#carboxylate ion
Photo

#carboxylic acid#boiling point#aldehydes#chemistry#solutions#ketone#alcohol#molecular mass#van der waals#force#attraction#carboxylate ion#hydrogen bond#intermolecular#intramolecular#noncavalent#interactions
1 note
·
View note
Text
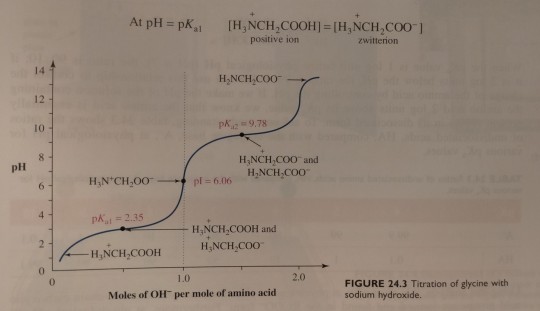
At this point, the concentration of the zwitterion equals that of the positively charged ion, and the pH of 2.35 equals the pKa value of the carboxyl group (pKa1):
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#concentration#zwitterion#ions#carboxyl#titration#glycine#sodium hydroxide#chemical reactions
0 notes
Text
(Recall from chapter 11 that the smaller the pKa the more acidic is the group. At lower pH, carboxylic acids are found in the RCOOH form and amines are found in the RNH3+ form. At higher pH, the opposite is true; carboxylic acids are present as the salt RCOO- and amines are present as uncharged RNH2. Figure 24.3 on p. 1061 shows how this looks at different pH.)
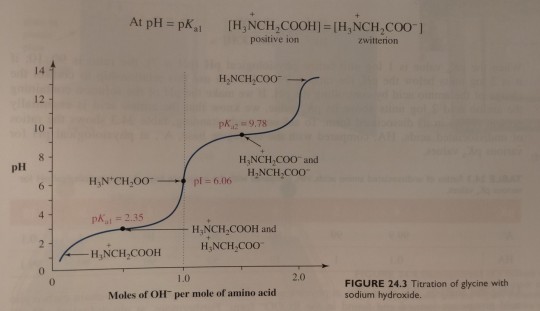
Next, the solution is titrated with 1.00 M NaOH; the volume of base added and the pH of the resulting solution are recorded and then plotted as shown in figure 24.3. (...) By examining the titration curve (figure 24.3), you can see that the isoelectric point for glycine falls halfway between the pKa values for the carboxyl groups and the ammonium ion:
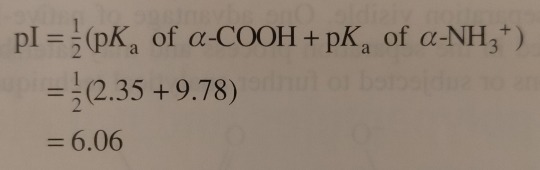
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#acidic#carboxylic acid#ph#salt#amine#titration#glycine#sodium hydroxide#carboxyl#ammonium#ions
1 note
·
View note
Note
Hi! Do you have any tips for studying chemistry? For some reason I cant seem to get all the formulas in my brain.
Hey!
My unhelpful but still favorite advice for shoving formulas into one's brain is to understand them 😅 A purely memorization-based approach is very bad for chemistry.
If the problem seems to be particularly understanding/ remembering formulas:
Ask yourself if this particular formula is just words turned into numbers and mathematical symbols. I think it may not work for everyone, but for example I found it easier to remember the literal definition of pH that is "the negative decimal logarithm of hydrogen ion concentration" rather than "pH = -log [H+]" bc otherwise I'd keep forgetting about the minus sign.
Check if you find deriving a formula from another formula easier than just memorizing it. Again, my personal example is I hate memorizing things so much I never really bothered to remember the equation that describes Ostwald's law of dilution - bc I knew I could easily, quickly, and painlessly derive it from the equilibrium constant for concentration + degree of dissociation (and I've done it so many times now it stuck in my brain anyway).
When all else fails, I turn to mnemotechnics. To this day I remember that Clapeyron's equation goes pV = nRT because many years ago someone on the internet shared a funny sentence whose words start with these 5 letters. The sillier the better.
If the issue is with chemistry in general:
Take it chapter by chapter. Chemistry, like most STEM subjects, is just blocks of knowledge upon blocks of knowledge. For example, if you want to learn electrolysis, you need to understand redox reactions first. Try to identify where the struggle begins and work from there.
Once you've picked a topic you want to work on, follow the reasoning in your textbook. If you get stuck, that might be a sign you're simply missing a piece of information from a previous chapter. If an example comes up, try to solve it along with the tips in the textbook.
If anything remains unclear, it's usually not the best idea to just leave it and move on. If the textbook becomes unhelpful, turn to the internet or maybe a friend. Otherwise, the next chapter may just turn out to be needlessly confusing.
Practice problems practice problems practice problems!! And not just the numerical ones. The theory-based ones where they ask you about reactions, orbitals, the properties of the elements etc. are important too.
Choose understanding over memorizing whenever possible.
Try to look at the big picture: the way certain concepts are intertwined, how one law may be a logical consequence of another law you learnt before, why some concepts are taught together, why you had to learn something else first to get to what you're studying now. Again, as an example, I think it's particularly fun to see towards the end of ochem, somewhere around the biomolecules: you need to integrate your knowledge of aromatic compounds, ketones and aldehydes, alcohols, carboxylic acids... Stack new information upon what you already know.
Study methods I'm a big fan of: spaced repetition, solving past papers (anything I can get my hands on tbh), flashcards for the things I absolutely have to memorize, exchanging questions and answers with a friend, watching related videos.
