#Biopharmaceuticals
Text

Biopharmaceutics
3 notes
·
View notes
Text
#biotech#pharmaceutical#biopharmaceuticals#t cells#car t cells#biotechnology#biotechtrends#pharmaceutical industry#Pharmarep#Leezettelopatic#pharmaceuticalrep#biotech and pharmaceuticals
3 notes
·
View notes
Photo

(via Plasma Fractionation Market Growth: Trends by Region (US, Russia, S. Korea, UK) [April 2024])
#plasmatherapy#plasmadonation#rarediseases#biopharmaceuticals#healthcareinnovation#biotechinvestment
1 note
·
View note
Text
Plasma Fractionation Market Growth: Trends by Region (US, Russia, S. Korea, UK) [April 2024]
Plasma Fractionation Market: Explore growth trends in the US, Russia, South Korea, and the UK. Discover top industry leaders and the future of plasma-derived therapies. (April 2024)
Plasma Fractionation Market: A Look at Global Leaders and Regional Trends (April 2024)
The plasma fractionation market plays a vital role in healthcare, transforming donated blood plasma into life-saving therapies. These treatments address a wide range of conditions, including immune deficiencies, chronic diseases, and blood clotting disorders. As the global demand for plasma-derived medications…
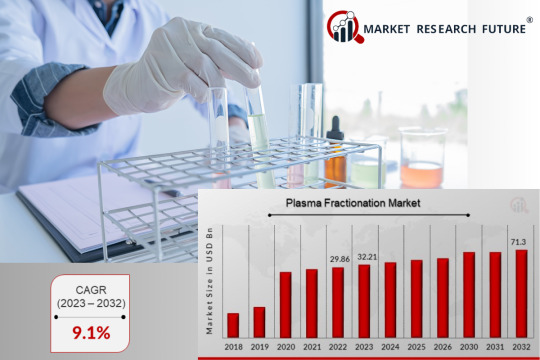
View On WordPress
#Biopharmaceuticals#BiotechInvestment#GlobalHealth#HealthcareInnovation#Immunotherapy#PersonalizedMedicine#PlasmaDonation#PlasmaShortage#PlasmaTherapy#RareDiseases
0 notes
Text


Products are getting better and better. How do you think?
#CEKG#Bioreactor#bioprocessing#upstream#VirusVaccines#AdenovirusVector#Protein-based#Vaccines#VLP#biotechnology#biotechindustry#biodefence#biofuels#biological#biopharmaceuticals
1 note
·
View note
Text
Powder compression, the primary technique for tablets manufacturing, involves applying pressure to force particles together in a confined space, creating a porous, solid specimen with defined geometry. This process occurs within a die using upper and lower punches to apply compressive force, resulting in a reduction in volume and the formation of bonds between particles. As a result, a compact, coherent tablet is produced.
The article:
#tablet manufacturing#manufacturer#industrial#pharmaceutical technology#pharmaceutical#pharmacy#physics#chemistry#biopharmaceuticals
1 note
·
View note
Text
PRISM MarketView Marks World Cancer Day

