#pharmacovigilance risk management jobs
Text
Pharmacovigilance Jobs: A Comprehensive Guide to the Best Pharmacovigilance Jobs in the Field
Pharmacovigilance Jobs: A Comprehensive Guide to the Best Pharmacovigilance Jobs in the Field
For those passionate about pharmacovigilance, this is a dream come true: a comprehensive guide to finding the best pharmacoivigilance jobs in the field! With so many options out there, it can be hard to know where to start – but don’t worry! This article provides helpful tips and tricks for jump-starting your career in pharmacovigilance. From industry info on job responsibilities and salary ranges to advice on setting yourself apart from other candidates, read on for an insider's look into breaking into this growing medical profession.
Pharmacovigilance Jobs: What are they?
Pharmacovigilance is the process of monitoring the side effects of medications. This includes both prescription and over-the-counter medications. Pharmacovigilance jobs are important because they help to ensure that the medications people take are safe. They also help to identify any potential risks associated with taking certain medications.
Pharmacovigilance jobs can be very important in protecting the public from the dangers of medication. They can also help to ensure that people have access to safe and effective medications. In many cases, pharmacovigilance jobs can help to prevent serious side effects from occurring.
The Career Path of a Pharmacovigilance Professional
The field of pharmacovigilance is a highly specialized and growing field within the pharmaceutical industry. The goal of pharmacovigilance professionals is to identify, assess and monitor any potential adverse drug effects (ADEs) that may occur in patients who are taking medication. ADEs can range from relatively minor issues such as nausea or headache, to more serious problems such as organ damage or death.
Pharmacovigilance professionals play a critical role in ensuring that the benefits of drug therapy outweigh any potential risks. They work with physicians, nurses and other healthcare professionals to identify any potential ADEs, and then work with regulatory agencies to ensure that the appropriate actions are taken to protect patients.
The career path of a pharmacovigilance professional can be a highly rewarding one. It can involve working in a variety of settings, including pharmaceutical companies, hospitals and regulatory agencies. There are many opportunities for advancement within the field, and pharmacovigilance professionals can expect to have a long and successful career in this rapidly growing field.
How to Land a Job in Pharmacovigilance
Pharmacovigilance is the monitoring of the effects of drugs after they have been released onto the market. This includes both the positive and negative effects of a drug, as well as any side effects that may occur. It is a critical part of ensuring that drugs are safe for public consumption, and it is also responsible for collecting data on drug use that can be used to improve future drug development.
So how does one go about getting a job in pharmacovigilance? The field is growing rapidly, so there are many opportunities available. But there are a few things that you need to know in order to give yourself the best chance of landing a job in this exciting and important industry.
First, pharmacovigilance is a research-based field. So if you want to work in pharmacovigilance, you need to be comfortable doing research and analyzing data. Pharmacovigilance jobs require strong analytical skills, so be sure to emphasize your quantitative skills in your resume and cover letters.
Second, pharmacovigilance jobs require excellent communication skills. As a pharmacovigilance professional, you will be working with doctors, patients, and other healthcare professionals. So it is important that you can communicate effectively with all types of people.
Third, pharmacovigilance jobs require an understanding of FDA regulations. The FDA is responsible for regulating the safety of drugs in the United States, and pharmacovigilance professionals must understand and comply with FDA regulations. If you don't have experience working with the FDA, be sure to highlight your experience complying with other regulatory agencies in your resume and cover letters.
Finally, pharmacovigilance jobs are often located in remote locations. So if you're not comfortable living in a rural area, you may want to consider another career path. However, if you are willing to relocate for a job opportunity in pharmacovigilance, there are many great opportunities available.
So those are some things to keep in mind if you're interested in working in pharmacovigilance. The field is growing rapidly, and it offers many opportunities for career growth. If you have the skills and experience that pharmaceutical companies are looking for, then be sure to emphasize those skills in your resume and cover letters. And don't forget to polish up your communication skills! Good luck!
The Skills You Need for a Career in Pharmacovigilance
Pharmacovigilance is the process of monitoring the effects of drugs on patients and reporting any adverse reactions. This is a critical role in ensuring that the public remains safe when taking medications. It is a fast-paced, ever-changing field that requires a range of skills.
The first skill you need for a career in pharmacovigilance is excellent attention to detail. You need to be able to accurately track and report any changes in a patient's condition. You must also be able to quickly identify any potential safety concerns.
In addition, pharmacovigilance professionals must have strong communication skills. They need to be able to effectively communicate with other health professionals, as well as with patients and their families. They must also be able to write clear and concise reports.
Finally, pharmacovigilance professionals must be able to work independently and be able to think critically. They must be able to analyze data and make sound decisions based on that data.
Continuing Education and Advancement Opportunities for Pharmacovigilance Professionals such as CCRPS Advanced Pharmacovigilance course
Pharmacovigilance (PV) is the science and activities related to the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problem. PV professionals play a critical role in ensuring that medications marketed to the public are safe.
The field of pharmacovigilance is constantly evolving, and PV professionals need to stay up-to-date on the latest advances in order to protect patients. One way to do this is by attending continuing education and advancement opportunities such as the CCRPS Advanced Pharmacovigilance course.
This course is designed for PV professionals who want to gain in-depth knowledge of the latest pharmacovigilance concepts and practices. It covers a variety of topics, including signal detection and investigation, product safety monitoring, and risk management.
Attendees will learn how to effectively detect and investigate potential safety issues with medications, as well as how to manage risks associated with their use. They will also have the opportunity to network with peers from around the world and discuss best practices in PV.
If you are a PV professional looking for an opportunity to deepen your knowledge and stay ahead of the curve, then the CCRPS Advanced Pharmacovigilance course is for you.
Pharmacovigilance is an important and specialized field within the pharmaceutical industry. Professionals in pharmacovigilance play a vital role in patient safety by monitoring the effects of medication and reporting any adverse reactions. If you’re interested in a career in pharmacovigilance, CCRPS can help you get started with our comprehensive certification program. Our course will provide you with the skills and knowledge you need to succeed in this exciting and challenging field. Enroll today and take the first step towards a rewarding career in pharmacovigilance!
#Pharmacovigilance Jobs Michigan#Pharmacovigilance Jobs#pharmacovigilance jobs#pharmacovigilance jobs remote#pharmacovigilance risk management jobs#pharmacovigilance entry level jobs#remote pharmacovigilance jobs#pharmacovigilance job salary#pharmacovigilance jobs salary#entry level pharmacovigilance jobs#pharmacovigilance job description#associate director pharmacovigilance jobs#indeed pharmacovigilance jobs#pharmacovigilance associate jobs#pharmacovigilance jobs boston#pharmacovigilance jobs entry level#pharmacovigilance jobs in usa#pharmacovigilance jobs in usa for indian#pharmacovigilance jobs nc#pharmacovigilance jobs near me#pharmacovigilance jobs nj#pharmacovigilance physician jobs#pharmacovigilance specialist jobs#remote pharmacovigilance jobs usa#accenture pharmacovigilance jobs#aggregate reporting in pharmacovigilance jobs#astrazeneca pharmacovigilance jobs#aurobindo pharmacovigilance jobs#bayer pharmacovigilance jobs#clinical research and pharmacovigilance jobs
0 notes
Text
"Unveiling the Future: How Data Science is Revolutionizing Upcoming Industries"
Data science continues to have a substantial impact on various industries, and its scope is expected to expand as new technologies emerge and businesses realize the potential of data-driven insights. Here are some upcoming industries where data science is likely to play a significant role:
Healthcare and Life Sciences: Data science can aid in personalized medicine, drug discovery, predictive analytics for patient outcomes, and healthcare operations optimization.
