#EU MDR
Text
Harnessing ISO Standards and EU MDR with Regulatory and Market Intelligence
Regulatory and Market Intelligence acts as a compass, guiding businesses through the complex maze of compliance requirements and shifting market trends. It involves the systematic gathering, analysis, and interpretation of regulatory information, market research, and competitor insights. By leveraging RMI, organizations gain a comprehensive understanding of ISO Standards, the EU MDR, and the industry landscape, thereby mitigating risks and capitalizing on emerging opportunities.
#MHRA Registration#US FDA Registration#UKCA Marking#EUDAMED Registration#UKRP#European Authorized Representative#FSC#Clinical Evaluation Report#UK Responsible Person#EAR#Free Sales Certificate#Regulatory and Market Intelligence#ISO Standards#EU MDR
0 notes
Text
#Medical Devices#IVDs#EU MDR#Regulatory Affairs#PMCF Plan#PMS system#Clinical Evaluation Report#EU Regulations#European Union#PMS#EU#EU MDR 2017/745
0 notes
Text
pour celleux qui cochent l'avant-dernière case, hésitez pas à raconter quelles étaient les choses à collectionner à la mode dans votre école 👀
#ce poll sortant de nulle part a été motivé par la série animée samuel sur arte mdr#j'ai eu un gros flashback pendant l'épisode 6#polls#blabla#je me rappelle qu'en dehors des feuilles diddl y a eu un gros retour des billes quand j'étais encore en primaire#aussi d'ailleurs si vous connaissez pas samuel get on it#c'est sur la chaîne courts toujours sur youtube#c'est trop bien
11 notes
·
View notes
Text
LE "I THOUGHT THAT WAS NON-DIEGETIC"... MOANED. si sexy de ta part le doc omg je meurs
#oui j'ai dis que j'allais pas parler + que ça de dw mais.#c'est genre pas un spoil#genre on m'a tag mon post sur le fait que ruby ÉVOQUE startrek en tant que spoil.#quand littéralement hier à 3h du mat j'ai vu quelqu'un parler D'UN THEME D'UN PERSO GENRE#c'est d'un autre calibre non jsp???? quelqu'un qui spoil juste l'annonce du retour d'un gros perso vs. qqn qui dit#“olalala comme star trek mdr”#mais bref#vous êtes pareil que les sunday girlies de succession je retiens je retiens#comparé à la fandom de saw qui genre pendant tout le mois où on devait attendre en france qu'il passe au ciné#j'ai pas eu un seul spoil. genre dont le caméo à la fin. je savais qu'il allait être là parce que le cast listing mais même après un mois de#la sortie de saw x le temps qu'il passe en france j'l'avais toujours pas su. ALLO. c'est vraiment selon les fandom le respect de pas spoi#tout le monde dès la minute de la sortie c'est fou ça#remember les gonz qui postait des screenshots de succession PENDANT QUE L'EPSIODE ETAIT EN TRAIN D'AIR genre allo#même pas 20min into l'ep qui sort je me faisais spoil des bails ça rendait fou#même si j'aurai bloqué le tag ça servait à rien la moitié taggais rien#BREF#ON S'EN FOUT#juste content que je follow pas vraiment de personne into dw ou du moins qui poste pas grand chose en rapport
2 notes
·
View notes
Text
soit il est bête soit je suis douée dans l'art du paraître et de la dissimulation soit il me prend pour une conne quand il me dit en substance "j'aime bien quand tu fais la jalouse" suivi de "c'est drôle tu fais genre tu l'es mais tu ne sais pas le faire" alors que si complètement et c'est pas marrant, je le suis ???
#ensuite il m'a dit qu'il voudrait que je le sois mais mdr ça va pas monsieur#je re rentre dans une phase où il me prend la tête#l'ami platonique redevient l'ami casse-tête#tout à l'heure j'ai eu une intuition: ça pue la merde#en même temps tout m'énerve aujourd'hui#du coup j'ai décidé de l'énerver aussi vu que y a que ça qui marche avec lui!!!#comportement d'adulte responsable 😎
11 notes
·
View notes
Text

Toujours dans la lancée de mes anciens élèves, y a quelques mois j'ai découvert aussi que l'une d'elles était devenu maman à 15 ans
#choquée#au début je pensais que c'était sa petite soeur mdr#mais non non après longue investigation c'est bien son bébé#bon après chacun fait ce qu'il veut hein#elle a l'air toute heureuse alors c'est le principal#mais c'est ouf quand même#après ça va être méchant et plein de préjugés ce que je vais dire#mais ça ne m'étonne qu'à moitié#je l'aimais pas trop cette élève#elle en avait rien à battre du français#et au début de l'année y a eu un cours où elle a passé l'heure à se foutre de ma gueule avec sa copine#à la récré jme suis mise à pleurer en salle des profs à cause d'elle#ça m'a rappelé des vieux souvenirs pourris du collège#cette période de merde#mais bon j'en ai parlé avec les profs et la CPE avec qui j'étais très proche#et j'ai été hyper soutenue#elle a convoqué les deux filles#et après j'ai plus jamais eu de problèmes#bref voilà pour la petite histoire#et cette fille est maintenant maman
11 notes
·
View notes
Note
est-ce que je devrais me mater vdf (la série) ? genre est-ce que ça vaut le coup ?