If by any chance you end up taking pchem, I have a post for that specifically.
I hope you can find something helpful here :) Good luck!
13 notes
·
View notes
Text
So I was thinking the one thing I can use my degree to contribute to this fandom with is to include whatever the fuck the fake chemical Ema mentions in turnabout corner is and it's actually funny as fuck. Anyway grain of salt, I'm still in the process of getting my degree and won't be certified scientist for a few years so like, I may and probably will fuck up but hey this is a fake chemical and it's mostly just cute wordplay so.
The chemical is hydroxyacelunodosetrase.
So I'm going to break down the boring bits first. So hydroxy is an OH group. Very common. Insanely. Basically just here to denote that this is a chemical to lay people because of how common this is. Acel is a little weird. But Ace normally means a hydrocarbon chain with a length of 2 and sometimes you do need to add on stuff for pronouncablity but I think it's a deliberate misspelling (because ace like ace attorney) of an acyl group which is a C=O group but honestly outside of very specific circumstances specific it's not thaaaat common in chemical names because it doesn't provide as much utility as denoting a more specific subset of acyl groups like a ketone, aldehyde, ester or carboxylic acid group but it is still used especially when denoting ions or free radicals or especially when bonded to a halogen. So the fact it's needing to be notated puts my lab alarm off screaming "shit look into this before using it, it could be dangerous" because that normally means its reactive as fuuuuuck. Acyl groups as a whole are pretty reactive, that double bond to oxygen likes very much to no longer be a double bond but there are a lot of configurations that will make it a little more stable. If it need to be notated as such it's probably not in one of those. Very funny given its used in context as a threat.
The only other part of it that is chemically coherent is the trase suffix. (Edit edit fuck I think what I'm thinking of is ase as a suffix which means enzyme. I do not know where the tr is coming from) It's I believe common in enzymes but I am not a biochemist nor very good at biochemistry and I cannot find a source on Google backing that up and it is just coming from my brain so I may well be wrong. What matters though is how it's pronounced. Which is the same as Tres. Which makes the last 2 bits make more sense. Chemistry uses Greek numbering. The rabbit hole of chemical naming is deep and I'm not getting into it because there are multiple naming systems and one of them is standardised and the other one is actually the thing everyone uses. But Uno is not a thing in chemical names. Neither is dose but it's probably ment to be like a dose as in a drug dose. Again not found in the name it's self but I think that's why it's spelt like that. But the upshot is that the last 3 parts are pronounced Uno dos Tres which is obviously 123 in Spanish. It's cute.
So basically in conclusion it's not a real thing at all. It's all word play and it's would probably be pretty nasty even if it wasn't. I think it's cute. I'm glad some thought was put into this fake chemical instead of just a keyboard spam.
24 notes
·
View notes
Text

This bugs me because it's wrong in the details but right overall. pKa, first of all, isn't the Acid dissociation constant: it's the negative base 10 logarithm of the acid dissociation constant, Ka, which is given by the following equation:

Where HA is the acid, H+ is the acidic protons it can give off, and a- is the conjugate base or the remainder of the molecule after the proton is given off.
(pH, to note, is also the negative base ten logarithm - in this case, of the amount of h+ ions in a particular solution, in the example springing off of this, the amount of H+ ions in monster energy).
If you have a solution that already has a ton of H+ protons in it, such as monster energy drink, and try to dissolve more acidic particles in it, well, the equilibrium is already very top heavy - so the H+ particles in HA, our acid, will be trying to squeeze into a crowded room, fail, and remain as the undissasociated acid, pushing the equilibrium constant to an even more tiny number than it already is for basically every organic acid. It's a much more complicated relationship than "pKa indicates roughly the pH around which acids can't dissociate" which unless I'm missing something is nonsense - you have to calculate the equilibria constants of these acids with the initial acidity factored it if you want to do anything relating these two values to each other.
It's frustrating because the overall conclusion is right!! for reasons not really related to what they said, the high pH of the monster energy does mean it's more difficult for the acid protons of these acids to dissociate!

This, however, is just flat out wrong. The lower the pKa value is, the easier it is for that proton to dissociate; this is how negative logarithms work. If you calculate the ratio up above and find that the amount of dissociated species to acid heavily favors the dissociated species, that is a very strong acid, and you will have pKa values that go negative. In most organic carboxylic acids (like those named here), we're looking at a pKa of around 5: meaning that if you dissolve the acid in pure neutral water, how many protons (and conjugate base ions) are in solution compared to the undissociated molecule, you'll get a ratio of about 1/100000 - in other words, a mixture that overwhelmingly does not dissociate.
For secondary/tertiary/so on deprotonations, it becomes progressively more difficult to get rid of those protons, because the conjugate base is already shouldering a negative charge and because the solution already has a concentration of protons. Thus, if you want to say "once this one dissociates then all of the other ones are already dissociated too" you really have to be looking at the highest pKa.

This part is just goofy. "The trapped acidity of the malic/citric/ascorbic acids is dumped into your mouth" that also happens when you eat the solid candy anyway...
#Like I get it you found the equilibrium esp acid equilibrium part of gen chem hard.#everyone did! but don't go calling yourself a chemist then say literally incorrect information just because you can find some pubmed links
3 notes
·
View notes
Text

SUBJECT
The superior physicochemical ambiance provided by Chitin/Carboxylated Chitosan for the formation of hydroxyapatite film
DEPARTMENT
Department of Chemical Engineering
PROFESSOR AND STUDENT
Professor:Ten-Chin Wen
Student:Wei-Cheng Li
ABSTRACT
Chitosan (CS) and chitin (CH) are two natural polysaccharides. Chitosan with different carboxylation degrees rendering specific zwitterionic properties. In this study, carboxylated chitosan (CCS) and CH polymeric matrix was mineralized to form an hydroxyapatite film.