Financial Services: Financial institutions use data science for fraud detection, risk assessment, algorithmic trading, customer behavior analysis, and credit scoring.
Retail and E-Commerce: Data science helps optimize inventory management, pricing strategies, recommendation systems, and customer segmentation for targeted marketing.
Energy and Utilities: The energy sector benefits from data analytics for smart grid management, predictive maintenance of equipment, and energy consumption optimization.
Manufacturing: Data science improves manufacturing processes through predictive maintenance, quality control, supply chain optimization, and demand forecasting.
Agriculture: Precision agriculture utilizes data science to optimize crop yield, resource allocation, pest control, and environmental monitoring.
Transportation and Logistics: Data science plays a role in route optimization, fleet management, demand forecasting, and autonomous vehicles.
Telecommunications: Data science assists in customer churn prediction, network optimization, and personalized service offerings.
Media and Entertainment: Content recommendation, audience segmentation, and analyzing viewer engagement are areas where data science is making an impact.
Real Estate: Data science helps in property price prediction, market trend analysis, and investment decision-making.
Environmental Conservation: Data science aids in monitoring and analyzing environmental data, including climate patterns, pollution levels, and habitat preservation.
Education: Data science can personalize learning experiences, assess student performance, and optimize educational resources.
Government and Public Services: Data-driven decision-making is becoming increasingly important for optimizing public services, policy formulation, and resource allocation.
Insurance: Insurers use data science for risk assessment, claims processing, fraud detection, and customized pricing.
Travel and Tourism: Data science enhances traveler experiences through personalized recommendations, pricing optimization, and destination insights.
Pharmaceuticals: Data science plays a role in drug discovery, clinical trials optimization, and pharmacovigilance.
Smart Cities: The concept of smart cities involves integrating data science for efficient urban planning, traffic management, energy consumption, and public services.
Cybersecurity: Data science helps in identifying and responding to cyber threats by analyzing patterns and anomalies in network data.
As technology continues to advance and businesses recognize the value of data-driven insights, certybox is creating a difference in providing the top professional courses along with job assistance. It's essential for professionals in the field to stay updated with the latest developments and tools to make the most of these opportunities.

5 notes
·
View notes
Text
Navitas Lifesciences Fresher Pharmacovigilance job vacancies for B Pharmacy, M Pharmacy, Pharm D, or BDS candidates
Navitas Lifesciences Pvt Ltd is a leading pharmaceutical company dedicated to ensuring the safety and efficacy of healthcare products. As they expand our team, we are excited to announce an opportunity for Fresher Pharmacovigilance Associates to join us in Chennai/Bangalore. If you are a recent graduate with a degree in Pharmacy (B.Pharm/M.Pharm), Pharm.D, or BDS, this is your chance to embark on a rewarding career in pharmacovigilance with a company committed to excellence and innovation.
Job Title: Pharmacovigilance Associate
Company: Navitas Lifesciences Pvt Ltd
Location: Chennai/Bangalore
Job Description:
Navitas Lifesciences Pvt Ltd is seeking enthusiastic and dedicated individuals to join our team as Pharmacovigilance Associates. As a Pharmacovigilance Associate, you will play a crucial role in ensuring the safety and efficacy of pharmaceutical products. Your responsibilities will include:
Reviewing and evaluating adverse event reports
Conducting thorough investigations into adverse events
Documenting all findings accurately and in compliance with regulatory requirements
Communicating with healthcare professionals and patients regarding safety concerns
Collaborating with cross-functional teams to assess and manage risks associated with pharmaceutical products
Participating in the development and implementation of pharmacovigilance processes and procedures
Staying updated with industry regulations and guidelines related to pharmacovigilance
[caption id="attachment_68553" align="aligncenter" width="1200"] fresher pharmacovigilance vacancies - navitas lifesciences Chennai/Bangalore[/caption]
Requirements:
Bachelor's or Master's degree in Pharmacy (B.Pharm/M.Pharm), Pharm.D, or BDS
Strong attention to detail and analytical skills
Excellent communication and interpersonal skills
Ability to work effectively in a team environment
Familiarity with pharmacovigilance principles and regulations is a plus
Freshers are welcome to apply
If you are passionate about pharmacovigilance and are looking to start your career in a dynamic and supportive environment, we encourage you to share your updated CV with us at [email protected]. Join us at Navitas Lifesciences Pvt Ltd and contribute to the advancement of healthcare through safety and innovation.
0 notes
Text
The Crucial Job of Clinical Data Management and Drug Safety Specialists

In the mind boggling scene of drug innovative work, two basic support points stand tall. clinical data management and drug safety specialists. Together, they structure the foundation of guaranteeing the safety and adequacy of drugs brought to showcase. How about we dive into the meaning of these jobs and the important commitments they make to the medical services industry.
Clinical Data Management (CDM):
Clinical data management is the careful course of gathering, cleaning, and overseeing data got during clinical preliminaries. It includes a progression of methodical moves toward guarantee the uprightness, precision, and classification of the data accumulated from these preliminaries. These means incorporate data assortment, database plan, approval, and quality control measures.
Key Elements of Clinical Data Management:
Data Assortment: CDM specialists are answerable for planning case report structures (CRFs) and electronic data catch (EDC) frameworks to gather precise and applicable data from clinical preliminary members.
Data Cleaning: They fastidiously survey and clean the gathered data to distinguish and correct any disparities or mistakes, guaranteeing the data's quality and dependability.
Database Management: CDM specialists create and keep up with databases to safely store and sort out clinical preliminary data, complying to administrative rules and industry guidelines.
Quality Control: They execute thorough quality control measures to approve the exactness, culmination, and consistency of the data, relieving any likely dangers or inconsistencies.
Administrative Consistence: CDM specialists guarantee consistence with administrative necessities and rules set out by administrative specialists like the Food and Drug Organization (FDA) and the European Medications Office (EMA).
Drug Safety Specialists:
Drug safety specialist, otherwise called pharmacovigilance experts, assume a vital part in checking and evaluating the safety profile of drug items all through their lifecycle. Their essential goal is to recognize, evaluate, and forestall unfavorable drug responses (ADRs) to protect patient wellbeing.
Key Elements of Drug Safety Specialists:
Unfriendly Occasion Revealing: They are answerable for gathering, assessing, and reporting unfavorable occasions and other safety-related data related with the utilization of restorative items.
