#Non-alcoholic Steatohepatitis Clinical Trials Market
Link
According to Nova one advisor's latest report, titled Non-Alcoholic Steatohepatitis Clinical Trials Market Size: Industry Trends, Share, Growth, Opportunity and Forecast 2022-2030," the global Non-Alcoholic Steatohepatitis Clinical Trials market reached a value of US$ 2.40 Billion in 2021. Looking forward, Nova one advisor expects the market to reach a value of US$ 4.1 Billion by 2030, exhibiting a CAGR of 6.4% during 2022-2030.
0 notes
Text
In-Depth Analysis of Non-Alcoholic Steatohepatitis Clinical Trials
Non-Alcoholic Steatohepatitis (NASH) represents a burgeoning public health concern, underscored by its escalating prevalence and significant disease burden. Clinical trials serve as pivotal platforms for elucidating the pathophysiology of NASH and evaluating novel therapeutic modalities. Our comprehensive analysis delves into the intricate landscape of NASH clinical trials, offering invaluable insights for researchers, clinicians, and stakeholders.
Current Landscape of NASH Clinical Trials
NASH clinical trials encapsulate a diverse array of investigational approaches, ranging from lifestyle interventions to pharmacological agents targeting various facets of NASH pathogenesis. The multifaceted nature of NASH necessitates a multifaceted approach in trial design and therapeutic intervention.
Key Trends and Developments
Pharmacological Interventions
Pharmacological interventions constitute a cornerstone in NASH clinical trials, with numerous agents targeting different pathophysiological pathways. From insulin sensitizers and lipid-lowering agents to anti-inflammatory and antifibrotic therapies, the armamentarium against NASH continues to expand.
For more regional insights into the NASH clinical trials market, download a free report sample
Lifestyle Modifications
Lifestyle modifications, encompassing dietary interventions, exercise regimens, and weight management strategies, are integral components of NASH clinical trials. Lifestyle interventions aim to ameliorate metabolic abnormalities and mitigate disease progression, often complementing pharmacological therapies.
Biomarker Development
The quest for reliable biomarkers for NASH diagnosis, prognostication, and therapeutic response assessment remains a paramount objective in clinical research. Biomarker development initiatives seek to identify non-invasive, surrogate markers reflective of NASH activity and severity, thereby facilitating clinical trial endpoints and patient stratification.
Challenges and Opportunities
Disease Heterogeneity
The heterogeneous nature of NASH poses a formidable challenge in clinical trial design and patient selection. Efforts to characterize distinct NASH phenotypes and identify biomarkers predictive of treatment response are essential for optimizing trial outcomes and therapeutic efficacy.
Endpoint Selection
Selecting appropriate clinical endpoints that accurately reflect NASH disease activity and progression remains a contentious issue in clinical trial design. Consensus regarding validated surrogate endpoints and clinically meaningful outcomes is imperative for advancing therapeutic development in NASH.
Regulatory Landscape
Navigating the evolving regulatory landscape surrounding NASH drug development presents inherent complexities and uncertainties. Close collaboration between regulatory agencies, industry stakeholders, and academic researchers is indispensable for streamlining trial protocols and expediting drug approval processes.
Future Directions
Personalized Medicine
The paradigm shift towards personalized medicine heralds a new era in NASH therapeutics, wherein treatment decisions are tailored to individual patient characteristics and disease phenotypes. Biomarker-driven approaches and genomic profiling hold promise in optimizing treatment selection and predicting therapeutic responses.
Combination Therapies
The synergistic effects observed with combination therapies underscore the potential for enhanced therapeutic efficacy in NASH management. Rational drug combinations targeting complementary pathophysiological pathways offer a promising avenue for mitigating disease progression and improving clinical outcomes.
Patient-Centric Trials
Adopting a patient-centric approach in clinical trial design and implementation is paramount for enhancing patient engagement, retention, and satisfaction. Incorporating patient-reported outcomes and integrating patient preferences into trial protocols can enhance the relevance and success of NASH clinical trials.
Conclusion
In conclusion, our comprehensive review delineates the dynamic landscape of NASH clinical trials, highlighting key trends, challenges, and future directions. By fostering collaborative research endeavors and embracing innovative approaches, we endeavor to accelerate therapeutic innovation and alleviate the burden of NASH on a global scale.
0 notes
Text
PRISM MARKETVIEW HIGHLIGHTS ALTIMMUNE’S POSITIVE TOPLINE RESULTS IN OBESITY DRUG TRIAL

NEW YORK, NY, December 05, 2023 - PRISM MarketView, a leading provider of unbiased market insight and company news, highlights Altimmune, Inc. (Nasdaq: ALT) which has reported positive topline results from its 48-week MOMENTUM Phase 2 obesity trial of pemvidutide.
The company’s study enrolled 391 subjects with obesity and at least one comorbidity. Over 50% of subjects achieved at least 15% weight loss and over 30% of subjects achieved at least 20% weight loss on the 2.4 mg dose. The company’s share price rose more than 40% in morning trading on Friday.
