharmacology lectures
usmle preparation videos
usmle step 1 videos download
usmle step 1 videos free
usmle step 2 obstetrics videos
usmle step 3 videos download
usmle videos for sale
usmle videos online
usmle world cs videos
usmle gynaecology lectures
usmle surgery videos
0 notes
Link
Clinical Significance
Guidelines by the Center for Nutrition Policy and Promotion recommend a daily sodium intake to be less than 2300 mg/d in the general population and 1500 mg/d among people at greater risk of cardiovascular diseases (ie, individuals aged >50 years, African Americans, and those who have hypertension, type 2 diabetes mellitus, or chronic kidney disease).1 The American Heart Association has recently emphasized goals to achieve “Ideal Cardiovascular Health” by 2020, and one of the dietary metrics is daily sodium intake of less than 1500 mg/d.2
Evidence concerning a possible beneficial effect of dietary sodium restriction on cardiovascular events and cardiovascular and all-cause mortality is largely indirect. Most of the studies testing a “low sodium diet” use surrogate markers for detecting sodium intake, such as 24-hour dietary recall, food questionnaires, and urinary sodium excretion. Moreover, most trials testing a low sodium diet offer dietary advice to restrict sodium and do not randomize patients to the exact same diets, with the only difference being a reduction in the sodium intake.
On the outcomes side, most evidence for the effects of sodium on cardiovascular-related outcomes relates to blood pressure. Although there are reasonable data to support that sodium restriction lowers blood pressure, the effects may be transient and inconsistent, with some individuals even having paradoxical increases in blood pressure. The degree of blood pressure lowering on average might be clinically trivial, approximately 2 mm Hg in normotensive individuals and approximately 4 mm Hg in hypertensive individuals. Finally, sodium restriction also has the adverse effects of activating the renin-angiotensin-aldosterone system, increasing catecholamines, and adversely affecting insulin and lipids.3 Whether a reduction in any of the surrogate markers will lead to a decrease in morbidity and mortality of the population needs to be established independently of these surrogates, and such evidence is scarce.4
Reaching a definitive position is further complicated because a large portion of evidence supporting low sodium diets comes from observational studies and nonrandomized trials.5 Among the randomized studies, many evaluated a large decrease in the sodium intake for a short period of time or a small decrease in the sodium intake for a long period of time. Both of these study designs represent situations that may not be directly applicable to real-life situations.
This review critically analyzes the data for low sodium diets, starting with surrogate markers such as blood pressure and other risk factors such as type 2 diabetes mellitus, and then moving to clinical outcomes such as cardiovascular morbidity and events, and cardiovascular and overall mortality.
Surrogate Markers
Much of the support for the idea that a low sodium diet leads to a lower blood pressure comes from the Dietary Approaches to Stop Hypertension (DASH) study.6 This study enrolled 412 participants and randomly assigned them to receive the control diet or the DASH diet. In both groups, the participants were assigned randomly to a high sodium diet (150 mmol/d), normal sodium diet (100 mmol/d), or low sodium diet (50 mmol/d) for 30 consecutive days and were then crossed over within their assigned groups. When the participants were shifted from a high sodium diet to a normal sodium diet, the systolic blood pressure decreased by 2.1 mm Hg (P < .001) in the control group and by 1.3 mm Hg (P = .03) in the DASH group. When they were shifted from a normal sodium diet to a low sodium diet, there was a further reduction in systolic blood pressure of 4.6 mm Hg in the control group (P < .001) and 1.7 mm Hg in the DASH group (P < .01). When compared with the controls, the DASH diet led to a lower systolic blood pressure of 7.1 mm Hg in participants without hypertension and 11.5 mm Hg in participants with hypertension. However, the DASH diet was significantly different from the control diet in terms of more fruits, vegetables, low-fat dairy foods, whole grains, poultry, fish, nuts, potassium, calcium, magnesium, dietary fiber, and protein, and less red meat, sweets, sugar-containing beverages, total and saturated fat, and cholesterol. Although the group on the DASH diet had a lower urinary sodium excretion, this does not necessarily imply that the benefit was being solely delivered by a dietary sodium reduction. In addition, this study did not evaluate the long-term effects of the intervention and the clinically relevant variables, such as mortality or morbidity.
Salt and Type 2 Diabetes Mellitus
In patients with type 2 diabetes mellitus, a low sodium diet has been associated with increased cardiovascular and all-cause mortality.7 Even moderate salt reduction may lead to increased activation of the sympathetic nervous system and renin-angiotensin-aldosterone system, and insulin resistance.
A cohort study7 enrolled 638 diabetic persons who were consistently followed for a period of 9.9 years. Their baseline urinary sodium excretion levels were 184 ± 73 mmol/24 hours, which remained constant throughout the study duration. Urinary sodium levels were inversely related to the all-cause mortality rate (P < .001) and cardiovascular mortality rate (sub-hazard ratio [HR], 0.65; confidence interval [CI], 0.44-0.95; P = .03). Each 100 mmol increase in the urinary sodium excretion led to a decrease in all-cause mortality of 28% (95% CI, 6-45; P = .02). This study implies a potential contraindication to a low sodium diet not only in those with type 2 diabetes mellitus but also by extension in the general population because of the widespread prevalence of type 2 diabetes mellitus. This leads to the question, are the current dietary guideline recommendations for a low sodium diet in the general population (including type 2 diabetes mellitus) appropriate? The limitation index of these data is that the results are based on a cohort study examining urinary sodium excretion levels versus a randomized controlled trial of patients receiving identical diets, with the only variation being the amount of sodium intake.
Patient-oriented Outcomes: Morbidity and Mortality
Congestive heart failure is characterized by various processes that lead to reduced renal perfusion and activation of the sympathetic nervous system and the renin-angiotensin-aldosterone system.8 This leads to preferential retention of water compared with sodium and can cause hyponatremia. Restricted dietary sodium intake may further exacerbate these processes, therefore precipitating hyponatremia.
A study9 enrolled 410 patients with congestive heart failure and followed them for 6 months to compare the dietary sodium intake levels with doses of diuretics in these patients. These patients were divided into 8 groups: group A received 1000 mL/d of fluid intake, 120 mmol/d, and 250 mg furosemide twice daily; group B received 1000 mL/d of fluid intake, 120 mmol/d, and 125 mg furosemide twice daily; group C received 1000 mL/d fluid intake, 80 mmol/d, and 250 mg furosemide twice daily; group D received 1000 mL/d fluid intake, 80 mmol/d, and 125 mg furosemide twice daily; group E received 2000 mL/d fluid intake, 120 mmol/d, and 250 mg furosemide twice daily; group F received 2000 mL/d fluid intake, 120 mmol/d, and 125 mg furosemide twice daily; group G received 2000 mL/d fluid intake, 80 mmol/d, and 250 mg furosemide twice daily; and group H received 2000 mL/d fluid intake, 80 mmol/d, and 125 mg furosemide twice daily for 30 days or more after discharge and for 180 days afterward. Group A showed the greatest statistically significant reduction in readmissions, brain natriuretic peptide, aldosterone, and plasma renin activity compared with the other groups (P < .001). Therefore, a normal sodium diet (2.8 g/d) and a higher dose of a diuretic (250 mg twice daily) yielded the best results as opposed to a low sodium diet (1.8 g/d) and a lower diuretic dose (125 mg twice daily).
The low sodium diet caused increased mortality and heart failure hospitalizations versus a normal sodium diet in patients with systolic heart failure. These results have been verified across multiple randomized controlled trials in patients with systolic heart failure.10,11,12,13 These findings can be explained partially on the basis of studies conducted in mice.14 It has been shown that the renin-angiotensin-aldosterone system has a central role in atherogenesis and that dietary salt intake plays a significant role in controlling this system. A study in rats has shown a 4-fold increase in plaque formation with a low sodium diet compared with a normal sodium diet, and this effect can be blocked by the use of angiotensin-converting enzyme inhibition, which suggests that it is mediated by the renin-angiotensin-aldosterone system. Effects observed with a high sodium diet include reduced vascular inflammation and atherogenesis and a modest increase in systolic blood pressure (5 ± 1 mm Hg). These data, although generated from mice, may explain the inverse relationship between dietary sodium intake and mortality rate.
The majority of data relating dietary sodium to cardiovascular health are based on its effects on blood pressure. Data from the 3 epidemiologic studies National Health And Nutrition Examination Survey (NHANES) I to III have been analyzed to assess the relationship between dietary sodium intake and cardiovascular mortality rates.15,16,17 NHANES I acquired information from 20,729 participants via interview and examination, and followed them using interview, tracking, and vital events registry. An inverse association was seen between dietary salt intake and all-cause mortality (lowest to highest salt intake quartile 23.18 to 19.01, P < .0001) and cardiovascular mortality (sodium 11.80 to 9.60, P < .0019; calories 12.80 to 8.94, P < .0002; sodium/calorie ratio 9.73 to 11.35, P = .017).15 Moreover, sodium intake was inversely associated with all-cause (P = .0069) and cardiovascular mortality (P = .086). NHANES II followed a similar population of 7154 participants for 13.7 years and yielded similar results. The sodium adjusted for calories and sodium/calorie ratio were both independently and inversely associated with cardiovascular mortality (P = .03 and P = .008, respectively; adjusted HR of cardiovascular mortality for sodium <2300 mg, 1.37; CI, 1.03-1.81; P = .033) and all-cause mortality (HR, 1.28; CI, 1.10-1.50; P = .003).16 However, these results did not hold true for participants aged more than 55 years, obese participants, and non-white participants. NHANES III was a cohort study based on 8699 participants who were followed using national vital entries registries for the outcomes of all-cause and cardiovascular mortality.17An inverse association between dietary sodium intake and cardiovascular mortality was shown (HR, 1.80; CI, 1.05-3.08; P = .03). Moreover, an inverse association of continuous sodium (per 1000 mg) intake with cardiovascular and all-cause mortality was observed with a 99% CI of 0.73 to 1.06 (P = .07) and 0.86 to 1.04 (P = .11), respectively. These findings question any potential survival advantage with a low sodium diet and indicate caution for population-wide sodium restriction.
Conversely, some studies have suggested a lower and higher mortality rate with a high sodium diet depending on the New York Heart Association (NYHA) functional class status. An observational study18 in 302 patients showed that patients with a daily urinary sodium excretion level >3 g had a reduced risk for a cardiovascular events (HR, 0.44; CI, 0.20-0.97) for NYHA functional class I/II congestive heart failure, but an increased risk (HR, 2.54; CI, 1.10-5.84) for NYHA III/IV congestive heart failure compared with a urinary sodium excretion level <3 g. This study was an observational study, in contrast to the randomized controlled trials indicating benefits of a normal sodium diet, and used urinary sodium levels as a measure of dietary sodium intake, which may be a reasonable surrogate marker for normal individuals but not for patients with congestive heart failure, who have severe renal excretory disturbances.
A large observational analysis of 2 cohorts,19 including 28,880 patients (from the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial [ONTARGET] and Telmisartan Randomized Assessment Study in ACE-intolerant Subjects With Cardiovascular Disease [TRANSCEND] trials), was conducted to investigate the effects of sodium and potassium intakes on the incidence of cardiovascular disease. The primary composite outcome measure was death related to cardiovascular causes, myocardial infarction, stroke, and hospitalization for congestive heart failure. The mean estimated baseline 24-hour urinary sodium excretion and standard deviation were 4.77 g (1.61) and 4 to 5.99 g, respectively, in the reference group. The patients were followed for an average of 56 months, during which the primary outcome was observed in 4729 patients (16.4%), with 2057 cardiovascular deaths, 1412 patients with myocardial infarction, 1282 patients with stroke, and 1213 patients hospitalized for congestive heart failure. In the control group, there was an incidence of 6.3% cardiovascular death, 4.6% myocardial infarction, 4.2% stroke, and 3.8% hospitalizations with congestive heart failure. In the cohort, a higher and lower urinary sodium excretion was associated with increased cardiovascular mortality. A higher baseline urinary sodium excretion had a statistically significant association with cardiovascular death (9.7% for 7-8 g/d; HR, 1.53; 95% CI, 1.26-1.86; and 11.2% for >8 g/d; HR, 1.66; 95% CI, 1.31-2.10), myocardial infarction (6.8%; HR, 1.48; 95% CI, 1.11-1.98 for >8 g/d), stroke (6.6%; HR, 1.48; 95% CI, 1.09-2.01 for >8 g/d), and hospitalization for congestive heart failure (6.5%; HR, 1.51; 1.12-2.05 for >8 g/d). Likewise, a lower urinary sodium excretion also had a statistically significant correlation with an increased risk of cardiovascular death (8.6%; HR, 1.19; 95% CI, 1.02-1.39 for 2-2.99 g/d; 10.6%; HR, 1.37; 95% CI, 1.09-1.73 for <2 g/d) and hospitalization for congestive heart failure (5.2%; HR, 1.23; 95% CI, 1.01-1.49 for 2-2.99 g/d). This cohort study suggests a J-shaped relationship between dietary sodium intake and cardiovascular risk factor; therefore, a higher and lower dietary sodium intake may be related to adverse cardiovascular outcomes. Moreover, the lowest cardiovascular event rates occurred in the moderate sodium excretion (4-5.99 g/d) and high potassium excretion (>3 g/d) groups. Thus, it seems that a normal sodium diet (4-6 g/d) in addition to a high potassium intake may be best for the general population.
Trials of Hypertension Prevention (TOHP) phases I and II were 2 large randomized controlled trials enrolling 2182 and 2382 patients, respectively. In TOHP I,20 the patients were randomized to 3 interventions, one of which was a low sodium diet; however, the low sodium diet and control groups were not given the exact same diets. Although a lowered dietary sodium intake, as measured by a urinary sodium excretion of 44 mmol/24 hours, was able to reduce the diastolic blood pressure by 0.9 mm Hg (P < .05) and systolic blood pressure by 1.7 mm Hg (P < .01), it may have been due to the diet that lowers urinary sodium and not necessarily the lower sodium content. In TOHP II,21 counseling was used to reduce the dietary sodium intake in the test group. During the study period, the urinary sodium excretion decreased 50 and 40 mmol/d at 6 and 36 months, respectively. This decrease in urinary sodium excretion was associated with a 2.9/1.6 mm Hg decrease in the intervention group (all groups, P < .001). However, this study treated the intervention group differently from the control group. The groups were not given the same diets, and the intervention group was counseled to reduce sodium in their diet. Moreover, the intervention group also was counseled to increase spices, which alone may have cardiovascular benefits. Thus, a lower urinary sodium does not necessarily indicate that the results are due to a lowered sodium intake.
Other Unintended Consequences Relevant to Cardiovascular Health
A Cochrane review based on 167 studies showed that a low sodium diet in normotensive whites leads to a small reduction in systolic blood pressure (−1.27 mm Hg; CI, −1.88 to −0.66; P = .0001), without significantly reducing diastolic blood pressure (−0.05 mm Hg; CI, −0.51 to 0.42; P = .85).22 However, a low sodium diet caused an increase in renin (P < .00001), aldosterone (P < .00001), noradrenaline (P < .00001), adrenaline (P < .0002), cholesterol (P < .001), and triglycerides (P < .0008). This meta-analysis included studies that were only 2 weeks long and did not use good screening measures for quality. Inclusion of trials with an acute reduction in dietary sodium intake may not fully elucidate its long-term effects. Despite this fact, the potential harmful effects of sodium reduction may outweigh its benefits, especially in those individuals who generally did not have a significant reduction in blood pressure (normotensive whites and Asians).
With a lack of consistent efficacy of a low sodium diet, and potential harm, the cost-effectiveness of such a worldwide approach to a low sodium diet is questionable. A major source of dietary iodine is through salt. Therefore, a low sodium diet could lead to worsening of thyroid diseases. Salt also gives palatability to food and possesses numerous antimicrobial effects. It is possible, at least theoretically, that food-borne infections could increase if we were to decrease the amount of salt in foods.
A low sodium diet may even be counterproductive from a public health perspective. In addition to possibly exacerbating, it may distract efforts from other, more worthwhile programs that have a stronger foundation in evidence.23 Here again, the possibility that a low sodium diet may lead to worsened cardiovascular survival rates is a concern. The potential population-level effects of such an extreme intervention, with the American Heart Association recommending a sodium intake of <1.5 g/d for all Americans,2 can be expected to lead to potentially negative results on morbidity and mortality.
Even if a low sodium diet was advisable, is it physiologically possible? Although a low sodium diet may have benefit, there is still evidence that states it is not possible to modify human sodium intake levels because of complex neurohumoral homeostatic mechanisms.24 This makes public health programs focusing on salt reduction in the general population potentially counterproductive. Whether or not dietary salt intake is physiologically determined is still not known.25 If it is physiologically determined, with an optimal dietary range, then any modification to the dietary intake may be risky.
Conclusions
There is no conclusive evidence that a low sodium diet reduces cardiovascular events in normotensive and pre-hypertensive or hypertensive individuals. On the contrary, there is sound evidence that a low sodium diet leads to a worse cardiovascular prognosis in patients with systolic congestive heart failure or type 2 diabetes mellitus. Worldwide sodium restriction, through its adverse effects on insulin resistance, may lead to an increase in the rates of type 2 diabetes mellitus. By potentially moving the food industry to produce lower-sodium products could lead to greater consumption of processed foods and greater incidence of metabolic syndrome. Other adverse effects also are possible with attempted sodium restrictions, whereas low sodium diets themselves may not be possible because of inherent physiologic regulation. Advising low sodium diets seems misguided and potentially dangerous and illustrates the problem of guidelines based on flawed studies using surrogate measures.
References
Dietary Guidelines for Americans, 2010. Alexandria, VA: US Department of Agriculture, Center for Nutrition Policy and Promotion. Available at: http://www.cnpp.usda.gov/Publications/DietaryGuidelines/2010/PolicyDoc/Chapter3.pdf. Accessed December 18, 2012.