Risk Appraisal: Drug safety specialists lead careful assessments of antagonistic occasions to survey the possible dangers and advantages of drug items, directing dynamic cycles.
Signal Identification: They use data mining methods and factual investigations to recognize potential safety flags or arising chances related with explicit drugs or drug classes.
Risk Management: In view of their appraisals, they create and carry out risk minimization techniques and chance management intends to alleviate potential safety concerns and advance patient results.
Administrative Announcing: Drug safety specialists guarantee ideal and exact revealing of safety data to administrative specialists, medical services experts, and people in general according to administrative necessities.
End:
All in all, clinical data management and drug safety specialists are fundamental resources in the drug business' mission to foster protected and successful therapies for different ailments. Their careful scrupulousness, administrative ability, and devotion to patient safety are major to the outcome of clinical preliminaries and the post-advertising observation of drug items. By teaming up intimately with different partners, including scientists, medical services experts, and administrative organizations, these experts contribute essentially to propelling medical services and further developing patient results around the world.
0 notes
Text
A Comprehensive Guide to Demystifying Clinical Data Management
Introduction:
The foundation of contemporary clinical research is clinical data management (CDM), which guarantees the confidentiality, accuracy, and integrity of the information gathered during clinical trials. With the goal of elucidating the intricacies of CDM, this guide provides information on its significance, procedures, optimal methodologies, and professional prospects in the pharmaceutical and healthcare sectors.
Gain a comprehensive grasp of clinical data management (CDM) and its importance in clinical research, from data collection to database locking. Examine its importance in guaranteeing data quality, adhering to legal requirements, and producing trustworthy clinical evidence.
Clinical Data Management Best Practices:
Learn about tried-and-true CDM best practices, such as quality control measures, data management plans (DMPs), and standard operating procedures (SOPs). Discover how to put these strategies into effect to reduce mistakes, guarantee consistency, and expedite data processing.
Technological Developments in CDM:
Learn about the most recent developments in technology that are influencing the area of CDM, including risk-based monitoring (RBM) tools, clinical trial management systems (CTMS), and electronic data capture (EDC) systems. Recognize the ways in which these technologies improve compliance, efficiency, and data quality.
Important Elements of Clinical Data Management:
Examine the fundamental elements of CDM, such as data entry techniques, validation, cleansing, coding, and database architecture. Recognize the roles that each element plays in maintaining the overall accuracy and dependability of clinical trial data.
Standards and Regulatory Compliance:
Handle the complicated regulatory environment that surrounds clinical data management, including the International Conference on Harmonization (ICH) standards, Good Clinical Practice (GCP) recommendations, and data privacy laws like HIPAA and GDPR. Recognize how crucial compliance is to preserving participant anonymity and data integrity.
Explore a variety of job options in clinical data management (CDM), including positions as a quality assurance specialist, clinical database programmer, clinical data manager, and data coordinator. Learn about the abilities, credentials, and licenses needed to succeed in these positions.
Conclusion:
For clinical trial data to be of the highest quality, integrity, and compliance with regulations, clinical data management is essential. Professionals can enhance their careers in this dynamic sector of clinical research and contribute to the success of such undertakings by comprehending its procedures, best practices, and technological developments.
For more info:-
clinical research data management
clinical data management
pharmacovigilance certification
0 notes
Text
Unlocking a Career in Drug Safety: Embark on a Pharmacovigilance Course
In the intricate world of pharmaceuticals, where medications hold the power to heal and sustain, the significance of pharmacovigilance cannot be overstated. Pharmacovigilance, the vigilant monitoring of drug safety, stands as a cornerstone of patient well-being, safeguarding individuals from adverse drug reactions (ADRs). Embarking on a pharmacovigilance course offers a gateway to a fulfilling career in this dynamic and essential field.
Delving into the Realm of Pharmacovigilance
A pharmacovigilance course immerses individuals in the intricacies of drug safety, equipping them with the knowledge and skills to navigate the multifaceted aspects of this field. Through comprehensive coursework, participants gain insights into:
The fundamental principles of pharmacovigilance
The regulatory framework governing drug safety
Methods for detecting and assessing ADRs
Strategies for risk management and signal detection
Effective communication of pharmacovigilance findings
The Whiteboard Academy: A Beacon of Pharmacovigilance Education
The Whiteboard Academy distinguishes itself as a leading provider of pharmacovigilance courses, offering a range of programs tailored to diverse career aspirations. Their curriculum, meticulously designed by industry experts, ensures that participants gain a thorough understanding of pharmacovigilance principles and practices. The Whiteboard Academy's pharmacovigilance courses are renowned for:
In-depth coverage of pharmacovigilance concepts
Hands-on training in ADR detection and management
Exposure to real-world case studies and simulations
Guidance on navigating regulatory requirements
Untapping Career Opportunities in Pharmacovigilance
Completion of a pharmacovigilance course opens doors to a plethora of rewarding career opportunities in the healthcare and pharmaceutical sectors. With the growing emphasis on patient safety, pharmacovigilance professionals are increasingly sought after by:
Pharmaceutical companies
Clinical research organizations (CROs)
Regulatory agencies
Healthcare institutions
Investing in a Pharmacovigilance Course: A Catalyst for Personal and Professional Growth
Enrolling in a pharmacovigilance course represents an investment in personal and professional growth. By acquiring expertise in this critical field, individuals can:
Contribute to the improvement of patient safety
Gain a competitive edge in the job market
Pursue a career in a rapidly growing industry
Make a meaningful impact on public health
Conclusion
Venturing into the realm of pharmacovigilance through a comprehensive course empowers individuals to become champions of patient safety. The Whiteboard Academy's commitment to providing exceptional pharmacovigilance education equips participants with the knowledge, skills, and confidence to excel in this dynamic field. Embrace the opportunity to safeguard patient well-being and embark on a fulfilling career in pharmacovigilance.
0 notes
Text
Why Should You Consider a Career in Medical Coding?
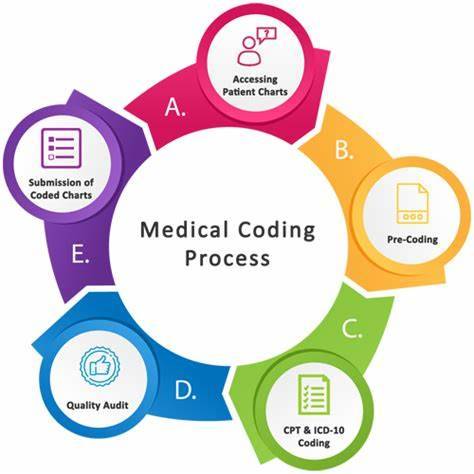
Introduction
Are you looking for a rewarding career in the healthcare industry that doesn't require years of medical school? If so, medical coding might be the perfect choice for you. In this blog, we'll explore the exciting world of medical coding, its importance in clinical research, and why you should seriously consider it as a career option. Let's dive in!
