#Lutetium 177 PSMA
Text
Discover the transformative potential of nuclear medicine therapy and its role in reshaping cancer care in India.
0 notes
Text
Prostate Cancer Treatment Market - Future Growth Prospects for the Global Leaders
The latest market report published by Credence Research, Inc. “Global Prostate Cancer Treatment Market: Growth, Future Prospects, and Competitive Analysis, 2022 – 2030. The market for prostate cancer treatment is witnessing substantial growth, with projected revenues to rise from USD 12,415.6 million in 2022 to an estimated USD 21,425.39 million by 2030. This projection signifies a compound annual growth rate (CAGR) of 8.11% from 2023 to 2030. With an increasing global demand for effective treatment methods, the opportunities within this market are expanding.
Prostate cancer is a prevalent malignancy affecting men globally. It originates in the prostate gland, a small walnut-shaped organ that produces seminal fluid. Early detection and timely intervention are crucial for improving patient outcomes. To stay ahead in the battle against prostate cancer, it's essential to comprehend the latest trends and treatments.
Prostate cancer is one of the most common forms of cancer in men, and as a result, there is a significant demand for effective treatments and therapies. In recent years, there has been a notable shift towards more targeted and personalized approaches to prostate cancer treatment. This includes the use of precision medicine, immunotherapy, and advanced radiation therapy techniques, among others. Additionally, ongoing research and development efforts are leading to the discovery of novel drugs and therapies that hold promise in improving patient outcomes.
Prostate Cancer Treatment Market opportunities are steadily emerging as advancements in medical technology and research continue to unfold. With an increasing prevalence of prostate cancer cases globally, the demand for effective treatment options is on the rise. This presents a significant opportunity for pharmaceutical companies, biotechnology firms, and healthcare providers to develop innovative therapies that target specific molecular pathways involved in prostate cancer progression. Moreover, there is immense potential within the field of precision medicine, where personalized treatment approaches based on genetic profiling can revolutionize patient outcomes.
Key Treatment Modalities
1. Surgery
Surgical intervention, such as radical prostatectomy, remains a primary treatment option for localized prostate cancer. It involves the removal of the prostate gland. Advancements in minimally invasive techniques, including robot-assisted surgery, have reduced recovery times and improved outcomes.
2. Radiation Therapy
Radiation therapy, using high-energy X-rays or protons, is another vital component of prostate cancer treatment. Innovations like intensity-modulated radiation therapy (IMRT) and stereotactic body radiation therapy (SBRT) allow for precise targeting of cancer cells while minimizing damage to surrounding tissues.
3. Hormone Therapy
Hormone therapy, also known as androgen deprivation therapy (ADT), is often used in combination with surgery or radiation. It aims to lower testosterone levels, which can fuel prostate cancer growth. Recent advancements in hormonal therapies have enhanced their effectiveness and reduced side effects.
Emerging Therapies
1. Immunotherapy
Immunotherapy has gained momentum as a promising treatment avenue for advanced prostate cancer. Checkpoint inhibitors, such as pembrolizumab and nivolumab, have shown potential in boosting the immune system's ability to combat cancer cells.
2. Targeted Therapies
Precision medicine has revolutionized cancer treatment. Targeted therapies, like enzalutamide and abiraterone, specifically target pathways involved in prostate cancer growth. These drugs have extended survival rates and improved quality of life for many patients.
3. Radiopharmaceuticals
Radiopharmaceuticals like Lutetium-177 PSMA have emerged as a game-changer in the treatment of metastatic castration-resistant prostate cancer (mCRPC). They deliver highly targeted radiation to cancer cells, offering new hope for patients.
The Role of Research and Clinical Trials
Research plays a pivotal role in advancing prostate cancer treatment. Clinical trials offer patients access to cutting-edge therapies and contribute to the development of future treatments. Stay informed about ongoing trials and potential breakthroughs that could shape the future of prostate cancer care.
Browse 245 pages report Prostate Cancer Treatment Market By Treatment Type (Chemotherapy, Biological Therapy, Hormone Therapy) By Distribution (Hospital Pharmacies, Drug Stores & Retail Pharmacies, Online Pharmacies) - Growth, Future Prospects & Competitive Analysis, 2016 – 2030 https://www.credenceresearch.com/report/prostate-cancer-treatment-market
List of the prominent players in the Prostate Cancer Treatment Market:
Johnson & Johnson Services, Inc.
