#color-enhanced scanning electron microscope
Photo

A color-enhanced scanning electron microscope image of mushroom spores. When ingested, this fungus causes euphoria, hallucinations, and altered perception of time.
PHOTOGRAPH BY TED KINSMAN, SCIENCE PHOTO LIBRARY
#ted kinsman#photographer#science photo library#national geographic#color-enhanced scanning electron microscope#mushroom spores#micro photography#fungus#fungi#nature
48 notes
·
View notes
Text

I posted 7,930 times in 2022
That's 1,430 more posts than 2021!
30 posts created (0%)
7,900 posts reblogged (100%)
Blogs I reblogged the most:
@astercrash
@an-unimpressed-jackalope
@apollothrace
@youandwhoseamy
@beawisefooland
I tagged 2,249 of my posts in 2022
#star trek - 148 posts
#umineko - 59 posts
#comix - 52 posts
#ds9 - 50 posts
#utena - 45 posts
#friends at the table - 40 posts
#life is strange - 38 posts
#disco elysium - 34 posts
#music - 26 posts
#goncharov - 25 posts
My Top Posts in 2022:
#5
The eyes have been used to signify a perverse capacity - honed to perfection in the history of science tied to militarism, capitalism, colonialism, and male supremacy - to distance the knowing subject from everybody and everything in the interests of unfettered power. The instruments of visualization in multinationalist, postmodernist culture have compounded these meanings of disembodiment. The visualizing technologies are without apparent limit. The eye of any ordinary primate like us can be endlessly enhanced by sonography systems, magnetic reasonance imaging, artificial intelligence-linked graphic manipulation systems, scanning electron microscopes, computed tomography scanners, color-enhancement techniques, satellite surveillance systems, home and office video display terminals, cameras for every purpose from filming the mucous membrane lining the gut cavity of a marine worm living in the vent gases on a fault between continental plates to mapping a planetary hemisphere elsewhere in the solar system. Vision in this technological feast becomes unregulated gluttony; all seems not just mythically about the god trick of seeing everything from nowhere, but to have put the myth into ordinary practice. And like the god trick, this eye fucks the world to make techno-monsters.
- Donna Haraway, Situated Knowledges
Since reading this Letterboxd review, I’ve been thinking all day about how Mamoru Oshii put Donna Haraway in Ghost In The Shell 2, presumably in tribute to her Cyborg Manifesto, but he could as well have put her in Patlabor 2: The Movie in tribute to her Situated Knowledges. Anyway, took the excuse to post my favourite paragraph from maybe my favourite philosophy essay.
22 notes - Posted February 3, 2022
#4
Listening to the Umineko soundtrack on shuffle is wild because you'll go from thumping final showdown electronica to baroque hold music to something that makes you weep openly on the bus to M. Zakky's Monochrome Clock.
33 notes - Posted October 6, 2022
#3
Dreamt you could buy hormones over at the counter at Forbidden Planet (British comics and nerd shit store) but they had to route the payment through a nearby pharmacy and I got anxious about inconveniencing the folks in the queue behind me
48 notes - Posted April 7, 2022
#2
Sometimes I make little noises and no one can stop me. They try so hard but the noises just get littler.
365 notes - Posted June 29, 2022
My #1 post of 2022
Gotta say, never unfollowing all those long abandoned Tumblr accounts is really suddenly starting to pay off.
26,118 notes - Posted November 14, 2022
Get your Tumblr 2022 Year in Review →
4 notes
·
View notes
Link
0 notes
Text
Lanthanum Hexaboride (LaB6) Ceramics Parts

Lanthanum hexaboride (LaB6) also called lanthanum boride, and LaB6 is an inorganic chemical, a boride of lanthanum. It is a refractory ceramic material with a dark purple appearance that has a melting point of 2210°C, and is insoluble in water and hydrochloric acid. When bombarded with ions, its physical appearance is altered, and it has a green color in place of its usual dark purple color.
Lanthanum hexaboride (LaB6) also called lanthanum boride, and LaB6 is an inorganic chemical, a boride of lanthanum. It is a refractory ceramic material with a dark purple appearance that has a melting point of 2210°C, and is insoluble in water and hydrochloric acid. When bombarded with ions, its physical appearance is altered, and it has a green color in place of its usual dark purple color.