Vipin K. Garg, Ph.D., President and Chief Executive Officer of Altimmune, commented, “This is an important day for Altimmune and we couldn’t be more pleased with these results. We believe the magnitude of weight loss, robust reductions in triglycerides, LDL cholesterol and blood pressure, together with the safety profile observed in this trial, could potentially differentiate pemvidutide from the other incretin-based therapies. If approved, we believe pemvidutide could offer an important option for obesity patients, including those with risk factors for cardiovascular disease.”
Dr. Scott Harris, Chief Medical Officer of Altimmune, said, “The level of weight loss achieved at 48 weeks in this trial has been shown to reverse the key complications of obesity. Moreover, the trajectory of weight loss at the end of treatment with the 2.4 mg dose suggests the potential for greater weight loss with continued treatment.”
Pemvidutide is a novel, investigational, peptide-based GLP-1/glucagon dual receptor agonist in development for the treatment of obesity and metabolic dysfunction-associated steatohepatitis (MASH), formerly known as non-alcoholic steatohepatitis (NASH). The drug has exhibited an excellent safety profile to date, particularly cardiac-related safety.
Activation of the GLP-1 and glucagon receptors is believed to mimic the complementary effects of diet and exercise on weight loss, with GLP-1 suppressing appetite and glucagon increasing energy expenditure.

About Altimmune
Altimmune is a clinical-stage biopharmaceutical company focused on developing treatments for obesity and liver diseases. The Company’s lead product candidate, pemvidutide, is a GLP-1/glucagon dual receptor agonist that is being developed for the treatment of obesity and MASH, formerly known as NASH. In addition, Altimmune is developing HepTcell™, an immunotherapeutic designed to achieve a functional cure for chronic hepatitis B. For more information, please visit www.altimmune.com.
About PRISM MarketView:
Established in 2020, PRISM MarketView is dedicated to the monitoring and analysis of small cap stocks in burgeoning sectors. We deliver up-to-the-minute financial market news, provide comprehensive investor tools and foster a dynamic investor community. Central to our offerings are proprietary indexes that observe emerging sectors, including biotech, clean energy, next-generation tech, medical devices and beyond. Visit us at prismmarketview.com and follow us on Twitter.
PRISM MarketView does not provide investment advice.
Contact:
PRISM MarketView
[email protected]
646-863-6341
SOURCE: PRISM MarketView
#press release#prism mediawire#stock market#investing#prismdigitalmedia#prismmarketview#nasdaq#healthcare#pharmaceutical#ALT#Altimmune
0 notes
Text
Non-Alcoholic Steatohepatitis Clinical Trials Market: Global Industry Analysis, Size, Share, Growth, Trends And Forecast 2022 to 2032
According to Future Market Insights’ Non-Alcoholic Steatohepatitis Clinical Trials Market Study research, global sales were stable at US$ 2.5 billion in 2021. The anticipated market growth from 2022 to 2032 is anticipated to be 7%, which is much greater than the increase in the past. With a CAGR of roughly 11.2% from 2022 to 2032, Phase 3 is anticipated to generate the largest revenue for the Non-Alcoholic Steatohepatitis Clinical Trials Market.
Market for Non-Alcoholic Steatohepatitis Clinical Trials from 2017 to 2021 in terms of revenue In contrast to the Demand Forecast for 2022 to 2032
According to a study on the Non-Alcoholic Steatohepatitis Clinical Trials Market conducted by Future Market Insights, a provider of market research and competitive intelligence, from 2017 to 2021, the market value of this disease increased at a compound annual growth rate (CAGR) of about 6.2%, with the USA, the UK, China, and Japan accounting for a sizable portion of the global market.
Request Sample @
https://www.futuremarketinsights.com/reports/sample/rep-gb-15802
Moreover, advancing technology, an ageing population, an increase in the prevalence of chronic diseases, and an increase in surgical operations are the main drivers of the expansion. Because of this, the market for non-alcoholic steatohepatitis clinical trials is anticipated to expand at a CAGR.
How did the market for non-alcohol steatohepatitis clinical trials perform during the pandemic?
Trials were put on hold as a result of the pandemic because of decreased patient participation in clinical research and supply chain disruptions. Virtual participants and COVID-compliant screening, however, allowed some organisations to complete the trials. As an illustration, Novartis conducted a phase two trial of a particular medicine for non-alcoholic steatohepatitis after the drug was recognised as a ground-breaking treatment in the United States. In phase 3a, semaglutide will be started in patients with non-alcoholic steatohepatitis (NASH) in 2021. The outcomes of a phase two proof-of-concept experiment in NASH were given by Novo Nordisk and Gilead Sciences. In the US, NASH is the second most common reason for liver transplants. Hepatocellular carcinoma is also connected to NASH.
The results of a phase two proof-of-concept study in NASH were revealed by Nordisk and Gilead Sciences. The second most common reason for liver transplants in the US is NASH. Several studies have found a connection between NASH and hepatocellular cancer growth.
Ask An Analyst @
https://www.futuremarketinsights.com/ask-question/rep-gb-15802
Analysis by country
The USA Clinical Trials for Non-Alcoholic Steatohepatitis: Market Research
One of the most prevalent chronic liver diseases in the USA is NAFLD. In the USA, roughly 20% of adults are thought to have NASH and about 25% are thought to have NAFLD. Given these worrying figures, the Non-Alcoholic Steatohepatitis Clinical Trials market in the USA is predicted to grow at a CAGR of 7.5% from 2022 to 2032, reaching a valuation of US$ 1.9 billion.
