Text
Is Your Product a Medical Device? Know The Difference!
This is an exciting time to be a part of the healthcare innovation sector. Its advancement, in today’s time, is more than ever before. Many medical devices and consumer healthcare products are making their way into the market.
In contrast to the typical consumer healthcare products, like earbuds, nutrition bars, and oral hygiene products; several sophisticated and high-tech consumer products (like Fitbit) are now being introduced.
Due to their advanced features and offerings, some consumer products might inadvertently fall under the category of a medical device, which is regulated by different regulatory bodies in different countries, and by the FDA in the US.
Typically, any product that treats, diagnoses, cures, mitigates, prevents disease, or affects the body’s structure or function can be classified as a medical device. T
he FDA gives a detailed definition for a medical device which can be referred to here- Definition.
More often than not, there is an explicit distinction between a consumer product and a medical device, however, in some cases, the distinction is implicit.
In the long run, it is always better to have a clear idea about your product type.
Let’s take a few examples here to understand the difference:
A pill dispenser, which dispenses pills to a patient, could be mistaken as a consumer healthcare product, but it is a medical device because it releases medication at specified times and intends to improve patient medication compliance
A fitness tracker that simply tracks physical activity level and informs about the calories burned, steps taken, etc, is not a medical device as long as claims that the device is intended to manage a patient’s condition, treat a disease, or provide information that enables the user in making health-related decisions, are not made
A software accessory that wirelessly collects, records, and transmits biometric data (including blood pressure, weight, activity level, and pulse oximeter readings) from a variety of home-monitoring devices (including fitness bands) to healthcare professionals for analysis, is a medical device, as the data will assist in self-management of health condition, or for determining the need of medical intervention
If you are developing a healthcare product and are not sure if it is a medical device or a consumer product, here is the complete guide to determine whether your product is a medical device or not.
Another way of determining if your product comes under the consumer product or medical device category is to understand the risks posed by the product, implicit and explicit claims made for the product, and target user, use cases, and settings.
If you are still unsure about how to classify your device, then we are here to assist you.
At Elexes we do a regulatory application so that you can hear directly from the FDA, whether or not your device is a medical device.
If your product is determined to be a consumer product, it can be sold to consumers right away. But, if it is determined to be a medical device, you would be required to go through an extensive regulatory process.
Making this determination sooner rather than later will save you a lot of time, and money, and not to mention, a headache, in the long run. So pause and ask yourself, “Is my product a medical device?" If you don't have the answer to this, you can simply contact us at [email protected]
0 notes
Text
Elexes has a strong history of helping dietary supplements and nutritional labels for foods in crafting supplement fact sheets. Not just this, At Elexes, we have also helped several cosmetic brands to get regulatory approvals.
Here, we are going to discuss one such case.
Brief:
A few months ago, two companies reached out to us. One was a cosmetic company and the other was a food product company.
Requirement of cosmetic company:
The cosmetic company client got a warning from the FDA about making drug-related claims, so they wanted to make all their product labels FDA, TGA, and MHRA compliant.
Outcome
For the cosmetic company:
We reviewed all the labels, manuals, content on the website, and their advertising as well as promotional material to ensure that they are complying with applicable regulations.
We retracted and reworded many claims which implied that the cosmetic product was affecting the structure or function of the body, or that it had a therapeutic or pharmacological action.
Some redacted or restructured claims were:
“The XY anti-wrinkle cream rejuvenates your skin and makes you look 20 years younger”
“The hair shampoo makes your hair stronger”
“The mouthwash reduces plaque or tartar”.
These were replaced by acceptable cosmetic claims such as -
“The XY cream moisturizes skin, covers up age spots, and makes you look younger”
“The hair shampoo adds body to hair and makes them fuller and thicker”,
“The mouth fresh reduces bad breath or mouth odor”
Requirement of food company:
The food company approached us to create several FDA-compliant fact sheets.
Outcome:
We made 20 supplement fact sheets for the food company. The only information that we needed from the client was their supplement material specification and formulation sheet.
For creating the supplement fact sheets, we took into account the FDA’s daily recommended values, leveraged our experience with similar data, and decided what special regulations need to be taken into account for pregnant women and children.
Our turnaround time for all the 20 fact sheets was 5 days.
Elexes takes great pride and pleasure in helping food and cosmetics companies to prevent FDA or global regulatory warning letters or notices by providing proactive solutions for complying with regulations.
Wondering what all services we offer for Food and Cosmetic industry?
You can check our -
Food Services & Cosmetic Services Page to know more.
If you have any query contact us at [email protected]
Food and Cosmetics
1 note
·
View note
Text
Patient Preference Information of FDA (Streamlining Patient Feedbacks)

The Patient Preference Information (PPI) initiative, established as part of the FDA Reauthorization Act of 2017, emphasizes the importance of patient input in healthcare decision-making, particularly regarding medical device development and evaluation. This initiative aims to centralize patient feedback to enhance understanding of diseases and improve device design and usage.