New York, N.Y., February 01, 2024 - PRISM MarketView, a leading provider of unbiased market insight and company news, today recognizes the tremendous contributions of companies striving to develop treatments for cancer, a complex disease driven by numerous factors. It is a leading cause of death worldwide, accounting for nearly 10 million deaths in 2020, and is the second leading cause of death in the United States.
To mark World Cancer Day, which will be held on February 4, PRISM MarketView highlights emerging companies working to improve the lives of cancer patients and their families by developing and commercializing innovative new treatments for cancer.
Panbela Therapeutics, Inc.
Biopharmaceutical company, Panbela Therapeutics, is developing disruptive therapeutics for patients with urgent unmet medical needs. The company's lead product candidates are Ivospemin (SBP-101), which has completed a Phase Ia/Ib clinical trial for the treatment of patients with metastatic pancreatic ductal adenocarcinoma. The company has entered a research agreement with the Johns Hopkins University School of Medicine for the development of Ivospemin. Panbela recently announced it has reached 50% enrollment for its global randomized, double-blind placebo-controlled ASPIRE clinical trial to evaluate ivospemin in combination with gemcitabine and nab-Paclitaxel in patients with metastatic pancreatic ductal adenocarcinoma. Panbela’s market cap stands at approximately $672,341.
Lisata Therapeutics, Inc.
Lisata Therapeutics, Inc., is developing and commercializing innovative therapies for the treatment of solid tumors. Its lead oncology product candidate is LSTA1, which is in Phase 1b/2a and 2b clinical studies for the treatment of solid tumor, including metastatic pancreatic ductal adenocarcinoma (mPDAC), in combination with a range of anti-cancer regimens. The company recently announced it had treated the first patient in a Phase 2a trial evaluating LSTA1 in patients with newly diagnosed glioblastoma multiforme (GBM). LSTA1 has been granted orphan drug designation by the FDA for malignant glioma. Lisata’s market cap stands at $21.163 million.
Rigel Pharmaceuticals, Inc.
Biotechnology company, Rigel Pharmaceuticals, is developing therapies that enhance the lives of patients with cancer. The company's commercialized products include Rezlidhia, a non-intensive monotherapy for the treatment of adult patients with relapsed or refractory (R/R) acute myeloid leukemia (AML). The company has entered a strategic development collaboration with The University of Texas MD Anderson Cancer Center for the development of REZLIDHIA (Olutasidenib) in acute myeloid leukemia (AML) and other hematologic cancers. The company recently announced a collaboration with CONNECT, an international collaborative network of pediatric cancer centers, to evaluate REZLIDHIA® in combination with temozolomide as maintenance therapy in newly diagnosed pediatric and young adult patients with high-grade glioma. Rigel’s market cap is currently $210.116 million.
AIM ImmunoTech
AIM ImmunoTech is an immuno-pharma company focused on the research and development of therapeutics to treat multiple types of cancers. The company's lead product candidate is Ampligen, which the company is evaluating for the treatment of renal cell carcinoma, malignant melanoma, non-small cell lung, ovarian, breast, colorectal, prostate and pancreatic cancer. The company recently announced is had enrolled the first subject in a Phase 1b/2 clinical trial combining AIM’s Ampligen® (rintatolimod) with AstraZeneca’s anti-PD-L1 immune checkpoint inhibitor Imfinzi® (durvalumab) for the treatment of late-stage pancreatic cancer. AIM ImmunoTech’s market cap is $20.851 million.
Essa Pharma
Essa Pharma, a clinical stage pharmaceutical company, is focused on the development of small molecule drugs for the treatment of prostate cancer. The company recently presented updated dose escalation data from its Phase 1/2 study evaluating masofaniten in combination with enzalutamide for the treatment of prostate cancer at the 2024 ASCO Genitourinary Cancers Symposium. It has collaboration agreements in place with Bayer Consumer Care AG; Janssen Research & Development, LLC; and Astellas Pharma Inc. and its share price has been trending upwards since October 2023. Essa Pharma’s market cap is currently sitting at $364.35 million.
About PRISM MarketView:
Established in 2020, PRISM MarketView is dedicated to the monitoring and analysis of small cap stocks in burgeoning sectors. We deliver up-to-the-minute financial market news, provide comprehensive investor tools and foster a dynamic investor community. Central to our offerings are proprietary indexes that observe emerging sectors, including biotech, clean energy, next-generation tech, medical devices and beyond. Visit us at prismmarketview.com and follow us on Twitter.
PRISM MarketView does not provide investment advice.
Media Contact
Company Name: Prism MarketView
Email: [email protected]
Phone: 646-863-6341
Website: https://prismmarketview.com
#press release#prism mediawire#stock market#investing#prismdigitalmedia#prismmarketview#healthcare#nasdaq#worldcancerday#biopharmaceuticals#Pharmaceutical
0 notes
Text
Single-Use Bioprocessing Market in the Quest for Efficient Biopharmaceutical Production
The global single-use bioprocessing market size is projected to reach USD 80.13 million by 2030, registering a compound annual growth rate (CAGR) of 16.24% over the forecast period, according to a new report by Grand View Research, Inc. The demand for single-use bioprocessing offerings is driven by the commercial advantages offered, including a reduction in costs and time required for bioprocessing operations. Originally used for monoclonal antibody production, single-use technologies are also gaining traction for cell and gene therapy manufacturing. As a result, broadening the scope of applications in biomanufacturing operations is likely to drive industry growth.

