#bohr model
Photo

Niels Bohr was born on October 7, 1885. A Danish physicist who made foundational contributions to understanding atomic structure and quantum theory, for which he received the Nobel Prize in Physics in 1922, Bohr was also a philosopher and a promoter of scientific research. He developed the Bohr model of the atom, in which he proposed that energy levels of electrons are discrete and that the electrons revolve in stable orbits around the atomic nucleus but can jump from one energy level (or orbit) to another. Although the Bohr model has been supplanted by other models, its underlying principles remain valid. He conceived the principle of complementarity: that items could be separately analysed in terms of contradictory properties, like behaving as a wave or a stream of particles.
#niels bohr#physics#quantum theory#atoms#bohr model#atomic structure#chemistry#science#nobel prize#nobel prize winners#science birthdays#science history#on this day#on this day in science history
2 notes
·
View notes
Text
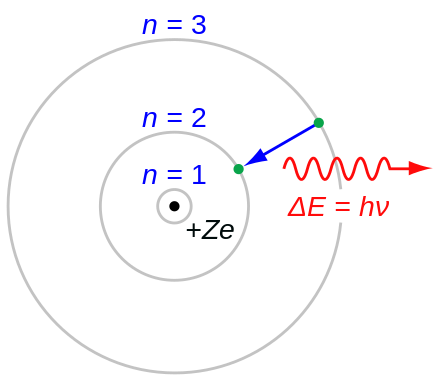
Happy birthday to Niels Bohr, whose breakthrough model of the atom is the one you see in your high school textbooks…and that model has been replaced several times over since then, whereas your textbooks have not
0 notes
Text
Heisenberg's uncertainty principle (idea 2/2 behind quantum mechanics)
So Heisenberg says that the more precisely/accurately we know the momentum of a particle, the less precisely/accurately we know the particle's position, and vice versa. This is because of particle-wave duality, so it's just the nature of things, it's not because of the accuracy limits of our measuring instruments. We know that light isn't just a wave because if light were just a wave, the photoelectric effect wouldn't happen. If light were just a wave, we don't have threshold frequencies because we'd just need to shine a light of any frequency on an electron long enough to excite it and eject it.
Heisenberg reasoned that light acts particle-like because light is a wave packet. In a wave packet, there are many waves of different de Broglie wavelengths. Sometimes they're in phase and sometimes they're out of phase. When they're in phase, you get constructive interference that forms a photon and when they're out of phase, you get destructive interference that cancels out the waves (observed as "there is space between the wave packets"). So what you get is a beam of photons like this:
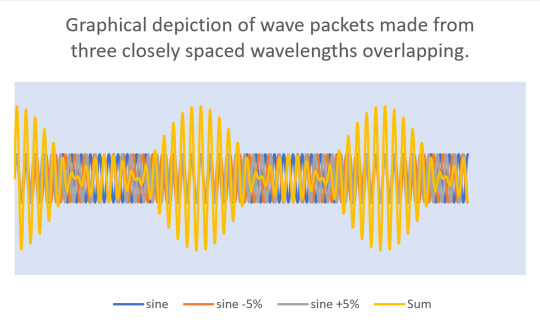
In other words, it's impossible to have a single frequency of light. If light behaves like particles, the only way that's possible is if no matter how good we are at isolating frequencies of light, what we end up with is a wave packet - a spread of frequency, an uncertainty in frequency.
Bohr and Rydberg were thinking of the electron in hydrogen like it emits or absorbs a single frequency of light as it moves between distinct energy levels, like the electron has a definite energy. But Heisenberg says that the photon must have a spread of frequencies to exist as a photon, and if there's a spread of frequencies of EMR emitted or absorbed by the electron, then the electron also must have a spread of energies, an uncertainty in energy in the atom because frequency is directly proportional to energy. This means that the energy in E = hv can't be known exactly.
Heisenberg's uncertainty is a consequence of the fact that to measure/observe stuff at the atomic level, we must disturb it (e.g. bounce light off of it to see it) and that disturbance is always significant at the atomic level. In contrast, such a disturbance would be insignificant for the most part for things at the macroscopic level. Thus, Bohr's model can't be correct because it says that electron momentum and therefore energy is always well defined. So we need a new model.