The FDA is currently soliciting public input on areas of patient preference that could impact various stages of medical device development and evaluation. PPI can be conducted by various stakeholders including patient groups, industry members, researchers, and healthcare providers. It helps in evaluating a device's benefit-risk profile and is essential for patient-centered product development.
Submission of PPI can be done electronically via the Federal eRulemaking Portal or through paper submission to the FDA. Submitters have the option to keep their information confidential. PPI feedback is sought on parameters such as conducting clinical studies, post-market follow-up, and enhancing device usability.
PPI is seen as a valuable dataset for future development stages and requires active regulatory and quality involvement to ensure continuous improvement in device technologies based on patient preferences.
Read the complete story here:
https://www.elexes.com/patient-preference-information-submission-ppi/
#fda#regulatory#cemarking#iso13485#medical device#healthcare#qualityassurance#clinicaltrials#pma#denovo#510k#patient#technologies#patientpreferance ppi medicaldevices
0 notes
Text
Quality Objectives | How to write a good quality objective?

Quality Objectives (QO) are goals set by top management to monitor and improve a company's Quality Management System (QMS).
They stem from the Quality Policy and translate it into measurable goals.
QOs are crucial for:
⦿ Assessing progress
⦿ Ensuring compliance
⦿ Enhancing customer satisfaction
Key points for writing effective Quality Objectives:
1. Clarity and Conciseness: Objectives should be clear, concise, achievable, and measurable.
2. SMART Criteria: They should be Specific, Measurable, Achievable, Realistic, and Time-bound.
3. Alignment with Quality Policy: Objectives must align with the Quality Policy and reflect its aims.
4. Relevance and Timeframe: Ensure objectives are relevant to the specific timeframe and achievable within it.
5. Departmental Focus: Objectives can be set for specific departments to monitor their goals, contributing to the overall organizational objectives.
Examples of meaningful Quality Objectives for a medical device company include achieving a low incoming inspection rejection rate, maintaining high customer satisfaction levels, and obtaining regulatory authorization for sales in specific regions like Canada.
For tailored Quality Objectives, companies can customize them based on product lines or specific products. Additionally, consulting with regulatory or quality partners like Elexes can help in designing objectives aligned with certification and compliance standards like ISO 13485, MDSAP, or 21 CFR Part 820.
Overall, Quality Objectives are essential for driving continuous improvement, ensuring compliance, and enhancing overall product quality and customer satisfaction within a company's Quality Management System.
Check out the full story here:
#fda#healthcare#quality#Regulaory#medtech#nb#audit#Consulting#innovation#CER CEMarking Regulatory Quality MedicalDevice Healthcare#quality objective#medical devices#medicaldevice#medicaldeviceregulations#medicaldevicequality qualitymanagementsystems quality management
0 notes
Text
Quality Sytems: Facilitating continuous improvement!

Continuous improvement refers to the ongoing effort to enhance processes, products, or services incrementally over time. Achieving continuous improvement involves implementing a quality management system (QMS) that goes beyond mere documentation.
A robust QMS fosters good practices across all aspects of a company's operations related to developing, manufacturing, processing, and distributing medical devices. While there are standard requirements that companies must adhere to, the specific implementation of a QMS varies based on factors such as the types of devices manufactured, in-house processes versus outsourcing, company size, and short- and long-term objectives.
Setting up and implementing a QMS involves several steps:
1. Identify the target market and regulatory requirements to comply with.
2. Generate Standard Operating Procedures (SOPs) based on company activities.
3. Draft SOPs by regulatory requirements and define roles and responsibilities.
4. Train personnel on SOPs and ensure compliance in day-to-day operations.
5. Follow applicable SOPs and generate associated records.
Effective implementation of a QMS improves efficiency by consistently meeting customer and regulatory requirements. Internal audits monitor implementation effectiveness and drive improvements. External audits by regulatory bodies assess compliance and may lead to ISO 13485 certification. A typical audit cycle includes an initial audit, surveillance audits in subsequent years, and a recertification audit every three years.
Implementing a QMS typically takes six months, but it can be expedited with additional resources and planning. For medical device manufacturers, establishing a pre-production QMS is beneficial for laying a foundation during the design, development, and testing phases.
Aligning strategies with regulatory requirements is crucial for continuous improvement. Therefore, implementing a QMS is essential for companies, especially those developing new technologies, to ensure quality and compliance.
Read the complete article: Quality Sytems: Facilitating continuous improvement!
0 notes
Text
Access to De Novo Summaries - A treasure for manufacturers

The De Novo pathway, offered by the FDA, is a risk-based classification process for novel medical devices lacking predicate devices.
Although historically underutilized due to stringent requirements and costs, recent trends indicate growing interest. In 2018, a record 44 De Novo requests were granted.
To streamline this pathway, the FDA proposed a rule in 2018 for clearer guidelines and transparency. Commissioner Scott Gottlieb aims to enhance efficiency and expects increased usage. Since its inception, 235 novel devices have been authorized via De Novo.