The unique properties of lanthanum hexaboride crystals provide stable electron-emitting media with work functions near 2.70 eV. The low work function yields higher currents at lower cathode temperatures than tungsten, which means greater brightness, or current at the beam focus, and longer life. Typically, LaB6 cathodes exhibit 10 times the brightness and 50 times the service life of tungsten cathodes. In electron microscope applications, these characteristics translate to more beam current in a smaller spot at the sample, improved resolution, and less frequent cathode replacement.
Lanthanum Hexaboride Applications:
Thermionic emission (cathode)
Plasma source for plasma-enhanced coating(PECVD)
Vacuum electron beam welding machine
Electron beam surface reforming device
Electron beam lithography device
Transmission electron microscope
Scanning electron microscope
Surface analysis device
Radiotherapy devices

Lanthanum Hexaboride Properties:
Properties
Unit
Lanthanum Hexaboride
Purity
%
>99.5
Density
g/cm3
>4.30
Structure
/
Monocrystalline
Vickers hardness
HV
1065
Shore hardness
HS
HS
Thermal conductivity
W/mK
15
Electrical Conductivity
S/m
1.83*10^6
Flexural strength
MPa
165
Innovacera supplies our customers with a high-quality Lanthanum Hexaboride ceramic parts.
Read the full article
0 notes
Photo


Scanning Electron Micrograph of Butterfly Wing Scales
Color-enhanced scanning electron micrograph (SEM) of scales from the wing of an Emerald Swallowtail (Papilio palinurus) butterfly.
The scales are modified hairs, or setae, and are made of chitin, a common substance in insect exoskeletons. The iridescence of the colors of a butterfly's wings is produced by the diffraction of light by the microscopic structure of these scales.
Explore More Butterfly SEMs
Layers of wing scales are transparent but tiny ridges on the scales break up and reflect light (diffraction) to give the scales their shimmering iridescent appearance.
© Eye of Science / Science Source
55 notes
·
View notes
Text
Conserving the Surtout de Table: Cut Glass
Written by Sarah Barack, Head of Conservation, Senior Objects Conservator
Thanks to a generous gift from the Smithsonian Women’s Committee, Cooper Hewitt’s spectacular surtout de table centerpiece was the focus of a recent technical study and conservation treatment by the museum’s conservation department. In this series of posts, conservators will be sharing the results of the technical study, as well as more information on the making of the piece and its conservation.
Transparent and colorless, the glass dishes mounted along the tiered servers were meant to hold fruit and other items, allowing the delectable treats to seemingly float above the glittering surtout de table. And yet the decorative patterns cut into the bowls carry forward the play of light that bounces across the surtout’s gilded surfaces and reflects off the mirrors below. This refraction, or bending, of light is enhanced by the composition of the glass itself, which included lead. Leaded glass has a higher refractive index (RI) than glass without lead (the refractive index indicates how fast light travels through a substance, when compared to how fast it travels in a vacuum). Leaded glass also disperses light as it passes through the medium, which means that the light is separated into the various wavelengths that comprise white light. Altogether, you notice a greater sparkle or brilliance when looking at leaded glass, and glass workers took full advantage of these properties by cutting the material to create patterns that maximize these effects.
Detail of the surtout de table, featuring the cut-glass dishes and Three Graces centerpiece.
Leaded glass is often associated with the English glassmaker (or glass factory owner) George Ravenscroft, as he was awarded a patent to produce leaded glass in England in 1674. Eventually companies such as Waterford in Ireland became known for their exquisite cut crystal products.
Conservators must understand the materials—and manufacture—of objects they hope to preserve through study and treatment. Glass composition can be determined through sampling (usually the removal of a minute amount of original material) and conducting analysis on, for instance, a Scanning Electron Microscope (SEM) with Energy Dispersive Spectroscopy (EDS). But a low-cost technology and inexpensive way to quickly determine whether or not glass has a significant lead content is to use ultra-violet (UV) light. Leaded glass will often fluoresce, or produce, a distinct blue-ish color when illuminated under UV light.
The leaded glass of the surtout de table fluorescing blue under UV light.
Three of the glass dishes associated with the surtout were damaged at some point in their history, and it was necessary to stabilize this damage through conservation treatment. As leaded glass has a high RI, conservators must also make sure that whatever adhesive will be used to repair the glass has a similar RI. If the RI of the adhesive varies greatly from the original glass, cracks will remain visible (even if the adhesive is helping to make sure that the damage is stable). The closer the RI of two material becomes, the less visible the difference between them. Therefore, adhesive used to repair broken glass will be almost invisible if the RI is appropriate. We often use a two-part colorless epoxy resin that was formulated for glass conservation—meaning it has a useful RI and presents very good aging properties. Any glues that yellow within 10 to 15 years or less are avoided, especially when treating a colorless leaded glass object.
