#Medical Device Regulatory Consultants in India
Text
Medical Device Regulatory Consultants in India
Medical device regulatory consultants in India, medical device registration & approval services by CliniExperts. For medical device regulatory services; call +91 7672005050
#Medical Device Regulatory Consultants#Medical Device Regulatory Consultants in India#Medical Device Regulatory Services
0 notes
Text
Top Market Research Consultant Companies In India

Lifesciences Intellipedia is a comprehensive platform that provides research and intelligence services to businesses operating in the life sciences sector. The platform is designed to help companies make informed decisions by providing reliable and updated information on market trends, competitive analysis and industry developments.
We cover a range of sectors within the life sciences industry including biotechnology, pharmaceuticals, medical devices, diagnostics and healthcare. The platform provides a variety of research and intelligence services, including market research, competitive intelligence, regulatory intelligence, and patent analysis. Why are you waiting? Get in touch with our top market research consultant companies in India and visit our Lifescience Intellipedia website to start your new business with us.
2 notes
·
View notes
Text
opportunity to work remotely as a Senior Regulatory Affairs Associate at Parexel. Learn about the qualifications, responsibilities, and locations available in India, including Remote, Hyderabad, and Mumbai.
About Parexel: Parexel is a renowned consulting firm specializing in helping biopharmaceutical and medical device companies navigate regulatory challenges. Join a dynamic team focused on innovation and collaboration, with opportunities for continuous learning and career growth.
Role Overview: Senior Regulatory Affairs Associate
Qualifications:
Around 4-8 years of relevant experience in drug product life cycle management
Good understanding of global regulatory frameworks, including EU/US procedures
Experience in handling CMC-related queries and submissions
Working knowledge of Regulatory Information Management Systems like Veeva Vault
Strong communication and mentoring skills
Ability to work independently and collaborate effectively with cross-functional teams
Responsibilities:
Around 4-8 years of relevant experience in handling of pre and post approval life cycle management of drug products (small molecules as well as biologics) in various markets.
Good understanding of regulatory framework, including regional trends, for various types of applications and procedures
Contribute to preparation (including authoring where relevant) and delivery of simple, and with experience, increasingly more complex regulatory maintenance submissions from either a global and/or regional perspective.
Working knowledge of EU/US regulatory procedures including post approval requirements. Knowledge of ROW markets regulatory legislations would be an added advantage
Experience in handling CMC related health authority queries
Good understanding of regulatory framework, including regional trends, for various types of applications and procedures for small and large molecules across all regions as well as knowledge of global pharmaceutical legislation and guidance specifically linked to regulatory CMC aspects in the ICH countries.
Preparation and review of Marketing Authorization Applications & Variations for various types of medicinal products (Orals & Parenterals) for filing in EU through different types of procedures (DCP/MRP/National Procedures).
Preparation of documentation for different types of Variation procedures like Super grouping,
Grouping and Work-sharing to the Marketing Authorizations.
Regulatory review of DMFs, batch records, specifications, and stability data to ensure their compliance with the regulatory requirements.
Providing regulatory impact assessment for change proposals and identification of required documentation for EU submissions
Liaise closely with cross-functional members with aligned product responsibilities.
Execute and maintain submission delivery plans, submission content plans, and proactively provide status updates to designated stakeholders.
To prepare, review and submit safety variations to Health Authorities and perform post Approval CMC related updates.
Working experience in Regulatory Information Management Systems like Veeva Vault.
Strong communications skills and ability to guide and mentor team members
[caption id="attachment_52372" align="aligncenter" width="930"] Parexel Regulatory Affairs Job Vacancies - Senior Regulatory Affairs Associate[/caption]
Success Profile: Are you a communicator, detail-oriented problem solver with a strategic mindset? Parexel values individuals who can build relationships, drive results, and contribute to strategic regulatory initiatives.
How to Apply: Interested candidates can apply for the Remote Senior Regulatory Affairs Associate position at Parexel through the official job listing here.
0 notes
Text
Navigating Liposuction Clinics in Delhi: Cost, Procedures, and Considerations for Liposuction Surgery in Delhi

Introduction: Liposuction surgery in Delhi has become increasingly popular in recent years, offering individuals a way to sculpt and contour their bodies effectively. As the capital city of India, Delhi boasts numerous clinics and medical facilities specializing in liposuction procedures. However, navigating through the myriad of options can be daunting, especially when considering factors like cost, safety, and quality of care. In this comprehensive guide, we'll delve into the world of liposuction clinics in Delhi, exploring the surgery costs, procedures, and essential considerations for anyone considering liposuction surgery in the bustling metropolis.
Understanding Liposuction Surgery: Before diving into the specifics of liposuction clinics in Delhi, it's essential to grasp the fundamentals of liposuction surgery itself. Liposuction, also known as lipoplasty or body contouring surgery, is a cosmetic procedure designed to remove excess fat from specific areas of the body. Common target areas include the abdomen, thighs, hips, buttocks, arms, and chin. The surgery involves the use of a cannula, a thin tube connected to a vacuum device, which is inserted through small incisions in the skin to suction out fat cells, resulting in a smoother and more contoured appearance.
Liposuction Surgery Cost in Delhi: One of the primary concerns for individuals considering liposuction surgery in Delhi is the cost associated with the procedure. The cost of liposuction surgery can vary significantly depending on several factors, including the extent of the treatment area, the amount of fat to be removed, the surgeon's experience and expertise, the clinic's location and reputation, and the type of liposuction technique used. Liposuction Surgeon cost in Delhi
In Delhi, liposuction surgery costs typically range from INR 50,000 to INR 3,00,000 or more, depending on these factors. Clinics in upscale neighborhoods or those with advanced technology and facilities may charge higher prices. Additionally, the complexity of the procedure and the surgeon's skill level can also impact the overall cost. It's essential for individuals considering liposuction surgery in Delhi to consult with multiple clinics, compare quotes, and inquire about any additional fees or expenses involved to make an informed decision.
Finding the Right Liposuction Clinic in Delhi: When searching for a liposuction clinic in Delhi, several key factors should be taken into consideration to ensure a safe and successful surgical experience. These factors include:
Watch This Video:-https://youtu.be/EWB0HtqQK0Q?si=UEAgIJFi_B2r3G8T
Board Certification and Accreditation: Choose a clinic that is staffed by qualified and experienced plastic surgeons who are board-certified and have undergone specialized training in liposuction procedures. Additionally, ensure that the clinic is accredited by reputable medical organizations or regulatory bodies, indicating adherence to high standards of patient care and safety.
Reputation and Reviews: Research the reputation of the clinic and read reviews from previous patients to gauge their satisfaction levels and overall experience. Positive testimonials and recommendations can provide valuable insights into the quality of care and results offered by the clinic.
Technology and Facilities: Opt for a clinic equipped with state-of-the-art technology and modern facilities to ensure optimal outcomes and minimize the risk of complications. Advanced liposuction techniques, such as laser-assisted or ultrasound-assisted liposuction, may offer enhanced precision, faster recovery times, and superior results.
Consultation and Customization: Schedule a consultation with the surgeon to discuss your goals, concerns, and expectations regarding liposuction surgery. A reputable clinic will provide personalized treatment plans tailored to your unique needs and preferences, taking into account factors such as your anatomy, skin elasticity, and desired outcome.
