#anodizing aluminium
Link
Today, with constant expansions to meet diverse needs for the changing markets, we are transforming into a one-stop solution provider inclusive design, engineering, and production until finished goods. We are using high-efficiency manufacturing equipment to meet extreme quality services and fast delivery.
0 notes
Link
Alufinish is your proficient partner when it comes to products and processes for the chemical surface treatment of aluminium, steel and zinc. Alufinish, one of our trusted partners, supplies us with an extensive range of chemical surface treatments for metals. Learn more via our website.
1 note
·
View note
Photo

Nicolas Pelzer - Cockpit Rule - True Sided, 2017
180 x 65 x 38 cm
44 notes
·
View notes
Photo

Modern Exterior
An illustration of a large, one-story, mixed-siding home with a shed roof and a metal roof.
#kynar coated metal wall panels#stone: bedface exposed sandstone#clear anodized aluminium doors#electostatically coated metal doors#exterior#modern interpretation of mid-century ranch#accessible design
0 notes
Photo

Aluminium Anodized Name Plates
0 notes
Text
#anodizedaluminiumsheetsuppliersindubai#anodized aluminium sheets#anodizedaluminiumsheetsuppliersinUAE
0 notes
Text

Expertly Designed Omichef Kadhai
#kadhai#kitchenware#cooking#cookware#hard anodized kadhai#cookingappliances#aluminium kadhai#omichef#cooking utensis#designed#product#product design#brand#stylish kadhai#product designing#photography#photooftheday#photoart
1 note
·
View note
Text
#aluminium bottle#aluminium bottles#bottles#bottle#anodized aluminium bottles for perfumes packaging
0 notes
Text
What is Aluminium Anodizing and How Does It Work
Aluminium anodizing is a process used to increase the thickness of the natural oxide layer on thesurface of aluminum.
0 notes
Photo

Flat Panel Closet in London
#Inspiration for a small contemporary gender-neutral dressing room remodel with flat-panel cabinets and dark wood cabinets anodized aluminium#interior design details#metal work#mews#dark veneer joinery
0 notes
Text
Which metals expand when freezing?

You’ve probably heard that all metals expand when heated. But is that true? Let’s take a closer look at the science behind the metal expansion.
All metals have what’s called a “lattice structure.” This means that the atoms that make up the metal are arranged in a repeating pattern. When a metal is heated, the atoms vibrate more and more, until they eventually break free from the lattice structure. This is what causes the metal to expand.
Not all metals expand at the same rate, though. For example, lighter metals like aluminum expand more than heavier metals like iron. This is because it takes less energy to break apart the lattice structure of more lightweight metals.
There are some exceptions to this rule, though. For example, while iron is a heavier metal, it expands more than aluminum when heated. This is because iron has what’s called a “body-centred cubic” lattice structure, while aluminum has a “face-centred cubic” lattice structure. The body-centred cubic structure is less stable than the face-centred cubic structure, so it breaks apart more easily when heated.
So, is it true that all metals expand when heated? Yes! All metals have a lattice structure, and when that lattice structure is broken apart by heat, the metal expands. Some metals expand more than others, depending on their weight and lattice structure. Now you know the science behind why all metals expand when heated!
But which metals expand when freezing?
It’s a well-known fact that water expands when it freezes. But did you know that metals also expand when they freeze? Here, we’ll look closely at why this is the case. We’ll also examine which metals expand the most when they freeze and why this expansion can sometimes be problematic.
The thermal expansion of solids is a physical property that refers to the tendency of matter to change in shape, area, or volume in response to a change in temperature. Thermal expansion occurs when molecules in a material absorb energy from their surroundings, causing them to vibrate and move further apart — this increase in intermolecular spacing results in an overall increase in the size of the material.
Most metals expand when they freeze because their atoms are relatively free to move around. This means that when the temperature decreases and the atoms have less energy, they move more slowly and take up more space. As a result, the metal expands.
However, not all metals expand when they freeze. For example, iron contracts when it freezes because its atoms are tightly held together by strong interatomic forces. While iron is an exception, most other metals expand when they freeze — some of them expand quite significantly!
Here are some examples of common metals and alloys and how much they expand when they freeze:
* Aluminum: 12%
* Brass: 17%
* Copper: 16%
* Gold: 14%
* Silver: 18%
* Steel: 11%
* Tin: 26%
* Zinc: 27%
As you can see, some metals expand quite a bit when they freeze! Unfortunately, this expansion can sometimes cause problems, mainly if the metal is used in construction or engineering applications. That’s because when the metal expands, it stresses the material it’s attached to and can cause cracks or breaks. In extreme cases, this expansion can even lead to catastrophic failures.
So, why do metals expand when frozen?
It’s all thanks to their atomic structure! Most metals have relatively free atoms to move around, which means that they will expand when the temperature decreases and they have less energy.
Who are we?
We are an aluminum enthusiast and an aluminum metals supplier in Canada and the USA.
We offer premium, raw & anodized Aluminum sheets, Aluminum nameplates, assorted colour aluminum wire, cold or hot rolled Aluminum Plate, Gold, Black, Silver, and Purple Aluminum Foil and Aluminum Coil for sale in our warehouse, ready to ship today.
Call us today, Toll-Free: 866–860–0652, if you need further information.
1 note
·
View note
Text
Hindalco Aluminium Sheet