Whelton, P.K., Appel, L.J., Sacco, R.L. et al. Sodium, blood pressure, and cardiovascular disease: further evidence supporting the American heart association sodium reduction recommendations.Circulation. 2012; 126: 2880–2889
Alderman, M.H. Reducing dietary sodium: the case for caution. JAMA. 2010; 303: 448–449
Ciani, O., Buyse, M., Garside, R. et al. Comparison of treatment effect sizes associated with surrogate and final patient relevant outcomes in randomised controlled trials: meta-epidemiological study. (f457)BMJ. 2013; 346
Graudal, N.A., Galloe, A.M., and Garred, P. Effects of sodium restriction on blood pressure, renin, aldosterone, catecholamines, cholesterols and triglyceride. JAMA. 1998; 279: 1383–1391
Sacks, F.M., Svetkey, L.P., Vollmer, W.M...., and DASH-Sodium Collaborative Research Group. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001; 344: 3–10
Ekinci, E.I., Clarke, S., Thomas, M.C. et al. Dietary salt intake and mortality in patients with type 2 diabetes. Diabetes Care. 2011; 34: 703–709
Gupta, D., Georgiopoulou, V.V., Kalogeropoulos, A.P. et al. Dietary sodium intake in heart failure.Circulation. 2012; 126: 479–485
Paterna, S., Parrinello, G., Cannizzaro, S. et al. Medium term effects of different dosage of diuretic, sodium, and fluid administration on neurohormonal and clinical outcome in patients with recently compensated heart failure. Am J Cardiol. 2009; 103: 93–102
Licata, G., Di Pasquale, P., Parrinello, G. et al. Effects of high-dose furosemide and small-volume hypertonic saline solution infusion in comparison with a high dose of furosemide as bolus in refractory congestive heart failure: long-term effects. Am Heart J. 2003; 145: 459–466
Paterna, S., Di Pasquale, P., Parrinello, G. et al. Changes in brain natriuretic peptide levels and bioelectrical impedance measurements after treatment with high-dose furosemide and hypertonic saline solution versus high-dose furosemide Alone in refractory congestive heart failure. J Am Coll Cardiol. 2005; 45: 1997–2003
Paterna, S., Fasullo, S., Parrinello, G. et al. Short-term effects of hypertonic saline solution in acute heart failure and long-term effects of a moderate sodium restriction in patients with compensated heart failure with New York heart Association Class III (Class C) (SMAC-HF study). Am J Med Sci. 2011;342: 27–37
Paterna, S., Gaspare, P., Fasullo, S. et al. Normal-sodium diet compared with low- sodium diet in compensated congestive heart failure: is sodium an old enemy or a new friend?. Clin Sci (London). 2008; 114: 221–230
Tikellis, C., Pickering, R.J., Tsorotes, D. et al. Activation of the renin-angiotensin system mediates the effects of dietary salt intake on atherogenesis in the apolipoprotein E knockout mouse. Hypertension. 2012; 60: 98–105
Alderman, M.H., Cohen, H., and Madhavan, S. Dietary sodium intake and mortality: the National Health and Nutrition Examination Survey (NHANES I). Lancet. 1998; 351: 781–785
Cohen, H.W., Hailpern, S.M., Fang, J., and Alderman, M.H. Sodium intake and mortality in the NHANES II: follow-up study. Am J Med. 2006; 119: e7–e14
Cohen, H.W., Hailpern, S.M., and Alderman, M.H. Sodium intake and mortality follow-up in the Third National Health and Nutrition Examination Survey (NHANES III). J Gen Intern Med. 2008; 23: 1297–1302
Lennie, T.A., Song, E.K., Wu, J.R. et al. Three gram sodium intake is associated with longer event-free survival only in patients with advanced heart failure. J Card Fail. 2011; 17: 325–330
O'Donnell, M.J., Yusuf, S., Mente, A. et al. Urinary sodium and potassium excretion and risk of cardiovascular events. J Am Med Assoc. 2011; 306: 2229–2238
TOHP group. The effects of nonpharmacologic interventions on blood pressure of persons with high normal levels. Results of the Trials of Hypertension Prevention, Phase I. JAMA. 1992; 267: 1213–1220
TOHP group. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high-normal blood pressure. The Trials of Hypertension Prevention, phase II. The Trials of Hypertension Prevention Collaborative Research Group. Arch Intern Med. 1997; 157: 657–667
Graudal, N.A., Hubeck-Graudal, T., and Jurgens, G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride (Review).(CD004022)Cochrane Database Syst Rev. 2011; 11
Lucan, S.C. Attempting to reduce sodium intake might do harm and distract from a greater enemy.(e3)Am J Public Health. 2013; 103
McCarron, D.A., Drüeke, T.B., and Stricker, E.M. Science trumps politics: urinary sodium data challenge US dietary sodium guideline. Am J Clin Nutr. 2010; 92: 1005–1006
McCarron, D.A., Geerling, J.C., Kazaks, A.G., and Stern, J.S. Can dietary sodium intake be modified by public policy?. Clin J Am Soc Nephrol. 2009; 4: 1878–1882
#diet#low sodium diet#Nutrition#electrolytes#family medicine#best practices#controversy#cardiology#sodium#heart disease#Hypertension#internal medicine#Integrative Medicine
1 note
·
View note
Text
caduet tablets Uses, Dosage, Side Effects, Precautions & Warnings
Drug Online
caduet Generic drug of the Therapeutic class: Cardiology and angiology
Active ingredients: Amlodipine , Atorvastatin
what is caduet medication?
This medication is supplied as blue, oval-shaped, film-coated tablets debossed with “Pfizer” on one side and “CDT 101” on the other side.
Boxes of 7, 10, 14, 20, 28, 30, 50, 56, 60, 90, 100 or 200 tablets.
Not all presentations may be marketed.
what is caduet medication used for and indication?
CADUET is indicated for the prevention of cardiovascular events in hypertensive patients with 3 associated cardiovascular risk factors, with normal to moderately elevated cholesterol without established coronary artery disease, and in whom, according to current recommendations, the concomitant use of amlodipine and a low dose of atorvastatin is suitable .
CADUET should be used when the response to regimen and other non-pharmacological measures is inadequate.
caduet dosage
Oral route
The usual starting dose is 5 mg / 10 mg once daily.
If tighter blood pressure control is required, a dosage of 10 mg / 10 mg once daily may be administered.
The tablets can be taken at any time of the day, with or without food.
CADUET can be used alone or in combination with other antihypertensive drugs, but it must not be used in combination with other calcium channel blockers or another statin.
The combination of CADUET and fibrates should generally be avoided (see sections 4.4 and Interactions with other medicinal products and other forms of interaction ).
Patients with renal impairment : No dosage adjustment is necessary in patients with impaired renal function (see sections 4.4 and Pharmacokinetic properties ).
Patients with hepatic impairment : CADUET is contraindicated in patients with active hepatic disease.
Children / Adolescents : The safety and efficacy of CADUET have not been established in children / adolescents. Therefore, the use of CADUET is not recommended in this population.
Elderly : It does not appear necessary to adjust the dose in the elderly (see section Pharmacokinetic properties ).
Combination with other medicinal products : In combination with ciclosporin, the dose of atorvastatin should not exceed 10 mg (see section Interactions with other medicinal products and other forms of interaction ).
Contraindications
CADUET is contraindicated in patients:
Being hypersensitive to dihydropyridines *, to amlodipine, to atorvastatin or to any of the excipients of this medicine,
have active liver disease or a persistent and unexplained increase in serum transaminases exceeding 3 times the upper limit of normal,
In women who are pregnant, breastfeeding or of childbearing potential and not using reliable contraceptive methods (see section Pregnancy and breast-feeding ),
In combination with itraconazole, ketoconazole, telithromycin (see section Interactions with other medicinal products and other forms of interactions ),
having severe hypotension,
Having shock (including cardiogenic shock),
Having an obstruction of the efferent pathway to the left ventricle (for example severe aortic stenosis),
With haemodynamically unstable heart failure after acute myocardial infarction.
* amlodipine is a calcium channel blocker derived from dihydropyridine.
HOW TO TAKE CADUET?
Always take this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if in doubt.
Adults
The recommended starting dose of CADUET is one 5 mg / 10 mg tablet per day. Your doctor may increase the dose if necessary to 1 tablet of CADUET 10 mg / 10 mg per day.
The tablets should be swallowed whole with a glass of water. They can be taken at any time of the day, with or without food. However, it is best to take them at the same time each day.
Continue to follow your doctor’s dietary advice, especially to eat a low fat diet, avoid tobacco, and exercise regularly.
If you have the impression that the effect of CADUET tablets is too strong or too weak, talk to your doctor or pharmacist.
Use in children and adolescents
This medication is not recommended for children and adolescents.
If you take more CADUET 10 mg / 10 mg film-coated tablets than you should
If you accidentally take too many CADUET tablets (more than your usual dose), ask your doctor or the nearest hospital for advice. Take any remaining tablets, the carton or the whole carton with you so that hospital staff can quickly identify which medicine you have taken.
If you forget to take CADUET 10 mg / 10 mg film-coated tablets
If you forget to take your dose of CADUET, take your next dose at the normal time.
Do not take a double dose to make up for the dose you forgot to take.
If you stop taking CADUET 10 mg / 10 mg film-coated tablets
Do not stop taking CADUET unless your doctor tells you to.
If you have any further questions on the use of this medicine or want to stop taking your treatment, ask your doctor or pharmacist for more information.
How To Store Caduet?
Keep this medication out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the blister, the carton and the vial after “EXP”. The expiration date refers to the last day of that month.
Store at a temperature not exceeding 30 ° C.
Do not throw away any medicines via a wastewater treatment plant or with household waste. Ask your pharmacist how to throw away the medicines you no longer use. These measures will help protect the environment.
caduet side effects
The safety of CADUET has been evaluated in double-blind, placebo-controlled studies in 1092 patients treated for concomitant hypertension and dyslipidemia. During clinical trials with CADUET, no specific adverse events specific to this combination were observed.
Adverse events were limited to those previously reported for amlodipine and / or atorvastatin (see table of adverse events below).
In controlled clinical trials, discontinuation of treatment due to clinical adverse events or laboratory abnormalities was observed in 5.1% of patients treated with amlodipine and atorvastatin versus 4.0% of patients treated with amlodipine and atorvastatin. patients taking a placebo.
The adverse events below, listed according to MedDRA system organ classification and frequency order, relate to amlodipine and atorvastatin individually.
Very common: ³ 1/10, common: ³ 1/100 and <1/10, infrequent: ³ 1/1000 and <1/100, rare: ³ 1/10000 and <1/1000, very rare: < 1 / 10,000, frequency not known (cannot be estimated from the available data).
MedDRA
System organ classes
Side effects
Frequency
Amlodipine
Atorvastatin
Infections and infestations
Nasopharyngitis
–
Frequent
Blood and lymphatic system disorders
Leukopenia
Very rare
–
Thrombocytopenia
Very rare
Rare
Immune system disorders
hypersensitivity
Very rare
Frequent
Anaphylaxis
–
Very rare
Metabolism and nutrition disorders
Hyperglycemia *
Very rare
Frequent
Weight gain
Infrequent
Infrequent
Weightloss
Infrequent
–
Hypoglycemia
–
Infrequent
Anorexia
–
Infrequent
Psychiatric disorders
Insomnia
Infrequent
Infrequent
Mood disorders (including anxiety)
Infrequent
–
Nightmares
–
Infrequent
Depression
Infrequent
Frequency not known
Confusion
Rare
–
Disorders of the system nervous
Drowsiness
Frequent
–
Dizziness
Frequent
Infrequent
Headache (especially at the start of treatment)
Frequent
Frequent
Tremors
Infrequent
–
Hypoesthesias, paresthesias
Infrequent
Infrequent
Syncope
Infrequent
Hypertonia
Very rare
–
Peripheral neuropathy
Very rare
Rare
Amnesia
–
Infrequent
Dysgeusia
Infrequent
Infrequent
Extrapyramidal syndrome
Frequency not known
–
Eye disorders
Blurred vision
–
Infrequent
Visual disturbances (including diplopia)
Infrequent
Rare
Ear and labyrinth disease
Tinnitus
Infrequent
Infrequent
Hearing loss
–
Very rare
Cardiac disorders
Palpitations
Frequent
–
Angina pectoris
Rare
–
Myocardial infarction
Very rare
–
Arrhythmia (including bradycardia, ventricular tachycardia and atrial fibrillation)
Very rare
–
Vascular disorders
Flushing
Frequent
–
Hypotension
Infrequent
–
Vasculitis
Very rare
–
Respiratory, thoracic and mediastinal disorders
Pharyngolaryngeal pain
–
Frequent
Epistaxis
–
Frequent
Dyspnea
Infrequent
–
Rhinitis
Infrequent
–
Cough
Very rare
–
Interstitial lung disease, especially during long-term treatment
Frequency not known
Gastrointestinal disorders
Gingival hyperplasia
Very rare
–
Nausea
Frequent
Frequent
Upper and lower abdominal pain
Frequent
Infrequent
Vomiting
Infrequent
Infrequent
Dyspepsia
Infrequent
Frequent
Changes in intestinal transit (including diarrhea and constipation)
Infrequent
–
Dry mouth
Infrequent
–
Dysgeusia
Infrequent
–
Diarrhea, constipation, gas
–
Frequent
Gastritis
Very rare
–
Pancreatitis
Very rare
Infrequent
Eructation
–
Infrequent
Hepatobiliary disorders
Hepatitis
Very rare
Infrequent
Cholestasis
–
Rare
Jaundice
Very rare
–
Hepatic insufficiency
–
Very rare
Skin and subcutaneous tissue disorders
Bullous dermatosis including erythema multiforme
Very rare
Rare
Angioedema
Very rare
–
Erythema multiforme
Very rare
–
Alopecia
Infrequent
Infrequent
Purpura
Infrequent
–
Skin discoloration
Infrequent
–
Pruritus
Infrequent
Infrequent
Eruption
Infrequent
Infrequent
Hyperhidrosis
Infrequent
–
Exanthema
Infrequent
–
Urticaria
Very rare
Infrequent
Angioneurotic edema
Very rare
Rare
Exfoliative dermatitis
Very rare
–
Photosensitivity
Very rare
–
Stevens-Johnson syndrome
Very rare
Rare
Bullous erythroderma with epidermolysis
–
Rare
Musculoskeletal and connective tissue disorders
Swelling of the joints (including swelling of the ankles)
Frequent
Frequent
Arthralgia, myalgia
(see section Warnings and precautions for use )
Infrequent
Frequent
Muscle cramps, muscle spasms
Infrequent
Frequent
Back pain
Infrequent
Frequent
Neck pain
–
Infrequent
Pain in extremity
–
Frequent
Muscle fatigue
–
Infrequent
Myositis (see section Warnings and precautions for use )
–
Rare
Rhabdomyolysis, myopathy
(see section Warnings and precautions for use )
–
Rare
Tendinopathies, in rare cases tendon rupture
–
Rare
Immune-mediated necrotizing myopathy
–
Frequency not known
Kidney and urinary tract disorders
Urination disorder, nocturia, pollakiuria
Infrequent
–
Reproductive system and breast disorders
Incapacity
Infrequent
Infrequent
Gynecomastia
Infrequent
Very rare
General disorders and administration site conditions
Edema
Frequent
Infrequent
Peripheral edema
–
Infrequent
Tired
Frequent
Infrequent
Chest pain
Infrequent
Infrequent
Asthenia
Infrequent
Infrequent
Pain
Infrequent
–
Discomfort
Infrequent
Infrequent
Fever
–
Infrequent
Investigations
Increased liver enzymes: alanine aminotransferase, aspartate aminotransferase (mainly related to cholestasis)
Very rare
Frequent
Blood CK increased (see section 4.4 )
–
Frequent
Leukocyturia
–
Infrequent
* Diabetes: The frequency depends on the presence or absence of risk factors (fasting blood sugar ³ 5.6 mmol / l, BMI> 30 kg / m², increased triglyceride levels, history of high blood pressure).
caduet Interactions
Taking other medications
Tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription. Some medicines may interact with CADUET 10 mg / 10 mg film-coated tablets. This interaction may cause one or both of the medicines to be less effective, or on the contrary, this interaction may increase the risk or severity of side effects, including major muscle disorders known as rhabdomyolysis and myopathy (see section 4). .
Some medicines may interact with CADUET 10 mg / 10 mg film-coated tablets:
certain antibiotics such as rifampicin or certain “macrolide” antibiotics such as erythromycin, clarithromycin, telithromycin, fusidic acid or certain drugs used to treat infections caused by fungi such as ketoconazole, itraconazole,
medicines used to regulate lipid levels: fibrates, such as gemfibrozil or colestipol,
medicines used to regulate your heart rhythm, such as amiodarone, diltiazem and verapamil,
medicines used to treat or prevent seizures such as carbamazepine, phenobarbital, phenytoin, fosphenytoin, primidone,
medicines used to change how your immune system works, such as cyclosporin
protease inhibitors such as ritonavir, indinavir, nelfinavir used in the treatment of HIV,
medicines used to treat depression, such as nefazodone and imipramine,
medicines used to treat mental disorders, such as neuroleptics,
medicines used to treat heart failure, such as beta blockers
medicines used to treat high blood pressure, such as angiotensin II inhibitors, converting enzyme inhibitors, verapamil and diuretics,
alpha blockers used to treat high blood pressure or prostate problems
other medicines known to interact with CADUET 10 mg / 10 mg film-coated tablets such as ezetimibe (which lowers cholesterol), warfarin (which decreases blood clotting), oral contraceptives, stiripentol (an anticonvulsant used for treatment of epilepsy), cimetidine (used for heartburn and stomach ulcers), phenazone (a pain reliever), antacids (containing aluminum or magnesium, used to relieve problems stomach), and amifostine (used to treat cancer),
sildenafil (used in the treatment of erectile dysfunction, impotence),
dantrolene and baclofen (muscle relaxants),
steroids,
over-the-counter St. John’s Wort products.