What is Medical Coding?
Medical coding is like the language of healthcare. It involves transforming medical information such as diagnoses, procedures, and treatments into universal codes. These codes are used for various purposes, including billing, insurance claims, and clinical research. Essentially, medical coders are responsible for ensuring that the healthcare system runs smoothly by accurately documenting patient records.
Why Choose a Career in Medical Coding?
1. In-Demand Career: The healthcare industry is constantly growing, and with it, the demand for skilled medical coders. Hospitals, clinics, insurance companies, and research institutions are always in need of qualified professionals to handle their coding needs.
2. Short Training Period: Unlike many other healthcare careers that require years of education, you can become a medical coder relatively quickly. Numerous institutes offer courses and training programs in medical coding that can be completed in a matter of months.
3. Diverse Opportunities: Medical coding isn't limited to just one type of job. You can find opportunities in various settings, including hospitals, private practices, pharmaceutical companies, and research organizations. If you want to explore related fields, you can also transition into areas like pharmacovigilance, drug regulatory affairs, or clinical data management.
4. Stability and Job Security: The healthcare industry is known for its stability, and medical coding is no exception. As long as there are healthcare services, there will be a need for medical coders. This translates to job security and peace of mind in your career.
5. Work-Life Balance: Many medical coding jobs offer excellent work-life balance. You'll typically work regular hours in a comfortable office setting, allowing you to maintain a healthy work-life balance.
6. Good Earning Potential: While salaries can vary based on location and experience, medical coders generally earn a competitive wage. With experience and additional certifications, you can increase your earning potential even further.
7. Contributing to Healthcare: By ensuring accurate coding, you help maintain the integrity of patient records, improve patient care, and support clinical research, pharmacovigilance, drug regulatory affairs, and clinical data management.
The Role of Medical Coding in Clinical Research
Now, let's delve into the connection between medical coding and clinical research. Clinical research plays a vital role in advancing healthcare treatments and therapies. It involves testing new drugs, medical devices, and treatment protocols to ensure their safety and effectiveness. Medical coding is an essential part of this process for several reasons:
1. Data Accuracy: Accurate coding ensures that the data collected during clinical trials is reliable. Researchers rely on this data to make informed decisions about the safety and efficacy of new treatments.
2. Regulatory Compliance: Regulatory agencies, such as the FDA, require precise documentation of clinical trial data. Medical coding helps maintain compliance with these regulations, which is critical for getting new drugs and treatments approved.
3. Patient Safety: Proper coding helps identify any adverse events or side effects experienced by patients during clinical trials. This information is crucial for patient safety and determining the risks and benefits of a new treatment.
4. Data Analysis: Medical coding simplifies the process of data analysis by categorizing information into standardized codes. This makes it easier for researchers to identify trends and draw conclusions from the data.
How to Start Your Career in Medical Coding?
1. Take a course: Look for reputable institutes or online courses that offer medical coding training. These courses cover topics like anatomy, medical terminology, and coding systems such as ICD-10 and CPT.
2. Get Certified: While certification isn’t always required, it can significantly boost your job prospects. Consider obtaining certifications like Certified Professional Coder (CPC) or Certified Coding Specialist (CCS) through recognized organizations.
3. Gain Experience: Entry-level positions may require some on-the-job experience. Look for internships or entry-level coding jobs to build your skills and resume.
4. Stay Updated: Medical coding guidelines and regulations can change, so it’s essential to stay current. Attend workshops, and seminars, and continue your education to remain competitive in the field.
5. Network: Join professional organizations such as the American Health Information Management Association (AHIMA) or the American Academy of Professional Coders (AAPC) to connect with other professionals in the industry.
Conclusion
In conclusion, a career in medical coding offers stability, good earning potential, and diverse opportunities within the healthcare industry. Moreover, it plays a crucial role in clinical research, contributing to the development of new and better treatments for various medical conditions. If you're interested in healthcare, have an eye for detail, and enjoy working in a structured environment, medical coding could be the perfect career choice for you. Consider enrolling in a reputable training program or course to kickstart your journey into this rewarding field. Your future as a medical coder awaits!
#medical coding institute#medical coding training#medical coding course#Clinical research training#Clinical Research Institute#Clinical research course#Pharmacovigilance course#Pharmacovigilance training institute#Pharmacovigilance jobs#Clinical data management course#Clinical data management training institute#Clinical research management
0 notes
Text
Pharmacovigilance Training & Placements
Pharmacovigilance is the science and activities related to the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problems. It plays a crucial role in ensuring the safety and efficacy of medications and is an important aspect of the pharmaceutical industry. Enhance your career prospects through comprehensive Pharmacovigilance Training.
If you're interested in pharmacovigilance training and placements, here are some steps you can follow:
1. Education: Obtain a relevant educational background in pharmacy, medicine, life sciences, or a related field. A bachelor's or master's degree in pharmacy, pharmacology, or toxicology is often preferred. Enroll in a Pharmacovigilance course online to learn about the science and practices of drug safety.
2. Training programs: Look for specialized training programs or courses that focus on pharmacovigilance. These programs can provide you with a comprehensive understanding of pharmacovigilance principles, regulations, and practices. Great Online Training offers a comprehensive online pharmacovigilance training program designed for professionals in the pharmaceutical, biotechnology, and medical device industries. The program covers essential topics such as adverse event reporting, signal detection, risk management, and regulatory compliance. Students can access the course materials at their own pace and receive a certificate upon successful completion. Join a Pharmacovigilance course with placement to kickstart your career in the pharmaceutical industry.
3. Internships or entry-level positions: Seek internships or entry-level positions in pharmaceutical companies, contract research organizations (CROs), regulatory authorities, or pharmacovigilance service providers. These opportunities can provide hands-on experience and exposure to the field. Earn a Pharmacovigilance Certificate Course to demonstrate your expertise in drug safety and regulatory compliance.
4. Certification: Consider obtaining relevant certifications to enhance your credentials. For individuals interested in pursuing a career in pharmacovigilance, there are reputable online training programs available, such as those offered by Great Online Training, that provide comprehensive courses and resources to help learners develop the necessary skills and knowledge to excel in this field. Take advantage of the Pharmacovigilance free online courses with certificate to enhance your skills and knowledge in drug safety.
5. Networking: Build a professional network by attending conferences, workshops, and industry events related to pharmacovigilance. Networking can help you connect with professionals in the field and explore potential job opportunities. Acquire in-depth knowledge and skills in drug safety with a Diploma in Pharmacovigilance.
6. Stay updated: Pharmacovigilance regulations and practices evolve over time. Stay updated with the latest guidelines, regulatory requirements, and emerging trends in pharmacovigilance through continuous learning and professional development. Obtain a Pharmacovigilance Certification to validate your proficiency in ensuring drug safety and patient welfare.
7. Job search: Utilize online job portals, professional networking platforms, and pharmaceutical industry websites to search for pharmacovigilance job openings. Additionally, consider reaching out to recruitment agencies specializing in the pharmaceutical industry.
