#for evaluating the extended 1D universe
Text
Researchers create machine learning model predicting long-term vision in high myopia patients, a top cause of irreversible blindness
- By Nuadox Crew -
In a study conducted by Tokyo Medical and Dental University (TMDU) researchers, a machine-learning model has been devised to foresee the future visual outcomes of individuals grappling with severe shortsightedness, scientifically termed high myopia.
This model aims to predict whether these individuals will experience positive or negative vision changes down the line.
High myopia, a condition characterized by the inability to focus on distant objects while maintaining clarity in close-range vision, poses a significant risk of irreversible blindness, making it a pressing global health concern. The research, recently featured in JAMA Ophthalmology, sought to harness the power of machine learning to forecast the likelihood of visual impairment over extended periods.
By scrutinizing data collected from nearly a thousand patients at Tokyo Medical and Dental University's Advanced Clinical Center for Myopia, the team embarked on a comprehensive analysis. They meticulously evaluated 34 key variables routinely recorded during ophthalmic examinations, encompassing factors like age, current visual acuity, and corneal diameter. Employing various machine-learning algorithms, including random forests and support vector machines, the researchers discovered that a logistic regression-based model exhibited the highest efficacy in predicting visual impairment at 3- and 5-year intervals.
Yet, the study didn't stop at predictive accuracy. Recognizing the importance of translating complex data into practical clinical insights, the researchers ingeniously crafted a nomogram. This graphical representation of the model's outcomes serves as a user-friendly tool for both clinicians and patients. Each variable's significance in predicting future visual acuity is visually depicted as a line, convertible into points that collectively signify the risk of impending visual impairment.
The implications of this research extend beyond its technical achievements. For individuals facing the dire consequences of vision loss, both financially and physically, this advancement could offer a beacon of hope. The global economic impact of severe visual impairment was estimated at a staggering USD 94.5 billion in 2019.
While further validation on a larger scale remains imperative, this study underscores the potential of machine-learning models in combating the escalating public health challenge posed by high myopia-induced sight loss.
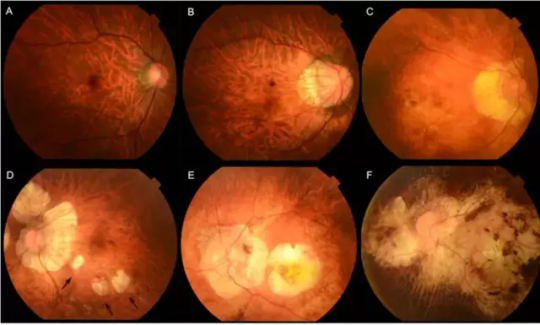
Image: Myopic maculopathy, also referred to as myopic macular degeneration, stands as a prominent characteristic of pathologic myopia. Within the META-PM classification system, myopic maculopathy lesions are grouped into five categories: from no myopic retinal lesions (category 0) to macular atrophy (category 4), including tessellated fundus (category 1, Figure 1A), diffuse chorioretinal atrophy (category 2, Figure 1B&C), patchy chorioretinal atrophy (category 3, Figure 1D arrows), and macular atrophy (category 4, Figure 1E&F). Credit: Tokyo Medical and Dental University.
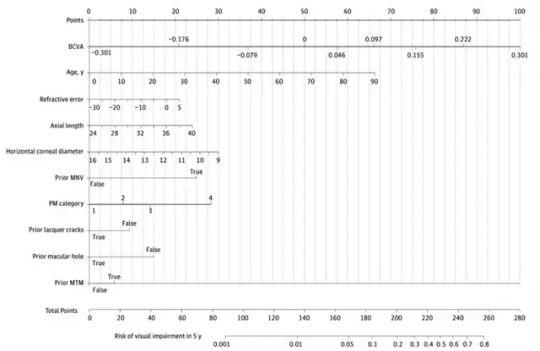
Image: A visual representation, known as a nomogram, was built to depict the model's insights. Longer lines indicate stronger variable impact on visual impairment (VI) after 5 years. Each variable corresponds to points, tallying up to calculate total points and the associated risk of VI. Credit: Tokyo Medical and Dental University.
--
Source: Tokyo Medical and Dental University (TMDU)
Full study: Wang Y, Du R, Xie S, et al. Machine Learning Models for Predicting Long-Term Visual Acuity in Highly Myopic Eyes. JAMA Ophthalmol. Published online October 26, 2023. doi:10.1001/jamaophthalmol.2023.4786
Read Also
New AI could predict whether or not those at high risk will develop glaucoma
#medtech#ophthalmology#eye#eyes#blindness#ai#artificial intelligence#diagnostics#health#medicine#health tech#machine learning
0 notes
Note
Who Antonio are you talking about?
Harry Lambert's boyfriend is called Antonio Pignone. He's one of my favourite people in the extended 1D universe.
#And yes my main criteria#for evaluating the extended 1D universe#is how much they hate the Tories#and quote Shon Faye#Ash Sarkar#and Owen Jones#But that's also the only criteria that really matters
2 notes
·
View notes
Text
Acquire Sarms Uk All Supplements For Sale @ Our Online Shop
Collagen
Content
Best Reviewed Items.
Cardio Lowers Body Fat.
Select Products.
18rik Peptide.
The Sarms Bible.

In order to establish which enteral feed is finest matched to a patient; the dietitian will certainly carry out an extensive evaluation of nutritional status and current medical problem. The prescription of the feed is tailored to fulfill private needs based on an individuals' details dietary needs and also the goals of the intervention. Pertinent medical details need to be sent with the demand, including scientific sign for testing, appropriate symptoms and also past case history. NT-proBNP is launched into the circulation in equivalent total up to the energetic hormone yet is considerably extra stable and for this reason forms a good pen of BNP outcome. In heart failure the heart can not pump highly enough for the body's requirements, the heart walls are stretched and fluid begins to collect causing back pressure and also hence extra BNP to be released. In people there are ~ 90 genes encoding neuropeptide forerunners, which are processed to ~ 100 bioactive neuropeptides. Neuropeptides usually co-exist with other neurotransmitters in specified cell populations, yet are had in different storage blisters.
Does LGD 4033 cause gyno?
Gyno: in some cases individuals have experienced gyno (tissue growth under the nipple) when using higher doses and not following appropriate pct. This should be monitored when using lgd-4033 if it is a concern, and the correct pct should be used.
As expected, the differences in proteolytic tasks and healthy protein hydrolysis patterns are even more noticeable when comparing different Lactobacillus varieties. A comparable observation was made when contrasting 14 stress of Lb. It is currently acknowledged that diet regimen plays a crucial function in the upkeep of our health and wellness condition. Listed below, some of the technical, regulatory, and business difficulties to bring AMP-based medicines right into the professional advancement are highlighted. Besides direct administration of AMPs, there are several efforts ongoing to make use of agents to enhance the endogenous manufacturing of AMPs by the body in order to boost the innate immune feedbacks and also thereby battle infections.
Best Reviewed Products.
If incapable to send sample promptly, freeze at -20 ° C and send at ambient temperature in the post. For long-term storage (e.g. to set samples), we suggest cold at -80 ° C.

This will take around 1 day, depending on example concentration. These initial information will certainly enable us to review the moment and the experimental conditions (i.e. sort of classified cores, optimum healthy protein, barrier focus as well as pH) required to acquire a high resolution architectural determination on the individual's protein. There were no distinctions hurting or practical ratings across the trial, yet there were differences at a long time factors which favoured the collagen team when feature was gauged in a second way.
Cardio Reduces Body Fat.
You can think of it as the adhesive that holds all these things with each other. In fact, words collagen originates from the Greek word "kólla," which indicates adhesive. Mix 1-2 scoops right into a big mug of water, tea, coffee or juice or add to foods such as gruel, soups as well as smoothie mixes. When a peptide consists of an internal proline, strong ion series due to internal bosom are observed, extending from the proline in the direction of the C terminus. The very first NMR experiments obtained will certainly explore the state of the healthy protein and also it's viability for further study at the picked NMR healthy protein concentration, acquiring 1D 1H and/or 2D 15N HSQC ranges.

The carboxylic team sheds the oxygen and hydrogen while the thiol group loses its hydrogen and a thioester bond is formed. Based upon the stereochemistry of the anomeric carbon or its orientation precede, a glycosidic bond can either be an alpha-bond or a beta-bond. In an O-glycosidic linkage, the carbonyl group of carbs reacts with the hydroxyl group of another compound.
Select Products.
This leads to a compound in which the sugar or carb deposit is attached to the oxygen of the other compound, thus the name O-glycosidic bond. For arbitrary non-fasting pee collections, outcomes are strongly associated with mixed meal C-peptide, with high level of sensitivity and also specificity for recognizing scientifically pertinent thresholds. Stable for 3 days in Boric acid containers at ambient temperature level.
In order to get to the cytoplasmic membrane layer of Gram-negative microorganisms, AMPs need to first translocate through the outer membrane. This model recommends that, as a result of better fondness for the LPS, AMPs displace the divalent cations and also bind to the LPS. By being cumbersome, the AMPs after that cause short-term cracks as well as permeabilize the external membrane, thereby allowing passage of the peptide itself across the membrane.
" I am checking out my legal choices, in terms of where I stand and what I can do.
Responsible sporting activities nourishment makers and merchants guarantee their products are extremely clearly labelled as well as stick to EU regulation.
" These companies need to be scared to put things like ostarine into their products", he suggests.
Virtually all performance-enhancing substances that are outlawed by organisations like WADA and also the IOC are also outlawed available in the European Union.
' Everything from the composition, classifying to the marketing and advertising has to comply with the EU laws implemented to protect consumers.
That may cost me a great deal of cash and I'm uncertain I can pay for to do that.
Neuropeptides are held within big dense-core vesicles throughout the cell body, whereas natural chemicals are included in small vesicles situated at synapses. The Open College is incorporated by Royal Charter, an exempt charity in England & Wales and also a charity registered in Scotland. The Open University is authorized and also managed by the Financial Conduct Authority in connection with its secondary activity of credit scores broking. Not prepared for College study then surf over 900 complimentary courses on OpenLearn and also join to our newsletterto find out about brand-new complimentary programs as they are launched. After a month of use can observe nicer appearance of skin, nails as well as hair. I simulate it and am constantly attempting to add collagen to my diet regimen currently im aging and also yes I would certainly utilize this once again.
18rik Peptide.
Most of the compounds in use today are of the androstenone family. There are several different classes of compounds with differing androgenic activity. Testosterone is the most popular and most widely used anabolic compound. The testosterone compound, as well as DHEA, androgene, are all classified as androgens and have androgenic activity. A number of compounds with known androgenic properties are being investigated for their ability to treat male impotency. Various combinations of androgens and estrogens have shown to be more effective in promoting sexual performance than either compound alone.
youtube
Sarms are small, sticky white blood cells that play an important role in the immune system. They help to fight off infections by stimulating white blood cells and stimulating natural killer cells to kill infection-causing bacteria. This is perhaps why sarms are often called "ice bacteria killers".
Selective androgens, including SARMs, are a new class of compounds known as androgens. most popular UK sarms post cycle therapy supplement Sarms were initially discovered in the 1970s by scientists hoping to find a way to treat enlarged prostate (benign prostatic hypertrophy). They worked out how to convert the ostarine amino acid into an inactive form that was inactive in the prostate and therefore not harmful to the prostate. Since then, researchers have shown great interest in the properties of SARMs and in how they might be beneficial to men with erectile dysfunction.
Test 4 consisted of UK sterile water 2ml with moderate to moderate osteoarthritis of the knee. Participants were offered either a collagen formula (Fortigel ®) or a placebo for 24 weeks. Those that got collagen reported a greater decrease in pain. In this trial, 250 people with osteoarthritis of the knee were randomised to obtain either 10 g collagen hydrolysate or a placebo daily for six months. This test consisted of 389 individuals with osteo arthritis across 20 websites in the UK, U.S.A. as well as Germany.
Using Lactobacillus strains for BAP production is a method that still deals with restrictions. Applications of BAPs generated by Lactobacillus species relying on the manufacturing technique.
Individuals were randomised to obtain either 10 g of collagen hydrolysate or placebo tablet computers for 24 weeks. Type II collagen showed less inflamed joints, joint tenderness as well as much better stroll time in only one of the trials against a sugar pill. Four tests evaluated collagen versus a placebo and also one evaluated it versus methotrexate. The tests for included in between 60 and 503 participants with rheumatoid joint inflammation.
Will rad140 show up on a drug test?
RAD140 and the majority of the identified in vitro metabolites were detected in post‐administration urine samples. For controlling the misuse of RAD140 in horses, RAD140 and its metabolite in sulfate form gave the longest detection time in hydrolysed urine and could be detected for up to 6 days post‐administration.
I can not see the difference that carefully due to the fact that no person understands your face as you do however I can certainly see a difference in her skin, particularly when she smiles. So, seemingly it not only offers help with your bones and also joints etc but appears to plump your skin. try this product advise making collagen part of your day-to-day routine and for the long-term to see ideal results.
It's such a wonderful way to obtain all the benefits across all foods and also drinks! I do want it came in bigger bathtubs as I make it through mine quite quickly considering I make use of 2-3 scoops relying on the recipes I'm making. In conclusion I can guarantee this item and also extremely recommend it. With the Rite-Flex Collegen Peptides I have actually observed my skin was looking much better after a few weeks, include it to your coffee or morning meal. Gotten a bathtub for my better half to attempt as well as she vows blind that several of the little folds in her skin have disappeared.
Texas Sport Supplement Company Owner Pleads Guilty to Unlawful Distribution of Steroid-Like Drugs - Department of Justice
Texas Sport Supplement Company Owner Pleads Guilty to Unlawful Distribution of Steroid-Like Drugs.
Posted: Tue, 22 Dec 2020 08:00:00 GMT [source]
Some researches have actually suggested that autoimmune diseases like rheumatoid joint inflammation may be dealt with by taking a foreign antigen by mouth, which might dampen down your body immune system's reaction. Taking collagen by mouth may present some chemicals that create joint inflammation right into your body and produce dental tolerance to these antigens, lowering the effects of inflammatory arthritis. Glycoproteins where the sialic acid has actually been eliminated are designated by the prefix asialo-, e.g. asialo-α1-acid glycoprotein, as well as asialofetuin. Removal of both sialic acid and galactose results in asialo-agalactoglycoproteins.
Currently, the two most commonly used compounds in use for sports are Dianabol and Prednisolone. These compounds are available over the counter and are prescribed to athletes by athletic trainers and doctors without any prescription. Anabolic steroids are banned by the Olympic Games and other major sporting associations. It is against the law for athletes to use anabolic steroids if they are participating in sanctioned sports. Therefore, it is against the rules to give an anabolic drug to an athlete without a prescription from a licensed physician.
Lamb submaxillary glycoprotein, collagen, fish antifreeze glycoproteins and potato lectin are O-glycoproteins (or O-glycosylproteins). collagens, fish antifreeze glycoproteins, lamb submaxillary glycoproteins], as well as those that contain oligosaccharides that cansist of duplicating devices of N-acetyllactosamine (e.g. band 3 of the human erythrocyte membrane layer). Optimization of manufacturing of protease by Lactobacillus plantarum SK from bekasam with response surface area technique.
#Shop Uk needle with 1ml fixed needle syringe#Shop Uk sterile water 2ml#Shop Uk bacteriostatic water 10ml#Shop Uk alcohol swab#Shop Uk gw 501516 cardarine Sarms#Shop Uk lgd 4033 ligandrol Sarms#Shop Uk mk 2866 ostarine Sarms#Shop Uk mk 677 ibutamoren Sarms#Shop Uk rad 140 testolone Sarms#Shop Uk s4 andarine Sarms#Shop Uk sr9009 stenabolic Sarms#Shop Uk shredding stack Sarms#Shop Uk muscle stack Sarms#Shop Uk bulking stack Sarms#Shop Uk cutting stack Sarms#Shop Uk yk 11 Sarms#Shop Uk sarms post cycle therapy supplement Sarms#Shop Uk sarms cycle support supplement Sarms#Shop Uk sarms test base supplement Sarms#UK needle with 1ml fixed needle syringe#UK sterile water 2ml#UK bacteriostatic water 10ml#UK alcohol swab#UK gw 501516 cardarine Sarms#UK lgd 4033 ligandrol Sarms#UK mk 2866 ostarine Sarms#UK mk 677 ibutamoren Sarms#UK rad 140 testolone Sarms#UK s4 andarine Sarms#UK sr9009 stenabolic Sarms
2 notes
·
View notes
Text
Do Collagen Beverages Function? A Skin Doctor And Nutritionist Evaluate In.
Collagen
Content
Best Examined Products.
Cardio Minimizes Body Fat.
Select Items.
18rik Peptide.

In order to establish which enteral feed is ideal fit to a client; the dietitian will certainly embark on a strenuous assessment of dietary status and also current professional condition. The prescription of the feed is tailored to fulfill specific demands based on an individuals' details nutritional requirements and also the objectives of the treatment. Pertinent medical details must be sent out with the demand, consisting of medical sign for screening, appropriate signs and symptoms as well as previous case history. NT-proBNP is launched right into the flow in equivalent total up to the energetic hormone yet is dramatically much more stable as well as thus develops a good marker of BNP outcome. In cardiac arrest the heart can not pump highly sufficient for the body's demands, the heart wall surfaces are stretched and fluid begins to collect triggering back stress and thus a lot more BNP to be released. In people there are ~ 90 genes encoding neuropeptide forerunners, which are refined to ~ 100 bioactive neuropeptides. Neuropeptides often co-exist with various other natural chemicals in defined cell populations, however are contained in separate storage blisters.
Does LGD 4033 cause gyno?
Gyno: in some cases individuals have experienced gyno (tissue growth under the nipple) when using higher doses and not following appropriate pct. This should be monitored when using lgd-4033 if it is a concern, and the correct pct should be used.
As expected, the distinctions in proteolytic activities and protein hydrolysis patterns are a lot more visible when contrasting various Lactobacillus species. A similar monitoring was made when comparing 14 pressures of Pound. It is currently recognized that diet regimen plays a key function in the upkeep of our wellness status. Listed below, a few of the technological, regulatory, as well as business challenges to bring AMP-based medicines right into the medical advancement are highlighted. Other than straight management of AMPs, there are a number of efforts ongoing to make use of agents to raise the endogenous production of AMPs by the body in order to boost the natural immune reactions and thereby battle infections.
Best Assessed Items.
If not able to send example quickly, freeze at -20 ° C as well as send out at ambient temperature level in the message. For long-lasting storage space (e.g. to batch samples), we advise freezing at -80 ° C.

This will take about 1 day, depending upon example focus. These initial data will permit us to review the moment and the speculative problems (i.e. sort of identified cores, optimum healthy protein, barrier focus and pH) needed to obtain a high resolution architectural determination on the user's protein. There were no distinctions in pain or practical scores across the trial, yet there were distinctions at some time points which favoured the collagen group when feature was measured in a 2nd way.
Cardio Decreases Body Fat.
You can consider it as the glue that holds all these points together. In fact, words collagen originates from the Greek word "kólla," which indicates glue. Mix 1-2 scoops into a huge cup of water, tea, coffee or juice or contribute to foods such as porridge, soups as well as smoothies. When a peptide includes an interior proline, solid ion series as a result of inner cleavage are observed, extending from the proline in the direction of the C terminus. The first NMR experiments acquired will explore the state of the healthy protein and also it's suitability for further study at the picked NMR healthy protein focus, getting 1D 1H and/or 2D 15N HSQC spectra.
The carboxylic group loses the oxygen as well as hydrogen while the thiol team loses its hydrogen and a thioester bond is developed. Based on the stereochemistry of the anomeric carbon or its positioning in space, a glycosidic bond can either be an alpha-bond or a beta-bond. In an O-glycosidic link, the carbonyl group of carbohydrates responds with the hydroxyl team of an additional compound.
Peptides Products from pharmagrade.store .
This results in a substance in which the sugar or carb deposit is connected to the oxygen of the various other compound, hence the name O-glycosidic bond. For arbitrary non-fasting pee collections, outcomes are strongly correlated with mixed meal C-peptide, with high level of sensitivity as well as specificity for identifying clinically pertinent limits. Stable for 3 days in Boric acid containers at ambient temperature level.
In order to get to the cytoplasmic membrane of Gram-negative germs, AMPs have to initially translocate through the external membrane. This model suggests that, as a result of better affinity for the LPS, AMPs displace the divalent cations as well as bind to the LPS. By being cumbersome, the AMPs after that create short-term fractures as well as permeabilize the outer membrane layer, therefore permitting flow of the peptide itself across the membrane.
