#eCTD Submissions
Text
#NeeS#eCTD Submissions#eCTD software#non-eCTD electronic Submission#eCTD 4.0#Regulatory Submissions#eCTD transitioning
0 notes
Text
Bring forth trust in your business to engage in Foreign Supplier Verification Program
Taking authentic service in each service domain is a must for all business professionals so that they should not repent on self-business development decisions. Everyone has a strong desire to select the imperative business to get the most affirmative result. In other words, one has to select the business that is free from recession invasion. Whenever you keep this concern in your mind, the drug supplement and development business are in a top priority.

Establishing the outlet of this business is not possible for everyone as one should have to maintain some safety protocol. To develop such a business, one should have the sure availability of this document to propose their business in a new direction. To cater results in the same direction, one should have to take Ectd submissions. Anyway, it is the standard way to submit different kinds of applications. After all, you go through how can amendments, supplements, and reports should be delivered to the drug evaluation and research center.
Thereby, it is expected to develop such a verified document so that nobody can object to the decisions of the medical shop. It is not easy to submit such type of document with full approval possibilities. So, you should have to search out those companies who are offering you such service. Throughout the global region, you have the availability of many service providers. But, you cannot select any random name.
Keep all confusion to one side and you can last your search with us. We are one of the popular destinations to make your service process most authentic and reliable. We are known as the alpha green resource for making favorable results to maintain integrity, trust, and respect in your business. Our professionals deal in a wide range of services and offer you Foreign Supplier Verification Program to let your business true effort to cater to customer’s needs. View our website to know more information.
0 notes
Text
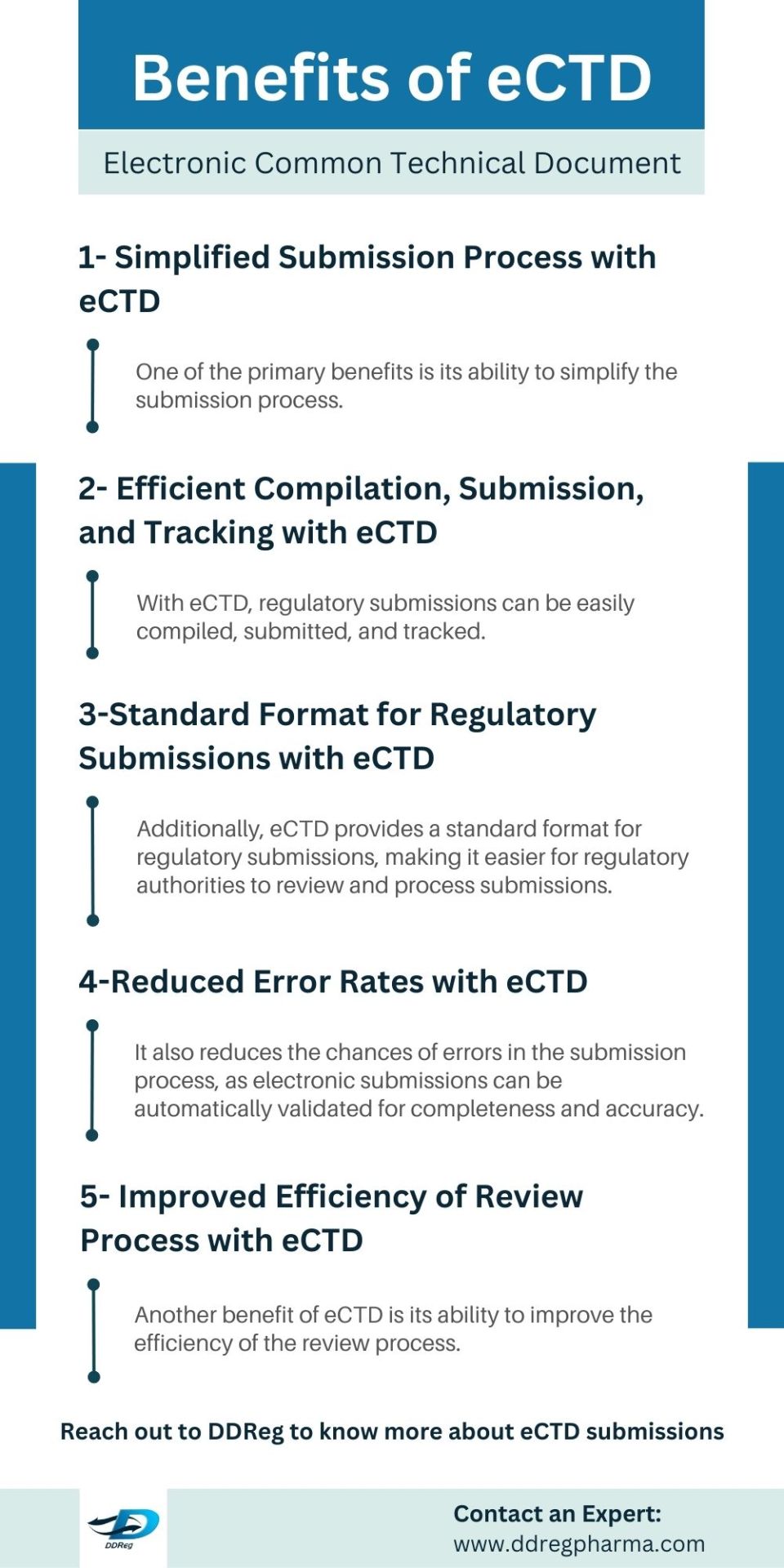
The Benefits of eCTD- DDreg Pharma
The eCTD offers numerous advantages compared to traditional paper-based submissions. It simplifies the submission process by allowing easy compilation, submission, and tracking of regulatory submissions. Electronic submissions reduce errors and improve review efficiency, leading to faster approvals and cost savings for pharmaceutical companies. To learn more about eCTD submission, read our blog.
0 notes
Text
Global Regulatory Publishing and eCTD submission Services | MakroCare