It can be challenging to separate patients into distinct fibrosis phases in NASH trials because to pathologist bias. In order to achieve more accurate and consistent biopsy analysis, Sagimet Biosciences is adopting digital histopathology in its Stage IIb non-alcoholic steatohepatitis (NASH) investigation. Digital pathology for NASH involves histological imaging of biopsy samples, which are subsequently assessed using artificial intelligence (AI) to find fibrosis alterations. Sagimet is collaborating with HistoIndex, a Singapore-based diagnostic company, in the Phase IIb FASCINATE-2 trial (NCT04906421) to research TVB-2640.
Market Segments Covered in Non-Alcoholic Steatohepatitis Clinical Trials Market Analysis
By Study Design:
Interventional
Observational
Expanded Access
By Phase:
Phase 1
Phase 2
Phase 3
Phase 4
Request Customization @
https://www.futuremarketinsights.com/customization-available/rep-gb-15802
By Region:
North America
Latin America
Asia-Pacific (APAC)
Middle East and Africa (MEA)
Europe
0 notes
Text
Non-alcoholic Steatohepatitis Clinical Trials Market Trends Analysis Report By Phase, Study Design, Region And Forecast To 2030 : Grand View Research Inc.
Non-alcoholic Steatohepatitis Clinical Trials Market Trends Analysis Report By Phase, Study Design, Region And Forecast To 2030 : Grand View Research Inc.
San Francisco, 6 Apr 2022: The Report Non-alcoholic Steatohepatitis Clinical Trials Market Size, Share & Trends Analysis Report By Phase (Phase I, II, III, IV), By Study Design (Interventional, Expanded Access), By Region (APAC, Europe), And Segment Forecasts, 2022 – 2030
The global non-alcoholic steatohepatitis clinical trials market size is expected to reach USD 4.2 billion by 2030,…

View On WordPress
#Non-alcoholic Steatohepatitis Clinical Trials Industry#Non-alcoholic Steatohepatitis Clinical Trials Market#Non-alcoholic Steatohepatitis Clinical Trials Market 2022#Non-alcoholic Steatohepatitis Clinical Trials Market 2030#Non-alcoholic Steatohepatitis Clinical Trials Market Revenue#Non-alcoholic Steatohepatitis Clinical Trials Market Share#Non-alcoholic Steatohepatitis Clinical Trials Market Size
0 notes
Link
The global non-alcoholic steatohepatitis clinical trials market size was valued at USD 2.40 billion in 2020, and is predicted to be worth around USD 4.1 billion by 2030, registering a CAGR of 6.4% during the forecast period 2022 to 2030.
0 notes
Text
Signs Agreement with Vetbiolix for Development of Piclidenoson for Pets
New Post has been published on https://depression-md.com/signs-agreement-with-vetbiolix-for-development-of-piclidenoson-for-pets/
Signs Agreement with Vetbiolix for Development of Piclidenoson for Pets

All pre-clinical, clinical, and regulatory development work to be conducted and financially covered by Vetbiolix
Veterinary market has potential for a shorter path to regulatory approval and product revenues
PETACH TIKVA, Israel, June 28, 2021–(BUSINESS WIRE)–Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE:CFBI), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address inflammatory, cancer and liver diseases, today announced it has signed a development and commercialization agreement with Vetbiolix, a France-based veterinary biotech company, for the development of Piclidenoson for the treatment of osteoarthritis in companion animals including dogs and cats.
Vetbiolix will have the exclusive right to Piclidenoson in the veterinary osteoarthritis market for two years, during which time Vetbiolix will conduct proof-of-concept studies and cover all associated costs. If the studies yield positive data and Vetbiolix exercises its option to obtain the license from Can-Fite, then Vetbiolix will be obligated to pay Can-Fite upfront and milestone payments, in addition to royalties on sales upon regulatory approval for veterinary use.
The canine osteoarthritis market is projected to reach $3 billion by 2024. According to Grand View Research, the broader global companion animal health market is estimated at a value of $20 billion in 2021 and is expected to grow to $27 billion by 2028.
Current treatments for canine osteoarthritis include oral non-steroidal anti-inflammatory drugs (NSAIDs) which only treat symptoms and carry significant harmful side effects, and an injectable disease modifying osteoarthritis drug (DMOAD) that targets the progression of the disease. Piclidenoson, an oral drug that has a favorable safety profile in humans and in animal studies, offers a potentially safe and effective oral treatment for canine osteoarthritis.
“The veterinary market is a significant opportunity where our drugs may have an impact. Both the size of the market and the shorter timelines to regulatory approval have the potential to result in milestone and royalty revenues for Can-Fite. We believe Piclidenoson’s safety and efficacy data in over 1,000 humans, as well as preclinical data from small animals, indicate it may offer relief to the growing number of companion animals with osteoarthritis,” stated Can-Fite VP of Business Development, Dr. Sari Fishman.