A recent FDA update introduced summary documents for De Novo devices, providing objective evidence for decision-making and aiding future 510(k) submissions.
These summaries offer manufacturers valuable insights, although full De Novo submissions must adhere to all regulatory requirements.
Elexes supports medical device manufacturers in De Novo submissions, ensuring compliance and approval. For more information, contact [email protected] or [email protected].
You can read the full article: Access to De Novo Summaries | A Treasure for manufacturers
0 notes
Text
Comprehensive CER - A key component to CE Marking

Whether applying for a CE Marking or already possessing one, compliance with MEDDEV 2.7/1 Revision 4 (June 2016) for creating or updating a Clinical Evaluation Report (CER) is crucial.
Failure to comply can halt CE certification processes. Common pitfalls leading to non-compliance include:
1. Inadequate Evaluator Qualification: The CER Evaluator must have 10 years of relevant experience, familiarity with similar devices or clinical areas, and a signed conflict of interest declaration. Separation of authorship and evaluation roles is essential, with the Evaluator typically requiring a minimum of 10 years' professional experience or 5 years with a higher education degree.
2. Unsubstantiated Equivalence: Any claims of equivalence with preexisting CE Marked devices must be thoroughly justified, accounting for differences in safety. A detailed analysis of competitors and device relationships is vital.
3. Missing Risk-Benefit Analysis (RBA): An RBA ensures a holistic approach to device safety, aligning with ISO 14971:2012 requirements. Comprehensive risk assessment and RBAs help preemptively address regulatory concerns, ensuring a robust CER.
4. Superficial Post-Market Data Utilization: Regulatory databases like FDA MAUDE and EUDAMED should be fully leveraged to gather device performance data. Proper analysis of regulatory intelligence databases strengthens the CER, anticipating and addressing common issues observed.
The CER should be continuously updated with post-market stage risk awareness. Elexes assists medical device manufacturers in creating organized Technical Files, with a comprehensive CER being a critical component.
Read the Full Article: Comprehensive CER – A Key Component to CE Marking
For inquiries, contact [email protected].
#medical devices#cer#clinical evaluation#post market regulations#iso standard#eudamed#fda#fdaapproval#us fda
0 notes
Text
Clinical Evaluation Report (CER) | Everything To Know About CER

A Clinical Evaluation Report (CER) is essential for assessing the safety and performance of medical devices, particularly in the European Union (EU) under the Medical Device Regulation (MDR) and the In Vitro Diagnostic Medical Device Regulation (IVDR). It provides evidence based on clinical data gathered throughout the device's lifecycle, aiming to demonstrate that its benefits outweigh its risks and meet its intended use.
A typical CER includes device description, clinical data, state-of-the-art analysis, risk assessment, clinical evaluation plan, data analysis, conclusions, and references. The report's content and structure may vary based on device type, classification, and regulatory requirements.
Developing a CER involves understanding regulatory requirements, identifying scope and objectives, collecting clinical data, conducting a literature review, performing risk assessment, analyzing data, drafting the report, expert review, updating, submitting, document control, and periodic review.
Updates to a CER may be triggered by post-market surveillance data, new clinical studies, scientific literature, device design or labeling changes, regulatory requests, risk assessment changes, post-market surveillance plan updates, annual product reviews, or product recalls.
Maintaining a robust system for tracking CER changes and having knowledgeable individuals oversee updates is crucial.
For further assistance, contact [email protected].
Read the complete article: Clinical Evaluation Report (CER)
0 notes
Text
Medical Device Import/Expert In USA (Regulations for Medical Device Importers & Exporters)

The article Medical Device Import/Expert In USA (Regulations for Medical Device Importers & Exporters) discusses the import and export requirements for medical devices in the United States, emphasizing the importance of compliance with FDA regulations to ensure safety and efficacy.
It highlights the responsibilities of foreign establishments, initial importers, and distributors, outlining specific requirements such as establishment registration, quality system compliance, labeling, and reporting obligations.
For medical device importation, the FDA's Center for Devices and Radiological Health (CDRH) oversees compliance with relevant regulations, including premarket notification and medical device reporting.
The article also addresses import inspection procedures and specifications, such as device registration and listing.
Regarding export requirements, the FDA issues export certificates to demonstrate compliance with US regulations, which are requested by foreign governments or customers. The article explains the types of export certificates available, including certificates to foreign governments, certificates of exportability, and certificates for non-clinical research use only.
Furthermore, it discusses other export documents issued by the FDA, such as export permit letters and simple notifications, along with recordkeeping requirements for exported devices.
Overall, the article provides comprehensive guidance for medical device importers and exporters, underscoring the importance of compliance with FDA regulations. It also promotes Elexes Medical Consulting as a trusted partner for navigating import and export requirements in the medical device industry.
