Photo




i am actually proper made up with my chemistry homework for the christmas holidays; we had to make our own revision guide c:
411 notes
·
View notes
Text
Tests for Negative Ions
non-metals form negative ions (anions)
unknown substances can be detected using the following tests:
to test for carbonates, this is done by a adding dilute acid and if it fizzes and the gas turns lime water cloudy then carbonate ions are present. (acid + carbonate > salt + water + carbon dioxide)
to test for halides (chloride, bromide or iodide), add dilute nitric acid (HNO3) followed by silver nitrate solution (AgNO3)
chloride, Cl- gives a white precipitate of silver chloride
bromide, Br- gives a cream precipitate of silver bromide
iodide, I- gives a yellow precipitate of silver iodide

to test for a sulfate ion SO4 2-, add dilute HCl followed by barium chloride solution, BaCl2
a white precipitate of barium sulfate means the original compound was a sulfate
22 notes
·
View notes
Text
Tests for Positive Ions
ionic substances can be identified by testing for the separate ions present
compounds made from metals all have a metal ion (the ‘cation’) bonded with either a non-metal ion or compound ion (the ‘anion’).
metals always form positive ions and can be identified using flame tests and precipitate tests

you can test for different substances by dipping a clean wire loop into a sample of the compound, putting it in a flame and seeing what colour it goes
barium, Ba2+ gives a green flame (BaG)
lithium Li+ gives a crimson flame (LiC)
calcium, Ca2+ gives a red flame (CaR)
sodium, Na+ gives a yellow flame (NaY)
potassium, K+ gives a lilac flame (KiL)

sometimes there will be no obvious colour with a flame test, so instead a precipitate test can be done by adding sodium hydroxide solution and a precipitate will be formed
copper, Cu2+ forms a blue precipitate
iron (iii), Fe3+ forms a brown precipitate (iron three looks like wee - if you're dehydrated)
iron (ii), Fe2+ forms a green precipitate (iron two looks like duck poo)
magnesium, Mg2+ and aluminium Al3+ ions both give white precipitates however aluminium precipitate redissolves in excess NaOH (sodium hydroxide) to form a colourless solution - magnesium remains solid
#c3#gcse#gcsechemistry#quantative chemistry#further analysis#revision#exams#chemistry#positive ions#unit 3#gcserevision#science#further#triple science
12 notes
·
View notes
Text
Limestone Products

limestone (calcium carbonate) is a naturally occurring resource that provides essential building materials
it can be used for:
neutralising acidic lakes and soils
buildings and roads
making cement - by heating in a kiln with powdered clay
making mortar - by mixing cement, sand and water
making concrete - by mixing cement with sand and water and gravel
making glass - by heating with sand and sodium carbonate
advantages of limestone products:
limestone is widely available and cheaper than granite or marble
it's an easy rock to cut
concrete is a quick and easy way of constructing buildings
limestone products don't rot and can't be gnawed away by insects or rodents
they're fire resistant
concrete doesn't corrode
the quarrying and associated businesses provide jobs and bring money into the local economy
disadvantages of limestone products:
concrete buildings are unattractive
concrete has a low tensile strength and can crack
limestone quarrying permanently changes the landscape and destroys habitats of animals and birds
quarrying also creates a lot of noise and air pollution
quarrying and making products uses energy, likely to come from burning fossil fuels, which contributes to global warming
#gcsechemistry#chemistry#gcserevision#limestone#products#revision#c1#calcium carbonate#jobs#concrete#cement#science#core science#glass#global warming#rock
11 notes
·
View notes
Text
The Limestone Cycle