Story continues
Matthieu Roquette, President at Vetbiolix commented, “The quality of preclinical and clinical data generated by Can-Fite on Piclidenoson and its pharmacological profile make this highly selective A3 Adenosine Receptor Agonist a drug candidate likely to meet the unmet veterinary medical need to date in the management of osteoarthritis pathology in dogs and cats. Moreover, the mechanism of action of Piclidenoson makes this product a strong candidate for a large spectrum of inflammatory disease indications affecting Pets. We are aiming to enter in veterinary regulatory development by the end of 2022 based on clinical proof of concept data we will generate within the next 12 months.”
In 2019, the U.S. Patent and Trademark Office issued to the Can-Fite patent #10,265,337 titled “Use of A3 Adenosine Receptor Agonist in Osteoarthritis Treatment” for Piclidenoson in the treatment of osteoarthritis in mammals.
About Vetbiolix
Vetbiolix develops innovative products for treatment and prevention of diseases affecting pets. As pharmaceutical and biotech companies research novel molecules and compounds for human medicine, tests in different species often reveal exciting possibilities for pets. Vetbiolix has developed a unique approach focused on turning this potential into innovative prescription medicines and care products for pets. To date, veterinarians have still few therapeutics and real preventive care products at their disposal that have been specifically developed and approved for pets. Along with a virtual VetBiotech organization, Vetbiolix exclusively focuses on clinical developments of prescription medicines, diagnostics, nutraceuticals and care products for pets, thanks to its qualified external R&D partners in Europe & the US. Vetbiolix is supported by the Eurasanté Bio-Incubator, the northern France health cluster ranked among the top 20 best European incubators fostering pharm/biotech start-up development (Labiotech.eu 2019).
About Can-Fite BioPharma Ltd.
Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CFBI) is an advanced clinical stage drug development Company with a platform technology that is designed to address multi-billion dollar markets in the treatment of cancer, liver, inflammatory disease and COVID-19. The Company’s lead drug candidate, Piclidenoson, is currently in a Phase III trial for psoriasis and a Phase II study in the treatment of moderate COVID-19. Can-Fite’s liver drug, Namodenoson, is headed into a Phase III trial for hepatocellular carcinoma (HCC), the most common form of liver cancer, and a Phase IIb trial for the treatment of non-alcoholic steatohepatitis (NASH). Namodenoson has been granted Orphan Drug Designation in the U.S. and Europe and Fast Track Designation as a second line treatment for HCC by the U.S. Food and Drug Administration. Namodenoson has also shown proof of concept to potentially treat other cancers including colon, prostate, and melanoma. CF602, the Company’s third drug candidate, has shown efficacy in the treatment of erectile dysfunction. These drugs have an excellent safety profile with experience in over 1,500 patients in clinical studies to date. For more information please visit: www.can-fite.com.
Forward-Looking Statements
This press release may contain forward-looking statements, about Can-Fite’s expectations, beliefs or intentions regarding, among other things, market risks and uncertainties, its product development efforts, business, financial condition, results of operations, strategies or prospects. In addition, from time to time, Can-Fite or its representatives have made or may make forward-looking statements, orally or in writing. Forward-looking statements can be identified by the use of forward-looking words such as “believe,” “expect,” “intend,” “plan,” “may,” “should” or “anticipate” or their negatives or other variations of these words or other comparable words or by the fact that these statements do not relate strictly to historical or current matters. These forward-looking statements may be included in, but are not limited to, various filings made by Can-Fite with the U.S. Securities and Exchange Commission, press releases or oral statements made by or with the approval of one of Can-Fite’s authorized executive officers. Forward-looking statements relate to anticipated or expected events, activities, trends or results as of the date they are made. Because forward-looking statements relate to matters that have not yet occurred, these statements are inherently subject to risks and uncertainties that could cause Can-Fite’s actual results to differ materially from any future results expressed or implied by the forward-looking statements. Many factors could cause Can-Fite’s actual activities or results to differ materially from the activities and results anticipated in such forward-looking statements. Factors that could cause our actual results to differ materially from those expressed or implied in such forward-looking statements include, but are not limited to: our history of losses and needs for additional capital to fund our operations and our inability to obtain additional capital on acceptable terms, or at all; uncertainties of cash flows and inability to meet working capital needs; the impact of the COVID-19 pandemic; the initiation, timing, progress and results of our preclinical studies, clinical trials and other product candidate development efforts; our ability to advance our product candidates into clinical trials or to successfully complete our preclinical studies or clinical trials; our receipt of regulatory approvals for our product candidates, and the timing of other regulatory filings and approvals; the clinical development, commercialization and market acceptance of our product candidates; our ability to establish and maintain strategic partnerships and other corporate collaborations; the implementation of our business model and strategic plans for our business and product candidates; the scope of protection we are able to establish and maintain for intellectual property rights covering our product candidates and our ability to operate our business without infringing the intellectual property rights of others; competitive companies, technologies and our industry; statements as to the impact of the political and security situation in Israel on our business; and risks and other risk factors detailed in Can-Fite’s filings with the SEC and in its periodic filings with the TASE. In addition, Can-Fite operates in an industry sector where securities values are highly volatile and may be influenced by economic and other factors beyond its control. Can-Fite does not undertake any obligation to publicly update these forward-looking statements, whether as a result of new information, future events or otherwise.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210628005218/en/
Contacts
Can-Fite BioPharma
Motti Farbstein
[email protected]
+972-3-9241114
Source link
0 notes
Text
The Global NASH Biomarker Market to Surge at CAGR of 21.28% by 2028
Triton Market Research presents the Global Non-Alcoholic Steatohepatitis (NASH) Biomarker Market segmented by Industry Verticals (Hospital, Academic Research Key, Pharma & CRO Industry, Diagnostic Lab), Type (Oxidative stress Biomarker, Apoptosis Biomarker, Hepatic fibrosis Biomarker, Serum Biomarker, Other Types), and by Geography (Asia-Pacific, North America, Middle East and Africa, Latin America, Europe). It further discusses the Market Summary, Industry Outlook, Key Insights, Impact of COVID-19, Porter’s Five Forces Analysis, Market Attractiveness Index, Vendor Scorecard, Key Market Strategies, Drivers, Restraints, Opportunities, Competitive Landscape, Research Methodology & Scope, Global Market Size, Forecasts & Analysis (2021-2028).