In case you missed the link to the full article: Medical Device Import/Expert In USA (Regulations for Medical Device Importers & Exporters)
0 notes
Text
De Novo paving the path for Technological Advancements
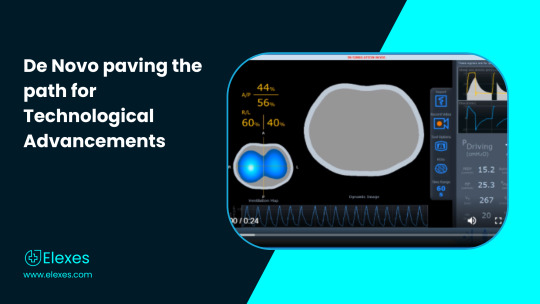
While there existed many computerized and digital tomographs with specific applications (Trauma CT, Cardiac CT, CT used in Nuclear Medicine / PET), there has never been one with an application in the respiratory space. Electrical impedance tomography (EIT) is a noninvasive, non-radiologic imaging modality that is useful for the assessment of lung disorders during mechanical ventilation. EIT offers potentially important benefits over standard imaging modalities since it is portable in nature and has non-radiological characteristics, which makes it conducive for medical use and diagnostic applications.
Device Classification
🔘 According to the FDA, any new device which is not in commercial distribution will be automatically classified as Class III devices irrespective of the level of risk associated with the device. This comes after the post amendment of FD&C Act (Medical Devices Act), May 28, 1976.
🔘 Since Electrical Impedance Tomograph (EIT) is a non- commercial device developed after May 28, 1976, it is classified as a Class III device. Since no predicates existed with regard to ventilator electrical impedance tomograph, the next step was to go for a De Novo Classification.

Devices can be classified into Class I or Class II under section 513(i) of the FD&C Act i.e. the reclassification of devices.
Devices Reclassification by De Novo Process
The De Novo process provides a pathway to classify novel medical devices for which general controls alone, or general and special controls, provide reasonable assurance of safety and effectiveness for the intended use, but for which there is no legally marketed predicate device. De Novo classification is a risk-based classification process.
De Novo Classification
De Novo classification is beneficial to the consumers as they can gain access to innovative devices which go through just the right amount of regulatory rigour.
Timpel Inc. is the company which is registered under De Novo for reclassification of Ventilator Electrical Impedance Tomograph device. It is the first of its kind that cleared De Novo and is now approved by the FDA as a Class II device under the Device Classification Name: Ventilator Electrical Impedance Tomograph.
The features of this device are listed as follows:
➡️ Intuitive: Integrated clinical decision support tools
➡️ Realtime: High temporal resolution
➡️ Easy to install, simple to use
➡️ Portable, can benefit multiple patients
➡️ Comfortable ergonomic belts
➡️ Non-Invasive in nature
➡️ Portable for bed-side access, no relocation of patient is required
➡️ Radiation Free: Can be used frequently
➡️ Provides continuous, real-time images
➡️ Operator independent (supervision not necessary)
Special Control Tests for De Novo Classification for Class II devices
Since the device includes a wide variety of features, the special controls of the FDA were considered for the device to ensure that the device is safe and effective for human use. Technical tests according to amended 21 CFR part 868 (Anesthesiological Devices) are mentioned in the table below along with the standards.
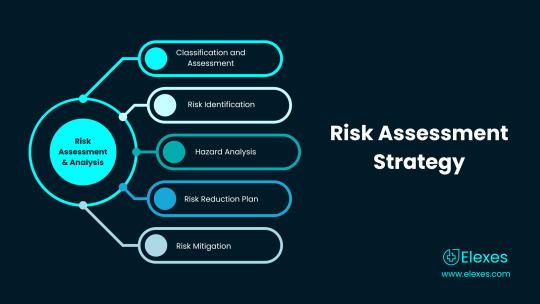
Risk and Mitigation steps associated with the device
Tissue Reaction (Adverse in nature) - Biocompatibility testing/evaluation (ISO 10993-1:2018)
Electromagnetic Interferences - Electromagnetic Compatibility Testing (ISO 60601-1: 2018)
Communicable/Non Communicable infections - Proper Packaging and labeling implementation (ISO 16142-1:2016)
Image distortion due to hardware and software malfunction - Software verification and Validation (IEC 62304:2006)Risk Analysis (ISO 14971:2007)Non Clinical Performance Testing (60068-2:2018
Thermal and/or electrical shock injury - Mechanical and Thermal safety Testing (ISO 60068-2)
Additional tests to be carried out under De Novo are:
Guidance for image interpretation
Instructions for reprocessing
Plethysmography accuracy testing
Benefits of De Novo Classification
Upon getting approved by the FDA under De Novo classification, the device was assigned as the generic type ‘Ventilator Electrical Impedance Tomograph’ and was defined as a non-radiological and non-invasive ventilator device that provides an assessment to the variations in local impedances within a cross-section of a patient’s thorax. This is quite a remarkable achievement due to the following reasons:
This De Novo approval of Timple Inc. paves the path for bringing more such innovative Ventilator Tomographs to the market.