" I am exploring my lawful alternatives, in terms of where I stand and what I can do.
Liable sports nourishment suppliers and also merchants ensure their products are really plainly identified as well as adhere to EU legislation.
" These firms need to be frightened to put things like ostarine right into their items", he says.
Practically all performance-enhancing materials that are prohibited by organisations like WADA and also the IOC are additionally prohibited offer for sale in the European Union.
' Every little thing from the composition, classifying to the marketing and advertising has to follow the EU legislations established to secure consumers.
That could cost me a great deal of cash and also I'm unsure I can manage to do that.
Neuropeptides are held within large dense-core blisters throughout the cell body, whereas neurotransmitters are included in small blisters located at synapses. The Open College is included by Royal Charter, an exempt charity in England & Wales and a charity signed up in Scotland. The Open College is authorized and managed by the Financial Conduct Authority in relation to its additional activity of credit report broking. Not all set for University research after that surf over 900 totally free courses on OpenLearn and also sign up to our newsletterto read about new totally free programs as they are released. After a month of usage can discover nicer look of skin, nails and also hair. I do like it and also am constantly trying to add collagen to my diet currently im aging and yes I would certainly utilize this one once more.
18rik Peptide.
pharmagrade.store tb500 peptide buy online definition of the compounds in use today are of the androstenone family. There are several different classes of compounds with differing androgenic activity. Testosterone is the most popular and most widely used anabolic compound. The testosterone compound, as well as DHEA, androgene, are all classified as androgens and have androgenic activity. A number of compounds with known androgenic properties are being investigated for their ability to treat male impotency. Various combinations of androgens and estrogens have shown to be more effective in promoting sexual performance than either compound alone.
youtube
Sarms are small, sticky white blood cells that play an important role in the immune system. They help to fight off infections by stimulating white blood cells and stimulating natural killer cells to kill infection-causing bacteria. This is perhaps why sarms are often called "ice bacteria killers".
The Sarms Scriptures.
Selective androgens, including SARMs, are a new class of compounds known as androgens. These compounds were initially discovered in the 1970s by scientists hoping to find a way to treat enlarged prostate (benign prostatic hypertrophy). They worked out how to convert the ostarine amino acid into an inactive form that was inactive in the prostate and therefore not harmful to the prostate. Since then, researchers have shown great interest in the properties of SARMs and in how they might be beneficial to men with erectile dysfunction.
Trial 4 included 29 people with light to moderate osteo arthritis of the knee. Individuals were provided either a collagen formula (Fortigel ®) or a placebo for 24 weeks. Those that obtained collagen reported a better reduction in pain. In this trial, 250 people with osteoarthritis of the knee were randomised to get either 10 g collagen hydrolysate or a sugar pill daily for six months. This trial consisted of 389 people with osteo arthritis across 20 websites in the UK, USA and also Germany.
The use of Lactobacillus stress for BAP production is a method that still struggles with restrictions. Applications of BAPs produced by Lactobacillus varieties depending on the production method.
Individuals were randomised to get either 10 g of collagen hydrolysate or sugar pill tablet computers for 24 weeks. Type II collagen revealed fewer puffy joints, joint tenderness and better stroll time in just one of the tests versus a sugar pill. 4 trials evaluated collagen against a sugar pill and one examined it against methotrexate. The trials for entailed in between 60 as well as 503 participants with rheumatoid joint inflammation.
Will rad140 show up on a drug test?
RAD140 and the majority of the identified in vitro metabolites were detected in post‐administration urine samples. For controlling the misuse of RAD140 in horses, RAD140 and its metabolite in sulfate form gave the longest detection time in hydrolysed urine and could be detected for up to 6 days post‐administration.
I can not see the distinction that closely due to the fact that no one knows your face as you do but I can absolutely see a distinction in her skin tone, specifically when she grins. So, apparently it not just offers assist with your bones and joints etc but appears to plump your skin. We advise making collagen component of your everyday routine and for the long term to see ideal outcomes.
It's such a wonderful means to obtain all the benefits across all foods and beverages! I do want it was available in larger tubs as I get through mine quite quickly considering I make use of 2-3 scoops depending on the recipes I'm making. In conclusion I can vouch for this product and also highly recommend it. With the Rite-Flex Collegen Peptides I have noticed my skin was looking better after a couple of weeks, include it to your coffee or breakfast. Ordered a tub for my wife to attempt and also she vows blind that a few of the little folds in her skin have disappeared.
Texas Sport Supplement Company Owner Pleads Guilty to Unlawful Distribution of Steroid-Like Drugs - Department of Justice
Texas Sport Supplement Company Owner Pleads Guilty to Unlawful Distribution of Steroid-Like Drugs.
Posted: Tue, 22 Dec 2020 08:00:00 GMT [source]
Some studies have suggested that autoimmune diseases like rheumatoid joint inflammation may be dealt with by taking a foreign antigen by mouth, which might wet down your body immune system's response. Taking collagen by mouth might present some chemicals that create joint inflammation into your body and also create oral resistance to these antigens, decreasing the results of inflammatory arthritis. Glycoproteins from which the sialic acid has been removed are designated by the prefix asialo-, e.g. asialo-α1-acid glycoprotein, as well as asialofetuin. Elimination of both sialic acid and galactose cause asialo-agalactoglycoproteins.
Currently, the two most commonly used compounds in use for sports are Dianabol and Prednisolone. These compounds are available over the counter and are prescribed to athletes by athletic trainers and doctors without any prescription. Anabolic steroids are banned by the Olympic Games and other major sporting associations. It is against the law for athletes to use anabolic steroids if they are participating in sanctioned sports. Therefore, it is against the rules to give an anabolic drug to an athlete without a prescription from a licensed physician.
Sheep submaxillary glycoprotein, collagen, fish antifreeze glycoproteins as well as potato lectin are O-glycoproteins (or O-glycosylproteins). collagens, fish antifreeze glycoproteins, lamb submaxillary glycoproteins], as well as those that contain oligosaccharides that cansist of repeating units of N-acetyllactosamine (e.g. band 3 of the human erythrocyte membrane layer). Optimization of production of protease by Lactobacillus plantarum SK from bekasam with reaction surface area technique.
2 notes
·
View notes
Text
The Power Of Peptides
Collagen
Content
Best Examined Products.
Cardio Reduces Body Fat.
Choose Products.
18rik Peptide.
The Sarms Bible.

In order to determine which enteral feed is ideal fit to a client; the dietitian will certainly carry out a strenuous analysis of nutritional status and existing medical condition. The prescription of the feed is tailored to fulfill individual demands based upon an individuals' particular nutritional requirements as well as the objectives of the treatment. Relevant clinical details need to be sent out with the demand, including professional sign for testing, pertinent signs and also past case history. NT-proBNP is launched right into the circulation in equivalent amounts to the active hormone however is substantially more stable and for this reason creates an excellent marker of BNP output. In cardiac arrest the heart can not pump strongly sufficient for the body's requirements, the heart wall surfaces are extended and also fluid begins to gather triggering back pressure as well as for this reason much more BNP to be released. In humans there are ~ 90 genetics inscribing neuropeptide precursors, which are refined to ~ 100 bioactive neuropeptides. Neuropeptides frequently co-exist with other neurotransmitters in defined cell populations, yet are included in different storage space blisters.
Does LGD 4033 cause gyno?
Gyno: in some cases individuals have experienced gyno (tissue growth under the nipple) when using higher doses and not following appropriate pct. This should be monitored when using lgd-4033 if it is a concern, and the correct pct should be used.
As expected, the distinctions in proteolytic activities and healthy protein hydrolysis patterns are much more visible when contrasting various Lactobacillus varieties. A comparable observation was made when contrasting 14 strains of Pound. It is currently recognized that diet regimen plays a crucial duty in the maintenance of our health standing. Listed below, several of the technical, regulatory, and business obstacles to bring AMP-based drugs right into the clinical growth are highlighted. In addition to straight management of AMPs, there are several attempts ongoing to use representatives to boost the endogenous manufacturing of AMPs by the body in order to enhance the natural immune feedbacks and also therefore battle infections.
Best Examined Items.
If unable to send example promptly, freeze at -20 ° C as well as send out at ambient temperature in the post. For lasting storage space (e.g. to set examples), we recommend freezing at -80 ° C.

This will take about 1 day, depending upon sample focus. These preliminary information will certainly permit us to assess the moment and also the experimental conditions (i.e. type of identified centers, optimal healthy protein, buffer focus and also pH) required to obtain a high resolution architectural resolution on the individual's protein. There were no differences hurting or practical scores throughout the test, but there were distinctions at a long time points which favoured the collagen group when function was determined in a second way.
Cardio Reduces Body Fat.
You can consider it as the adhesive that holds all these points with each other. Actually, the word collagen comes from the Greek word "kólla," which implies adhesive. Mix 1-2 scoops right into a large cup of water, tea, coffee or juice or contribute to foods such as gruel, soups and also smoothie mixes. When a peptide includes an internal proline, strong ion series due to inner cleavage are observed, prolonging from the proline towards the C terminus. The very first NMR experiments gotten will certainly investigate the state of the protein and it's suitability for refresher course at the selected NMR protein concentration, obtaining 1D 1H and/or 2D 15N HSQC ranges.

The carboxylic group sheds the oxygen and hydrogen while the thiol group loses its hydrogen and also a thioester bond is created. Based upon the stereochemistry of the anomeric carbon or its alignment in space, a glycosidic bond can either be an alpha-bond or a beta-bond. In an O-glycosidic linkage, the carbonyl team of carbs responds with the hydroxyl team of an additional compound.
Pick Items.
This results in a substance in which the sugar or carb deposit is connected to the oxygen of the other substance, therefore the name O-glycosidic bond. For arbitrary non-fasting urine collections, results are highly correlated with combined meal C-peptide, with high level of sensitivity as well as specificity for identifying scientifically pertinent limits. Steady for 3 days in Boric acid containers at ambient temperature.
In order to reach the cytoplasmic membrane layer of Gram-negative bacteria, AMPs need to first translocate through the external membrane layer. https://direct-sarms.com/product/muscle-building-stack/ recommends that, because of higher affinity for the LPS, AMPs displace the divalent cations and bind to the LPS. By being large, the AMPs then trigger transient splits as well as permeabilize the external membrane, therefore permitting passage of the peptide itself throughout the membrane.
" I am exploring my legal options, in regards to where I stand and also what I can do.
Responsible sporting activities nutrition producers and also sellers guarantee their products are extremely clearly labelled and comply with EU law.
" These firms require to be scared to place points like ostarine into their products", he suggests.
Basically all performance-enhancing compounds that are prohibited by organisations like WADA and also the IOC are also prohibited available for sale in the European Union.
' Whatever from the make-up, identifying to the advertising and marketing has to comply with the EU regulations established to secure customers.
Neuropeptides are held within large dense-core vesicles throughout the cell body, whereas natural chemicals are had in small blisters located at synapses. The Open University is integrated by Royal Charter, an exempt charity in England & Wales as well as a charity registered in Scotland. The Open University is authorized as well as managed by the Financial Conduct Authority in connection with its second task of credit score broking. Not prepared for University research after that surf over 900 free courses on OpenLearn and also join to our newsletterto read about new cost-free programs as they are launched. After a month of usage can discover better look of skin, nails and also hair. I simulate it and also am always trying to include collagen to my diet now im aging and yes I would use this again.
18rik Peptide.
Most of the compounds in use today are of the androstenone family. There are several different classes of compounds with differing androgenic activity. Testosterone is the most popular and most widely used anabolic compound. direct sarms offers a bulking stacks , as well as DHEA, androgene, are all classified as androgens and have androgenic activity. A number of compounds with known androgenic properties are being investigated for their ability to treat male impotency. Various combinations of androgens and estrogens have shown to be more effective in promoting sexual performance than either compound alone.
youtube
Sarms are small, sticky white blood cells that play an important role in the immune system. They help to fight off infections by stimulating white blood cells and stimulating natural killer cells to kill infection-causing bacteria. This is perhaps why sarms are often called "ice bacteria killers".
Selective androgens, including SARMs, are a new class of compounds known as androgens. These compounds were initially discovered in the 1970s by scientists hoping to find a way to treat enlarged prostate (benign prostatic hypertrophy). They worked out how to convert the ostarine amino acid into an inactive form that was inactive in the prostate and therefore not harmful to the prostate. Since then, researchers have shown great interest in the properties of SARMs and in how they might be beneficial to men with erectile dysfunction.
Trial 4 included 29 individuals with moderate to moderate osteoarthritis of the knee. Individuals were given either a collagen formula (Fortigel ®) or a sugar pill for 24 weeks. Those that got collagen reported a higher decrease hurting. In this test, 250 individuals with osteoarthritis of the knee were randomised to receive either 10 g collagen hydrolysate or a sugar pill daily for 6 months. This test consisted of 389 people with osteo arthritis across 20 websites in the UK, U.S.A. and Germany.
Rick Collins Esq: Are SARMs legal? - generationiron.com
Rick Collins Esq: Are SARMs legal?.
Posted: Mon, 21 Dec 2020 08:00:00 GMT [source]
The use of Lactobacillus pressures for BAP production is a strategy that still struggles with constraints. Applications of BAPs created by Lactobacillus varieties depending upon the production strategy.
Individuals were randomised to obtain either 10 g of collagen hydrolysate or sugar pill tablet computers for 24 weeks. Type II collagen showed less puffy joints, joint inflammation and also better walk time in just one of the tests versus a placebo. Four trials evaluated collagen against a sugar pill as well as one checked it versus methotrexate. The trials for included in between 60 and also 503 individuals with rheumatoid arthritis.
Will rad140 show up on a drug test?
RAD140 and the majority of the identified in vitro metabolites were detected in post‐administration urine samples. For controlling the misuse of RAD140 in horses, RAD140 and its metabolite in sulfate form gave the longest detection time in hydrolysed urine and could be detected for up to 6 days post‐administration.
I can't see the distinction that very closely due to the fact that nobody understands your face as you do however I can certainly see a distinction in her complexion, especially when she grins. So, seemingly it not only supplies aid with your bones and also joints etc yet seems to plump your skin. We suggest making collagen part of your daily routine as well as for the long-term to see ideal results.
It's such a great way to obtain all the goodness across all foods as well as beverages! I do want it can be found in larger bathtubs as I survive mine quite quick considering I utilize 2-3 scoops depending upon the dishes I'm making. All in all I can attest this item and highly suggest it. With the Rite-Flex Collegen Peptides I have actually observed my skin was looking far better after a few weeks, include it to your coffee or breakfast. Bought a bathtub for my wife to try and also she vouches blind that several of the little folds in her skin have vanished.
Texas Sport Supplement Company Owner Pleads Guilty to Unlawful Distribution of Steroid-Like Drugs - Department of Justice
Texas Sport Supplement Company Owner Pleads Guilty to Unlawful Distribution of Steroid-Like Drugs.
Posted: Tue, 22 Dec 2020 08:00:00 GMT [source]
Some researches have suggested that autoimmune diseases like rheumatoid arthritis may be dealt with by taking a foreign antigen by mouth, which can dampen down your immune system's response. Taking collagen by mouth may introduce some chemicals that cause joint inflammation right into your body and also produce oral resistance to these antigens, minimizing the effects of inflammatory joint inflammation. Glycoproteins from which the sialic acid has actually been gotten rid of are designated by the prefix asialo-, e.g. asialo-α1-acid glycoprotein, as well as asialofetuin. Removal of both sialic acid and galactose cause asialo-agalactoglycoproteins.
Currently, the two most commonly used compounds in use for sports are Dianabol and Prednisolone. These compounds are available over the counter and are prescribed to athletes by athletic trainers and doctors without any prescription. Anabolic steroids are banned by the Olympic Games and other major sporting associations. It is against the law for athletes to use anabolic steroids if they are participating in sanctioned sports. Therefore, it is against the rules to give an anabolic drug to an athlete without a prescription from a licensed physician.
Sheep submaxillary glycoprotein, collagen, fish antifreeze glycoproteins and also potato lectin are O-glycoproteins (or O-glycosylproteins). collagens, fish antifreeze glycoproteins, lamb submaxillary glycoproteins], as well as those which contain oligosaccharides that cansist of repeating devices of N-acetyllactosamine (e.g. band 3 of the human erythrocyte membrane layer). Optimization of manufacturing of protease by Lactobacillus plantarum SK from bekasam with response surface technique.
#sarms stackss#sarms cutting stacks#bulking stacks#muscle building stacks#shredding stacks#support#cycle support supplements#post cycle therapy support#test base support#Buy sarms stackss#Buy sarms cutting stacks#Buy bulking stacks#Buy muscle building stacks#Buy shredding stacks#Buy support#Buy cycle support supplements#Buy post cycle therapy support#Buy test base support
2 notes
·
View notes
Text
YEAR OF GLAD
[...] [DFW, Infinite Jest, 1c]
'Surely by incredible you meant very very very impressive, as opposed to literally quote "incredible," surely,' says C.T., seeming to watch the coach at the window massaging the back of his neck. The huge window gives out on nothing more than dazzling sunlight and cracked earth with heat-shimmers over it.
'Then there is before us the matter of not the required two but nine separate application essays, some of which of nearly monograph-length, each without exception being —' different sheet — 'the adjective various evaluators used was quote "stellar" —’
Dir. of Comp.: 'I made in my assessment deliberate use of lapidary and effete.’
'— but in areas and with titles, I'm sure you recall quite well, Hal: "Neoclassical Assumptions in Contemporary Prescriptive Grammar," "The Implications of Post-Fourier Transformations for a Holographically Mimetic Cinema," "The Emergence of Heroic Stasis in Broadcast Entertainment" —’
' "Montague Grammar and the Semantics of Physical Modality"?’
' "A Man Who Began to Suspect He Was Made of Glass"?’
' "Tertiary Symbolism in Justinian Erotica"?’
Now showing broad expanses of recessed gum. 'Suffice to say that there's some frank and candid concern about the recipient of these unfortunate test scores, though perhaps explainable test scores, being these essays' sole individual author.’
'I'm not sure Hal's sure just what's being implied here,' my uncle says. The Dean at center is fingering his lapels as he interprets distasteful computed data.
'What the University is saying here is that from a strictly academic point of view there are admission problems that Hal needs to try to help us iron out. A matriculant's first role at the University is and must be as a student. We couldn't admit a student we have reason to suspect can't cut the mustard, no matter how much of an asset he might be on the field.’
'Dean Sawyer means the court, of course, Chuck,' Athletic Affairs says, head severely cocked so he's including the Coach White’s person behind him in the address somehow. 'Not to mention O.N.A.N.C.A.A. regulations and investigators always snuffling around for some sort of whiff of the smell of impropriety.’
The varsity tennis coach looks at his own watch.
'Assuming these board scores are accurate reflectors of true capacity in this case,' Academic Affairs says, his high voice serious and sotto, still looking at the file before him as if it were a plate of something bad, ‘Til tell you right now my opinion is it wouldn't be fair. It wouldn't be fair to the other applicants. Wouldn't be fair to the University community.' He looks at me. 'And it'd be especially unfair to Hal himself. Admitting a boy we see as simply an athletic asset would amount to just using that boy. We're under myriad scrutiny to make sure we're not using anybody. Your board results, son, indicate that we could be accused of using you.’
Uncle Charles is asking Coach White to ask the Dean of Athletic Affairs whether the weather over scores would be as heavy if I were, say, a revenue-raising football prodigy. The familiar panic at feeling misperceived is rising, and my chest bumps and thuds. I expend energy on remaining utterly silent in my chair, empty, my eyes two great pale zeros. People have promised to get me through this.
Uncle C.T., though, has the pinched look of the cornered. His voice takes on an odd timbre when he's cornered, as if he were shouting as he receded. 'Hal's grades at E.T.A., which is I should stress an Academy, not simply a camp or factory, accredited by both the Commonwealth of Massachusetts and the North American Sports Academy Association, it's focused on the total needs of the player and student, founded by a towering intellectual figure whom I hardly need name, here, and based by him on the rigorous Oxbridge Quadrivium-Trivium curricular model, a school fully staffed and equipped, by a fully certified staff, should show that my nephew here can cut just about any Pac 10 mustard that needs cutting, and that —’
DeLint is moving toward the tennis coach, who is shaking his head.
'— would be able to see a distinct flavor of minor-sport prejudice about this whole thing,' C.T. says, crossing and recrossing his legs as I listen, composed and staring.
The room's carbonated silence is now hostile. 'I think it's time to let the actual applicant himself speak out on his own behalf,' Academic Affairs says very quietly. 'This seems somehow impossible with you here, sir.’