Request a Free Sample:
https://www.tritonmarketresearch.com/reports/non-alcoholic-steatohepatitis-biomarker-market
Triton’s report estimates the global non-alcoholic steatohepatitis (NASH) biomarker market to advance in revenue at a CAGR of 21.28% during the phase of 2021-2028.
NASH refers to a more severe form of non-alcoholic fatty liver disease, which can develop into disorders like cirrhosis and hepatocellular carcinoma. A NASH biomarker is used in several verticals, including hospitals, diagnostic labs, and pharma, among others.
Factors such as growth opportunities in the developing regions and the surge in awareness pertaining to non-alcoholic fatty liver disease generate new opportunities for the NASH biomarker market. The emerging regions are offering several opportunities to NASH therapeutics manufacturers due to the improvement in healthcare infrastructure, rise in prevalence of NASH, and surge in demand for healthcare services. Along with this, the growing geriatric population in these nations is also likely to surge the need for NASH therapeutics, driving the studied market’s growth.
However, the side effects of NASH therapeutics and the unavailability of specialized diagnostic tests for non-alcoholic fatty liver disease are negatively impacting the growth of the NASH biomarker market.
North America holds the largest market for the non-alcoholic steatohepatitis (NASH) biomarker market, and is anticipated to continue its stronghold till 2028. In the United States, the market is primarily driven by the rise in the prevalence of diabetes and obesity among the population. Further, the expected launch of pipeline molecules, along with the early diagnosis of NASH, is another set of factors motivating the NASH biomarker market’s growth. Above all, the favorable reimbursement policies in the healthcare sector are also likely to boost the studied market to a growth path across North America.
The key players established in the NASH biomarker market are Siemens Healthineers, Bristol Myers Squibb Company, Allergan, Merck & Co, Novo Nordisk, Zydus Cadila, Gilead Sciences Inc, Novartis AG, AstraZeneca, Genfit SA, Pfizer Inc, Viking Therapeutics, and Madrigal Pharmaceuticals.
The threat of new players in the market is high as several companies focus on conducting clinical trials. Moreover, numerous small and medium players are expected to enter the market by collaborating with larger companies, increasing the threat of new entrants. Furthermore, several new drugs for the treatment of NASH are in the pipeline, with multiple diagnostic tests likely to gain approval. This is estimated to increase the level of competition among existing market players in the next few years.
Contact Us: [email protected]
Phone: +44 7441 911839
#Non-alcoholic Steatohepatitis Biomarker#NASH#lifesciences#healthcare#wellness#global insights#triton market research
0 notes
Text
Non-Alcoholic Steatohepatitis (NASH) Disease - Global Clinical Trials Review, H1, 2021 published on
https://www.sandlerresearch.org/non-alcoholic-steatohepatitis-nash-disease-global-clinical-trials-review-h1-2021.html
Non-Alcoholic Steatohepatitis (NASH) Disease - Global Clinical Trials Review, H1, 2021
Non-Alcoholic Steatohepatitis (NASH) Disease – Global Clinical Trials Review, H1, 2021
Summary
GlobalData’s clinical trial report, “Non-Alcoholic Steatohepatitis (NASH) Disease – Global Clinical Trials Review, H1, 2021” provides an overview of Non-Alcoholic Steatohepatitis (NASH) Clinical trials scenario. This report provides top line data relating to the clinical trials on Non-Alcoholic Steatohepatitis (NASH) . Report includes an overview of trial numbers and their average enrollment in top countries conducted across the globe. The report offers coverage of disease clinical trials by region, country (G7 & E7), phase, trial status, end points status and sponsor type. Report also provides prominent drugs for in-progress trials (based on number of ongoing trials). GlobalData Clinical Trial Reports are generated using GlobalData’s proprietary database – Pharma – Clinical trials database. Clinical trials are collated from 80+ different clinical trial registries, conferences, journals, news etc across the globe. Clinical trials database undergoes periodic update by dynamic process.
The report enhances the decision making capabilities and helps to create an effective counter strategies to gain competitive advantage.
Note: Certain sections in the report may be removed or altered based on the availability and relevance of data for the indicated disease.