O U I, je dirais même plus : il faut. rend-toi immédiatement sur youtube. (sans te commander <3)
en plus la s1 va très très vite à voir, au départ c'est des petits épisodes de 5 minutes bricolés vite fait et qui avaient pas d'histoire derrière, et au fil du temps ça avance vers un énorme plot c'est assez fou. je te dirais de te faire une idée sur la saison 1, et de voir si tu veux continuer ? (sachant que les personnages sont encore mieux dans la s2, notamment JUDITH my love ♥)
#je me sens presque + vdf bitch que kaamelott bitch c'est dire#je dig trop le fait que l'univers s'est enrichi à un point assez dingue mdr le point culminant en un film c'est vraiment tout ce#qu'ils méritent snirf#en plus y'a de la représentation et c'est DROLE af#french culture at its peak à mes yeux#c'est vraiment un bijou d'écriture en plus#on dirait trop pas au début parce que c'est vraiment fabriqué avec 3 bouts de bois ksjksjksj#mais après ils ont eu ankama derrière alors ils avaient + de moyen notamment en saison 4#asks#vdf#julz
13 notes
·
View notes
Note
C'est fou comment les edelstans ont le flair pour commenter avec le même discours chaque post qui ne lui lèche pas les bottes.
C'est le Dedel-flair, dès que Dedel est "menacée" par n'importe quel quidam sur internet qui ose citer le canon et dire que Faerghus/Rhea c'est pas caca, ils interviennent pour restaurer l'ordre dans la galaxie.
Plus sérieusement, d'autres en ont déjà parlé et plus développé que moi, mais je souscris à leur analyse, personne ne déteste canon!Dedel autant que les Dedelstans.
Mais bon, c'est la règle du fandom, si t'aimes pas le canon, tu ponds tes headcanons - soit tu grinces des dents quand on mentionne le canon et tu passes ton chemin, soit tu fais chier tout le monde pour rappeler que ton headcanon est le meilleur de tous, mais en contrepartie tu passes pour un guignol.
#anon#replies#french post#j'ai quand même eu raquie en anon qui avait les hémorroides après le post sur Rhea et ses cookies#'gna gna gna c'est faux c'est pas la vrai Rhea Rhea Caca et elle est toujours en mode vénère Pat c'est mon dieu etc etc'#c'est just un reblog d'un fanart d'une personne qui a dessiné le perso faisant des cookies mdr#j'en suis à me demander si certains blogs n'ont pas été visés parce qu'ils disaient trop de bien des gens que Dedel n'aime pas#ou développaient des headcanon sur autre chose que la vie fantastique de dedel et ses amies#FE16
5 notes
·
View notes
Text
Regulatory and Market Intelligence
Regulatory and Market Intelligence refers to the process of gathering, analyzing, and utilizing information related to government regulations and market trends. It involves monitoring changes in laws, policies, and industry dynamics to help businesses make informed decisions, stay compliant with regulations, and seize opportunities in the marketplace.
#Regulatory and Market Intelligence#EUDAMED Registration#Post-Market Surveillance#ISO Standards#Clinical Evaluation Report#Technical Files#EC Representative#EU MDR#US FDA 510K#FDA Label#EC Rep#EU CE Marking#Cosmetics Registration
0 notes
Text
#Post-market clinical investigation#Medical Devices#EU MDR#Clinical Investigations#GCP#EAR#PMCF#Regulatory Affairs#EU MDR 2017/745
0 notes
Text
b. qui a traduit du CÉLINE DION en LATIN pour le film Bis Repetita (parce que la scénariste est sa pote) ça me turbofume de rire genre c’est vraiment à ça qu’on passe ses soirées quand on a une vie sociale et l’agreg ?
#mdr c’était une séance privée pour les prépas y’a eu un tollé d’applaudissements et de cris quand y’a eu son nom au générique#ils ont pas le droit de sortir la chanson séparément parce qu’ils ont pas les droits mais on va exiger les paroles#prépa talk
0 notes
Text
PMCF Medical Device
Cetas Healthcare is one of the top global Syndicated Research with an exclusive focus in the medical device industry. We offer PMCF Surveys, syndicated reports, PMCF Medical Device, Syndicated Market Research, clinical data & Custom Market Research solutions, and more to our clients. With extensive practice in the Cardiovascular space, we also have prior experience in several therapy areas. We work regularly with top global medical device companies like GE Healthcare, Boston Scientific, Medtronic, Baxter, B Braun, BD, etc., helping them with their product development & management needs. Our offices are located in Singapore, Netherlands, USA, and India.