Single-use Bioprocessing Market Report Highlights
The simple & peripheral elements segment held the largest share in 2023 due to the significant adoption of these products as a result of a variety of customizable options available for bioprocessing applications
The upstream bioprocessing workflow segment accounted for the largest share in 2023. Continuous developments and betterment in technologies for upstream bioprocessing are driving the segment growth
North America was the leading region in 2023 due to the high R&D spending and growth of the biopharmaceutical manufacturing sector in the region
Furthermore, the presence of key players, such as Thermo Fisher Scientific, Inc. and Danaher Corp., is driving the regional market
The biopharmaceutical manufacturers end-use segment dominated the industry in 2023 and accounted for the maximum revenue share. This was due to the high demand for biologics and heavy investments in cell & gene therapy manufacturing
For More Details or Sample Copy please visit link @: Single-use Bioprocessing Market Report
Furthermore, strategic initiatives from key players are expanding the industry's growth prospects. For instance, in July 2021, Cytiva and Pall Corp. announced investment plans for capacity expansion over two years. Among other key products, more than USD 300 million were invested in single-use technologies, such as bioreactor bags for cell expansion, used to make personalized therapies and syringe filters for scientific research. Similarly, the growing adoption of single-use equipment for in-house and contract manufacturing has opened new avenues for the flow of investments in this space. The industry is witnessing significant advancements in several product portfolios, including disposable probes and sensors, stirring systems, bioreactor designs, and filtration technologies, which are expected to contribute to strong revenue growth.
The benefits offered by single-use bioprocessing systems have enabled biopharmaceutical manufacturers to offer their products faster to the market by introducing multi-product facilities, entering into partnerships, or outsourcing pipeline products for contract development and manufacturing. For instance, in January 2021, Sartorius AG partnered with RoosterBio, a leading supplier of human Mesenchymal Stem/Stromal Cells (hMSC). This collaboration aimed at advancing cell & gene therapy manufacturing by leveraging the single-use manufacturing technologies from Sartorius AG. The COVID-19 pandemic has generated new growth opportunities for key stakeholders in the industry.
#Single-Use Bioprocessing#Biomanufacturing#Bioprocessing Technology#Biopharmaceuticals#Disposable Technology#Sustainable Bioprocessing#Flexible Manufacturing#Bioproduction#BioProcess Containers#Bioreactors#Downstream Processing
0 notes
Text
Biopharmaceuticals Industry Drives High Growth Through Innovation In Cell And Gene Therapies
The biopharmaceuticals industry develops medicines from living cells and organisms to treat a wide range of medical conditions. Biopharmaceuticals, also known as biologics, include vaccines, blood components, allergenics, somatic cells, gene therapies, tissues, and recombinant therapeutic proteins. They can be composed of sugars, proteins, nucleic acids, or complex combinations of these substances, or may be living cells or tissues. The global biopharmaceutical industry is increasingly focusing on new cell and gene therapies to treat cancer and genetic diseases. Cell therapy often uses stem cells to replace or repair damaged tissues and cells, while gene therapy aims to fix defects by manipulating genes inside patient's cells and tissues. Major biopharmaceutical companies are making substantial investments in R&D for cutting-edge cell and gene therapies with the potential to treat previously untreatable medical conditions.
The global biopharmaceuticals Market is estimated to be valued at US$ 397.24 Bn in 2023 and is expected to exhibit a CAGR of 9.4% over the forecast period 2023 to 2030, as highlighted in a new report published by Coherent Market Insights.
Market Dynamics:
The growth of the global biopharmaceuticals market is driven significantly by continued innovation in cell and gene therapies. According to the report by CMI, biopharma companies invested over $100 billion globally in R&D for cell and gene therapies in 2021 alone. Cell therapies have shown promising results in cancer immunotherapy by leveraging the immune system to fight tumors. Additionally, gene therapies aim to treat genetic diseases such as hemophilia at their root cause by fixing the defective genes. The high success rate of recent gene therapy clinical trials is fueling further investments in this area. While cell and gene therapies offer potential cures, their development requires substantial funding and brings high risks. Overall, continued biomedical advances are critical to maximize the healthcare outcomes from the multi-billion dollar biopharma industry.
SWOT Analysis
Strength: The biopharmaceuticals market is dominated by large companies with widespread R&D facilities and huge financial resources. These large players hold a major share of the market and have strong brand recognition. Biopharmaceuticals have minimal side effects compared to traditional drugs. They can be customized for individual patients which improves treatment effectiveness.
Weakness: Biopharmaceuticals have a lengthy and complex development process making them highly capital intensive. The development of biologics requires specialized expertise and infrastructure which increases costs significantly. Some biopharmaceuticals also have short shelf lives and require specialized storage and distribution networks.