Astellas Pharma, Inc.
Eli Lilly and Company
Sanofi
Ipsen Pharma
Bayer AG
AstraZeneca
Valeant Pharmaceuticals International, Inc.
Merck & Co., Inc.
Pfizer Inc.
Conclusion
The landscape of prostate cancer treatment is continually evolving, offering new hope to patients worldwide. By staying informed about the latest advancements, breakthrough therapies, and clinical trials, we can collectively work towards a future where prostate cancer is a manageable condition. Join us in the pursuit of better treatments and improved outcomes for those affected by this disease. Together, we can outrank the challenges posed by prostate cancer.
Why to Buy This Report-
The report provides a qualitative as well as quantitative analysis of the global Prostate Cancer Treatment Market by segments, current trends, drivers, restraints, opportunities, challenges, and market dynamics with the historical period from 2016-2020, the base year- 2021, and the projection period 2022-2028.
The report includes information on the competitive landscape, such as how the market's top competitors operate at the global, regional, and country levels.
Major nations in each region with their import/export statistics
The global Prostate Cancer Treatment Market report also includes the analysis of the market at a global, regional, and country-level along with key market trends, major player analysis, market growth strategies, and key application areas.
Browse Full Report: https://www.credenceresearch.com/report/prostate-cancer-treatment-market
Visit: https://www.credenceresearch.com/
Related Report: https://www.credenceresearch.com/report/synthetic-ovulation-stimulants-market
Related Report: https://www.credenceresearch.com/report/transfer-membrane-market
Browse Our Blog: https://www.linkedin.com/pulse/prostate-cancer-treatment-market-top-players-size-share-singh-uh35c
About Us -
Credence Research is a viable intelligence and market research platform that provides quantitative B2B research to more than 10,000 clients worldwide and is built on the Give principle. The company is a market research and consulting firm serving governments, non-legislative associations, non-profit organizations, and various organizations worldwide. We help our clients improve their execution in a lasting way and understand their most imperative objectives. For nearly a century, we’ve built a company well-prepared for this task.
Contact Us:
Office No 3 Second Floor, Abhilasha Bhawan, Pinto Park, Gwalior [M.P] 474005 India
0 notes
Text
Best Nuclear Medicine Treatment in Trivandrum, India | KIMSHEALTH Hospital
Nuclear medicine is a medical specialty involving the application of radioactive substances in the diagnosis and treatment of disease

Procedures & Treatments
Nuclear medicine, in a sense, is “radiology done inside out” or “endoradiology” because it records radiation emitting from within the body rather than radiation that is generated by external sources like X-rays. In addition, nuclear medicine scans differ from radiology as the emphasis is not on imaging anatomy but the function and for such reason, it is called a physiological imaging modality.
Single Photon Emission Computed Tomography or SPECT and Positron Emission Tomography or PET scans are the two most common imaging modalities in nuclear medicine
HEART:
Myocardial viability PET and SPECT
Adenosine Stress perfusion scan
Stress Dobutamine Myocardial MIBI perfusion SPECT
Stress and Rest myocardial perfusion SPECT
MUGA scan
BRAIN:
FDG brain PET CT
F-18 DOPA PET CT
Brain perfusion SPECT
Ictal and interIctal SPECT
Trodat SPECT
Tumour Viability Study
SKELETAL SYSTEM:
Three Phase Bone scan
Whole body Bone scan
F18 Fluoride Bone PET CT.
FDG PET prosthesis/infection Imaging.