limestone (calcium carbonate) is a naturally occurring resource that provides essential building materials
the reaction cycle:
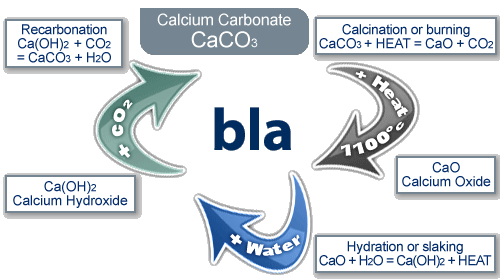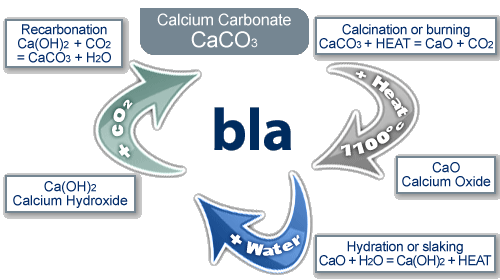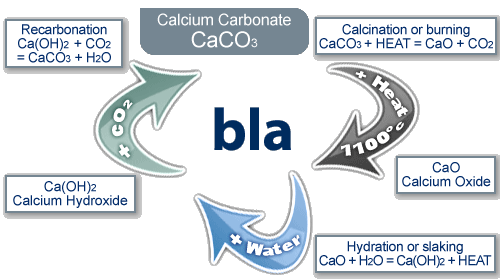
when limestone (calcium carbonate) is heated it thermally decomposes to make calcium oxide and carbon dioxide
calcium oxide reacts with water to produce calcium hydroxide
if more water is added to calcium hydroxide, calcium hydroxide solution is created, commonly know as lime water
limewater can be used as a test for carbon dioxide, because when calcium hydroxide reacts with CO₂, it turns back to calcium carbonate and gives off water. this can be seen as limewater going cloudy
#limestone#cycle#limestone cycle#gcsechemistry#revision#science#gcserevision#exams#calcium carbonate#c1#gcse#gcsescience#reactions
21 notes
·
View notes
Text
Balancing Equations
a chemical equation is balanced when then number of atoms of each type on each side of the equation is the same. so if there are 12 hydrogens on the left, there must be 12 hydrogens on the right side because of the law of conservation of mass - you can't make or destroy atoms during a chemical reaction.
how to balance an equation:
find out how many atoms of each type are on each side of the equation - it can help to use a table
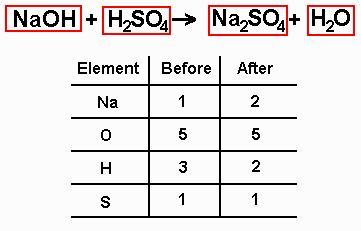
pick a starting element and multiply the number on the side which doesn't have enough atoms of that type by the number required to bring it up to the same as the other side, and put this number (the coefficient) at the start of the chemical formula concerned
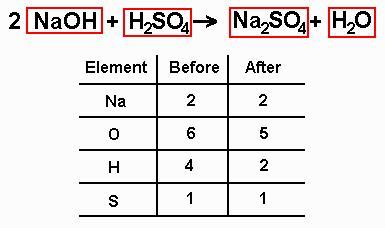
repeat until all the elements are balanced
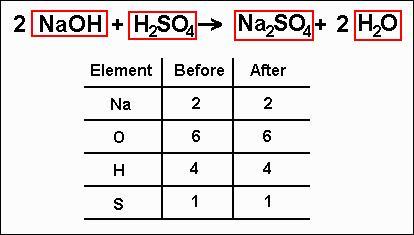
never change the subscripts of the atoms
it is usually easier to leave balancing oxygen and hydrogen to the last steps as these elements are often in more than one chemical on each side, so it's hard to know where to start
practice questions:
1. __NaCl + __BeF2 --> __NaF + __BeCl2
2. __FeCl3 + __Be3(PO4)2 --> __BeCl2 + __FePO4
3. __AgNO3 + __LiOH --> __AgOH + __LiNO3
4. __CH4 + __O2 --> __CO2 + __H2O
5. __Mg + __Mn2O3 --> __MgO + __Mn
1. 2 NaCl + 1 BeF2 --> 2 NaF + 1 BeCl2
2. 2 FeCl3 + 1 Be3(PO4)2 --> 3 BeCl2 + 2 FePO4
3. 1 AgNO3 + 1 LiOH --> 1 AgOH + 1 LiNO3
4. 1 CH4+ 2 O2 --> 1 CO2 + 2 H2O
5. 3 Mg + 1 Mn2O3 --> 3 MgO + 2 Mn
#gcsechemistry#balancing equations#chemistry#equations#gcse#revision#chemistry revision#c1#gcserevision#elements#atoms#science#core science
155 notes
·
View notes
Text
i think i lost an electron i’d better keep an ion that
485K notes
·
View notes
Text
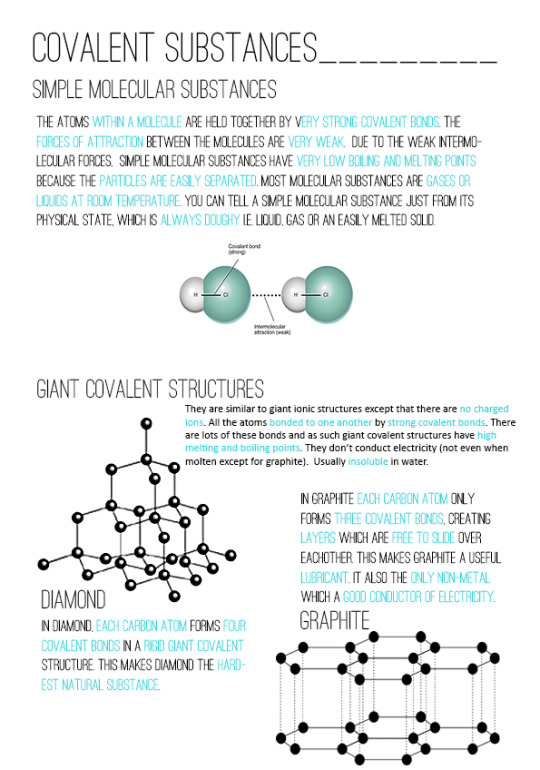
Covalent Bonding