Safety and Aftercare: Prioritize safety and post-operative care when selecting a liposuction clinic in Delhi. Inquire about the clinic's safety protocols, infection control measures, and anesthesia options to ensure a smooth and complication-free surgery. Additionally, ask about the availability of follow-up appointments and aftercare services to monitor your progress and address any concerns during the recovery period. Best Plastic Surgeon in Delhi
Conclusion: Liposuction surgery in Delhi offers individuals a transformative solution to achieve their desired body contouring goals. By understanding the cost, procedures, and essential considerations associated with liposuction surgery, individuals can make informed decisions when selecting a clinic and embarking on their cosmetic journey. Whether seeking to eliminate stubborn fat deposits or enhance their overall appearance, choosing a reputable and experienced liposuction clinic in Delhi is paramount to achieving safe, satisfying, and long-lasting results.
#drhiranmayijha#eternavisionandaesthetics#liposuctionsurgeryindelhi#liposuction#consultnow#youtube#liposuction cost in delhi#liposuctiontreatment#liposuctionprocedure
0 notes
Text
Streamlining Medical Device Safety: CDSCO’s New Online PSUR Guidelines in India
Introduction: CDSCO has revolutionized safety reporting for medical devices and in-vitro diagnostic devices in India. As of March 19, 2024, all manufacturers must submit safety reports online through the Sugam portal. This blog explores the significance of these reports, their components, and the streamlined submission process.
What is Periodic Safety Update Reports (PSURs)?
Periodic Safety Update Reports (PSURs) are vital for investigational medical devices, demonstrating post-sale performance. Integral to Post-Market Surveillance (PMS), these reports monitor device safety and efficacy.
Key Components of PSURs:
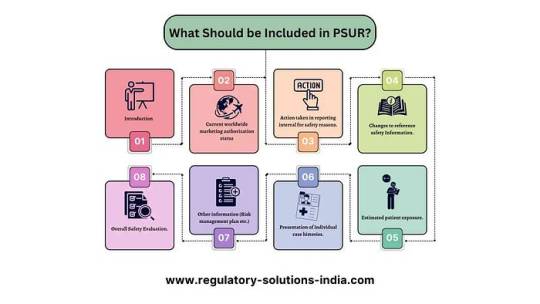
PSUR Submission Requirements:
Manufacturers and importers of investigational medical devices are responsible for timely and accurate PSUR submissions. Reports are due biannually for the first two years, annually thereafter, or as needed based on health requirements. All submissions must now be made through the Sugam portal.
Conclusion:
PSURs are more than paperwork; they are essential for post-sale medical device safety. By meticulously analyzing safety data, companies and regulators ensure devices meet safety standards and perform effectively. This continuous monitoring fosters trust in medical technology.
Regulatory Solutions India (RSI): RSI specializes in regulatory consultancy for medical devices. Contact us for assistance in navigating PSUR submissions and ensuring compliance with evolving regulations.
What is a PSUR, and why is it important for medical device manufacturers?
A PSUR is a critical report demonstrating the post-sale performance of investigational medical devices. It ensures devices remain safe and effective for patient use.
2. What information should be included in a PSUR?
PSURs should detail device intended use, global authorization status, safety actions, updates, usage estimates, research findings, and overall safety reviews.
3. Who is responsible for submitting PSURs, and when should they be submitted?
Manufacturers and importers must submit PSURs. Reports are due biannually for the first two years, annually thereafter, or as needed based on health requirements.
4. How has the submission process for PSURs changed in India?
As of March 19, 2024, CDSCO mandates online PSUR submissions through the Sugam portal, streamlining the process and ensuring greater organization.
5. Why are PSURs considered crucial for maintaining trust in medical technology?
PSURs play a critical role in ensuring investigational medical devices remain safe post-sale. By monitoring safety data, companies uphold patient and healthcare professional trust in medical technology.
0 notes
Text
Fumigation Products Market: Global Industry Analysis and Forecast 2023 – 2030
Fumigation Products Market size is projected to reach USD 1,124.77 Million by 2028 from an estimated USD 839.92 Million in 2021, growing at a CAGR of 4.26% globally.
The fumigation method is generally utilized for the operation of pest control. The fumigation process includes the removal of pests by using the fumes of numerous chemicals. It removes pests such as bacteria, weeds, nematodes, fungi, and other hard-to-remove pests. Fumigation products are highly poisonous. They produce noxious gases when exposed to air and moisture. The chemicals used for fumigation such as carbon dioxide sulfuryl fluoride, aluminum phosphide, and magnesium phosphide. There are three types of fumigations which are solid, liquid, and gas. Additionally, the Fumigation products can remove pests at all stages of their lifecycles, from eggs to full-grown adults. It is usually done by devices such as ultra-low volume ULV foggers, aerosol disinfector, mini fogging machines, medical fogger machines, and others.
Get Full PDF Sample Copy of Report: (Including Full TOC, List of Tables & Figures, Chart) @
https://introspectivemarketresearch.com/request/15859
The latest research on the Fumigation Products market provides a comprehensive overview of the market for the years 2023 to 2030. It gives a comprehensive picture of the global Fumigation Products industry, considering all significant industry trends, market dynamics, competitive landscape, and market analysis tools such as Porter's five forces analysis, Industry Value chain analysis, and PESTEL analysis of the Fumigation Products market. Moreover, the report includes significant chapters such as Patent Analysis, Regulatory Framework, Technology Roadmap, BCG Matrix, Heat Map Analysis, Price Trend Analysis, and Investment Analysis which help to understand the market direction and movement in the current and upcoming years. The report is designed to help readers find information and make decisions that will help them grow their businesses. The study is written with a specific goal in mind: to give business insights and consultancy to help customers make smart business decisions and achieve long-term success in their particular market areas.
Leading players involved in the Fumigation Products Market include:
Rentokil Initial plc, Solvay S.A., Detia Degesch GmbH, Industrial Fumigant Company LLC, Royal Agro Organic Pvt. Ltd., UPI-USA, National Fumigants, Corteva Agriscience, JAFFER Group of Companies, AMVAC Chemical Corporation and other major players.
If You Have Any Query Fumigation Products Market Report, Visit:
https://introspectivemarketresearch.com/inquiry/15859
Segmentation of Fumigation Products Market:
By Type
Phosphine
Chloropicrin
Telone
Metam Sodium
DimethylDisulfide
1,3-Dichloropropene
Others
By Form
Solid Fumigation Products
Liquid Fumigation Products
Gas Fumigation Products
By Treatment Method
Magnesium Phosphide Fumigation Products
Aluminum Phosphide Fumigation Products
Sulfuryl Fluoride Fumigation Products
Carbon Dioxide (Co2) Fumigation Products
Others
By End User
Residential Fumigation Products
Agricultural Fumigation Products
Warehouses/ Storage Fumigation Products
Others
By Regions: -
North America (US, Canada, Mexico)
Eastern Europe (Bulgaria, The Czech Republic, Hungary, Poland, Romania, Rest of Eastern Europe)
Western Europe (Germany, UK, France, Netherlands, Italy, Russia, Spain, Rest of Western Europe)
Asia Pacific (China, India, Japan, South Korea, Malaysia, Thailand, Vietnam, The Philippines, Australia, New Zealand, Rest of APAC)
Middle East & Africa (Turkey, Bahrain, Kuwait, Saudi Arabia, Qatar, UAE, Israel, South Africa)
South America (Brazil, Argentina, Rest of SA)
What to Expect in Our Report?
(1) A complete section of the Fumigation Products market report is dedicated for market dynamics, which include influence factors, market drivers, challenges, opportunities, and trends.