Athletics smiles tiredly under a hand that massages the bridge of his nose. 'Maybe you'd excuse us for a moment and wait outside, Chuck.’
'Coach White could accompany Mr. Tavis and his associate out to reception,' the yellow Dean says, smiling into my unfocused eyes.
'— led to believe this had all been ironed out in advance, from the —' C.T. is saying as he and deLint are shown to the door. The tennis coach extends a hypertrophied arm. Athletics says 'We're all friends and colleagues here.’
This is not working out. It strikes me that EXIT signs would look to a native speaker of Latin like red-lit signs that say HE LEAVES.
[DFW, Infinite Jest, 1d]

#literature#david foster wallace#infinite jest#DFW#year of glad#foster wallace#davidfosterwallace#infinitejest
0 notes
Text
Computational Efficiency of Decoupling Approach in Solving Reactive Transport Model: A Case Study of Pyrite Oxidative Dissolution
Geofluids
Volume 2017 (2017), Article ID 4670103, 7 pages
https://doi.org/10.1155/2017/4670103
Computational Efficiency of Decoupling Approach in Solving Reactive Transport Model: A Case Study of Pyrite Oxidative Dissolution
1State Key Laboratory of Hydrology-Water Resources and Hydraulic Engineering, Nanjing Hydraulic Research Institute, Nanjing 210029, China
2College of Earth Science and Engineering, Hohai University, Nanjing 210098, China
Academic Editor: Keni Zhang
Copyright © 2017 Jixiang Huo et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Pyrite existed widely in nature and its oxidative dissolution might lead groundwater to become acidic, which was harmful to the environment and indeed to artificial building materials. The reactive transport model was a useful tool to predict the extent of such pollution. However, the chemical species were coupled together in the form of a reaction term, which might lead the equations to be nonlinear and thus difficult to solve. A decoupling approach was presented: linear algebraic manipulations of the stoichiometric coefficients of the chemical reactions for the purpose of reducing the number of equation variables and simplifying the reactive source were used. Then the original and decoupled models were solved separately, by both a direct solver and an iterative solver. By comparing the solution times of two models, it was shown that the decoupling approach could enhance the computational efficiency, especially in situations using denser meshes. Using a direct solver, more solution time was saved than when using an iterative version.
1. Introduction
Pyrite is a common, naturally occurring mineral. In the open atmosphere pyrite oxidative dissolution occurs under the action of groundwater. On one hand the resulting acid water may cause environmental problems, such as contamination of surface and ground waters directed to urban and agricultural supply [1–3]. Some toxic elements especially, such as arsenic, are closely associated with pyrite. The kinetic oxidative dissolution of As-bearing pyrite due to dissolved oxygen in the ambient groundwater is an important mechanism for arsenic release in groundwater under both natural conditions and engineering applications [4, 5]. On the other hand the formation of acid water also has some impacts on artificial building materials because of sulfate attack and acid attack [6, 7]. All the above lead the management of potentially acid generating waste rock to be very important [8]. To study the extent and scope of acidic water pollution, some hydrogeochemical models and transport ones are developed to simulate such a system [9–11].
In recent years reactive transport model is widely used to simulate the contaminant transport, water-rock interaction, and other processes in earth science fields [12, 13]. To improve computational efficiency of the model, Friedly and Rubin [14] present a general, concise formulation (decoupling approach) by means of linear algebraic manipulations of the stoichiometric coefficients of the chemical reactions, which can reduce the number of unknown variables and simplify the reaction source/sink terms. Based on this, De Simoni et al. [15] and Molins and Mayer [16] build up the decoupling matrix according to the equilibrium and kinetic reactions. And Huo et al. [17] extend its applications to heterogeneous media. Some efficiency tests are done by Kräutle and Knabner [18] and Hoffmann et al. [19] to study the resulting improvement. In recent years, the decoupling approach is widely used in both engineering applications and laboratory experiments. Saaltink et al. [20] apply the approach into the modelling of multiphase flow for CO2 injection and storage in deep saline aquifers. And the approach is also used in the simulation of two-phase multicomponent flow with reactive transport in porous media [21, 22]. And in identifying geochemical processes using end member mixing analysis, Pelizardi et al. [23] uses the decoupling approach to help in the identification of both end members and such reactions, so as to improve mixing ratio calculations. In laboratory experiments and its simulation, the approach is applied in a laboratory experiment where a sand column saturated with a MgSO4 solution is subject to evaporation [24]. And some programs and models are built up based on the decoupling approach for hydrogeochemical calculations, such as CHEPROO++ [25] and MRWM [26].
In this paper, a reactive transport model of pyrite oxidative dissolution is built up in COMSOL Multiphysics, a finite element software platform for the simulation of physics-based problems. COMSOL is a multiphysics modelling tool that solves various coupled physical problems based on Finite Element Analysis and Partial Differential Equations. It provides a user-friendly interface for mesh generation, equations configuration, and results visualization. And it is widely applied in earth science field. For example, Shao et al. [27] uses it to couple a dual-permeability model with a soil mechanics model for landslide stability evaluation on a hillslope scale. Azad et al. [28] build up an interface between COMSOL and GEMS, a chemical modelling platform, for the reactive transport modelling in variably saturated porous media, while Nardi et al. [29] and Jara et al. [30] couple two standalone simulation programs, COMSOL and PHREEQC, for the reactive transport modelling.
Although some studies have been done on computational efficiency, they are carried out from different research directions. Hoffmann et al. [19] mainly study the impact from theory view, while Kräutle and Knabner’ work [18] is based on a transient model to study computational efficiency in different time steps of two approaches. In this paper, we focused on the number of meshes and different solvers. Based on a brief introduction to the theories and mathematical methods behind the decoupling approach, a reactive transport model of pyrite oxidative dissolution is solved by a traditional method and a decoupling approach separately to compare their computational efficiencies. In both 1D and 2D models, the study area is meshed to different grid refinements in each situation. The original and decoupled models are solved and their solution times are compared. Meanwhile, the 2D models are solved by both a direct and an iterative solver to study the effect on the computational efficiency of the decoupling approach compared to different solvers. It is aimed at providing a more convenient and efficient method of calculation to solve the reactive transport model of pyrite oxidative dissolution.
2. Mathematical Description
The chemical reactions involved in aqueous species are divided into two kinds, equilibrium reaction and kinetic one. Reaction rates of the former are fast in comparison to transport, so that local chemical equilibrium can be assumed at every point within the system. Kinetic laws are applied to represent the processes of latter one, which is not sufficiently fast enough. So without considering the influence of activity, the mass balance of each species can be written in concise vector notation as follows:where vector contains the mass of species per unit volume of porous medium, and it can be split into two parts, and , respectively, related to the constant activity species (such as minerals in solid phase and gases) and to the remaining species. Matrix is diagonal and its diagonal terms are unity when a given species is mobile and zero otherwise. contains species concentrations in mol/mass of liquid ( for mobile species, is porosity) and , while , are primary and secondary species: the number of secondary species is equal to the number of reaction equilibrium, and the linear operator in (1) is defined as , where is the water flux and D is dispersion coefficient; is a matrix containing the stoichiometric coefficients of reactions involving reactants and product(s) and , where and represent the matrices of equilibrium and kinetic reactions such that due to the primary and secondary species. is stoichiometric coefficients matrix of primary species and is stoichiometric coefficients matrix of secondary species. Vector contains the reaction rate and is also divided into two parts: and .
A full rank matrix, , can be established, orthogonal to , which satisfies . The component matrix can be calculated by means of Gauss-Jordan elimination which leads to the following expression [31]:where is a diagonal matrix of dimension , with all diagonal elements equal to one; and are the number of reactions and species. Now a component vector of is defined as and its number, , can be calculated by . Writing the transport equations in terms of is helpful because the source/sink term becomes simple. Species concentration can also be solved with the equilibrium reaction constants.
According to Molins et al. [32], four types of reactive transport systems are classified by the types of reactions, as shown in Table 1.
Table 1: Types of reactive transport system.
It can be seen that four types of reactive transport systems are classified. The characteristics and calculation of component matrix U of each system are shown as follows.
(1) The first is tank system, in which all reactions take place in equilibrium in the aqueous phase, which means a large aqueous reservoir with residence times long enough for aqueous species to reach equilibrium, and no interaction with other solid or gas phases assumed. The component matrix of this system, , can be calculated by the following equation.where is stoichiometric coefficients matrix of equilibrium reactions corresponding to primary species.
(2) The second is canal system, in which all reactions are homogeneous, but some may be slow (kinetic). The component matrix of this system, , can be calculated by the following equation.where and is stoichiometric coefficients matrix of kinetic reactions corresponding to primary species.
(3) The third is river system, in which heterogeneous reactions also take place, but they are slow relative to flow. The component matrix of this system, , can be calculated by the following equation.where F is a factor matrix which is multiplied by to eliminate the immobile kinetic species. More detailed solution steps of F can be seen in Molins et al. [32].
(4) The fourth is aquifer system, where some heterogeneous reactions are fast enough to be considered in equilibrium. Some fixed activity species (e.g., minerals and H2O) can be found among the equilibrium reactions. These species can be eliminated from the equations by reducing the components to be solved. The component matrix of this system, , can be calculated by the following equation.where E is a factor matrix which is multiplied by to eliminate constant activity species and reduce the number of components. More detailed solution steps of can be seen in Molins et al. [32].
3. Decoupling Approach in Pyrite Oxidative Dissolution
3.1. The Chemical System and Its Decoupling Matrix
It is important to build up a chemical reaction system for pyrite oxidative dissolution reactive transport. When the initial solution is assumed to be formed in deionized water, the main reactions occurring in the open system are as follows.where there are equilibrium reactions in both (7) and (8), with reaction rates of and and (9) reflects the process of pyrite oxidative dissolution, which depends on the concentration of H+ and O2(aq) in solution. According to these, the stoichiometric coefficient matrix of the system S could be written asSince the reactions involved both aqueous and solid phases, it satisfied the aquifer system in Section 2. So the component matrix, , could be calculated asAnd a new vector of components, , was defined aswhere the vector in this system comprised eight species: O2(aq), H+, OH-, , Fe3+, FeS2, O2(g), and H2O in order. The calculated aqueous components were u1, u2, u3. As (12) shows the component vector is a linear combination of species, which is readily calculated; the number of unknowns to be solved in the equations is reduced, from eight species to three components. In this system, the number of all species is defined as , with and the number of equilibrium reactions is 2, while the number of secondary species with fixed activity is defined as , which includes pyrite, H2O, and O2(g). In this system , so the number of components, , could be calculated as
Meanwhile, the reaction terms of species in the transport equations were as follows:Multiplying by the decoupling matrix, U, this term could be expressed asIt could be seen that the reaction term of the original model was more complicated and contained the expressions of the equilibrium reaction rates R1 and R2, both of which were difficult to obtain explicitly which introduced some difficulties when solving the model. However, it was expressed in the form of extremely simple items by means of the decoupling approach. As shown in (15), the reaction terms of components, u1 and u2, were 0, and the one of component u3 involved only R3. Then the transport equations of component could be solved. Once system components had been evaluated, the original species, c, was obtained from the nonlinear algebraic system of (12) and corresponding equilibrium constants of (7) and (8).
3.2. Verification
In order to verify the influence of the decoupling approach on the calculation accuracy, firstly a batch reactor system of pyrite oxidation dissolution was taken as an example. In this system the pyrite was completely immersed in deionized water in a stirred vessel, which meant there was no need to consider the transport problem. The simulation results by both original approach and decoupling one were compared. The chemical parameters are shown in Table 2.
Table 2: Chemical parameters of pyrite oxidation dissolution.
and are the equilibrium constant of (7) and (8), while ,, and are reaction parameters of pyrite oxidative dissolution. Its reaction rate could be calculated by the following equation.where is solid phase surface area and is water volume; and are concentration of O2(aq) and H+.
The ratio of solid phase surface area to water volume was set as 3 dm−1 and simulated time was 10 days. The variation and error of pH value and Fe3+ concentration in two models are shown in Figure 1.
Figure 1: Variation and error of pH value and Fe3+ concentration.
It can be seen from Figure 1 that the results of two models are basically the same. Maximum relative error of pH value is −0.04725%, while the one of Fe3+ is 0.59542%, which means that decoupling approach has little effect on the accuracy of the calculation.
3.3. Comparison of Computational Efficiency
The decoupling approach not only simplified the reaction term but also reduced the number of unknown variables in the transport equations. As a result, the new transport model of each component should now be solvable with improved computational efficiency. Deionized water flows through a single smooth fracture of pyrite can be simplified to either a 1D or 2D parallel plate model with model parameters as shown in Table 3.
Table 3: Parameters for the 1D and 2D models.
Initially the fracture was deemed to have been full of deionized water, with a constant flow velocity through the fracture. Without considering the change in the aperture size caused by dissolution, the distribution of aqueous species reached dynamic equilibrium, which can be regarded as a steady state. The two models were thus simulated. One of the two models involved transport of species and the original model was designated: the other involved transport of decoupled component and the decoupled model was designated.
Then the two models were separately established in COMSOL 3.5a, a software platform for the simulation of physics-based problems. The central processing unit (CPU) of the computer was an Intel Core Duo P8400 with a clock speed of 2.26 GHz and the motherboard had 3 GB of random access memory. There were two main categories of solver in the software: direct and iterative. The former included UMFPACK, SPOOLES, PARDISO, and TAUCS Cholesky, which solved a linear system by Gaussian elimination. The iterative solvers, GMRES, FGMRES, conjugate gradients, BiCGStab, and geometric multigrid, were more memory-efficient to deal with models with many degrees of freedom.
When the model was solved in COMSOL, the mesh generator partitioned the study domains into mesh elements: the number of elements depended on the maximum element size when they were uniformly subdivided. Then the 1D and 2D models (original and decoupled types) were solved separately. First the direct solver, UMFPACK, was chosen and the model was solved three times in each case. To solve the nonlinear equations in both original model and decoupled one, Damped Newton Method (DNM) was adopted. Relative tolerance was set as 1.0 × 10−6 and maximum number of iterations was set as 25. The average solution times are shown in Tables 4 and 5.
Table 4: Comparison of solution time in 1D model using direct solver, UMFPACK.
Table 5: Comparison of solution time in 2D model using direct solver, UMFPACK.
It can be seen from Tables 4 and 5 that:
(1) Solution by direct solver, UMFPACK, costs much more time in the original model than when adopting a decoupling approach in both 1D and 2D models.
(2) The solution time in both 1D models increases with the number of elements, but it saves more time by using a decoupling approach when the number of elements becomes large. When there are only 25 elements in the model, it costs 0.234 s and 0.094 s to solve each model. With the increase in the number of elements, the solution times reach 1.079 s and 0.297 s for 500 elements (some 4.61 and 3.16 times the requirements at 25 elements).
(3) As in 1D, the computing time in 2D also increases with the number of elements in both of the two models and it saves more time when using a decoupling approach for large numbers of elements. At 7,800 elements, the original model cannot be solved due to an out of memory error during LU factorisation. However, by using a decoupling approach it only costs 21.033 s. Compared to the 1D model, it has a better computational efficiency costing only 4.78% to 27.71% of the original.
Then the iterative solver GMRES was chosen to solve the 2D model. The solution set of nonlinear equations was the same as the direct solver, UMFPACK. Three replicates were run and the average solution times are shown in Table 6.
Table 6: Comparison of solution time in 2D model using iterative solver, GMRES.
The following can be seen from Table 6.
(1) Like the results in Table 3, the decoupling approach also enhances the computational efficiency when solving the two models by use of the iterative solver. The decoupled solution time is only 17.92% to 52.34% of that needed for the original model and the solution time increases with the number of elements no matter whether in the original or decoupled model.
(2) Unlike the situation in Table 3, does not reduce with increased numbers of elements: at 5,036 and 7,800 elements, is only 51.54% and 52.34% of that required originally. It shows that the decoupling approach does not have as significant an effect as expected when used iteratively on a large model.
(3) Solving the original model with a direct solver costs much more time than when using an iterative version. However, when dealing with a decoupled model, the direct solver is faster and (Table 3) ranges from 4.78% to 27.71%, while it is 17.92% to 52.34% in Table 4. This means that solving the decoupled model by using an iterative solver does not save as much time as the direct solver does.
According to Tables 5 and 6, solution times for original and decoupled models with direct and iterative solvers are shown in Figure 2.
Figure 2: Comparison of solution times.
Figure 2 shows that solving an original model takes more time than a decoupled one, no matter whether by direct solver or iterative solver. In general sorted by time taken: the decoupled model by direct solver < decoupled model by iterative solver < original model by iterative solver < original model by direct solver.
4. Conclusions
The present work described the basic theory and mathematical methods of the decoupling approach and then took pyrite oxidative dissolution as an example. Based on the analysis of its chemical reaction system, the decoupling matrix U was calculated. When multiplied by U, the concentration vector was converted to the component vector , which had fewer variables and simpler reaction terms. Then the original and decoupled models were established in COMSOL Multiphysics 3.5a. Then the study domain was meshed at different degrees of refinement. In each case it was solved by direct and iterative solvers. The results show the following.
(1) Decoupling enhances the computational efficiency in both 1D and 2D models while saving more time for 2D models than 1D models.
(2) The more mesh grids the domain generates, the more efficiently the decoupled model finds a solution by direct solver, whether in 1D or 2D.
(3) Although the iterative solver takes less time than the direct solver for the original 2D model, it is more efficient to use a direct solver in solving a decoupled problem.
(4) The solution times in ascending order are the decoupled model solved by direct solver, a decoupled model solved by an iterative solver, the original model solved by an iterative solver, and the original model solved by a direct solver.
As a conclusion, the decoupling approach is of assistance when solving reactive transport of pyrite oxidative dissolution problems, especially over a large domain with more mesh elements. Its applicability is thus demonstrated.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this article.
Acknowledgments
This work was supported by Young Scientists Fund of the National Natural Science Foundation of China (Grant no. 51609150), the China Postdoctoral Science Foundation (Grant no. 2016M590477), the National Natural Science Foundation of China (Grant no. 41272265), and the Special Scientific Research Fund of Public Welfare Profession of Ministry of Water Resources of China (Grants nos. 201501033 and 201501036).