CS grafted carboxylated group at pH 6, 8, and 10 for products denoted as CCS6, CCS8, CCS10. It was the better degrees of carboxylated, the higher ion conductivity. CCS with zwitterion helped ions movement. Based on thermogravimetric analysis, thermal cracking temperature of the amide group on chitin increased after mineralization. In the TEM image, the PILP behavior was found, and formed hexagonal hydroxyapatite beside chitin. The Young's modulus of hydroxyapatite evaluated by AFM was 5.19±0.06GPa. Meanwhile, Ca/P ratio by EDX analysis was 1.65, similar to bones.
Original URL: 15-Student:Department of Chemical Engineering【Wei-Cheng Li】-Undergraduate Research , NCKU https://en.ur.ncku.edu.tw/book/15-Student:Department+of+Chemical+Engineering%E3%80%90Wei-Cheng+Li%E3%80%91/
The copyright belongs to the author. For commercial reprints, please contact the author for authorization, and for non-commercial reprints, please indicate the source.
2 notes
·
View notes
Text
What is the Procedure of Tollens Reagent?
Tollens test, also known as the silver mirror test, is a chemical test used to distinguish between aldehydes and ketones. This test is based on the oxidation-reduction reaction between aldehydes and reagent, which results in the formation of a silver mirror on the inner surface of the reaction vessel.
Principle of Tollens Test
The principle behind the Tollens test lies in the fact that aldehydes are readily oxidized to carboxylic acids, while Tollen’s reagent, which is an alkaline solution of silver nitrate, acts as an oxidizing agent. When an aldehyde is present in the solution, it reduces Tollen’s reagent, causing elemental silver to precipitate and form a mirror-like coating.
Tollens reagent, also known as silver mirror reagent, is a solution that contains silver ions in an alkaline medium. It is prepared by adding silver nitrate to a solution of sodium hydroxide until a slight precipitate of silver oxide is formed. The precipitate is then dissolved by adding ammonia solution drop by drop until the solution becomes colorless.
Tollens Reagent Preparation
To prepare Tollens reagent, follow these steps:
Dissolve 5 grams of silver nitrate (AgNO3) in 50 mL of distilled water.
In a separate container, dissolve 5 grams of sodium hydroxide (NaOH) in 100 mL of distilled water.
Slowly pour the sodium hydroxide solution into the silver nitrate solution while stirring.
A brown precipitate of silver oxide (Ag2O) will form.
Add dilute ammonia solution drop by drop to the brown precipitate until it dissolves completely and the solution turns colorless.
Test the resulting solution with litmus paper to ensure it is slightly alkaline. If necessary, adjust the pH by adding more sodium hydroxide or dilute ammonia solution.
Tollens Test Procedure
The Tollens test procedure is as follows:
Take a small quantity of the unknown compound and dissolve it in water or ethanol, depending on its solubility.
Transfer this solution into a clean test tube.
Add a few drops of Tollens reagent to the test tube.
Gently heat the mixture by placing the test tube in a water bath or by using a Bunsen burner.
Observe the reaction mixture to form a silver mirror on the test tube’s inner surface.
The appearance of a silver mirror indicates a positive Tollens test, confirming the presence of an aldehyde or alpha hydroxy ketone.
Benefits of Tollens Test
The Tollens test offers several benefits in organic chemistry:
It provides a simple and reliable method for detecting the presence of aldehydes and alpha hydroxy ketones.
The formation of a silver mirror is a visual confirmation, making the test easy to interpret.
It does not require expensive equipment and can be performed using basic laboratory apparatus.
The Tollens test can be used qualitatively as well as quantitatively for determining the concentration of aldehydes in a given sample.
Limitations of Tollens Test
While the Tollens test is a valuable tool, it does have some limitations:
It only detects aldehydes and alpha hydroxy ketones, not other functional groups.
The reaction requires the presence of an acidic hydrogen atom adjacent to the carbonyl group.
It may not work efficiently for highly reactive aldehydes or alpha hydroxy ketones.
The test can yield false-positive results if reducing agents other than aldehydes or alpha hydroxy ketones are present in the solution.
In conclusion, the Tollens test is a useful chemical test that provides a simple and reliable method for detecting the presence of aldehydes and alpha hydroxy ketones. By observing the formation of a silver mirror, chemists can confirm these compounds in a sample. The Tollens test has its limitations, but when used appropriately, it can provide valuable information in organic chemistry analysis.
Keen on effortlessly mastering concepts, as explained above? Dive into our Tutoroot Blog section for simplified learning. Enhance your understanding of subjects and have your questions clarified through Tutoroot’s online tuition. Immerse yourself in the world of Tutoroot’s online home tuitions by scheduling a FREE DEMO session today.