When it comes to placements, the demand for skilled pharmacovigilance professionals is generally high, considering the importance of drug safety and regulatory compliance.
Pharmaceutical companies, CROs, regulatory agencies, and pharmacovigilance service providers often have dedicated pharmacovigilance departments or teams. These organizations can provide opportunities for placements in roles such as drug safety associate, pharmacovigilance specialist, medical safety officer, or pharmacovigilance manager.
Keep in mind that the availability of pharmacovigilance training programs and job opportunities may vary based on your location and the pharmaceutical industry landscape in your region. It's essential to research and explore the specific opportunities and requirements in your desired area. Choose a reputable Pharmacovigilance Institute to receive comprehensive training and education in drug safety.
#pharmacovigilance training#pharmacovigilance free online courses with certificate#pharmacovigilance course online#pharmacovigilance course with placement#pharmacovigilance certificate course#diploma in pharmacovigilance#pharmacovigilance certification#pharmacovigilance institute
0 notes
Video
youtube
Informed Consent ( IC ) Waiver in Clinical Trials I Clinical Research
What is a waiver of informed consent?
Waiver of Documentation of Informed Consent (45 CFR 46.117)
This means that the study team must provide a subject with the required consent information, but the study team is not required to obtain the subject's signature on the informed consent document.
What is a waiver in clinical trials?
A Protocol Waiver may be defined as the sponsor approval for a prospective protocol deviation. Such deviations can often involve (but may not be limited to) the active recruitment to a study of one or more participants who do not meet the inclusion/exclusion criteria as detailed in the study protocol.
What is the criteria for waiver of documentation of informed consent?
For research that is FDA-regulated, a waiver of documentation of consent may be granted only: When the research presents no more than minimal risk of harm to participants and involves no procedures for which written consent is normally required outside the research context.
What is the difference between a waiver and informed consent?
A waiver is legal document releasing or relinquishing a known right, claim, or privilege. In this context, it is the relinquishment to pursue a claim in a certain set of defined circumstances. Informed consent is a written acknowledgement that a participant understands the risks inherent in a particular activity.
Inform consent:- A process by which a subject voluntarily confirms his or her willingness to participate in a particular trial, after having been informed of all aspects of the trial that are relevant to the subject’s decision to participate
Inform consent Waiver:- It is a process in which informed consent is not obtained from subjects, or that eliminates or alters some (or all) of the elements of informed consent as set forth in Federal regulations (45 CFR 46.116d)
The IRB must ensure that the following 4 criteria are met prior to approving a waiver or alteration of consent:
The research poses no more than minimal risk to subjects
The research does not adversely affect the rights and welfare
The research not practicable without the waiver
Whenever appropriate. The subject will be provided with pertinent information after participation
Siro Clinical Research Institute. www.siroinstitute.com
Post Graduate Diploma in Clinical Research. Pharmacovigilance, Clinical Trials, Clinical Data Management, Clinical Operation, Medical writing.
#Pharmacovigilance #CDM #data #Clinicalresearch, #regulatoryaffairs, #medicalcoding, #clinicalSAS #management #health #comment #pune
#clinicalresearch #medicaldevices #career #opportunity #oncology
#safety #pharmajobs #gpat #pharmacy #mbbs #jobs #jobsearch #training #healthandsafety #nowhiring #healthcare #clinical #opportunities #doctor #medicine #hiring #bpharmajobs #coding #regulatoryaffairs #medical #nursing
#clinicalresearch #medicaldevices #career #opportunity #oncology #safety #pharmajobs #gpat #pharmacy #mbbs #jobs #jobsearch # #training #healthandsafety #nowhiring
0 notes
Text
Pharmacovigilance Job Opportunities In Top Companies
The pharmaceutical and healthcare sector has grown tremendously in the past few years. Out of which, pharmacovigilance is coming out as a viable option for students to build a stable career. The career growth and opportunities in Pharmacovigilance are immense. Pharmacovigilance aims to gather, understand, assess, and prevent adverse effects of a drug or medicinal product. This is done by various processes such as
1. Individual Case Safety Reports (ICSR).
2. Clinical Review of Safety Reports.
3. Cohort Event Monitoring.
4. Longitudinal Electronic Patient Reports.
5. Periodic Safety Update Reports (PSUR).
6. Spontaneous Recording.
7. Record Linkage.
8. Expedited Report.
A candidate in pharmacovigilance performs the following functions:
1. Reporting adverse events received from customers or healthcare bodies.
2. Reducing the risk of serious side effects.
3. Creating and reviewing serious adverse effects reports.
4. Performing safety audits.

Types of Companies Hiring in Pharmacovigilance
Pharmacovigilance provides a bright career option in terms of both growth and salary. The starting position in the domain is a pharmacovigilance associate or drug safety associate. The companies that hire pharmacovigilance professionals are:
1. Pharmaceutical companies.
2. Contract research organizations.
3. Biotechnology companies.
4. Dedicated pharmacovigilance centers.
5. Regulatory authorities.
6. Knowledge process outsourcing companies.
The above companies offer pharmacovigilance jobs for the following profiles:
1. Drug safety associate.
2. Pharmacovigilance associate.
3. Case processing expert.
4. Safety associate.
5. Clinical safety scientist.
6. Pharmacovigilance scientist.
Top 20 Pharmacovigilance Companies in India
1. Novartis
Novartis is headquartered in Basel, Switzerland and based out of Hyderabad in India. The company is also a CRO for pharmacovigilance and clinical data management.
2. Parexel
Parexel is one of the oldest and most popular CROs based out of Hyderabad in India. The company also operates in Chandigarh and Bangalore. It provides jobs in pharmacovigilance, regulatory affairs, clinical data management, medical writing, and so on.
Talk to us now for best suited
Pharmacovigilance course
3. Syneos Health
The INC Research and inVentiv Health merged to form a new company called Syneos Health. It is a well-known company from the US which is based out of Hyderabad, Pune and Delhi in India. It provides job opportunities in pharmacovigilance, clinical data management, medical writing, and so on.
4. Cognizant
Cognizant is headquartered in the US and is based out of Hyderabad, Mumbai, Pune, and Kolkata in India. It offers services in pharmacovigilance and clinical data management. Cognizant has numerous job opportunities for pharmacy graduates and postgraduates. Cognizant is one of the world's leading professional services firms, transforming clients' business, operating, and technology models for the digital world.
5. Accenture
Accenture is headquartered in Dublin, Ireland and based out of Bangalore, Chennai, and Hyderabad in India. It is the first big IT company to introduce pharmacovigilance services in India
6. Tata Consultancy Services (TCS)
TCS is a multinational company headquartered in Mumbai, India. It also operates in Pune, Nagpur, Indore, and Hyderabad. The company hires professionals for pharmacovigilance, clinical data management and regulatory affairs. TCS is increasing its Research and Development team for the Pharma Division and they are actively recruiting Pharmacovigilance Associates and Clinical Researchers.