Similar devices in the future shall have a lesser regulatory burden and can be easily accessed by the users.
The device can now be used as a predicate by other manufacturers, making similar device, for 510(k) clearance of their products which means the manufacturers do not have to submit a separate De Novo or Pre Market approval form for their products.
The De Novo classification has reclassified the device as a Class II device with special controls thus making it self-sufficient in mitigating the residual risks, since the general control functions cannot mitigate some of the specific residual risks.
Conclusion
De Novo classification is not just a device reclassification process for medical devices but it is an application that the manufacturers can use in order to commercialize their products.
This process clearly enables the manufacturer to know about their products genuinity and reduces the regulatory burdens which are commonly associated with other classification processes.
The effort of one can lessen the efforts of different manufacturers for their devices. Also, these manufacturer’s can pursue new angles of modifications and improvement for better accessibility and treatment opportunities.
To do a DeNovo certification the sponsor can wait for the FDA to get back stating not substantially equivalent (NSE) determination in response to a 510(k) submission or the manufacturer can determine that there is no legally marketed predicate device yet, to produce substantial equivalence and apply for a DeNovo.
Sponsor’s or Manufacturers can also contact professional service firms like Elexes Medical Consulting to acquire the assistance for such submissions and obtaining an approval from the FDA. Feel free contact [email protected] with any questions or comments on this content.
1 note
·
View note
Text
FDA - Medical Devices Advisory Committee

The U.S. Food and Drug Administration (FDA) has 31 advisory committees responsible for the evaluation and regulation of drugs, medical devices, and biologics.
The advisory committee protects and promotes public health while meeting the requirements established in the Federal Advisory Committee Act.
Committees are either mandated by statute or established at the discretion of the Department of Health and Human Services.
The advisory committee has multiple roles in developing and evaluating New Drugs, Biologics, and Medical Devices.
Their primary goal lies in assisting the FDA to evaluate applications for Pre-Market Approvals (PMAs) of medical devices, New Drug Applications (NDAs), and Product Licensing Agreements (PLAs) for biologics. The committees include chairperson, members, a consumer, industry and sometimes a patient representative.
Medical Devices Advisory Committee
Medical Devices Advisory Committee supports the FDA to protect and promote public health. The committee consists of 18 panels with a maximum of 159 standing members.
The selection of members is carried out by the Commissioner or designee from among authorities in Clinical and Administrative Medicine, Engineering, Biological, and Physical Sciences, etc.
The panel advises the FDA on the safety and efficacy of the medical devices except for the Medical Device Dispute Resolution Panel.
According to the respective specialty area, the panel advises the commissioner of Food and Drugs regarding recommended classification or reclassification of devices into one of three regulatory categories.
For recommendations of medical devices with respect to regulatory aspects, the committee reviews and evaluates the issues related to clinical study designs on the safety and effectiveness of marketed and investigational medical devices.
Overall, the 17 panels except for the Dispute Resolution Panel (DRP) of the medical device advisory committee, advise the Commissioner in accomplishing the constraints in assuring the safety and effectiveness of the medical devices in accordance with their specialty ****area and authorization.
The advisory committee also plays a major supportive role to agency professionals and influence agency decisions. FDA calls meetings with the committee or with the advisory panel as necessary. Generally, each of the panels meet once a year or as per the requirements by the FDA and important regulatory decisions or discussions are carried out in these meetings.
It is important that Manufacturers have a regulatory and quality team that stays abreast of decisions concerning their products and any such decisions or discussions are preemptively taken into account for a speedy FDA clearance, approval or compliance activities.
0 notes
Text
510(k) Premarket Notification - A Passport for Market Entry
Every company who aims to market their medical device in the US, be it a Class I, II or III. device, must get FDA’s approval. There are only a few devices that are 510(k) exempt.
To know if your medical device is 510(k) or PMA exempt, you must check our article Is your medical device 510(k) exempt? Understanding the classification of medical devices!
Today, we are going to discuss about - 510(k) Premarket Notification. But before we start the discussion, you must check out our guide on PMA -
Premarket Approvals | PMA Basics, supplements, amendments, QMS & More…
Premarket Notification is a premarket submission that FDA demands before approving a medical device. After reviewing this submission, will FDA send a letter to the submitter of 510(k) stating whether or not the medical device has been found to be Substantially Equivalent (SE). If the device is SE, the submitting company/individual can then market their device in the US.
The submitter is required to establish SE with respect to a legally marketed device, that
- has been legally marketed prior to May 28, 1976
- has been found to be SE via 510(k)
- \is a de-novo device without any premarket notification requirements
Check the complete guide on PMA - Here
Need assistance with your PMA submission? You can contact us at [email protected]
0 notes
Text
Software Precertification Program: An Innovative Approach to Expedite Patient Access for SaMD

The Software FDA Pre-Cert Program is a pathway that comprises a regulatory model which is more visible than the current model to assess the safety and effectiveness, without inhibiting patient access, of software technologies which are being used as Medical Devices.