CADUET may lower your blood pressure further if you are already taking other medicines to treat your high blood pressure.
If you are taking or have recently taken any other medicines, including medicines obtained without a prescription, talk to your doctor or pharmacist.
INTERACTIONS OF CADUET 10 MG / 10 MG WITH FOOD AND DRINK
Food and drinks
CADUET 10 mg / 10 mg film-coated tablets can be taken at any time of the day, with or without food.
Grapefruit juice
Do not take more than one or two glasses of grapefruit juice per day as large amounts of grapefruit juice may affect the effects of CADUET 10 mg / 10 mg film-coated tablets.
Alcohol
Avoid drinking too much alcohol while taking CADUET 10 mg / 10 mg film-coated tablets. See also section 2 “Take special care with CADUET 10 mg / 10 mg film-coated tablets” for more details.
Drive and use machines
Do not drive or use machines if you feel dizzy after taking this medicine.
Do not drive or use machines if you feel dizzy after taking this medicine.
Do not drive or use machines if you feel dizzy after taking this medicine.
Warnings and Precautions
Heart failure
Liver function monitoring
Clinical signs of hepatic dysfunction
Increased transaminases
Heavy alcohol consumption
Hepatic insufficiency
History of liver disease
Predisposing factor to the occurrence of rhabdomyolysis
Subject over 70 years of age
Unexplained muscle pain
Muscle cramp
Muscular weakness
Increase in CPK
Rhabdomyolysis
History of hemorrhagic stroke
History of lacunar infarction
Interstitial lung disease
Risk of diabetes
Woman of childbearing age
Heart failure
Patients with heart failure should be treated with caution. In a long-term placebo-controlled study in patients with severe heart failure (NYHA class III and IV), the reported incidence of pulmonary edema was higher in the amlodipine group compared to the placebo group. (see section Pharmacodynamic properties). Calcium channel blockers including amlodipine should be used with caution in patients with congestive heart failure because they may increase the risk of cardiovascular events and mortality.
Liver effects
Liver function tests should be performed before the start of treatment and then regularly after initiation of treatment, as well as in the event of signs or symptoms suggestive of liver damage. In the event of elevation of the serum transaminase level, monitoring is necessary until normalization.
A persistent increase in ALT or AST exceeding 3 times the upper limit of normal (ULN), should lead to discontinuation of treatment.
The half-life of amlodipine is increased and its AUC (Area Under the Curve) is greater in patients with hepatic impairment; dosage recommendations have not been established.
Due to the presence of atorvastatin, CADUET should be used with caution in patients who consume large amounts of alcohol, in patients with hepatic impairment and / or a history of liver disease.
Muscle effects
Like other HMG-CoA reductase inhibitors, atorvastatin can affect skeletal muscles and lead to myalgia, myositis and myopathies. These muscle damage may rarely progress to rhabdomyolysis, characterized by high levels of creatine kinase (CK) (more than 10 times the ULN), myoglobinemia and myoglobinuria which can lead to kidney failure, and in some cases be fatal.
Regular testing of CK or other muscle enzymes is not recommended in asymptomatic patients treated with statins. However, dosing of CKs is recommended before initiating statin therapy in patients with predisposing factors to rhabdomyolysis as well as in those with muscle symptoms during statin therapy (see below).
Very rare cases of autoimmune-mediated necrotizing myopathies (IMNM) have been reported during or after treatment with certain statins. IMNM is clinically characterized by proximal muscle weakness and increased serum creatine kinase, which persist despite discontinuation of statin therapy.
Before initiation of treatment
CADUET should be prescribed with caution in patients with predisposing factors to rhabdomyolysis. Before starting treatment with a statin, creatine kinase (CK) levels should be checked in the following situations:
Renal failure.
Hypothyroidism.
Personal or family history of genetic muscle diseases.
Personal history of muscle toxicity during treatment with a statin or a fibrate.
History of liver disease and / or excessive alcohol consumption.
In elderly patients (> 70 years), the need for these measures should be assessed, depending on the presence of other factors predisposing to rhabdomyolysis.
Situations in which increased plasma concentrations may occur, due to interactions (see section 4.5) and use in special populations including genetic polymorphisms (see section 5.2). ).
In these situations, a regular reassessment of the benefit / risk of the treatment, as well as regular clinical monitoring is recommended.
If the initial CK level is significantly elevated (more than 5 times the ULN) treatment should not be started.
Creatine kinase measurement
Creatine kinase (CK) should not be measured after heavy exercise or in the presence of another possible cause of increased CK, as this will make interpretation of the results difficult. In the event of a significant elevation of CK (more than 5 times the ULN) before treatment, this should be systematically checked again within 5 to 7 days to confirm the results.
During treatment
It is recommended that patients be asked to promptly report any unexplained muscle pain, muscle cramps or weakness, particularly if accompanied by malaise or fever.
· If symptoms appear when a patient is under treatment with CADUET, a CK levels should be measured; if the CK level is significantly elevated (more than 5 times the ULN), treatment should be discontinued.
· If muscular symptoms are severe and cause daily discomfort, discontinuation of treatment should be considered, even if CK levels did not exceed 5 times the ULN.
· If symptoms resolve and CK levels return to normal, the reintroduction of CADUET may be considered at the lowest dose and under close supervision.
Treatment with CADUET should be discontinued in the event of a clinically significant increase in CK level (> 10 times the ULN) or if rhabdomyolysis is diagnosed or suspected.
Amlodipine has no effect on laboratory parameters.
Combinations with other drugs
As with other statins, the risk of rhabdomyolysis is increased when CADUET is administered in combination with certain drugs which may increase the plasma concentration of atorvastatin, such as strong inhibitors of CYP3A4 or protein transporters (eg, cyclosporin, telithromycin, clarithromycin, delavirdine, stiripentol, ketoconazole, voriconazole, itraconazole, posaconazole, determovir and HIV protease inhibitors including ritonavir, lopinavir, atazanavir, indinavir, darunavir, tipranavir, etc / ritonavir). The risk of myopathy may also be increased in combination with gemfibrozil and other fibrates, antivirals used in the treatment of hepatitis C (HCV) (boceprevir, telaprevir, elbasvir / grazoprevir), erythromycin, niacin, l ‘ ezetimibe or colchicine. Therapeutic alternatives (not exhibiting these interactions) should be considered as far as possible.
In the event that the combination of these medicinal products with CADUET is necessary, the benefit / risk of concomitant treatments should be carefully assessed and appropriate clinical monitoring is recommended (see section Interactions with other medicinal products and other forms of interactions).
CADUET should not be administered simultaneously with fusidic acid in systemic form, and for up to 7 days after stopping treatment with fusidic acid. In patients where the use of systemic fusidic acid is considered essential, statin therapy should be discontinued for the duration of fusidic acid therapy. Cases of rhabdomyolysis (some fatal) have been reported in patients receiving fusidic acid and a statin in combination (see section Interactions with other medicinal products and other forms of interactions). Patients should be informed of the need to seek immediate medical attention if they experience symptoms of muscle weakness, pain, or muscle tenderness.
Statin therapy can be restarted seven days after the last dose of fusidic acid.
In exceptional circumstances, when prolonged treatment with systemic fusidic acid is required, e.g. for the treatment of severe infections, the need for co-administration of CADUET and fusidic acid should only be considered in cases of per case and under close medical supervision.
Prevention of stroke by aggressively lowering cholesterol levels (SPARCL study)
In a posteriori analysis performed in subgroups of patients with recent stroke or transient ischemic attack (TIA) but without coronary artery disease, a higher frequency of hemorrhagic stroke was observed in treated patients. per 80 mg atorvastatin compared to patients on placebo.
This high risk is particularly observed in patients who have already had a hemorrhagic stroke or a lacunar infarction at the inclusion of the study.
In patients with a history of hemorrhagic stroke or lacunar infarction, the benefit / risk balance of atorvastatin 80 mg is uncertain. Therefore, the potential risk of occurrence of haemorrhagic stroke should be carefully assessed before any initiation of treatment (see section Pharmacodynamic properties).
Interstitial lung disease
Cases of interstitial lung disease have exceptionally been reported with certain statins, particularly during long-term therapy .
Symptoms may include dyspnea, a non-productive cough, and altered general condition (fatigue, weight loss, and fever). If interstitial lung disease is suspected in a patient, statin therapy should be discontinued.
Diabetes
There is some evidence that statins, as a pharmacological class, increase blood sugar. In some patients at high risk of developing diabetes, statins may cause hyperglycaemia requiring the initiation of diabetes treatment. This risk is nevertheless compensated by the reduction in the vascular risk under statins and therefore it should not be a reason for stopping statins. Patients at risk (fasting blood glucose between 5.6 and 6.9 mmol / l, BMI> 30 kg / m², increased triglyceride levels, arterial hypertension) should be monitored clinically and biologically in accordance with national recommendations.
PREGNANCY & BREAST-FEEDING & FERTILITY
Caduet is contraindicated during pregnancy and lactation.
Women of childbearing age
Women of childbearing potential should use reliable contraceptive measures during treatment ( see Contraindications ).
Pregnancy
The safety of atorvastatin has not been established in pregnant women. No controlled clinical trials have been performed in pregnant women treated with atorvastatin. Following intrauterine exposure to HMG-CoA reductase inhibitors, birth defects have rarely been reported. Studies in animals have shown reproductive toxicity ( see Preclinical safety ).
Treatment of the mother with atorvastatin may reduce the fetal level of mevalonate, which is a precursor of cholesterol biosynthesis. Atherosclerosis is a chronic process, and stopping a lipid-lowering drug during pregnancy should generally have little effect on the long-term risk associated with primary hypercholesterolemia.
For these reasons, Caduet should not be used during pregnancy, or in a woman planning to become pregnant or in whom pregnancy is suspected. Caduet treatment should be withheld during pregnancy or until it has been determined that the woman is not pregnant ( see Contraindications ).
If pregnancy is discovered during treatment, it should be stopped immediately.
Feeding with milk
Amlodipine is excreted in breast milk. The proportion of maternal dose received by the infant has been estimated at an interquartile range of 3-7%, with a maximum of 15%. The effect of amlodipine on infants is unknown. The excretion of atorvastatin or its metabolites in human milk is not known. In rats, the plasma concentrations of atorvastatin and its metabolites are similar to those found in milk ( see Preclinical safety ). Due to the possibility of serious side effects, women treated with Caduet should not breast-feed their infants ( see Contraindications ). Atorvastatin is contraindicated during breastfeeding ( see Contraindications ).
Fertility
No effect of atorvastatin on fertility has been demonstrated in studies conducted in male or female animals ( see Preclinical Safety ).
Reversible biochemical changes in the sperm head have been reported in some patients treated with calcium channel blockers. There is insufficient clinical data regarding the potential effect of amlodipine on fertility. In a study carried out in rats, adverse effects were detected on male fertility ( see Preclinical safety ).
What happens if I overdose from Caduet?
No information is available regarding overdose of Caduet in humans.
Amlodipine:
For amlodipine, experience with intentional overdose in humans is limited. Massive overdose could cause extensive peripheral vasodilation and possibly reflex tachycardia. Marked and possibly prolonged systemic hypotension up to and including shock with fatal outcome has been reported. Any hypotension resulting from acute intoxication requires monitoring in an intensive cardiological care unit. A vasoconstrictor can be used to restore vascular tone and blood pressure. Since amlodipine is highly protein bound, dialysis is unlikely to provide any benefit.
Atorvastatin:
There is no specific treatment for atorvastatin overdose. In the event of overdose, the patient should receive symptomatic treatment and supportive measures should be implemented as needed. Liver function and serum CK concentrations should be monitored. Due to the strong binding of the drug to plasma proteins, hemodialysis is not expected to significantly increase the clearance of atorvastatin.
What should I do if I miss a dose?
If you forget to take CADUET 5/ 10 mg -5/ 10 mg film-coated tablets:
If you forget to take your dose of CADUET, take your next dose at the normal time.
Do not take a double dose to make up for the dose you forgot to take.
What is Forms and Composition ?
SHAPES and PRESENTATIONS
5 mg / 10 mg film-coated tablet (oval, debossed with “Pfizer” on one side and “CDT 051” on the other side; white) and 10 mg / 10 mg (oval, debossed with “Pfizer “On one side and” CDT 101 “on the other side; blue): Boxes of 30 and 90, in blisters.
COMPOSITION
p cpAmlodipine (INN) as amlodipine besilate5 mgor10 mgAtorvastatin (INN) as atorvastatin calcium trihydrate10 mg
Excipients (common): Core: calcium carbonate, croscarmellose sodium, microcrystalline cellulose, pregelatinized corn starch, polysorbate 80, hydroxypropylcellulose, colloidal anhydrous silica, magnesium stearate. Film- coating : 5 mg / 10 mg tablet: Opadry II white 85F28751 (polyvinyl alcohol, titanium dioxide E171, macrogol 3000, talc). 10 mg / 10 mg tablet: Opadry II blue 85F10919 (polyvinyl alcohol, titanium dioxide E171, macrogol 3000, talc, indigo carmine aluminum lake E132).
NOT’s
Edrug-online contains comprehensive and detailed information about drugs available in the medical field, and is divided into four sections:
general information:
Includes a general description of the drug, its use, brand names, FAQs, and relevant news and articles
Additional information:
General explanation about dealing with the medicine: how to take the medicine, the doses and times of it, the start and duration of its effectiveness, the recommended diet during the period of taking the medicine, the method of storage and storage, recommendations in cases for forgetting the dose and instructions to stop taking the drug and take additional doses.
Special warnings:
For pregnant and breastfeeding women, the elderly, boys and drivers, and use before surgery.
Side effects:
It treats possible side effects and drug interactions that require attention and its effect on continuous use.
The information contained in this medicine is based on medical literature, but it is not a substitute for consulting a doctor.
The post caduet tablets Uses, Dosage, Side Effects, Precautions & Warnings appeared first on Drug Online.
from Drug Online
https://bit.ly/3lxi9nO
via Edrug Online
from Faculty of Medicine
https://bit.ly/3hIQsGt
via Internal Medicine
0 notes
Text
caduet tablets Uses, Dosage, Side Effects, Precautions & Warnings
Drug Online
caduet Generic drug of the Therapeutic class: Cardiology and angiology
Active ingredients: Amlodipine , Atorvastatin
what is caduet medication?
This medication is supplied as blue, oval-shaped, film-coated tablets debossed with “Pfizer” on one side and “CDT 101” on the other side.
Boxes of 7, 10, 14, 20, 28, 30, 50, 56, 60, 90, 100 or 200 tablets.
Not all presentations may be marketed.
what is caduet medication used for and indication?
CADUET is indicated for the prevention of cardiovascular events in hypertensive patients with 3 associated cardiovascular risk factors, with normal to moderately elevated cholesterol without established coronary artery disease, and in whom, according to current recommendations, the concomitant use of amlodipine and a low dose of atorvastatin is suitable .
CADUET should be used when the response to regimen and other non-pharmacological measures is inadequate.
caduet dosage
Oral route
The usual starting dose is 5 mg / 10 mg once daily.
If tighter blood pressure control is required, a dosage of 10 mg / 10 mg once daily may be administered.
The tablets can be taken at any time of the day, with or without food.
CADUET can be used alone or in combination with other antihypertensive drugs, but it must not be used in combination with other calcium channel blockers or another statin.
The combination of CADUET and fibrates should generally be avoided (see sections 4.4 and Interactions with other medicinal products and other forms of interaction ).
Patients with renal impairment : No dosage adjustment is necessary in patients with impaired renal function (see sections 4.4 and Pharmacokinetic properties ).
Patients with hepatic impairment : CADUET is contraindicated in patients with active hepatic disease.
Children / Adolescents : The safety and efficacy of CADUET have not been established in children / adolescents. Therefore, the use of CADUET is not recommended in this population.
Elderly : It does not appear necessary to adjust the dose in the elderly (see section Pharmacokinetic properties ).
Combination with other medicinal products : In combination with ciclosporin, the dose of atorvastatin should not exceed 10 mg (see section Interactions with other medicinal products and other forms of interaction ).
Contraindications
CADUET is contraindicated in patients:
Being hypersensitive to dihydropyridines *, to amlodipine, to atorvastatin or to any of the excipients of this medicine,
have active liver disease or a persistent and unexplained increase in serum transaminases exceeding 3 times the upper limit of normal,
In women who are pregnant, breastfeeding or of childbearing potential and not using reliable contraceptive methods (see section Pregnancy and breast-feeding ),
In combination with itraconazole, ketoconazole, telithromycin (see section Interactions with other medicinal products and other forms of interactions ),
having severe hypotension,
Having shock (including cardiogenic shock),
Having an obstruction of the efferent pathway to the left ventricle (for example severe aortic stenosis),
With haemodynamically unstable heart failure after acute myocardial infarction.
* amlodipine is a calcium channel blocker derived from dihydropyridine.
HOW TO TAKE CADUET?
Always take this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if in doubt.
Adults
The recommended starting dose of CADUET is one 5 mg / 10 mg tablet per day. Your doctor may increase the dose if necessary to 1 tablet of CADUET 10 mg / 10 mg per day.
The tablets should be swallowed whole with a glass of water. They can be taken at any time of the day, with or without food. However, it is best to take them at the same time each day.
Continue to follow your doctor’s dietary advice, especially to eat a low fat diet, avoid tobacco, and exercise regularly.
If you have the impression that the effect of CADUET tablets is too strong or too weak, talk to your doctor or pharmacist.
Use in children and adolescents
This medication is not recommended for children and adolescents.