References
J. A. Grande, R. Beltrán, A. Sáinz, J. C. Santos, M. L. De La Torre, and J. Borrego, “Acid mine drainage and acid rock drainage processes in the environment of Herrerías Mine (Iberian Pyrite Belt, Huelva-Spain) and impact on the Andevalo Dam,”Environmental Geology, vol. 47, no. 2, pp. 185–196, 2005.View at Publisher·View at Google Scholar·View at Scopus
J. A. Grande, M. Santisteban, M. L. de la Torre, T. Valente, and E. Pérez-Ostalé, “Characterisation of AMD Pollution in the Reservoirs of the Iberian Pyrite Belt,”Mine Water and the Environment, vol. 32, no. 4, pp. 321–330, 2013.View at Publisher·View at Google Scholar·View at Scopus
P. M. Heikkinen, M. L. Räisänen, and R. H. Johnson, “Geochemical characterisation of seepage and drainage water quality from two sulphide mine tailings impoundments: Acid mine drainage versus neutral mine drainage,”Mine Water and the Environment, vol. 28, no. 1, pp. 30–49, 2009.View at Publisher·View at Google Scholar·View at Scopus
M. M. Rahman, M. Bakker, C. H. L. Patty et al., “Reactive transport modeling of subsurface arsenic removal systems in rural Bangladesh,”Science of the Total Environment, vol. 537, pp. 277–293, 2015.View at Publisher·View at Google Scholar·View at Scopus
S. Fakhreddine, J. Lee, P. K. Kitanidis, S. Fendorf, and M. Rolle, “Imaging geochemical heterogeneities using inverse reactive transport modeling: An example relevant for characterizing arsenic mobilization and distribution,”Advances in Water Resources, vol. 88, pp. 186–197, 2016.View at Publisher·View at Google Scholar·View at Scopus
A. Rodrigues, J. Duchesne, B. Fournier, B. Durand, P. Rivard, and M. Shehata, “Mineralogical and chemical assessment of concrete damaged by the oxidation of sulfide-bearing aggregates: Importance of thaumasite formation on reaction mechanisms,”Cement and Concrete Research, vol. 42, no. 10, pp. 1336–1347, 2012.View at Publisher·View at Google Scholar·View at Scopus
T. Schmidt, A. Leemann, E. Gallucci, and K. Scrivener, “Physical and microstructural aspects of iron sulfide degradation in concrete,”Cement and Concrete Research, vol. 41, no. 3, pp. 263–269, 2011.View at Publisher·View at Google Scholar·View at Scopus
M. F. Lengke, A. Davis, and C. Bucknam, “Improving management of potentially acid generating waste rock,”Mine Water and the Environment, vol. 29, no. 1, pp. 29–44, 2010.View at Publisher·View at Google Scholar·View at Scopus
C. Kohfahl and A. Pekdeger, “Rising groundwater tables in partly oxidized pyrite bearing dump-sediments: Column study and modelling approach,”Journal of Hydrology, vol. 331, no. 3-4, pp. 703–718, 2006.View at Publisher·View at Google Scholar·View at Scopus
B. Blunden and B. Indraratna, “Pyrite oxidation model for assessing ground-water management strategies in acid sulfate soils,”Journal of Geotechnical and Geoenvironmental Engineering, vol. 127, no. 2, pp. 146–157, 2001.View at Publisher·View at Google Scholar·View at Scopus
R. Abbassi, F. Khan, and K. Hawboldt, “Prediction of minerals producing acid mine drainage using a computer-assisted thermodynamic chemical equilibrium model,”Mine Water and the Environment, vol. 28, no. 1, pp. 74–78, 2009.View at Publisher·View at Google Scholar·View at Scopus
C. I. Steefel, D. J. DePaolo, and P. C. Lichtner, “Reactive transport modeling: An essential tool and a new research approach for the Earth sciences,”Earth and Planetary Science Letters, vol. 240, no. 3-4, pp. 539–558, 2005.View at Publisher·View at Google Scholar·View at Scopus
C. I. Steefel, C. A. Appelo, and B. Arora, “Reactive transport codes for subsurface environmental simulation,”Computational Geosciences, vol. 19, no. 3, pp. 445–478, 2015.View at Publisher·View at Google Scholar·View at MathSciNet
J. C. Friedly and J. Rubin, “Solute transport with multiple equilibrium‐controlled or kinetically controlled chemical reactions,”Water Resources Research, vol. 28, no. 7, pp. 1935–1953, 1992.View at Publisher·View at Google Scholar·View at Scopus
M. De Simoni, J. Carrera, X. Sánchez-Vila, and A. Guadagnini, “A procedure for the solution of multicomponent reactive transport problems,”Water Resources Research, vol. 41, no. 11, Article ID W11410, pp. 1–16, 2005.View at Publisher·View at Google Scholar·View at Scopus
S. Molins and K. U. Mayer, “Coupling between geochemical reactions and multicomponent gas and solute transport in unsaturated media: A reactive transport modeling study,”Water Resources Research, vol. 43, no. 5, Article ID W05435, 2007.View at Publisher·View at Google Scholar·View at Scopus
J.-X. Huo, H.-Z. Song, and Z.-W. Wu, “Multi-component reactive transport in heterogeneous media and its decoupling solution,”Journal of Contaminant Hydrology, vol. 166, pp. 11–22, 2014.View at Publisher·View at Google Scholar·View at Scopus
S. Kräutle and P. Knabner, “A new numerical reduction scheme for fully coupled multicomponent transport-reaction problems in porous media,”Water Resources Research, vol. 41, no. 9, Article ID W09414, pp. 1–17, 2005.View at Publisher·View at Google Scholar·View at Scopus
J. Hoffmann, S. Kräutle, and P. Knabner, “A general reduction scheme for reactive transport in porous media,”Computational Geosciences, vol. 16, no. 4, pp. 1081–1099, 2012.View at Publisher·View at Google Scholar·View at MathSciNet
M. W. Saaltink, V. Vilarrasa, F. De Gaspari, O. Silva, J. Carrera, and T. S. Rötting, “A method for incorporating equilibrium chemical reactions into multiphase flow models for CO2 storage,”Advances in Water Resources, vol. 62, pp. 431–441, 2013.View at Publisher·View at Google Scholar·View at Scopus
E. Ahusborde, M. Kern, and V. Vostrikov, “Numerical simulation of two-phase multicomponent flow with reactive transport in porous media: application to geological sequestration of CO2,” in Proceedings of the ESAIM: Proceedings and Surveys, vol. 50, pp. 21–39.
E. Ahusborde and M. El Ossmani, “A sequential approach for numerical simulation of two-phase multicomponent flow with reactive transport in porous media,”Mathematics and Computers in Simulation, vol. 137, pp. 71–89, 2017.View at Publisher·View at Google Scholar·View at MathSciNet·View at Scopus
F. Pelizardi, S. A. Bea, J. Carrera, and L. Vives, “Identifying geochemical processes using End Member Mixing Analysis to decouple chemical components for mixing ratio calculations,”Journal of Hydrology, vol. 550, pp. 144–156, 2017.View at Publisher·View at Google Scholar
P. Gamazo, M. W. Saaltink, J. Carrera, L. Slooten, S. A. Bea, and M. Gran, “Modeling the influence of MgSO4 invariant points on multiphase reactive transport process during saline soil evaporation,”Physics and Chemistry of the Earth, vol. 64, pp. 57–64, 2013.View at Publisher·View at Google Scholar·View at Scopus
F. d. Gaspari, “Mixing and speciation algorithms for geochemical and reactive transport problems,” 2015.
J. Soler-Sagarra, L. Luquot, L. Martínez-Pérez, M. W. Saaltink, F. De Gaspari, and J. Carrera, “Simulation of chemical reaction localization using a multi-porosity reactive transport approach,”International Journal of Greenhouse Gas Control, vol. 48, pp. 59–68, 2016.View at Publisher·View at Google Scholar·View at Scopus
W. Shao, T. Bogaard, and M. Bakker, “How to Use COMSOL Multiphysics for Coupled Dual-permeability Hydrological and Slope Stability Modeling,”Procedia Earth and Planetary Science, vol. 9, pp. 83–90, 2014.View at Publisher·View at Google Scholar
V. J. Azad, C. Li, C. Verba, J. H. Ideker, and O. B. Isgor, “A COMSOL-GEMS interface for modeling coupled reactive-transport geochemical processes,”Computers & Geosciences, vol. 92, pp. 79–89, 2016.View at Publisher·View at Google Scholar·View at Scopus
A. Nardi, A. Idiart, P. Trinchero, L. M. De Vries, and J. Molinero, “Interface COMSOL-PHREEQC (iCP), an efficient numerical framework for the solution of coupled multiphysics and geochemistry,”Computers & Geosciences, vol. 69, pp. 10–21, 2014.View at Publisher·View at Google Scholar·View at Scopus
D. Jara, J.-R. d. Dreuzy, and B. Cochepin, “TReacLab, An object-oriented implementation of non-intrusive splitting methods to couple independent transport and geochemical software,”Computers & Geosciences.
M. W. Saaltink, C. Ayora, and J. Carrera, “A mathematical formulation for reactive transport that eliminates mineral concentrations,”Water Resources Research, vol. 34, no. 7, pp. 1649–1656, 1998.View at Publisher·View at Google Scholar·View at Scopus
S. Molins, J. Carrera, C. Ayora, and M. W. Saaltink, “A formulation for decoupling components in reactive transport problems,”Water Resources Research, vol. 40, no. 10, pp. W103011–W1030113, 2004.View at Publisher·View at Google Scholar·View at Scopus
Source
http://www.hindawi.com/journals/geofluids/2017/4670103/
from
http://taxi.nearme.host/computational-efficiency-of-decoupling-approach-in-solving-reactive-transport-model-a-case-study-of-pyrite-oxidative-dissolution/
from NOVACAB - Blog http://novacabtaxi.weebly.com/blog/computational-efficiency-of-decoupling-approach-in-solving-reactive-transport-model-a-case-study-of-pyrite-oxidative-dissolution
0 notes
Text
Computational Efficiency of Decoupling Approach in Solving Reactive Transport Model: A Case Study of Pyrite Oxidative Dissolution
Geofluids
Volume 2017 (2017), Article ID 4670103, 7 pages
https://doi.org/10.1155/2017/4670103
Computational Efficiency of Decoupling Approach in Solving Reactive Transport Model: A Case Study of Pyrite Oxidative Dissolution
1State Key Laboratory of Hydrology-Water Resources and Hydraulic Engineering, Nanjing Hydraulic Research Institute, Nanjing 210029, China
2College of Earth Science and Engineering, Hohai University, Nanjing 210098, China
Academic Editor: Keni Zhang
Copyright © 2017 Jixiang Huo et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Pyrite existed widely in nature and its oxidative dissolution might lead groundwater to become acidic, which was harmful to the environment and indeed to artificial building materials. The reactive transport model was a useful tool to predict the extent of such pollution. However, the chemical species were coupled together in the form of a reaction term, which might lead the equations to be nonlinear and thus difficult to solve. A decoupling approach was presented: linear algebraic manipulations of the stoichiometric coefficients of the chemical reactions for the purpose of reducing the number of equation variables and simplifying the reactive source were used. Then the original and decoupled models were solved separately, by both a direct solver and an iterative solver. By comparing the solution times of two models, it was shown that the decoupling approach could enhance the computational efficiency, especially in situations using denser meshes. Using a direct solver, more solution time was saved than when using an iterative version.
1. Introduction
Pyrite is a common, naturally occurring mineral. In the open atmosphere pyrite oxidative dissolution occurs under the action of groundwater. On one hand the resulting acid water may cause environmental problems, such as contamination of surface and ground waters directed to urban and agricultural supply [1–3]. Some toxic elements especially, such as arsenic, are closely associated with pyrite. The kinetic oxidative dissolution of As-bearing pyrite due to dissolved oxygen in the ambient groundwater is an important mechanism for arsenic release in groundwater under both natural conditions and engineering applications [4, 5]. On the other hand the formation of acid water also has some impacts on artificial building materials because of sulfate attack and acid attack [6, 7]. All the above lead the management of potentially acid generating waste rock to be very important [8]. To study the extent and scope of acidic water pollution, some hydrogeochemical models and transport ones are developed to simulate such a system [9–11].
In recent years reactive transport model is widely used to simulate the contaminant transport, water-rock interaction, and other processes in earth science fields [12, 13]. To improve computational efficiency of the model, Friedly and Rubin [14] present a general, concise formulation (decoupling approach) by means of linear algebraic manipulations of the stoichiometric coefficients of the chemical reactions, which can reduce the number of unknown variables and simplify the reaction source/sink terms. Based on this, De Simoni et al. [15] and Molins and Mayer [16] build up the decoupling matrix according to the equilibrium and kinetic reactions. And Huo et al. [17] extend its applications to heterogeneous media. Some efficiency tests are done by Kräutle and Knabner [18] and Hoffmann et al. [19] to study the resulting improvement. In recent years, the decoupling approach is widely used in both engineering applications and laboratory experiments. Saaltink et al. [20] apply the approach into the modelling of multiphase flow for CO2 injection and storage in deep saline aquifers. And the approach is also used in the simulation of two-phase multicomponent flow with reactive transport in porous media [21, 22]. And in identifying geochemical processes using end member mixing analysis, Pelizardi et al. [23] uses the decoupling approach to help in the identification of both end members and such reactions, so as to improve mixing ratio calculations. In laboratory experiments and its simulation, the approach is applied in a laboratory experiment where a sand column saturated with a MgSO4 solution is subject to evaporation [24]. And some programs and models are built up based on the decoupling approach for hydrogeochemical calculations, such as CHEPROO++ [25] and MRWM [26].
In this paper, a reactive transport model of pyrite oxidative dissolution is built up in COMSOL Multiphysics, a finite element software platform for the simulation of physics-based problems. COMSOL is a multiphysics modelling tool that solves various coupled physical problems based on Finite Element Analysis and Partial Differential Equations. It provides a user-friendly interface for mesh generation, equations configuration, and results visualization. And it is widely applied in earth science field. For example, Shao et al. [27] uses it to couple a dual-permeability model with a soil mechanics model for landslide stability evaluation on a hillslope scale. Azad et al. [28] build up an interface between COMSOL and GEMS, a chemical modelling platform, for the reactive transport modelling in variably saturated porous media, while Nardi et al. [29] and Jara et al. [30] couple two standalone simulation programs, COMSOL and PHREEQC, for the reactive transport modelling.
Although some studies have been done on computational efficiency, they are carried out from different research directions. Hoffmann et al. [19] mainly study the impact from theory view, while Kräutle and Knabner’ work [18] is based on a transient model to study computational efficiency in different time steps of two approaches. In this paper, we focused on the number of meshes and different solvers. Based on a brief introduction to the theories and mathematical methods behind the decoupling approach, a reactive transport model of pyrite oxidative dissolution is solved by a traditional method and a decoupling approach separately to compare their computational efficiencies. In both 1D and 2D models, the study area is meshed to different grid refinements in each situation. The original and decoupled models are solved and their solution times are compared. Meanwhile, the 2D models are solved by both a direct and an iterative solver to study the effect on the computational efficiency of the decoupling approach compared to different solvers. It is aimed at providing a more convenient and efficient method of calculation to solve the reactive transport model of pyrite oxidative dissolution.
2. Mathematical Description
The chemical reactions involved in aqueous species are divided into two kinds, equilibrium reaction and kinetic one. Reaction rates of the former are fast in comparison to transport, so that local chemical equilibrium can be assumed at every point within the system. Kinetic laws are applied to represent the processes of latter one, which is not sufficiently fast enough. So without considering the influence of activity, the mass balance of each species can be written in concise vector notation as follows:where vector contains the mass of species per unit volume of porous medium, and it can be split into two parts, and , respectively, related to the constant activity species (such as minerals in solid phase and gases) and to the remaining species. Matrix is diagonal and its diagonal terms are unity when a given species is mobile and zero otherwise. contains species concentrations in mol/mass of liquid ( for mobile species, is porosity) and , while , are primary and secondary species: the number of secondary species is equal to the number of reaction equilibrium, and the linear operator in (1) is defined as , where is the water flux and D is dispersion coefficient; is a matrix containing the stoichiometric coefficients of reactions involving reactants and product(s) and , where and represent the matrices of equilibrium and kinetic reactions such that due to the primary and secondary species. is stoichiometric coefficients matrix of primary species and is stoichiometric coefficients matrix of secondary species. Vector contains the reaction rate and is also divided into two parts: and .
A full rank matrix, , can be established, orthogonal to , which satisfies . The component matrix can be calculated by means of Gauss-Jordan elimination which leads to the following expression [31]:where is a diagonal matrix of dimension , with all diagonal elements equal to one; and are the number of reactions and species. Now a component vector of is defined as and its number, , can be calculated by . Writing the transport equations in terms of is helpful because the source/sink term becomes simple. Species concentration can also be solved with the equilibrium reaction constants.
According to Molins et al. [32], four types of reactive transport systems are classified by the types of reactions, as shown in Table 1.
Table 1: Types of reactive transport system.
It can be seen that four types of reactive transport systems are classified. The characteristics and calculation of component matrix U of each system are shown as follows.
(1) The first is tank system, in which all reactions take place in equilibrium in the aqueous phase, which means a large aqueous reservoir with residence times long enough for aqueous species to reach equilibrium, and no interaction with other solid or gas phases assumed. The component matrix of this system, , can be calculated by the following equation.where is stoichiometric coefficients matrix of equilibrium reactions corresponding to primary species.
(2) The second is canal system, in which all reactions are homogeneous, but some may be slow (kinetic). The component matrix of this system, , can be calculated by the following equation.where and is stoichiometric coefficients matrix of kinetic reactions corresponding to primary species.
(3) The third is river system, in which heterogeneous reactions also take place, but they are slow relative to flow. The component matrix of this system, , can be calculated by the following equation.where F is a factor matrix which is multiplied by to eliminate the immobile kinetic species. More detailed solution steps of F can be seen in Molins et al. [32].
(4) The fourth is aquifer system, where some heterogeneous reactions are fast enough to be considered in equilibrium. Some fixed activity species (e.g., minerals and H2O) can be found among the equilibrium reactions. These species can be eliminated from the equations by reducing the components to be solved. The component matrix of this system, , can be calculated by the following equation.where E is a factor matrix which is multiplied by to eliminate constant activity species and reduce the number of components. More detailed solution steps of can be seen in Molins et al. [32].
3. Decoupling Approach in Pyrite Oxidative Dissolution
3.1. The Chemical System and Its Decoupling Matrix
It is important to build up a chemical reaction system for pyrite oxidative dissolution reactive transport. When the initial solution is assumed to be formed in deionized water, the main reactions occurring in the open system are as follows.where there are equilibrium reactions in both (7) and (8), with reaction rates of and and (9) reflects the process of pyrite oxidative dissolution, which depends on the concentration of H+ and O2(aq) in solution. According to these, the stoichiometric coefficient matrix of the system S could be written asSince the reactions involved both aqueous and solid phases, it satisfied the aquifer system in Section 2. So the component matrix, , could be calculated asAnd a new vector of components, , was defined aswhere the vector in this system comprised eight species: O2(aq), H+, OH-, , Fe3+, FeS2, O2(g), and H2O in order. The calculated aqueous components were u1, u2, u3. As (12) shows the component vector is a linear combination of species, which is readily calculated; the number of unknowns to be solved in the equations is reduced, from eight species to three components. In this system, the number of all species is defined as , with and the number of equilibrium reactions is 2, while the number of secondary species with fixed activity is defined as , which includes pyrite, H2O, and O2(g). In this system , so the number of components, , could be calculated as
Meanwhile, the reaction terms of species in the transport equations were as follows:Multiplying by the decoupling matrix, U, this term could be expressed asIt could be seen that the reaction term of the original model was more complicated and contained the expressions of the equilibrium reaction rates R1 and R2, both of which were difficult to obtain explicitly which introduced some difficulties when solving the model. However, it was expressed in the form of extremely simple items by means of the decoupling approach. As shown in (15), the reaction terms of components, u1 and u2, were 0, and the one of component u3 involved only R3. Then the transport equations of component could be solved. Once system components had been evaluated, the original species, c, was obtained from the nonlinear algebraic system of (12) and corresponding equilibrium constants of (7) and (8).
3.2. Verification
In order to verify the influence of the decoupling approach on the calculation accuracy, firstly a batch reactor system of pyrite oxidation dissolution was taken as an example. In this system the pyrite was completely immersed in deionized water in a stirred vessel, which meant there was no need to consider the transport problem. The simulation results by both original approach and decoupling one were compared. The chemical parameters are shown in Table 2.
Table 2: Chemical parameters of pyrite oxidation dissolution.
and are the equilibrium constant of (7) and (8), while ,, and are reaction parameters of pyrite oxidative dissolution. Its reaction rate could be calculated by the following equation.where is solid phase surface area and is water volume; and are concentration of O2(aq) and H+.
The ratio of solid phase surface area to water volume was set as 3 dm−1 and simulated time was 10 days. The variation and error of pH value and Fe3+ concentration in two models are shown in Figure 1.
Figure 1: Variation and error of pH value and Fe3+ concentration.
It can be seen from Figure 1 that the results of two models are basically the same. Maximum relative error of pH value is −0.04725%, while the one of Fe3+ is 0.59542%, which means that decoupling approach has little effect on the accuracy of the calculation.
3.3. Comparison of Computational Efficiency
The decoupling approach not only simplified the reaction term but also reduced the number of unknown variables in the transport equations. As a result, the new transport model of each component should now be solvable with improved computational efficiency. Deionized water flows through a single smooth fracture of pyrite can be simplified to either a 1D or 2D parallel plate model with model parameters as shown in Table 3.
Table 3: Parameters for the 1D and 2D models.
Initially the fracture was deemed to have been full of deionized water, with a constant flow velocity through the fracture. Without considering the change in the aperture size caused by dissolution, the distribution of aqueous species reached dynamic equilibrium, which can be regarded as a steady state. The two models were thus simulated. One of the two models involved transport of species and the original model was designated: the other involved transport of decoupled component and the decoupled model was designated.
Then the two models were separately established in COMSOL 3.5a, a software platform for the simulation of physics-based problems. The central processing unit (CPU) of the computer was an Intel Core Duo P8400 with a clock speed of 2.26 GHz and the motherboard had 3 GB of random access memory. There were two main categories of solver in the software: direct and iterative. The former included UMFPACK, SPOOLES, PARDISO, and TAUCS Cholesky, which solved a linear system by Gaussian elimination. The iterative solvers, GMRES, FGMRES, conjugate gradients, BiCGStab, and geometric multigrid, were more memory-efficient to deal with models with many degrees of freedom.