Altogether, the glass dishes help enliven the appearance of the surtout, and show us how elaborate and sumptuous the entire ensemble must have looked.
Cooper Hewitt’s surtout de table, on display in the exhibition Tablescapes: Designs for Dining (on view October 5, 2018–April 14, 2019).
from Cooper Hewitt, Smithsonian Design Museum http://bit.ly/2D55hAF
via IFTTT
1 note
·
View note
Text
HOW A PHOTOCOPY Equipment Functions
A photocopier (also acknowledged as a copier or copy device) is a equipment that tends to make copies of files and other visual pictures onto paper or plastic film rapidly and cheaply. Most modern photocopiers use a technology called xerography, a dry method that makes use of electrostatic charges on a light-weight-sensitive photoreceptor to first attract and then transfer toner particles (a powder) onto paper in the type of an picture. Warmth, force or a mix of the two is then employed to fuse the toner on to the paper. Copiers can also use other technologies this sort of as ink jet, but xerography is regular for business office copying. Earlier versions included the Gestetner stencil duplicator, invented by David Gestetner in 1881.
Industrial xerographic office photocopying was launched by Xerox in 1959,[1][2] and it progressively changed copies produced by Verifax, Photostat, carbon paper, mimeograph devices, and other duplicating devices.
Photocopying is broadly utilized in the company, education, and govt sectors. Although there have been predictions that photocopiers will sooner or later grow to be obsolete as info personnel boost their use of electronic doc development, storage and distribution, and rely significantly less on distributing true pieces of paper, as of 2015, photocopiers proceed to be extensively utilized. In the 2010s, there is a convergence in some substantial-conclude devices in between the roles of a photocopier, a fax device, a scanner, and a personal computer community-connected printer into a multi-operate printer. Reduced-end machines that can copy and print in color have increasingly dominated the property-office industry as their costs fell steadily by means of 2017. Higher-conclude colour photocopiers able of dealing with heavy responsibility cycles and big-format printing remain a costlier specialty for print and layout outlets.
Contents
one Heritage
one.1 Colour photocopiers
1.2 Digital technological innovation
2 How it operates (utilizing xerography)
three Copyright troubles
4 Counterfeiting
5 Health concerns
six Forensic identification
seven See also
eight References
nine More reading through
Heritage
Chester Carlson, the inventor of photocopying, was initially a patent legal professional, as effectively as a component-time researcher and inventor. His task at the patent business office in New York needed him to make a large number of copies of essential papers. Carlson, who was arthritic, discovered this to be a distressing and tiresome method. This determined him to carry out experiments with photo conductivity. Carlson utilised his kitchen area for his "electrophotography" experiments, and, in 1938, he utilized for a patent for the approach. He made the first photocopy employing a zinc plate covered with sulfur. ΦΩΤΟΤΥΠΙΚΑ ΜΗΧΑΝΗΜΑΤΑ "ten-22-38 Astoria" have been composed on a microscope slide, which was placed on leading of a lot more sulfur and under a brilliant light-weight. Soon after the slide was eliminated, a mirror picture of the phrases remained. Carlson attempted to promote his creation to some businesses, but failed since the process was nonetheless underdeveloped. At the time, numerous copies had been most commonly manufactured at the point of document origination, employing carbon paper or guide duplicating machines, and individuals did not see the require for an digital machine. Among 1939 and 1944, Carlson was turned down by above 20 organizations, including IBM and Standard Electric—neither of which believed there was a significant marketplace for copiers.
In 1944, the Battelle Memorial Institute, a non-earnings organization in Columbus, Ohio, contracted with Carlson to refine his new process. Over the next five many years, the institute executed experiments to enhance the process of electrophotography. In 1947, Haloid Corporation (a tiny New York-based mostly company and vendor of photographic paper) approached Battelle to acquire a license to build and market a copying device based mostly on this technology.[2]
Haloid felt that the phrase "electrophotography" was as well difficult and did not have good remember benefit. Following consulting a professor of classical language at Ohio State University, Haloid and Carlson altered the identify of the method to "xerography," which was derived from Greek phrases that intended "dry composing." Haloid known as the new copier devices "Xerox Machines" and, in 1948, the term "Xerox" was trademarked. Haloid at some point changed its name to Xerox Company.
