#cdsco sugam portal
Text
Medical Device Registration process in India

For medical devices manufactured in India, obtaining a CDSCO manufacturing license is compulsory. This registration ensures compliance with regulatory standards, verifying the safety and quality of the device for use in healthcare. Get in touch with PSR Compliance, a top CDSCO consultant in India.
#Medical Device Manufacturing License#Medical device consulting#Medical Device Registration#Medical Device Registration in India#CDSCO Import License#CDSCO Consultant#CDSCO Certificate#CDSCO Registration#cdsco sugam portal
0 notes
Text
CDSCO Registration For Medical Device
#cdsco guidelines#medical device cdsco#cdsco online#cdsco md online#cdsco sugam portal#cdsco sugam login#sugam portal cdsco#cdsco registration#cdsco license#cdsco registration process
0 notes
Text
Streamlining Medical Device Safety: CDSCO’s New Online PSUR Guidelines in India
Introduction: CDSCO has revolutionized safety reporting for medical devices and in-vitro diagnostic devices in India. As of March 19, 2024, all manufacturers must submit safety reports online through the Sugam portal. This blog explores the significance of these reports, their components, and the streamlined submission process.
What is Periodic Safety Update Reports (PSURs)?
Periodic Safety Update Reports (PSURs) are vital for investigational medical devices, demonstrating post-sale performance. Integral to Post-Market Surveillance (PMS), these reports monitor device safety and efficacy.
Key Components of PSURs:
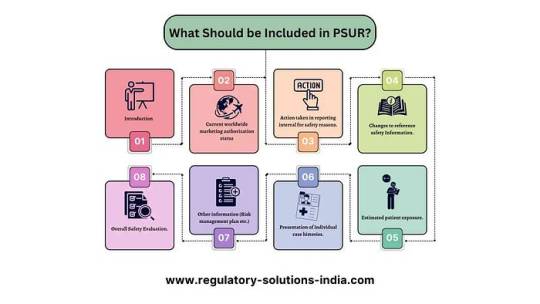
PSUR Submission Requirements:
Manufacturers and importers of investigational medical devices are responsible for timely and accurate PSUR submissions. Reports are due biannually for the first two years, annually thereafter, or as needed based on health requirements. All submissions must now be made through the Sugam portal.
Conclusion:
PSURs are more than paperwork; they are essential for post-sale medical device safety. By meticulously analyzing safety data, companies and regulators ensure devices meet safety standards and perform effectively. This continuous monitoring fosters trust in medical technology.
Regulatory Solutions India (RSI): RSI specializes in regulatory consultancy for medical devices. Contact us for assistance in navigating PSUR submissions and ensuring compliance with evolving regulations.
What is a PSUR, and why is it important for medical device manufacturers?
A PSUR is a critical report demonstrating the post-sale performance of investigational medical devices. It ensures devices remain safe and effective for patient use.
2. What information should be included in a PSUR?
PSURs should detail device intended use, global authorization status, safety actions, updates, usage estimates, research findings, and overall safety reviews.
3. Who is responsible for submitting PSURs, and when should they be submitted?
Manufacturers and importers must submit PSURs. Reports are due biannually for the first two years, annually thereafter, or as needed based on health requirements.
4. How has the submission process for PSURs changed in India?
As of March 19, 2024, CDSCO mandates online PSUR submissions through the Sugam portal, streamlining the process and ensuring greater organization.
5. Why are PSURs considered crucial for maintaining trust in medical technology?
PSURs play a critical role in ensuring investigational medical devices remain safe post-sale. By monitoring safety data, companies uphold patient and healthcare professional trust in medical technology.
0 notes
Text
Demystifying CDSCO Cosmetic Import Registration in India
The cosmetics industry in India has experienced remarkable growth in recent years, with consumers increasingly demanding a wide array of beauty and personal care products. In order to maintain safety standards and protect consumers, the Central Drugs Standard Control Organization (CDSCO) has implemented stringent regulations governing the import of cosmetics. This blog post aims to shed light on the CDSCO Cosmetic Import Registration process, offering insights into the steps and requirements for businesses looking to import cosmetics into India.