When the model was solved in COMSOL, the mesh generator partitioned the study domains into mesh elements: the number of elements depended on the maximum element size when they were uniformly subdivided. Then the 1D and 2D models (original and decoupled types) were solved separately. First the direct solver, UMFPACK, was chosen and the model was solved three times in each case. To solve the nonlinear equations in both original model and decoupled one, Damped Newton Method (DNM) was adopted. Relative tolerance was set as 1.0 × 10−6 and maximum number of iterations was set as 25. The average solution times are shown in Tables 4 and 5.
Table 4: Comparison of solution time in 1D model using direct solver, UMFPACK.
Table 5: Comparison of solution time in 2D model using direct solver, UMFPACK.
It can be seen from Tables 4 and 5 that:
(1) Solution by direct solver, UMFPACK, costs much more time in the original model than when adopting a decoupling approach in both 1D and 2D models.
(2) The solution time in both 1D models increases with the number of elements, but it saves more time by using a decoupling approach when the number of elements becomes large. When there are only 25 elements in the model, it costs 0.234 s and 0.094 s to solve each model. With the increase in the number of elements, the solution times reach 1.079 s and 0.297 s for 500 elements (some 4.61 and 3.16 times the requirements at 25 elements).
(3) As in 1D, the computing time in 2D also increases with the number of elements in both of the two models and it saves more time when using a decoupling approach for large numbers of elements. At 7,800 elements, the original model cannot be solved due to an out of memory error during LU factorisation. However, by using a decoupling approach it only costs 21.033 s. Compared to the 1D model, it has a better computational efficiency costing only 4.78% to 27.71% of the original.
Then the iterative solver GMRES was chosen to solve the 2D model. The solution set of nonlinear equations was the same as the direct solver, UMFPACK. Three replicates were run and the average solution times are shown in Table 6.
Table 6: Comparison of solution time in 2D model using iterative solver, GMRES.
The following can be seen from Table 6.
(1) Like the results in Table 3, the decoupling approach also enhances the computational efficiency when solving the two models by use of the iterative solver. The decoupled solution time is only 17.92% to 52.34% of that needed for the original model and the solution time increases with the number of elements no matter whether in the original or decoupled model.
(2) Unlike the situation in Table 3, does not reduce with increased numbers of elements: at 5,036 and 7,800 elements, is only 51.54% and 52.34% of that required originally. It shows that the decoupling approach does not have as significant an effect as expected when used iteratively on a large model.
(3) Solving the original model with a direct solver costs much more time than when using an iterative version. However, when dealing with a decoupled model, the direct solver is faster and (Table 3) ranges from 4.78% to 27.71%, while it is 17.92% to 52.34% in Table 4. This means that solving the decoupled model by using an iterative solver does not save as much time as the direct solver does.
According to Tables 5 and 6, solution times for original and decoupled models with direct and iterative solvers are shown in Figure 2.
Figure 2: Comparison of solution times.
Figure 2 shows that solving an original model takes more time than a decoupled one, no matter whether by direct solver or iterative solver. In general sorted by time taken: the decoupled model by direct solver < decoupled model by iterative solver < original model by iterative solver < original model by direct solver.
4. Conclusions
The present work described the basic theory and mathematical methods of the decoupling approach and then took pyrite oxidative dissolution as an example. Based on the analysis of its chemical reaction system, the decoupling matrix U was calculated. When multiplied by U, the concentration vector was converted to the component vector , which had fewer variables and simpler reaction terms. Then the original and decoupled models were established in COMSOL Multiphysics 3.5a. Then the study domain was meshed at different degrees of refinement. In each case it was solved by direct and iterative solvers. The results show the following.
(1) Decoupling enhances the computational efficiency in both 1D and 2D models while saving more time for 2D models than 1D models.
(2) The more mesh grids the domain generates, the more efficiently the decoupled model finds a solution by direct solver, whether in 1D or 2D.
(3) Although the iterative solver takes less time than the direct solver for the original 2D model, it is more efficient to use a direct solver in solving a decoupled problem.
(4) The solution times in ascending order are the decoupled model solved by direct solver, a decoupled model solved by an iterative solver, the original model solved by an iterative solver, and the original model solved by a direct solver.
As a conclusion, the decoupling approach is of assistance when solving reactive transport of pyrite oxidative dissolution problems, especially over a large domain with more mesh elements. Its applicability is thus demonstrated.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this article.
Acknowledgments
This work was supported by Young Scientists Fund of the National Natural Science Foundation of China (Grant no. 51609150), the China Postdoctoral Science Foundation (Grant no. 2016M590477), the National Natural Science Foundation of China (Grant no. 41272265), and the Special Scientific Research Fund of Public Welfare Profession of Ministry of Water Resources of China (Grants nos. 201501033 and 201501036).
References
J. A. Grande, R. Beltrán, A. Sáinz, J. C. Santos, M. L. De La Torre, and J. Borrego, “Acid mine drainage and acid rock drainage processes in the environment of Herrerías Mine (Iberian Pyrite Belt, Huelva-Spain) and impact on the Andevalo Dam,”Environmental Geology, vol. 47, no. 2, pp. 185–196, 2005.View at Publisher·View at Google Scholar·View at Scopus
J. A. Grande, M. Santisteban, M. L. de la Torre, T. Valente, and E. Pérez-Ostalé, “Characterisation of AMD Pollution in the Reservoirs of the Iberian Pyrite Belt,”Mine Water and the Environment, vol. 32, no. 4, pp. 321–330, 2013.View at Publisher·View at Google Scholar·View at Scopus
P. M. Heikkinen, M. L. Räisänen, and R. H. Johnson, “Geochemical characterisation of seepage and drainage water quality from two sulphide mine tailings impoundments: Acid mine drainage versus neutral mine drainage,”Mine Water and the Environment, vol. 28, no. 1, pp. 30–49, 2009.View at Publisher·View at Google Scholar·View at Scopus
M. M. Rahman, M. Bakker, C. H. L. Patty et al., “Reactive transport modeling of subsurface arsenic removal systems in rural Bangladesh,”Science of the Total Environment, vol. 537, pp. 277–293, 2015.View at Publisher·View at Google Scholar·View at Scopus
S. Fakhreddine, J. Lee, P. K. Kitanidis, S. Fendorf, and M. Rolle, “Imaging geochemical heterogeneities using inverse reactive transport modeling: An example relevant for characterizing arsenic mobilization and distribution,”Advances in Water Resources, vol. 88, pp. 186–197, 2016.View at Publisher·View at Google Scholar·View at Scopus
A. Rodrigues, J. Duchesne, B. Fournier, B. Durand, P. Rivard, and M. Shehata, “Mineralogical and chemical assessment of concrete damaged by the oxidation of sulfide-bearing aggregates: Importance of thaumasite formation on reaction mechanisms,”Cement and Concrete Research, vol. 42, no. 10, pp. 1336–1347, 2012.View at Publisher·View at Google Scholar·View at Scopus
T. Schmidt, A. Leemann, E. Gallucci, and K. Scrivener, “Physical and microstructural aspects of iron sulfide degradation in concrete,”Cement and Concrete Research, vol. 41, no. 3, pp. 263–269, 2011.View at Publisher·View at Google Scholar·View at Scopus
M. F. Lengke, A. Davis, and C. Bucknam, “Improving management of potentially acid generating waste rock,”Mine Water and the Environment, vol. 29, no. 1, pp. 29–44, 2010.View at Publisher·View at Google Scholar·View at Scopus
C. Kohfahl and A. Pekdeger, “Rising groundwater tables in partly oxidized pyrite bearing dump-sediments: Column study and modelling approach,”Journal of Hydrology, vol. 331, no. 3-4, pp. 703–718, 2006.View at Publisher·View at Google Scholar·View at Scopus
B. Blunden and B. Indraratna, “Pyrite oxidation model for assessing ground-water management strategies in acid sulfate soils,”Journal of Geotechnical and Geoenvironmental Engineering, vol. 127, no. 2, pp. 146–157, 2001.View at Publisher·View at Google Scholar·View at Scopus
R. Abbassi, F. Khan, and K. Hawboldt, “Prediction of minerals producing acid mine drainage using a computer-assisted thermodynamic chemical equilibrium model,”Mine Water and the Environment, vol. 28, no. 1, pp. 74–78, 2009.View at Publisher·View at Google Scholar·View at Scopus
C. I. Steefel, D. J. DePaolo, and P. C. Lichtner, “Reactive transport modeling: An essential tool and a new research approach for the Earth sciences,”Earth and Planetary Science Letters, vol. 240, no. 3-4, pp. 539–558, 2005.View at Publisher·View at Google Scholar·View at Scopus
C. I. Steefel, C. A. Appelo, and B. Arora, “Reactive transport codes for subsurface environmental simulation,”Computational Geosciences, vol. 19, no. 3, pp. 445–478, 2015.View at Publisher·View at Google Scholar·View at MathSciNet
J. C. Friedly and J. Rubin, “Solute transport with multiple equilibrium‐controlled or kinetically controlled chemical reactions,”Water Resources Research, vol. 28, no. 7, pp. 1935–1953, 1992.View at Publisher·View at Google Scholar·View at Scopus
M. De Simoni, J. Carrera, X. Sánchez-Vila, and A. Guadagnini, “A procedure for the solution of multicomponent reactive transport problems,”Water Resources Research, vol. 41, no. 11, Article ID W11410, pp. 1–16, 2005.View at Publisher·View at Google Scholar·View at Scopus
S. Molins and K. U. Mayer, “Coupling between geochemical reactions and multicomponent gas and solute transport in unsaturated media: A reactive transport modeling study,”Water Resources Research, vol. 43, no. 5, Article ID W05435, 2007.View at Publisher·View at Google Scholar·View at Scopus
J.-X. Huo, H.-Z. Song, and Z.-W. Wu, “Multi-component reactive transport in heterogeneous media and its decoupling solution,”Journal of Contaminant Hydrology, vol. 166, pp. 11–22, 2014.View at Publisher·View at Google Scholar·View at Scopus
S. Kräutle and P. Knabner, “A new numerical reduction scheme for fully coupled multicomponent transport-reaction problems in porous media,”Water Resources Research, vol. 41, no. 9, Article ID W09414, pp. 1–17, 2005.View at Publisher·View at Google Scholar·View at Scopus
J. Hoffmann, S. Kräutle, and P. Knabner, “A general reduction scheme for reactive transport in porous media,”Computational Geosciences, vol. 16, no. 4, pp. 1081–1099, 2012.View at Publisher·View at Google Scholar·View at MathSciNet
M. W. Saaltink, V. Vilarrasa, F. De Gaspari, O. Silva, J. Carrera, and T. S. Rötting, “A method for incorporating equilibrium chemical reactions into multiphase flow models for CO2 storage,”Advances in Water Resources, vol. 62, pp. 431–441, 2013.View at Publisher·View at Google Scholar·View at Scopus
E. Ahusborde, M. Kern, and V. Vostrikov, “Numerical simulation of two-phase multicomponent flow with reactive transport in porous media: application to geological sequestration of CO2,” in Proceedings of the ESAIM: Proceedings and Surveys, vol. 50, pp. 21–39.
E. Ahusborde and M. El Ossmani, “A sequential approach for numerical simulation of two-phase multicomponent flow with reactive transport in porous media,”Mathematics and Computers in Simulation, vol. 137, pp. 71–89, 2017.View at Publisher·View at Google Scholar·View at MathSciNet·View at Scopus
F. Pelizardi, S. A. Bea, J. Carrera, and L. Vives, “Identifying geochemical processes using End Member Mixing Analysis to decouple chemical components for mixing ratio calculations,”Journal of Hydrology, vol. 550, pp. 144–156, 2017.View at Publisher·View at Google Scholar
P. Gamazo, M. W. Saaltink, J. Carrera, L. Slooten, S. A. Bea, and M. Gran, “Modeling the influence of MgSO4 invariant points on multiphase reactive transport process during saline soil evaporation,”Physics and Chemistry of the Earth, vol. 64, pp. 57–64, 2013.View at Publisher·View at Google Scholar·View at Scopus
F. d. Gaspari, “Mixing and speciation algorithms for geochemical and reactive transport problems,” 2015.
J. Soler-Sagarra, L. Luquot, L. Martínez-Pérez, M. W. Saaltink, F. De Gaspari, and J. Carrera, “Simulation of chemical reaction localization using a multi-porosity reactive transport approach,”International Journal of Greenhouse Gas Control, vol. 48, pp. 59–68, 2016.View at Publisher·View at Google Scholar·View at Scopus
W. Shao, T. Bogaard, and M. Bakker, “How to Use COMSOL Multiphysics for Coupled Dual-permeability Hydrological and Slope Stability Modeling,”Procedia Earth and Planetary Science, vol. 9, pp. 83–90, 2014.View at Publisher·View at Google Scholar
V. J. Azad, C. Li, C. Verba, J. H. Ideker, and O. B. Isgor, “A COMSOL-GEMS interface for modeling coupled reactive-transport geochemical processes,”Computers & Geosciences, vol. 92, pp. 79–89, 2016.View at Publisher·View at Google Scholar·View at Scopus
A. Nardi, A. Idiart, P. Trinchero, L. M. De Vries, and J. Molinero, “Interface COMSOL-PHREEQC (iCP), an efficient numerical framework for the solution of coupled multiphysics and geochemistry,”Computers & Geosciences, vol. 69, pp. 10–21, 2014.View at Publisher·View at Google Scholar·View at Scopus
D. Jara, J.-R. d. Dreuzy, and B. Cochepin, “TReacLab, An object-oriented implementation of non-intrusive splitting methods to couple independent transport and geochemical software,”Computers & Geosciences.
M. W. Saaltink, C. Ayora, and J. Carrera, “A mathematical formulation for reactive transport that eliminates mineral concentrations,”Water Resources Research, vol. 34, no. 7, pp. 1649–1656, 1998.View at Publisher·View at Google Scholar·View at Scopus
S. Molins, J. Carrera, C. Ayora, and M. W. Saaltink, “A formulation for decoupling components in reactive transport problems,”Water Resources Research, vol. 40, no. 10, pp. W103011–W1030113, 2004.View at Publisher·View at Google Scholar·View at Scopus
Source
http://www.hindawi.com/journals/geofluids/2017/4670103/
from TAXI NEAR ME http://taxi.nearme.host/computational-efficiency-of-decoupling-approach-in-solving-reactive-transport-model-a-case-study-of-pyrite-oxidative-dissolution/
0 notes
Text
Advective Displacement Method for the Characterisation of Pore Water Chemistry and Transport Residence in Claystone
Abstract
The advective displacement method applies large hydraulic gradients to a confined rock core sample to yield small aliquots of the preserved in situ pore water, applicable to aquitard rocks with hydraulic conductivities as low as 10−14 m/s. Examples from argillaceous rocks indicate that only minor artefacts are induced and that analytical methods optimized for small aliquots provide a comprehensive chemical and isotopic characterisation. Multicomponent transport properties are derived from extending experimental time and using a traced artificial pore water for injection. Examples include quantification of the anion exclusion effect that can even resolve a small difference between transport properties for chloride and bromide in claystone. Controls by mineral saturation and cation exchange processes are also constrained by data from this approach. Technical details are provided for construction, material selection, components, sensors, and analytical issues.
1. Introduction
Properties and performance of hydraulically tight clayey rock formations are of interest in the context of cap rocks for oil, gas, and carbon dioxide confinement, as pore water archives for past hydrogeochemical evolution, as hydrocarbon source rock for shale gas, and as confining units for radioactive and chemotoxic waste ([1, 2], editorial of this volume). In contrast to tight crystalline rocks, porosity and water content are substantial, in the range of a few to about twenty volume percent, and hydraulic conductivities are typically <10−12 m/s. The focus of this paper is on a particular method from which to gain insight into pore water chemistry, hydraulic properties, transport of dissolved species, and reactive transport (water/rock interaction) that may also be coupled to the hydraulic properties. The motivation is to gain process understanding in the context of deep geologic disposal of radioactive waste, but the methods and results are transferable to the other aforementioned fields of research.
Our understanding of pore water chemistry and transport of dissolved species is based on models that bridge measureable quantities (fluxes, concentrations, and bulk properties) and the nanometric pore scale that is only very selectively observable. A milestone was the Pearson et al. [3] data digest on Opalinus Clay based on laboratory work and field tests performed in the Underground Rock Laboratory at Mont Terri, Switzerland (https://www.mont-terri.ch). The thermodynamically based concepts of aqueous speciation, mineral equilibria (and buffering), interdependencies such as pH-PCO2-carbonate-sulphate-ion exchanger [4], and electrostatic effects exerted by certain clay minerals (commonly named “anion exclusion” based on some variant of Poisson-Boltzmann theory) offer a sufficient level of complexity for the discussion of results presented in this paper. Significant progress has been made regarding details, and more constraining data have been elaborated [5–8].
Sophisticated reactive transport models (e.g., Crunchflow, Flotran, and Phreeqc-Comsol coupling) and inexpensive computation power are available to simulate fully described laboratory experiments that are necessary as testing cases for both implementation of complex and coupled processes (benchmark-type exercises) and testing of alternative conceptualisations (choice of processes and simplifying boundary/initial conditions). An example of such a data set and its comparative modelling by increasing degrees of complexity is that of a reactive transport core infiltration experiment in compacted bentonite [9].
The focus of this contribution is on the technical details of the experimental approach because much hinges on design details and material selection for equipment, on details of sample preparation, on the ability to analyse very small samples, and on keeping experiments stable for months to years.
2. Materials and Methods
2.1. Advective Displacement Method and Sample Requirement
During the 1990s when characterisation of pore water in tight clayey rock formations was advancing, direct sampling techniques included rock squeezing, long-term borehole sampling, and an indirect fingerprinting method by aqueous leaching [3]. As an alternative, a so-called advective displacement method was tested whereby the injection of an artificial pore water on one side of a sample core was used to displace the in situ pore water out of the other end of the sample [10]. A first pore water displacement experiment with Opalinus Clay started in 2002, and it is still running as of 2017. The original apparatus was constructed at the Paul Scherrer Institut (Laboratory for Waste Management), Switzerland, for the purpose of studying radionuclide transport in synthetic fractures, and this prototype was moved to the University of Bern and subsequently modified and replaced by new equipment designed and constructed in-house.
The advective displacement method implicitly assumes the following:(i)A cylindrical rock sample with planar ends perpendicular to the cylinder axis(ii)A well-preserved rock sample, with respect to water content and the reactive mineral components (pyrite), for example, sealed in moderate vacuum and stored refrigerated or at in situ temperature of the formation(iii)No disturbing effects from drilling fluid (particularly if stable isotope data should be acquired), or other cutting fluids, or from compressed gas when dry drilling is employed (for some argillaceous rocks). The outermost near-surface part may be turned off efficiently on a lathe(iv)Minimization of exposure to atmosphere at all stages(v)Approximation of 1D column transport. This is normally met by samples taken perpendicular to bedding but may not be met for samples parallel to bedding and that show distinct layering. In the latter case, a more permeable subvolume may be tested preferentially.
A number of potentially disturbing effects are difficult to control:(i)Volumetric strain is associated with the unloading of a sample when sampling a deep formation.(ii)Outgassing will occur in case gas saturation is reached during unloading.(iii)The presence of a gas phase at in situ condition will result in gas escape and potentially some displacement of pore water.(iv)An adjustment of pore water chemistry from in situ temperature to storage or experimental temperature will occur (carbonate-sulphate system).(v)Microbial activity may start at sample surfaces and may persist during an experiment.
2.2. Properties and Processes That Can Be Studied by Advective Displacement Experiments
Diverse processes, chemical, physical, and transport properties, can be determined by imposing a wide range of hydraulic and chemical boundary conditions for advective displacement experiments.Hydraulic conductivity K [m/s] is calculated very accurately from sample dimensions (l [m], A [m2]), average hydraulic head (h [m]), and average volumetric flow rate (Q [m3/s]) for a time span of interest, . A lower practical limit is 10−14 m/s if fluid chemistry is also of interest, or even 10−15 m /s if only conductivity is to be determined.The temperature dependence of hydraulic conductivity can be determined accurately with a thermostat-equipped device.Changes of hydraulic conductivity as function of total stress of a sample or clogging of flow-paths by transport of micro particles or by precipitation/dissolution of secondary minerals (fluid-rock interaction) can also be recorded.The in situ pore water can be displaced with an artificial pore water, and the first few small samples represent pore water composition, subject to some artefacts.Extending pore water displacement to an advection experiment with tracers, breakthrough of these can be analysed and transport properties determined, such as anions versus deuterated water, or reactive components such as cations, or dissolved organic carbon species.In very reactive systems (soluble salts), changes in porosity and/or pore structure will affect hydraulic properties, and this can be monitored.Anion-accessible porosity ratio can be determined as the ratio between the anion concentration obtained from an aqueous extract back-calculated to the total porosity (water content) of a sample and that measured in the outflow. This assumes that the anion-accessible porosity is represented by the porosity that is also involved in advective flow.Because outflow can be continuously sampled, the saturation state with respect to potentially controlling minerals (precipitation or dissolution) can be evaluated as a function of percolation time. These are clues for potentially controlling mineral equilibria (carbonates, sulphates, but also ion-exchange).Postmortem analysis of a sample core may yield additional constraints apart from physical properties, such as a longitudinal distribution of the cation population on the clay exchanger, or mineral zonation in reactive systems.Redox-sensitive systems may be studied by imposing special precautions during sample preparation and sampling of the outflow and by introducing redox-sensitive tracers.Two-phase flow phenomena or the initial saturation state may also be tested (pore water uptake).