In 1949, Xerox Company launched the 1st xerographic copier called the Design A.[3] Xerox became so profitable that, in North The us, photocopying arrived to be commonly identified as "xeroxing." Xerox has actively fought to avert "Xerox" from getting to be a genericized trademark. Whilst the word "Xerox" has appeared in some dictionaries as a synonym for photocopying, Xerox Corporation usually requests that this kind of entries be modified, and that folks not use the expression "Xerox" in this way. Some languages incorporate hybrid phrases, such as the commonly used Polish phrase kserokopia ("xerocopy"), even although comparatively number of photocopiers are of the Xerox brand name.
In the early 1950s, Radio Company of The usa (RCA) released a variation on the procedure called Electrofax, whereby pictures are fashioned immediately on specially coated paper and rendered with a toner dispersed in a liquid.
Throughout the 1960s and by means of the 1980s, Savin Corporation developed and bought a line of liquid-toner copiers that implemented a engineering dependent on patents held by the organization.
Before the prevalent adoption of xerographic copiers, photo-direct copies made by devices such as Kodak's Verifax have been employed. A main obstacle connected with the pre-xerographic copying technologies was the higher cost of materials: a Verifax print essential supplies costing US$.15 in 1969, whilst a Xerox print could be produced for $.03 including paper and labor. The coin-operated Photostat equipment still discovered in some general public libraries in the late sixties produced letter-size copies for $.twenty five each, at a time when the bare minimum wage for a US worker was $1.sixty five per hour the Xerox devices that replaced them typically billed $.ten.
Xerographic copier producers took gain of a high perceived-worth of the 1960s and early nineteen seventies, and marketed paper that was "specially made" for xerographic output. By the end of the 1970s, paper producers created xerographic "runability" one of the requirements for most of their business office paper manufacturers.
DADF or Duplex Computerized Document feeder - Canon IR6000
Some units sold as photocopiers have replaced the drum-dependent process with inkjet or transfer movie technological innovation.
Amongst the crucial benefits of photocopiers over before copying technologies are their capacity:
to use plain (untreated) place of work paper,
to apply duplex (or two-sided) printing,
In a position to scan numerous internet pages routinely with an ADF, and
at some point, to kind and/or staple output.
Color photocopiers
Colored toner grew to become available in the nineteen fifties, though complete-color copiers had been not commercially available till 3M unveiled the Shade-in-Colour copier in 1968, which used a dye sublimation process rather than standard electrostatic technologies. The first electrostatic shade copier was introduced by Xerox (the 6500) in 1973. Colour photocopying is a concern to governments, as it facilitates counterfeiting currency and other files: for more details, see Counterfeiting part.
Electronic engineering
There is an escalating craze for new photocopiers to adopt electronic technology, therefore replacing the more mature analog technologies. With digital copying, the copier properly is made up of an built-in scanner and laser printer. This design has several rewards, this sort of as computerized picture quality enhancement and the ability to "build work" (that is, to scan webpage pictures independently of the procedure of printing them). Some digital copiers can purpose as large-pace scanners this kind of versions normally offer you the potential to deliver documents via electronic mail or to make them offered on file servers.
A wonderful advantage of electronic copier technologies is "computerized electronic collation." For instance, when copying a established of 20 pages twenty times, a digital copier scans each webpage only when, then uses the stored data to generate 20 sets. In an analog copier, possibly each website page is scanned 20 times (a total of 400 scans), making one particular set at a time, or 20 individual output trays are utilised for the 20 sets.
Low-finish copiers also use digital technologies, but are inclined to consist of a common Pc scanner coupled to an inkjet or lower-end laser printer, the two of which are significantly slower than their counterparts in higher-conclude copiers. Nonetheless, low-stop scanner-inkjets can offer shade copying at a reduced acquire price but with a much increased price for each copy. The value of electronics is this sort of that blended scanner-printers sometimes have developed-in fax machines. (See Multifunction printer.)