MakroCare Regulatory Publishing and eCTD Submission Services help you to reduce the cost and effort involved in converting paper-based documentation into eCTD.
0 notes
Text
Data Security and Confidentiality in eCTD Publishing Tools: Safeguarding Sensitive Information
In the current era of digitalization life sciences has seen a major change in the way that regulatory submissions are developed and presented. Electronic Common Technical Document (eCTD) publishing has transformed the process of submission by making it faster and more efficient. However, with the ease that digital technologies offer comes the vital responsibility of ensuring security and privacy.
This article will dive into the crucial role of security for data in eCTD publishing and discuss methods to safeguard sensitive information during the entire submission process.
The Imperative of Data Security
Security of data isn't an unimportant checkbox on the list of regulatory compliance. The eCTD Software format permits biotech and pharmaceutical companies to prepare electronic submissions of their application that has numerous advantages like a reduction in paper use speedier reviews and improved collaboration. However, it also presents risks if not managed properly.
• Protection of intellectual Property: Pharmaceutical companies invest large amounts of money for developing and research. Data security is essential to prevent unauthorised access to confidential information, thereby protecting intellectual property rights of the company.
• Patient Privacy: Data from clinical trials and patient information should be handled with utmost security to ensure privacy for patients and to comply with laws on protection of data.
• Regulation Compliance: A lot of regulatory eCTD Submission Software agencies require a strict adherence to standards regarding data security in their submission specifications. Failure to adhere to these standards can lead to delays or even rejections.
• Reputation and Trust: Keeping secure and confidential data builds trust with regulators and partners as well as others. Any breach could damage the image of a company irreparably.
Strategies for Safeguarding Sensitive Information
• Secure encryption: Use strong encryption methods to safeguard the data during transport and in rest. By using encryption, even in the event of unauthorized access the data is unreadable and inaccessible.
• Access Controls: Set strict access control that restricts the access of users to view or edit sensitive information. Multi-factor authentication adds a new layer of protection to the user's access.
• Audit Trails: Keep precise audit trails that record every step performed inside the eCTD publishing system. This does not just increase accountability, but also assists in identifying any suspicious actions.