We tested in one or another way all of the potential applications mentioned above and are actively pursuing chemicophysical coupling phenomena, experimentally and by modelling approaches [11, 12]. Some select details are addressed in the technical description below.
2.3. Technical Description and Equipment
The principle design is that of a triaxial cell, but with a fixed length for the sample specimen (Figure 1). A rigid steel cylinder serves as pressure vessel, with a confining medium (water) that exerts a quasi-isostatic pressure on a cylindrical sample. Quasi-isostatic refers to a small supported area that reduces the cross section on which confining pressure acts. Working with a confining pressure ensures a self-sealing system against the interior of a sample, a more reliable option compared to press-fits, glued-in samples, or other mechanical confinements.
Figure 1: Advective displacement apparatus, (a) technical drawing, (b) schematic, © rock sample (white) with titanium coupling (green) and insert (dark grey) with details of O-ring seals and capillary for fluid injection (centre) and connection to confining fluid (off-centre), (d) insert with small O-ring sealing directly on the capillary for fluid injection, and (e) core sample mounted between inserts before lowering into pressure vessel. Inner diameter of this pressure vessel is 120 mm; the diameter of the sample core is 101 mm.
The sample assembly (Figure 1(e)) comprises the cylindrical rock sample sandwiched between coupling pieces made of titanium. Porous filter discs are placed between the sample and the coupling pieces. The assembly is sealed against the confining medium by an inner layer of Teflon tape covered by a latex sleeve (details below). The assembly becomes self-sealing once the confining pressure is ramped up.
The sample assembly is mounted between two steel inserts (Figures 1(a) and 1(b)) and lowered into the pressure cylinder (Figure 1(e)). Conventional O-rings seal the enclosed confining medium against the pressure vessel and against the sample assembly (Figures 1© and 1(d)). A spindle (Figure 1(a)) adjusts to the length of a sample assembly, and normally no extra force is imposed initially on the sample by the spindle.
An artificial pore water is injected and collected with thin PEEK (polyether ether ketone) capillaries (1/16′′ OD, 1.6 mm). These capillaries are fixed and fed through the sliding inserts by means of Swagelok fittings (Figure 1©), and an additional small O-ring is sealing the capillary at the contact point of the sample assembly and the inserts (Figure 1(d)) to avoid any stagnant dead volume of the percolating fluid.
Porous filter discs are a critical component that are required to distribute and collect the in-flow and outflow to and from a sample. A number of considerations determine the choice of filter material: minimizing pore volume is commonly desirable as well as chemical inertness. Porous Teflon is limited in stiffness and will collapse slowly when used at high confining pressures (above 2 MPa). A clever work-around is to use a thin porous Teflon filter with 3–5 small titanium frits as inserts that support the load. In this way, a filter thickness of 0.5 mm or less may be achieved with a diameter up to 120 mm. Alternatives may be woven filters made from very thin titanium wire, or a grooved adapter surface in case of stiff rock like granite. Thick filters (>1 mm) are commonly not practical in applications for low-permeability rocks due to the significant dead volume compared to transport rates.
Steel cylinders for the pressure vessel are easiest manufactured from ready-made seamless precision steel tubes, honed, and centred on the inner diameter, such as used for hydraulic applications. Only an outer thread has to be machined such that caps can be screwed on at top and base (Figure 1(a)). Maximum pressure is limited by the maximum hoop stress that can be applied to the cylinder. A safe design is to hold maximum pressure of bottled argon or helium, for example, 20 MPa, but 50–100 MPa may also be reached with conventional O-ring sealing techniques. All our plumbing not contacted by pore fluids (for hydraulics) are made from stainless steel Swagelok components, using 6 mm and 1/8′′ (3.2 mm) product lines, and needle valves. These components are rated >20 MPa pressure.
Chemically inert materials are used for all fluid-wetted parts, including capillary tubing, valves, and fluid containers. Components from analytical equipment are readily available and include PEEK tubing, PEEK valves (used in high pressure liquid chromatography, e.g., Vici/Valco, Rheodyne, IDEX), and internally coated stainless steel tanks (sold as sampling cylinders, e.g., from Swagelok).
Sampling of aliquots from the outflow is conveniently done by small syringes directly connected to the outflow capillary; this protects samples from atmosphere. Syringe friction is significant (up to 0.1 MPa for a 1 ml standard O-ring sealed plastic syringe) when working at small hydraulic gradients. Alternatively, a gas-pressured back-pressure system can be implemented.
Controls include generation of the hydraulic confining pressure by bottled argon in a gas/liquid tank partially filled with the confining medium. This simple system also provides an effective buffering against pressure changes induced by temperature changes. The infiltrating artificial pore water is easiest driven at constant-pressure condition imposed by bottled He in a polymer-coated steel cylinder, with a capillary connecting to the infiltration apparatus via an injection valve. Using a 6-port two-position valve allows switching between two different fluids (e.g., one traced), or refilling the fluid reservoir at pressure, with a HPLC pump, for example. Helium is a good choice because of its small solubility and small pressure-dependence of solubility. Using argon or nitrogen leads to degassing in the experimental core that is subjected to a substantial hydraulic gradient.
We monitor all pressures and temperatures via data acquisition but do not use computer-control for long-term experiments, computers being far less reliable compared to hydraulic systems.
The pressure vessel may conveniently be heated or cooled by an outer heater jacket or cooling coil. A copper coil (or any other tubing material) in combination with a circulation heater/cooler is an easy way to thermostat or moderately heat or cool an experiment (indicated in Figure 1(a)).
Main operational safety issues relate to compressed gases, compressed liquids, and electric components. Most of our equipment is designed to hold full bottled gas pressure (20 MPa), except for fluid/gas reservoirs that are generally rated to somewhat lower pressures (12–34 MPa for Swagelok sampling cylinders). Safety heads or pressure relieve valves protect such containers according to regulatory requirements.
A number of parameters for the outflowing fluid are conveniently measured in-line, including pH, electric conductivity (EC), and redox potential (Eh). Except for conductivity, electrode measurements cannot be performed continuously over extended time, but small flow-through cells can be connected to a stream switching valve and activated for select periods of time, bracketed by calibration before and after in case of pH. Convenient cells for EC are those used in ion-chromatography instruments, for example, as the one used by Metrohm (Figure 2(a)) that can be connected directly to 1/16′′ PEEK capillaries without dead volume and in conjunction with a Metrohm conductivity meter connected to data acquisition. These small two-electrode EC cells are designed for relatively low conductivities (large cell constant of ca. 10) and the conductivity meter has to be capable of using combinations of conductivity range and cell constants beyond what is common practice. Small flow-through cells for pH are scarce on the market, and we fabricate ours in-house from POM (polyoxymethylene) and semi-micro-combination electrodes with tip diameters of 2-3 mm, immersion depth of 4–6 mm, and a flushed volume of 20–30 μl (Figure 2(b)). It is critical to provide a relatively soft seal above the electrode tip, and the mounting is very fragile. Using a 6-port stream switching valve, the ports for flushing and calibration can be set up in combination with the fluid outflow from the experiment.
Figure 2: Flow-through cells for (a) electric conductivity and (b) pH.
Tests with measuring Eh in-line have been a mixed success. More often than not, only partly reducing conditions are obtained even over several weeks of flow-through time, but never as low as might be expected from the sulphate/sulphide/pyrite/Fe-carbonate (or silicate) buffer imposed by most argillaceous rocks. This is likely due to the experimental set-up being not sufficiently gas-tight without converting to a more sophisticated construction. We made some tests using a very small-volume (10–20 μl) cell from Metrohm used normally as a three-electrode amperometric detector, just using a Ag/AgCl flat-base reference electrode, and either an Au or Pt measuring electrode.
2.4. Sample Preparation and Setting Up an Experiment
A requirement is a sample with preserved moisture content and that has been exposed to atmosphere for as little as possible. A cylindrical segment is dry-cut (or wet if permissible) with planar surfaces that are parallel and perpendicular to the cylinder axis. Total sample mass and dimensions are measured. Off-cuts are kept for determining chemicophysical parameters as a precharacterisation. We modified a mitre saw normally used for wood by fitting a diamond blade used normally for dry cutting. This is extremely efficient, precise, and inexpensive and cuts all materials but requires an industrial vacuum device for dust collection.
The sample core is placed between titanium coupling pieces (Figure 3), with filters in between. In the example shown (Figure 3(a)), a 1 mm thick porous titanium filter is used (85 mm DM) with a Teflon ring to match the sample diameter of 101 mm. The assembly is sealed against confining pressure by an inner layer of Teflon tape (Figure 3©) covered by a latex sleeve (Figure 3(d)), sealed against the coupling parts with silicone sealant, and taped to ensure a tight initial seal.
Figure 3: Stages of sample preparation: (a) rock core with porous filters at top and base, (b) placed between titanium couplings, © wrapped with Teflon tape, and (d) covered with latex sleeve. See text for details.
Starting an advective displacement experiment includes loading the sample assembly into the pressure vessel (Figure 1(e)), filling in the confining fluid (water), and ramping up confining pressure, typically 1-2 MPa above the desired injection pressure. Tightness of the sealing system of the rock sample (Figure 3) can be affirmed by leaving the system pressurized overnight, but without in-flow and outflow capillaries connected. We use the injection valve to evacuate the dead space in the filter disc and capillaries, switching directly from vacuum to the pressurized artificial pore water to start fluid injection. Likewise, the dead space in the fluid outflow part can be evacuated or flushed with argon with the help of a switching valve, before connecting to the sampling syringe. It may take several days until the head space is flushed with the displaced pore water, and arrival of outflow is first indicated by a jump in the electrical conductivity measured in-line (Figure 2(a)). Typical flow rates range from 0.05 to 0.5 ml/day for argillaceous rock samples of 8–12 cm length and hydraulic heads of 2–8 MPa, corresponding to head gradients of – m/m.
2.5. Tracers Used in Advective Displacement Experiments
A good frame of reference for transport is the use of a water tracer (deuterium, tritium) in all experiments. An anionic tracer should also be used, because anions get transported faster compared to water in clayey rocks due to a distinct anion exclusion effect that reduces the cross-sectional area available for transport and, hence, increases the average linear velocity. Choices are limited if the in situ anionic minor components are of interest (Br−, I−, and low-molecular-weight organic acids). The anions contained in the undisturbed pore water can be observed as breakout tracers that elute from in situ concentration towards zero if the artificial pore water is tracer-free. Of concern are potential microbially induced redox reactions that may affect concentrations of anionic complexes such as sulphate and nitrate, particularly in the presence of dissolved natural organic matter. Many more choices of radionuclide tracers are available when working in a hot-laboratory environment.
Optical tracers, such as fluorescent dyes, are of little use in clay-rich rocks because these large molecules become retarded by sorption and/or potentially by ion filtration. It is safest to use tracers that resemble common pore water components, because testing for the suitability of a tracer at low transport rates is extremely time consuming.
2.6. Analytical Techniques
Sampling strategy for pore water displacement aims at collecting 3–5 small samples that are largely unaffected by the injected artificial pore water, such that ideally a concentration plateau can be confirmed and the onset of the anion tracer breakthrough detected. Sampling for obtaining breakthrough behaviour of tracers requires frequent early samples for inert tracers (water, anions) and far less frequent samples for late times or reactive components. A combination of in-line measurements and laboratory measurements of the sampled aliquots needs to be tailored to each experiment.
The parameters of interest involve those that can be measured in-line by electrodes with very small flow-through cells (electric conductivity, pH, and redox potential, details above). Those measured on small aliquots in automated analysers include ion chromatography (major anions, cations, and some carboxylic acids), ICP-OES (Si, Al, and some minor components such as Sr), carbon IR analyser (TIC, TOC, and NPOC), micro titration (total alkalinity), and cavity ring-down spectroscopy (CRDS, water stable isotopes, and some others). Analytical techniques need to be optimized for small sample volumes of 0.5–2 ml, but still allowing for the measurement of most of the desired parameters listed above. Key are an optimized dilution strategy, an appropriate workflow, and optimized autosamplers handling small-volume vials, apart from trained analytical personnel. More details on our analytical instruments and how this is implemented are given in some of our publicly accessible technical reports [13, 14].
2.7. Beyond Conventional Core Infiltration
We recently built core infiltration equipment from polymer plastics such that running experiments can be recorded periodically by X-ray computed tomography, and so progress of fluid-rock interaction can be monitored, for example. We first used this in combination with medical X-ray CT and samples of compacted bentonite reacting with cementitious fluids [15, 16]. The pressure vessel was fabricated from carbon fibre fabric, with fibre directions oriented to hold hoop stress and longitudinal stress. Threaded sleeves were made from fabric-reinforced epoxy polymer and glued onto the carbon fibre cylinder. Other components were machined from POM (polyoxymethylene). The maximum pressure is 5–10 MPa, limited by O-ring seals that are difficult to implement due to lack of stiffness and some creep.
A miniature version of a core infiltration cell was produced as a prototype for synchrotron X-ray CT experiments under geologic conditions, for example, fit for elevated pressure and temperature. The original cell was fabricated from a thick-walled optical glass cylinder (and later an aluminium cylinder) and caps made of reinforced PEEK [17] or stainless steel. The cell was designed for 20 MPa confining pressure and an operation temperature up to 200°C.
3. Results and Discussion
3.1. Anion Exclusion Effect and Controls on Pore Water Composition in Opalinus Clay
Anion exclusion effects are well known from soils, compacted bentonite, and argillaceous rocks [18] and are pronounced even in rocks that are not overly rich in clays, such as argillaceous limestone. The effect is largely electrostatic caused by clay surfaces (inner, outer) with permanent negative charge, such as in smectites, illite, and illite/smectite mixed layers. The effect is stronger at low ionic strength and vanishes at very high salinities as would be expected from Poisson-Boltzmann theory. One corollary is that salinities calculated form aqueous leaching and water content measurements may underestimate true salinities by a factor of two or more, and this is relevant for the evaluation of speciation, mineral saturation, and sorption.
Mäder et al. [10] and Mäder and Waber [19] presented one of the first advective displacement experiments with a sample from Opalinus Clay (proposed host rock for radioactive waste in Switzerland) including some preliminary data of early aliquots and showed a partial breakthrough of deuterated water and bromide that indicated an earlier breakthrough for bromide relative to water (as δ2H, Figure 4). This experiment started in March 2002 and is still ongoing. Complete data for the first 2.5 years represent the percolation of 3 pore volumes (based on total water content) and during this time a full breakthrough of deuterated water and bromide was observed. The artificial pore water contained 9.3 g/l chloride, 1.4 g/l of sulphate, and 2.3 g/l of bromide as tracer. This was somewhat more saline than the earliest sample aliquots of the outflow (9.0 g/l Cl, 1.4 g/l SO4), but the associated ionic strength effects caused by such a gradient on anion exclusion are minor. The earliest sample aliquots contained 30–37 mg/l of bromide, representing the pore water [10]. Modelling of the bromide and water tracer breakthrough with the one-dimensional advection-dispersion equation (Figure 4(b)) resulted in a ratio of linear average velocity for bromide relative to deuterated water of 1.67 and experiment-scale Péclet numbers of 7.4 (Br) and 4.65 (δ2H). The anion exclusion effect derived from modelling and expressed as chloride-accessible porosity fraction is 0.6 assuming this simple two-porosity model. The hydraulic conductivity of this sample (perpendicular to bedding) is m/s. Full details of the modelling are given in an unpublished technical report (Mäder et al. 2008).
Figure 4: (a) Breakthrough of deuterated water and bromide versus time expressed as pore volume (water content); (b) modelled breakthrough curves with optimum values for Péclet numbers and the ratio of the anion average linear velocity relative to water, . The dashed lines are for a model including small mixing cells at both ends of the sample, representing the filter discs.
Equilibrium speciation modelling of the early extracted aliquots (data tables in [10]) reveals a slight oversaturation with respect to calcite (SI = 0.2) that can be corrected for a presumed CO2-outgassing effect, and this yields a partial pressure of CO2 of 10−2.6–10−2.9 bar. Laboratory pH of the early aliquots of 7.4–7.5 are in agreement with one measured in-line of 7.5, just after taking a first sample aliquot. Of note are substantial initial concentrations of organic carbon (60 mg/l), and this was also observed in subsequent advective displacement experiments [13, 14] as well as in pore water squeezing [20]. Improved analytical capabilities revealed in later experiments that approximately half of the dissolved TOC is present in form of carboxylic acids like acetate, formate, and lactate. These concentrations are decreasing gradually with time below those seen in aqueous extracts back-calculated to pore water concentration but remain higher than measured from some boreholes used for long-term sampling of pore water [21]. The exact nature and mechanism of the production and release of carboxylic acids in these rocks and the relationship to the solid organic matter are not yet well understood.
Another distinct feature of this first experiment and all subsequent experiments with Opalinus Clay and adjacent clay-rich units is that the outflow appears to be buffered by celestite (Sr-sulphate) with calculated saturation indices within ±0.05 for most analyses, while sulphate is evolving gradually from the concentrations seen in the earliest extracts towards that in the artificial pore water. Partial data for Opalinus Clay is given by Mäder et al. [10], and sulphate elution from a different rock type is illustrated in Figure 5(b). The ubiquitous presence of celestite could not be demonstrated in these rocks and it is therefore possible that buffering by celestite may not operate due to its initial presence but may be controlled by precipitation at least near the outflow region of the Opalinus Clay sample. Mechanisms are not yet entirely clear, but the relevant ion activity product [Sr2+]·[] may increase due to either a source for sulphate (small amount of pyrite oxidation) or a source for strontium, being displaced from the clay exchanger, for example. While the intrinsic coupling of carbonate/sulphate/ion-exchange (linking pH, alkalinity, ) had been intensely discussed [3, 5, 6], the role of strontium in this context had been given far less attention.
Figure 5: (a) Breakthrough of bromide, chloride, and sulphate in an argillaceous limestone, with experimental time converted to pore volumes (water content); (b) elution of select cations for the same experiment. The composition of the injected artificial pore water (APW) is indicated on the right side.
3.2. Ion-Specific Transport Properties in an Argillaceous Limestone: Small Difference between Br− and Cl−
There is some debate about possible small differences between the transport properties (diffusion coefficient) of bromide and chloride. A measured bromide/chloride ratio near that of seawater is used as a strong indication of a marine origin of such a water. In the context of characterising pore water composition, tracer profiles, and water/rock interaction in a clay-rich Mesozoic sequence ([13], Effingen Member at Gösgen, Switzerland), one of the advective displacement experiments started with a mismatch in salinity between the injected artificial pore water and the in situ pore water as seen in the earliest aliquots. The injected pore water contained almost twice as much chloride as the earliest extracts (17 g/l versus 9 g/l) with sulphate values of 1.9 g/l versus 1.1 g/l. The clay content of this argillaceous limestone is 20% (6% illite/smectite mixed layers), the water-content porosity 5.6%, and the hydraulic conductivity m/s. The anion-accessible porosity fraction is 0.64, obtained by comparing the concentration of the early extracted aliquots with those of aqueous leachates back-calculated to the water content. The experiment was continued for 420 days. Full details are given in Mazurek et al. [13].
While the relatively strong salinity gradient due to the injection of a much more saline water has an effect on the anion exclusion extent, both bromide and chloride should be affected to the same extent. The breakthrough data for Br− and Cl− (Figure 5(a)) show a small but distinct and well-resolved difference between the two anions, with chloride being eluted a bit earlier than bromide. Such a behaviour demonstrates that there is indeed a small difference between the effective diffusion coefficients of Cl− and Br− in such a rock. Bromide elutes initially at 35 mg/l and is gradually flushed out until below detection limit after two pore volumes of transport. The breakout data is inverted and presented as breakthrough in the figure. In contrast to conservative anions, sulphate clearly elutes as a reactive component (Figure 5(b)). This data has not yet been modelled, one objective being the quantification of the difference in the apparent diffusion coefficient of bromide and chloride. Given enough geologic time, even a small difference in transport properties should lead to deviations from a seawater ratio simply by out-diffusion against a freshwater boundary, for example.