Mathematically, Heisenberg's uncertainty principle looks like this in one dimension (x), where h/4*pi is the lowest amount of uncertainty you can get:
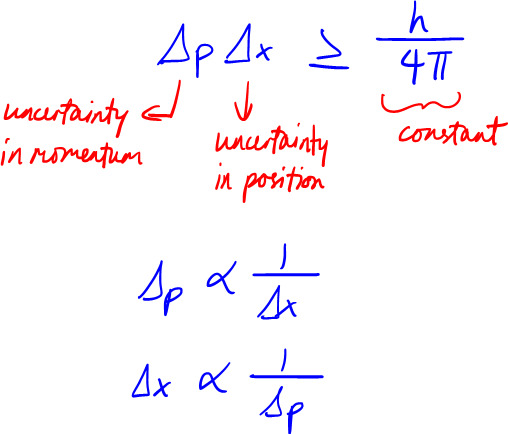
Basic derivation:

Useful uncertainty principle example here
0 notes
Text

#hydrogen#atom#electric#charge#bohrs#model#rydberg#cpnstant#physics#chemistry#solutions#planck constant
3 notes
·
View notes
Text
the plum pudding model is a fascinating name for a baby girl
#i'm omw to visit niels bohr's silly little office where he used to work 😍😍 and this model came to mind ^#i mean he didn't agree with me that it's a good name but whatever 🙄 ok solar system freak#🧷
4 notes
·
View notes
Text
wooo just finished my chem resource sheet and i’m feeling absolutely demolished
bohr models, molar mass, lewis dots + structures, balancing equations & more
2 notes
·
View notes
Text

You have an uncanny knack for NUCLEAR PHYSICS,
knowledge is knowing that the rutherford model on the shirt, while revolutionary in identifying that atoms have a positively charged nucleus, is hilariously outdated and niels bohr actually won the nobel prize for his model of the atom a few years after this model was made, and if jade knows nuclear physics she would know this
wisdom is knowing that having the rutherford model on a shirt is fun anyway
#mine's a black one with light blue models and says Physics and it's very ugly and i got it for free#zahro reads homestuck#fr the gold foil experiment is sooooooo. To Me. darling baby#plum pudding model WHO#knowledge is also knowing that the bohr model is also out of date because it doesnt take the heisenberg uncertainty principle into account
1 note
·
View note
Text
As a tank it’s easier to protect healers because they’re generally going to be behind me or nearby,
Dps can be just about anywhere though, they’re like quantum electron orbitals, I have an idea of where they might be based on the type but when observed they can be behind my shield, three streets back on top of a building, or knee deep in enemy lines doing their cirque de solei audition
And to be clear I’m not complaining, I just try to make a general good defense, keep tabs on the healers and help the dps when they wander by
#overwatch#overwatch 2#if you don’t know what a quantum orbital is you can look up the difference between the Bohr and quantum models of the atom#but also you really don’t have to do that#tanking#sigma main#I hope this post finds people who understand this#maevith
2 notes
·
View notes
Text

Bohr such a dedicated apprentice he literally postponed his honeymoon to save Rutherford's atomic model and suggested he and his wife write a thesis together on how to fix his model so it wouldn't be discarded.