Graft viability imaging
GASTROINTESTINAL:
Salivary scan
GE Reflux scan
Gastric Emptying Time
RBC — GI Bleed scan
Meckel’s scan
Whole body DOTA PET CT
HEPATOBILIARY/LIVER — SPLEEN:
Liver Spleen scan
RBC Blood pool scan
Hepatobiliary (HIDA) scan
MAA Sunt
GENITOURINARY:
Diuretic DTPA Renal scan
GFR Estimation
EC Renal scan (ERPF)
DMSA Cortical Renal scan
Captopril Renal scan
VU Reflux study
Renal Transplant study
Lymphoscintigraphy for Chyluria
Whole body PSMA PET CT
ENDOCRINE:
Technetium Thyroid scan
MIBI Thyroid scan
MIBI Parathyroid Scan
MIBG scan
Octreotide SPECT CT
ONCOLOGY:
Whole body FDG PET CT
Whole body Gallium 68 DOTA scan
Whole body Gallium 68 PSMA scan
F-18 Choline PET CT
Whole body iodine scan
Post therapy scan
F-18 DOPA PET CT
Senthinal lymphoscintrigrapy
Scintimammography
HSA scan for protein leak
INFECTION IMAGING:
Whole body FDG PET infection Imaging
Gallium 67 scan
99mTc Ubiquicidin Imaging
RESPIRATORY:
Lung Perfusion Scan
Ventilation — Perfusion (V/Q) scan
Quantitative lung ventilation
Mucociliary clearance study
Ventilation
BLOOD:
Lymphoscintigraphy for Lymphoedema.
Sentinel Node Mapping
RADIONUCLIDE THERAPY:
Radio lodine treatment for Thyrotoxicosis ( < 10 mci)
Radio lodine treatment for Thyrotoxicosis ( > 10 mci)
Radioiodine therapy for Ca. Thyroid ( <100 mci )
Radioiodine therapy for Ca. Thyroid ( >100 mci )
rTSH Radioiodine therapy for Ca thyroid
177 Lutetium DOTA therapy for Neuroendocrine tumors.
177 Lutetium PSMA therapy for Ca. Prostate
Samarium Skeletal Pain Palliative Therapy
90 Yttrium Radiation Synovectomy.
131 Iodine Lipidiol TARE therapy for HCC.
90 Yttrium Lipidiol TARE therapy
Strontium 89 skeletal pain palliative therapy
PET SCAN (OUTSIDE ) REVIEW
CT CONTRAST (PET CT)
omnipaque
visipaque
oral contrast (PET CT)
RBC BLOOD POOL
F CHOLINE
0 notes
Text
#superspecialitycare#Outcome of Lu177 PSMA Therapy in India#Cost of Lutetium or Lu177 PSMA Therapy in India
0 notes
Text

Lutetium-177 PSMA therapy is a new, cutting-edge treatment for prostate cancer that is showing great promise. This therapy uses a radioactive isotope called lutetium-177, which targets and kills cancer cells while sparing healthy tissue. Lutetium-177 PSMA therapy is currently being used in clinical trials and has been shown to be effective in treating prostate cancer. This treatment is non-invasive just like Actinium (Ac225) PSMA Therapy and has very few side effects, making it an attractive option for patients with this disease.
TAG- Outcome of Lu177 PSMA Therapy in India, Cost of Lutetium or Lu177 PSMA Therapy in India
0 notes
Text
Trends | Future and Growth for Prostate Health Market
Prostate health is an important concern for men. The prostate is a small gland that is part of the male reproductive system. It is located just below the bladder and in front of the rectum. Its primary function is to produce fluid that transports sperm during ejaculation. Prostate health is important for a number of reasons.
An enlarged prostate can cause discomfort and can lead to urinary problems such as frequent urination, difficulty urinating, or a weak urine stream. An enlarged prostate can also increase the risk of prostate cancer. Other risks for prostate cancer include age, family history, and ethnicity.
There are a number of steps men can take to promote prostate health and reduce their risk of prostate cancer. Eating a balanced diet and engaging in regular physical activity can help keep the prostate healthy. Additionally, men should have a yearly prostate exam to check for any signs of prostate cancer.
According to a research report "Prostate Health Market by Disease Indication (Prostate Cancer, PARP Inhibitors, Cytotoxic Drug, Benign Prostate Hyperplasia (BPH), Tamsulosin, 5 Alpha Reductase, Prostatitis, OTC, Prescription (Rx), & Region (NA, Europe, APAC) - Global Forecasts to 2026" published by MarketsandMarkets, the global prostate health market is projected to reach USD 48.9 billion by 2026 from USD 31.8 billion in 2021, at a CAGR of 9.0% during the forecast period.
Request for assumptions & how numbers were triangulated.
https://www.marketsandmarkets.com/requestsampleNew.asp?id=107055093
Market Growth Drivers
Growing prevalence of prostate cancer and BPH
Market Growth Opportunities
Emerging economies (such as China, India, Brazil, and Mexico) are projected to offer significant growth opportunitie
Market Challenges
Low awareness regarding prostate health among men
Industry Trends
This represents an annual growth of about 7% and a total increase of 58% from 2010. Obesity and Benign Prostatic Hyperplasia (BPH) are closely associated.