If you take more CADUET 10 mg / 10 mg film-coated tablets than you should
If you accidentally take too many CADUET tablets (more than your usual dose), ask your doctor or the nearest hospital for advice. Take any remaining tablets, the carton or the whole carton with you so that hospital staff can quickly identify which medicine you have taken.
If you forget to take CADUET 10 mg / 10 mg film-coated tablets
If you forget to take your dose of CADUET, take your next dose at the normal time.
Do not take a double dose to make up for the dose you forgot to take.
If you stop taking CADUET 10 mg / 10 mg film-coated tablets
Do not stop taking CADUET unless your doctor tells you to.
If you have any further questions on the use of this medicine or want to stop taking your treatment, ask your doctor or pharmacist for more information.
How To Store Caduet?
Keep this medication out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the blister, the carton and the vial after “EXP”. The expiration date refers to the last day of that month.
Store at a temperature not exceeding 30 ° C.
Do not throw away any medicines via a wastewater treatment plant or with household waste. Ask your pharmacist how to throw away the medicines you no longer use. These measures will help protect the environment.
caduet side effects
The safety of CADUET has been evaluated in double-blind, placebo-controlled studies in 1092 patients treated for concomitant hypertension and dyslipidemia. During clinical trials with CADUET, no specific adverse events specific to this combination were observed.
Adverse events were limited to those previously reported for amlodipine and / or atorvastatin (see table of adverse events below).
In controlled clinical trials, discontinuation of treatment due to clinical adverse events or laboratory abnormalities was observed in 5.1% of patients treated with amlodipine and atorvastatin versus 4.0% of patients treated with amlodipine and atorvastatin. patients taking a placebo.
The adverse events below, listed according to MedDRA system organ classification and frequency order, relate to amlodipine and atorvastatin individually.
Very common: ³ 1/10, common: ³ 1/100 and <1/10, infrequent: ³ 1/1000 and <1/100, rare: ³ 1/10000 and <1/1000, very rare: < 1 / 10,000, frequency not known (cannot be estimated from the available data).
MedDRA
System organ classes
Side effects
Frequency
Amlodipine
Atorvastatin
Infections and infestations
Nasopharyngitis
–
Frequent
Blood and lymphatic system disorders
Leukopenia
Very rare
–
Thrombocytopenia
Very rare
Rare
Immune system disorders
hypersensitivity
Very rare
Frequent
Anaphylaxis
–
Very rare
Metabolism and nutrition disorders
Hyperglycemia *
Very rare
Frequent
Weight gain
Infrequent
Infrequent
Weightloss
Infrequent
–
Hypoglycemia
–
Infrequent
Anorexia
–
Infrequent
Psychiatric disorders
Insomnia
Infrequent
Infrequent
Mood disorders (including anxiety)
Infrequent
–
Nightmares
–
Infrequent
Depression
Infrequent
Frequency not known
Confusion
Rare
–
Disorders of the system nervous
Drowsiness
Frequent
–
Dizziness
Frequent
Infrequent
Headache (especially at the start of treatment)
Frequent
Frequent
Tremors
Infrequent
–
Hypoesthesias, paresthesias
Infrequent
Infrequent
Syncope
Infrequent
Hypertonia
Very rare
–
Peripheral neuropathy
Very rare
Rare
Amnesia
–
Infrequent
Dysgeusia
Infrequent
Infrequent
Extrapyramidal syndrome
Frequency not known
–
Eye disorders
Blurred vision
–
Infrequent
Visual disturbances (including diplopia)
Infrequent
Rare
Ear and labyrinth disease
Tinnitus
Infrequent
Infrequent
Hearing loss
–
Very rare
Cardiac disorders
Palpitations
Frequent
–
Angina pectoris
Rare
–
Myocardial infarction
Very rare
–
Arrhythmia (including bradycardia, ventricular tachycardia and atrial fibrillation)
Very rare
–
Vascular disorders
Flushing
Frequent
–
Hypotension
Infrequent
–
Vasculitis
Very rare
–
Respiratory, thoracic and mediastinal disorders
Pharyngolaryngeal pain
–
Frequent
Epistaxis
–
Frequent
Dyspnea
Infrequent
–
Rhinitis
Infrequent
–
Cough
Very rare
–
Interstitial lung disease, especially during long-term treatment
Frequency not known
Gastrointestinal disorders
Gingival hyperplasia
Very rare
–
Nausea
Frequent
Frequent
Upper and lower abdominal pain
Frequent
Infrequent
Vomiting
Infrequent
Infrequent
Dyspepsia
Infrequent
Frequent
Changes in intestinal transit (including diarrhea and constipation)
Infrequent
–
Dry mouth
Infrequent
–
Dysgeusia
Infrequent
–
Diarrhea, constipation, gas
–
Frequent
Gastritis
Very rare
–
Pancreatitis
Very rare
Infrequent
Eructation
–
Infrequent
Hepatobiliary disorders
Hepatitis
Very rare
Infrequent
Cholestasis
–
Rare
Jaundice
Very rare
–
Hepatic insufficiency
–
Very rare
Skin and subcutaneous tissue disorders
Bullous dermatosis including erythema multiforme
Very rare
Rare
Angioedema
Very rare
–
Erythema multiforme
Very rare
–
Alopecia
Infrequent
Infrequent
Purpura
Infrequent
–
Skin discoloration
Infrequent
–
Pruritus
Infrequent
Infrequent
Eruption
Infrequent
Infrequent
Hyperhidrosis
Infrequent
–
Exanthema
Infrequent
–
Urticaria
Very rare
Infrequent
Angioneurotic edema
Very rare
Rare
Exfoliative dermatitis
Very rare
–
Photosensitivity
Very rare
–
Stevens-Johnson syndrome
Very rare
Rare
Bullous erythroderma with epidermolysis
–
Rare
Musculoskeletal and connective tissue disorders
Swelling of the joints (including swelling of the ankles)
Frequent
Frequent
Arthralgia, myalgia
(see section Warnings and precautions for use )
Infrequent
Frequent
Muscle cramps, muscle spasms
Infrequent
Frequent
Back pain
Infrequent
Frequent
Neck pain
–
Infrequent
Pain in extremity
–
Frequent
Muscle fatigue
–
Infrequent
Myositis (see section Warnings and precautions for use )
–
Rare
Rhabdomyolysis, myopathy
(see section Warnings and precautions for use )
–
Rare
Tendinopathies, in rare cases tendon rupture
–
Rare
Immune-mediated necrotizing myopathy
–
Frequency not known
Kidney and urinary tract disorders
Urination disorder, nocturia, pollakiuria
Infrequent
–
Reproductive system and breast disorders
Incapacity
Infrequent
Infrequent
Gynecomastia
Infrequent
Very rare
General disorders and administration site conditions
Edema
Frequent
Infrequent
Peripheral edema
–
Infrequent
Tired
Frequent
Infrequent
Chest pain
Infrequent
Infrequent
Asthenia
Infrequent
Infrequent
Pain
Infrequent
–
Discomfort
Infrequent
Infrequent
Fever
–
Infrequent
Investigations
Increased liver enzymes: alanine aminotransferase, aspartate aminotransferase (mainly related to cholestasis)
Very rare
Frequent
Blood CK increased (see section 4.4 )
–
Frequent
Leukocyturia
–
Infrequent
* Diabetes: The frequency depends on the presence or absence of risk factors (fasting blood sugar ³ 5.6 mmol / l, BMI> 30 kg / m², increased triglyceride levels, history of high blood pressure).
caduet Interactions
Taking other medications
Tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription. Some medicines may interact with CADUET 10 mg / 10 mg film-coated tablets. This interaction may cause one or both of the medicines to be less effective, or on the contrary, this interaction may increase the risk or severity of side effects, including major muscle disorders known as rhabdomyolysis and myopathy (see section 4). .
Some medicines may interact with CADUET 10 mg / 10 mg film-coated tablets:
certain antibiotics such as rifampicin or certain “macrolide” antibiotics such as erythromycin, clarithromycin, telithromycin, fusidic acid or certain drugs used to treat infections caused by fungi such as ketoconazole, itraconazole,
medicines used to regulate lipid levels: fibrates, such as gemfibrozil or colestipol,
medicines used to regulate your heart rhythm, such as amiodarone, diltiazem and verapamil,
medicines used to treat or prevent seizures such as carbamazepine, phenobarbital, phenytoin, fosphenytoin, primidone,
medicines used to change how your immune system works, such as cyclosporin
protease inhibitors such as ritonavir, indinavir, nelfinavir used in the treatment of HIV,
medicines used to treat depression, such as nefazodone and imipramine,
medicines used to treat mental disorders, such as neuroleptics,
medicines used to treat heart failure, such as beta blockers
medicines used to treat high blood pressure, such as angiotensin II inhibitors, converting enzyme inhibitors, verapamil and diuretics,
alpha blockers used to treat high blood pressure or prostate problems
other medicines known to interact with CADUET 10 mg / 10 mg film-coated tablets such as ezetimibe (which lowers cholesterol), warfarin (which decreases blood clotting), oral contraceptives, stiripentol (an anticonvulsant used for treatment of epilepsy), cimetidine (used for heartburn and stomach ulcers), phenazone (a pain reliever), antacids (containing aluminum or magnesium, used to relieve problems stomach), and amifostine (used to treat cancer),
sildenafil (used in the treatment of erectile dysfunction, impotence),
dantrolene and baclofen (muscle relaxants),
steroids,
over-the-counter St. John’s Wort products.
CADUET may lower your blood pressure further if you are already taking other medicines to treat your high blood pressure.
If you are taking or have recently taken any other medicines, including medicines obtained without a prescription, talk to your doctor or pharmacist.
INTERACTIONS OF CADUET 10 MG / 10 MG WITH FOOD AND DRINK
Food and drinks
CADUET 10 mg / 10 mg film-coated tablets can be taken at any time of the day, with or without food.
Grapefruit juice
Do not take more than one or two glasses of grapefruit juice per day as large amounts of grapefruit juice may affect the effects of CADUET 10 mg / 10 mg film-coated tablets.
Alcohol
Avoid drinking too much alcohol while taking CADUET 10 mg / 10 mg film-coated tablets. See also section 2 “Take special care with CADUET 10 mg / 10 mg film-coated tablets” for more details.
Drive and use machines
Do not drive or use machines if you feel dizzy after taking this medicine.
Do not drive or use machines if you feel dizzy after taking this medicine.
Do not drive or use machines if you feel dizzy after taking this medicine.
Warnings and Precautions
Heart failure
Liver function monitoring
Clinical signs of hepatic dysfunction
Increased transaminases
Heavy alcohol consumption
Hepatic insufficiency
History of liver disease
Predisposing factor to the occurrence of rhabdomyolysis
Subject over 70 years of age
Unexplained muscle pain
Muscle cramp
Muscular weakness
Increase in CPK
Rhabdomyolysis
History of hemorrhagic stroke
History of lacunar infarction
Interstitial lung disease
Risk of diabetes
Woman of childbearing age
Heart failure
Patients with heart failure should be treated with caution. In a long-term placebo-controlled study in patients with severe heart failure (NYHA class III and IV), the reported incidence of pulmonary edema was higher in the amlodipine group compared to the placebo group. (see section Pharmacodynamic properties). Calcium channel blockers including amlodipine should be used with caution in patients with congestive heart failure because they may increase the risk of cardiovascular events and mortality.
Liver effects
Liver function tests should be performed before the start of treatment and then regularly after initiation of treatment, as well as in the event of signs or symptoms suggestive of liver damage. In the event of elevation of the serum transaminase level, monitoring is necessary until normalization.
A persistent increase in ALT or AST exceeding 3 times the upper limit of normal (ULN), should lead to discontinuation of treatment.
The half-life of amlodipine is increased and its AUC (Area Under the Curve) is greater in patients with hepatic impairment; dosage recommendations have not been established.
Due to the presence of atorvastatin, CADUET should be used with caution in patients who consume large amounts of alcohol, in patients with hepatic impairment and / or a history of liver disease.
Muscle effects
Like other HMG-CoA reductase inhibitors, atorvastatin can affect skeletal muscles and lead to myalgia, myositis and myopathies. These muscle damage may rarely progress to rhabdomyolysis, characterized by high levels of creatine kinase (CK) (more than 10 times the ULN), myoglobinemia and myoglobinuria which can lead to kidney failure, and in some cases be fatal.
Regular testing of CK or other muscle enzymes is not recommended in asymptomatic patients treated with statins. However, dosing of CKs is recommended before initiating statin therapy in patients with predisposing factors to rhabdomyolysis as well as in those with muscle symptoms during statin therapy (see below).
Very rare cases of autoimmune-mediated necrotizing myopathies (IMNM) have been reported during or after treatment with certain statins. IMNM is clinically characterized by proximal muscle weakness and increased serum creatine kinase, which persist despite discontinuation of statin therapy.
Before initiation of treatment
CADUET should be prescribed with caution in patients with predisposing factors to rhabdomyolysis. Before starting treatment with a statin, creatine kinase (CK) levels should be checked in the following situations:
Renal failure.
Hypothyroidism.
Personal or family history of genetic muscle diseases.
Personal history of muscle toxicity during treatment with a statin or a fibrate.
History of liver disease and / or excessive alcohol consumption.
In elderly patients (> 70 years), the need for these measures should be assessed, depending on the presence of other factors predisposing to rhabdomyolysis.
Situations in which increased plasma concentrations may occur, due to interactions (see section 4.5) and use in special populations including genetic polymorphisms (see section 5.2). ).
In these situations, a regular reassessment of the benefit / risk of the treatment, as well as regular clinical monitoring is recommended.
If the initial CK level is significantly elevated (more than 5 times the ULN) treatment should not be started.
Creatine kinase measurement
Creatine kinase (CK) should not be measured after heavy exercise or in the presence of another possible cause of increased CK, as this will make interpretation of the results difficult. In the event of a significant elevation of CK (more than 5 times the ULN) before treatment, this should be systematically checked again within 5 to 7 days to confirm the results.
During treatment
It is recommended that patients be asked to promptly report any unexplained muscle pain, muscle cramps or weakness, particularly if accompanied by malaise or fever.
· If symptoms appear when a patient is under treatment with CADUET, a CK levels should be measured; if the CK level is significantly elevated (more than 5 times the ULN), treatment should be discontinued.
· If muscular symptoms are severe and cause daily discomfort, discontinuation of treatment should be considered, even if CK levels did not exceed 5 times the ULN.
· If symptoms resolve and CK levels return to normal, the reintroduction of CADUET may be considered at the lowest dose and under close supervision.
Treatment with CADUET should be discontinued in the event of a clinically significant increase in CK level (> 10 times the ULN) or if rhabdomyolysis is diagnosed or suspected.
Amlodipine has no effect on laboratory parameters.
Combinations with other drugs
As with other statins, the risk of rhabdomyolysis is increased when CADUET is administered in combination with certain drugs which may increase the plasma concentration of atorvastatin, such as strong inhibitors of CYP3A4 or protein transporters (eg, cyclosporin, telithromycin, clarithromycin, delavirdine, stiripentol, ketoconazole, voriconazole, itraconazole, posaconazole, determovir and HIV protease inhibitors including ritonavir, lopinavir, atazanavir, indinavir, darunavir, tipranavir, etc / ritonavir). The risk of myopathy may also be increased in combination with gemfibrozil and other fibrates, antivirals used in the treatment of hepatitis C (HCV) (boceprevir, telaprevir, elbasvir / grazoprevir), erythromycin, niacin, l ‘ ezetimibe or colchicine. Therapeutic alternatives (not exhibiting these interactions) should be considered as far as possible.
In the event that the combination of these medicinal products with CADUET is necessary, the benefit / risk of concomitant treatments should be carefully assessed and appropriate clinical monitoring is recommended (see section Interactions with other medicinal products and other forms of interactions).
CADUET should not be administered simultaneously with fusidic acid in systemic form, and for up to 7 days after stopping treatment with fusidic acid. In patients where the use of systemic fusidic acid is considered essential, statin therapy should be discontinued for the duration of fusidic acid therapy. Cases of rhabdomyolysis (some fatal) have been reported in patients receiving fusidic acid and a statin in combination (see section Interactions with other medicinal products and other forms of interactions). Patients should be informed of the need to seek immediate medical attention if they experience symptoms of muscle weakness, pain, or muscle tenderness.
Statin therapy can be restarted seven days after the last dose of fusidic acid.
In exceptional circumstances, when prolonged treatment with systemic fusidic acid is required, e.g. for the treatment of severe infections, the need for co-administration of CADUET and fusidic acid should only be considered in cases of per case and under close medical supervision.
Prevention of stroke by aggressively lowering cholesterol levels (SPARCL study)
In a posteriori analysis performed in subgroups of patients with recent stroke or transient ischemic attack (TIA) but without coronary artery disease, a higher frequency of hemorrhagic stroke was observed in treated patients. per 80 mg atorvastatin compared to patients on placebo.
This high risk is particularly observed in patients who have already had a hemorrhagic stroke or a lacunar infarction at the inclusion of the study.
In patients with a history of hemorrhagic stroke or lacunar infarction, the benefit / risk balance of atorvastatin 80 mg is uncertain. Therefore, the potential risk of occurrence of haemorrhagic stroke should be carefully assessed before any initiation of treatment (see section Pharmacodynamic properties).
Interstitial lung disease
Cases of interstitial lung disease have exceptionally been reported with certain statins, particularly during long-term therapy .
Symptoms may include dyspnea, a non-productive cough, and altered general condition (fatigue, weight loss, and fever). If interstitial lung disease is suspected in a patient, statin therapy should be discontinued.