7. Vigimedsafe
Vigimedsafe is headquartered in the US and based out of Hyderabad in India. It provides services in pharmacovigilance under various brackets such as aggregate reporting, signal detection, narrative writing, and so on. It is also a great company for freshers to learn and excel in the domain.
8. Makrocare
Makrocare is another old CRO based out of Hyderabad in India. The company offers opportunities in pharmacovigilance, clinical research, medical writing, regulatory affairs, and clinical data management.
9. Indegene
Indegene is a CRO headquartered in Bangalore, India. The company also finds its presence in the US, UK, China, and Japan. It offers job opportunities in pharmacovigilance, clinical data management, medical writing, and so on.
10. Freyr Solutions
Freyr Solutions is a leading company based out of Hyderabad in India. It provides end-to-end services in pharmacovigilance, regulatory affairs, and clinical data management.
11. iSafety
iSafety is another leading life science organization which provides opportunities in pharmacovigilance and clinical data management.
Apart from the above, the other companies hiring for pharmacovigilance are:
12. Dr Reddy’s Laboratories.
13. Aurobindo Pharma Limited.
14. BioClinica.
15. Inventive.
16. Genpact.
17. Quintiles.
18. HCL.
19. Novo Nordisk.
20. Kinapse.
21. Syngene.
Pharmacovigilance Job Locations in India
Pharmacovigilance services are provided by huge multinational giants based out of India with their services functional in the country. These companies have a global foothold in the pharmacovigilance domain and are based in metro cities in India. If you are interested in building a career in pharmacovigilance, you might have to consider moving to the following cities
1. Hyderabad.
2. Bangalore.
3. Mumbai
4. Pune
5. Chandigarh
6. Delhi
7. Chennai
To Sum It Up
Pharmacovigilance is a promising career for healthcare students. It offers a great salary along with stable career growth. The companies providing pharmacovigilance services are prominent all over the world with their headquarters in the US or Europe. In India, these companies are based out of metro and Tier-1 cities such as Bangalore, Hyderabad, Mumbai, and so on. If you wish to build a stable career in pharmacovigilance. For more information and to get trained click on Pharmacovigilance Course
0 notes
Text
Online Pharmacovigilance Courses in Pune
This online training for pharmacovigilance provides you with a comprehensive overview of pharmacovigilance, including its principles, regulatory requirements, and current trends. These Online Pharmacovigilance Courses in Pune are the best pharmacovigilance courses in India. This course is Live- Instructor-led online training, where you attend a class every day in any part of the world. The advantage of online pharmacovigilance courses is you get every class recording lifetime access. Class notes and assignments are shared based on the topics covered. This course is suitable for Fresh graduates in science student and working professionals also who want to grow their careers. Our trainers are professional and they have 10 years of experience in this field. Our Expert Trainer will answers all the questions related to the studies. After class, if you have doubts then the trainer can be connected at any time via email or phone.
The purpose of this course is to provide a comprehensive overview of pharmacovigilance and its responsibilities. Participants will be able to describe basic principles for pharmacovigilance in different countries and regions as well as learn about international pharmacovigilance standards. Pharmacovigilance is the study and monitoring of the effects of drugs. This course will provide you with a comprehensive understanding of the pharmacovigilance process, as well as its importance in protecting public health. Pharmacovigilance is the process of monitoring the safety of pharmaceutical products. The internet has made it easier for people to study pharmacovigilance courses online. Online pharmacy courses are a great way to learn more about the industry. They will teach you about the different aspects of this job and how to work in it. These courses will provide you with all the necessary skills for working in this industry. You will be able to find a job in the field and make a good living if you complete these courses.
There are many advantages to studying online pharmacovigilance courses. One of these advantages is that you can study at your own place and at your own time as long as you have an internet connection.
What you will learn in these courses?
1. Introduction to Pharmacology, Clinical Trials, and Pharmacovigilance
2. Pharmacovigilance Programme in India
3. Methodologies in Pharmacovigilance
4. Adverse Drug Reactions and Safety Reports
5. Narrative Writing in Pharmacovigilance
6. Management Systems and Drug Dictionaries in Pharmacovigilance
7. Special Case Scenarios in Pharmacovigilance
8. Seriousness & Expectedness & Causality Assessment Criteria
9. Overview of Aggregate Safety Reports
10. Signal Detection and Risk management
11. Overview of Argus Database
This online training for pharmacovigilance provides you with a comprehensive overview of pharmacovigilance, including its principles, regulatory requirements, and current trends. These Online Pharmacovigilance Courses in Pune are the best pharmacovigilance courses in India. This course is Live- Instructor-led online training, where you attend a class every day in any part of the world. The advantage of online pharmacovigilance courses is you get every class recording lifetime access. Class notes and assignments are shared based on the topics covered. This course is suitable for Fresh graduates in science student and working professionals also who want to grow their careers. Our trainers are professional and they have 10 years of experience in this field. Our Expert Trainer will answers all the questions related to the studies. After class, if you have doubts then the trainer can be connected at any time via email or phone.
The purpose of this course is to provide a comprehensive overview of pharmacovigilance and its responsibilities. Participants will be able to describe basic principles for pharmacovigilance in different countries and regions as well as learn about international pharmacovigilance standards. Pharmacovigilance is the study and monitoring of the effects of drugs. This course will provide you with a comprehensive understanding of the pharmacovigilance process, as well as its importance in protecting public health. Pharmacovigilance is the process of monitoring the safety of pharmaceutical products. The internet has made it easier for people to study pharmacovigilance courses online. Online pharmacy courses are a great way to learn more about the industry. They will teach you about the different aspects of this job and how to work in it. These courses will provide you with all the necessary skills for working in this industry. You will be able to find a job in the field and make a good living if you complete these courses.
There are many advantages to studying online pharmacovigilance courses. One of these advantages is that you can study at your own place and at your own time as long as you have an internet connection.
What you will learn in these courses?
1. Introduction to Pharmacology, Clinical Trials, and Pharmacovigilance
2. Pharmacovigilance Programme in India
3. Methodologies in Pharmacovigilance
4. Adverse Drug Reactions and Safety Reports
5. Narrative Writing in Pharmacovigilance
6. Management Systems and Drug Dictionaries in Pharmacovigilance
7. Special Case Scenarios in Pharmacovigilance
8. Seriousness & Expectedness & Causality Assessment Criteria
9. Overview of Aggregate Safety Reports
10. Signal Detection and Risk management
11. Overview of Argus Database
1 note
·
View note
Text
IQVIA is hiring a Safety Aggregate Report Specialist 2 (exclusively in Kolkata).
Work Type: Full-Time
Workplace Duties:
Work Summary: IQVIA is seeking a Safety Aggregate Report Specialist to work in Kolkata, India. Managing safety aggregate reports, literature surveillance, signal management operations, and benefit risk management paperwork are among your responsibilities in this position.
Crucial Roles:
Assume responsibility for the SARA deliverables and make sure that service level agreements (SLAs) are followed.