Finasteride and Dutasteride (among other 5-ARIs) convert testosterone to dihydrotestosterone. These drugs also decrease the synthesis of several neuroactive steroids, but the modulation of the neuroendocrine stress response may lead to depression. Other side effects of 5-ARIs are gynecomastia, impotence, and reduced libido and ejaculate volume.
Download an Illustrative Overview:
https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=107055093
Some of the prominent players operating in the prostate health market are Eli Lilly and Company (US), Pfizer Inc. (US), Merck & Co., Inc. (US), GlaxoSmithKline plc. (UK), Abbott (US), and Astellas Pharma Inc. (Japan), and other players.
Research Developments
In March 2022, Lantheus Holdings, Inc. (US) collaborated with Novartis AG (Switzerland) to include PYLARIFY (piflufolastat F18) in prostate cancer clinical trials with Pluvicto (lutetium Lu 177 vipivotide tetraxetan) to support prostate cancer clinical development.
In March 2022, Novartis AG (Switzerland) received approval for PluvictoTM (lutetium Lu 177 vipivotide tetraxetan) (formerly referred to as 177Lu-PSMA-617) by FDA for the treatment of adult patients with a certain type of advanced cancer called prostate-specific membrane antigen–positive metastatic castration-resistant prostate cancer (PSMA-positive mCRPC).
In January 2021, Pharex Tamsulosin for the treatment of benign prostatic hyperplasia was introduced by PHAREX Health Corporation (Philippines) in collaboration with the Philippine Urological Association (PUA).
In October 2020, Israeli medical device manufacturer Butterfly Medical raised USD 7 million in a Series B round to develop an anatomically shaped nitinol implant positioned in the prostatic urethra to relieve BPH symptoms
Content Source:
1. https://www.prnewswire.com/news-releases/prostate-health-market-worth-48-9-billion-by-2026--exclusive-report-by-marketsandmarkets-301443961.html
1 note
·
View note
Text
World-first clinical trial to prevent prostate cancer deaths
World-first clinical trial to prevent prostate cancer deaths
Australian medical scientists are working to prevent deaths from prostate cancer as a world-first clinical trial reaches its second phase.
Researchers are testing the effectiveness of an experimental therapy, known as Lutetium-177-PSMA, combined with immunotherapy.
If found to be effective, it could establish a new global standard of care for patients.
Prostate cancer is the most common cancer in…

View On WordPress
0 notes
Link
#Nuclear Medicine Therapy in India#Transarterial Radioembolisation#I 131 MIBG Therapy#Actinium Ac 225 Therapy#Targeted Alpha PRRT#Lutetium 177 PSMA#Lu177 PSMA therapy#Lu psma therapy#targeted alpha psma therapy
0 notes
Text
Lutetium 177 Prostate Cancer Therapy | Nuclear Medicine Therapy
Experience optimized Prostate Cancer Therapy with Lutetium 177 at Nuclear Medicine Therapy. Our specialized approach in nuclear medicine offers tailored treatments, ensuring targeted solutions and enhanced patient outcomes for Prostate Cancer. Explore our innovative therapies today.