#tollenstestreaction#tollenstest#tollensreagentformula#tollenstestprinciple#tollensreagentpreparation
0 notes
Text
1,3,6-HEXANETRICARBONITRILE CAS#: 1772-25-4
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product Name1,3,6-HEXANETRICARBONITRILEIUPAC Namepyridihexane-1,3,6-tricarbonitrileMolecular StructureCAS Registry Number 1772-25-4MDL NumberMMFCD00131222Synonyms1,3,6-Hexanetricarbonitrile1772-25-4hexane-1,3,6-tricarbonitrile1,3,6-TRICYANOHEXANE4-CyanosuberonitrileSJY3YNQ3SIDTXSID4041234HSDB 5855UNII-SJY3YNQ3SIEINECS 217-199-7(+/-)-1,3,6-HEXANETRICARBONITRILE, TECH. , 80+%SCHEMBL1323168CHEMBL3183751DTXCID2021234BAA77225Tox21_300976MFCD00129792AKOS0221827011,3,6-TRICYANOHEXANE s12371NCGC00248239-01NCGC00254878-01BS-21827CAS-1772-25-4CS-0198995H1504NS00025841Q27289244Molecular FormulaC9H11N3Molecular Weight161.2InChIInChI=1S/C9H11N3/c10-6-2-1-4-9(8-12)5-3-7-11/h9H,1-5H2InChI KeyLNLFLMCWDHZINJ-UHFFFAOYSA-NIsomeric SMILESC(CC#N)CC(CCC#N)C#N
Patent InformationPatent IDTitlePublication DateCN115784927Preparation method of alkane trinitrile2023US2021/408602ELECTROLYTE AND ELECTROCHEMICAL DEVICE2021US4128571Thermal conversion of 4-cyano-suberonitrile to acrylonitrile1978US5132427Process for the preparation of amines1992
Physical Data
AppearanceLight yellow to yellow oily liquid
Boiling Point, °CPressure (Boiling Point), Torr186 - 2000.2
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Frequency (NMR Spectroscopy), MHzChemical shifts1Hchloroform-d1Chemical shifts13Cchloroform-d1Chemical shifts1Hchloroform-d1400
Route of Synthesis (ROS)
Route of Synthesis (ROS) of 1,3,6-Hexanetricarbonitrile CAS 1772-25-4
ConditionsYieldWith water; sodium chloride In dimethyl sulfoxide at 160℃; Reagent/catalyst; Temperature; Solvent;Experimental Procedure4-8 Preparation of 1,3,6-hexane trinitrileTake 50 grams of 2,5-dicyano-2-cyanoethyl-pentanoic acid ethyl ester prepared in Example 1, add 100 grams of DMSO, add 8 grams of water, 2.5 grams of NaCl, and raise the temperature to 160°C.After the gas no longer overflowed, the DMSO and water were removed by concentration under reduced pressure, and then the distillation was continued to obtain a product of 1 mmHg, a fraction at 195-196°C of 32.0 g, a yield of 92.8%, and a GC purity of 99.5%.92.8%
Safety and Hazards
Pictogram(s)SignalWarningGHS Hazard StatementsH302 (97.62%): Harmful if swallowed H332 (92.86%): Harmful if inhaled Precautionary Statement CodesP261, P264, P270, P271, P301+P317, P304+P340, P317, P330, and P501(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
TransportationUnder the room temperature and away from lightHS CodeStorageUnder the room temperature and away from lightShelf Life1 yearMarket Price
DruglikenessLipinski rules componentMolecular Weight161.206logP0.107HBA3HBD0Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)71.37Rotatable Bond (RotB)5Matching Veber Rules2
Use Pattern1,3,6-HEXANETRICARBONITRILE CAS#: 1772-25-4 is an important electrolyte additive, and the composition of the electrolyte restricts the application of positive and negative electrode materials at high voltages. Traditional organic carbonates, such as linear carbonates like DEC, DMC, EMC, and cyclic carbonates like PC, EC, tend to undergo decomposition at high voltages . Therefore, the development of novel organic solvents with a wide electrochemical window, high lithium salt solubility, and low toxicity has become a key focus in the development of high-voltage electrolytes. Nitrile-based organic solvents typically possess a wide electrochemical window, high anodic stability, low viscosity, and high boiling points, among other excellent characteristics . Additionally, the decomposition products of solvents containing nitrile groups are generally carboxylates, aldehydes, or corresponding organic amines, eliminating the generation of toxic CN- ions during usage . Nitrile solvents demonstrate a broad electrochemical window and are considered promising new organic solvents. However, in terms of the electrochemical performance of lithium-ion batteries, nitrile solvents still face compatibility issues with the negative electrode. The formation of a mixed system with carbonate solvents or the addition of mixed salts like LiBOB can partially alleviate this issue.
Read the full article
0 notes
Text
Innovative Hydrophilics: Super Absorbent Polymers in Hygiene Products

Super Absorbent Polymers: Revolutionizing Absorbency Applications
What are Super Absorbent Polymers?
Super absorbent polymers (SAPs), also known as superabsorbent materials, are advanced materials whose main characteristic is the ability to absorb and retain extremely large amounts of a liquid relative to their own mass. They are cross-linked polymers that can absorb hundreds of times their own weight in water, aqueous solutions, or biological fluids.
Chemistry and Structure of SAPs
SAPs are produced via polymerization of acrylic acid or acrylamide. These monomers contain polar carboxyl or amide groups which provide hydrophilicity allowing the polymers to absorb water. The monomers are cross-linked during polymerization which creates a three-dimensional network structure. This cross-linked structure prevents the absorbed liquid from leaving the polymer network, even under pressure. Common cross-linkers used are N,N'-methylenebisacrylamide or trimethylolpropane triacrylate which form covalent bonds between the linear or partially branched acrylic acid chains.
Applications in Hygiene Products
One of the largest applications of SAPs is in disposable hygiene products such as diapers, feminine hygiene products, and adult incontinence products. SAP particles are incorporated into the absorbent core of these products where they can soak up large volumes of fluid, many times their own weight, and lock the fluid away from the skin. This improves comfort and prevents leakage compared to previous designs using just cellulosic fluff pulp or tissue. SAPs transformed these industries by allowing for thinner, drier, and more convenient products.