0 notes
Text
science side of tumblr who do you think would win in a fight bohr or brahe and justify your answer
#i think theyre equally matched in quirkiness#tychonic sustem has the same vibes as bohrs hydrogen model
0 notes
Text
Carbon bohr model

The number of electrons that can be accommodated in a shell is given by the formula: Now, let us find the position of these electrons inside the atom. This means that 14 electrons are revolving around the nucleus of the Silicon atom. Therefore, in the Silicon atom, number of electrons = Atomic Number = 14 Now, let us try to find the number of electrons in the Silicon atom, Here, p + represents proton and n° represents neutron. Therefore, Number of neutrons in Silicon = 28 – 14 = 14Īs the neutrons and protons are located in the nucleus of an atom, the nucleus of Silicon can now be drawn as follows: Number of neutrons = Atomic mass (rounding it up to the nearest whole number) –Īs mentioned in the previous section, the atomic number of Silicon is 28.086, rounding it up to the nearest whole number gives 28. Now, finding the number of neutrons in the Silicon atom, Therefore, number of protons in Silicon = Atomic Number of Silicon = 14 In the case of Silicon, the atomic number = 14 The atomic number of an atom is always equal to the number of protons that an atom contains. To draw the Bohr model of silicon we shall first identify the number of atomic species present in this atom.įirst, let us find out the number of protons in the Silicon atom. Silicon is represented by the symbol Si.The electronic configuration of Silicon is 1s 22s 22p 63s 23p 2.The facts that can be derived from it are: The above-shown Silicon box represents its various properties. Silicon belongs to the carbon family and is located in the 14 th group of the Periodic table. The Silicon atom has 14 protons and 14 neutrons, and 14 electrons revolve around its nucleus in three shells viz. The farthest shell or outermost energy level of an atom is called the valence shell and the electrons that are located in this shell are known as valence electrons. When an electron gets excited it jumps from the lower energy level to the higher energy level. The shell closest to the nucleus is said to have the least energy, also known as the ground state while the one located farthest from the nucleus has the maximum amount of energy associated with it. The shells are also known as energy levels. with the lowest value assigned to the shell located closest to the nucleus. Multiple numbers of shells are present in an atom depending upon the number of electrons it contains.Īccording to the Bohr model of the atom, the shells are named as K, L, M, N, etc., or 1, 2, 3, 4, etc. Shells: These are the circular paths surrounding the atoms in which the electrons are said to revolve around the nucleus.As per the Bohr-Rutherford model, the electrons revolve around the nucleus in fixed orbits or shells. Electrons: These are the negatively charged subatomic particles that surround the nucleus of the atom.Neutrons: These are the neutral subatomic particles that are present inside the nucleus.Proton: This name was given by Ernst Rutherford to the positively charged species existing inside the nucleus.It comprises positively charged protons and neutral neutrons. Nucleus: It is the heart of an atom, located right at its center.To understand the atomic structure better we first need to comprehend the different parts and particles that constitute an atom. The different atomic particles such as electrons, protons, and neutrons are shown at their predicted positions. This model is used to illustrate the atomic composition in pictorial form. The Bohr model is also known as the Bohr-Rutherford model as it was developed as a modification of the Rutherford model.

0 notes
Text
Long considered myth, freakishly large rogue waves are very real and can split apart ships and even damage oil rigs. Using 700 years' worth of wave data from more than a billion waves, scientists at the University of Copenhagen and University of Victoria have used artificial intelligence to find a formula for how to predict the occurrence of these maritime monsters. The new knowledge can make shipping safer.
Stories about monster waves, called rogue waves, have been the lore of sailors for centuries. But when a 26-meter-high rogue wave slammed into the Norwegian oil platform Draupner in 1995, digital instruments were there to capture and measure the North Sea monster. It was the first time that a rogue had been measured and provided scientific evidence that abnormal ocean waves really do exist.
Since then, these extreme waves have been the subject of much study. And now, researchers from the University of Copenhagen's Niels Bohr Institute have used AI methods to discover a mathematical model that provides a recipe for how—and not least when—rogue waves can occur.
With the help of enormous amounts of big data about ocean movements, researchers can predict the likelihood of being struck by a monster wave at sea at any given time.
Continue Reading.