Know more at:
0 notes
Text
1.516. Tag - 05. Februar 2021
So, hier jetzt ein kurzer medizinischer Exkurs zu der Therapie, der ich mich hier aussetze. Seit einigen Jahren wird Lutetium-177 zur Therapie gegen neuroendokrine Tumore wie beispielsweise in der Prostata oder Bauchspeicheldrüse eingesetzt. Dazu wird Lu-177 an ein Eiweißmolekül, eine so genannte Fähre, gekoppelt und gelangt damit direkt in den Tumor. Lu-177 ist ein Betastrahler (Beta-Minus-Zerfall: Aussendung eines Elektrons und eines Elektron-Antineutrinos) mit der sehr geringen Reichweite von etwa zwei Millimeter, was bedeutet, dass das gesunde Gewebe praktisch nicht geschädigt wird. Die 225Actinium-PSMA-Therapie ist eine noch neuartigere, aber vielversprechende Therapie für das metastasierte, kastrationsresistente Prostatakarzinom. Sie ist vor allem für Patienten gedacht, die nach bereits erfolgter Lutetium-177-PSMA-Therapie ein fortschreiten der Tumorerkrankung aufweisen. Das Prinzip der Therapie basiert darauf, dass ein therapeutisch wirksamer Strahler (Actinium-225) an eine spezielle Spürsubstanz gebunden wird, die diesen Strahler gezielt zu den Tumorzellen des Prostatakarzinoms trägt, dort bindet und in die Zellen aufgenommen wird. Durch die lokale Bestrahlung kommt es dann zur selektiven Zerstörung der Tumorzellen bei weitgehender Schonung des umliegenden Gewebes. Die Spürsubstanz bindet dabei an das sogenannte Prostata-spezifische Membranantigen (PSMA), welches um ein vielfaches höher auf Zellen des Prostatakarzinoms und seiner Metastasen vorkommt. Dieser Bindungsmechanismus wird bereits sehr erfolgreich in der PSMA PET-CT-Diagnostik und bei der 177Lutetium-PSMA-Therapie genutzt. Die 225Actinium-PSMA-Therapie ist noch nicht zugelassen und wurde bisher nur an einer kleinen Anzahl von Patienten durchgeführt. Insbesondere das Nebenwirkungsprofil ist noch nicht umfassend bekannt. Die bis jetzt festgestellten signifikanten Nebenwirkungen sind: Einschränkung der Speicheldrüsenfunktion; Auswirkungen auf das Blutsystem und Einschränkung der Nierenfunktion. Im Vergleich zu der bereits durchgeführten 177Lutetium-PSMA-Liganden-Therapie, die eine Behandlung mit beta-Strahlern darstellt, handelt es sich hierbei um die Gabe einer mit einem radioaktiven alpha-Strahler markierten Substanz. Hierbei handelt es sich um einen höher energetischen Strahler, welcher eine höhere Rate von Doppelstrangbrüchen in den Tumorzellen hervorrufen kann und somit zum Zelltod der Tumorzelle führt. Ein weiterer Vorteil ist die kürzere Reichweite von lediglich ca. 0,04 mm, was eine Schonung des umgebenden Gewebes bedeutet. Die 225Actinium-PSMA-Therapie wird im Rahmen einer individuellen Heilmaßnahme während eines kurzen stationären Aufenthalts durchgeführt. Das soll erst einmal reichen. Heute geht es mir leider überhaupt nicht gut. Vielleicht ist dieses Actinium-225 doch ziemlich hart und verursacht eben ziemlich starke Knochenschmerzen. Keine Ahnung. Aber da muss ich durch. Jetzt steht die Ganzkörperszintigrafie an, vor der ich ziemlich großen Respekt habe. Man hat da das Gefühl, dass einem ein großer und sehr schwerer Granit-Block direkt aufs Gesicht fällt. Ist natürlich nicht so und ich begegne dem, indem ich mir eine Maske über die Augen ziehe. 30 Minuten lang. Da spürt man übrigens im Liegen auch sämtliche Knochen, weil sich dieses dämliche Gehirn nur darauf konzentriert. Ich werde es überstehen, aber mich nie daran gewöhnen. 48 Stunden nach meiner Litetium- und Actinium-Infusion kann ich entlassen werden. Das wäre also 13.00 Uhr am Samstag, dem 06.02.21. Da mache ich wieder 3 ganz große Kreuze. Auch, wenn ich dann erfahren werde, ob, wie und wann es weitergeht. Die Ganzkörperszintigrafie habe ich gegen 12.15 Uhr überstanden und ich muss sagen, es verlief eben nicht so extrem wie es beschrieben habe. Ist eben jedesmal anders und diesmal war es weitestgehend easy. Ich habe auch währenddessen die Schwester zugetextet. Den Ausdruck der Szintigrafie seht ihr hier unten. Deutlich erkennbar in der Vorder-/Rückansicht, dass die Tumorlast zurückgedrängt wurde. Keine Ahnung, ob hier das Actinium 225 schon gewirkt hat. Ich werde nachfragen! Ciao.


Foyer der Zentralklinik Bad Berka. Jawohl, der Pfeil zeigt genau, wo es hingeht. Nämlich „RAUS“ und ab nach Hause - am 06. Februar 2021, 12.45 Uhr.