(2) Another broad section of the research study is reserved for regional analysis of the Fumigation Products market where important regions and countries are assessed for their growth potential, consumption, market share, and other vital factors indicating their market growth.
(3) Players can use the competitive analysis provided in the report to build new strategies or fine-tune their existing ones to rise above market challenges and increase their share of the Fumigation Products market.
(4) The report also discusses competitive situation and trends and sheds light on company expansions and merger and acquisition taking place in the Fumigation Products market. Moreover, it brings to light the market concentration rate and market shares of top three and five players.
(5) Readers are provided with findings and conclusion of the research study provided in the Fumigation Products Market report.
Our study encompasses major growth determinants and drivers, along with extensive segmentation areas. Through in-depth analysis of supply and sales channels, including upstream and downstream fundamentals, we present a complete market ecosystem.
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
Acquire This Reports: -
https://introspectivemarketresearch.com/checkout/?user=1&_sid=15859
About us:
Introspective Market Research (introspectivemarketresearch.com) is a visionary research consulting firm dedicated to assisting our clients to grow and have a successful impact on the market. Our team at IMR is ready to assist our clients to flourish their business by offering strategies to gain success and monopoly in their respective fields. We are a global market research company, that specializes in using big data and advanced analytics to show the bigger picture of the market trends. We help our clients to think differently and build better tomorrow for all of us. We are a technology-driven research company, we analyse extremely large sets of data to discover deeper insights and provide conclusive consulting. We not only provide intelligence solutions, but we help our clients in how they can achieve their goals.
Contact us:
Introspective Market Research
3001 S King Drive,
Chicago, Illinois
60616 USA
Ph no: +1-773-382-1047
Email: [email protected]
#Fumigation Products#Fumigation Products Market#Fumigation Products Market Size#Fumigation Products Market Share#Fumigation Products Market Growth#Fumigation Products Market Trend#Fumigation Products Market segment#Fumigation Products Market Opportunity#Fumigation Products Market Analysis 2023
0 notes
Text
Medical Device Registration process in India

For medical devices manufactured in India, obtaining a CDSCO manufacturing license is compulsory. This registration ensures compliance with regulatory standards, verifying the safety and quality of the device for use in healthcare. Get in touch with PSR Compliance, a top CDSCO consultant in India.
#Medical Device Manufacturing License#Medical device consulting#Medical Device Registration#Medical Device Registration in India#CDSCO Import License#CDSCO Consultant#CDSCO Certificate#CDSCO Registration#cdsco sugam portal
0 notes
Text
Medical Clothing Market: Global Industry Analysis and Forecast 2023 – 2030
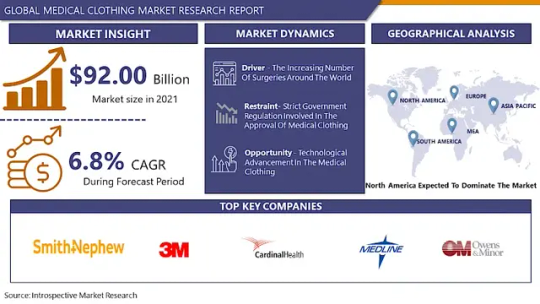
The Global Market for Medical Clothing Estimated at USD 98.35 Billion In The Year 2022, Is Projected To Reach A Revised Size Of USD 167.72 Billion By 2030, Growing At A CAGR Of 6.9 % Over The Forecast Period 2023-2030.
The medical clothing market encompasses a wide range of garments designed specifically for healthcare professionals and patients alike. These garments serve various purposes, including infection control, protection against hazardous substances, and maintaining hygiene standards in medical settings. With advancements in materials and technology, medical clothing has evolved to offer improved comfort, functionality, and performance.
The global medical clothing market has witnessed steady growth in recent years, driven by increasing awareness regarding infection control measures, growing concerns about healthcare-associated infections (HAIs), and stringent regulations mandating the use of protective clothing in healthcare facilities. Additionally, the COVID-19 pandemic has further underscored the importance of personal protective equipment (PPE), including medical clothing, in preventing the spread of infectious diseases.
Get Full PDF Sample Copy of Report: (Including Full TOC, List of Tables & Figures, Chart) @
https://introspectivemarketresearch.com/request/16612
The latest research on the Medical Clothing market provides a comprehensive overview of the market for the years 2023 to 2030. It gives a comprehensive picture of the global Medical Clothing industry, considering all significant industry trends, market dynamics, competitive landscape, and market analysis tools such as Porter's five forces analysis, Industry Value chain analysis, and PESTEL analysis of the Medical Clothing market. Moreover, the report includes significant chapters such as Patent Analysis, Regulatory Framework, Technology Roadmap, BCG Matrix, Heat Map Analysis, Price Trend Analysis, and Investment Analysis which help to understand the market direction and movement in the current and upcoming years. The report is designed to help readers find information and make decisions that will help them grow their businesses. The study is written with a specific goal in mind: to give business insights and consultancy to help customers make smart business decisions and achieve long-term success in their particular market areas.
Leading players involved in the Medical Clothing Market include:
3M (US), Smith+Nephew (UK), LynkTrac Technologies LLC (US), Cardinal Health (US), Superior Group of Companies (US), Mölnlycke Health Care AB (Sweden), Ansell Ltd. (Australia), Medline Industries, Inc. (US), Plasti Surge Industries Pvt (India), Owens & Minor, Inc. (US), Healing Hands (New Jersey), Tronex International Inc. (US), Henry Schein, Inc. (US), Carhartt, Inc. (US), Landau Uniforms (US), and Other Major Players
Market Driver:
One significant driver of the medical clothing market is the rising emphasis on infection control measures. Healthcare facilities worldwide are increasingly prioritizing infection prevention and control to minimize the risk of HAIs and ensure patient and staff safety. As a result, there is a growing demand for medical clothing equipped with features such as antimicrobial properties, fluid resistance, and barrier protection. Manufacturers are innovating to develop advanced textiles and garment designs that offer enhanced protection without compromising comfort or breathability.
Market Opportunity:
An emerging market opportunity lies in the integration of smart technologies into medical clothing. With the proliferation of wearable sensors, IoT devices, and electronic textiles, there is immense potential to develop smart medical garments capable of monitoring vital signs, detecting pathogens, and providing real-time health data to healthcare providers. Smart clothing could revolutionize patient monitoring, enabling early detection of health issues and improving overall healthcare outcomes. Furthermore, the adoption of telemedicine and remote patient monitoring platforms presents a significant opportunity for the development of medical clothing with integrated connectivity features, facilitating seamless data transmission and remote healthcare delivery.
If You Have Any Query Medical Clothing Market Report, Visit:
https://introspectivemarketresearch.com/inquiry/16612
Segmentation of Medical Clothing Market:
By Products
Gowns
Coveralls
Other
By Usage
Reusable
Disposable
By Distribution channel
Online
Offline
By End Users
Hospitals
Specialty Clinics
Ambulatory Centres
Home Care Settings
Others
By Regions: -
North America (US, Canada, Mexico)
Eastern Europe (Bulgaria, The Czech Republic, Hungary, Poland, Romania, Rest of Eastern Europe)
Western Europe (Germany, UK, France, Netherlands, Italy, Russia, Spain, Rest of Western Europe)
Asia Pacific (China, India, Japan, South Korea, Malaysia, Thailand, Vietnam, The Philippines, Australia, New Zealand, Rest of APAC)
Middle East & Africa (Turkey, Bahrain, Kuwait, Saudi Arabia, Qatar, UAE, Israel, South Africa)
South America (Brazil, Argentina, Rest of SA)
What to Expect in Our Report?