Chloride obviously elutes before bromide (Figure 5) indicating that either the transport-accessible pore volumes are different, the ion size/mobilities are different, or some form of different chemical interactions exists. The electrophoretic mobility of chloride [22] and thus the self-diffusion coefficient in water (e.g., [23]) is smaller than that for bromide by ca. 2%. On the other hand, the ionic radius (crystallographic/effective) of chloride (0.167/0.181 nm) is distinctly smaller than that for bromide (0.182/0.196 nm), but the hydrated radii are quite similar [24], compared to bromide being somewhat larger (ca. 0.330 versus 0.320 nm, but overlapping data range), with a smaller hydration number for bromide (ca. 6 versus 8). If ion size is key, then the larger bromide may be retarded relative to chloride due to a “tortuosity effect” (pore size and shape, pore throat, size). The same type of preferential transport of chloride was observed by Al et al. [25] examining different ways of extracting dissolved pore water components from Opalinus Clay (and other clay rocks). These authors also discuss the potential role of ion pair formation that may be different between the two species.
The elution of cations (Figure 5(b)) is still far from equilibrium after percolation of four pore volumes. It is evident that Ca and Mg are displaced from the clay exchanger, and Na is retained. It should be possible to model such an experiment with a multicomponent reactive transport model and so gain more insight into the ion-exchange process and its coupling to the sulphate and carbonate system.
3.3. Water Stable Isotope Data Obtained from Advective Displacement Experiments
Advective displacement is not an efficient method for obtaining substantial amounts of water stable isotope data such as required to establish formation-scale profiles. More efficient are diffusive exchange, distillation, or possibly squeezing. Profile data from diffusive exchange and squeezing and three samples from advective displacement are compared (Figure 6). The profile is from the Schlattingen geothermal well including a clay-rich Mesozoic sequence in Northern Switzerland. A full mineralogical and chemicophysical data set and methodology are included in Wersin et al. 2013 and 2016 [14, 26], except for the results from advective displacement experiments that are only partially included. Two units are examined in detail, Opalinus Clay and the overlaying Brown Dogger, both of Lower Jurassic age. Samples for diffusive exchange and squeezing that are located closest to the advective displacement samples are marked by coloured borders around symbols in Figure 6. Diffusive exchange data define a linear trend inclined relative to the global meteoric water line. For Opalinus Clay, near-by samples subjected to squeezing and advective displacement are located on this trend but are shifted to more negative δ18O (by 0.5) relative to diffusive exchange, and also slightly more negative δ2H in case of advective displacement. For Brown Dogger, advective displacement data are also shifted to more negative values and so is one squeezing experiment but not the other one.
Figure 6: Water stable isotope composition of samples from advective displacement, diffusive exchange, and pore water squeezing from samples of Opalinus Clay (OPA) and Brown Dogger (BD). Samples from similar depth as advective displacement samples are correlated by different borders around symbols (black, orange, and dotted). Errors on δ18O are 0.15–0.2 and 1.5–2 for δ2H, but precision is much better for samples measured in the same analytical sample series. DiffEx: diffusive exchange; SQ: squeezing; AD: advective displacement; GMWL: global meteoric water line.
A likely reason for the isotope shift seen in the advective displacement experiment is that the sample cores were cut with a diamond saw using a small amount of tap water (composition indicated in Figure 6) that was blown off afterwards. At that time, the intention was not to derive reliable isotopic data, and our dry cutting facility was not yet implemented. It appears that by applying a more appropriate sample preparation water stable isotope data consistent with diffusive exchange may be obtained from advective displacement. The focus on the water stable isotopes in advective displacement is normally on using an artificial pore water with a large positive δ2H in order to detect and record breakthrough, and this is not very sensitive to the exact value in the initial samples.
4. Conclusions
Equipment developed for advective displacement and core infiltration experiments is relatively complex but based on simple hydraulic components, a robust design, and augmented by a careful choice of fluid-wetted materials. Key is a well-organized strategy for sampling, sample preservation, and sample preparation.
Advective displacement and its extension as a core infiltration experiment can yield a large amount of data to constrain the composition of the pore water preserved in a sample and transport properties derived from breakthrough of tracers. Elution of reactive components subject to ion-exchange or buffering by mineral equilibria (such as shown to operate for strontium and sulphate by celestite) requires extended experimental times of >1 year for such tight clayey rocks. Analytical methods have to strike a compromise between sample size (time resolution) and the number of components to be analysed. In-line analysis by electrodes (EC, pH, and Eh) in very small-volume flow-through cells is possible, but meaningful Eh measurements likely require a more elaborate protection from gas-diffusion and sample conditioning. Experiments with multiple tracers allow direct comparison to resolve small differences in transport properties of conservative components, such as shown to exist for chloride and bromide.
A comparison of pore water squeezing and advective displacement is currently hampered by very few available data gathered from the exact same rock samples. Both methods do induce certain artefacts. One issue is the observed salinity gradient obtained from subsequent squeezing aliquots [20], a consequence of anion exclusion, that render the composition of a squeezed aliquot sensitive to the chosen squeezing conditions. Both methods appear to mobilize organic compounds and this is evident in substantial concentrations of low-molecular-weight organic acids (early aliquots in advective displacement) that are much higher than what can be recovered in aqueous extracts or during long-term borehole sampling. Advective displacement is a destruction-free method whereas squeezing may provoke strain-induced dissolution of minerals such as carbonates. The extent of anion exclusion (chloride) obtained by both methods so far from clay-rich rocks of interest in Switzerland is in reasonable agreement (ca. 0.4–0.6 for anion-accessible/total porosity).
Possible improvements to sample preparation include efficient removal of the outer cylindrical surface by turning it on a lathe and by accurate dry cutting with diamond cutting facilities. This is expected to yield accurate water isotope composition for the early extracts that represent most closely the preserved pore water. In comparison to through-diffusion experiments, core infiltration also provides information on hydraulic properties, and feedback of changes in porosity/permeability to transport can be resolved precisely.
Conflicts of Interest
The author declares that there are no conflicts of interest.
Acknowledgments
All new equipment was built in-house at our machine shop. Adrian Liechti and Thomas Siegenthaler provided their expertise and a number of students helped to assemble “the beasts.” Thomas Gimmi performed the tracer transport modelling calculations for the first Opalinus Clay experiment. Nagra (National Cooperative for the Disposal of Radioactive Waste) partially supported and funded this research. Nick Waber was a very constructive discussion partner about pore water chemistry.
Geological Sciences, University of Bern, Baltzerstrasse 3, 3067 Bern, Switzerland
Copyright © 2018 Urs Mäder. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Source
http://www.hindawi.com/journals/geofluids/2018/8198762/
from TAXI NEAR ME http://taxi.nearme.host/advective-displacement-method-for-the-characterisation-of-pore-water-chemistry-and-transport-residence-in-claystone/
from NOVACAB https://novacabtaxi.tumblr.com/post/171301231781
0 notes
Text
Advective Displacement Method for the Characterisation of Pore Water Chemistry and Transport Residence in Claystone
Abstract
The advective displacement method applies large hydraulic gradients to a confined rock core sample to yield small aliquots of the preserved in situ pore water, applicable to aquitard rocks with hydraulic conductivities as low as 10−14 m/s. Examples from argillaceous rocks indicate that only minor artefacts are induced and that analytical methods optimized for small aliquots provide a comprehensive chemical and isotopic characterisation. Multicomponent transport properties are derived from extending experimental time and using a traced artificial pore water for injection. Examples include quantification of the anion exclusion effect that can even resolve a small difference between transport properties for chloride and bromide in claystone. Controls by mineral saturation and cation exchange processes are also constrained by data from this approach. Technical details are provided for construction, material selection, components, sensors, and analytical issues.
1. Introduction
Properties and performance of hydraulically tight clayey rock formations are of interest in the context of cap rocks for oil, gas, and carbon dioxide confinement, as pore water archives for past hydrogeochemical evolution, as hydrocarbon source rock for shale gas, and as confining units for radioactive and chemotoxic waste ([1, 2], editorial of this volume). In contrast to tight crystalline rocks, porosity and water content are substantial, in the range of a few to about twenty volume percent, and hydraulic conductivities are typically <10−12 m/s. The focus of this paper is on a particular method from which to gain insight into pore water chemistry, hydraulic properties, transport of dissolved species, and reactive transport (water/rock interaction) that may also be coupled to the hydraulic properties. The motivation is to gain process understanding in the context of deep geologic disposal of radioactive waste, but the methods and results are transferable to the other aforementioned fields of research.
Our understanding of pore water chemistry and transport of dissolved species is based on models that bridge measureable quantities (fluxes, concentrations, and bulk properties) and the nanometric pore scale that is only very selectively observable. A milestone was the Pearson et al. [3] data digest on Opalinus Clay based on laboratory work and field tests performed in the Underground Rock Laboratory at Mont Terri, Switzerland (https://www.mont-terri.ch). The thermodynamically based concepts of aqueous speciation, mineral equilibria (and buffering), interdependencies such as pH-PCO2-carbonate-sulphate-ion exchanger [4], and electrostatic effects exerted by certain clay minerals (commonly named “anion exclusion” based on some variant of Poisson-Boltzmann theory) offer a sufficient level of complexity for the discussion of results presented in this paper. Significant progress has been made regarding details, and more constraining data have been elaborated [5–8].
Sophisticated reactive transport models (e.g., Crunchflow, Flotran, and Phreeqc-Comsol coupling) and inexpensive computation power are available to simulate fully described laboratory experiments that are necessary as testing cases for both implementation of complex and coupled processes (benchmark-type exercises) and testing of alternative conceptualisations (choice of processes and simplifying boundary/initial conditions). An example of such a data set and its comparative modelling by increasing degrees of complexity is that of a reactive transport core infiltration experiment in compacted bentonite [9].
The focus of this contribution is on the technical details of the experimental approach because much hinges on design details and material selection for equipment, on details of sample preparation, on the ability to analyse very small samples, and on keeping experiments stable for months to years.
2. Materials and Methods
2.1. Advective Displacement Method and Sample Requirement
During the 1990s when characterisation of pore water in tight clayey rock formations was advancing, direct sampling techniques included rock squeezing, long-term borehole sampling, and an indirect fingerprinting method by aqueous leaching [3]. As an alternative, a so-called advective displacement method was tested whereby the injection of an artificial pore water on one side of a sample core was used to displace the in situ pore water out of the other end of the sample [10]. A first pore water displacement experiment with Opalinus Clay started in 2002, and it is still running as of 2017. The original apparatus was constructed at the Paul Scherrer Institut (Laboratory for Waste Management), Switzerland, for the purpose of studying radionuclide transport in synthetic fractures, and this prototype was moved to the University of Bern and subsequently modified and replaced by new equipment designed and constructed in-house.
The advective displacement method implicitly assumes the following:(i)A cylindrical rock sample with planar ends perpendicular to the cylinder axis(ii)A well-preserved rock sample, with respect to water content and the reactive mineral components (pyrite), for example, sealed in moderate vacuum and stored refrigerated or at in situ temperature of the formation(iii)No disturbing effects from drilling fluid (particularly if stable isotope data should be acquired), or other cutting fluids, or from compressed gas when dry drilling is employed (for some argillaceous rocks). The outermost near-surface part may be turned off efficiently on a lathe(iv)Minimization of exposure to atmosphere at all stages(v)Approximation of 1D column transport. This is normally met by samples taken perpendicular to bedding but may not be met for samples parallel to bedding and that show distinct layering. In the latter case, a more permeable subvolume may be tested preferentially.
A number of potentially disturbing effects are difficult to control:(i)Volumetric strain is associated with the unloading of a sample when sampling a deep formation.(ii)Outgassing will occur in case gas saturation is reached during unloading.(iii)The presence of a gas phase at in situ condition will result in gas escape and potentially some displacement of pore water.(iv)An adjustment of pore water chemistry from in situ temperature to storage or experimental temperature will occur (carbonate-sulphate system).(v)Microbial activity may start at sample surfaces and may persist during an experiment.
2.2. Properties and Processes That Can Be Studied by Advective Displacement Experiments
Diverse processes, chemical, physical, and transport properties, can be determined by imposing a wide range of hydraulic and chemical boundary conditions for advective displacement experiments.Hydraulic conductivity K [m/s] is calculated very accurately from sample dimensions (l [m], A [m2]), average hydraulic head (h [m]), and average volumetric flow rate (Q [m3/s]) for a time span of interest, . A lower practical limit is 10−14 m/s if fluid chemistry is also of interest, or even 10−15 m /s if only conductivity is to be determined.The temperature dependence of hydraulic conductivity can be determined accurately with a thermostat-equipped device.Changes of hydraulic conductivity as function of total stress of a sample or clogging of flow-paths by transport of micro particles or by precipitation/dissolution of secondary minerals (fluid-rock interaction) can also be recorded.The in situ pore water can be displaced with an artificial pore water, and the first few small samples represent pore water composition, subject to some artefacts.Extending pore water displacement to an advection experiment with tracers, breakthrough of these can be analysed and transport properties determined, such as anions versus deuterated water, or reactive components such as cations, or dissolved organic carbon species.In very reactive systems (soluble salts), changes in porosity and/or pore structure will affect hydraulic properties, and this can be monitored.Anion-accessible porosity ratio can be determined as the ratio between the anion concentration obtained from an aqueous extract back-calculated to the total porosity (water content) of a sample and that measured in the outflow. This assumes that the anion-accessible porosity is represented by the porosity that is also involved in advective flow.Because outflow can be continuously sampled, the saturation state with respect to potentially controlling minerals (precipitation or dissolution) can be evaluated as a function of percolation time. These are clues for potentially controlling mineral equilibria (carbonates, sulphates, but also ion-exchange).Postmortem analysis of a sample core may yield additional constraints apart from physical properties, such as a longitudinal distribution of the cation population on the clay exchanger, or mineral zonation in reactive systems.Redox-sensitive systems may be studied by imposing special precautions during sample preparation and sampling of the outflow and by introducing redox-sensitive tracers.Two-phase flow phenomena or the initial saturation state may also be tested (pore water uptake).
We tested in one or another way all of the potential applications mentioned above and are actively pursuing chemicophysical coupling phenomena, experimentally and by modelling approaches [11, 12]. Some select details are addressed in the technical description below.
2.3. Technical Description and Equipment
The principle design is that of a triaxial cell, but with a fixed length for the sample specimen (Figure 1). A rigid steel cylinder serves as pressure vessel, with a confining medium (water) that exerts a quasi-isostatic pressure on a cylindrical sample. Quasi-isostatic refers to a small supported area that reduces the cross section on which confining pressure acts. Working with a confining pressure ensures a self-sealing system against the interior of a sample, a more reliable option compared to press-fits, glued-in samples, or other mechanical confinements.
Figure 1: Advective displacement apparatus, (a) technical drawing, (b) schematic, (c) rock sample (white) with titanium coupling (green) and insert (dark grey) with details of O-ring seals and capillary for fluid injection (centre) and connection to confining fluid (off-centre), (d) insert with small O-ring sealing directly on the capillary for fluid injection, and (e) core sample mounted between inserts before lowering into pressure vessel. Inner diameter of this pressure vessel is 120 mm; the diameter of the sample core is 101 mm.
The sample assembly (Figure 1(e)) comprises the cylindrical rock sample sandwiched between coupling pieces made of titanium. Porous filter discs are placed between the sample and the coupling pieces. The assembly is sealed against the confining medium by an inner layer of Teflon tape covered by a latex sleeve (details below). The assembly becomes self-sealing once the confining pressure is ramped up.
The sample assembly is mounted between two steel inserts (Figures 1(a) and 1(b)) and lowered into the pressure cylinder (Figure 1(e)). Conventional O-rings seal the enclosed confining medium against the pressure vessel and against the sample assembly (Figures 1(c) and 1(d)). A spindle (Figure 1(a)) adjusts to the length of a sample assembly, and normally no extra force is imposed initially on the sample by the spindle.
An artificial pore water is injected and collected with thin PEEK (polyether ether ketone) capillaries (1/16′′ OD, 1.6 mm). These capillaries are fixed and fed through the sliding inserts by means of Swagelok fittings (Figure 1(c)), and an additional small O-ring is sealing the capillary at the contact point of the sample assembly and the inserts (Figure 1(d)) to avoid any stagnant dead volume of the percolating fluid.
Porous filter discs are a critical component that are required to distribute and collect the in-flow and outflow to and from a sample. A number of considerations determine the choice of filter material: minimizing pore volume is commonly desirable as well as chemical inertness. Porous Teflon is limited in stiffness and will collapse slowly when used at high confining pressures (above 2 MPa). A clever work-around is to use a thin porous Teflon filter with 3–5 small titanium frits as inserts that support the load. In this way, a filter thickness of 0.5 mm or less may be achieved with a diameter up to 120 mm. Alternatives may be woven filters made from very thin titanium wire, or a grooved adapter surface in case of stiff rock like granite. Thick filters (>1 mm) are commonly not practical in applications for low-permeability rocks due to the significant dead volume compared to transport rates.
Steel cylinders for the pressure vessel are easiest manufactured from ready-made seamless precision steel tubes, honed, and centred on the inner diameter, such as used for hydraulic applications. Only an outer thread has to be machined such that caps can be screwed on at top and base (Figure 1(a)). Maximum pressure is limited by the maximum hoop stress that can be applied to the cylinder. A safe design is to hold maximum pressure of bottled argon or helium, for example, 20 MPa, but 50–100 MPa may also be reached with conventional O-ring sealing techniques. All our plumbing not contacted by pore fluids (for hydraulics) are made from stainless steel Swagelok components, using 6 mm and 1/8′′ (3.2 mm) product lines, and needle valves. These components are rated >20 MPa pressure.
Chemically inert materials are used for all fluid-wetted parts, including capillary tubing, valves, and fluid containers. Components from analytical equipment are readily available and include PEEK tubing, PEEK valves (used in high pressure liquid chromatography, e.g., Vici/Valco, Rheodyne, IDEX), and internally coated stainless steel tanks (sold as sampling cylinders, e.g., from Swagelok).
Sampling of aliquots from the outflow is conveniently done by small syringes directly connected to the outflow capillary; this protects samples from atmosphere. Syringe friction is significant (up to 0.1 MPa for a 1 ml standard O-ring sealed plastic syringe) when working at small hydraulic gradients. Alternatively, a gas-pressured back-pressure system can be implemented.
Controls include generation of the hydraulic confining pressure by bottled argon in a gas/liquid tank partially filled with the confining medium. This simple system also provides an effective buffering against pressure changes induced by temperature changes. The infiltrating artificial pore water is easiest driven at constant-pressure condition imposed by bottled He in a polymer-coated steel cylinder, with a capillary connecting to the infiltration apparatus via an injection valve. Using a 6-port two-position valve allows switching between two different fluids (e.g., one traced), or refilling the fluid reservoir at pressure, with a HPLC pump, for example. Helium is a good choice because of its small solubility and small pressure-dependence of solubility. Using argon or nitrogen leads to degassing in the experimental core that is subjected to a substantial hydraulic gradient.
We monitor all pressures and temperatures via data acquisition but do not use computer-control for long-term experiments, computers being far less reliable compared to hydraulic systems.
The pressure vessel may conveniently be heated or cooled by an outer heater jacket or cooling coil. A copper coil (or any other tubing material) in combination with a circulation heater/cooler is an easy way to thermostat or moderately heat or cool an experiment (indicated in Figure 1(a)).
Main operational safety issues relate to compressed gases, compressed liquids, and electric components. Most of our equipment is designed to hold full bottled gas pressure (20 MPa), except for fluid/gas reservoirs that are generally rated to somewhat lower pressures (12–34 MPa for Swagelok sampling cylinders). Safety heads or pressure relieve valves protect such containers according to regulatory requirements.