3 notes
·
View notes
Text
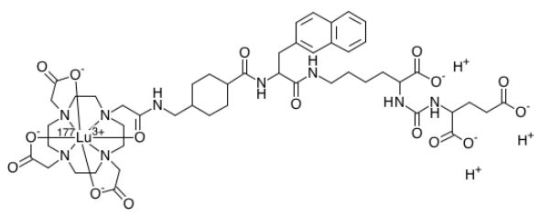
Lutetium Lu 177 vipivotide tetraxetan
Lutetium Lu 177 vipivotide tetraxetan
Lutetium Lu 177 vipivotide tetraxetan
FDA APPROVED 2022/3/23, Pluvicto
To treat prostate-specific membrane antigen-positive metastatic castration-resistant prostate cancer following other therapies
FormulaC49H65N9O16. Lu. 3HCAS1703749-62-5Mol weight1214.0819
Antineoplastic, Radioactive agent DiseaseProstate cancer (PSMA positive)
ルテチウム(177Lu)ビピボチドテトラキセタン;
UNII-G6UF363ECX, WHO…
View On WordPress
#APPROVALS 2022#ルテチウム(177Lu)ビピボチドテトラキセタン#FDA 2022#Lutetium Lu 177 vipivotide tetraxetan#PROSTRATE CANCER
0 notes
Text
FDA Approves Pluvicto (177Lu-PSMA-617)
Novartis and Advanced Accelerator Applications, the radioligand business of Novartis, has announced that their investigational treatment, Pluvicto™, was approved by the FDA. Pluvicto, lutetium Lu 177 vipivotide tetraxetan (formerly referred to as 177Lu-PSMA-617) is the first approved targeted radioligand therapy for the treatment of men with progressive, PSMA‑positive metastatic castration-resistant prostate cancer.
Specifically, the approval is only for the treatment of men with advanced prostate cancer that is metastatic (spread outside the prostate gland), castrate resistant (no longer responding to primary hormone therapy), and be prostate-specific membrane antigen (PSMA) positive. The approval also includes the requirement that the men should have already been treated with other anticancer treatments (androgen receptor pathway inhibition and taxane-based chemotherapy).
To confirm that the prostate cancer is PSMA positive, meaning that the tumor expresses the target, PSMA, along with the approval of Pluvicto, the FDA also approved a complementary diagnostic imaging agent, Locametz. Locametz is a radiolabeling method using gallium-68 to confirm and identify PSMA-positive lesions. Not all prostate cancer expresses PSMA. Since Pluvicto uses PSMA as a target, it will only be effective for those PSMA positive cancers.
The approval was based on pivotal Phase III VISION trial, where men with pre-treated PSMA-positive mCRPC who received Pluvicto plus standard of care had a statistically significant reduction in risk of death over those just treated with the standard of care (SOC).
The most common adverse events (all grades) in the Pluvicto arm of the study were fatigue (43%), dry mouth (39%), nausea (35%), anemia (low red blood cell counts) (32%), decreased appetite (21%), and constipation (20%).
Novartis also is running two additional Phase III studies evaluating Pluvicto in earlier stages of treatment for men with metastatic prostate cancer.
0 notes
Text
Lutetium-177 PSMA therapy is a new, cutting-edge treatment for prostate cancer that is showing great promise. This therapy uses a radioactive isotope called lutetium-177, which targets and kills cancer cells while sparing healthy tissue. Lutetium-177 PSMA therapy is currently being used in clinical trials and has been shown to be effective in treating prostate cancer. This treatment is non-invasive just like Actinium (Ac225) PSMA Therapy and has very few side effects, making it an attractive option for patients with this disease.
TAG- Outcome of Lu177 PSMA Therapy in India, Cost of Lutetium or Lu177 PSMA Therapy in India
0 notes
Text
Radioligand Therapy (RLT) Market Segmentation and Forecast Analysis up to 2025
Radioligand Therapy (RLT) Market: Introduction
· Radioligand therapy (RLT) is a targeted therapeutics option for a cancer treatment. Radiopharmaceutical is infused or injected into a peripheral vein. Ligands are labelled with radioactive isotopes, mostly beta-emitters, such as lutetium-177. High rate of long-lasting tumor remission and stabilization can be achieved using radioligand therapy (RLT).