(1) A complete section of the Medical Clothing market report is dedicated for market dynamics, which include influence factors, market drivers, challenges, opportunities, and trends.
(2) Another broad section of the research study is reserved for regional analysis of the Medical Clothing market where important regions and countries are assessed for their growth potential, consumption, market share, and other vital factors indicating their market growth.
(3) Players can use the competitive analysis provided in the report to build new strategies or fine-tune their existing ones to rise above market challenges and increase their share of the Medical Clothing market.
(4) The report also discusses competitive situation and trends and sheds light on company expansions and merger and acquisition taking place in the Medical Clothing market. Moreover, it brings to light the market concentration rate and market shares of top three and five players.
(5) Readers are provided with findings and conclusion of the research study provided in the Medical Clothing Market report.
Our study encompasses major growth determinants and drivers, along with extensive segmentation areas. Through in-depth analysis of supply and sales channels, including upstream and downstream fundamentals, we present a complete market ecosystem.
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
Acquire This Reports: -
https://introspectivemarketresearch.com/checkout/?user=1&_sid=16612
About us:
Introspective Market Research (introspectivemarketresearch.com) is a visionary research consulting firm dedicated to assisting our clients to grow and have a successful impact on the market. Our team at IMR is ready to assist our clients to flourish their business by offering strategies to gain success and monopoly in their respective fields. We are a global market research company, that specializes in using big data and advanced analytics to show the bigger picture of the market trends. We help our clients to think differently and build better tomorrow for all of us. We are a technology-driven research company, we analyse extremely large sets of data to discover deeper insights and provide conclusive consulting. We not only provide intelligence solutions, but we help our clients in how they can achieve their goals.
Contact us:
Introspective Market Research
3001 S King Drive,
Chicago, Illinois
60616 USA
Ph no: +1-773-382-1047
Email: [email protected]
#Medical Clothing#Medical Clothing Market#Medical Clothing Market Size#Medical Clothing Market Share#Medical Clothing Market Growth#Medical Clothing Market Trend#Medical Clothing Market segment#Medical Clothing Market Opportunity#Medical Clothing Market Analysis 2023
0 notes
Text
The Importance of ISO 13485 Certification in the Medical Device Industry
/ Uncategorized / By Factocert Mysore

Introduction: ISO 13485 Certification in India
ISO 13485 Certification in India is a crucial component of the jigsaw. This certification guarantees that producers follow stringent quality management systems in a sector where accuracy and safety are essential requirements. Join us as we explore the significance of ISO 13485 certification in India and learn how it plays a vital role in saving lives every day, whether you are already familiar with it or are just beginning your path in the medical device sector. So buckle up, and let’s go on this enlightening journey together!
Understanding ISO 13485 certification in India and its Significance
It is impossible to overstate the significance of ISO certification for the medical device sector. You must uphold stringent quality standards to guarantee your gadgets are reliable and secure. The ISO 13485 certification in India standard is among the most significant ones for medical equipment.
The worldwide ISO 13485 certification in India standard was created exclusively for medical equipment. It outlines the specifications for a thorough quality management system, including design and development, manufacturing, storage and distribution, customer support, and post-market monitoring.
Implementing an ISO 13485-based quality management system has numerous advantages. It helps ensure your devices comply with all relevant regulatory requirements. Additionally, it can enhance cooperation and communication within your business and with clients and suppliers. By using ISO 13485, you can save on costs related to rework or scrap while simultaneously increasing product quality and consistency.
Although ISO 13485 certification in India accreditation has several advantages, the most significant is its promotion of patient safety. Establishing a quality management system based on this standard commits you to creating safe and reliable medical devices. The fact that it offers producers a competitive edge in the market is arguably the most significant advantage. ISO 13485:2016 medical devices demonstrate to potential customers that a manufacturer is committed to quality and safety. It aids in improving a manufacturer’s internal processes and procedures.
The ISO 13485 certification in India may help medical device manufacturers streamline their processes, provide better customer service, and grow their clientele.
ISO 13485 certification in India Requirement
Although implementing ISO 13485 certification in India may seem complicated or intimidating, it helps eliminate some of the random regulations and methods in the medical device industry.
A quality management system (QMS) must be designed and kept up to date and contain documentation, internal audits, and remedial measures.
Risk management system: Teams must also establish a risk management plan to detect and assess any hazards associated with the medical device throughout its lifespan.
Verification of compliance: According to ISO 13485, businesses must ensure that their products meet customer and legal requirements. This involves upholding traceability and record-keeping mechanisms to ensure that items are recognized and tracked along the supply chain.
Organizations should set up and maintain a system for controlling non-conforming items to identify and deal with issues immediately.
The medical device sector could start to experience some harmonization and uniformity of systems and procedures as ISO 13485 certification in India is increasingly adopted globally by businesses and governmental organizations. Due to this standardization, the industry will become more organized, and significant inventions will have a more accessible and quicker path to market.
Why Factocert for ISO 13485 Certification in India
We provide the best ISO consultants Who are knowledgeable and provide the best solution. And to know how to get ISO certification. Kindly reach us at [email protected]. work according to ISO standards and help organizations implement ISO certification in India with proper documentation.
For more information, visit ISO 13485 Certification in India.
RELATED LINKS
ISO Certification in India
ISO 9001 Certification in India
ISO 14001 Certification in India
ISO 45001 Certification in India
ISO 27001 Certification in India
ISO 22000 Certification in India
HALAL Certification in India
ISO 13485 Certification in India
RELATED ARTICLE
ISO CONSULTANT IN INDIA
0 notes
Text
Exploring the Dynamic Landscape of Clinical Research Companies in India
Introduction: Clinical Research Companies in India plays a pivotal role in advancing medical science, ensuring the safety and efficacy of healthcare products, and improving patient outcomes. India has emerged as a significant player in the global clinical research landscape, offering a diverse range of services across various therapeutic areas. In this article, we delve into the thriving ecosystem of clinical research companies in India, with a focus on Bangalore, a prominent hub in the country's research and development sector.
Clinical Research Companies in India: India boasts a robust network of clinical research organizations (CROs) catering to the pharmaceutical, biotechnology, medical device, cosmetic, and nutraceutical industries. These companies provide a wide array of services, including clinical trial management, regulatory affairs, data management, pharmacovigilance, and quality assurance. Some notable clinical research companies operating in India include QuintilesIMS, ICON, PPD, Syneos Health, and Parexel.
Clinical Research Companies in Bangalore: Clinical research companies in Bangalore, often referred to as the Silicon Valley of India, is home to numerous clinical research companies leveraging the city's vibrant ecosystem and skilled workforce. Companies like Biocon, Strides Pharma Science, and Anthem Biosciences are leading players in clinical research and drug development, conducting trials across therapeutic areas such as oncology, cardiology, neurology, and infectious diseases.
Medical Device Clinical Research Companies in India: The Medical device clinical research companies in India sector in India has witnessed rapid growth, prompting the emergence of specialized clinical research companies focused on medical device trials. These companies conduct feasibility studies, regulatory consulting, clinical trial management, and post-market surveillance for medical devices. Some prominent players in this domain include Siro Clinpharm, DiagnoSearch Life Sciences, and Axon Clinical Research.