94 notes
·
View notes
Text

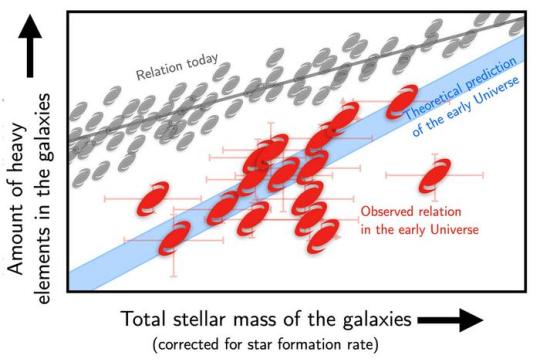
Astronomers discover newborn galaxies with the James Webb Space Telescope
With the launch of the James Webb Space Telescope, astronomers are now able to peer so far back in time that we are approaching the epoch where we think that the first galaxies were created. Throughout most of the history of the Universe, galaxies seemingly tend to follow a tight relation between how many stars they have formed, and how many heavy elements they have formed. But for the first time we now see signs that this relation between the amount of stars and elements does not hold for the earliest galaxies. The reason is likely that these galaxies simply are in the process of being created, and have not yet had the time to create the heavy elements.
The Universe is teeming with galaxies — immense collections of stars and gas — and as we peer deep into the cosmos, we see them near and far. Because the light has spent more time reaching us, the farther away a galaxy is, we are essentially looking back through time, allowing us to construct a visual narrative of their evolution throughout the history of the Universe.
Observations have shown us that galaxies through the last 12 billion years — that is, 5/6 of the age of the Universe — have been living their life in a form of equilibrium: There appears to be a fundamental, tight relation between on one hand how many stars they have formed, and on the other hand how many heavy elements they have formed. In this context, “heavy elements”, means everything heavier than hydrogen and helium.
This relation makes sense, because the Universe consisted originally only of these two lightest elements. All heavier elements, such as carbon, oxygen, and iron, was created later by the stars.
James Webb peers deeper
The very first galaxies should therefore be “unpolluted” by heavy elements. But until recently we haven’t been able to look so far back in time. In addition to being far away, the reason is that the longer light travels through space, the redder it becomes. For the most distant galaxies you have to look all the way into the infrared part of the spectrum, and only with the launch of James Webb did we have a telescope big and sensitive enough to see so far.
And the space telescope did not disappoint: Several has James Webb broken its own record for the most distant galaxy, and now it finally seems that we are reaching the epoch where some of the very first galaxies were created.
In a new study, published today in the scientific journal Nature Astronomy, af team of astronomers from the Danish research center Cosmic Dawn Center at the Niels Bohr Institute and DTU Space in Copenhagen, has discovered what seems indeed to be some of the very first galaxies which are still in the process of being formed.
“Until recently it has been near-impossible to study how the first galaxies are formed in the early Universe, since we simply haven’t had the adequate instrumentation. This has now changed completely with the launch of James Webb,” says Kasper Elm Heintz, leader of the study and assistant professor at the Cosmic Dawn Center.
Fundamental relation breaks down
The relationship between the total stellar mass of the galaxy and the amount of heavy elements is a bit more complex than that. How fast the galaxy produces new stars also has something to say. But if you correct for that, you get a beautiful, linear relationship: The more massive the galaxy, the more heavy elements.
But this relation is now being challenged by the latest observations.
“When we analyzed the light from 16 of these first galaxies, we saw that they had significantly less heavy elements, compared to what you’d expect from their stellar masses and the amount of new stars they produced,” says Kasper Elm Heintz.
In fact the galaxies turned out to have, on average, four times less amounts of heavy elements that in the later Universe. These results are in stark contrast to the current model where galaxies evolve in a form of equilibrium throughout most of the history of the Universe.
Predicted by theories
The result is not entirely surprising though. Theoretical models of galaxy formation, based on detailed computer programs, do predict something similar. But now we’ve seen it!
The explanation, as proposed by the autors in the article, is simply that we are witnessing galaxies in the process of being created. Gravity has gathered the first clumps of gas, which have begun to form stars.
If the galaxies then lived their lives undisturbed, the stars would quickly enrich them with heavy elements. But in between the galaxies at that time were large amounts of fresh, unpolluted gas, streaming down to the galaxies faster than the stars can keep up.
“The result gives us the first insight into the earliest stages of galaxy formation which appear to be more intimately connected with the gas in between the galaxies than we thought.