Understanding the CDSCO's Role
The CDSCO, operating under the Ministry of Health and Family Welfare in India, plays a pivotal role in regulating cosmetics, pharmaceuticals, and medical devices. Its primary mission is to safeguard public health by ensuring that imported cosmetics adhere to the prescribed standards of quality, safety, and efficacy.
The Importance of CDSCO Cosmetic Import Registration
CDSCO Cosmetic Import Registration is a crucial step for businesses seeking to import cosmetics into India. This registration serves several vital purposes:
Safety Assurance: CDSCO registration ensures that imported cosmetics do not contain harmful ingredients and comply with safety standards, thereby protecting consumers from potential health risks.
Quality Control: It enforces stringent quality control measures on imported cosmetics, ensuring that they meet the necessary standards for efficacy and labeling.
Consumer Trust: CDSCO registration helps build consumer trust by demonstrating a commitment to product safety and regulatory compliance.
The CDSCO Cosmetic Import Registration Process
The CDSCO Cosmetic Import Registration process involves several key steps:
Applicant Eligibility: Only entities registered in India, such as manufacturers, importers, or authorized agents, are eligible to apply for CDSCO Cosmetic Import Registration.
Formulation Approval: Before importing, the formulation of the cosmetic product must be approved by the Drug Controller General of India (DCGI). A comprehensive dossier containing details about the product's composition, manufacturing process, and safety data must be submitted.
Application Submission: Applicants must submit an application for cosmetic import registration through the SUGAM portal, along with the required documents and fees.
Review and Inspection: CDSCO reviews the application and may conduct inspections to ensure compliance with safety and quality standards. Any discrepancies or non-compliance issues are addressed during this phase.
Labeling Compliance: Imported cosmetics must adhere to specific labeling requirements, including ingredient lists, manufacturing dates, and usage instructions, as outlined in the Cosmetic Rules, 2020.
Grant of Registration: If the application meets all requirements and passes inspections, CDSCO grants cosmetic import registration, permitting the legal importation and sale of the product in India.
Renewal: Cosmetic import registration typically has a validity of three years and must be renewed before expiry to continue importing and selling the product.
Conclusion
The CDSCO Cosmetic Import Registration process in India is integral to ensuring the safety and quality of cosmetics available in the market. By adhering to regulatory guidelines and standards set by CDSCO, importers not only comply with the law but also build trust with consumers. For businesses looking to enter the Indian cosmetics market, navigating this process diligently is essential to ensure their products meet all the necessary requirements for import registration. In doing so, they contribute to the overall safety and satisfaction of consumers in India's thriving cosmetics industry.
0 notes
Text
Navigate the CDSCO registration process in India effortlessly with ASC Group. By signing up on the Sugam CDSCO portal and submitting an online application with specified registration purposes and essential details, we handle all the necessary documentation and liaise with authorities on your behalf. Count on us to expedite your market entry, saving you precious time. With our extensive experience and proven track record in regulatory affairs, ASC Group ensures excellence and compliance in the competitive healthcare industry. For all your CDSCO registration needs, feel free to contact ASC Group, and benefit from our reliable services.
0 notes
Text
Clinical Trial Application in India
The evaluation of the safety and effectiveness of novel drugs or treatments is made possible through clinical trials, which are a crucial component of the drug development process. The regulatory body in charge of approving clinical trials in India is the Central Drugs Standard Control Organisation (CDSCO), which also ensures that the trials protect participants' safety and well-being by upholding the highest ethical and scientific standards.
The Sugam portal, an online mechanism for submitting and processing clinical trial applications in India, was launched by the CDSCO to streamline the clinical trial approval procedure. Applicants can submit their applications through the Sugam portal, check on the status of those applications, and get fast updates on that status.