Opportunity: The rapidly aging global population is increasing the demand for therapies to treat chronic diseases. Biopharmaceuticals are increasingly being used to treat conditions like cancer, immunological disorders, and cardiovascular diseases. Developing economies in Asia Pacific and Latin America offer huge market potential due to rising healthcare expenditure.
Threats: Price controls and drug reimbursement policies enacted by various governments pose pricing challenges. Stricter regulations for approval and safety monitoring also increase compliance costs. Expiry of patents for blockbuster drugs results in competition from cheaper biosimilars. Outsourcing of manufacturing and lax regulatory oversight in some nations threatens quality assurance.
Key Takeaways
The global biopharmaceuticals market growth is expected to witness high growth over the forecast period supported by rising demand for specialty drugs and biologics globally. The global biopharmaceuticals Market is estimated to be valued at US$ 397.24 Bn in 2023 and is expected to exhibit a CAGR of 9.4% over the forecast period 2023 to 2030.
The North American region currently dominates the market owing to advanced research capabilities and strong presence of leading players. However, Asia Pacific is emerging as the fastest growing regional market due to growing health awareness, increasing healthcare spending, and improving research infrastructure in countries like China, India, and South Korea. The presence of contract manufacturing hubs in India and China coupled with improving research capabilities is attracting significant investments from global pharmaceutical giants. Furthermore, rising affluence, increased awareness, and favorable government policies are driving healthcare spending upwards in major Asian economies. Countries like India, China, South Korea, and Singapore are actively promoting the development of biologics and biosimilars.
Key players
Key players operating in the biopharmaceuticals market are Accenture, Teleperformance SE, Infosys Limited (Infosys BPM), WNS (Holdings) Ltd., HCL Technologies Limited, AMDOCS, CBRE Group Inc., Sodexo, NCR Corporation, TTEC Holdings, Inc., Wipro Limited, and Capgemini. These companies offer integrated services and solutions across the value chain to help pharmaceutical companies optimize operations and reduce costs.
Get more insights on this topic: https://www.newsstatix.com/biopharmaceuticals-market-industry-insights-trends-biopharmaceuticals-market/ Explore more information on this topic, Please visit: https://filmik.in/molecular-cytogenetics-the-future-of-genomic-research/
#Biopharmaceuticals#Biopharmaceuticals Market#Biopharmaceuticals Market size#Biopharmaceuticals Market share#Biopharmaceuticals Market demand#Biopharmaceuticals Market analysis
0 notes
Text
Understanding the Dynamics of the Fill Finish Manufacturing Market
Almost every day, we rely on pharmaceuticals like ear drops, eye drops, nasal sprays, and vaccines to be sterile, hygienic, and free from contamination. Because these parenteral products are injected or introduced to delicate mucus membranes, adding something non-sterile to your eye, or nose, or injected into your muscle, skin, or vein, can have serious adverse effects, such as a fever or infection. This requires a special manufacturing process called aseptic processing, or fill-finish manufacturing, which addresses risks through a range of cleaning, sterilization, and isolation practices. It is expected that the fill-finish manufacturing market will continue to grow in the areas of fill-finish and lyophilization to support the rising parenteral drug product manufacturing.
Fill-finish operations, including all the processes and activities that get the pharmaceutical component ready for distribution and commercialization as a Drug Product (DP), cover a significant portion of the whole biopharmaceutical manufacturing, in terms of both process complexity and processing costs. Many pharmaceutical companies have shifted their focus from developing blockbuster drugs to creating treatments for rare diseases, also known as orphan drugs. Often these rare disease treatments require parenteral drug formulations. The increase in rare disease treatment development and the increase in prefilled syringe usage for sterile-fill finish biologics contribute to the increasing popularity and need for sterile-fill finish manufacturing operations. As the demand for sterile fill-finish operations rises, pharmaceutical companies are partnering with contract manufacturers that can support the fill-finish production needs of their unique parenteral product.
0 notes
Text
Europe Biopharmaceutical Processing Equipment and Consumables Market by Product Type {Filtration, Chromatography [Consumables, Equipment], Disposable Bioreactors, Cell Culture Media, Shakers, Services), Application (Vaccine, mAb, R&D), and End User - Forecast to 2030
#Europe Biopharmaceutical Processing Equipment and Consumables Market#Biopharmaceutical Processing Equipment and Consumables Market#Biopharmaceutical Processing Equipment#biopharma#biopharmaceuticals#bioprocessing#biotechnology
0 notes
Text
3 Key Challenges for Biopharma Marketers Trying to Reach Healthcare Providers
Discover the top three challenges faced by biopharma marketers when targeting healthcare providers, and learn how to overcome them.
0 notes
Text
The Evolving Landscape of Healthcare Cold Chain Logistics in 2024
The healthcare cold chain logistics market is booming, driven by the need for efficient and sustainable transportation of temperature-sensitive medical products
The Booming Landscape of Healthcare Cold Chain Logistics
The healthcare industry relies heavily on the secure and efficient transportation of temperature-sensitive medical products. This intricate network is known as the healthcare cold chain logistics market, expected to reach a staggering USD 23.65 billion by 2030 at a CAGR of 7.89%.
Market Segmentation: A Multifaceted Approach
The market…