Use in Agriculture and Horticulture
The water storage and slow release properties of SAPs make them applicable for use in agriculture and horticulture. They can absorb and retain irrigation water, fertilizers, or pesticides in the soil allowing plants to access these at a controlled rate. SAP fertilizers reduce leaching and increase nutrient uptake efficiency. They are also included in growing media like peat or coco peat to enhance moisture retention for container crops and aid seed germination. SAPs minimize watering needs while improving plant growth.
Medical and Wound Care Applications
Given their ability to swell in contact with fluids, SAPs have applications in the biomedical field as superabsorbent wound dressings. They can absorb wound exudate to help keep the wound site moist which promotes healing. Their liquid absorbency helps prevent skin maceration around the wound. Some SAP wound dressings even incorporate antimicrobial agents. Other medical uses of SAPs include their incorporation into tampons, surgical sponges, and implant materials.
Environmental Remediation
Due to their absorbency, SAPs have potential applications for environmental remediation. They can remove pollutants from aqueous solutions through absorption. For example, researchers have investigated using SAPs to absorb oils, solvents, heavy metals and radioactive elements from contaminated water and soils. Cross-linked with metal chelating ligands, they show promise for wastewater treatment applications by sequestering metal ions. SAPs are also studied for use in containment booms for oil spill cleanups.
0 notes
Text
Magnesium Citrate: Uses, Side Effects, and Everything You Need To Know
Magnesium citrate is a dietary supplement that combines magnesium with citric acid. This mineral is vital for various bodily functions, and the citrate component aids absorption. Widely used to relieve constipation and boost magnesium levels, it comes in liquid or capsule form.
There are, however, some side effects and dosage considerations to keep in mind. Consulting healthcare professionals before use is crucial for personalized guidance. The following are some essential facts you should consider before purchasing this product.
Compound of Magnesium Citrate
The chemical formula for magnesium citrate is Mg3(C6H5O7)2. This formula reflects the combination of three magnesium (Mg) ions with two citrate ions (C6H5O7). The citric acid (C6H8O7) is a weak organic acid with three acidic carboxyl groups, and when it complexes with magnesium ions, it forms magnesium citrate. The formulation of the compound may vary depending on the salt form or hydration state.
Forms of Magnesium Citrate
Magnesium citrate is commonly available in different forms, mainly differing in their physical state or delivery method. The two primary forms are liquid (solution) and capsules.
Liquid Magnesium Citrate:
Liquid magnesium citrate is a solution that generally comes in a bottle.
It is often used as a laxative to relieve constipation and soften the stool due to its ability to draw water into the intestines.
This form is convenient for those who prefer a liquid option or have difficulty swallowing capsules.
Capsules or Tablets:
Magnesium citrate is also available in capsule or tablet form.
Capsules may be preferred by those who find them more comfortable to swallow and want a more controlled and measured dosage.
Tablets are another option, though they might take longer to break down in the digestive system than capsules.
Chemical Properties of Magnesium Citrate
Magnesium citrate possesses several chemical properties that assist in its functionality and applications. Here are some fundamental chemical properties of magnesium citrate:
Complex Formation: Magnesium citrate emerges by the chelation or complexation of magnesium ions with citrate ions derived from citric acid. Citric acid is a tricarboxylic acid with three carboxyl groups, and each magnesium citrate molecule typically involves the coordination of one magnesium ion with two citrate ions.
Solubility: Magnesium citrate is generally soluble in water, enabling its absorption in the digestive system. This solubility contributes to the effectiveness of magnesium citrate as a dietary supplement and as a laxative when used to relieve constipation.
Bioavailability: The citrate component in magnesium citrate enhances the bioavailability of magnesium. It means the body can absorb and utilize magnesium more efficiently than magnesium supplements.
Acid-Base Properties: Citric acid is a part of the magnesium citrate molecule and contributes acidic properties. In solution, magnesium citrate may exhibit slightly acidic characteristics due to the presence of citric acid.
Laxative Effect: Magnesium citrate’s laxative properties are attributed to its ability to draw water into the intestines, softening the stool and promoting bowel movements.
Hydration State: Magnesium citrate supplements may exist in different hydration states depending on the product. The number of water molecules associated with the magnesium citrate complex can vary.
It’s necessary to consider these chemical properties when using magnesium citrate, especially in healthcare and dietary applications. As with any supplement, understanding its chemical characteristics can provide insights into its behavior in the body and guide appropriate usage.
Uses of Magnesium Citrate
Magnesium citrate is a versatile dietary supplement with several uses, addressing magnesium deficiency and promoting digestive health. Here are some common uses:
Medical Uses:
Magnesium Deficiency: The primary use of this substance is to supplement magnesium levels in the body. A magnesium deficiency can lead to various health issues, including muscle cramps, fatigue, and irregular heartbeat.
Constipation Relief: One of the primary uses of magnesium citrate is a laxative to relieve constipation. It facilitates stool softening and encourages bowel movements by attracting water into the intestines.
Colon Cleansing: In medical settings, healthcare professionals may prescribe magnesium citrate as part of colon cleansing procedures before specific medical examinations or surgeries.
Kidney Stone Prevention: Some studies suggest that adequate magnesium intake may help prevent the formation of certain types of kidney stones. As a magnesium source, it may contribute to this preventive effect.
Muscle Function and Relaxation: Magnesium is essential for proper muscle function and may be used to support muscle health and relaxation. Athletes and individuals with muscle cramps may consider magnesium supplementation.
Bone Health: Magnesium is a crucial component for bone health, working concurrently with calcium and vitamin D. Supplementing with magnesium citrate may contribute to overall bone health.
Cardiovascular Health: Magnesium plays a role in maintaining normal heart rhythm and blood pressure. Adequate magnesium levels, supported by magnesium citrate supplementation, may contribute to cardiovascular health.