• Secure Infrastructure: Select eCTD publishing tools built on secured infrastructure, frequently updated to fix security holes and are armed with intrusion prevention systems and firewalls.
• Users Training: Offer extensive training for employees who are engaged within participating in the eCTD publishing procedure. Be sure that they are aware the security protocols and best practices for avoiding unintentional breach.
• Data Minimization: Only include the information required in submissions, thus reducing the chance of disclosing sensitive information. This improves the efficiency of the process of reviewing.
• Vendor Due Diligence: When you are using an external third party eCTD software for publishing, perform thorough due diligence regarding the security measures they use and their certifications.
• Periodic Audits and Evaluations: Perform periodic security assessments and audits in order to discover vulnerabilities and then take action to address these.
The Final Thought!!
The life sciences industry continues to adopt eCTD Publishing Tools for submissions to regulatory agencies and submissions, the need to secure sensitive data has never been more important. Data breaches can result in a variety of implications, from regulatory repercussions and irreparable harm to a business's reputation.
When they prioritize the security and confidentiality of data pharmaceutical and biotech companies will be able to traverse the cyber world with confidence, build trust with regulatory agencies as well as others, and guarantee the continuous success of their data submissions.
0 notes
Text
Parexel are hiring for multiple positions in Regulatory Affairs- Submission Publishing - Apply Now
We at Parexel are hiring for multiple positions in Regulatory Affairs- Submission Publishing.
We are looking for candidates with 2-4 yrs of experience in Submission Publishing willing to work in night shift and having knowledge in Paper, Nees and eCTD formats (US, EU, Saudi, Canada etc).
1.Regulatory activities include troubleshooting as well as dispatching the submissions to health authorities…

View On WordPress
0 notes
Text

USFDA Drug Master Files are submissions made to USFDA for human drug products which provide confidential and detailed information about the facilities, processes, or articles used in the manufacturing, processing, packaging, and storing. The DMF are required to be submitted in eCTD formats with exception of type III DMF. The DMF are not required by the regulation.
0 notes
Text

USFDA Drug Master Files are submissions made to USFDA for human drug products which provide confidential and detailed information about the facilities, processes, or articles used in the manufacturing, processing, packaging, and storing. The DMF are required to be submitted in eCTD formats with exception of type III DMF. The DMF are not required by the regulation.
0 notes
Text

USFDA Drug Master Files are submissions made to USFDA for human drug products which provide confidential and detailed information about the facilities, processes, or articles used in the manufacturing, processing, packaging, and storing. The DMF are required to be submitted in eCTD formats with exception of type III DMF. The DMF are not required by the regulation.
0 notes
Text

USFDA Drug Master Files are submissions made to USFDA for human drug products which provide confidential and detailed information about the facilities, processes, or articles used in the manufacturing, processing, packaging, and storing. The DMF are required to be submitted in eCTD formats with exception of type III DMF. The DMF are not required by the regulation.
0 notes
Text
#This article talks about the challenges faced by pharma companies and opportunities while transitioning from paper to electronic submissions#eCTD Submissions#China#eCTD Transition#Regulatory Submissions#NMPA#Freyr SUBMIT PRO#Paperless submissions#Paperless Regulatory Submissions#Pharmaceutical market
0 notes
Text

The transition from NeeS to eCTD involves converting non-standardized electronic regulatory document submissions to a globally accepted standard. This requires restructuring and formatting the documents according to eCTD specifications, resulting in improved efficiency, accuracy, and consistency of the submission process.
#regulatoryintelligence#regulatoryaffairs#regulatorycompliance#regulatoryconsulting#regulatorysolutions#regulatorystrategies#pharmaregulatoryaffairs#globalregulatoryaffairs
0 notes
Photo

eCTD Submission Certification Program - Part Time/Distance Learning,Enroll now for the “Certificate Course in eCTD Submissions” andGet Live training on eCTD software!!!
.
.
The eCTD specification has been developed to facilitate the Global electronic Submission, Review, and Lifecycle management of medicinal product dossiers for regulatory applications.