Oversee the creation and completion of numerous aggregate reports, such as line listings, RMPs, DSURs, PADERs, PBRERs/PSUR, and RMPs.
When necessary, draft answers to questions from the Pharmacovigilance Risk Assessment Committee (PRAC) and regulatory bodies.
Maintain continuous literature safety surveillance, including identification of ICSRs and aggregate data review, for both marketed and investigational products.
Assume the role of Signal Management Lead for post-marketing and clinical trial initiatives, establishing signaling protocols, carrying out signal identification tasks, and recording signals.
Interface with clients and collaborate with internal functional groups within Lifecycle Safety and other business units.
Take part in audits, support departmental objectives, and follow standard operating procedures (SOPs).
Qualifications:
Requires a bachelor's degree in a medical or scientific field.
Preferably two to three years of relevant work experience.
outstanding familiarity with Lifecycle Safety services and procedures combined with a readiness to pick up new abilities.
Understanding of global regulatory requirements such as Good Clinical Practice (GCP), Good Pharmacovigilance Practice (GVP), and International Conference of Harmonization (ICH) guidelines.
Proven ability to meet deadlines, manage competing priorities, and maintain high-quality standards.
Proficiency in Microsoft Office applications and familiarity with medical terminology.
Strong communication, organizational, and time management skills.
good judgment, self-reliance, and ability to make decisions.
Self-driven, adaptable, and possessing strong coaching and mentoring abilities.
strong project management, operational metrics, and leadership skills knowledge.
[caption id="attachment_47409" align="aligncenter" width="930"] IQVIA Jobs; PV Safety Aggregate Report Specialist 1[/caption]
About IQVIA: IQVIA is a world leader in clinical research services, technology solutions, and advanced analytics for the life sciences sector. Come along with us to positively influence healthcare around the world. Visit IQVIA Careers to learn more.
How to Apply:
Don't miss this opportunity to contribute to impactful work and grow your career at IQVIA. Apply now for the IQVIA Hiring Safety Aggregate Report Specialist 2 - Kolkata using the IQVIA PV Referral Link.
0 notes
Text
Career Opportunities in Clinical Research
What is Clinical Research?
Clinical research is a new arm of healthcare sciences opening wide range of career opportunities in India as well as for pharmaceutical & science graduates, masters. Pharmacovigilance is the most precise way for evaluation of the relevancy of Clinical Research.
The tests on drugs are conducted under observation whether a new innovative product performs as expected or not. There is ‘n’ number of innovative therapies generated in laboratories but very few of them survive & reach to the point of human testing. It represents a key research activity with the potential to improve the quality of health care and control costs through careful comparison of alternative treatments. Although many clinical trials are of high quality, a careful reader of the medical literature will notice that a large number have deficiencies in design, conduct, analysis, presentation, and/or interpretation of results. The Clinical Research Course of Fusion Technology Solutions is a best option to start a career in this evergreen field.

How to Start Career in Clinical Research?
Any fresher from pharmacy graduate or relevant science graduate can start their career as a clinical research professional after successful completion of the clinical research course. There are many institutes and hospitals carrying the certified clinical research training. Some institutes are also providing the placement guarantee after successful completion of the course. Fusion Technology Solutions is best institute in Pune provides best clinical research training with 100 % placement guarantee.
Candidates can also peruse the distance learning clinical research course while doing job or other education.
Profiles in Clinical Research
Clinical Research Associate (CRA)
Role- Monitor & Supervise individuals administering clinical trials. Assist with the research of pharmaceuticals, biologics and devices.
Clinical Research Coordinator (CRC),
Role- Directing clinical trials alongside Principal Investigators (PI) or Clinical Research Associates (CRAs) using good clinical practice (GCP).
Data Manager
Role- Prepare protocols, reviews regulations, and otherwise helps the Senior Manager to keep the trial on track.
Clinical Research Scientist
Role, conduct scientific tests and monitor the clinical trials.
The Clinical Quality Assurance Auditor (CQA)
Role- to inspect the documents and processes of the clinical trial to make sure that they comply with certain guidelines, known as the good clinical practice (GCP). The clinical QA auditor also needs to make sure that the trial is adhering to the standard operating procedures (SOP).
Clinical Safety Analyst
Role- to monitor, code, organize, and track the adverse events that happen during the trial, they would also be acting as an advocate for the patients.
The Medical Writer
Role- to write detail of clinical trials and prepare trial report which is going to be used by other pharmaceutical companies, hospitals, physicians, and even the public.
Research Assistant –
Role- work with the trial sponsor to ensure that patient progress is closely followed, that the recruitment of patients has produced a random sample that adverse results are properly recorded, and all other patient information is properly documented.
Regulatory Coordinator –
Role- make sure that regulations are followed & also ensures strict adherence to all codes of ethics, monitors procedures and then reports findings to the appropriate regulatory agencies.
Senior Regulatory Associate –
Role- make sure that documentation is in order and regulations are followed so that paperwork for the trial can be submitted to the FDA for approval.
Regulatory Specialist –
Role- to help with regulatory documentation and submission, ensures adherence to Standard Operating Procedures and otherwise assists the regulatory team with all compliance matters.
CTMS (A Clinical Trial Management System) Manager –
Role- to oversee the program that is keeping a trial organized also responsible for making sure that all elements of the trial are properly scheduled, performed and recorded, ensuring documentation of costs and risks related to the trial.
CTMS (Clinical Trial Management System) Associate –
Role- to assist the CTMS Manager in all duties. Updates the CTMS program when amendments are made and helps to uphold accuracy in reports.
1 note
·
View note
Text
How to Complete the Pharmacovigilance Course: A Complete Guide
Introduction:
In the pharmaceutical sector, taking a pharmacovigilance course provides access to a crucial area of medication safety and regulatory compliance. In addition to providing information on the importance of pharmacovigilance, course structure, important modules, career possibilities, and industry demands, the goal of this guide is to serve as a path for aspiring students.
Modules and Course Structure:
Gain an understanding of pharmacovigilance by exploring its underlying concepts and highlighting its function in tracking and evaluating adverse drug reactions (ADRs) after a product has been put on the market. Examine its significance for maintaining public health and adhering to regulations.
Selecting the Appropriate Path:
Learn about the standard format and modules of pharmacovigilance courses, which include topics like risk management plans, drug safety surveillance, regulations, signal detection, and pharmacovigilance systems. Recognize how these courses give students the knowledge and abilities they need.
Choosing the Correct Course:
When choosing a pharmacovigilance course, take into account factors like accreditation, length of the course, mode of delivery (online or in-person), relevance of the curriculum, faculty experience, and industry relationships.
Practical Training and Case Studies:
Learn the importance of these learning tools in pharmacovigilance courses, as they give students real-world examples to apply their theoretical knowledge. Examine how these elements improve your ability to think critically and make decisions.
Certification and Industry Recognition:
Recognize the importance of earning certification in pharmacovigilance from respectable organizations. Find out more about accredited certificates and how they affect credibility in the industry and job advancement.