A number of parameters for the outflowing fluid are conveniently measured in-line, including pH, electric conductivity (EC), and redox potential (Eh). Except for conductivity, electrode measurements cannot be performed continuously over extended time, but small flow-through cells can be connected to a stream switching valve and activated for select periods of time, bracketed by calibration before and after in case of pH. Convenient cells for EC are those used in ion-chromatography instruments, for example, as the one used by Metrohm (Figure 2(a)) that can be connected directly to 1/16′′ PEEK capillaries without dead volume and in conjunction with a Metrohm conductivity meter connected to data acquisition. These small two-electrode EC cells are designed for relatively low conductivities (large cell constant of ca. 10) and the conductivity meter has to be capable of using combinations of conductivity range and cell constants beyond what is common practice. Small flow-through cells for pH are scarce on the market, and we fabricate ours in-house from POM (polyoxymethylene) and semi-micro-combination electrodes with tip diameters of 2-3 mm, immersion depth of 4–6 mm, and a flushed volume of 20–30 μl (Figure 2(b)). It is critical to provide a relatively soft seal above the electrode tip, and the mounting is very fragile. Using a 6-port stream switching valve, the ports for flushing and calibration can be set up in combination with the fluid outflow from the experiment.
Figure 2: Flow-through cells for (a) electric conductivity and (b) pH.
Tests with measuring Eh in-line have been a mixed success. More often than not, only partly reducing conditions are obtained even over several weeks of flow-through time, but never as low as might be expected from the sulphate/sulphide/pyrite/Fe-carbonate (or silicate) buffer imposed by most argillaceous rocks. This is likely due to the experimental set-up being not sufficiently gas-tight without converting to a more sophisticated construction. We made some tests using a very small-volume (10–20 μl) cell from Metrohm used normally as a three-electrode amperometric detector, just using a Ag/AgCl flat-base reference electrode, and either an Au or Pt measuring electrode.
2.4. Sample Preparation and Setting Up an Experiment
A requirement is a sample with preserved moisture content and that has been exposed to atmosphere for as little as possible. A cylindrical segment is dry-cut (or wet if permissible) with planar surfaces that are parallel and perpendicular to the cylinder axis. Total sample mass and dimensions are measured. Off-cuts are kept for determining chemicophysical parameters as a precharacterisation. We modified a mitre saw normally used for wood by fitting a diamond blade used normally for dry cutting. This is extremely efficient, precise, and inexpensive and cuts all materials but requires an industrial vacuum device for dust collection.
The sample core is placed between titanium coupling pieces (Figure 3), with filters in between. In the example shown (Figure 3(a)), a 1 mm thick porous titanium filter is used (85 mm DM) with a Teflon ring to match the sample diameter of 101 mm. The assembly is sealed against confining pressure by an inner layer of Teflon tape (Figure 3(c)) covered by a latex sleeve (Figure 3(d)), sealed against the coupling parts with silicone sealant, and taped to ensure a tight initial seal.
Figure 3: Stages of sample preparation: (a) rock core with porous filters at top and base, (b) placed between titanium couplings, (c) wrapped with Teflon tape, and (d) covered with latex sleeve. See text for details.
Starting an advective displacement experiment includes loading the sample assembly into the pressure vessel (Figure 1(e)), filling in the confining fluid (water), and ramping up confining pressure, typically 1-2 MPa above the desired injection pressure. Tightness of the sealing system of the rock sample (Figure 3) can be affirmed by leaving the system pressurized overnight, but without in-flow and outflow capillaries connected. We use the injection valve to evacuate the dead space in the filter disc and capillaries, switching directly from vacuum to the pressurized artificial pore water to start fluid injection. Likewise, the dead space in the fluid outflow part can be evacuated or flushed with argon with the help of a switching valve, before connecting to the sampling syringe. It may take several days until the head space is flushed with the displaced pore water, and arrival of outflow is first indicated by a jump in the electrical conductivity measured in-line (Figure 2(a)). Typical flow rates range from 0.05 to 0.5 ml/day for argillaceous rock samples of 8–12 cm length and hydraulic heads of 2–8 MPa, corresponding to head gradients of – m/m.
2.5. Tracers Used in Advective Displacement Experiments
A good frame of reference for transport is the use of a water tracer (deuterium, tritium) in all experiments. An anionic tracer should also be used, because anions get transported faster compared to water in clayey rocks due to a distinct anion exclusion effect that reduces the cross-sectional area available for transport and, hence, increases the average linear velocity. Choices are limited if the in situ anionic minor components are of interest (Br−, I−, and low-molecular-weight organic acids). The anions contained in the undisturbed pore water can be observed as breakout tracers that elute from in situ concentration towards zero if the artificial pore water is tracer-free. Of concern are potential microbially induced redox reactions that may affect concentrations of anionic complexes such as sulphate and nitrate, particularly in the presence of dissolved natural organic matter. Many more choices of radionuclide tracers are available when working in a hot-laboratory environment.
Optical tracers, such as fluorescent dyes, are of little use in clay-rich rocks because these large molecules become retarded by sorption and/or potentially by ion filtration. It is safest to use tracers that resemble common pore water components, because testing for the suitability of a tracer at low transport rates is extremely time consuming.
2.6. Analytical Techniques
Sampling strategy for pore water displacement aims at collecting 3–5 small samples that are largely unaffected by the injected artificial pore water, such that ideally a concentration plateau can be confirmed and the onset of the anion tracer breakthrough detected. Sampling for obtaining breakthrough behaviour of tracers requires frequent early samples for inert tracers (water, anions) and far less frequent samples for late times or reactive components. A combination of in-line measurements and laboratory measurements of the sampled aliquots needs to be tailored to each experiment.
The parameters of interest involve those that can be measured in-line by electrodes with very small flow-through cells (electric conductivity, pH, and redox potential, details above). Those measured on small aliquots in automated analysers include ion chromatography (major anions, cations, and some carboxylic acids), ICP-OES (Si, Al, and some minor components such as Sr), carbon IR analyser (TIC, TOC, and NPOC), micro titration (total alkalinity), and cavity ring-down spectroscopy (CRDS, water stable isotopes, and some others). Analytical techniques need to be optimized for small sample volumes of 0.5–2 ml, but still allowing for the measurement of most of the desired parameters listed above. Key are an optimized dilution strategy, an appropriate workflow, and optimized autosamplers handling small-volume vials, apart from trained analytical personnel. More details on our analytical instruments and how this is implemented are given in some of our publicly accessible technical reports [13, 14].
2.7. Beyond Conventional Core Infiltration
We recently built core infiltration equipment from polymer plastics such that running experiments can be recorded periodically by X-ray computed tomography, and so progress of fluid-rock interaction can be monitored, for example. We first used this in combination with medical X-ray CT and samples of compacted bentonite reacting with cementitious fluids [15, 16]. The pressure vessel was fabricated from carbon fibre fabric, with fibre directions oriented to hold hoop stress and longitudinal stress. Threaded sleeves were made from fabric-reinforced epoxy polymer and glued onto the carbon fibre cylinder. Other components were machined from POM (polyoxymethylene). The maximum pressure is 5–10 MPa, limited by O-ring seals that are difficult to implement due to lack of stiffness and some creep.
A miniature version of a core infiltration cell was produced as a prototype for synchrotron X-ray CT experiments under geologic conditions, for example, fit for elevated pressure and temperature. The original cell was fabricated from a thick-walled optical glass cylinder (and later an aluminium cylinder) and caps made of reinforced PEEK [17] or stainless steel. The cell was designed for 20 MPa confining pressure and an operation temperature up to 200°C.
3. Results and Discussion
3.1. Anion Exclusion Effect and Controls on Pore Water Composition in Opalinus Clay
Anion exclusion effects are well known from soils, compacted bentonite, and argillaceous rocks [18] and are pronounced even in rocks that are not overly rich in clays, such as argillaceous limestone. The effect is largely electrostatic caused by clay surfaces (inner, outer) with permanent negative charge, such as in smectites, illite, and illite/smectite mixed layers. The effect is stronger at low ionic strength and vanishes at very high salinities as would be expected from Poisson-Boltzmann theory. One corollary is that salinities calculated form aqueous leaching and water content measurements may underestimate true salinities by a factor of two or more, and this is relevant for the evaluation of speciation, mineral saturation, and sorption.
Mäder et al. [10] and Mäder and Waber [19] presented one of the first advective displacement experiments with a sample from Opalinus Clay (proposed host rock for radioactive waste in Switzerland) including some preliminary data of early aliquots and showed a partial breakthrough of deuterated water and bromide that indicated an earlier breakthrough for bromide relative to water (as δ2H, Figure 4). This experiment started in March 2002 and is still ongoing. Complete data for the first 2.5 years represent the percolation of 3 pore volumes (based on total water content) and during this time a full breakthrough of deuterated water and bromide was observed. The artificial pore water contained 9.3 g/l chloride, 1.4 g/l of sulphate, and 2.3 g/l of bromide as tracer. This was somewhat more saline than the earliest sample aliquots of the outflow (9.0 g/l Cl, 1.4 g/l SO4), but the associated ionic strength effects caused by such a gradient on anion exclusion are minor. The earliest sample aliquots contained 30–37 mg/l of bromide, representing the pore water [10]. Modelling of the bromide and water tracer breakthrough with the one-dimensional advection-dispersion equation (Figure 4(b)) resulted in a ratio of linear average velocity for bromide relative to deuterated water of 1.67 and experiment-scale Péclet numbers of 7.4 (Br) and 4.65 (δ2H). The anion exclusion effect derived from modelling and expressed as chloride-accessible porosity fraction is 0.6 assuming this simple two-porosity model. The hydraulic conductivity of this sample (perpendicular to bedding) is m/s. Full details of the modelling are given in an unpublished technical report (Mäder et al. 2008).
Figure 4: (a) Breakthrough of deuterated water and bromide versus time expressed as pore volume (water content); (b) modelled breakthrough curves with optimum values for Péclet numbers and the ratio of the anion average linear velocity relative to water, . The dashed lines are for a model including small mixing cells at both ends of the sample, representing the filter discs.
Equilibrium speciation modelling of the early extracted aliquots (data tables in [10]) reveals a slight oversaturation with respect to calcite (SI = 0.2) that can be corrected for a presumed CO2-outgassing effect, and this yields a partial pressure of CO2 of 10−2.6–10−2.9 bar. Laboratory pH of the early aliquots of 7.4–7.5 are in agreement with one measured in-line of 7.5, just after taking a first sample aliquot. Of note are substantial initial concentrations of organic carbon (60 mg/l), and this was also observed in subsequent advective displacement experiments [13, 14] as well as in pore water squeezing [20]. Improved analytical capabilities revealed in later experiments that approximately half of the dissolved TOC is present in form of carboxylic acids like acetate, formate, and lactate. These concentrations are decreasing gradually with time below those seen in aqueous extracts back-calculated to pore water concentration but remain higher than measured from some boreholes used for long-term sampling of pore water [21]. The exact nature and mechanism of the production and release of carboxylic acids in these rocks and the relationship to the solid organic matter are not yet well understood.
Another distinct feature of this first experiment and all subsequent experiments with Opalinus Clay and adjacent clay-rich units is that the outflow appears to be buffered by celestite (Sr-sulphate) with calculated saturation indices within ±0.05 for most analyses, while sulphate is evolving gradually from the concentrations seen in the earliest extracts towards that in the artificial pore water. Partial data for Opalinus Clay is given by Mäder et al. [10], and sulphate elution from a different rock type is illustrated in Figure 5(b). The ubiquitous presence of celestite could not be demonstrated in these rocks and it is therefore possible that buffering by celestite may not operate due to its initial presence but may be controlled by precipitation at least near the outflow region of the Opalinus Clay sample. Mechanisms are not yet entirely clear, but the relevant ion activity product [Sr2+]·[] may increase due to either a source for sulphate (small amount of pyrite oxidation) or a source for strontium, being displaced from the clay exchanger, for example. While the intrinsic coupling of carbonate/sulphate/ion-exchange (linking pH, alkalinity, ) had been intensely discussed [3, 5, 6], the role of strontium in this context had been given far less attention.
Figure 5: (a) Breakthrough of bromide, chloride, and sulphate in an argillaceous limestone, with experimental time converted to pore volumes (water content); (b) elution of select cations for the same experiment. The composition of the injected artificial pore water (APW) is indicated on the right side.
3.2. Ion-Specific Transport Properties in an Argillaceous Limestone: Small Difference between Br− and Cl−
There is some debate about possible small differences between the transport properties (diffusion coefficient) of bromide and chloride. A measured bromide/chloride ratio near that of seawater is used as a strong indication of a marine origin of such a water. In the context of characterising pore water composition, tracer profiles, and water/rock interaction in a clay-rich Mesozoic sequence ([13], Effingen Member at Gösgen, Switzerland), one of the advective displacement experiments started with a mismatch in salinity between the injected artificial pore water and the in situ pore water as seen in the earliest aliquots. The injected pore water contained almost twice as much chloride as the earliest extracts (17 g/l versus 9 g/l) with sulphate values of 1.9 g/l versus 1.1 g/l. The clay content of this argillaceous limestone is 20% (6% illite/smectite mixed layers), the water-content porosity 5.6%, and the hydraulic conductivity m/s. The anion-accessible porosity fraction is 0.64, obtained by comparing the concentration of the early extracted aliquots with those of aqueous leachates back-calculated to the water content. The experiment was continued for 420 days. Full details are given in Mazurek et al. [13].
While the relatively strong salinity gradient due to the injection of a much more saline water has an effect on the anion exclusion extent, both bromide and chloride should be affected to the same extent. The breakthrough data for Br− and Cl− (Figure 5(a)) show a small but distinct and well-resolved difference between the two anions, with chloride being eluted a bit earlier than bromide. Such a behaviour demonstrates that there is indeed a small difference between the effective diffusion coefficients of Cl− and Br− in such a rock. Bromide elutes initially at 35 mg/l and is gradually flushed out until below detection limit after two pore volumes of transport. The breakout data is inverted and presented as breakthrough in the figure. In contrast to conservative anions, sulphate clearly elutes as a reactive component (Figure 5(b)). This data has not yet been modelled, one objective being the quantification of the difference in the apparent diffusion coefficient of bromide and chloride. Given enough geologic time, even a small difference in transport properties should lead to deviations from a seawater ratio simply by out-diffusion against a freshwater boundary, for example.
Chloride obviously elutes before bromide (Figure 5) indicating that either the transport-accessible pore volumes are different, the ion size/mobilities are different, or some form of different chemical interactions exists. The electrophoretic mobility of chloride [22] and thus the self-diffusion coefficient in water (e.g., [23]) is smaller than that for bromide by ca. 2%. On the other hand, the ionic radius (crystallographic/effective) of chloride (0.167/0.181 nm) is distinctly smaller than that for bromide (0.182/0.196 nm), but the hydrated radii are quite similar [24], compared to bromide being somewhat larger (ca. 0.330 versus 0.320 nm, but overlapping data range), with a smaller hydration number for bromide (ca. 6 versus 8). If ion size is key, then the larger bromide may be retarded relative to chloride due to a “tortuosity effect” (pore size and shape, pore throat, size). The same type of preferential transport of chloride was observed by Al et al. [25] examining different ways of extracting dissolved pore water components from Opalinus Clay (and other clay rocks). These authors also discuss the potential role of ion pair formation that may be different between the two species.
The elution of cations (Figure 5(b)) is still far from equilibrium after percolation of four pore volumes. It is evident that Ca and Mg are displaced from the clay exchanger, and Na is retained. It should be possible to model such an experiment with a multicomponent reactive transport model and so gain more insight into the ion-exchange process and its coupling to the sulphate and carbonate system.
3.3. Water Stable Isotope Data Obtained from Advective Displacement Experiments
Advective displacement is not an efficient method for obtaining substantial amounts of water stable isotope data such as required to establish formation-scale profiles. More efficient are diffusive exchange, distillation, or possibly squeezing. Profile data from diffusive exchange and squeezing and three samples from advective displacement are compared (Figure 6). The profile is from the Schlattingen geothermal well including a clay-rich Mesozoic sequence in Northern Switzerland. A full mineralogical and chemicophysical data set and methodology are included in Wersin et al. 2013 and 2016 [14, 26], except for the results from advective displacement experiments that are only partially included. Two units are examined in detail, Opalinus Clay and the overlaying Brown Dogger, both of Lower Jurassic age. Samples for diffusive exchange and squeezing that are located closest to the advective displacement samples are marked by coloured borders around symbols in Figure 6. Diffusive exchange data define a linear trend inclined relative to the global meteoric water line. For Opalinus Clay, near-by samples subjected to squeezing and advective displacement are located on this trend but are shifted to more negative δ18O (by 0.5) relative to diffusive exchange, and also slightly more negative δ2H in case of advective displacement. For Brown Dogger, advective displacement data are also shifted to more negative values and so is one squeezing experiment but not the other one.
Figure 6: Water stable isotope composition of samples from advective displacement, diffusive exchange, and pore water squeezing from samples of Opalinus Clay (OPA) and Brown Dogger (BD). Samples from similar depth as advective displacement samples are correlated by different borders around symbols (black, orange, and dotted). Errors on δ18O are 0.15–0.2 and 1.5–2 for δ2H, but precision is much better for samples measured in the same analytical sample series. DiffEx: diffusive exchange; SQ: squeezing; AD: advective displacement; GMWL: global meteoric water line.
A likely reason for the isotope shift seen in the advective displacement experiment is that the sample cores were cut with a diamond saw using a small amount of tap water (composition indicated in Figure 6) that was blown off afterwards. At that time, the intention was not to derive reliable isotopic data, and our dry cutting facility was not yet implemented. It appears that by applying a more appropriate sample preparation water stable isotope data consistent with diffusive exchange may be obtained from advective displacement. The focus on the water stable isotopes in advective displacement is normally on using an artificial pore water with a large positive δ2H in order to detect and record breakthrough, and this is not very sensitive to the exact value in the initial samples.
4. Conclusions
Equipment developed for advective displacement and core infiltration experiments is relatively complex but based on simple hydraulic components, a robust design, and augmented by a careful choice of fluid-wetted materials. Key is a well-organized strategy for sampling, sample preservation, and sample preparation.
Advective displacement and its extension as a core infiltration experiment can yield a large amount of data to constrain the composition of the pore water preserved in a sample and transport properties derived from breakthrough of tracers. Elution of reactive components subject to ion-exchange or buffering by mineral equilibria (such as shown to operate for strontium and sulphate by celestite) requires extended experimental times of >1 year for such tight clayey rocks. Analytical methods have to strike a compromise between sample size (time resolution) and the number of components to be analysed. In-line analysis by electrodes (EC, pH, and Eh) in very small-volume flow-through cells is possible, but meaningful Eh measurements likely require a more elaborate protection from gas-diffusion and sample conditioning. Experiments with multiple tracers allow direct comparison to resolve small differences in transport properties of conservative components, such as shown to exist for chloride and bromide.
A comparison of pore water squeezing and advective displacement is currently hampered by very few available data gathered from the exact same rock samples. Both methods do induce certain artefacts. One issue is the observed salinity gradient obtained from subsequent squeezing aliquots [20], a consequence of anion exclusion, that render the composition of a squeezed aliquot sensitive to the chosen squeezing conditions. Both methods appear to mobilize organic compounds and this is evident in substantial concentrations of low-molecular-weight organic acids (early aliquots in advective displacement) that are much higher than what can be recovered in aqueous extracts or during long-term borehole sampling. Advective displacement is a destruction-free method whereas squeezing may provoke strain-induced dissolution of minerals such as carbonates. The extent of anion exclusion (chloride) obtained by both methods so far from clay-rich rocks of interest in Switzerland is in reasonable agreement (ca. 0.4–0.6 for anion-accessible/total porosity).
Possible improvements to sample preparation include efficient removal of the outer cylindrical surface by turning it on a lathe and by accurate dry cutting with diamond cutting facilities. This is expected to yield accurate water isotope composition for the early extracts that represent most closely the preserved pore water. In comparison to through-diffusion experiments, core infiltration also provides information on hydraulic properties, and feedback of changes in porosity/permeability to transport can be resolved precisely.
Conflicts of Interest
The author declares that there are no conflicts of interest.
Acknowledgments
All new equipment was built in-house at our machine shop. Adrian Liechti and Thomas Siegenthaler provided their expertise and a number of students helped to assemble “the beasts.” Thomas Gimmi performed the tracer transport modelling calculations for the first Opalinus Clay experiment. Nagra (National Cooperative for the Disposal of Radioactive Waste) partially supported and funded this research. Nick Waber was a very constructive discussion partner about pore water chemistry.
Geological Sciences, University of Bern, Baltzerstrasse 3, 3067 Bern, Switzerland
Copyright © 2018 Urs Mäder. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Source
http://www.hindawi.com/journals/geofluids/2018/8198762/
from TAXI NEAR ME http://taxi.nearme.host/advective-displacement-method-for-the-characterisation-of-pore-water-chemistry-and-transport-residence-in-claystone/
0 notes