Scope
– The report provides a snapshot of the global clinical trials landscape
– Report provides top level data related to the clinical trials by Region, Country (G7 & E7), Trial Status, Trial Phase, Sponsor Type and End point status
– The report reviews top companies involved and enlists all trials (Trial title, Phase, and Status) pertaining to the company
– The report provides all the unaccomplished trials (Terminated, Suspended and Withdrawn) with reason for unaccomplishment
– The Report provides enrollment trends for the past five years
– Report provides latest news for the past three months
Note: Certain sections in the report may be removed or altered based on the availability and relevance of data for the indicated disease.
Reasons to Buy
– Assists in formulating key business strategies with regards to investment
– Helps in identifying prominent locations for conducting clinical trials which saves time and cost
– Provides top level analysis of Global Clinical Trials Market which helps in identifying key business opportunities
– Supports understanding of trials count and enrollment trends by country in global therapeutics market
– Aids in interpreting the success rates of clinical trials by providing a comparative scenario of completed and uncompleted (terminated, suspended or withdrawn) trials
– Facilitates clinical trial assessment of the indication on a global, regional and country level
Note: Certain sections in the report may be removed or altered based on the availability and relevance of data for the indicated disease.
0 notes
Text
Alzheimer’s Research UK starts AI-based drug hunt with Exscientia

Medical charity Alzheimer’s Research UK has teamed up with artificial intelligence specialist Exscientia to find new drug treatments for the devastating neurodegenerative disease.
The alliance will see Exscientia work with the charity’s Oxford Drug Discovery Institute (ODDI) to find therapeutics that target the neuroinflammation associated with Alzheimer’s disease (AD), focusing in particular on the NLRP3 inflammasome pathway.
Inflammasomes are a group of intracellular proteins associated with the inflammatory response, and prior research has linked the NLRP3 inflammasome to the aggregation of amyloid beta and tau protein – which together form the characteristic plaques and tangles in the brains of AD patients.
One recent study has pointed to NLRP3 as a potential a link between amyloid and tau disruption, so drugs inhibiting it may be able to address two AD pathologies in one molecule.
“Activation of the NLRP3 inflammasome has been shown to have an important role in AD pathogenesis,” said the two groups in a joint statement.
“While there have been other efforts to develop anti-inflammatory drugs for AD, targeting NLRP3 inflammasome inhibition in the brain is an innovative therapeutic approach.”
UK biotech Exscientia has signed a string of partnerships with pharma companies and other research-based organisations to apply its AI-based drug discovery platform.
The company hit the headlines last year when one partner – Japan’s Sumitomo Pharma – started clinical trials of a drug candidate for obsessive compulsive disorder, said to be the first medicine developed using AI to enter testing in humans.
It reckons its use of AI and machine learning can trim years off the current 12- to 15-year cycle from early research to marketed product.
The project with the ODDI will draw on research conducted over years by the Oxford group on the NLRP3 pathway, with its biology and screening expertise fed into Exscientia’s Centaur Chemist AI-powered drug design platform.
Dr John Davis, chief scientific officer of ODDI, said that the Exscientia partnership “will enable us to investigate multiple molecules in parallel and accelerate the project towards candidate declaration”.
He added: “Human genetic variation points towards a critical role for the body’s immune system in an individual’s risk of developing Alzheimer’s disease. It is vital that we develop treatments that target neuroinflammatory mechanisms underlying dementia.”
NLRP3 inflammasomes have emerged as something of a hot target in the biopharma sector over the last couple of years. Last September, Roche paid €380 million (around $460 million) to buy Inflazome and its pipeline of oral NLRP3 drugs, which are being developed for Parkinson’s disease, AD and peripheral inflammatory disorders like gout.
In 2019, Novartis also bought into the category via a $310 million takeover of IFM Therapeutics, which is developing NLRP3 drugs for atherosclerosis and non-alcoholic steatohepatitis (NASH), while UK startup NodThera raised $55 million last year for its own NLRP3 programme, eyeing NASH and diabetes.
The post Alzheimer’s Research UK starts AI-based drug hunt with Exscientia appeared first on .
from https://pharmaphorum.com/news/alzheimers-research-uk-starts-ai-based-drug-hunt-with-exscientia/
0 notes
Text
By 2026, Liver Fibrosis Treatment Market To Surpass US$ 28 Billion - Coherent Market Insights
Liver Fibrosis Treatment Market To exhibit a CAGR of 10.8% over the forecast period (2018 - 2026).
Request for Sample PDF Copy:
https://www.coherentmarketinsights.com/insight/request-pdf/2320
Description:
Liver fibrosis is characterized by repeated or continuous damage to liver resulting in an abnormally large amount of scar tissue in the liver. Fibrosis does not show any symptoms, however severe scarring results into cirrhosis, which further leads to cause symptoms. To detect the severity of disease, healthcare professionals conduct blood and imaging test, followed by liver biopsy, if required. In the possible treatment options for liver fibrosis, healthcare professionals focus on treating the cause, which often stops and slowdowns further scarring of liver and results in improvement of condition. The possible treatment options for liver fibrosis include use of antiviral drugs, removal or dissolve of a blockage in the bile ducts, lifestyle changes such as lower consumption of alcohol, control regime for obesity and overweight, and controlling blood sugar & lipid levels in people with nonalcoholic fatty liver.