Career Opportunities and Growth:
Learn about the various career pathways that are open to pharmacovigilance specialists. These include positions as regulatory affairs specialist, pharmacovigilance manager, pharmacovigilance scientist, and drug safety associate. Learn about the demands of the sector and your professional growth prospects.
Conclusion:
Starting a pharmacovigilance course is a crucial step in developing a fulfilling career in regulatory affairs and drug safety. Through a grasp of the subtleties of certification alternatives, course structures, and industry demands, learners may arm themselves with the knowledge and abilities required to succeed in an ever-evolving area.
For more info:-
pharmacovigilance course
pharmacovigilance training
pharmacovigilance courses online
pharmacovigilance certification
0 notes
Text
Pharmacovigilance Courses Shape a Defined Career Path
An Introduction to Pharmacovigilance
The pharmacovigilance industry mainly focuses on assessing the risks associated with pharmaceutical products as well as ensuring the safety and efficacy of medicines. However, pharmacovigilance course are an essentiality as they give the professionals the relevant qualification as well as experiences. . These skills may differ according to the job role of the professional. One normally needs to possess a bachelor’s degree to start their career in the industry. After their Clinical Research Courses, professionals can get many career opportunities in drug safety.
Two Main Career Paths in Pharmacovigilance
There are two career paths in the pharmacovigilance industry namely the management route and the technical roles. It is tough to climb up the career path due to the competition for senior roles, in the industry. However, pharmacovigilance courses could give one the adequate skills to get into senior roles. In the management career path, one could get job profiles such as drug safety officer, manager and director. Whereas, in the technical route, one could get job profiles such as risk management, signal detection and epidemiology. There are pharmacoepidemiology roles such as pharmacovigilance scientists, risk management specialists and vigilance managers. These could only be possible after pharmacovigilance courses.
The Career Path of a Pharmacovigilance Professional
Pharmacovigilance professionals start their career as a drug safety associate or pharmacovigilance officer. For that reason pharmacovigilance courses prepare them for their roles in the industry. The role of these professionals is to report side-effects of pharmaceutical products into a database and later risk management teams evaluate these reports. These candidates should be able to identify reasons for side-effects. Pharmacovigilance courses give one the skills and experiences to identify reasons for these adverse reactions or side effects.
Senior Job Profiles in the Pharmacovigilance Career
After gaining experience, professionals progress on to senior roles in drug safety. Their role is to identify and evaluate the health risks of pharmaceutical drugs. Pharmacovigilance courses enlighten professionals’ on the various responsibilities. Furthermore, these individuals are required to analyze risk or benefits of products and produce reports. They also make recommendations to drug safety physicians on several actions. For instance, they suggest alterations in prescribed dose to patients. Finally, these professionals also supervise the case processing professionals and signal detection teams. Pharmacovigilance courses give them experience to move on to senior positions
Role of a Pharmacovigilance Officer
A senior pharmacovigilance officer such as a specialist or management roles requires extensive knowledge in areas, such as medical writing, medical affairs, auditing or quality assurance. Pharmacovigilance courses give them the in-depth knowledge of these areas. Apart from monitoring adverse reactions, they collect information of medical products and adverse event reports sourced from various studies. This information is later submitted to the regulatory authorities. Pharmacovigilance courses inform them about their roles.
0 notes
Text
Balancing Benefit and Risk in Clinical Research
Introduction
The fields of medicine and healthcare are rapidly developing. Companies and research institutes play a vital role in advancing medical knowledge through clinical research. Clinical research surrounds various aspects, including medical coding, pharmacovigilance, drug regulatory affairs, and clinical data management. These fields are essential in ensuring the safety and effectiveness of new medical treatments. However, conducting clinical research comes with its own set of challenges, particularly when it comes to balancing the benefits and risks involved.

The Role of Clinical Research
Clinical research is the backbone of medical progress. It involves the systematic study of new drugs, medical devices, treatments, and procedures to determine their safety and efficacy. Companies and research institutes conduct clinical trials to gather data and evidence before these medical interventions are approved for widespread use.
Key Areas of Clinical Research
1. Medical Coding: Medical coding is like the language of healthcare. It involves translating medical records, diagnoses, and procedures into standardized codes. Accurate coding is crucial for proper billing and maintaining patient records.
2. Pharmacovigilance: This field focuses on monitoring the safety of drugs and vaccines post-approval. It helps identify and prevent adverse effects and ensures that patients receive safe medications.
3. Drug Regulatory Affairs: Drug regulatory affairs professionals work with regulatory agencies to ensure that new drugs meet safety and efficacy standards before they reach the market. They help companies navigate complex regulations.
4. Clinical Data Management: Managing clinical trial data is essential for maintaining the integrity of research. Data managers organize and validate information collected during trials.
Balancing Benefit and Risk
While clinical research is definitely important for medical progress, it also involves risks. Here are some ways in which companies and research institutes can strike a balance:
1. Ethical Considerations: Ethical guidelines and standards are the foundation of clinical research. Researchers must prioritize the well-being of participants and ensure that their rights and privacy are protected.
2. Informed Consent: Participants must provide informed consent before participating in a clinical trial. They should be fully aware of the potential risks and benefits, enabling them to make an informed decision.
3. Safety Monitoring: Continuous monitoring of participants' safety is essential. Any adverse events should be immediately reported and addressed.
4. Transparency: Transparency in reporting research findings is crucial. This includes disclosing both positive and negative results, helping to avoid biased information.
5. Regulatory Compliance: Companies and institutes must adhere to regulatory requirements in their respective fields, ensuring that the research meets high standards of safety and quality.
The Importance of Training
To ensure that clinical research is conducted responsibly, professionals in the field require acceptable training. Courses and training programs are available for medical coding, pharmacovigilance, drug regulatory affairs, and clinical data management. Proper training equips individuals with the knowledge and skills needed to conduct research while minimizing risks.
Job Placement in Clinical Research
For those interested in pursuing a career in clinical research, the job placement aspect is essential. Companies and institutes often offer placement opportunities for trained professionals, ensuring that they can apply their skills in real-world settings.
Conclusion
Balancing benefit and risk in clinical research is a complex but essential endeavor. Companies and research institutes play a critical role in advancing medical knowledge, but they must do so responsibly. Ethical considerations, informed consent, safety monitoring, transparency, and regulatory compliance are key factors in achieving this balance. Moreover, individuals interested in clinical research can benefit from training and job placement opportunities, enabling them to contribute to the field while ensuring the safety and well-being of patients. In this way, we can continue to make significant strides in healthcare while upholding the highest standards of ethics and safety.
#Medical billing and coding course#Pharmacovigilance jobs#Pharmacovigilance course#Pharmacovigilance training institute#Clinical data management course#Clinical data management training institute#Clinical research management#Clinical research training#Clinical Research Institute#Clinical research course#medical coding course#medical coding institute#medical coding training
0 notes