1 note
·
View note
Text
Lupine Publishers | Kinetic Isotherm Studies of Azo Dyes by Metallic Oxide Nanoparticles Adsorbent

Lupine Publishers | An archive of organic and inorganic chemical sciences
Abstract
We reported the synthesis of Cu4O3 nanoparticles fabricated by Camellia Sinensis (green tea) leaves extract as reducing and stabilizing agent and studied the azo dyes removal efficiency. The formation of copper oxide nanoparticles was confirmed after change in solution of salt and plant extract from green to pale yellow. Subsequently, the above said nanoparticles were characterized by SEM, XRD, FTIR, and UV spectrophotometer for size and morphology. The average particle size of copper oxide nanoparticle was found to be 17.26nm by XRD shrerrer equation, average grain diameter by SEM was calculated 8.5×10-2mm with spherical and oval shaped. UV spectroscopy range was between 200-400nm. These copper oxide nanoparticles were applied as azo dyes (Congo red and malachite green) degradation. Effect of reaction parameters were studied for optimum conditions. Kinetic models like Langmuir, Freundlich and elovich models were applied. Finally, concluded that these particles are effective degradation potential of azo dyes at about 70-75% from aqueous solution.
Keywords: Green Tea; Cu4O3; Green synthesis; XRD; Congored; Malachite Green
Background
With elevating improvement in technology, the Scientific developments are approaching to new horizons [1]. Besides supplementary needs, the stipulation of industrial wastewater has increased swiftly, supervened in the huge amount of wastewater including azo dyes. Azo dyes are the foremost group of commercial pollutants [2]. Azo dyes are class of synthetic dyes with a complex aromatic structure and contain two adjacent nitrogen bond (N=N), that can accompany color to materials [3]. Furthermore, the aromatic structures of dyes form them sturdy and not- biodegrade [4]. Textile consume prodigious quantities of hazardous chemicals particularly in dyeing operations. This work is constructed on malachite green and congored azo dyes. The toxic Habit of the azo dyes can be elaborated by fact that upon decomposition it breaks up into hazardous products [5]. The MG and CR azo dyes toxic dye which has been removed from water samples through the physical, chemical and biological methods. Azo dyes are toxic, probably cause aesthetic problems and mutagenic and carcinogenic effects on human health, so must be degraded [6]. Therefore, the adsorption method by using copper oxide metal nanoparticles for wastewater treatment comprised with azo dyes. Cu4O3 nanoparticle were applied as an adsorbent for the degradation of MG and CR dyes and its kinetic and isotherm studies. Biogenic technology is regarded an emerging advancement of the current time which has been utilized to synthesize nanoparticles of a desired shape and size by using plant extract [7]. Consequently, the synthesized nanoparticles using innovative techniques which is used as cost-friendly reagent and less reactive. The work symbolizes application of conventional physical and also chemical methods for decolorization of azo dyes. physical method includes osmosis, filtration, adsorption and flocculation. the chemical method (oxidation, electrolysis) and biological method (microorganism, enzymes) are also applicable [8]. Green technology deals with the manipulation of matter at size typically b/w 1-100nm range. Nanoparticles having high surface to volume ratio responsible for enhanced properties [9]. Specific area is appropriate for adsorption property and other relevant properties such as dye removal [10].
Azo dye normally has aromatic structure and N=N bond that’s why they are hardly biodegradable [11,12]. These dyes have also mutagenic and carcinogenic effect. Normally, conventional methods have considerably less potential of degradation. Copper oxide nanoparticles have efficient power of dyes removal [12-17]. Most probably, copper oxide are low cost and novel adsorbent of azodyes. Copper oxide nanoparticle has efficiency of azo dyes removal from wastewater [12]. Malachite green dye (C23H25N2 with molar mass364.911g/mol) is organic in nature. Its lethal dose is 80mg/kg the structure of malachite green dye is in Figure 1 below. Congo red an azo dye is sodium salt of 3,3′-bis structure. Congo red dye is water soluble, its solubility is enhanced in organic solvents. Its molecular formula is C32H22N6Na2O6S2 with molar mass of 696.665 g/mol [13- 14]. The structure is given below Figure 2. The Camellia synesis is evergreen small tree. The Camellia synesis leaves act as capping and reducing agent during the synthesis of metal nanoparticle. There are certain properties of green tea extract such as antitumor, antioxidant, anticoagulant, antiviral, blood pressure and lowering activity [18-22] (Figure 3). Plant extract has some chemicals like phenols, acid, vitamins, responsible for reduction of metal [23]. Camellia synesis leaves have polyphenols, catechins (ECG), OH groups which cause copper metal reduction (Table 1). Copper oxide Cu4O3 is known as paramelaconite material in tetragonal shape. Plants contain a wide range of secondary metabolites included phenolics help a vital role in the reduction of copper metal ions yielding nanoparticles [24]. Thus, ideally be used for the biosynthesis of nanoparticles. Copper oxide Cu4O3 is known as paramelaconite material in tetragonal shape. Copper nanoparticles synthesis by using green tea has Nano range particle size confirmed by characterization [25-28]. This is One-step processes in which no surfactants and other capping agents used.