Hindalco-India makes the AA 3003 sheets we have. All real HINDALCO aluminium sheet has ink stencil marks on each sheet that show the mill name, alloy temper, etc. If asked, we can send all of our deliveries with mill test certificates that match coil/heat numbers based on ASTM standards. Hindalco's cold-rolled sheets are finished to a high level of accuracy so that they meet international standards for thickness, tolerance, flatness, and size. It can be used in both commercial and general engineering applications because it has good metallurgical properties for further fabrication, anodizing properties, and a smooth surface.
0 notes
Text
Sixaluminium - Devasa+ (2)
The aluminum industry is a vital sector that plays a significant role in various industries, including construction, transportation, and manufacturing. Aluminium profile are widely used in these industries due to their lightweight, durable, and corrosion-resistant properties. These profiles are produced by aluminum profile manufacturers and aluminum extrusion companies, who are key players in the industry. Aluminium profile wholesalers play a crucial role in the distribution of aluminum profiles. They act as intermediaries between manufacturers and end-users, ensuring a smooth supply chain. These wholesalers offer a wide range of aluminum profiles, including standard profiles, conduit and trunking profiles, angle and corner profiles, and seamless aluminum extrusion . They provide a one-stop solution for customers, offering competitive prices and support throughout the purchasing process. By partnering with aluminum profile wholesalers, businesses can access a diverse range of profiles to meet their specific needs. Aluminium profile manufacturer and aluminum extrusion companies are responsible for the production and customization of aluminium manufacturer. They have the expertise and capabilities to design and manufacture profiles according to customer specifications. These manufacturers offer a wide range of profiles for architectural and industrial use, such as doors, windows, curtain walls, and other structural components. They utilize advanced techniques, including anodized aluminum, to enhance the appearance and durability of the profiles. By collaborating with aluminum profile manufacturers, businesses can obtain high-quality profiles tailored to their requirements.
1K notes
·
View notes
Text
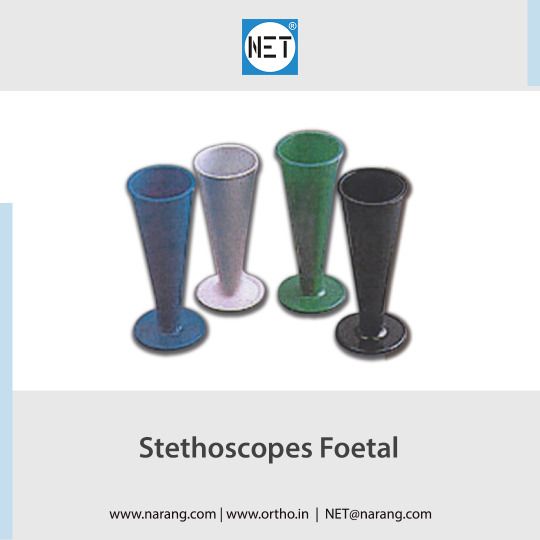
NET brand Foetal stethoscopes, including the Pinard foetal model, are available in both aluminium anodized and plastic variations ... https://www.narang.com/diagnostic-equipments-products/stethoscopes-spare-parts/ST11.php
2 notes
·
View notes
Text
Calcium sulphur batteries (uwu)
Okay, so, i've become interested in z-pinch studies for aerospace purposes (i'm really excited about the prospects, everything works on paper, but i naturally want to actually witness p+N14 fusion for above 0.01% of available protons before i go trying to get the materials to build a real liquid fueled SSTO fusion rocket, especially since there are thousands of folks way smarter than me who have presumably thought of this before and we don't have it yet, so yeah). Anyways, if i want the extremely large electricity input without making my electricity bill higher than a whole month's rent and getting my roommates mad at me, i'll need to collect solar or wind in a battery bank. Since lithium batteries are just about all immoral and expensive (yes i am writing this on a device powered by lithium batteries, it would be lovely if capitalists would take a hint and switch to things that just objectively perform better and are cheaper, but whatever), i figured this would be a nice excuse to experiment around with some new battery designs. Since all of them will require sulphur, i won't be able to really get into it before mid may due to some concerns about the smell and risks of getting sulphur powder everywhere (it's very yellow and hard to clean out), but i felt i might as well share my preliminary ideas. First off, in order to make the organic sulphur polymer, i'm looking to explore mostly citrate based polymers, perhaps with phenylalanine mixed in in order to both give more bulk as well as providing nitrogens for sulphenamides to form. Since i'll need urea later, i was also considering partially polymerizing urea with citric acid and adding that into the molten sulphur mix, but i'm less confident in the stability of that and a bit concerned about the potential noxious fumes produced. Regardless, that's the short of the sulphur cathode, details will definitely change after i refind that paper which went over a great way of preventing insoluble polysulphide production.
I'm also gonna experiment with anode material and even the ions i use. I know i said "calcium sulphur batteries" in the title, but due to how common aluminium is and how much easier magnesium is to work with (and the fact that their specific energies are higher), i'll also be considering those two. Even beyond that, there are so many potential anode materials, including even amorphous carbon and carbon nitrides which i'd love to test since there's just so much to improve on and i'd rather do a lot of experiments with cheap to make materials and potentially land on a great solution than accept something subpar because it took less effort. Anyways, of the materials i plan on using, there's magnesium sulphate, aluminium sulphate, calcium chloride, potentially other calcium salts (is the salt with taurine soluble in water? IDK, can't find an answer so i'll test it), charcoal, vegetable oil, urea, and phenylalanine. Those may seem like an unrelated hodgepodge of compounds, but they've been chosen because they're what i have/will soon have and they're also all extremely cheap. If the urea works out well in the battery, i may have to make this project a meme and attempt to make a z-pinch device with as much urine as possible (use it to make ammonia for the plasma, to make the batteries, and i'm sure there's some way to use urine in a capacitor (maybe just distilling off the water to use as a dielectric? idk, it's been a while since i tried making a capacitor)).
Anyway, i really didn't expect this long trainwreck of a post to end with discussions of urine, but what can you do? This is all probably nonsensical, even by my standards, but basically i want batteries and i think i can make them cheaper per megajoule of stored energy than the ones i could buy, even accounting for the inevitable failed experiments.
#utter nonsense#chemistry?#batteries#calcium sulphur batteries are cool i guess#z-pinch shit#almost certainly the beginning of a ton of failures#fortunately i should be able to afford all the chemicals with less than 1 month of income (after rent and utilities and whatnot)#sulphur is so cheap#so am i lol#idk if i want to attempt to make my own solar or wind farm or just buy some turbines or solar cells#turbines are pretty easy so i might build some myself#magnets are relatively cheap and i can use them for other things#and if you know where to look (trashcans behind the college) wire is free#and to make the turbine blades i can just take some sheet metal from the same dumpsters as the wire#alternatively i could just try charging the batteries during off-hours when electricity is super cheap#or making a simple biofuel engine#i should also look into making the capacitors#good bye!
2 notes
·
View notes
Text
Tonetouch from beoplay app