non-metals can bond covalently
covalent bonds are shared pairs of electrons
this only occurs in their outer shells
having a full outer shell makes them stable
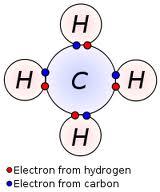
#covalent#bonding#covalent bonding#hydrocarbon#gcse#gcsechemistry#science#c1#c2#chemistry#revision#triple science
17 notes
·
View notes
Text
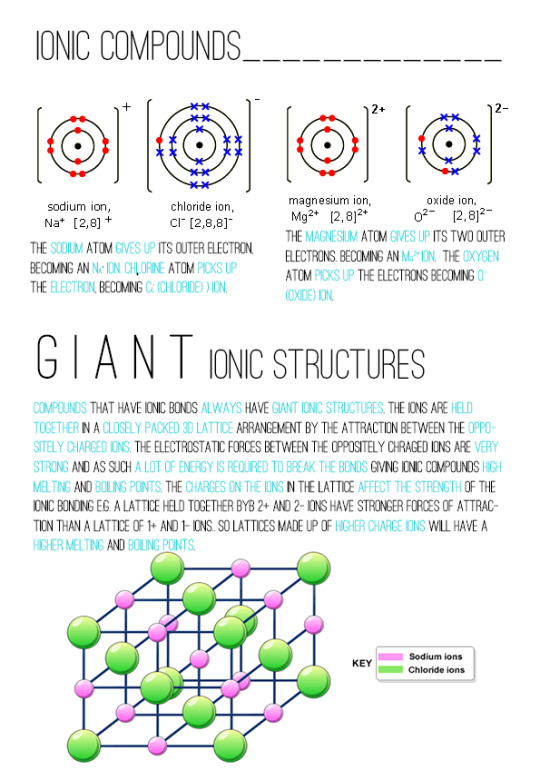
Ionic Compounds
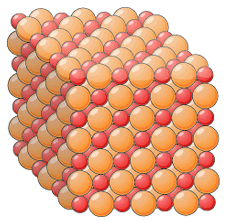
ionic compounds always form giant ionic lattices, packed in a regular arrangement
there are very strong electrostatic forces of attraction between oppositely charged ions in all directions
because of these strong attractions, it takes a lot of energy to overcome them, so ionic compounds have high melting and boiling points
when ionic compounds melt the ions are free to move and they'll carry an electric current
they also dissolve easily in water and the ions separate and free to move so carry electric currents
10 notes
·
View notes
Text
Ionic Bonding

ionic bonding is a way in which atoms chemically combine together to make compounds
a compound which is formed from a metal and a non-metal consists of ions
the metal atoms lose electrons to form positive ions and the non-metal atoms gain electrons to form negative ions
the opposite charges of each ion mean they are strongly attracted to each other
shells with only one electron are keen to get rid of their electron, whereas shells which are almost full are keen to gain electrons
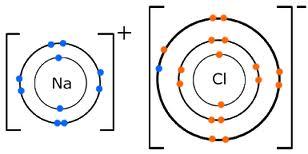
in sodium chloride the sodium atom gave an electron to the chlorine atom and now they are both ions with a charge of + or - 1
you can find out the charge formed on an ion by looking at the periodic table: the number of charges on an ion formed by a metal is equal to the group number - the number of charges on an ion formed by a non-metal is equal to the group number minus 8
11 notes
·
View notes
Text
Shells
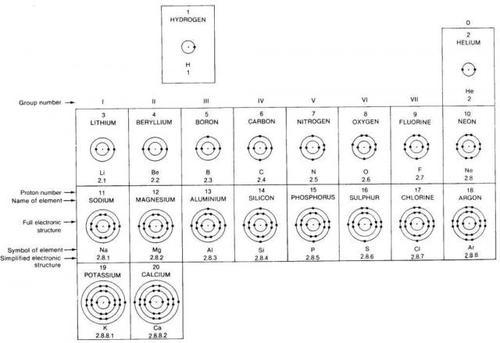
electrons always occupy shells (or energy levels) around the nucleus
shells closest to the nucleus are always filled first
the first shell can only contain two electrons
the next shells can contain a maximum of eight
electrons are more stable and less reactive when they have full outer shells
electrons without full outer shells want to react to fill it
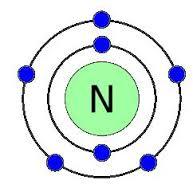
for example: nitrogen has 7 electrons, so it's electronic structure is 2,5