· Radioligand therapy (RLT) is a systemic therapy which is used in metastasized disease treatment. In radioligand therapies, radiopharmaceuticals bind to a tumor target.
· NET radioligand therapy is also known as peptide-receptor radionuclide therapy (PRRT). NET peptides targeting the somatostatin-receptor (SSTR) are used for treatment of neuroendocrine tumors.
· For prostate cancer treatment, radioligand specifically binds prostate-specific membrane antigen (PSMA). Prostate-specific membrane antigens (PSMA) are overexpressed in prostate cancer.
Request a PDF Brochure - https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=74355
Key Drivers of Global Radioligand Therapy (RLT) Market
· The global radioligand therapy market is likely to be driven by increase in footprint of pharmaceutical manufacturers in potential markets in Asia Pacific, Latin America, and Middle East & Africa
· Research & development of radioligand therapy candidate to treat a broad range of cancer types is expected to propel the global radioligand therapy treatment market during the forecast period. Endocyte's Lu-PSMA-617 is a radioligand therapy candidate which is currently under phase III clinical trial for treatment of prostate cancer.
Request for Analysis of COVID19 Impact on Radioligand Therapy (RLT) Market- https://www.transparencymarketresearch.com/sample/sample.php?flag=covid19&rep_id=74355
· Increase in prevalence of cancer across the globe is expected to fuel the market growth during the forecast period. According to the American Cancer Society, in 2019, around 174,650 new cases for prostate cancer were diagnosed in the U.S.
· Strategic acquisition by leading players operating in radioligand therapy is helping manufacturers in expanding cancer product pipeline. Thus, approval and commercialization of pipeline products in the near future is expected to propel the market during the forecast period.
· In October 2018, Novartis underwent purchase agreement with Endocyte to purchase Endocyte's Lu-PSMA-617 clinical product
Request For Custom Research - https://www.transparencymarketresearch.com/sample/sample.php?flag=CR&rep_id=74355
North America to Hold Major Share of Global Radioligand Therapy (RLT) Market
· North America is projected to lead the market during the forecast period. North America is the leading market for radioligand therapy (RLT) driven by high rate of adoption of radioligand therapy products after FDA approval. According to Novartis, Lutathera has been prescribed to over 1,100 patients in the U.S.
· Increase in awareness about the radioligand therapy and rise in patient base suffering from cancer in the U.S. are expected to drive the market in the region. Manufacturers are focused to enter into partnership with laboratories for research & development of radioligand therapy candidate which is anticipated to propel the market in the U.S.
Pre-Book Radioligand Therapy (RLT) Market Report - https://www.transparencymarketresearch.com/checkout.php?rep_id=74355<ype=S
· Governments in Europe have favorable reimbursement policies. Furthermore, increase in research laboratories, rise in prevalence of cancer, and increase in funding provided by governments are anticipated to propel the market in the region during the forecast period.
More Trending Reports by Transparency Market Research:
https://www.prnewswire.com/news-releases/technological-advancements-and-innovations-to-fuel-growth-of-soft-tissue-repair-market-from-2018-to-2026-tmr-301168624.html
https://www.prnewswire.com/news-releases/global-oral-contraceptive-pills-market-projected-to-expand-at-6-cagr-rising-number-of-unplanned-pregnancies-drives-market-demand-tmr-301171827.html
About Us
Transparency Market Research (TMR) is a market intelligence company, providing global business information reports and services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insight for thousands of decision makers. TMR’s experienced team of analysts, researchers, and consultants use proprietary data sources and various tools and techniques to gather and analyze information. Our business offerings represent the latest and the most reliable information indispensable for businesses to sustain a competitive edge.
Each TMR syndicated research report covers a different sector - such as pharmaceuticals, chemicals, energy, food & beverages, semiconductors, med-devices, consumer goods and technology. These reports provide in-depth analysis and deep segmentation to possible micro levels. With wider scope and stratified research methodology, TMR’s syndicated reports thrive to provide clients to serve their overall research requirement.
Contact
90 State Street, Suite 700
Albany, NY 12207
Tel: +1-518-618-1030
USA - Canada Toll Free: 866-552-3453
Website: https://www.transparencymarketresearch.com/
0 notes