Cosmetic Study Clinical Research Companies in India: The cosmetic industry in India is expanding, driven by changing consumer preferences and increasing awareness of skincare and beauty products. Cosmetic study clinical research companies in India companies specializing in cosmetic studies conduct safety assessments, efficacy trials, and consumer perception studies for skincare, haircare, and cosmetic products. Leading companies in this segment include Intertek, SGS India, and Eurofins Scientific.
Cosmetic Study Clinical Research Companies in Bangalore: Bangalore serves as a hub for cosmetic study Cosmetic study clinical research companies in Bangalore, offering state-of-the-art facilities and expertise in dermatology and cosmetic science. Companies like Vyome Therapeutics and Vyome Biosciences specialize in conducting clinical trials for skincare and dermatological products, contributing to the advancement of cosmetic science in India.
Nutraceutical Clinical Research Companies in India: With a growing emphasis on preventive healthcare and wellness, the nutraceutical industry has witnessed significant traction in India. Nutraceutical clinical research companies in India specializing in nutraceuticals conduct efficacy studies, safety assessments, and formulation development for dietary supplements, functional foods, and herbal products. Notable players in this domain include Nutriconnect, Arjuna Natural Pvt Ltd, and Bioneeds.
Nutraceutical Clinical Research Companies in Bangalore: Bangalore's thriving research ecosystem extends to Nutraceutical clinical research companies in Bangalore, which leverage the city's scientific expertise and infrastructure. Companies like Sristek Clinical Research Services and Auriga Research Pvt Ltd specialize in conducting clinical trials for nutraceutical products, driving innovation and evidence-based practice in the industry.
CROs in India: Clinical research organizations (CROs) play a vital role in facilitating clinical trials for pharmaceutical, biotechnology, and medical device companies. In India, CROs offer end-to-end services, from study design and protocol development to regulatory submission and post-marketing surveillance. Notable CRO’s in India operating in India include Siro Clinpharm, Lambda Therapeutic Research, Veeda Clinical Research, and GVK Biosciences.
CROs in Bangalore: Bangalore hosts several prominent CROs, providing comprehensive clinical research services to domestic and international clients. These CROs offer expertise in various therapeutic areas, advanced technologies, and a skilled workforce, making Bangalore an attractive destination for clinical trial outsourcing. Notable CRO’s in Bangalore include Ecron Acunova, Auriga Research Pvt Ltd, and Indegene.
Pharmacovigilance Companies in India: Pharmacovigilance companies in India play a crucial role in monitoring and assessing the safety of pharmaceutical products throughout their lifecycle. In India, pharmacovigilance companies offer services such as adverse event reporting, risk management, signal detection, and regulatory compliance. Leading pharmacovigilance companies in India include IQVIA, Novartis Pharmacovigilance, and Accenture.
Conclusion: India's clinical research sector continues to evolve, driven by technological advancements, regulatory reforms, and a skilled workforce. With a diverse range of services spanning pharmaceuticals, medical devices, cosmetics, and nutraceuticals, clinical research companies in India are poised for sustained growth and innovation. Bangalore, with its robust research ecosystem, emerges as a key destination for clinical research and development, further solidifying India's position as a global leader in healthcare innovation.
1 note
·
View note
Text
Home Healthcare Market Size to Reach Globally with Growing CAGR of 7.4% by 2032
The home healthcare market size was valued at USD 234.8 Billion in 2022 and is expected to reach USD 473.8 Billion by 2032 with a CAGR of 7.4%.
The home healthcare market has experienced significant growth in recent years, driven by factors such as an aging population, advancements in technology, and the increasing preference for in-home care. Home healthcare services encompass a wide range of medical and non-medical services provided to patients in their own homes, including nursing care, physical therapy, occupational therapy, and assistance with activities of daily living.
This industry’s focus on providing personalized and convenient care has made it an attractive option for individuals seeking alternatives to traditional hospital or nursing home settings. Additionally, the COVID-19 pandemic has further accelerated the adoption of home healthcare services as patients and healthcare providers alike seek to minimize exposure risks.
Recent developments in the home healthcare market include:
Telehealth Integration: The integration of telehealth services into home healthcare has become more prevalent, allowing patients to receive remote consultations, monitor vital signs, and communicate with healthcare providers from the comfort of their homes.
Technological Innovations: Advancements in wearable devices, remote monitoring technology, and digital health platforms have enhanced the quality of care delivered in the home setting while improving efficiency and reducing costs for both patients and providers.
Request Sample Report: https://datahorizzonresearch.com/request-sample-pdf/home-healthcare-market-2320
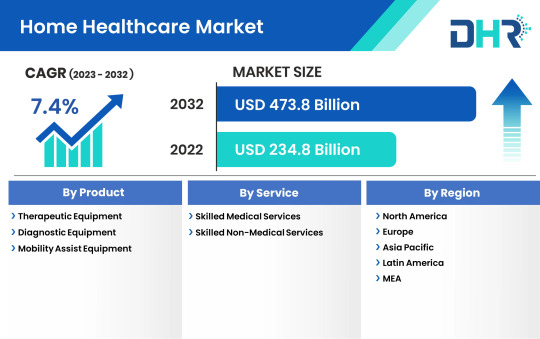
Expansion of Services: Home healthcare agencies are expanding their service offerings to meet the diverse needs of patients, including specialized care for chronic conditions, post-surgical recovery, palliative care, and mental health support.
Regulatory Changes: Regulatory changes and reimbursement policies have facilitated greater access to home healthcare services, leading to increased acceptance and utilization among patients and healthcare professionals.
Partnerships and Acquisitions: Strategic partnerships and acquisitions among home healthcare providers, technology companies, and healthcare systems have facilitated market expansion and enabled the delivery of comprehensive care solutions to patients at home.
Top Companies are:
· Amedisys Inc.
· Fresenius
· Amwell
· Roche
· Dickinson and Company
· ResMed
· Baxter International Inc.
· 3M
· B. Braun
· Maxim Healthcare Services
Market Segmentations:
By Product-
· Therapeutic Equipment
· Diagnostic Equipment
· Mobility Assist Equipment
By Service -
· Skilled Medical Service
· Skilled Non-Medical Services
Regional Analysis:
North America, particularly the United States, dominates the home healthcare market, boasting the largest market share. This prominence is primarily attributed to the increasing prevalence of chronic diseases and the aging demographic in the region. A significant portion of the American population expresses a strong preference for aging in the comfort of their own homes, surrounded by familial love and care, rather than in hospital settings. Consequently, the demand for home healthcare services has surged in the United States.
Meanwhile, the Asia-Pacific region is anticipated to witness the swiftest growth in the home healthcare market. This accelerated growth is fueled by the burgeoning elderly population across countries like China, Japan, and India. In these nations, there is a longstanding cultural tradition of individuals opting to remain in their homes post-retirement until the end of their lives.
Key highlights of the report include:
1. The report delivers thorough Market analysis, furnishing valuable insights to guide strategic decision-making.
2. The comprehensive research outlined in the study enhances the depth of your presentations and marketing strategies.
3. By offering crucial insights into key market competitors, the study empowers businesses with a strategic edge.
4. It delivers a precise assessment of evolving market dynamics, ensuring readers stay abreast of the latest industry trends.
5. With meticulous breakdowns of various market niches, the report facilitates informed decision-making processes.
0 notes
Text
Parexel Regulatory Affairs Associate Job Openings Bangalore
Parexel, a leading consulting firm playing a pivotal role in assisting biopharmaceutical and medical device companies in navigating the intricate regulatory landscape. With a commitment to collaboration, innovation, and continuous learning, Parexel is dedicated to bringing products to market faster and ensuring their sustained success.