Aims of Study
The main aim of the study was
To extract copper nanoparticles using camellia sinensis leaves
a) To characterize the copper NPs
b) To study its potential to degrade azodyes
c) To find out the effect of different experimental parameters on %degradation.
d) Kinetic study of adsorption of congored and malachite green dye
Method
Material and Method
The material used for the preparation of copper nanoparticles Cu4O3 includes copper sulfate (CuSO4.5H2O from Sigma Aldrich) and camellia sinensis leaves (from botanical garden of institute) for the preparation of green tea extract. All chemicals used were of analytical grade and pure (Figure 4).
Preparation of Green Tea Extract
Green tea leaves of 30g were taken and then washed with distilled water. further, the leaves were dried and then ground. The powder of green tea was used in the formation of extract [29]. The 100ml of deionized water was used. Later, the solution was boiled for 10 minutes and subsequently kept at low temperature after filtration.
Preparation of Cu4O3 Nanoparticles
A copper sulfate soln. of 50ml was added into 5ml of green tea extract. Magnetic stirrer was used for stirring. The color changed from green to pale yellow and finally dark brown confirmed the formation of nanoparticles. After the formation of nanoparticles, solution was centrifuged at the speed of 1000rpm for 20 mins. After the removal of supernatant copper oxide nanoparticles were dried and washed with ethanol. At the end calcination was performed at 500 degree for one hour and resultantly black colored particles were collected for characterization [27-29].
Results
Characterization of Cu4O3 Nanoparticles
UV spectrophotometer, X-ray diffractometer (XRD), Fourier transform infrared spectrophotometer (FTIR) and Scanning electron microscope (SEM) were used in order to characterize the size, shape, chemical and structural composition of Cu4O3 nanoparticles [30]. During the study, the green color soln. transformed into dark brown which confirm the formation of copper oxide nanoparticles.
X-Ray Diffraction Studies
The X-ray diffraction pattern of copper oxide nanoparticles were examined by x-ray diffractometer. To determine the intensity of copper oxide nanoparticles, the powder was added in the XRD cubes for analysis. The resultant pattern of the copper oxide nanoparticles was matched with JCPDS card number (033-0480), the peaks at 2θ intensity 28.09, 30.61, 36.14 and 44.14 and have 112, 103, 202 and 213 patterns respectively. However, average crystal size calculated by the Scherrer equation keeping lemda at 0.154 and FWHM value calculated 0.5 found was 17.2nm. The shapes of the particles of Cu4O3 nanoparticles in XRD was tetragonal [31-33].
Name and Formula
Reference code: 00-033-0480
Mineral name: Paramelaconite
Compound name: Copper OxideEmpirical formula: Cu4O3
Chemical formula: Cu4O3
Ultraviolet Spectroscopy:
The range at which copper oxide nanoparticles appeared was 200-400nm. The maximum absorption peak was confirmed at 280nm which confirmed the copper oxide nanoparticles (Figure 6).
FTIR Analysis:
In the current study, FTIR spectrum was examined to determine the copper nanoparticles functional group peaks. The overall peak was observed in ranged from 400 to 4000cm-1. The spectrum at peak 3310.7cm and 1611.2cm revealing the (Figure 7) presence of alcoholic group. The bands at 3310.7cm- 1, and 2850cm-1 another functional group present are listed in table below (Table 2).
SEM Analysis:
The average particle size of copper nanoparticle was analyzed by SEM model (JSM-6480). The range of grain of copper oxide nanoparticle was calculated about 8.5 ×10-2mm by SEM micrograph. The prepared copper oxide nanoparticles were well dispersed. It was observed that particles were smooth with a tetragonal shape (Figure 8).
Removal of Malachite Green and Congo Red Azo Dye by Cu4o3 Nanoparticles
Preparation of Standard Solution: In 1-liter distilled water, the dye was dissolved to prepare 1000ppm solution of malachite green and Congo red. From stock solution different concentrations of dyes were prepared. After dilution from 1000ppm solution to 100ppm solution was prepared. From that 150, 200, 250-ppm solution were prepared. Efficiency of Color removal was calculated by percentage degradation formula
% decolorization of dye= A-B /A×100.