This Beolit speaker also boasts of a redesigned speaker grill so it looks nicer while offering an enhanced sound performance that is distinctively Bang & Olufsen Signature Sound.īeolit 17 is currently sold in Bang & Olufsen stores, online on and from selected third party retailers. Use the connect button to make things more convenient for you. The BeoPlay App lets you connect, interact and update your devices, giving you total control at your fingertips. You can set the Alarm, Connect to play music, ToneTouch for personalization, or Remote to play/pause a song. It comes with a one-touch connect button that can be set to open certain functions. The Beolit 17 also features a scratch safe and non-slip tray for your phone. It’s portable and well-made with the premium grain leather plus anodized pearl-blasted aluminium. The speaker can be used for a maximum of 24 hours. It brings the classic Scandinavian feel together with the roughness of a gadget expected to last for a long time. You will notice the simple and minimalist principles used in designing the product. Thanks to the Beoplay App, you can play, pause or skip tracks with just a double-tap or a shake, change the sound profile in ToneTouch, and even set up the. If you may remember the Beolit 15 and Beolit 12, this one has a similar design. It’s easy to use and boasts of really smart features which you can access straight from a compatible Beoplay app. This is more than just a premium speaker. Two colors will be ready: Natural and Stone Grey. We also ensure you easy access to your product’s user guide.īang & Olufsen app replaces BeoSetup app for product set-up, BeoRemote app for operating your Bang & Olufsen TV and BeoPlay app for setting up and controlling your BeoPlay product.The Beolit 17 can be availed for NOK 4699 which is about $554 in the United States. taking its intelligence further, the ‘P2’ is supported by the beoplay app, which enables users to customize controls, settings and additional speakers. The Bang & Olufsen app guides you in how to use your product and how to get the most out of the product specific features.