The U.S. Food and Drug Administration (FDA) introduced Software Precertification program to address problems that the SaMD manufacturers are facing and provide suitable regulatory surveillance of software-based medical devices.
The Primary Goal of this program is to facilitate the development of software technologies and expedite patient access to these technologies.
Current Challenges
🟢 Lack of Transparency
Expectations between FDA and Manufacturers are not fully transparent. The Manufacturers of SaMD do not have a complete understanding of what is expected from them in terms of testing or data that they need to provide to the regulatory bodies in order to receive regulatory approvals or clearances.
🟢 Nature of SaMD
Unlike the hardware manufacturers who modify their products for a few months to years, SaMD manufacturers modify their products more often (a few weeks to months) in response to real-world performance and user feedback.
This problem is unique to SaMD because the software just by virtue of its nature needs to be updated every now and then for defect corrections and to make it a better fit for its users.
The FDA was discussing the FDA Pre-Cert program since 2017 and finally launched the Pre-Cert program at a pilot level in the year 2019. For the pilot program, manufacturers having a sturdy Culture of Quality and Organizational Excellence (CQOE) principles (hereafter referred to as “excellence principles”) were selected. The Excellence Principles are as follows:
☑️ Product Quality
☑️ Patient Safety
☑️ Clinical Responsibility
☑️ Cybersecurity Responsibility
☑️ Proactive Culture
The FDA applies the Total Product Lifecycle (TPLC) approach to empower the assessment and monitoring of the Software Product from its premarket development to post-market performance along with the continued exhibition of organization’s excellence. The goals of the Precertification Pilot Program are:
☑️ Assessment of the organizations for ensuring that they develop high-quality software products
☑️ Utilization of a streamlined premarket review that leverages unique postmarket opportunities to verify the safety, effectiveness and performance of the software product in the real world
Further, the program has been divided into four key components to outline the goals of the Precertification program.

Maintenance and Monitoring of FDA Pre-Cert Program Status
The Manufacturers, who are a part of the pre-cert program, shall trace and monitor their compliance with the excellence principles to ensure the safe and effective operation of their commercially distributed software (at various risk levels shown in the figure below) by responding appropriately to the post-market indicators.
Table1: Category of SaMD based on Risk Levels

Image Credit: FDA
The Software Precertification program benefits the organization’s ability to participate in a streamlined pre-market review and opportunities to collect and leverage real-world post-market data and ensure optimal quality of software products throughout their life cycle.
To make the best use of the FDA Pre-Cert program manufacturer’s must work with strong Regulatory Partners. For comments or questions please reach out to [email protected]. We at Elexes have a team of experts who can help you through the entire life cycle of medical device regulatory affairs. All you have to do is contact us.
NOTE: The FDA may modify the Pre-Cert Program based on the feedback collected from the Pre-cert Pilot Program.
#fda#regulatory#qualityassurance#iso13485#mdr#medicaldevice#healthcare#pma#denovo#precertification#medicalsoftware#health care#patientsafety#productquality#productdevelopment
0 notes
Text
IEC 60601-1 Evolution of Electrical Safety standards to match Device Development

In this blog, we are going to emphasize the pivotal role of standards, particularly focusing on the IEC 60601 series, in ensuring the safety and efficacy of medical devices, especially concerning their electrical components.
Here we will discuss the importance of these standards in product design and development within the medical device industry. The IEC, established in 1906, serves as the leading organization for developing international standards in the field of electrotechnology.
The IEC 60601 series encompasses various types of standards, including primary, collateral, and particular standards, each addressing different aspects of medical electrical equipment and systems.
The evolution of IEC 60601 over the years reflects advancements in technology and safety requirements in the medical field, with updates and revisions aimed at enhancing device safety and performance.
Global adoption of IEC 60601 standards is widespread, with countries across Europe, North America, Asia, and South America aligning with different editions of the standard to ensure the safety of medical devices marketed within their jurisdictions.
Despite the complexity associated with complying with these standards, the blog highlights the significant advantages they offer in terms of ensuring device safety and reliability for users worldwide. Ultimately, adherence to these standards contributes to the overall quality and safety of medical devices, benefiting both manufacturers and users alike.
Read the full story: https://www.elexes.com/iec-60601-1/
In case you need any help with the IEC 60601 compliance, all you have to do is contact our experts.
#fda#regulatory#iec certificate#medical device#electrical safety#productdevelopment#healthcare#iec60601 iecstandards isostandards medicalevices medicaldevice medical device
0 notes
Text
De Novo paving the path for Technological Advancements
While there existed many computerized and digital tomographs with specific applications (Trauma CT, Cardiac CT, CT used in Nuclear Medicine / PET), there has never been one with an application in the respiratory space.