Furthermore, research funding by various private research organizations to conduct research related to development of treatment for liver fibrosis and other fatty liver disease is expected to spur growth of liver fibrosis treatment market. For instance, in August 2018; professor from College of Science at Virginia Tech and Department of Pharmacology at the University of Virginia received US$ 400,000 grant from the Virginia Biosciences Health Research Corporation (VBHRC) with US$ 800,000 matching funds from Continuum Biosciences, Inc. This grant along with matching funds will help researchers to develop drugs to treat nonalcoholic steatohepatitis (NASH).
The global liver fibrosis treatment market size is expected to witness a CAGR of 10.8% over the forecast period (2018 – 2026) reaching a value of US$ 28.1 billion in 2026.
Global Liver Fibrosis Treatment Market Value (US$ Mn), by Region, 2026
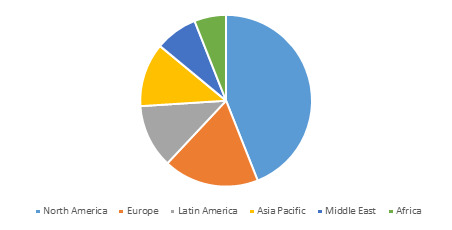
Liver Fibrosis Treatment | Coherent Market Insights
Source: Coherent Market Insights Analysis (2018)
Growing Research and Developments (R&D) Activities and New Developments for Liver Fibrosis Treatment are expected to Augment Market Growth
Liver fibrosis results from various chronic injuries and it often progresses to liver cirrhosis, liver failure, portal hypertension, and hepatocellular carcinoma. Moreover, liver transplantation is only treatment available for advanced stage liver fibrosis. Therefore, new treatment strategies are currently in clinical development stages to treat the liver fibrosis. These treatment option include inhibition of hepatic injury, inhibition of activation of Hematopoietic Stem Cells (HSCs), and inhibition of proliferation of HSCs, inhibition of deposition of type I collagen and proliferation of HSCs. Treatment options for liver fibrosis are usually depends on the underlying cause of the fibrosis. A healthcare professional can treat the underlying liver fibrosis illness conditions, to reduce the effects of liver disease.
Furthermore, key players in the liver fibrosis treatment market are engaged in pre-clinical and clinical studies to develop novel treatment therapies in liver fibrosis regime. For instance, in March 2018, the U.S. FDA granted Orphan Drug Designation for PTG-300: a subcutaneous injectable developed by Protagonist Therapeutics, Inc. The PTG-300 is currently in clinical development stage for the potential treatment of beta-thalassemia, and can also be used in the liver fibrosis treatment.
For instance, Gilead Sciences, Inc., in October 2017, released the Phase II result studies for Selonsertib: GS-0976 in Nonalcoholic Steatohepatitis (NASH) stage F3 liver fibrosis. These studies reported that, GS-0976 led to significant reductions in concentration of liver fat and fibrosis. Moreover, Gilead Sciences also contain 18 other pipeline molecules in the liver fibrosis treatment, which is further expected to boost company’s revenue in near future.
In April 2017, Tobira Therapeutics, Inc. initiated the clinical study to confirm the efficacy and safety of cenicriviroc (CVC) for the treatment of liver fibrosis in adult patients with NASH. The study is expected to complete in July 2019.
In April 2017, Bristol-Myers Squibb Company presented the Phase II clinical trial study of BMS-986036: a human fibroblast growth factor. The study results reported that BMS-986036 shows consistent improvement in liver fat, liver injury, and liver fibrosis in patients with Nonalcoholic Steatohepatitis (NASH). Increasing number of clinical trials are currently in process for the treatment of liver fibrosis, which are expected to augment the market growth over the forecast period.
Global Liver Fibrosis Treatment Market Share (%), By Treatment Type: 2018 & 2026
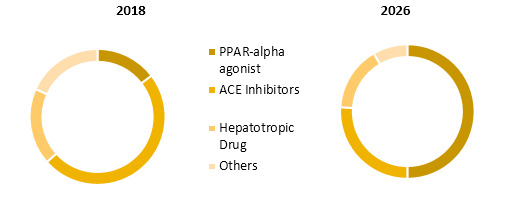
Liver Fibrosis Treatment | Coherent Market Insights
Source: Coherent Market Insights Analysis (2018)
North America market accounted for the largest market share, followed by Europe in 2018. This is owing to increasing burden of potential risk factors leading to liver fibrosis such as obesity, type 2 diabetic, metabolic fibrosis, alcoholic fibrosis, non-alcoholic steatohepatitis (NASH), non-alcoholic fatty liver disease (NAFLD), among others. For instance, according to the NASH Education Program, 2018 data findings, NAFLD is most common chronic liver condition in Western population of economies in the Europe and the U.S. region as compared to obesity and type 2 diabetes epidemics. Furthermore, the prevalence of NASH is expected to increase by 63% between 2015 and 2030, leading to NASH become a major cause of liver transplantation in the U.S. by 2020.
Furthermore, according to the World Health Organization (WHO), in 2016; more than 1.9 billion adults, 18 years and older were overweight, among which around 650 million were obese. According to Organisation for Economic Co-operation and Development (OECD): Obesity Update 2017 report, obesity levels are expected to be particularly high in the U.S., Mexico and U.K., where 47%, 39% and 35% of the population respectively are projected to be obese in 2030.