Diabetes
There is some evidence that statins, as a pharmacological class, increase blood sugar. In some patients at high risk of developing diabetes, statins may cause hyperglycaemia requiring the initiation of diabetes treatment. This risk is nevertheless compensated by the reduction in the vascular risk under statins and therefore it should not be a reason for stopping statins. Patients at risk (fasting blood glucose between 5.6 and 6.9 mmol / l, BMI> 30 kg / m², increased triglyceride levels, arterial hypertension) should be monitored clinically and biologically in accordance with national recommendations.
PREGNANCY & BREAST-FEEDING & FERTILITY
Caduet is contraindicated during pregnancy and lactation.
Women of childbearing age
Women of childbearing potential should use reliable contraceptive measures during treatment ( see Contraindications ).
Pregnancy
The safety of atorvastatin has not been established in pregnant women. No controlled clinical trials have been performed in pregnant women treated with atorvastatin. Following intrauterine exposure to HMG-CoA reductase inhibitors, birth defects have rarely been reported. Studies in animals have shown reproductive toxicity ( see Preclinical safety ).
Treatment of the mother with atorvastatin may reduce the fetal level of mevalonate, which is a precursor of cholesterol biosynthesis. Atherosclerosis is a chronic process, and stopping a lipid-lowering drug during pregnancy should generally have little effect on the long-term risk associated with primary hypercholesterolemia.
For these reasons, Caduet should not be used during pregnancy, or in a woman planning to become pregnant or in whom pregnancy is suspected. Caduet treatment should be withheld during pregnancy or until it has been determined that the woman is not pregnant ( see Contraindications ).
If pregnancy is discovered during treatment, it should be stopped immediately.
Feeding with milk
Amlodipine is excreted in breast milk. The proportion of maternal dose received by the infant has been estimated at an interquartile range of 3-7%, with a maximum of 15%. The effect of amlodipine on infants is unknown. The excretion of atorvastatin or its metabolites in human milk is not known. In rats, the plasma concentrations of atorvastatin and its metabolites are similar to those found in milk ( see Preclinical safety ). Due to the possibility of serious side effects, women treated with Caduet should not breast-feed their infants ( see Contraindications ). Atorvastatin is contraindicated during breastfeeding ( see Contraindications ).
Fertility
No effect of atorvastatin on fertility has been demonstrated in studies conducted in male or female animals ( see Preclinical Safety ).
Reversible biochemical changes in the sperm head have been reported in some patients treated with calcium channel blockers. There is insufficient clinical data regarding the potential effect of amlodipine on fertility. In a study carried out in rats, adverse effects were detected on male fertility ( see Preclinical safety ).
What happens if I overdose from Caduet?
No information is available regarding overdose of Caduet in humans.
Amlodipine:
For amlodipine, experience with intentional overdose in humans is limited. Massive overdose could cause extensive peripheral vasodilation and possibly reflex tachycardia. Marked and possibly prolonged systemic hypotension up to and including shock with fatal outcome has been reported. Any hypotension resulting from acute intoxication requires monitoring in an intensive cardiological care unit. A vasoconstrictor can be used to restore vascular tone and blood pressure. Since amlodipine is highly protein bound, dialysis is unlikely to provide any benefit.
Atorvastatin:
There is no specific treatment for atorvastatin overdose. In the event of overdose, the patient should receive symptomatic treatment and supportive measures should be implemented as needed. Liver function and serum CK concentrations should be monitored. Due to the strong binding of the drug to plasma proteins, hemodialysis is not expected to significantly increase the clearance of atorvastatin.
What should I do if I miss a dose?
If you forget to take CADUET 5/ 10 mg -5/ 10 mg film-coated tablets:
If you forget to take your dose of CADUET, take your next dose at the normal time.
Do not take a double dose to make up for the dose you forgot to take.
What is Forms and Composition ?
SHAPES and PRESENTATIONS
5 mg / 10 mg film-coated tablet (oval, debossed with “Pfizer” on one side and “CDT 051” on the other side; white) and 10 mg / 10 mg (oval, debossed with “Pfizer “On one side and” CDT 101 “on the other side; blue): Boxes of 30 and 90, in blisters.
COMPOSITION
p cp Amlodipine (INN) as amlodipine besilate 5 mg or 10 mg Atorvastatin (INN) as atorvastatin calcium trihydrate 10 mg
Excipients (common): Core: calcium carbonate, croscarmellose sodium, microcrystalline cellulose, pregelatinized corn starch, polysorbate 80, hydroxypropylcellulose, colloidal anhydrous silica, magnesium stearate. Film- coating : 5 mg / 10 mg tablet: Opadry II white 85F28751 (polyvinyl alcohol, titanium dioxide E171, macrogol 3000, talc). 10 mg / 10 mg tablet: Opadry II blue 85F10919 (polyvinyl alcohol, titanium dioxide E171, macrogol 3000, talc, indigo carmine aluminum lake E132).
NOT’s
Edrug-online contains comprehensive and detailed information about drugs available in the medical field, and is divided into four sections:
general information:
Includes a general description of the drug, its use, brand names, FAQs, and relevant news and articles
Additional information:
General explanation about dealing with the medicine: how to take the medicine, the doses and times of it, the start and duration of its effectiveness, the recommended diet during the period of taking the medicine, the method of storage and storage, recommendations in cases for forgetting the dose and instructions to stop taking the drug and take additional doses.
Special warnings:
For pregnant and breastfeeding women, the elderly, boys and drivers, and use before surgery.
Side effects:
It treats possible side effects and drug interactions that require attention and its effect on continuous use.
The information contained in this medicine is based on medical literature, but it is not a substitute for consulting a doctor.
The post caduet tablets Uses, Dosage, Side Effects, Precautions & Warnings appeared first on Drug Online.
from Drug Online https://bit.ly/3lxi9nO
via Edrug Online
from faculty of medicine
https://bit.ly/3gLbi6L
via Faculty of Medicine
0 notes
Text
caduet tablets Uses, Dosage, Side Effects, Precautions & Warnings
Drug Online
caduet Generic drug of the Therapeutic class: Cardiology and angiology
Active ingredients: Amlodipine , Atorvastatin
what is caduet medication?
This medication is supplied as blue, oval-shaped, film-coated tablets debossed with “Pfizer” on one side and “CDT 101” on the other side.
Boxes of 7, 10, 14, 20, 28, 30, 50, 56, 60, 90, 100 or 200 tablets.
Not all presentations may be marketed.
what is caduet medication used for and indication?
CADUET is indicated for the prevention of cardiovascular events in hypertensive patients with 3 associated cardiovascular risk factors, with normal to moderately elevated cholesterol without established coronary artery disease, and in whom, according to current recommendations, the concomitant use of amlodipine and a low dose of atorvastatin is suitable .
CADUET should be used when the response to regimen and other non-pharmacological measures is inadequate.
caduet dosage
Oral route
The usual starting dose is 5 mg / 10 mg once daily.
If tighter blood pressure control is required, a dosage of 10 mg / 10 mg once daily may be administered.
The tablets can be taken at any time of the day, with or without food.
CADUET can be used alone or in combination with other antihypertensive drugs, but it must not be used in combination with other calcium channel blockers or another statin.
The combination of CADUET and fibrates should generally be avoided (see sections 4.4 and Interactions with other medicinal products and other forms of interaction ).
Patients with renal impairment : No dosage adjustment is necessary in patients with impaired renal function (see sections 4.4 and Pharmacokinetic properties ).
Patients with hepatic impairment : CADUET is contraindicated in patients with active hepatic disease.
Children / Adolescents : The safety and efficacy of CADUET have not been established in children / adolescents. Therefore, the use of CADUET is not recommended in this population.
Elderly : It does not appear necessary to adjust the dose in the elderly (see section Pharmacokinetic properties ).
Combination with other medicinal products : In combination with ciclosporin, the dose of atorvastatin should not exceed 10 mg (see section Interactions with other medicinal products and other forms of interaction ).
Contraindications
CADUET is contraindicated in patients:
Being hypersensitive to dihydropyridines *, to amlodipine, to atorvastatin or to any of the excipients of this medicine,
have active liver disease or a persistent and unexplained increase in serum transaminases exceeding 3 times the upper limit of normal,
In women who are pregnant, breastfeeding or of childbearing potential and not using reliable contraceptive methods (see section Pregnancy and breast-feeding ),
In combination with itraconazole, ketoconazole, telithromycin (see section Interactions with other medicinal products and other forms of interactions ),
having severe hypotension,
Having shock (including cardiogenic shock),
Having an obstruction of the efferent pathway to the left ventricle (for example severe aortic stenosis),
With haemodynamically unstable heart failure after acute myocardial infarction.
* amlodipine is a calcium channel blocker derived from dihydropyridine.
HOW TO TAKE CADUET?
Always take this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if in doubt.
Adults
The recommended starting dose of CADUET is one 5 mg / 10 mg tablet per day. Your doctor may increase the dose if necessary to 1 tablet of CADUET 10 mg / 10 mg per day.
The tablets should be swallowed whole with a glass of water. They can be taken at any time of the day, with or without food. However, it is best to take them at the same time each day.
Continue to follow your doctor’s dietary advice, especially to eat a low fat diet, avoid tobacco, and exercise regularly.
If you have the impression that the effect of CADUET tablets is too strong or too weak, talk to your doctor or pharmacist.
Use in children and adolescents
This medication is not recommended for children and adolescents.
If you take more CADUET 10 mg / 10 mg film-coated tablets than you should
If you accidentally take too many CADUET tablets (more than your usual dose), ask your doctor or the nearest hospital for advice. Take any remaining tablets, the carton or the whole carton with you so that hospital staff can quickly identify which medicine you have taken.
If you forget to take CADUET 10 mg / 10 mg film-coated tablets
If you forget to take your dose of CADUET, take your next dose at the normal time.
Do not take a double dose to make up for the dose you forgot to take.
If you stop taking CADUET 10 mg / 10 mg film-coated tablets
Do not stop taking CADUET unless your doctor tells you to.
If you have any further questions on the use of this medicine or want to stop taking your treatment, ask your doctor or pharmacist for more information.
How To Store Caduet?
Keep this medication out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the blister, the carton and the vial after “EXP”. The expiration date refers to the last day of that month.
Store at a temperature not exceeding 30 ° C.
Do not throw away any medicines via a wastewater treatment plant or with household waste. Ask your pharmacist how to throw away the medicines you no longer use. These measures will help protect the environment.
caduet side effects
The safety of CADUET has been evaluated in double-blind, placebo-controlled studies in 1092 patients treated for concomitant hypertension and dyslipidemia. During clinical trials with CADUET, no specific adverse events specific to this combination were observed.
Adverse events were limited to those previously reported for amlodipine and / or atorvastatin (see table of adverse events below).
In controlled clinical trials, discontinuation of treatment due to clinical adverse events or laboratory abnormalities was observed in 5.1% of patients treated with amlodipine and atorvastatin versus 4.0% of patients treated with amlodipine and atorvastatin. patients taking a placebo.
The adverse events below, listed according to MedDRA system organ classification and frequency order, relate to amlodipine and atorvastatin individually.
Very common: ³ 1/10, common: ³ 1/100 and <1/10, infrequent: ³ 1/1000 and <1/100, rare: ³ 1/10000 and <1/1000, very rare: < 1 / 10,000, frequency not known (cannot be estimated from the available data).
MedDRA
System organ classes
Side effects
Frequency
Amlodipine
Atorvastatin
Infections and infestations
Nasopharyngitis
–
Frequent
Blood and lymphatic system disorders
Leukopenia
Very rare
–
Thrombocytopenia
Very rare
Rare
Immune system disorders
hypersensitivity
Very rare
Frequent
Anaphylaxis
–
Very rare
Metabolism and nutrition disorders
Hyperglycemia *
Very rare
Frequent
Weight gain
Infrequent
Infrequent
Weightloss
Infrequent
–
Hypoglycemia
–
Infrequent
Anorexia
–
Infrequent
Psychiatric disorders
Insomnia
Infrequent
Infrequent
Mood disorders (including anxiety)
Infrequent
–
Nightmares
–
Infrequent
Depression
Infrequent
Frequency not known
Confusion
Rare
–
Disorders of the system nervous
Drowsiness
Frequent
–
Dizziness
Frequent
Infrequent
Headache (especially at the start of treatment)
Frequent
Frequent
Tremors
Infrequent
–
Hypoesthesias, paresthesias
Infrequent
Infrequent
Syncope
Infrequent
Hypertonia
Very rare
–
Peripheral neuropathy
Very rare
Rare
Amnesia
–
Infrequent
Dysgeusia
Infrequent
Infrequent
Extrapyramidal syndrome
Frequency not known
–
Eye disorders
Blurred vision
–
Infrequent
Visual disturbances (including diplopia)
Infrequent
Rare
Ear and labyrinth disease
Tinnitus
Infrequent
Infrequent
Hearing loss
–
Very rare
Cardiac disorders
Palpitations
Frequent
–
Angina pectoris
Rare
–
Myocardial infarction
Very rare
–
Arrhythmia (including bradycardia, ventricular tachycardia and atrial fibrillation)
Very rare
–
Vascular disorders
Flushing
Frequent
–
Hypotension
Infrequent
–
Vasculitis
Very rare
–
Respiratory, thoracic and mediastinal disorders
Pharyngolaryngeal pain
–
Frequent
Epistaxis
–
Frequent
Dyspnea
Infrequent
–
Rhinitis
Infrequent
–
Cough
Very rare
–
Interstitial lung disease, especially during long-term treatment
Frequency not known
Gastrointestinal disorders
Gingival hyperplasia
Very rare
–
Nausea
Frequent
Frequent
Upper and lower abdominal pain
Frequent
Infrequent
Vomiting
Infrequent
Infrequent
Dyspepsia
Infrequent
Frequent
Changes in intestinal transit (including diarrhea and constipation)
Infrequent
–
Dry mouth
Infrequent
–
Dysgeusia
Infrequent
–
Diarrhea, constipation, gas
–
Frequent
Gastritis
Very rare
–
Pancreatitis
Very rare
Infrequent
Eructation
–
Infrequent
Hepatobiliary disorders
Hepatitis
Very rare
Infrequent
Cholestasis
–
Rare
Jaundice
Very rare
–
Hepatic insufficiency
–
Very rare
Skin and subcutaneous tissue disorders
Bullous dermatosis including erythema multiforme
Very rare
Rare
Angioedema
Very rare
–
Erythema multiforme
Very rare
–
Alopecia
Infrequent
Infrequent
Purpura
Infrequent
–
Skin discoloration
Infrequent
–
Pruritus
Infrequent
Infrequent
Eruption
Infrequent
Infrequent
Hyperhidrosis
Infrequent
–
Exanthema
Infrequent
–
Urticaria
Very rare
Infrequent
Angioneurotic edema
Very rare
Rare
Exfoliative dermatitis
Very rare
–
Photosensitivity
Very rare
–
Stevens-Johnson syndrome
Very rare
Rare
Bullous erythroderma with epidermolysis
–
Rare
Musculoskeletal and connective tissue disorders
Swelling of the joints (including swelling of the ankles)
Frequent
Frequent
Arthralgia, myalgia
(see section Warnings and precautions for use )
Infrequent
Frequent
Muscle cramps, muscle spasms
Infrequent
Frequent
Back pain
Infrequent
Frequent
Neck pain
–
Infrequent
Pain in extremity
–
Frequent
Muscle fatigue
–
Infrequent
Myositis (see section Warnings and precautions for use )
–
Rare
Rhabdomyolysis, myopathy
(see section Warnings and precautions for use )
–
Rare
Tendinopathies, in rare cases tendon rupture
–
Rare
Immune-mediated necrotizing myopathy
–
Frequency not known
Kidney and urinary tract disorders
Urination disorder, nocturia, pollakiuria
Infrequent
–
Reproductive system and breast disorders
Incapacity
Infrequent
Infrequent
Gynecomastia
Infrequent
Very rare
General disorders and administration site conditions
Edema
Frequent
Infrequent
Peripheral edema
–
Infrequent
Tired
Frequent
Infrequent
Chest pain
Infrequent
Infrequent
Asthenia
Infrequent
Infrequent
Pain
Infrequent
–
Discomfort
Infrequent
Infrequent
Fever
–
Infrequent
Investigations
Increased liver enzymes: alanine aminotransferase, aspartate aminotransferase (mainly related to cholestasis)
Very rare
Frequent
Blood CK increased (see section 4.4 )
–
Frequent
Leukocyturia
–
Infrequent
* Diabetes: The frequency depends on the presence or absence of risk factors (fasting blood sugar ³ 5.6 mmol / l, BMI> 30 kg / m², increased triglyceride levels, history of high blood pressure).
caduet Interactions
Taking other medications
Tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription. Some medicines may interact with CADUET 10 mg / 10 mg film-coated tablets. This interaction may cause one or both of the medicines to be less effective, or on the contrary, this interaction may increase the risk or severity of side effects, including major muscle disorders known as rhabdomyolysis and myopathy (see section 4). .
Some medicines may interact with CADUET 10 mg / 10 mg film-coated tablets:
certain antibiotics such as rifampicin or certain “macrolide” antibiotics such as erythromycin, clarithromycin, telithromycin, fusidic acid or certain drugs used to treat infections caused by fungi such as ketoconazole, itraconazole,
medicines used to regulate lipid levels: fibrates, such as gemfibrozil or colestipol,
medicines used to regulate your heart rhythm, such as amiodarone, diltiazem and verapamil,
medicines used to treat or prevent seizures such as carbamazepine, phenobarbital, phenytoin, fosphenytoin, primidone,
medicines used to change how your immune system works, such as cyclosporin
protease inhibitors such as ritonavir, indinavir, nelfinavir used in the treatment of HIV,
medicines used to treat depression, such as nefazodone and imipramine,
medicines used to treat mental disorders, such as neuroleptics,
medicines used to treat heart failure, such as beta blockers
medicines used to treat high blood pressure, such as angiotensin II inhibitors, converting enzyme inhibitors, verapamil and diuretics,
alpha blockers used to treat high blood pressure or prostate problems
other medicines known to interact with CADUET 10 mg / 10 mg film-coated tablets such as ezetimibe (which lowers cholesterol), warfarin (which decreases blood clotting), oral contraceptives, stiripentol (an anticonvulsant used for treatment of epilepsy), cimetidine (used for heartburn and stomach ulcers), phenazone (a pain reliever), antacids (containing aluminum or magnesium, used to relieve problems stomach), and amifostine (used to treat cancer),
sildenafil (used in the treatment of erectile dysfunction, impotence),
dantrolene and baclofen (muscle relaxants),
steroids,
over-the-counter St. John’s Wort products.