Electrical impedance tomography (EIT) is a noninvasive, non-radiologic imaging modality that is useful for the assessment of lung disorders during mechanical ventilation.
EIT offers potentially important benefits over standard imaging modalities since it is portable in nature and has non-radiological characteristics, which makes it conducive for medical use and diagnostic applications.
Device Classification
According to the FDA, any new device which is not in commercial distribution will be automatically classified as Class III devices irrespective of the level of risk associated with the device. This comes after the post amendment of FD&C Act (Medical Devices Act), May 28, 1976.
⊙ Since Electrical Impedance Tomograph (EIT) is a non- commercial device developed after May 28, 1976, it is classified as a Class III device. Since no predicates existed with regard to ventilator electrical impedance tomograph, the next step was to go for a De Novo Classification.
Devices can be classified into Class I or Class II under section 513(i) of the FD&C Act i.e. the reclassification of devices.
Devices can be reclassified by:
De Novo Process
The De Novo process provides a pathway to classify novel medical devices for which general controls alone, or general and special controls, provide reasonable assurance of safety and effectiveness for the intended use, but for which there is no legally marketed predicate device. De Novo classification is a risk-based classification process.
De Novo Classification
De Novo classification is beneficial to the consumers as they can gain access to innovative devices which go through just the right amount of regulatory rigour.
Timpel Inc. is the company which is registered under De Novo for reclassification of Ventilator Electrical Impedance Tomograph device.
It is the first of its kind that cleared De Novo and is now approved by the FDA as a Class II device under the Device Classification Name: Ventilator Electrical Impedance Tomograph
The features of this device are listed as follows:
➩ Intuitive: Integrated clinical decision support tools
➩ Realtime: High temporal resolution
➩ Easy to install, simple to use
➩ Portable, can benefit multiple patients
➩ Comfortable ergonomic belts
➩ Non-Invasive in nature
➩ Portable for bed-side access, no relocation of patient is required
➩ Radiation Free: Can be used frequently
➩ Provides continuous, real-time images
➩ Operator independent (supervision not necessary)
Special Control Tests for De Novo Classification for Class II devices
Since the device includes a wide variety of features, the special controls of the FDA were considered for the device to ensure that the device is safe and effective for human use.
Technical tests according to amended 21 CFR part 868 (Anesthesiological Devices) are mentioned in the table below along with the standards.
Risk and Mitigation steps associated with the device
➩ Tissue Reaction (Adverse in nature) – Biocompatibility testing/evaluation (ISO 10993-1:2018)
➩ Electromagnetic Interferences – Electromagnetic Compatibility Testing (ISO 60601-1: 2018)
➩ Communicable/Non Communicable infections – Proper Packaging and labeling implementation (ISO 16142-1:2016)
➩ Image distortion due to hardware and software malfunction – Software verification and Validation (IEC 62304:2006)Risk Analysis (ISO 14971:2007)Non Clinical Performance Testing (60068-2:2018
➩ Thermal and/or electrical shock injury – Mechanical and Thermal safety Testing (ISO 60068-2)
Additional tests to be carried out under De Novo are:
Guidance for image interpretation
Instructions for reprocessing
Plethysmography accuracy testing
Benefits of De Novo Classification
Upon getting approved by the FDA under De Novo classification, the device was assigned as the generic type ‘Ventilator Electrical Impedance Tomograph’ and was defined as a non-radiological and non-invasive ventilator device that provides an assessment to the variations in local impedances within a cross-section of a patient’s thorax.
This is quite a remarkable achievement due to the following reasons:
⊙ This De Novo approval of Timple Inc. paves the path for bringing more such innovative Ventilator Tomographs to the market.
⊙ Similar devices in the future shall have a lesser regulatory burden and can be easily accessed by the users.
⊙ The device can now be used as a predicate by other manufacturers, making similar device, for 510(k) clearance of their products which means the manufacturers do not have to submit a separate De Novo or Pre Market approval form for their products.
⊙ The De Novo classification has reclassified the device as a Class II device with special controls thus making it self-sufficient in mitigating the residual risks, since the general control functions cannot mitigate some of the specific residual risks.
Bottom Line
De Novo classification is not just a device reclassification process for medical devices but it is an application that the manufacturers can use in order to commercialize their products.
This process clearly enables the manufacturer to know about their products genuinity and reduces the regulatory burdens which are commonly associated with other classification processes.
The effort of one can lessen the efforts of different manufacturers for their devices. Also, these manufacturer’s can pursue new angles of modifications and improvement for better accessibility and treatment opportunities.
To do a DeNovo certification the sponsor can wait for the FDA to get back stating not substantially equivalent (NSE) determination in response to a 510(k) submission or the manufacturer can determine that there is no legally marketed predicate device yet, to produce substantial equivalence and apply for a DeNovo.
Sponsor’s or Manufacturers can also contact our professionals to acquire the assistance for such submissions and obtaining an approval from the FDA. Feel free contact at [email protected] for any questions or comments on this content.