View On WordPress
#biopharmaceuticals#blockchain#coldchain#coldchaintechnology#HealthcareInnovation#healthcarelogistics#logistics#pharmaceuticallogistics#sustainability#vaccinelogistics
0 notes
Text

At Manus Aktteva Biopharma LLP, we are an ISO 9001:2015 certified global sourcing enterprise based in India, and we serve a wide variety of institutional, private, and corporate clients throughout the globe with active pharmaceutical ingredients and intermediates, as well as other products like botanical extracts and phytochemicals. Thanks to our unique location, the decades of expertise of our team members, and our network of active pharmaceutical ingredient suppliers, we have become industry leaders for multinational organizations that source chemical and pharmaceutical ingredients from India.
1 note
·
View note
Text
SELLAS Advances Blood Cancer Treatment with “Tremendous Therapeutic Promise”

October 11, 2023
SELLAS Life Sciences Group, Inc. (Nasdaq: SLS) has reported two significant milestones this week as it pursues development of its drug candidate, SLS009 for hematological malignancies.
“SLS009 is a novel and highly selective CDK9 inhibitor which, to date, has shown tremendous therapeutic promise across multiple blood cancers.” - Angelos Stergiou, MD, ScD h.c., President and Chief Executive Officer of SELLAS

The company has dosed the first patient in a Phase Ib/II trial evaluating SLS009 (GFH009) in relapsed/refractory Peripheral T-cell Lymphomas (PTCL). SELLAS has also received Orphan Drug Designation (ODD) for SLS009, a novel and highly selective CDK9 inhibitor, for the treatment of acute myeloid leukemia (AML).
Angelos Stergiou, MD, ScD h.c., President and Chief Executive Officer of SELLAS, said, “We are pleased with the initiation of the Phase Ib/II trial of SLS009 in the underserved PTCL patient population. We are also honored to receive the ODD from the FDA. This designation underscores the potential of SLS009 to address a significant unmet medical need for patients with AML.”

Highlights - Acute Myeloid Leukemia
SLS009 is a highly selective CDK9 inhibitor that has already demonstrated a favorable safety profile, strong initial efficacy signals, and evidence of anti-tumor activity. It is currently being evaluated in an open-label, single-arm, multi-center Phase 2a study in patients with relapsed or refractory AML. Top-line data are expected by the end of this year.
ODD provides benefits to drug developers including assistance in the drug development process, tax credits for qualified clinical costs, exemptions from certain FDA fees and seven years of marketing exclusivity.