The Sugam portal has improved the efficiency, rigor, and transparency of clinical trial applications by digitizing the entire procedure. Additionally, it has sped up the application process, making the CDSCO's review more efficient.
Applicants must register and get a digital signature certificate (DSC), a secure digital key used to sign and authenticate electronic documents, before they may submit an application through the Sugam site. The application can be filed using the Sugam site once a DSC has been received.
The application must include in-depth details about the substance or treatment being tested, the study's design, and any safety and efficacy data discovered through preclinical research.
The CDSCO reviews the application to make sure it satisfies the requirements for clinical trials in terms of ethics and science, while also making sure participant safety and well-being are preserved.
After reviewing the application, the CDSCO may ask the applicant for more information or clarification. The CDSCO will issue an approval letter, permitting the applicant to move forward with the clinical research, once all issues have been addressed.
The Sugam portal's installation has enhanced India's clinical trial approval procedure, making it more effective, transparent, and accessible. The portal has also given the CDSCO the ability to guarantee that clinical trials uphold the highest ethical and scientific standards while protecting the safety and well-being of participants.
In conclusion, the Sugam site has been extremely helpful in encouraging the development of secure medications and medical procedures in India. The platform has made applications more effective and simplified, enabling the CDSCO to assess them more effectively by digitizing the clinical trial application and approval process in India. In the end, the Sugam portal has enhanced the Indian clinical trial process' openness, accessibility, and participant safety.
0 notes
Text
CDSCO starts online adverse event filing
From the desk of CDSCO- 01 March 2021 India’s Central Drugs Standard Control Organization (CDSCO) is switching to online serious adverse event (SAE) report filing via the Sugam online portal and is expected to stop accepting physical copies from 14 March. However, companies can continue to submit physical follow-ups for events that are already in the system. CDSCO has created manual and video…
View On WordPress
#cdsco#clinical research#clinical trials#dr nisha nair#india#indian pharma industry#indian pharmacovigilance#medical writing#pacific biosafety group#pharmacovigilance#san francisco bay area#san jose
1 note
·
View note
Text
Online Application For Issuance Of Veterinary Services
CDSCO has stated that all state licensing authorities and manufacturers submit Form 41 and Form 10 for veterinary vaccines using the Sugam portal. This has been done to streamline the normal submission process.
The applicants looking for such certificates or licenses can now apply in the sugam portal according to the checklist present in the modules. After March 2021, offline submission of applications through hard copy is not available as per CDSCO guidelines.
Sugam is one of the e-governance systems for discharging different functions carried out by CDSCO according to the Drugs and Cosmetics Act 1940. The software system is one of the online web portals where all applicants can put applications for NOCs, registration certificates, approvals, and permissions.
It offers a valuable interface for all applicants to track applications, download all permission that CDSCO has issued, and give responses to queries. It also helps the CDSCO officials process the applications online and create permissions online, as well as MIS reports. It consists of all step-by-step guidance for applicants of the Sugam portal with screenshots of the workflow for varied application submissions.
Before the Sugam portal, applicants have to submit applications in hard copy to various divisions in CDSCO. The timelines were also more than the existing scenario. Similarly, in earlier times, the fees for applications were made in the bank, and then the copy of challan or receipt was submitted to the CDSCO office. But now, in the SUGAM online portal, this fee is calculated as per the applications, and the payment is made online on the same portal.
Because of the Sugam portal, India is now a country that can compete with the International Council for Harmonization of technical requirements for Pharmaceuticals for Human Use regions.
Clini Experts consists of a group of smart working professionals with skills and knowledge of regulatory writing. We cater end to end services for complete client satisfaction. We Provides the best services like Clinical Trial Registration Consultants India, Drug product registration in India etc
0 notes
Text
Drug Licence Registration Process
The applicants can apply through the SUGAM online portal with all mandatory documents as well as fees. Then CDSCO will do the processing of this application on high priority according to the requirements of the drug license registration process.