Some of the key players operating in the liver fibrosis treatment market include Gilead Sciences, Inc., Merck & Co., Inc., Bristol-Myers Squibb, Johnson and Johnson, Novartis AG, Vertex Pharmaceuticals Incorporated, Pfizer Inc., FibroGen, Inc., and Pharmaxis Limited.
About Us:
Coherent Market Insights is a global market intelligence and consulting organization focused on assisting our plethora of clients achieve transformational growth by helping them make critical business decisions. We are headquartered in India, having office at global financial capital in the U.S. Our client base includes players from across all business verticals in over 150 countries worldwide. We do offer wide range of services such as Industry analysis, Consulting services, Market Intelligence, Customized research services and much more. We have expertise in many fields such as healthcare, chemicals and materials, Automation, semiconductors, electronics, energy, food and beverage, packaging and many more. Visit our website to know more.
Buy Report Here:
https://www.coherentmarketinsights.com/insight/buy-now/2320
Contact Us:
Mr. Shah
Coherent Market Insights
1001 4th Ave.
#3200
Seattle, WA 98154
Tel: +1-206-701-6702
Email: [email protected]
0 notes
Text
Non-Alcoholic Steatohepatitis (NASH) Market Revenue Analysis, Company Revenue Share, Global Forecast Till 2027
The global Non-alcoholic Steatohepatitis (NASH) Market is expected to reach USD 13.38 Billion by 2026, according to a new report by Reports and Data. This can be mainly associated with increasing consumption of fats globally. Based on statistics, Nonalcoholic Fatty Liver Disease (NAFFLD) affects around 80 million to 100 million Americans. NAFLD is expected to become the most common chronic liver condition globally in relation to the obesity and type 2 diabetes in the coming years. Estimates suggest that the incidence of NASH is projected to witness an increase of around 63% between 2015 and 2030.
Increase in the number of expected launch of pipeline drugs is also a significant factor stimulating market demand. The current statistics for the U.S. suggest that the healthcare cost associated with NASH is around USD 5 billion. Since the incidence of NASH is projected to rise significantly, estimates suggest that, if unchecked, the healthcare costs associated with NASH could rise up to USD 18 billion by 2030. Companies around the world have been focusing on bringing out new drugs in the market for the treatment of the disease.
North America is expected to be a key revenue generating region in the forecast period. The market is projected to grow at a CAGR of 53% in the forecast period. Increasing incidence of disease in the region has created a growth opportunity for the drug makers. Manufacturers have been focusing on bringing out new drugs. For instance: Recently in November 2018, Gilead Sciences, Inc. unveiled that its drug Selonsertib which is a dual anti-apoptotic and anti-inflammatory drug is currently undergoing Phase-3 trials for NASH compensated cirrhosis and NASH fibrosis.
Get a sample of the report @ https://www.reportsanddata.com/sample-enquiry-form/1111
Further key findings from the report suggest
Elafibranor is projected to be one of the fastest growing drugs of the NASH market. The segment is projected to grow at a CAGR of 55.4% in the forecast period. The drug which is an oral treatment is GENFIT’s lead pipeline product. It has been positioned as a first-in-class drug to treat NASH. The drug is currently being evaluated in the clinical Phase 3 study RESOLVE-IT.
North American market is forecasted to grow at a CAGR of 53% in the coming years. The disease is considered to be one of the leading causes of cirrhosis in adults in the U.S. High incidence of diabetes and obesity in the region are considered to be the main reason behind increasing incidence of NASH. Based on American Liver Foundation's National Medical Advisory Committee currently around 30 million Americans have been diagnosed with NASH. Additionally, around 90% of the NASH patients suffer from either obesity or diabetes.
Key participants include
Genfit, Intercept Pharmaceuticals, Gilead Sciences, Galmed Pharmaceuticals, Inventiva, Allergan and Tobira Therapeutics. Gilead Sciences is a key player in the NASH market. With a global presence in more than 35 countries, the company offers drugs including Selonsertib, Cilofexor and Firsocostat for the treatment of NASH.
Request a discount on the report @ https://www.reportsanddata.com/discount-enquiry-form/1111
For the purpose of this report, Reports and Data have segmented global NASH market on the basis of disease cause, drug type, end-user and region:
· Disease Cause Outlook (Revenue, USD Million; 2017-2027)
o Hypertension
o Heart Disease
o High Blood Lipid
o Type 2 Diabetes
o Obesity
· Drug Type Outlook (Revenue, USD Million; 2017-2027)
o Vitamin E & Pioglitazone
o Ocaliva
o Elafibranor
o Selonsertib
o Cenicriviroc
o Others
· End-user Outlook (Revenue, USD Million; 2017-2027)
o Hospitals
o Clinics
o Homecare settings
Request a customization of the report @ https://www.reportsanddata.com/request-customization-form/1111
· Regional Outlook
o North America
· U.S.
o Europe
· Germany
· UK
o Asia Pacific
· China
· India
· South-east Asia
o Latin America
· Brazil
o MEA
Thank you for reading our report. Please get in touch with us to know more about the report and customization of the report. Our team will ensure the report is tailored to meet your requirements.
Browse More Related Reports :
Actigraphy Sensors and Polysomnography Devices Market Analysis
Non-Invasive Brain Trauma Monitoring Devices Market Share
0 notes