.
Who Should Attend???
QC/QA Managers & Staff
-Documentation Department
-Regulatory Affairs Department - Responsible for CTD /DMF Preparation & Submission
-Research Chemist, Quality Control Chemist, CRO's involved in Documentation
-pharmacy Graduates with knowledge of CTD
.
Course Offered:
LIVE TRAINING SESSION,
E-LEARNING,
Hurry Up, Register now,
CALL/WHATSAPP For Enquiry: +91-9595750750
More info visit:https://bit.ly/2Vv1Ijp
#eCTDSubmissions#eCTDSubmissionstraining#pharmacareer#pharmatraining#regulatorytraining#regulatoryaffairscoursesinpune#regulatoryaffairs#girapune#FDAMandate#ectdsoftware#eCTDFormat
0 notes
Text
Best Practices for Implementing eCTD Submission Software in Your Organization
In the speedy world of drugs and biotechnology, the adoption of Electronic Common Technical Document (eCTD) submission software has become fundamental for smoothing out regulatory cycles. Proficient execution and combination of eCTD submission software can essentially improve efficiency and compliance.
In this blog entry, we will explore the accepted procedures for effectively carrying out eCTD Software inside your organization.
Grasp Administrative Prerequisites:
Before choosing and carrying out eCTD submission software, it's urgent to have an unmistakable comprehension of the administrative necessities well-defined for your district or target markets. Various nations might have special guidelines and assumptions, and your product ought to line up with these to guarantee fruitful entries.
Cross-Useful Cooperation:
Include all significant partners in the execution cycle, including administrative undertakings, IT, quality affirmation, and report supervisory crews. Cross-utilitarian cooperation guarantees that the product addresses the issues and assumptions of all divisions, prompting a smoother combination process.
Select a Complete Arrangement:
Pick eCTD submission software that offers an extensive set-up of highlights. The product ought to help report the executives, form control, electronic mark abilities, and incorporation with other existing frameworks. A comprehensive arrangement limits the requirement for different devices and advances a consistent work process.
Give Sufficient Preparation:
Put resources into thorough preparation for all clients engaged with the submission cycle. This incorporates administrative experts, record creators, and IT faculty. Appropriate preparation guarantees that clients can augment the capacities of the eCTD submission software and decreases the gamble of mistakes during entries.
Information Relocation Arranging:
If progressing from manual or heritage frameworks, plan for information movement cautiously. Guarantee that information from existing reports is precisely moved to the new framework. This incorporates metadata, rendition history, and some other important data. Exhaustive information relocation forestalls information irregularities and mistakes in entries.
Customization for Productivity:
Select eCTD submission software that takes into consideration customization to meet the particular necessities of your association. Customization choices ought to incorporate archive formats, submission layouts, and work process setups. Fitting the product to your association's work process upgrades effectiveness and client fulfillment.
The Bottom Line!!
All in all, effective execution of eCTD Submission Software requires cautious preparation, cooperation, and progressing obligation to progress.
By following these prescribed procedures, your drug or biotech association can improve its administrative cycles, upgrade compliance, and add to the effective conveyance of new medicines to showcase.
0 notes
Text
Healthcare Regulatory Affairs Outsourcing Market Partnering Deals of Key Players 2022 - 2028
The healthcare regulatory affairs outsourcing market is projected to reach US$ 14,996.35 million by 2028 from US$ 7,274.73 million in 2021; it is expected to grow at a CAGR of 10.9 % from 2021 to 2028.
The Healthcare Regulatory Affairs OutsourcingMarket research report by The Insight Partners includes market segmentation and overlays shadow upon the leading market players highlighting the favourable competitive landscape and trends prevailing over the years. This study provides information about the sales and revenue during the historic and forecasted period of 2021 to 2028. Understanding the segments helps in identifying the importance of different factors that aid the Healthcare Regulatory Affairs Outsourcingmarket growth.