Where A and B are absorbance of dye solution without nanoparticles and with particles respectively.
Mechanism of Azodye Degradation
50 microliter of the hydrogen peroxide H2O2 was added as the oxidizing agent to yield hydroxyl radical. Catalytic activity process mainly depends on the formation of superoxide anion radical and hydroxyl radical. The concentration of CR and MG dyes in aqueous solutions were measured by UV–vis spectrophotometer. A reducing agent H2O2 was added with adsorbent to check the adsorption capacity.
Effect of Experimental Parameters On % Degradation of Dye Removal
Time effect: Effect of time on percentage degradation of azo dyes was also studied by UV spectrophotometer. The samples of copper oxide NPs synthesized by green tea C-1, C-2(GT) were calculated. The time required for removal of above said dye was between (40-45min) and percentage removal was observed for all samples between 70-75%. The result of graphs clearly shows the time effect on color degradation of azo dye malachite green-MG and acid red 28-CR by using adsorbent copper oxides nanoparticles. The experimental conditions during experiment were kept constant just like temperature 308 kelvin and initial concentration of adsorbent was within ranges from 20- 250mg/l. Samples C-1, C-2 are samples codes synthesized by camellia sinensis leaves extract at different temperatures. In figure below C-1 sample is dye+ adsorbent +H202 and C-2 sample without reducing agent. It was concluded from graphs %degradation enhanced in presence of reducing agents. Figure 9 Effect of time by copper oxide nanoparticles samples C-1, C-2(Green tea mediated) on malachite green dye and Congo red dye calculated by ultraviolet spectrophotometer DB-20.
Adsorption Kinetics Studies: The kinetics of azo dye adsorption was carried under selecting optimum operating conditions. The kinetic parameters are helpful for the estimation of adsorption rate. A solution prepared by dissolving 20mg of adsorbent in 50ml of 10ppm dyes and continuously stirred.
Adsorption Kinetic Studies of Copper Oxide NPs: The pseudo-second-order model was found to explain the adsorption kinetics most effectively. The results indicated a significant potential of nanoparticles as an adsorbent for azo dye removal. The straight line shows that nanoparticles follow pseudo-second-order kinetics rather than first orde
Adsorption Reaction Isotherm Models
Langmuir Isotherm Model: The Langmuir isotherm is applicable for adsorption of a solute as monolayer adsorption on a surface having few numbers of identical sites. Langmuir isotherm model provide energies of adsorption onto the plain. That’s why, the Langmuir isotherm model is selected for adsorption capacity relating to monolayer surface of adsorbent. Adsorption process fits the Langmuir and pseudo-second-order models. Langmuir isotherm or single crystal surfaces describes well adsorption at low medium coverage, adsorption into multilayer is ruled out. Parameters of different models studied in this research are listed below in Table 3.
Freundlich Isotherm Model: The Freundlich isotherm model is suitable for the adsorption of dye on the adsorbent. Freundlich equation is stated below
In qe = Kf qm+ 1/n InCe
qe is the amount used of azo dye in unit of mg/g, Ce is the equilibrium concentration of the azo dye and Kf and n are constants factors affecting the capacity of adsorption and adsorption speed. The graph between lnqe versus ln Ce shows linearity. The adsorption reaction isotherms are fitted to models by linear square method. The result shows in this study that Langmuir model fit better than the Freundlich model. The adsorption activity of copper oxide nanoparticle samples prepared by green source were observed against the degradation of malachite green and congored azodyes (Figure15).
Discussion
In present we reported an eco-friendly and cost-efficient preparation of copper oxide nanoparticles by leaf extract of camellia sinensis. the characterization of particles were performed by SEM, UV, XRD, FTIR analysis. UV spectroscopy peak was observed at 280nm and a broadband observed which confirmed nanoparticles existence. The particle size was calculated by Scherrer equation was 17.26nm. The SEM results confirmed tetragonal shape of cu403 particles with grain average diameter 8.5×10-2nm, and FTIR spectra indicated the peaks of OH, C=C, C-H functional groups, which is due to thin coating of extract on nanoparticles. The calculated surface area of nanoparticles was 65m2/g. The %degradation of azo dyes malachite green and congored range were b/w70-75% at maximum 0.2g/l and 20mg/l dosage of adsorbent and dye. The optimum time was b/w 30-40mint, PH 3-4, temperature 70-80 Co for maximum degradation. The effect of different experimental parameters was studied on percentage degradation of dyes. The azo dyes congored and malachite green dyes adsorption isotherm models were studied. The reaction kinetics followed pseudo second order for both dyes rather than first order. The Langmuir model fit better with linearity rather than Freundlich, which confirmed by graph having r2 0.98,0.99and0.95 values for models. The elovich model also linear fit. In conclusion, copper oxide nanoparticles keep excellent azo dyes degradation potential.