CADUET may lower your blood pressure further if you are already taking other medicines to treat your high blood pressure.
If you are taking or have recently taken any other medicines, including medicines obtained without a prescription, talk to your doctor or pharmacist.
INTERACTIONS OF CADUET 10 MG / 10 MG WITH FOOD AND DRINK
Food and drinks
CADUET 10 mg / 10 mg film-coated tablets can be taken at any time of the day, with or without food.
Grapefruit juice
Do not take more than one or two glasses of grapefruit juice per day as large amounts of grapefruit juice may affect the effects of CADUET 10 mg / 10 mg film-coated tablets.
Alcohol
Avoid drinking too much alcohol while taking CADUET 10 mg / 10 mg film-coated tablets. See also section 2 “Take special care with CADUET 10 mg / 10 mg film-coated tablets” for more details.
Drive and use machines
Do not drive or use machines if you feel dizzy after taking this medicine.
Do not drive or use machines if you feel dizzy after taking this medicine.
Do not drive or use machines if you feel dizzy after taking this medicine.
Warnings and Precautions
Heart failure
Liver function monitoring
Clinical signs of hepatic dysfunction
Increased transaminases
Heavy alcohol consumption
Hepatic insufficiency
History of liver disease
Predisposing factor to the occurrence of rhabdomyolysis
Subject over 70 years of age
Unexplained muscle pain
Muscle cramp
Muscular weakness
Increase in CPK
Rhabdomyolysis
History of hemorrhagic stroke
History of lacunar infarction
Interstitial lung disease
Risk of diabetes
Woman of childbearing age
Heart failure
Patients with heart failure should be treated with caution. In a long-term placebo-controlled study in patients with severe heart failure (NYHA class III and IV), the reported incidence of pulmonary edema was higher in the amlodipine group compared to the placebo group. (see section Pharmacodynamic properties). Calcium channel blockers including amlodipine should be used with caution in patients with congestive heart failure because they may increase the risk of cardiovascular events and mortality.
Liver effects
Liver function tests should be performed before the start of treatment and then regularly after initiation of treatment, as well as in the event of signs or symptoms suggestive of liver damage. In the event of elevation of the serum transaminase level, monitoring is necessary until normalization.
A persistent increase in ALT or AST exceeding 3 times the upper limit of normal (ULN), should lead to discontinuation of treatment.
The half-life of amlodipine is increased and its AUC (Area Under the Curve) is greater in patients with hepatic impairment; dosage recommendations have not been established.
Due to the presence of atorvastatin, CADUET should be used with caution in patients who consume large amounts of alcohol, in patients with hepatic impairment and / or a history of liver disease.
Muscle effects
Like other HMG-CoA reductase inhibitors, atorvastatin can affect skeletal muscles and lead to myalgia, myositis and myopathies. These muscle damage may rarely progress to rhabdomyolysis, characterized by high levels of creatine kinase (CK) (more than 10 times the ULN), myoglobinemia and myoglobinuria which can lead to kidney failure, and in some cases be fatal.
Regular testing of CK or other muscle enzymes is not recommended in asymptomatic patients treated with statins. However, dosing of CKs is recommended before initiating statin therapy in patients with predisposing factors to rhabdomyolysis as well as in those with muscle symptoms during statin therapy (see below).
Very rare cases of autoimmune-mediated necrotizing myopathies (IMNM) have been reported during or after treatment with certain statins. IMNM is clinically characterized by proximal muscle weakness and increased serum creatine kinase, which persist despite discontinuation of statin therapy.
Before initiation of treatment
CADUET should be prescribed with caution in patients with predisposing factors to rhabdomyolysis. Before starting treatment with a statin, creatine kinase (CK) levels should be checked in the following situations:
Renal failure.
Hypothyroidism.
Personal or family history of genetic muscle diseases.
Personal history of muscle toxicity during treatment with a statin or a fibrate.
History of liver disease and / or excessive alcohol consumption.
In elderly patients (> 70 years), the need for these measures should be assessed, depending on the presence of other factors predisposing to rhabdomyolysis.
Situations in which increased plasma concentrations may occur, due to interactions (see section 4.5) and use in special populations including genetic polymorphisms (see section 5.2). ).
In these situations, a regular reassessment of the benefit / risk of the treatment, as well as regular clinical monitoring is recommended.
If the initial CK level is significantly elevated (more than 5 times the ULN) treatment should not be started.
Creatine kinase measurement
Creatine kinase (CK) should not be measured after heavy exercise or in the presence of another possible cause of increased CK, as this will make interpretation of the results difficult. In the event of a significant elevation of CK (more than 5 times the ULN) before treatment, this should be systematically checked again within 5 to 7 days to confirm the results.
During treatment
It is recommended that patients be asked to promptly report any unexplained muscle pain, muscle cramps or weakness, particularly if accompanied by malaise or fever.
· If symptoms appear when a patient is under treatment with CADUET, a CK levels should be measured; if the CK level is significantly elevated (more than 5 times the ULN), treatment should be discontinued.
· If muscular symptoms are severe and cause daily discomfort, discontinuation of treatment should be considered, even if CK levels did not exceed 5 times the ULN.
· If symptoms resolve and CK levels return to normal, the reintroduction of CADUET may be considered at the lowest dose and under close supervision.
Treatment with CADUET should be discontinued in the event of a clinically significant increase in CK level (> 10 times the ULN) or if rhabdomyolysis is diagnosed or suspected.
Amlodipine has no effect on laboratory parameters.
Combinations with other drugs
As with other statins, the risk of rhabdomyolysis is increased when CADUET is administered in combination with certain drugs which may increase the plasma concentration of atorvastatin, such as strong inhibitors of CYP3A4 or protein transporters (eg, cyclosporin, telithromycin, clarithromycin, delavirdine, stiripentol, ketoconazole, voriconazole, itraconazole, posaconazole, determovir and HIV protease inhibitors including ritonavir, lopinavir, atazanavir, indinavir, darunavir, tipranavir, etc / ritonavir). The risk of myopathy may also be increased in combination with gemfibrozil and other fibrates, antivirals used in the treatment of hepatitis C (HCV) (boceprevir, telaprevir, elbasvir / grazoprevir), erythromycin, niacin, l ‘ ezetimibe or colchicine. Therapeutic alternatives (not exhibiting these interactions) should be considered as far as possible.
In the event that the combination of these medicinal products with CADUET is necessary, the benefit / risk of concomitant treatments should be carefully assessed and appropriate clinical monitoring is recommended (see section Interactions with other medicinal products and other forms of interactions).
CADUET should not be administered simultaneously with fusidic acid in systemic form, and for up to 7 days after stopping treatment with fusidic acid. In patients where the use of systemic fusidic acid is considered essential, statin therapy should be discontinued for the duration of fusidic acid therapy. Cases of rhabdomyolysis (some fatal) have been reported in patients receiving fusidic acid and a statin in combination (see section Interactions with other medicinal products and other forms of interactions). Patients should be informed of the need to seek immediate medical attention if they experience symptoms of muscle weakness, pain, or muscle tenderness.
Statin therapy can be restarted seven days after the last dose of fusidic acid.
In exceptional circumstances, when prolonged treatment with systemic fusidic acid is required, e.g. for the treatment of severe infections, the need for co-administration of CADUET and fusidic acid should only be considered in cases of per case and under close medical supervision.
Prevention of stroke by aggressively lowering cholesterol levels (SPARCL study)
In a posteriori analysis performed in subgroups of patients with recent stroke or transient ischemic attack (TIA) but without coronary artery disease, a higher frequency of hemorrhagic stroke was observed in treated patients. per 80 mg atorvastatin compared to patients on placebo.
This high risk is particularly observed in patients who have already had a hemorrhagic stroke or a lacunar infarction at the inclusion of the study.
In patients with a history of hemorrhagic stroke or lacunar infarction, the benefit / risk balance of atorvastatin 80 mg is uncertain. Therefore, the potential risk of occurrence of haemorrhagic stroke should be carefully assessed before any initiation of treatment (see section Pharmacodynamic properties).
Interstitial lung disease
Cases of interstitial lung disease have exceptionally been reported with certain statins, particularly during long-term therapy .
Symptoms may include dyspnea, a non-productive cough, and altered general condition (fatigue, weight loss, and fever). If interstitial lung disease is suspected in a patient, statin therapy should be discontinued.
Diabetes
There is some evidence that statins, as a pharmacological class, increase blood sugar. In some patients at high risk of developing diabetes, statins may cause hyperglycaemia requiring the initiation of diabetes treatment. This risk is nevertheless compensated by the reduction in the vascular risk under statins and therefore it should not be a reason for stopping statins. Patients at risk (fasting blood glucose between 5.6 and 6.9 mmol / l, BMI> 30 kg / m², increased triglyceride levels, arterial hypertension) should be monitored clinically and biologically in accordance with national recommendations.
PREGNANCY & BREAST-FEEDING & FERTILITY
Caduet is contraindicated during pregnancy and lactation.
Women of childbearing age
Women of childbearing potential should use reliable contraceptive measures during treatment ( see Contraindications ).
Pregnancy
The safety of atorvastatin has not been established in pregnant women. No controlled clinical trials have been performed in pregnant women treated with atorvastatin. Following intrauterine exposure to HMG-CoA reductase inhibitors, birth defects have rarely been reported. Studies in animals have shown reproductive toxicity ( see Preclinical safety ).
Treatment of the mother with atorvastatin may reduce the fetal level of mevalonate, which is a precursor of cholesterol biosynthesis. Atherosclerosis is a chronic process, and stopping a lipid-lowering drug during pregnancy should generally have little effect on the long-term risk associated with primary hypercholesterolemia.
For these reasons, Caduet should not be used during pregnancy, or in a woman planning to become pregnant or in whom pregnancy is suspected. Caduet treatment should be withheld during pregnancy or until it has been determined that the woman is not pregnant ( see Contraindications ).
If pregnancy is discovered during treatment, it should be stopped immediately.
Feeding with milk
Amlodipine is excreted in breast milk. The proportion of maternal dose received by the infant has been estimated at an interquartile range of 3-7%, with a maximum of 15%. The effect of amlodipine on infants is unknown. The excretion of atorvastatin or its metabolites in human milk is not known. In rats, the plasma concentrations of atorvastatin and its metabolites are similar to those found in milk ( see Preclinical safety ). Due to the possibility of serious side effects, women treated with Caduet should not breast-feed their infants ( see Contraindications ). Atorvastatin is contraindicated during breastfeeding ( see Contraindications ).
Fertility
No effect of atorvastatin on fertility has been demonstrated in studies conducted in male or female animals ( see Preclinical Safety ).
Reversible biochemical changes in the sperm head have been reported in some patients treated with calcium channel blockers. There is insufficient clinical data regarding the potential effect of amlodipine on fertility. In a study carried out in rats, adverse effects were detected on male fertility ( see Preclinical safety ).
What happens if I overdose from Caduet?
No information is available regarding overdose of Caduet in humans.
Amlodipine:
For amlodipine, experience with intentional overdose in humans is limited. Massive overdose could cause extensive peripheral vasodilation and possibly reflex tachycardia. Marked and possibly prolonged systemic hypotension up to and including shock with fatal outcome has been reported. Any hypotension resulting from acute intoxication requires monitoring in an intensive cardiological care unit. A vasoconstrictor can be used to restore vascular tone and blood pressure. Since amlodipine is highly protein bound, dialysis is unlikely to provide any benefit.
Atorvastatin:
There is no specific treatment for atorvastatin overdose. In the event of overdose, the patient should receive symptomatic treatment and supportive measures should be implemented as needed. Liver function and serum CK concentrations should be monitored. Due to the strong binding of the drug to plasma proteins, hemodialysis is not expected to significantly increase the clearance of atorvastatin.
What should I do if I miss a dose?
If you forget to take CADUET 5/ 10 mg -5/ 10 mg film-coated tablets:
If you forget to take your dose of CADUET, take your next dose at the normal time.
Do not take a double dose to make up for the dose you forgot to take.
What is Forms and Composition ?
SHAPES and PRESENTATIONS
5 mg / 10 mg film-coated tablet (oval, debossed with “Pfizer” on one side and “CDT 051” on the other side; white) and 10 mg / 10 mg (oval, debossed with “Pfizer “On one side and” CDT 101 “on the other side; blue): Boxes of 30 and 90, in blisters.
COMPOSITION
p cp Amlodipine (INN) as amlodipine besilate 5 mg or 10 mg Atorvastatin (INN) as atorvastatin calcium trihydrate 10 mg
Excipients (common): Core: calcium carbonate, croscarmellose sodium, microcrystalline cellulose, pregelatinized corn starch, polysorbate 80, hydroxypropylcellulose, colloidal anhydrous silica, magnesium stearate. Film- coating : 5 mg / 10 mg tablet: Opadry II white 85F28751 (polyvinyl alcohol, titanium dioxide E171, macrogol 3000, talc). 10 mg / 10 mg tablet: Opadry II blue 85F10919 (polyvinyl alcohol, titanium dioxide E171, macrogol 3000, talc, indigo carmine aluminum lake E132).
NOT’s
Edrug-online contains comprehensive and detailed information about drugs available in the medical field, and is divided into four sections:
general information:
Includes a general description of the drug, its use, brand names, FAQs, and relevant news and articles
Additional information:
General explanation about dealing with the medicine: how to take the medicine, the doses and times of it, the start and duration of its effectiveness, the recommended diet during the period of taking the medicine, the method of storage and storage, recommendations in cases for forgetting the dose and instructions to stop taking the drug and take additional doses.
Special warnings:
For pregnant and breastfeeding women, the elderly, boys and drivers, and use before surgery.
Side effects:
It treats possible side effects and drug interactions that require attention and its effect on continuous use.
The information contained in this medicine is based on medical literature, but it is not a substitute for consulting a doctor.
The post caduet tablets Uses, Dosage, Side Effects, Precautions & Warnings appeared first on Drug Online.
from Drug Online https://bit.ly/3lxi9nO
via Edrug Online
0 notes
Text
Pressure Injectable PICC Kits Market : Future Growth Analysis, Demand Forecast, and Future Outlook
Pressure Injectable PICC Kits Market: Introduction
A peripherally inserted central catheter (PICC) is a small gauge catheter inserted peripherally. It is suitable for long-term use and there are no restrictions on age or gender. A PICC is a long, thin, flexible IV line or catheter. It is placed in a vein in the arm and guided up into a larger vein near the heart.
View Report : https://www.transparencymarketresearch.com/pressure-injectable-picc-kits-market.html
A PICC can have one or more openings called lumens. It may stay in for several weeks or months. PICCs are usually quite long to accommodate different lengths of insertion. These are frequently trimmed prior to insertion to prevent dislodgement and for easy handling. PICCs are not advisable in patients with renal failure and impending need for dialysis, in whom preservation of upper-extremity veins is needed for fistula or graft implantation. The infection might have a local inflammation which is uncommon with PICC-related infection caused by coagulase-negative staphylococci, as this pathogen incites little local or systemic inflammation. The patient should also be examined for fever or other signs of sepsis.
Get PDF Sample Copy of Report: (Including TOC, List of Tables & Figures, Chart) : https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=74074
Key Drivers of Global Pressure Injectable PICC Kits Market
Increased precision of PICC and CVC placement with tip location devices
Tip position verification of peripherally inserted central catheters (PICCs) is essential to the use of the catheter. The ideal tip position of a central venous catheter provides reliable venous access with optimal therapeutic delivery, while minimizing short- and long-term complications.
Ideal position limits have evolved and narrowed over time, making successful placement difficult and unreliable when depending exclusively on the landmark technique
For patients with atrial fibrillation (AF), ECG is used to detect the PICC tip position during catheterization and X-ray is done to confirm the tip position as the “gold standard” after PICC insertion
When the catheter enters the lower one third of superior vena cava, the amplitude of the f wave reaches the maximum. There is no statistical difference between X-ray PICC tip position verification and IC-ECG PICC tip position verification among patients with atrial fibrillation.
Increase in cases of infection due to usage of PICC
According to the NCBI, the most common organisms associated with hospital-acquired CRBSIs (in order of most to least common) are coagulase-negative Staphylococci, Staphylococcus aureus, Enterococci, and Candida
The cumulative incidence of CRBSIs for PICCs is 1.1 per 1000 PICC-days. However, it is found to be higher in inpatient setting (2.1 per 1000 PICC-days). This could be because patients managed on an outpatient basis are healthier in general, and their catheter is accessed less frequently.
There is still question about whether PICCS or centrally inserted venous catheters (CICCs) have lower infection rates.
Infection rates are higher for PICCs placed in the antecubital fossa compared to those placed in the upper arm.
North America to Account for Major Share of Global Market
North America is expected to account for major share of the global Pressure Injectable PICC Kits Market during the forecast period. The market in North America is likely to grow at a rapid pace in the near future due to rise in prevalence of chronic kidney dialysis and cancer. Moreover, increase in health care reach in untapped markets is expected to spur market growth during the forecast period.