0 notes
Text
CLIA Database: A Centralized Database of Clinical Tests & Instruments

Diagnosis plays a vital role in curing a disease. If the diagnosis itself is wrong, the disease cannot be treated. Osteosarcoma, a dangerous but common form of bone cancer which mostly affects the population of children and young adults (15 - 24), is often being misdiagnosed as growing pains or muscle strains, according to the Bone Cancer Research Trust (BCRT).
Due to improved awareness about the importance of diagnosis, Clinical Laboratories been gaining more interest now than ever. However, Clinical Laboratories in the States were reported with the wrong diagnosis and poor quality of services, prior to 1988.
The US Congress conducted several investigations of testing performed in the Physician Office Laboratories (POLs) and other labs.
One major initiate was -The Clinical Laboratory Improvement Amendments (CLIA) which was passed by the Congress in 1988 to set standards designed for improving the quality in all laboratory testing which also includes specifications for quality control and assurance, patient test management, personnel and proficiency testing.
Approximately 260,000 laboratory entities are covered under CLIA. Labs and POLs operating in New York or Washington are exempt from CLIA. The CLIA database contains information about commercially marketed in-vitro systems and tests.
The database is updated weekly and has been maintained by the FDA since January 31, 2000. Prior to 2000, the CDC (Center for Diseases Control and Prevention) used to maintain the database.
Types of CLIA certificates:
● Certificate of Waiver (COW) - Issued for performing only waived tests.
● Certificate for Provider who Performed Microscopy (PPM) procedures -Issued when a microscopy procedure (among a list of procedures included under this certificate) is to be performed on a patient.
● Certificate of Registration - Issued to the laboratory to conduct nonwaived testing until the laboratory is inspected to determine its compliance with the CLIA regulations.
● Certificate of Compliance (COC) - It is issued once the State Department of Health conducts the inspection and determines that the laboratory is compliant with all applicable CLIA requirements.
● Certificate of Accreditation (COA) - It is issued on the basis of the laboratory’s competence assessment by an accreditation organization approved by CMS.
NOTE: All types of certificates are valid for 2 years.
● Waived test - Simple laboratory examinations and procedures that have an insignificant risk of an erroneous result. Few of the waived tests include: Blood Glucose monitoring, Platelet Count, and Urine qualitative dipstick analysis
● Non-Waived test - It is a moderate and/or high complexity testing. Non-waived tests include: Hematology and Toxicology
A non-waived is further categorized into PPM and Moderate/High Complexity. PPM tests are different from moderate or high complexity tests. A test or device is classified as moderate or high, based on 7 criteria.
The decision for classification of device or test is based on a ‘scorecard’. Each criterion can be marked as 1, 2 or 3 based on the level of complexity (3 being the highest and 1 being least complex).
All the scores are then added to get an aggregate score. If the total score is 12 or less, it is considered as a moderate test. If the total score is greater than 12 it is considered as high complexity.
The 7 criteria for classification are as follows:
● Knowledge (scientific and technical knowledge)
● Training and experience
● Reagents and materials preparation
● Characteristics of operational steps
● Calibration, quality control, and proficiency testing materials
● Test system troubleshooting and equipment maintenance
● Interpretation and judgment.
To get a CLIA certification the Sponsor needs to fill CMS116 form and submit it to the respective State Agency. You can also contact professional service firms like Elexes Medical Consulting to assist in submitting for assisting in the certification process.
0 notes
Text
Modernization of 510(k) – A major milestone to safer and better healthcare
The FDA regulates over 190,000 distinct medical devices, with approximately 3,000 low and moderate-risk devices receiving FDA's 510(k) clearance on average annually, accounting for 80% of total approvals. The 510(k) pathway, established in 1976, undergoes constant refinement by the FDA to ensure practicality and transparency.
In November 2018, as part of its Medical Device Safety Action Plan, the FDA announced changes to the 510(k) clearance pathway.
Commissioner Scott Gottlieb emphasized the need to promote innovation and safety by encouraging reliance on modern predicate devices or objective performance criteria.
Notably, 20% of 510(k)s were approved based on predicates older than ten years, prompting the FDA to modernize the process by making certain devices ineligible as predicates and requiring updated data submissions.
The FDA is also developing an alternate 510(k) pathway based on contemporary standards rather than outdated predicates, aiming to foster innovation and facilitate access to advanced technology.
Key implications for medical device manufacturers include the elimination of old predicates, the requirement for objective testing evidence aligned with modern technology, and the FDA's plans to finalize guidance emphasizing safety and performance criteria.
Read Full Article: Modernization of 510(k) – A major milestone to safer and better healthcare
As regulations evolve, companies can rely on regulatory partners like Elexes to stay updated and navigate changes effectively. For more information, interested parties can contact Elexes at [email protected].
#fda#510k#medical device#regulatory#quality#modernization#510k medical device#medical devices#medicaldevicecompany 510k submission
0 notes