At the heart of the BeoPlay App are advanced digital sound algorithms developed by our acoustic team to make sure to deliver our Bang and Olufsen signature sound. To truly personalize your listening experience, you can use the Beoplay App to adapt the sound profile of your speaker using ToneTouch. The BeoPlay app lets you connect, interact and update your devices, giving you total control at your fingertips. Each B&O product has its own individually tuned presets, plus, you can select different listening presets to match your mood or activity. To make sure that users do not leave their earphones behind, Beoplay.
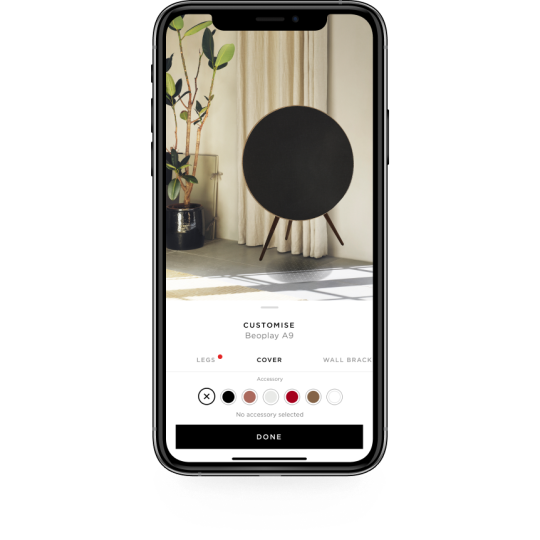
The ToneTouch feature lets you vary the tonality and sound staging to find just the right sound. Users simply choose one of the preset sound profiles in Beoplay App on their smartphone or Apple Watch, such as working out, commuting, listening to podcasts or relaxing, or they can adjust the tonality and staging with the playful and intuitive B&O PLAY ToneTouch interface. The Bang & Olufsen app will notify you about updates for your products. The M5 streaming speaker supports Apple AirPlay, Chromecast Built-in, Bluetooth, Beolink Multi-room and Spotify Connect technologies so you can access a world of music from your own little corner of the planet. Beoplay App: You can enhance your listening experience with the free Beoplay App for Android, iOS, and watchOS. Simply feel your way through different tonality and staging settings to find the sound that suits you. The Bang & Olufsen app guides you step-by-step through the setup of your product, and helps you personalize your product and music expereince.Įasy access to product specific sound settings of your Bang & Olufsen product, giving you beautiful sound no matter what you are listing to. ToneTouch: Beolit 17 also works with Beoplay App features such as ToneTouch that gives playful, intuitive, and easy personalisation to your music experience. The app showcases the striking Bang & Olufsen products connected to your Bang & Olufsen account. Enjoy our signature design as you turn your smartphone into a Bang & Olufsen-designed control centre.

8 notes
·
View notes