Position Title: Regulatory Affairs Associate
Location: Bengaluru, India
Job Description
About the Role
As a Regulatory Affairs Associate at Parexel, you will contribute significantly to the success of biopharmaceutical and medical device companies by navigating the complex regulatory landscape. Leveraging your scientific, technical, and regulatory expertise, you will collaborate closely with clients to develop and implement regulatory strategies, facilitating the expedited market entry and longevity of their products.
[caption id="attachment_23555" align="aligncenter" width="1200"] Parexel Regulatory Affairs Associate Job Openings Bangalore[/caption]
Key Accountabilities:
Project Execution:
Works effectively within a team environment
Works within broad project guidelines as directed by the Project Lead or Technical SME
With the guidance of the project Technical SME, demonstrates the ability to prioritize work to achieve specified project outcomes
Capitalizes on opportunities to improve one’s own performance and seeks feedback from the Project Lead and colleagues
Applies information provided by the Project Lead or senior colleagues to complete assigned project activities
Produces quality work that meets the expectations of project lead and the client
May serve as a Project Lead for small scale projects or a Work Stream Lead on larger projects
When serving as a Project Lead with clear guidance and support from Line Manager
Responsible for project planning and set-up and routinely interacts with the assigned Project Specialist (PS) or Project
Manager (PM) to appropriately control the project (e.g. project set-up, forecasting and financial entries, invoicing, etc.)
Functions as the main client contact and ensures accurate project reporting is in place
Ensures that the project team delivers to meet the client expectations for quality and timeliness
Ensures that appropriate risk identification and issue-escalation procedures are in place
Ensures project specific training compliance of the project team
Ensures and/or manages project financials including provision of accurate revenue forecasts
Ensures that the project team understand and work to the scope of the contract
Identifies new opportunities through Change In Scope or add-on business from existing work
Ensures timely project close-out activities are completed
Consulting Activities and Relationship Management:
Follows and implements the organization’s consulting models and methodologies under the guidance of the project lead and/or Technical SME
Delivers a limited range of consulting services within personal area of expertise under the guidance of the Project Lead and/or Technical SME
Completes assigned activities within project scope and objectives under the direction of the Project Lead and/or Technical SME
Identifies project and internal issues to senior colleagues and Project Lead and/or Technical SME
Interacts professionally at all working levels within a client organization and within PAREXEL
Identifies project and/or client needs to the Project Lead and/or Technical SME
Interactions result in clients expressing satisfaction with service provided
Business Development:
Begin networking within the industry (i.e. maintain contacts and relationships with clients once engagements are complete)
Communicates potential new business lead to PC management and account managers
PAREXEL-related Activities:
Meets established metrics as specified in scorecard on an annual basis
Completes basic job-related responsibilities, including
timesheets, expense reports, maintenance of CVs, training compliance, project deliverable archiving, participation in internal initiatives/projects
Defines self-development activities with the support of management in order to keep current within the industry
Skills:
Project management knowledge
Client-focused approach to work
Results orientation
Teamwork and collaboration skills
Consulting skills
Excellent interpersonal and intercultural communication skills, both written and verbal
Critical thinking and problem-solving skills
Proficiency in local language and extensive working knowledge of the English language
Knowledge and Experience: Initial years of experience in an industry-related environment
Education: Minimum of a Bachelor’s Degree in a Scientific or Technical Discipline
APPLY ONLINE
0 notes
Text
Can you outline the process for obtaining CE Mark certification?
/ Uncategorized / By Factocert Mysore

Introduction: CE mark certification in Saudi Arabia
CE Mark Certification in Saudi Arabia In today’s global market, ensuring compliance with international standards and regulations is paramount for businesses aiming to expand their reach. Obtaining CE Mark certification in Saudi Arabia is essential for products destined for the European market.
This certification complies with European Union (EU) regulations, allowing products to be legally sold within the European Economic Area (EEA). This blog post will delve into the intricacies of obtaining CE Mark certification in Saudi Arabia, offering valuable insights for businesses navigating this process.
CE Mark Certification:
The CE Mark certification in Saudi Arabia, which stands for Conformité Européenne, signifies that a product meets the essential requirements of relevant EU directives. While Saudi Arabia is not a member of the EU, businesses exporting to Europe or seeking to align with international standards often pursue CE Mark Certification certification in Saudi Arabia to ensure market access and competitiveness. This certification applies to various products, including machinery, electronics, medical devices, etc.
Key Steps in Obtaining CE Mark Certification in Saudi Arabia:
Determine Applicability: The first step is to ascertain whether your product falls within the scope of CE Mark Certification certification in Saudi Arabia requirements. This involves identifying the relevant EU directives applicable to your product category.
Conduct Product Testing: Products seeking CE Mark Certification certification in Saudi Arabia must undergo rigorous testing to assess conformity with applicable EU standards. Testing may encompass various aspects such as safety, performance, and environmental impact. It’s essential to engage accredited testing laboratories capable of conducting tests according to EU regulations.
Compile Technical Documentation: Detailed technical documentation is crucial to the CE Mark Certification certification in Saudi Arabia process. This documentation should provide comprehensive information about the product’s design, manufacturing processes, test results, and compliance with relevant directives. Ensuring accuracy and completeness in documentation is vital to streamline the certification process.
Assess Conformity: Following product testing and documentation compilation, a conformity assessment must be conducted to verify compliance with EU requirements. Depending on the product category, this assessment may involve self-declaration of conformity or engagement with a notified body accredited by EU authorities.
Affix the CE Mark certification in Saudi Arabia: Upon completing the conformity assessment, it can be affixed to the product or packaging. This mark signifies that the product meets the essential health, safety, and environmental protection requirements for distribution within the EEA.
Navigating Regulatory Requirements in Saudi Arabia:
While CE Mark certification certification in Saudi Arabia originates from EU regulations, businesses operating in Saudi Arabia must also consider local regulatory requirements. Saudi Arabian authorities may impose additional rules or standards for imported goods, necessitating thorough compliance assessments. CE Mark certification in India
Engaging with Regulatory Experts:
Navigating the complexities of CE Mark certification in Saudi Arabia regulatory requirements can take time and effort for businesses.
Seeking guidance from regulatory experts or consultants with expertise in international compliance can facilitate a smoother certification process. These professionals can assist in interpreting regulations, preparing documentation, and liaising with relevant authorities.
Benefits of CE Mark Certification:
CE Mark certification in Saudi Arabia offers numerous benefits for businesses exporting to the European market. Some key advantages include:
Enhanced Market Access: CE Mark certification in Saudi Arabia grants access to the lucrative European market, expanding business opportunities and customer reach.
Competitive Advantage: Certified products comply with stringent EU standards, instilling consumer confidence and enhancing competitiveness.
Legal Compliance: CE Mark certification in Saudi Arabia ensures adherence to EU regulations, mitigating the risk of non-compliance penalties and market entry barriers.
Reputation and Trust: Certification signifies a commitment to quality and safety, fostering trust and credibility with stakeholders, including customers, partners, and regulatory authorities. CE Mark certification in Singapore.
Conclusion:
Obtaining CE Mark certification in Saudi Arabia requires careful planning, adherence to regulatory requirements, and comprehensive documentation. By understanding the key steps involved and leveraging the expertise of regulatory professionals, businesses can successfully achieve certification and unlock access to the European market.