REQUEST FOR COVID19 IMPACT ANALYSIS – https://www.transparencymarketresearch.com/sample/sample.php?flag=covid19&rep_id=74074
Key Players Operating in Global Market
The global Pressure Injectable PICC Kits Market is highly concentrated due to presence of a few key players. A large number of manufacturers hold major share in the market in their respective regions. This has led to surge in the number of distributors and suppliers in emerging markets. Leading players operating in the global Pressure Injectable PICC Kits Market are:
AngioDynamics, Inc.
C. R. Bard Inc.
Teleflex Incorporated
B. Braun Melsungen AG
Medtronic plc
Vygon S.A
Cook Medical, Inc.
Becton, Dickinson and Company
Boston Scientific Corporation
Argon Medical Devices, Inc.
Medical Component, Inc.
Theragenics Corporation
Global Pressure Injectable Kit (PICC) Market: Research Scope
Global Pressure Injectable Kit (PICC) Market, by Product Type
Power Injected PICC
Conventional PICC
Global Pressure Injectable Kit (PICC) Market, by Design Type
Single Lumen
Double Lumens
Triple/Multiple Lumens
Global Pressure Injectable Kit (PICC) Market, by Catheter
Valved (positive pressure: valve opens outward)
Non-valved (negative pressure: valve opens inward)
Global Pressure Injectable Kit (PICC) Market, by End-user
Hospitals
Nursing Facilities
Home Care
Others
Global Pressure Injectable Kit (PICC) Market, by Region
North AmericaU.S.
Canada
EuropeGermany
France
U.K.
Italy
Spain
Asia PacificChina
Japan
India
Australia & New Zealand
Rest of Asia Pacific
Latin AmericaBrazil
Mexico
Rest of Latin America
Middle East & AfricaGCC Countries
South Africa
Rest of Middle East & Africa
More Trending Reports by Transparency Market Research – 1. https://www.prnewswire.com/news-releases/global-surgical-microscopes-market-to-exhibit-promising-cagr-of-10-for-2019-2027-increased-investments-for-medical-and-healthcare-research-to-fuel-market-growth-observes-tmr-301007369.html
2. https://www.biospace.com/article/diabetic-foot-ulcers-treatment-market-surge-in-number-of-amputations-due-to-diabetic-foot-ulcers-to-propel-the-market/
0 notes
Text
Pressure Injectable PICC Kits Market Growth Prospects, Trends and Forecast up to 2027
A peripherally inserted central catheter (PICC) is a small gauge catheter inserted peripherally. It is suitable for long-term use and there are no restrictions on age or gender. A PICC is a long, thin, flexible IV line or catheter. It is placed in a vein in the arm and guided up into a larger vein near the heart. A PICC can have one or more openings called lumens. It may stay in for several weeks or months. PICCs are usually quite long to accommodate different lengths of insertion.
Read Report Overview -
https://www.transparencymarketresearch.com/pressure-injectable-picc-kits-market.html
These are frequently trimmed prior to insertion to prevent dislodgement and for easy handling. PICCs are not advisable in patients with renal failure and impending need for dialysis, in whom preservation of upper-extremity veins is needed for fistula or graft implantation. The infection might have a local inflammation which is uncommon with PICC-related infection caused by coagulase-negative staphylococci, as this pathogen incites little local or systemic inflammation. The patient should also be examined for fever or other signs of sepsis.
Key Drivers of Global Pressure Injectable PICC Kits Market
Increased precision of PICC and CVC placement with tip location devices
Tip position verification of peripherally inserted central catheters (PICCs) is essential to the use of the catheter. The ideal tip position of a central venous catheter provides reliable venous access with optimal therapeutic delivery, while minimizing short- and long-term complications.
Ideal position limits have evolved and narrowed over time, making successful placement difficult and unreliable when depending exclusively on the landmark technique
For patients with atrial fibrillation (AF), ECG is used to detect the PICC tip position during catheterization and X-ray is done to confirm the tip position as the “gold standard” after PICC insertion
When the catheter enters the lower one third of superior vena cava, the amplitude of the f wave reaches the maximum. There is no statistical difference between X-ray PICC tip position verification and IC-ECG PICC tip position verification among patients with atrial fibrillation.
Request a PDF Sample on Pressure Injectable PICC Kits Market Report -
https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=74074
Increase in cases of infection due to usage of PICC
According to the NCBI, the most common organisms associated with hospital-acquired CRBSIs (in order of most to least common) are coagulase-negative Staphylococci, Staphylococcus aureus, Enterococci, and Candida
The cumulative incidence of CRBSIs for PICCs is 1.1 per 1000 PICC-days. However, it is found to be higher in inpatient setting (2.1 per 1000 PICC-days). This could be because patients managed on an outpatient basis are healthier in general, and their catheter is accessed less frequently.
There is still question about whether PICCS or centrally inserted venous catheters (CICCs) have lower infection rates.
Infection rates are higher for PICCs placed in the antecubital fossa compared to those placed in the upper arm.
North America to Account for Major Share of Global Market
North America is expected to account for major share of the global Pressure Injectable PICC Kits Market during the forecast period. The market in North America is likely to grow at a rapid pace in the near future due to rise in prevalence of chronic kidney dialysis and cancer. Moreover, increase in health care reach in untapped markets is expected to spur market growth during the forecast period.
Expanding Operations in Future? To Get the Perfect Launch Ask for a Custom Pressure Injectable PICC Kits Market Report.
Pre Book this Report -
https://www.transparencymarketresearch.com/checkout.php?rep_id=74074<ype=S
Key Players Operating in Global Market
The global Pressure Injectable PICC Kits Market is highly concentrated due to presence of a few key players. A large number of manufacturers hold major share in the market in their respective regions. This has led to surge in the number of distributors and suppliers in emerging markets. Leading players operating in the global Pressure Injectable PICC Kits Market are:
AngioDynamics, Inc., C. R. Bard Inc., Teleflex Incorporated, B. Braun Melsungen AG, Medtronic plc, Vygon S.A, Cook Medical, Inc., Becton, Dickinson and Company, Boston Scientific Corporation, Argon Medical Devices, Inc., Medical Component, Inc., Theragenics Corporation.
0 notes
Text
The Great Statin Debate — Why Cholesterol Is Misunderstood
youtube
The health editor for the Daily Mail recently published an article touting the merits of statin cholesterol-lowering drugs and, worse, eschewing the “deadly propaganda of the statin deniers.”1
Pointing to an analysis published in BMJ, which suggested 200,000 patients may have stopped taking statins due to negative media reports about the drugs,2 the article attacks those who question statins’ merits and claims the notion that statins reduce the risk of a major cardiac event as “indisputable scientific fact.”3
The real story is far from black and white, however, which is why the great statin debate continues — and experts in the field continue to speak out against statins in an attempt to clear the widespread myths about cholesterol and your health.
Are Concerns Over Statins ‘Fake News’?
The Daily Mail examined what it said amounted to “fake news” on statins, including the idea that having high cholesterol is harmless. The fact is, “high cholesterol” as defined by many health organizations is not one in the same with the levels of high cholesterol that can actually harm your health.
Here the article points to familial hypercholesterolaemia,4 an inherited condition characterized by abnormally high cholesterol, which tends to be resistant to lowering with lifestyle strategies like diet and exercise. I have long stated that the only group of people who may benefit from a cholesterol-lowering medication are those with genetic familial hypercholesterolemia. This is the vast minority of people taking these drugs, probably far less than one in 1000.
However, according to the U.S. Centers for Disease Control and Prevention (CDC), these drugs are also indicated for anyone who has already had a heart attack or stroke or been diagnosed with peripheral arterial disease, has an LDL cholesterol of 190 mg/dL or higher, or is between the ages of 40 and 75 with an LDL level of 70 mg/dL or higher and diabetes or a high risk of developing heart disease or stroke.5
In short, a staggering number of Americans are “eligible” for cholesterol-lowering drugs. According to the CDC, that number is more than 78 million Americans, who are either eligible for the drugs or already taking them.6 Yet, the Daily Mail article pointed out that “millions of middle-aged people who would benefit from taking statins, don’t,” which could be “because they’ve been led to believe that the drugs don’t work.”7
There’s More to Heart Disease Than Cholesterol
Statins are effective at lowering cholesterol, but whether this is the panacea for helping you avoid heart disease and extend your life span is a question worthy of closer scrutiny.
Such has been done by Dr. Malcolm Kendrick, a British physician and author of “Doctoring Data: How to Sort Out Medical Advice from Medical Nonsense,” “The Great Cholesterol Con” and “A Statin Nation: Damaging Millions in a Brave New Post-Health World” — and also one of the “statin deniers” targeted by the Daily Mail. Kendrick is among those who believe cholesterol does not cause heart disease — and he fires back at the Daily Mail article in the video above.
Kendrick states the most concerning risk factors for cardiovascular disease are actually insulin resistance, Type 2 diabetes and the chronic inflammation associated with these conditions, along with factors such as how you eat — whether you’re rushing or taking your time — and other stress-related factors, both physical and psychological.
He believes the conventional LDL/cholesterol hypothesis is flawed, in part because damage of the interior layers of your arteries precedes heart disease,8 and this damage can be induced by a number of factors, including smoking, high blood pressure, elevated blood sugar and inflammation.
Once the artery is damaged, cholesterol-rich plaque begins to build up as a protective mechanism. Problems arise when the rate of damage and resultant blood clot formation outpace or outstrip your body’s ability to repair. As noted by Kendrick, “For good health, you want to maintain a balance between the blood being too ready to clot, and the blood not clotting when you need it to.”9
So, what factors might lead to a situation in which the arterial damage is greater than your body’s ability to repair it? Kendrick’s “short list” includes over 30 factors alone, which include:
Use of certain drugs, including oral steroids, omeprazole, Avastin and thalidomide
Diseases such as Cushing’s disease, Kawasaki disease, rheumatoid arthritis, systemic lupus erythematosus, chronic kidney disease and acute renal failure, sickle cell disease, malaria and Type 2 diabetes, as well as bacterial and viral infections
Acute physical and mental stress, and chronic mental stress
Heavy metal exposure, including lead and mercury
Certain nutritional deficiencies, including vitamins B and C deficiencies
Without Cholesterol in Your Body, You Would Die
Another “statin denier” outed by the Daily Mail is Zoe Harcombe, Ph.D., nutritional researcher, author and public speaker. She states, “It is virtually impossible to explain how vital cholesterol is to the human body. If you had no cholesterol in your body you would be dead.”10
Your liver manufactures most, about 80 percent, of the cholesterol your body requires, which in and of itself suggests your body cannot survive without it. The remaining 20 percent comes from your diet. However, dietary cholesterol is absorbed at a rate of 20 to 60 percent, depending on the individual, and if you consume less, your body will compensate by making more and vice versa.
Contrary to popular belief, cholesterol is a crucial molecule necessary for optimal health, and not nearly the damaging culprit it’s been made out to be. Since cholesterol is a fatty substance, it does not travel well through your water-based bloodstream. Hence it is encapsulated in a lipoprotein.
As noted by Harcombe, the notion that there is good and bad cholesterol is also wrong. Low-density lipoprotein (LDL) and high-density lipoprotein (HDL) are not even actually cholesterol; they’re carriers and transporters of cholesterol, triglycerides (fat), phospholipids and proteins. “LDL would more accurately be called the carrier of fresh cholesterol and HDL would more accurately be called the carrier of recycled cholesterol,” she says.11
Ivor Cummins, a biochemical engineer with a background in medical device engineering and leading teams in complex problem-solving, similarly likens the very low-density lipoprotein (VLDL) your liver makes to a boat that shuttles not only cholesterol but also triglycerides through your bloodstream to your tissues.
The VLDL will dock onto receptors in your muscle tissue, where it releases triglycerides to be used for energy. If your triglycerides are high, it means you’re eating too many net carbohydrates, because it’s actually sugar that causes triglycerides to rise, not dietary fat.
Once the VLDL has dropped off the triglycerides to be burnt for energy (or stored as fat if you’re not using the energy due to inactivity), the VLDL becomes a low-density lipoprotein (LDL), which in conventional thinking is a “bad” kind of cholesterol.
High-density lipoprotein (HDL) is colloquially known as “good” cholesterol, and the HDL is indeed beneficial in that it acts as a master manager, helping protect the LDL against oxidation and transporting triglycerides and cholesterol in and out of the VLDL. In a healthy person, the LDL will be reabsorbed by the liver after about two days, where it gets broken up and recycled.
As a general rule, a high-sugar diet will cause damaged LDLs to rise, beneficial HDLs to drop, triglycerides and, often, total cholesterol to rise. Coming full circle, all of these are conventional indicators of atherosclerosis or inflammation in your arteries that can precipitate a heart attack.
For Those at Low Risk, Eating an Apple a Day Will Lower Your Heart Attack Risk as Much as a Statin
youtube
Dr. Aseem Malhotra, an interventional cardiologist consultant in London, U.K., is the third “statin denier” attacked by the Daily Mail. He gained quite a bit of publicity after the publication of his peer-reviewed editorial in BMJ in 2013, which argued that you should ignore advice to reduce your saturated fat intake, because it’s actually increasing your risk for obesity and heart disease.12
In addition to defending the merits of healthy saturated fats, Malhotra highlights the risks of statin drugs, noting that more than half of statin users stop using the drugs within a year, most citing side effects as the reason.13
Fatigue, nausea, joint and muscle pain and increases in blood sugar have all been associated with statin drug use, most of which cease when the drugs are stopped. He also points out that unhealthy diet, including excess sugar consumption, is the true culprit in heart disease:
“Over 80 percent of CVD [cardiovascular disease] is attributable to environmental factors, notably unhealthy diet and also smoking, alcohol and physical inactivity. Diet has primacy, accounting for a larger burden of CVD disease and death than tobacco, alcohol and inactivity combined. For those at low risk eating an apple a day has an equivalent risk reduction for myocardial infarction [heart attack] as taking a statin.”14,15
Statins Increase Diabetes Risk
Statins have been shown to increase your risk of diabetes via a number of different mechanisms. The most important one is that they increase insulin resistance, which can be extremely harmful to your health. Secondly, statins increase your diabetes risk by raising your blood sugar. Statins work by preventing your liver from making cholesterol.
As a result, your liver returns the sugar to your bloodstream, which raises your blood sugar levels. These drugs also rob your body of certain valuable nutrients, which can also impact your blood sugar levels. Two nutrients in particular, vitamin D and CoQ10, are both needed to maintain ideal blood glucose levels.
Importantly, statins deplete your body of CoQ10, vitamin K2, dolichol and selenium, thereby threatening your heart and overall health even further. Statins’ ability to lower the risk of minor heart attacks may actually be related to their ability to lower C-reactive protein, far more so than the lowering of cholesterol.
Researchers with the Erasmus Medical Center in the Netherlands recently analyzed data from more than 9,500 patients. Those who had ever used statins had a 38 percent higher risk of Type 2 diabetes, with the risk being higher in those with impaired glucose homeostasis and those who were overweight or obese.16
The researchers concluded, “Individuals using statins may be at higher risk for hyperglycemia, insulin resistance and eventually Type 2 diabetes. Rigorous preventive strategies such as glucose control and weight reduction in patients when initiating statin therapy might help minimizing the risk of diabetes.”
But a far better strategy may be preventing insulin resistance in the first place, by avoiding statin drugs and eating a healthy diet. According to Malhotra and a colleague:17
“In young adults, preventing insulin resistance could prevent 42 percent of myocardial infarctions, a larger reduction than correcting hypertension (36 percent), low high-density lipoprotein cholesterol (HDL-C) (31 percent), body mass index (BMI) (21 percent) or LDL-C (16 percent).18
It is plausible that the small benefits of statins in the prevention of CVD come from pleiotropic effects which are independent of LDL-lowering. The focus in primary prevention should therefore be on foods and food groups that have a proven benefit in reducing hard endpoints and mortality.”
How to Identify and Lower Your Heart Disease Risk
Rather than focusing on cholesterol, two tests that are far more important for assessing your CVD risk are the serum ferritin and gamma-glutamyl transpeptidase (GGT) tests.
The GGT test can be used as a screening marker for excess free iron and is a great indicator of your sudden cardiac death risk. The recommended, ideal levels, of ferritin and GGT are as follows. For more information about these tests, read “Cholesterol Does Not Cause Heart Disease.”
• Ferritin — Adult men and nonmenstruating women: 30 to 40 nanograms per milliliter (ng/mL) or 75 to 100 nanomoles per liter (nmol/L).
The most commonly used threshold for iron deficiency in clinical studies is 12 to 15 ng/mL (30 to 37 nmol/L). You do not want to be below 20 ng/mL (50 nmol/L) or above 80 ng/mL (200 nmol/L). High iron during pregnancy is also problematic; having a level of 60 or 70 ng/mL (150 or 175 nmol/L) is associated with greater odds of poor pregnancy outcomes.
• GGT — Below 16 units per liter (U/L) for men and below 9 U/L for women. Above 25 U/L for men and 18 U/L for women, your risk of chronic disease increases significantly.
In order to protect yourself against heart disease, here are a number of suggestions that can help you lower your insulin resistance and restore your insulin sensitivity, among other heart-protective mechanisms:
Avoid environmental pollutants and toxins, including smoking, vaping, heavy metals, herbicides and pesticides, especially glyphosate.
Minimize your exposure to electromagnetic fields and wireless radiation from cellphones, Wi-Fi, routers, smart meters and more, as this kind of radiation has been shown to cause serious free radical damage and mitochondrial dysfunction.
Eat an unprocessed whole food-based diet low in net carbs and high in healthy fats. A ketogenic diet — which is very low in net carbohydrates and high in healthy fats — is key for boosting mitochondrial function.
When your body is able to burn fat for fuel, your liver creates water-soluble fats called ketones that burn far more efficiently than carbs, thereby creating fewer reactive oxygen species and secondary free radicals. Ketones also decrease inflammation and improve glucose metabolism.19
Eat nitrate-rich foods to help normalize your blood pressure. Good sources include arugula, cilantro, rhubarb, butter leaf lettuce, mesclun mixed greens, beet greens, fresh beet juice, kvass (fermented beet juice) and fermented beet powder.