0 notes
Text
Cosmetic Product Registration
In a recent series of upgrades, CDSCO decided to shift the process of cosmetic product registration online. Obviously to keep pace with the digitization of most processes, the result was the inception of SUGAM portal.
#cosmetic product registration#cosmetic product registration online#cosmetic labeling requirements india#license for cosmetics#cosmetic registration
0 notes
Text
Purposes For Which CDSCO Registration Can be Obtained
The Central Drugs Standard Control Organization (CDSCO) is the National Regulatory Authority for Indian pharmaceuticals and medical devices under the Directorate General of Health Services, Ministry of Health and Family Welfare in India. It is the responsibility of CDSCO for approving new drugs and conduct of clinical trials in India under the Drugs and Cosmetics Act, 1940 (“Act”) and Drugs and Cosmetics Rules, 1945 (“Rules”). It also lays down standards for drugs and controls the quality of imported drugs and cosmetics in the country for bringing about uniformity in the enforcement of the can grant cdsco registration for different purposes. The different purposes for which an applicant can register under the CDSCO portal are:
Cosmetics Registration
Import or Manufacture of drugs
Export NOC (Zone)
Test License
Ethics Committee Registration
Formulation R&D Organization
Blood Bank Registration
Blood Product Registration
Dual Use NOC (Trader)
BA/BE Approved Sites
Sponsors (BA/BE and CT)
#cdsco guidelines#medical device cdsco#cdsco online#cdsco md online#cdsco sugam portal#cdsco sugam login#sugam portal cdsco#cdsco registration#cdsco license#cdsco registration process
0 notes
Text
CDSCO SUGAM Registration & online Login
SUGAM is a single window interface to access online services offered by CDSCO. This portal allows users to update and upload data on their website related to license of drugs & medical device manufacturing facilities in India. SUGAM portal is a single window interface. To apply for any form user, you need to register on the portal first. The comprehensive database of SUGAM portal includes details of manufacturer, manufacturing site etc. The applications which are submitted on SUGAM online portal are approved/rejected by CDSCO. For the registration process applicants need to submit necessary documents on an online portal.
SUGAM online portal allows us to avail ourselves of the following services:
Online submission
Review
Grant of license /permission.
Steps involved in CDSCO SUGAM Registration process:
Fill in the details given in the registration form and get the login credentials.
Open the link https://cdscoonline.gov.in/CDSCO/homepage and sign up.
After signing up provide the purpose of registration
Upload necessary documents.
After online registration, applicants need to submit hard copies to CDSCO for the further verification process.
Read More - CDSCO SUGAM Portal
#cdsco sugam login#cdsco online#sugam online applications#sugam portal registration#cdsco sugam#sugam cdsco#cdscoonline#cdsco sugam online
0 notes
Text
What is CDSCO SUGAM?