Sample PDF showcases the content structure and the nature of the information included in the report which presents a qualitative and quantitative analysis - https://www.theinsightpartners.com/sample/TIPRE00007611/
Global Healthcare Regulatory Affairs Outsourcing Market: Regional Analysis
The report provides a detailed overview of the industry including both qualitative and quantitative information. It provides an overview and forecast of the global market based on various segments. It also provides market size and forecast estimates from the year 2019 to 2028 with respect to five major regions, namely; North America, Europe, Asia-Pacific (APAC), the Middle East and Africa (MEA), and South America. The Healthcare Regulatory Affairs Outsourcing market by each region is later sub-segmented by respective countries and segments. The report covers the analysis and forecast of 18 countries globally along with the current trend and opportunities prevailing in the region.
The report has been curated after observing and studying various factors that determine regional growth such as economic, environmental, social, technological, and political status of the particular region. Analysts have studied the data of revenue, production, and manufacturers of each region. This section analyses region-wise revenue and volume for the forecast period of 2019 to 2028. These analyses will help the reader to understand the potential worth of investment in a particular region.
MARKET SEGMENTATION
Healthcare Regulatory Affairs Outsourcing Market – by Service Type
Regulatory & Scientific Strategy Development
Medical & Scientific Writing
eCTD & e-Submissions
Data Management Services
Life Cycle Management Services
Pharmacovigilance
Chemistry Manufacturing & Controls (CMC) Services
Regulatory Labelling
Regulatory Artwork Services
Healthcare Regulatory Affairs Outsourcing Market – by End User
Pharmaceutical Companies
Biotechnology Companies
Medical Devices Companies
Medical Device Software (SaMD)
Medical Device Materials & Biomaterials
Medical Device Biomarkers and In vitro Diagnostics (IVD)
Medical Device Substance-based
Medical Device of Combination Product (DDC)
Have a 15-minute-long discussion with the lead analyst and author of the report in a time slot decided by you. You will be briefed about the contents of the report and queries regarding the scope of the document will be addressed as well - https://www.theinsightpartners.com/speak-to-analyst/TIPHE100001274
Major Key Points of Healthcare Regulatory Affairs Outsourcing Market
Healthcare Regulatory Affairs OutsourcingMarket Overview
Healthcare Regulatory Affairs OutsourcingMarket Competition
Healthcare Regulatory Affairs OutsourcingMarket, Revenue and Price Trend
Healthcare Regulatory Affairs OutsourcingMarket Analysis by Application
Company Profiles and Key Figures in Healthcare Regulatory Affairs OutsourcingMarket
Market Dynamics
Methodology and Data Source
Companies Profiled in this report includes:
KLIFO
ProPharma Group
Arriello Ireland Ltd.
DRA CONSULTING OY
Asphalion S.L
Parexel International Corporation
IQVIA Inc.
Pharmalex Gmbh
ProductLife Group
Voisin Consulting Life Sciences (VCLS)
Azierta Contract Science Support Consulting
Leading market players and manufacturers are studied to help give a brief idea about them in the report. The challenges faced by them and the reasons they are on that position is explained to help make a well informed decision. Competitive landscape of Brown Sugar market is given presenting detailed insights into the company profiles, developments, merges, acquisitions, economic status and best SWOT analysis.
NOTE: Our team is studying Covid-19 and its impact on various industry verticals and wherever required we will be considering Covid-19 analysis of markets and industries. Cordially get in touch for more details.
Immediate delivery of our off-the-shelf reports and prebooking of upcoming studies, through flexible and convenient payment methods - https://www.theinsightpartners.com/buy/TIPRE00007611/
About Us:
The Insight Partners is a one stop industry research provider of actionable intelligence. We help our clients in getting solutions to their research requirements through our syndicated and consulting research services. We specialize in industries such as Semiconductor and Electronics, Aerospace and Defense, Automotive and Transportation, Biotechnology, Healthcare IT, Manufacturing and Construction, Medical Device, Technology, Media and Telecommunications, Chemicals and Materials.
Contact Us:
If you have any queries about this report or if you would like further information, please
Contact Person: Sameer Joshi
E-mail: [email protected]
Phone: +1-646-491-9876
0 notes