#pmcf#pmcf surveys#pmcf services#pmcf solutions#PMCF Medical Device#PMCF#PMCF studies#pmcf report#post market clinical follow up#eu medical device regulation#mdr medical device
0 notes
Text

Nu10 specializes in developing eu mdr software as a medical device solutions that meet the highest standards for medical devices. Our state-of-the-art software products are meticulously designed to adhere to the rigorous regulatory requirements of the European market. With Nu10's expertise in software development and regulatory compliance, we ensure that medical device manufacturers can confidently bring their innovative products to market, contributing to improved patient outcomes and healthcare advancements.
0 notes
Text
Selection of a Notified Body and Their Process for your new device authority?
#notified body#notified body for medical devices#list of notified bodies#ce notified body#eu notified body#mdr notified body list#list of notified bodies for medical devices
0 notes
Text

EU MDR Medical Device Labeling Requirements
The European Union (EU) Medical Device Regulation (MDR), implemented on May 26, 2021, mandates specific requirements regarding the labeling of medical devices. Medical device manufacturers must comply with these new regulations or risk facing fines, sanctions, or lost access to the EU market.
Labeling Requirements
The manufacturer of medical devices (MD) is responsible for accurate and complete labeling of everything they export. Every MD should have a label indicating the product description and safety and performance details relevant to the end-user.
The label should appear on the product itself or the packaging. Crucial details include the following:
Product name
Serial number
Product identification, contents of packaging, purpose
Manufacturer’s details
Contact address and details of authorized representative (for manufacturers based outside of the EU)
Instructions
Warnings or precautions
Importers are responsible for ensuring that the medical device is labeled in accordance with MDR Article 13 and accompanied by the required instructions for use. The importer’s EUDAMED SRN (Single Registration Number) must also be clearly indicated on the label. This identifies them as an economic operator in the EU market.
Language
There are 24 languages in the EU, all of which are considered official in their specific locations. Information should be available in the user’s preferred language.
Sterility
This symbol usually appears on medical devices that are sterilized. Most IVDs are not sterilized and would not need this symbol. Many of the medical device importer must look after about the sterilized labelling for the device they import.
Placement
MDR Annex I mandates that the label appears on the product’s surface or the packaging of each unit or multiple units. It should also appear on the manufacturer’s website and be updated. Further, the information shall be provided on the device itself and must be indelible and clearly legible. If it’s not practicable or appropriate, some or all details may appear on the packaging for each unit and/or on the packaging of multiple devices, as in the case of contact lenses or implants.
Symbols
Symbols are crucial for labels based on the MDR and In-Vitro Devices Regulations (IVDR). Here are some of the important symbols that appear on the label:
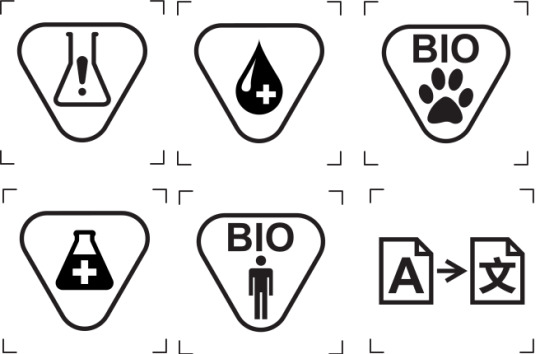
Some medical devices require symbols that indicate the presence of certain substances. There are separate symbols for devices that contain human blood and plasma derivatives and products that contain a medicinal substance.
There are also different symbols for devices that contain biological products from animals and humans.
Hazardous Substance
Medical devices require a symbol for hazardous substances. IVDs don’t need to use the symbol.
eLabeling
The EU mandated the use of electronic instructions for use (eIFUs) in March 2013, which is a more environmentally-friendly practice.
All the eLabels must follow the requirements under the MDR. According to EU regulations, eIFU can only be used in the following devices:
Devices and accessories used exclusively by professionals
Software
Devices with integrated screen display
Fixed installed medical device
Implantable devices
Further, medical devices classified as Class I and Class IIa or products with low to medium risks do not require instructions.
Importance of Labels on Medical Devices
Medical devices are crucial to healthcare; hence, they should be appropriately handled and labeled accordingly. Important details about the product should be on the label, as well as clear instructions for use.
More Information? GrowthImports

We provide non-EU medical and in-vitro device manufacturers with independent European-wide importing services compliant with Medical Device Regulation (MDR) and In-Vitro Diagnostic Regulation (IVDR) requirements.
Import your medical goods Into Europe with our hassle-free market access importing service while maintaining flexibility, compliance and increased quality standards. With over 30 years of combined experience, GrowthImports is dedicated to ensure the facilitation of a compliant and smooth international MDR/IVDR compliant importing process in the European market.
0 notes