This is one of the first James Webb observations on this topic, so we’re still waiting to see what the larger, more comprehensive observations that are currently being carried out can tell us.
There is no doubt that we will shortly have a much clearer understanding of how galaxies and the first structures began their formation during the first billion years after the Big Bang,” Kasper Elm Heintz concludes.
The study is published in Nature Astronomy.
TOP IMAGE....The big galaxy in the foreground is named LEDA 2046648, and is seen just over a billion years back in time, while most of the others lie even farther away, and hence are seen even further back in time. CREDIT ESA/Webb, NASA & CSA, A. Martel.
CENTRE IMAGE....This plot shows the observed galaxies in an “element-stellar mass diagram”: The farther to the right a galaxy is, the more massive it is, and the farther up, the more heavy elements it contains. The gray icons represent galaxies in the present-day Universe, while the red show the new observations of early galaxies. These ones clearly have much less heavy elements than later galaxies, but agree roughly with theoretical predictions, indicated by the blue band. Credit: Kasper Elm Heintz, Peter Laursen.
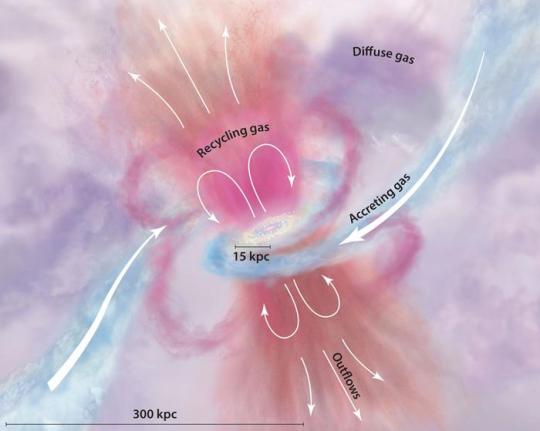
LOWER IMAGE....Diffuse gas from intergalactic space plummets toward the center, sparking star formation and becoming part of the galaxy’s rotating disk. When stars die, they return their gas to the galaxy (and the intergalactic space), now enriched with heavy elements. Credit: Tumlinson et al. (2017).
45 notes
·
View notes
Text

1 note
·
View note
Text
This book I got to scan for otherkin talks about quantum physics-based magic. I literally cannot escape quantum physics-based magic systems. Can Guilty Gear just like give me a moment, ok, for getting me consistently into this mess. I need to throttle Dr. Paradigm. My brain is getting so wrinkled from having to learn about quantum theory and particle theory and Planck's Constant and Heisenberg's Principle and Bohr models... At least it's in English this time. I GUESS.
#textpost#I started doing the damn Khan Academy course on all this nonsense because I just know I'll have to deal with it more later#Might as know what the hell I'm reading about this time. Save me hours of researching I could spend PUMMELING PARADIGM#I don't really understand it as much as I wish I could but if I can at least ID the terms and the basic stuff then that's fine#If I know what I'm looking at then that's enough to bullshit my way through a project#Translating Japanese requires a lot of brain power but the only time I've ever had to really strain my neurons was#with Paradigms story from the Overture Material Collection#Reading about pseudoscience magic in a second language polar opposite your native one is like. Oh god why#I actually got headaches trying to figure that one out. It has minor errors. Some day I'll fix them
25 notes
·
View notes
Text
Christopher Nolan’s highly-anticipated movie “Oppenheimer,” set for release July 21, 2023, depicts J. Robert Oppenheimer and his role in the development of the atomic bomb. But while the Manhattan Project wouldn’t have been possible without the work of many accomplished female scientists, the only women seen in the movie’s trailer are either hanging laundry, crying or cheering the men on.The only women featured in the official trailer for Christopher Nolan’s ‘Oppenheimer’ are crying, hanging laundry or supporting the men.
As a physics professor who studies ways to support women in STEM – science, technology, engineering and math – fields and a film studies professor who worked as a screenwriter in Hollywood, we believe the trailer’s depiction of women reinforces stereotypes about who can succeed in science. It also represents a larger trend of women’s contributions in science going unrecognized in modern media.