Get plenty of nonexercise movement each day; walk more and incorporate higher intensity exercise as your health allows.
Intermittently fast. After you’ve become accustomed to intermittently fasting for 16 to 18 hours, you can try a stricter fast once or twice a week, when you eat a 300- to 800-calorie meal loaded with detox supporting nutrients, followed by a 24-hour fast. So, in essence, you’re then only eating one 300- to 800-calorie meal in 42 hours.
If you have heart disease, consider enhanced external counterpulsation (EECP). To find a provider, see EECP.com.20
If you have heart disease, you may also consider taking g-strophanthin, an adrenal hormone that helps create more parasympathetic nervous system neurotransmitters, thereby supporting your parasympathetic nervous system. It also helps flush out lactic acid. Strophanthus is the name of the plant, the active ingredient of which is called g-strophanthin in Europe, and ouabain in the United States.
Get sensible sun exposure to optimize your vitamin D status and/or take an oral vitamin D3 supplement with magnesium and vitamin K2.
Implement heart-based wellness practices such as connecting with loved ones and practicing gratitude.
from Articles http://articles.mercola.com/sites/articles/archive/2019/03/19/why-are-statins-bad-for-you.aspx
source https://niapurenaturecom.tumblr.com/post/183557908421
0 notes
Text
The Great Statin Debate Why Cholesterol Is Misunderstood
The health editor for the Daily Mail recently published an article touting the merits of statin cholesterol-lowering drugs and, worse, eschewing the "deadly propaganda of the statin deniers."1
Pointing to an analysis published in BMJ, which suggested 200,000 patients may have stopped taking statins due to negative media reports about the drugs,2 the article attacks those who question statins' merits and claims the notion that statins reduce the risk of a major cardiac event as "indisputable scientific fact."3
The real story is far from black and white, however, which is why the great statin debate continues — and experts in the field continue to speak out against statins in an attempt to clear the widespread myths about cholesterol and your health.
Are Concerns Over Statins 'Fake News'?
The Daily Mail examined what it said amounted to "fake news" on statins, including the idea that having high cholesterol is harmless. The fact is, "high cholesterol" as defined by many health organizations is not one in the same with the levels of high cholesterol that can actually harm your health.
Here the article points to familial hypercholesterolaemia,4 an inherited condition characterized by abnormally high cholesterol, which tends to be resistant to lowering with lifestyle strategies like diet and exercise. I have long stated that the only group of people who may benefit from a cholesterol-lowering medication are those with genetic familial hypercholesterolemia. This is the vast minority of people taking these drugs, probably far less than one in 1000.
However, according to the U.S. Centers for Disease Control and Prevention (CDC), these drugs are also indicated for anyone who has already had a heart attack or stroke or been diagnosed with peripheral arterial disease, has an LDL cholesterol of 190 mg/dL or higher, or is between the ages of 40 and 75 with an LDL level of 70 mg/dL or higher and diabetes or a high risk of developing heart disease or stroke.5
In short, a staggering number of Americans are "eligible" for cholesterol-lowering drugs. According to the CDC, that number is more than 78 million Americans, who are either eligible for the drugs or already taking them.6 Yet, the Daily Mail article pointed out that "millions of middle-aged people who would benefit from taking statins, don't," which could be "because they've been led to believe that the drugs don't work."7
There's More to Heart Disease Than Cholesterol
Statins are effective at lowering cholesterol, but whether this is the panacea for helping you avoid heart disease and extend your life span is a question worthy of closer scrutiny.
Such has been done by Dr. Malcolm Kendrick, a British physician and author of "Doctoring Data: How to Sort Out Medical Advice from Medical Nonsense," "The Great Cholesterol Con" and "A Statin Nation: Damaging Millions in a Brave New Post-Health World" — and also one of the "statin deniers" targeted by the Daily Mail. Kendrick is among those who believe cholesterol does not cause heart disease — and he fires back at the Daily Mail article in the video above.
Kendrick states the most concerning risk factors for cardiovascular disease are actually insulin resistance, Type 2 diabetes and the chronic inflammation associated with these conditions, along with factors such as how you eat — whether you're rushing or taking your time — and other stress-related factors, both physical and psychological.
He believes the conventional LDL/cholesterol hypothesis is flawed, in part because damage of the interior layers of your arteries precedes heart disease,8 and this damage can be induced by a number of factors, including smoking, high blood pressure, elevated blood sugar and inflammation.
Once the artery is damaged, cholesterol-rich plaque begins to build up as a protective mechanism. Problems arise when the rate of damage and resultant blood clot formation outpace or outstrip your body's ability to repair. As noted by Kendrick, "For good health, you want to maintain a balance between the blood being too ready to clot, and the blood not clotting when you need it to."9
So, what factors might lead to a situation in which the arterial damage is greater than your body's ability to repair it? Kendrick's "short list" includes over 30 factors alone, which include:
Use of certain drugs, including oral steroids, omeprazole, Avastin and thalidomide
Diseases such as Cushing's disease, Kawasaki disease, rheumatoid arthritis, systemic lupus erythematosus, chronic kidney disease and acute renal failure, sickle cell disease, malaria and Type 2 diabetes, as well as bacterial and viral infections
Acute physical and mental stress, and chronic mental stress
Heavy metal exposure, including lead and mercury
Certain nutritional deficiencies, including vitamins B and C deficiencies
Without Cholesterol in Your Body, You Would Die
Another "statin denier" outed by the Daily Mail is Zoe Harcombe, Ph.D., nutritional researcher, author and public speaker. She states, "It is virtually impossible to explain how vital cholesterol is to the human body. If you had no cholesterol in your body you would be dead."10
Your liver manufactures most, about 80 percent, of the cholesterol your body requires, which in and of itself suggests your body cannot survive without it. The remaining 20 percent comes from your diet. However, dietary cholesterol is absorbed at a rate of 20 to 60 percent, depending on the individual, and if you consume less, your body will compensate by making more and vice versa.
Contrary to popular belief, cholesterol is a crucial molecule necessary for optimal health, and not nearly the damaging culprit it's been made out to be. Since cholesterol is a fatty substance, it does not travel well through your water-based bloodstream. Hence it is encapsulated in a lipoprotein.
As noted by Harcombe, the notion that there is good and bad cholesterol is also wrong. Low-density lipoprotein (LDL) and high-density lipoprotein (HDL) are not even actually cholesterol; they're carriers and transporters of cholesterol, triglycerides (fat), phospholipids and proteins. "LDL would more accurately be called the carrier of fresh cholesterol and HDL would more accurately be called the carrier of recycled cholesterol," she says.11
Ivor Cummins, a biochemical engineer with a background in medical device engineering and leading teams in complex problem-solving, similarly likens the very low-density lipoprotein (VLDL) your liver makes to a boat that shuttles not only cholesterol but also triglycerides through your bloodstream to your tissues.
The VLDL will dock onto receptors in your muscle tissue, where it releases triglycerides to be used for energy. If your triglycerides are high, it means you're eating too many net carbohydrates, because it's actually sugar that causes triglycerides to rise, not dietary fat.
Once the VLDL has dropped off the triglycerides to be burnt for energy (or stored as fat if you're not using the energy due to inactivity), the VLDL becomes a low-density lipoprotein (LDL), which in conventional thinking is a "bad" kind of cholesterol.
High-density lipoprotein (HDL) is colloquially known as "good" cholesterol, and the HDL is indeed beneficial in that it acts as a master manager, helping protect the LDL against oxidation and transporting triglycerides and cholesterol in and out of the VLDL. In a healthy person, the LDL will be reabsorbed by the liver after about two days, where it gets broken up and recycled.
As a general rule, a high-sugar diet will cause damaged LDLs to rise, beneficial HDLs to drop, triglycerides and, often, total cholesterol to rise. Coming full circle, all of these are conventional indicators of atherosclerosis or inflammation in your arteries that can precipitate a heart attack.
For Those at Low Risk, Eating an Apple a Day Will Lower Your Heart Attack Risk as Much as a Statin
Dr. Aseem Malhotra, an interventional cardiologist consultant in London, U.K., is the third "statin denier" attacked by the Daily Mail. He gained quite a bit of publicity after the publication of his peer-reviewed editorial in BMJ in 2013, which argued that you should ignore advice to reduce your saturated fat intake, because it's actually increasing your risk for obesity and heart disease.12
In addition to defending the merits of healthy saturated fats, Malhotra highlights the risks of statin drugs, noting that more than half of statin users stop using the drugs within a year, most citing side effects as the reason.13
Fatigue, nausea, joint and muscle pain and increases in blood sugar have all been associated with statin drug use, most of which cease when the drugs are stopped. He also points out that unhealthy diet, including excess sugar consumption, is the true culprit in heart disease:
"Over 80 percent of CVD [cardiovascular disease] is attributable to environmental factors, notably unhealthy diet and also smoking, alcohol and physical inactivity. Diet has primacy, accounting for a larger burden of CVD disease and death than tobacco, alcohol and inactivity combined. For those at low risk eating an apple a day has an equivalent risk reduction for myocardial infarction [heart attack] as taking a statin."14,15
Statins Increase Diabetes Risk
Statins have been shown to increase your risk of diabetes via a number of different mechanisms. The most important one is that they increase insulin resistance, which can be extremely harmful to your health. Secondly, statins increase your diabetes risk by raising your blood sugar. Statins work by preventing your liver from making cholesterol.
As a result, your liver returns the sugar to your bloodstream, which raises your blood sugar levels. These drugs also rob your body of certain valuable nutrients, which can also impact your blood sugar levels. Two nutrients in particular, vitamin D and CoQ10, are both needed to maintain ideal blood glucose levels.
Importantly, statins deplete your body of CoQ10, vitamin K2, dolichol and selenium, thereby threatening your heart and overall health even further. Statins' ability to lower the risk of minor heart attacks may actually be related to their ability to lower C-reactive protein, far more so than the lowering of cholesterol.
Researchers with the Erasmus Medical Center in the Netherlands recently analyzed data from more than 9,500 patients. Those who had ever used statins had a 38 percent higher risk of Type 2 diabetes, with the risk being higher in those with impaired glucose homeostasis and those who were overweight or obese.16
The researchers concluded, "Individuals using statins may be at higher risk for hyperglycemia, insulin resistance and eventually Type 2 diabetes. Rigorous preventive strategies such as glucose control and weight reduction in patients when initiating statin therapy might help minimizing the risk of diabetes."
But a far better strategy may be preventing insulin resistance in the first place, by avoiding statin drugs and eating a healthy diet. According to Malhotra and a colleague:17
"In young adults, preventing insulin resistance could prevent 42 percent of myocardial infarctions, a larger reduction than correcting hypertension (36 percent), low high-density lipoprotein cholesterol (HDL-C) (31 percent), body mass index (BMI) (21 percent) or LDL-C (16 percent).18
It is plausible that the small benefits of statins in the prevention of CVD come from pleiotropic effects which are independent of LDL-lowering. The focus in primary prevention should therefore be on foods and food groups that have a proven benefit in reducing hard endpoints and mortality."
How to Identify and Lower Your Heart Disease Risk
Rather than focusing on cholesterol, two tests that are far more important for assessing your CVD risk are the serum ferritin and gamma-glutamyl transpeptidase (GGT) tests.
The GGT test can be used as a screening marker for excess free iron and is a great indicator of your sudden cardiac death risk. The recommended, ideal levels, of ferritin and GGT are as follows. For more information about these tests, read "Cholesterol Does Not Cause Heart Disease."
• Ferritin — Adult men and nonmenstruating women: 30 to 40 nanograms per milliliter (ng/mL) or 75 to 100 nanomoles per liter (nmol/L).
The most commonly used threshold for iron deficiency in clinical studies is 12 to 15 ng/mL (30 to 37 nmol/L). You do not want to be below 20 ng/mL (50 nmol/L) or above 80 ng/mL (200 nmol/L). High iron during pregnancy is also problematic; having a level of 60 or 70 ng/mL (150 or 175 nmol/L) is associated with greater odds of poor pregnancy outcomes.
• GGT — Below 16 units per liter (U/L) for men and below 9 U/L for women. Above 25 U/L for men and 18 U/L for women, your risk of chronic disease increases significantly.
In order to protect yourself against heart disease, here are a number of suggestions that can help you lower your insulin resistance and restore your insulin sensitivity, among other heart-protective mechanisms:
Avoid environmental pollutants and toxins, including smoking, vaping, heavy metals, herbicides and pesticides, especially glyphosate.
Minimize your exposure to electromagnetic fields and wireless radiation from cellphones, Wi-Fi, routers, smart meters and more, as this kind of radiation has been shown to cause serious free radical damage and mitochondrial dysfunction.
Eat an unprocessed whole food-based diet low in net carbs and high in healthy fats. A ketogenic diet — which is very low in net carbohydrates and high in healthy fats — is key for boosting mitochondrial function.
When your body is able to burn fat for fuel, your liver creates water-soluble fats called ketones that burn far more efficiently than carbs, thereby creating fewer reactive oxygen species and secondary free radicals. Ketones also decrease inflammation and improve glucose metabolism.19
Eat nitrate-rich foods to help normalize your blood pressure. Good sources include arugula, cilantro, rhubarb, butter leaf lettuce, mesclun mixed greens, beet greens, fresh beet juice, kvass (fermented beet juice) and fermented beet powder.
Get plenty of nonexercise movement each day; walk more and incorporate higher intensity exercise as your health allows.
Intermittently fast. After you've become accustomed to intermittently fasting for 16 to 18 hours, you can try a stricter fast once or twice a week, when you eat a 300- to 800-calorie meal loaded with detox supporting nutrients, followed by a 24-hour fast. So, in essence, you're then only eating one 300- to 800-calorie meal in 42 hours.
If you have heart disease, consider enhanced external counterpulsation (EECP). To find a provider, see EECP.com.20
If you have heart disease, you may also consider taking g-strophanthin, an adrenal hormone that helps create more parasympathetic nervous system neurotransmitters, thereby supporting your parasympathetic nervous system. It also helps flush out lactic acid. Strophanthus is the name of the plant, the active ingredient of which is called g-strophanthin in Europe, and ouabain in the United States.
Get sensible sun exposure to optimize your vitamin D status and/or take an oral vitamin D3 supplement with magnesium and vitamin K2.
Implement heart-based wellness practices such as connecting with loved ones and practicing gratitude.
from
http://articles.mercola.com/sites/articles/archive/2019/03/19/why-are-statins-bad-for-you.aspx
source http://niapurenaturecom.weebly.com/blog/the-great-statin-debate-why-cholesterol-is-misunderstood
0 notes
Text
Dialysis Unit Profit Primarily From Small Percentage of Privately Insured Patients
MedicalResearch.com Interview with:

Dr. Childers
Chris Childers, MD, PhD
Division of General Surgery
David Geffen School of Medicine at UCLA
Los Angeles, CA 90095
MedicalResearch.com: What is the background for this study?
Response: Patients with end-stage renal disease – poorly functioning kidneys – often have to receive dialysis. This typically requires a patient to visit an outpatient clinic several times a week to have their blood filtered by a machine. Over the past few years, two for-profit companies have increased their control over the outpatient dialysis market – DaVita and Fresenius. Combined they control approximately ¾ of the market. A number of concerns have been raised against these for-profit companies suggesting that the quality of care they deliver may be worse than the care delivered at not-for-profit companies. But, because they control so much of the market and because patients have to receive dialysis so frequently, patients may not have much choice in the clinic they visit.
Medicare covers patients who are 65 years or older and also patients on dialysis regardless of age. Medicare pays a fixed rate for dialysis which they believe is adequate to cover the clinics' costs. However, if a patient also has private insurance, the insurer is required to pay for dialysis instead of Medicare. Whereas Medicare rates are fixed by the federal government, private insurers have to negotiate the price they pay, and may pay much more as a result.
MedicalResearch.com: What are the main findings?
Response: Our study found that only 10% of patients cared for in DaVita's clinics have private insurance but these patients account for one-third of the company’s overall revenue. We found that, on average, government payers (such as Medicare) paid an average of $248/treatment, but private insurers paid $1,041/treatment – over 4 times the amount. DaVita makes a large profit each year in large part because of capitalizing on these privately insured patients.
MedicalResearch.com: What should readers take away from your report?
Response: Previous research has shown that the reason healthcare is so expensive in the United States is because the prices we pay for services are much higher than other developed nations. We believe the dialysis market epitomizes this concept. Two patients with the same disease can pay prices that differ by a factor of 4 depending on their insurance. This undoubtedly has downstream effects on all Americans. If your insurer is required to pay $1000/treatment ($150,000/year) for some of its members, those costs will eventually trickle down in the form of higher premiums.
MedicalResearch.com: What recommendations do you have for future research as a result of this work?
Response: Future research should look at potential strategies for reducing the prices paid by private insurers for dialysis. The two broad approaches to this would include increasing competition or government intervention to cap prices. Efforts are needed to understand what strategies would be most effective, while ensuring patient access to care and safety.
No disclosures
Citation:
Childers CP, Dworsky JQ, Kominski G, Maggard-Gibbons M. A Comparison of Payments to a For-Profit Dialysis Firm From Government and Commercial Insurers. JAMA Intern Med. Published online May 13, 2019. doi:10.1001/jamainternmed.2019.0431
The information on MedicalResearch.com is provided for educational purposes only, and is in no way intended to diagnose, cure, or treat any medical or other condition. Always seek the advice of your physician or other qualified health and ask your doctor any questions you may have regarding a medical condition. In addition to all other limitations and disclaimers in this agreement, service provider and its third party providers disclaim any liability or loss in connection with the content provided on this website.
Read the full article
0 notes