Migraine Prevention: Some individuals find relief from migraines through magnesium supplementation. While the evidence is not conclusive, magnesium citrate may be considered by those looking for non-pharmacological approaches to migraine prevention.
Non-Medical Uses:
Food Additive: It acts as an acidity regulator and emulsifying agent in food products like processed meats, cheeses, and beverages.
Anti-Seizure Agent: In veterinary medicine, it controls seizures in animals with epilepsy.
Fire Retardant: Magnesium citrate finds application in fire-retardant materials due to its ability to release water vapor when heated, suppressing flames.
Side Effects of Magnesium Citrate
While magnesium citrate is generally safe when used as directed, excessive intake can lead to side effects. Consult with a healthcare professional before starting magnesium citrate supplementation. Common side effects may include:
Diarrhea: One of the most common side effects of magnesium citrate is diarrhea. It has a laxative effect, and excessive intake can lead to loose stools or diarrhea. Adjusting the dosage may help alleviate this side effect.
Nausea and Abdominal Cramping: Some individuals may experience nausea or abdominal cramping when taking magnesium citrate. It is more likely to occur at higher doses.
Electrolyte Imbalance: Prolonged or excessive use of magnesium citrate can potentially lead to electrolyte imbalances, affecting levels of other minerals in the body, such as calcium and potassium.
Dehydration: Diarrhea caused by magnesium citrate can lead to dehydration if fluid compensation loss is not enough by increased water intake.
Kidney Issues: Individuals with kidney problems should use magnesium supplements with caution, as excessive magnesium intake can be harmful to those with impaired kidney function.
Allergic Reactions: Some people may be allergic to magnesium citrate and experience rash, itching, swelling, or other symptoms. Seek medical attention if you observe any signs of an allergic reaction.
Interaction with Medications: Magnesium citrate can interact with certain medications, including antibiotics, diuretics, and drugs for heart conditions. Consult with a healthcare professional if you are taking other medications.
Most people can safely use magnesium citrate as directed for short-term purposes, such as relieving constipation. If you experience persistent or severe side effects, it’s advisable to seek medical attention promptly.
Precautions using Magnesium Citrate
Dosage:
Follow instructions carefully: Always stick to the recommended dosage on the label or as a doctor’s prescription. Taking too much magnesium citrate can cause unpleasant side effects.
Start low and adjust gradually: If you’re new to magnesium citrate, start with a lower dose and increase further as needed. It can help minimize side effects like diarrhea.
Medical conditions and medications:
Talk to your doctor before taking magnesium citrate if you have any underlying medical conditions: This includes kidney disease, liver disease, diabetes, or heart problems. It’s crucial to check with your doctor to avoid potential complications, as It can interact with certain medications.
Be aware of potential interactions: Certain medications like diuretics, antibiotics, and heart medications can interact with magnesium citrate. Discuss your current medications with your doctor before starting magnesium citrate.
Other precautions:
Long-term use of magnesium citrate without consulting your doctor is not advisable.: Long-term use of magnesium citrate can lead to magnesium overdosage, which can be harmful.
Choose reputable brands: When buying magnesium citrate supplements, opt for reputable brands and check the product label for quality assurance.
Safety, knowledge, and caution are required when using magnesium citrate. By following these precautions and consulting your doctor when necessary, you can reap the potential benefits of magnesium citrate while minimizing risks.
Conclusion
As we explore the applications and benefits of magnesium citrate, it is clear that this compound plays a critical role in supporting overall health and well-being. Whether addressing magnesium deficiency, promoting digestive health, or venturing into diverse industrial applications, magnesium citrate is a versatile and essential component.
For those seeking a reliable source of high-quality magnesium citrate, Annexe Chem Pvt. Ltd. rises as a leading provider in India. We offer exceptional products and ensure stringent quality standards, committed to excellence in the chemical and pharmaceutical industry. Contact us today to experience the best quality and reliability in magnesium citrate supply.
Choose Annexe Chem Pvt. Ltd. for excellence that surpasses expectations.
0 notes
Text
The actual structure of the acetate ion lies somewhere between these two forms, with the because charge delocalised over the two O atoms and the carboxylate carbon atom.
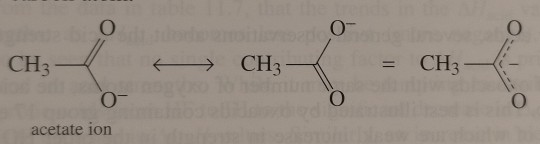
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#chemical structure#acetate#ion#anion#oxygen#delocalized#carboxylate
0 notes
Text
Decoding C.O. Citric Acid: A Versatile Solution for Various Industries
Introduction
In the intricate world of modern industries, citric acid emerges as a versatile and indispensable compound with a myriad of applications. Commonly abbreviated as C.O. citric acid, this organic acid is derived from citrus fruits and has found its way into diverse sectors due to its multifunctional properties. From the realms of food and beverages to pharmaceuticals, cosmetics, and even cleaning products, C.O. citric acid stands as a cornerstone ingredient, playing a pivotal role in enhancing products and processes. This exploration delves into the diverse applications and significance of C.O. citric acid across industries, shedding light on its characteristics, benefits, and wide-ranging utility.

Characteristics and Production
C.O. citric acid is a weak organic acid with a sour taste and a crystalline appearance. It is naturally found in citrus fruits such as lemons, limes, and oranges, but its widespread applications necessitate large-scale production that often involves fermentation processes using microorganisms like Aspergillus niger. The fermentation approach yields a more cost-effective and environmentally friendly production method compared to chemical synthesis.