#shells#electrons#energy levels#chemistry#c1#revision#gcse#chemistryrevision#gcsechemistry#nitrogen#react#periodic table#elements#science#core science
10 notes
·
View notes
Text
Element Information
on the periodic table you can find this information for each different element:
element name
element symbol
atomic number
atomic mass (weight)

you can use this information to work out how many electrons, protons and neutrons an atom has and then draw an atomic diagram.
atomic number tells you the amount of protons in the nucleus
because atoms have no charge, this is also the number of electrons in the atom
atomic mass tells you the weight of the entire nucleus and from this you can work out how many neutrons are in the nucleus, but taking away the atomic number from the atomic mass
so carbon has 6 protons, therefore 6 electrons and 12-6=6 so it also has 6 neutrons

#carbon#chemistry#element#information#elements#periodic table#gcse#revision#c1#electrons#neutrons#protons#shells#gcsechemistry#gcserevision#chemistryrevision
9 notes
·
View notes
Text
The Periodic Table
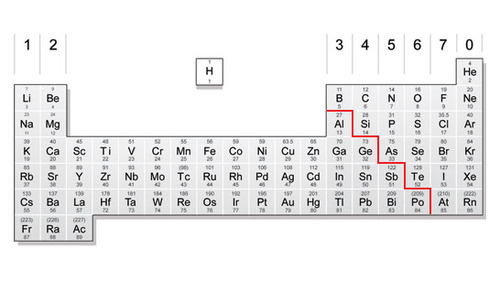
the periodic table is a logical organisation of elements
the vertical columns are called groups and elements in the same column have similar properties because they have the same amount of electrons on their outer shell
the horizontal rows are called periods and elements in each period have the same number of shells
for example:- Nitrogen is in group 5 and period 2 so it has 2 shells with 5 electrons on the outer shell
group 0 are the noble gases - these all have full outer shells so are stable and very unreactive
the red 'stepped' line separates the metals from the non metals
the middle block are transition metals
#gcse#gcsechemistry#c1#nitrogen#gcserevision#revision#science#chemistry#periodic table#elements#metals#non metals#school
19 notes
·
View notes
Text
Elements, Compounds and Mixtures
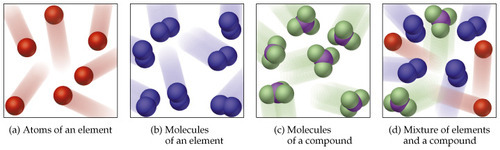
elements consist of one type of atom, e.g. Carbon (C) and Iron (Fe)
molecules are formed when two or more atoms join together with chemical bonds e.g. Oxygen (O2) because it goes around in pairs
compounds are molecules that contain at least two different elements e.g. Water (H20)
a mixture is a substance made by combining two or more different elements in such a way that no chemical reaction occurs. it is usually easily separated back into original elements using techniques like distillation and chromatography e.g. Hydrocarbons
#chemistry#gcse#revision#molecules#elements#compounds#mixtures#school#chemistryrevision#triple science#c1#core science
5 notes
·
View notes
Text
The Atom
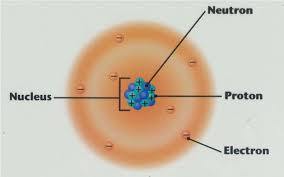
atoms are amazingly tiny
they're the building blocks of everything
atoms have a small nucleus surrounded by shells containing electrons
the nucleus is in the middle of the atom and contains protons and neutrons
protons are positively charged
neutrons are neutral (they have no charge)
the electrons move around the nucleus
electrons are negatively charged
overall, atoms have no charge because the number of protons equals the number of electrons
#c1#the atom#atoms#chemistry#gcse#revision#gcserevision#school#science#core science#nucleus#proton#neutron#electron#charge#unit 1
7 notes
·
View notes