#cdsco sugam login#cdsco online#sugam online applications#sugam portal registration#cdsco sugam#sugam cdsco#cdscoonline#cdsco sugam online
0 notes
Text
CDSCO Import License for Medical Devices in India | Operon Strategist
#cdsco import license#medical device import license india#medical import license#import license for medical devices in india#sugam portal cdsco#cdsco guidelines#medical device cdsco#requirement for import export license in india
0 notes
Text
CDSCO Import License for Medical Devices in India
#cdsco import license#medical device import license india#medical import license#import license for medical devices in india#sugam portal cdsco#cdsco guidelines#medical device cdsco#requirement for import export license in india
0 notes
Text
Cdsco Import License For Medical Devices
All medical devices entering India must comply with the Indian Medical Device Regulation set forth by the Central Drugs Standard Control Organization (CDSCO). All manufacturers, importers, and distributors must register their medical devices and obtain an import license from CDSCO. The CDSCO Import License also relies on other factors outside of the device itself, including the manufacturer’s location, manufacturing standards, and the quality and safety standards of the factory. CDSCO Import License of medical devices is mandatory for any industry or an individual who plans to import medical devices in India. CDSCO Import license of medical devices is regulated in India. Any industry or individual with a license (wholesale and manufacturing license issued under central drug standard control organization (CDSCO), Drugs and cosmetics act, 1940 can import medical devices into India. A foreign manufacturer exporting to India must obtain an Import License through a registered importer dealing with the products. The import license granted under Drugs and Cosmetics Rules, 1945 reflects that the imported products conform to set standards through testing. The CDSCO also provides a Certificate of import license for all therapeutic goods such as pharmaceuticals for human use, biological, blood and blood components, Medical devices, diagnostic kits etc. The CDSCO is authorized to Approve, Prohibit, and Monitor Clinical Trials on Medical Devices in India. The regulation aims to balance the benefits and risks of medical devices and arrive at an evidence-based approach. CDSCO module to register and license Manufacturers, Wholesalers, Testing Laboratories and Other related applications like renewal, temporary permits, amendment etc. The Drugs and Cosmetics Act and Rules discharge all functions to specific categories of new drugs. The Licensing Authority shall grant the import licenses after considering the recommendations of the Import Licensing committee, which is held responsible for reviewing applications relating to import license and fixation of required norms.
We help you import medical devices from all over the world. Our services include:
Obtain the manufacturing and local commercial registration – CDSCO Import License and MSME License.
Obtain Drug Master File (DMF).
Obtain Certificate of Free Sale, GMP Certification for your plant, the appointment of a local agent and representative to represent you in India.
Prepare the product dossier for registration and renewals.
Label evaluation for compliance with Indian labelling requirements.
If you want to launch your product in India, you need to know importation procedures and requirements. To help you understand the process, we have created this guide on importing medical devices in India.
CDSCO Import of medical devices in India Requirements:
Form41 (Registration Certificate) is required as per requirements of the Drugs & Cosmetic Act.
Wholesale Drug License in Form 20B & 21B.
The firm must be registered with the CDSCO Sugam Online System.
Process for Obtaining CDSCO Import License:
For Obtaining an Import License in Form 10, an application in Form 8 and Form 9 is required. Since 1 April 2016, All Applications Should be made through the CDSCO’s Sugam Portal. For more information on the CDSCO’s – Portal for CDSCO Import License and Registration Certificate.
The Different purposes for which an applicant can register under the CDSCO portal are:
Cosmetics Registration.
Import or Manufacture of drugs.
Export NOC (Zone).
Test License.
Ethics Committee Registration.
Formulation R&D Organization.
Blood Bank Registration.
Blood Product Registration.
Dual Use NOC (Trader).
BA/BE Approved Sites.
Sponsors (BA/BE and CT).
Important license and forms for CDSCO:
Medical Device Registration Certification.
Import Authorization.
Import Test License.
Manufacturing Test License.
Approval for a clinical trial.
Market approval following a successful clinical trial.
Permission to produce following the completion of a satisfactory clinical trial.
Retail sale of a notified medical device.
Wholesale sale of a notified medical device.
Manufacturing license for a notified medical device.
License to lend (manufactured in facility owned by third party).
Document required CDSCO License:
Id proof.
Rent agreement/ownership proof.
Undertaking issued by a government authority.
Manufacturing license or wholesale License in case of import.
Copy of Approved CDSCO.
Form 40.
IEC code.
CDSCO registration with an export and Import license is mandatory for all medical products such as pharmaceuticals, including diabetic drug manufacturers, registered medical device distributors, wholesalers, and manufacturers.
You were getting a CDSCO import license for medical devices made easy. Apply now and get your license issued with the lowest price guarantee. Get the best solution for the CDSCO import license with a 100% on-time guarantee. Operon Strategist Assist you. they Are provide best medical device regulatory consultancy
#cdsco import license#medical device import license india#medical import license#import license for medical devices in india#sugam portal cdsco#cdsco guidelines#medical device cdsco#requirement for import export license in india
0 notes