Highlights - Peripheral T-cell Lymphomas
SELLAS’ Phase Ib/II study is an open-label, single-arm trial of up to 95 patients, evaluating safety and efficacy. The study is fully funded by GenFleet and is being conducted in China.
The company stated that collaborating with GenFleet amplifies the potential of its highly selective CDK9 inhibitor in multiple indications and reflects the companies’ joint commitment to delivering this groundbreaking treatment to cancer patients globally.
SELLAS and GenFleet Therapeutics (Shanghai), Inc. have entered into an exclusive license agreement that grants rights to SELLAS for the development and commercialization of SLS009 (GFH009), a highly selective small molecule CDK9 inhibitor, across all therapeutic and diagnostic uses worldwide outside of Greater China (mainland China, Hong Kong, Macau, and Taiwan).
About SELLAS Life Sciences: SELLAS is a late-stage clinical biopharmaceutical company focused on the development of novel therapeutics for a broad range of cancer indications. SELLAS’ lead product candidate, galinpepimut-S (GPS), is licensed from Memorial Sloan Kettering Cancer Center and targets the WT1 protein, which is present in an array of tumor types. GPS has potential as a monotherapy and combination with other therapies to address a broad spectrum of hematologic malignancies and solid tumor indications. The Company is also developing SLS009 (formerly GFH009), a small molecule, highly selective CDK9 inhibitor, which is licensed from GenFleet Therapeutics (Shanghai), Inc., for all therapeutic and diagnostic uses in the world outside of Greater China. For more information on SELLAS, please visit www.sellaslifesciences.com.
#press release#prism mediawire#stock market#investing#nasdaq#biopharmaceuticals#cancer treatment#healthcare#medical#SELLAS Life Sciences#sls
0 notes
Text
Clinical Trials : Holistic Exploration of the Current State and Future Outlook
The global clinical trials market size is expected to reach USD 123.5 billion by 2030, expanding at a CAGR of 6.49 from 2024 to 2030, according to a new report by Grand View Research, Inc. An increase in the volume and complexity of clinical trials has been witnessed lately, which plays an important role in the R&D of new drugs and products. The market witnessed a decline of 6% in 2020 owing to the COVID-19 pandemic. However, the market is projected to recover from 2021 onwards. In addition, clinical trials have become increasingly costly, adding to the overall cost of developing a drug.

Clinical Trials Market Report Highlights
The phase III clinical trials segment dominated the market with a 53.3% share in 2023. This can be attributed to the complexity of this phase
The interventional studies segment dominated the market in 2023. It is one of the most prominent methods used in clinical trials in the study design segment owing to the increasing demand for the intervention for clinical trials by researchers
North America held 50.3% of the market share in 2023. Favorable government initiatives and the presence of a large number of players in the U.S. that offer advanced services are responsible for market growth
Asia Pacific region is anticipated to grow at the fastest CAGR over the forecast period owing to the increasing patient pool and cost-efficient services.
For More Details or Sample Copy please visit link @: Clinical Trials Market Report
The increasing need for developing new drugs for chronic diseases, such as cancer, respiratory disorders, diabetes, cardiovascular diseases, and others, is creating immense pressure on the healthcare industry. The COVID-19 pandemic and the increasing demand for developing a suitable treatment are driving the market. The high number of people affected by the disease further depicts an increasing need for therapeutics & vaccines. Currently, there are 288 therapeutics and 106 vaccines under development, out of which, nearly 7.0% of therapeutics are in Phase IV, 21.0% in Phase III, and 43.0% & 13.0% in Phase II & Phase I, respectively.
The pandemic has resulted in the global disruption of traditional onsite clinical trials. Hence, regulatory bodies worldwide have undertaken various initiatives for fast-tracking clinical trials for the development of innovative solutions. One such instance is Solidarity, an international clinical trial launched by the WHO to find effective treatment against COVID-19. Although the pandemic has forced many medical device & drug developers to revise the approach to such crises, integrating best practices within clinical trial procedures & adapting to virtual trials, which can support the continuous development of therapeutics.
ClinicalTrials #HealthcareResearch #MedicalInnovation #DrugDevelopment #PatientRecruitment #Biopharmaceuticals #ClinicalResearch #RegulatoryCompliance #DataManagement #PatientEngagement #PrecisionMedicine #TherapeuticTrials #CROs #ClinicalResearchOrganizations #GlobalHealth #ClinicalStudyDesign #PharmaceuticalIndustry #BiotechResearch #ClinicalEndpoints #HealthTechIntegration
#Clinical Trials#Healthcare Research#Medical Innovation#Drug Development#Patient Recruitment#Biopharmaceuticals#Clinical Research#Regulatory Compliance#Data Management#Patient Engagement#Precision Medicine#Therapeutic Trials#CROs#Clinical Research Organizations#Global Health#Clinical Study Design#Pharmaceutical Industry#Biotech Research#Clinical End-points#HealthTech Integration
0 notes