The versatility of C.O. citric acid lies in its unique molecular structure, which encompasses three carboxylic acid functional groups. This structure renders it an excellent chelating agent, a quality that influences its applications in various industries.
Food and Beverage Industry
One of the most prominent domains where C.O. citric acid finds its utility is the food and beverage industry. Its role as a natural preservative, acidulant, and flavor enhancer has made it a staple ingredient in a wide array of products. As a natural preservative, it extends the shelf life of products by inhibiting the growth of microorganisms. Its acidulant properties contribute to the tangy and refreshing taste of many foods and beverages, including soft drinks, jams, and candies.
Beyond its taste-enhancing role, C.O. citric acid also has a significant impact on food texture. It is employed to regulate the gelling properties of pectin, a substance commonly used in jams and jellies. Moreover, its chelating properties enable it to bind with metal ions, preventing discoloration and maintaining the visual appeal of fruits and vegetables.
Pharmaceutical and Cosmetic Applications
In the pharmaceutical realm, C.O. citric acid serves as an integral component in the formulation of effervescent tablets. When combined with sodium bicarbonate, it creates a chemical reaction that releases carbon dioxide, resulting in the characteristic fizziness of effervescent medications. This not only enhances patient compliance but also improves the solubility and bioavailability of certain drugs.
The cosmetic industry also benefits from C.O. citric acid's multifaceted properties. It is utilized as a pH adjuster in various cosmetic formulations, ensuring that products maintain an optimal pH level for skin compatibility. Additionally, its exfoliating properties make it a valuable ingredient in skincare products, aiding in the removal of dead skin cells and promoting a smoother complexion.
Cleaning and Household Products
C.O. citric acid's chelating abilities find practical application in cleaning products. It acts as a descaling agent, effectively removing mineral deposits and limescale from surfaces and appliances. This property is harnessed in household cleaners, dishwasher detergents, and even descaling solutions for coffee machines. Its eco-friendly nature and biodegradability contribute to the growing trend of using C.O. citric acid-based cleaning alternatives.
Conclusion
In conclusion, C.O. citric acid stands as a versatile and valuable solution that transcends industry boundaries. Its unique molecular structure and multifunctional properties have granted it a significant role in an array of sectors. From its application in food and beverages, where it preserves, enhances flavor, and maintains texture, to its use in pharmaceuticals, cosmetics, and cleaning products, where its properties enhance solubility, adjust pH, and combat limescale, C.O. citric acid demonstrates its versatility at every turn.
The prevalence of C.O. citric acid in these diverse industries underscores its importance as a transformative ingredient. As industries continue to evolve and prioritize environmentally friendly and effective solutions, the significance of C.O. citric acid is only poised to grow. Its natural origin, wide-ranging benefits, and compatibility with multiple processes make it a cornerstone of modern production and product formulation.
0 notes
Text
What is EDTA? What are the applications of EDTA?
EDTA or Ethylenediaminetetraacetic acid is a weak acid used as a chelating agent. Ethylenediamine tetra acetic acid contains an amino carboxylic group with the formula C10H16N2O8. It is a versatile chemical compound with a wide range of applications across various industries.
EDTA is considered an anthropogenic compound with the ability to sequester various polyvalent cations, use to minimize metal contaminants, and facilitate enzymatic reactions that could be inhibited by heavy metal traces.
How Does EDTA Work?
EDTA is a versatile compound with strong chelating properties that grabs metallic cations such as lead or calcium from the process. Its chemical structure contains four carboxylic acid groups and two amine groups, which allow it to form strong bonds with metal ions, preventing them from reacting with other substances, If the bond is weak, other chemicals can break this bond to form their own compounds. EDTA is the most widely used chelating agent for the removal of toxic heavy metal ions in wastewater treatment.
EDTA can react with metallic cations, creating a stable rigid compound that can be easily excreted from the application system. Inorganic Contaminants may react with metallic ions, forming their own compounds due to their ability to form a strong covalent bond with those metal compounds.
EDTA has antibacterial activity and metal chelation of the ligand upon gram-negative bacteria has been reported. EDTA works by forming stable complexes with metal ions through a process called chelation.
Applications Of EDTA
Wastewater treatment: EDTA used as a chelating agent for the removal of toxic heavy metal ions in wastewater treatment.
Textile industry: Prevents metal ion impurities from modifying the colours of dyed products.
Pulp & paper industry: It inhibits the ability of metal ions, especially Manganese from catalysing the disproportionation of hydrogen peroxide in chlorine-free bleaching.
Laundry: Reduces water hardness by dissolving scales of Ca2+, Mg2+, and cations, which are then less likely to interfere with soaps and detergents
‘Scrubbing’: Removes hydrogen sulphide from gas streams. This conversion is achieved by oxidizing the hydrogen sulphide to elemental sulphur, which is non-volatile.
Laboratory: Widely used for scavenging metal ions.
Cosmetics: Used in shampoos, cleaners, and other personal care products as a sequestering agent
Chemtex Speciality Limited is a pioneering speciality chemical manufacturer, The best EDTA manufacturer in India, We Tetrasodium EDTA (4Na), Disodium EDTA (2Na), EDTA Trisodium (3Na) and Pure Acid form.
Disodium EDTA (2Na) – Disodium salt of EDTA is used as sequestering / chelating agent, that helps in cleaning and descaling from metal surfaces. Also, it finds extensive usage in the removal of iron oxide/hydroxide and calcium carbonate, other organic substances and microbial slimes.