Lise Meitner: A pioneering role model in physics
The Manhattan Project would not have been possible without the work of physicist Lise Meitner, who discovered nuclear fission. Meitner used Einstein’s E=MC² to calculate how much energy would be released by splitting uranium atoms, and it was that development that would prompt Einstein to sign a letter urging President Franklin Roosevelt to begin the United States’ atomic research program.
Einstein called Meitner the “Madame Curie of Germany” and was one of a pantheon of physicists, from Max Planck to Niels Bohr, who nominated Meitner for a Nobel Prize 48 times during her lifetime.
Meitner never won. Instead, the prize for fission went to Otto Hahn, her male lab partner of 30 years in Berlin. Hahn received the news of his nomination under house arrest in England, where he and other German scientists were being held to determine how far the Third Reich had advanced with its atomic program.
Of Jewish descent, Meitner had been forced to flee the Nazis in 1938 and refused to use this scientific discovery to develop a bomb. Rather, she spent the rest of her life working to promote nuclear disarmament and advocating for the responsible use of nuclear energy.
Meitner was not the only woman who made a significant contribution during this time. But the lack of physics role models like Meitner in popular media leads to real-life consequences. Meitner doesn’t appear as a character in the film, as she was not part of the Manhattan Project, but we hope the script alludes to her groundbreaking work.
A lack of representation
Only around 20% of the undergraduate majors and Ph.D. students in physics are women. The societal stereotypes and biases, expectation of brilliance, lack of role models and chilly culture of physics discourage many talented students from historically marginalized backgrounds, like women, from pursuing physics and related disciplines.
Societal stereotypes and biases influence students even before they enter the classroom. One common stereotype is the idea that genius and brilliance are important factors to succeed in physics. However, genius is often associated with boys, and girls from a young age tend to shy away from fields associated with innate brilliance.
Studies have found that by the age of 6, girls are less likely than boys to believe they are “really, really smart.” As these students get older, often the norms in science classes and curricula tend not to represent the interests and values of girls. All of these stereotypes and factors can influence women’s perception of their ability to do physics.
Research shows that at the end of a yearlong college physics course sequence, women with an “A” have the same physics self-efficacy as men with a “C”. A person’s physics self-efficacy is their belief about how good they are at solving physics problems – and one’s self-efficacy can shape their career trajectory.
Women drop out of college science and engineering majors with significantly higher grade-point averages than men who drop out. In some cases, women who drop out have the same GPA as men who complete those majors. Compared to men, women in physics courses feel significantly less recognized for their accomplishments. Recognition from others as a person who can excel in physics is the strongest predictor of a student’s physics identity, or whether they see themselves as someone who can excel in physics.
More frequent media recognition of female scientists, such as Meitner, could vicariously influence young women, who may see them as role models. This recognition alone can boost young women’s physics self-efficacy and identity.
When Meitner started her career at the beginning of the 20th century, male physicists made excuses about why women had no place in a lab – their long hair might catch fire on Bunsen burners, for instance. We like to believe we have made progress in the past century, but the underrepresentation of women in physics is still concerning.
Diversity as an asset to science
If diverse groups of scientists are involved in brainstorming challenging problems, not only can they devise better, future-oriented solutions, but those solutions will also benefit a wider range of people.
Individuals’ lived experiences affect their perspectives – for example, over two centuries ago, mathematician Ada Lovelace imagined applications far beyond what the original inventors of the computer intended. Similarly, women today are more likely to focus on applications of quantum computers that will benefit their communities. Additionally, physicists from Global South countries are more likely to develop improved stoves, solar cells, water purification systems or solar-powered lamps. The perspectives that diverse groups bring to science problems can lead to new innovations.
Our intention is not to disparage the “Oppenheimer” movie, but to point out that by not centering media attention on diverse voices – including those of women in physics like Meitner – filmmakers perpetuate the status quo and stereotypes about who belongs in physics. Additionally, young women continue to be deprived of exposure to role models who could inspire their academic and professional journeys'
74 notes
·
View notes