CE Mark certification in Saudi Arabia not only facilitates market entry but also signifies a commitment to quality, safety, and regulatory compliance, bolstering the reputation and competitiveness of businesses on a global scale.
Why Factocert for CE Mark Certification in Saudi Arabia
We provide the best ISO consultants Who are knowledgeable and provide the best solution. And to know how to get ISO certification. Kindly reach us at [email protected]. work according to ISO standards and help organizations implement ISO certification in Saudi Arabia with proper documentation.
For more information, visit CE Mark Certification in Saudi Arabia
Related links:
· ISO certification in Saudi Arabia
· ISO 9001 certification in Saudi Arabia
· ISO 14001 certification in Saudi Arabia
· ISO 45001 certification in Saudi Arabia
· ISO 27001 certification in Saudi Arabia
· ISO 22000 certification in Saudi Arabia
Related Articles
ISO Consultant in Saudi Arabia
0 notes
Text
Top Career Opportunities After B Pharm in India

Pursuing a Bachelor of Pharmacy (B.Pharm) degree is a significant achievement that opens up various career paths in the healthcare and pharmaceutical industries. This degree provides a solid foundation in pharmaceutical science, preparing graduates for a range of roles in drug therapy management, patient care, and medication distribution, among others.
In this blog, we'll explore the diverse career opportunities that await B.Pharm graduates, focusing on those emerging from B.Pharm colleges in Mangalore, a city known for its educational excellence in this field.
Pharmacist
The most direct career path for B.Pharm graduates is becoming a pharmacist. Pharmacists are essential healthcare professionals who dispense medications prescribed by doctors, advise patients on how to use their medications safely, and provide immunizations. They work in various settings, including community pharmacies, hospitals, and clinics. In addition to dispensing medications, pharmacists can conduct health and wellness screenings and manage chronic diseases.
Pharmaceutical Industry Roles
Graduates can pursue various roles in the pharmaceutical industry, such as in research and development (R&D), quality control, and marketing. R&D roles involve developing new drugs and improving existing ones, requiring a blend of scientific knowledge and innovation. Quality control positions ensure that pharmaceutical products are safe and effective, adhering to strict regulations. Meanwhile, marketing roles involve promoting new drugs to healthcare professionals and the public, requiring strong communication skills and a deep understanding of the pharmaceutical market.
Clinical Research Associate
A clinical research associate (CRA) monitors the progress of clinical trials, working closely with doctors and researchers to ensure trials are conducted ethically and the data collected is accurate. CRAs are critical in bringing new drugs to market by ensuring clinical trials adhere to regulatory standards and protocols. This career requires attention to detail, strong organizational skills, and a commitment to patient safety and ethical standards.
Drug Inspector
Drug inspectors ensure that pharmaceutical products are manufactured, stored, and distributed according to regulatory standards. They inspect facilities, review manufacturing processes, and test samples to ensure health and safety regulations compliance. This role is vital for maintaining the integrity of the pharmaceutical supply chain and protecting public health.
Regulatory Affairs Specialist
Regulatory affairs specialists work at the intersection of science, law, and business, ensuring that pharmaceutical products comply with regulatory standards. They prepare and submit documentation required for regulatory approval of new drugs and oversee compliance with regulatory requirements throughout a product's lifecycle. This career path requires a deep understanding of regulatory guidelines, strong analytical skills, and effective communication abilities.
Sales and Marketing in the Pharmaceutical Industry
Sales and marketing professionals in the pharmaceutical industry play a crucial role in promoting drugs and medical devices to healthcare professionals and institutions. This requires an in-depth understanding of pharmaceutical products, excellent communication skills, and a strategic approach to sales. These roles often involve working closely with doctors, pharmacists, and hospital staff to inform them about the benefits and uses of various pharmaceutical products.
Entrepreneurship
With a solid foundation in pharmaceutical science, B.Pharm graduates can also venture into entrepreneurship by starting their pharmacies, pharmaceutical companies, or consultancy services. This path requires business acumen, strategic planning, and a deep understanding of the pharmaceutical market.
Conclusion
A B.Pharm degree opens the door to various career opportunities in the healthcare and pharmaceutical industries. Whether you're interested in patient care, research, regulatory affairs, or entrepreneurship, there's a path for you.
Graduates from B.Pharm colleges in Mangalore, in particular, are well-positioned to take advantage of these opportunities, thanks to the quality education and training they receive.
As the demand for healthcare professionals continues to grow, the future looks bright for B.Pharm graduates, offering both rewarding careers and the chance to impact public health significantly.
1 note
·
View note
Text
Medical Clothing Market: Global Industry Analysis and Forecast 2023 – 2030

The Global Market for Medical Clothing Estimated at USD 98.35 Billion In The Year 2022, Is Projected To Reach A Revised Size Of USD 167.72 Billion By 2030, Growing At A CAGR Of 6.9 % Over The Forecast Period 2023-2030.
The medical clothing market encompasses a wide range of garments designed specifically for healthcare professionals and patients alike. These garments serve various purposes, including infection control, protection against hazardous substances, and maintaining hygiene standards in medical settings. With advancements in materials and technology, medical clothing has evolved to offer improved comfort, functionality, and performance.
The global medical clothing market has witnessed steady growth in recent years, driven by increasing awareness regarding infection control measures, growing concerns about healthcare-associated infections (HAIs), and stringent regulations mandating the use of protective clothing in healthcare facilities. Additionally, the COVID-19 pandemic has further underscored the importance of personal protective equipment (PPE), including medical clothing, in preventing the spread of infectious diseases.
Get Full PDF Sample Copy of Report: (Including Full TOC, List of Tables & Figures, Chart) @
https://introspectivemarketresearch.com/request/16612
The latest research on the Medical Clothing market provides a comprehensive overview of the market for the years 2023 to 2030. It gives a comprehensive picture of the global Medical Clothing industry, considering all significant industry trends, market dynamics, competitive landscape, and market analysis tools such as Porter's five forces analysis, Industry Value chain analysis, and PESTEL analysis of the Medical Clothing market. Moreover, the report includes significant chapters such as Patent Analysis, Regulatory Framework, Technology Roadmap, BCG Matrix, Heat Map Analysis, Price Trend Analysis, and Investment Analysis which help to understand the market direction and movement in the current and upcoming years. The report is designed to help readers find information and make decisions that will help them grow their businesses. The study is written with a specific goal in mind: to give business insights and consultancy to help customers make smart business decisions and achieve long-term success in their particular market areas.
Leading players involved in the Medical Clothing Market include:
3M (US), Smith+Nephew (UK), LynkTrac Technologies LLC (US), Cardinal Health (US), Superior Group of Companies (US), Mölnlycke Health Care AB (Sweden), Ansell Ltd. (Australia), Medline Industries, Inc. (US), Plasti Surge Industries Pvt (India), Owens & Minor, Inc. (US), Healing Hands (New Jersey), Tronex International Inc. (US), Henry Schein, Inc. (US), Carhartt, Inc. (US), Landau Uniforms (US), and Other Major Players
Market Driver:
One significant driver of the medical clothing market is the rising emphasis on infection control measures. Healthcare facilities worldwide are increasingly prioritizing infection prevention and control to minimize the risk of HAIs and ensure patient and staff safety. As a result, there is a growing demand for medical clothing equipped with features such as antimicrobial properties, fluid resistance, and barrier protection. Manufacturers are innovating to develop advanced textiles and garment designs that offer enhanced protection without compromising comfort or breathability.