Trisodium EDTA (3Na) – It is used as a disinfectant, colour retention agent, anticoagulant for blood collection, preservative, antioxidant, chelating agent in personal care products and flavouring agent for foods. It is used to minimize metal ion contaminants, and to facilitate enzymatic reactions that could be inhibited by heavy metal traces.
Tetrasodium EDTA (4Na) – Tetrasodium EDTA is also used as a chelating agent, with the ability to sequester various polyvalent cations, that bind calcium, magnesium and other metal ions, minimizing metal ion contamination and facilitating enzymatic reactions that could be inhibited by heavy metal traces. EDTA Tetrasodium Salt also works as a slime dispersant and is even effective in reducing scale deposits and water hardness.
Key Features & Benefits
• Optimum Chelating efficiency
• Prevents metal contamination
• Sequesters magnesium, calcium and other metal ions.
• Cost-effective
Chemtex is one of the leading EDTA Manufactures in India. EDTA is the most widely used chelating agent for the removal of toxic heavy metal ions in various industrial applications such as water treatment, F&B, Laboratory processes, etc.
Follow More: https://www.chemtexltd.com/products-and-solutions/performance-chemicals/general-chemicals/edta/
0 notes
Text

Alkyl Halide
Alkyl Halide- Alkanes that include one or more halogens, commonly known as haloalkanes or alkyl halides, are the main source of these chemical compounds. Alkyl halides fall under the umbrella term of halocarbons. Alkyl halides, also known as haloalkanes, are created when halogen atoms—such as those from fluorine, chlorine, bromine, or iodine—replace hydrogen atoms in an aliphatic hydrocarbon. Alkanes, alkenes, alcohols, and carboxylic acids are only a few examples of the organic precursors that can be used to make them. Alkyl halides often have hydrogen atoms bonded to the alkyl group's sp3 hybridized carbon atom. The following are some different criteria that can be used to categorize alkyl halides.
Halogen Atom Count
Here, whether they have one, two, or more halogen atoms in their structure is the fundamental factor determining their classification. Under this heading, we have: Mono Holakane, Dihaloakane, Trihaloalkane.
The Halogen Atom's Position Across the Carbon Atom Chain
The halogen atom's position on the carbon atom chain determines the categorization.
Primary alkyl halide- The carbon that is connected to the halogen family in these haloalkanes will only be joined to one other alkyl group. No matter how strongly a large group is connected to it.
Secondary alkyl halide- The carbon atom that is connected to the halogen atom in this sort of haloalkane is joined directly to the other two alkyl groups, which may or may not be the same.
Tertiary alkyl halide- This particular class of haloalkanes has three alkyl groups directly linked to the carbon atom that bears the halogen element. This alkyl group may include both the same and distinct alkyl groups.
Alkyl Halide Properties-
Alkyl halides have no color when they are present in their purest form. However, when bromides and iodides are exposed to light, they become colored. Many volatile halogen substances smell sweet. Solids or liquids are higher members. As the size and quantity of electrons rise, the attraction becomes stronger. As the mass of halogen atoms and the quantity of carbon atoms both rise, so does the density. Energy is needed to overcome the attraction between the haloalkane molecules and break the hydrogen bonds between the water molecules for haloalkanes in order to dissolve haloalkanes in water.
Chemical Reaction
Three different types of haloalkane chemical reactions exist:
Nucleophilic substitution reaction- A nucleophile combines with haloalkane in this kind of reaction, which has a partial positive charge on the carbon atom bound to the halogen. Following a substitution reaction, the leaving group halogen atom departs as a halide ion. The substitution process is known as a nucleophilic substitution reaction because a nucleophile started it.
Elimination Reaction- A hydrogen atom from the -carbon atom and a halogen atom from the -carbon atom will be eliminated when a haloalkane with a hydrogen atom is heated with an alcoholic solution of potassium hydroxide. As a result, one of the products is an alkene. Elimination is frequently referred to as a "elimination reaction" since the -hydrogen atom is engaged in the process.
Reactions with Metals- When certain metals are combined with the majority of organic chlorides, bromides, and iodides, compounds with carbon-metal bonds are produced. These substances are referred to as organometallic substances. Haloalkanes and magnesium metal are combined to create the product in dry ether.
Uses Of Alkyl Halide- In nature, halogen-containing organic molecules are abundant, and some of them have clinical use. These groups of substances have useful uses in both daily life and industry. They serve as starting materials for the synthesis of a wide variety of organic compounds as well as solvents for generally nonpolar molecules. Some completely fluorinated substances are thought to be possible blood substitutes in operations.
Every student who is studying for the NEET should thoroughly understand the fundamentals of alkyl halide in order to better understand topics that are connected to it. The Best NEET coaching in Bangalore gives students notes on all crucial subjects and topics, including Alkyl Halide, to assist them pass the exam on their first try.
0 notes
Text
Monovalent cations such as the potassium ion can form electrostatic bonds with the carboxylic groups of many organic acids (Figure 13.17A). (...) Divalent ions such as the calcium ion form electrostatic bonds with pectates (Figure 13.17B) and the carboxylic groups of polygalacturonic acid.
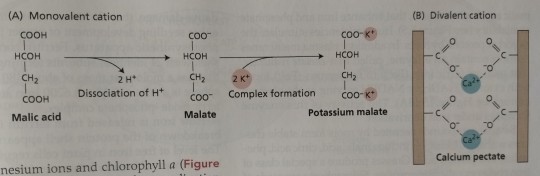

"Plant Physiology and Development" int'l 6e - Taiz, L., Zeiger, E., Møller, I.M., Murphy, A.
#book quotes#plant physiology and development#nonfiction#textbook#malic acid#malate#potassium malate#calcium pectate#cation#monovalence#divalence#electrostatic bonds
0 notes