Market Opportunity:
An emerging market opportunity lies in the integration of smart technologies into medical clothing. With the proliferation of wearable sensors, IoT devices, and electronic textiles, there is immense potential to develop smart medical garments capable of monitoring vital signs, detecting pathogens, and providing real-time health data to healthcare providers. Smart clothing could revolutionize patient monitoring, enabling early detection of health issues and improving overall healthcare outcomes. Furthermore, the adoption of telemedicine and remote patient monitoring platforms presents a significant opportunity for the development of medical clothing with integrated connectivity features, facilitating seamless data transmission and remote healthcare delivery.
If You Have Any Query Medical Clothing Market Report, Visit:
https://introspectivemarketresearch.com/inquiry/16612
Segmentation of Medical Clothing Market:
By Products
Gowns
Coveralls
Other
By Usage
Reusable
Disposable
By Distribution channel
Online
Offline
By End Users
Hospitals
Specialty Clinics
Ambulatory Centres
Home Care Settings
Others
By Regions: -
North America (US, Canada, Mexico)
Eastern Europe (Bulgaria, The Czech Republic, Hungary, Poland, Romania, Rest of Eastern Europe)
Western Europe (Germany, UK, France, Netherlands, Italy, Russia, Spain, Rest of Western Europe)
Asia Pacific (China, India, Japan, South Korea, Malaysia, Thailand, Vietnam, The Philippines, Australia, New Zealand, Rest of APAC)
Middle East & Africa (Turkey, Bahrain, Kuwait, Saudi Arabia, Qatar, UAE, Israel, South Africa)
South America (Brazil, Argentina, Rest of SA)
What to Expect in Our Report?
(1) A complete section of the Medical Clothing market report is dedicated for market dynamics, which include influence factors, market drivers, challenges, opportunities, and trends.
(2) Another broad section of the research study is reserved for regional analysis of the Medical Clothing market where important regions and countries are assessed for their growth potential, consumption, market share, and other vital factors indicating their market growth.
(3) Players can use the competitive analysis provided in the report to build new strategies or fine-tune their existing ones to rise above market challenges and increase their share of the Medical Clothing market.
(4) The report also discusses competitive situation and trends and sheds light on company expansions and merger and acquisition taking place in the Medical Clothing market. Moreover, it brings to light the market concentration rate and market shares of top three and five players.
(5) Readers are provided with findings and conclusion of the research study provided in the Medical Clothing Market report.
Our study encompasses major growth determinants and drivers, along with extensive segmentation areas. Through in-depth analysis of supply and sales channels, including upstream and downstream fundamentals, we present a complete market ecosystem.
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
Acquire This Reports: -
https://introspectivemarketresearch.com/checkout/?user=1&_sid=16612
About us:
Introspective Market Research (introspectivemarketresearch.com) is a visionary research consulting firm dedicated to assisting our clients to grow and have a successful impact on the market. Our team at IMR is ready to assist our clients to flourish their business by offering strategies to gain success and monopoly in their respective fields. We are a global market research company, that specializes in using big data and advanced analytics to show the bigger picture of the market trends. We help our clients to think differently and build better tomorrow for all of us. We are a technology-driven research company, we analyse extremely large sets of data to discover deeper insights and provide conclusive consulting. We not only provide intelligence solutions, but we help our clients in how they can achieve their goals.
Contact us:
Introspective Market Research
3001 S King Drive,
Chicago, Illinois
60616 USA
Ph no: +1-773-382-1047
Email: [email protected]
#Medical Clothing#Medical Clothing Market#Medical Clothing Market Size#Medical Clothing Market Share#Medical Clothing Market Growth#Medical Clothing Market Trend#Medical Clothing Market segment#Medical Clothing Market Opportunity#Medical Clothing Market Analysis 2023
0 notes
Text
Review and Approval Process for FDA Certification in Pune

FDA Certification in Pune - is a crucial regulatory requirement for businesses operating in the food, drug, and medical device industries in Pune, India. The Food and Drug Administration (FDA) of India sets stringent standards and guidelines to ensure the safety, efficacy, and quality of products available in the market. Obtaining FDA certification signifies compliance with these regulations, instilling trust among consumers and stakeholders.
In Pune, a vibrant hub for various industries, FDA certification holds immense significance for manufacturers, distributors, and exporters. It not only assures adherence to national standards but also opens doors to national and international markets, facilitating trade and fostering growth.
Navigating the FDA certification process in Pune requires a comprehensive understanding of regulatory frameworks, documentation requirements, and quality management systems. From preparing meticulous documentation to implementing robust quality control measures, businesses must demonstrate a commitment to upholding the highest standards of safety and quality.
FDA Implementation in Pune
Understanding FDA Regulations: FDA Implementation in Turkey - Businesses need to familiarize themselves with the specific regulations and guidelines provided by the FDA of India pertaining to their industry and product category.
Documentation Preparation: Comprehensive documentation is essential, including product information, manufacturing processes, quality control measures, and safety data. Businesses must ensure that all documentation meets FDA requirements.
Quality Management System (QMS) Implementation: Establishing a robust Quality Management System is crucial to ensure that manufacturing processes comply with FDA standards. This includes implementing Good Manufacturing Practices (GMP) and other quality control measures.
Staff Training and Awareness: Employees need to be trained on FDA regulations and the importance of compliance. This includes training on hygiene practices, handling of raw materials, and adherence to standard operating procedures.
Overview of FDA Audit Process in Pune
Preparation: The company being audited should prepare well in advance by reviewing FDA regulations, gathering necessary documentation, and ensuring that all facilities and processes are in compliance.
Notification: FDA Audit in Botswana - The FDA or an authorized auditing body will typically notify the company of the audit in advance, specifying the date, scope, and objectives of the audit.
On-site Inspection: Auditors will conduct an on-site inspection of the company's facilities, including manufacturing plants, storage areas, laboratories, and offices. They will examine equipment, processes, and infrastructure to ensure compliance with FDA regulations.
Documentation Review: Auditors will review documentation related to product manufacturing, quality control, testing procedures, employee training, and more. This includes batch records, standard operating procedures (SOPs), quality manuals, and validation reports.
FDA Services in Pune
Regulatory Consulting: Consulting services provide guidance on navigating FDA regulations, interpreting guidelines, and ensuring compliance with applicable laws. Consultants can assist with regulatory strategy development, document preparation, and submission processes.
Documentation Assistance: FDA services in Pune include assistance with preparing and reviewing regulatory documentation required for product registration, approval, or certification. This may include product dossiers, technical files, labeling, and packaging materials.
Quality Management Systems (QMS) Implementation: Service providers offer support in establishing and maintaining robust QMS frameworks aligned with FDA requirements. This includes developing standard operating procedures (SOPs), conducting internal audits, and implementing corrective and preventive actions (CAPA).
Good Manufacturing Practices (GMP) Compliance: Consultants provide guidance on adhering to GMP standards to ensure the quality, safety, and efficacy of manufactured products. This includes assessing manufacturing facilities, processes, and personnel training programs.
How to get FDA consultant in Pune for my Business
If you're seeking for an FDA Certification consultant in Pune to ensure compliance with international standards and enhance business operations, B2Bcert Consultants can be a wonderful choice. There are several reasons to choose B2Bcert as your FDA Certification consultant in Pune, but the main one is their commitment to providing excellent services at fair costs. Corporate environments place a premium on budgets, and B2Bcert distinguishes itself by offering solutions at a reasonable cost without compromising the quality of its advisory services.
0 notes