Conclusion
In present we reported an eco-friendly and cost-efficient preparation of copper oxide nanoparticles by leaf extract of camellia Sinensis. According to kinetic study it proved that Cu4O3 NPs keep excellent adsorption capability for MG and CR azo dyes.
https://lupinepublishers.com/chemistry-journal/pdf/AOICS.MS.ID.000174.pdf
https://lupinepublishers.com/chemistry-journal/fulltext/kinetic-isotherm-studies-of-azo-dyes-by-metallic-oxide-nanoparticles-adsorbent.ID.000174.php
For more Lupine Publishers Open Access Journals Please visit our website: https://lupinepublishersgroup.com/
For more Open Access Journal on Chemistry articles Please Click Here:
https://lupinepublishers.com/chemistry-journal/
To Know More About Open Access Publishers Please Click on Lupine Publishers
Follow on Linkedin : https://www.linkedin.com/company/lupinepublishers
Follow on Twitter : https://twitter.com/lupine_online
0 notes
Text
The eyes have been used to signify a perverse capacity - honed to perfection in the history of science tied to militarism, capitalism, colonialism, and male supremacy - to distance the knowing subject from everybody and everything in the interests of unfettered power. The instruments of visualization in multinationalist, postmodernist culture have compounded these meanings of disembodiment. The visualizing technologies are without apparent limit. The eye of any ordinary primate like us can be endlessly enhanced by sonography systems, magnetic reasonance imaging, artificial intelligence-linked graphic manipulation systems, scanning electron microscopes, computed tomography scanners, color-enhancement techniques, satellite surveillance systems, home and office video display terminals, cameras for every purpose from filming the mucous membrane lining the gut cavity of a marine worm living in the vent gases on a fault between continental plates to mapping a planetary hemisphere elsewhere in the solar system. Vision in this technological feast becomes unregulated gluttony; all seems not just mythically about the god trick of seeing everything from nowhere, but to have put the myth into ordinary practice. And like the god trick, this eye fucks the world to make techno-monsters.
- Donna Haraway, Situated Knowledges
Since reading this Letterboxd review, I’ve been thinking all day about how Mamoru Oshii put Donna Haraway in Ghost In The Shell 2, presumably in tribute to her Cyborg Manifesto, but he could as well have put her in Patlabor 2: The Movie in tribute to her Situated Knowledges. Anyway, took the excuse to post my favourite paragraph from maybe my favourite philosophy essay.
25 notes
·
View notes
Photo

Leishmaniasis and Red Blood Cells . Color enhanced scanning electron micrograph (SEM) showing a Leishmaniasis parasite and red blood cells. Leishmaniasis is a protozoan parasite disease of vertebrates spread by the bite of the female sandfly (Leishmania sp.). There are at least 18 different types of leishmaniasis and it exists almost everywhere in the world. After infection, an inflamed swelling develops at the area of the sandfly bite. Leishmaniasis can present itself in three ways… cutaneous, mucocutaneous, or visceral. The cutaneous form presents with skin ulcers while the mucocutaneous form presents with ulcers of the skin, mouth, and nose. The visceral form starts with skin ulcers; later it presents with fever, anemia, and enlarged spleen and liver. Risk factors include poverty, malnutrition, deforestation, and urbanization. Tissue specimens, such as from skin sores (for cutaneous leishmaniasis) or from bone marrow (for visceral leishmaniasis), can be examined for the parasite under a standard light microscope. Magnification: 6,000x when printed at 10 cm wide. . © Eye of Science/Science Source . . Follow @atomstalk . . . . #microbiologist #microalgae #fungusfriday #fungusphotography #microscopic #microscope #microscopy #microscopes #microworld #redbloodcells #redbloodcell #fungus https://www.instagram.com/p/CCGmFqHjPKB/?igshid=869v2uhhns5a
#microbiologist#microalgae#fungusfriday#fungusphotography#microscopic#microscope#microscopy#microscopes#microworld#redbloodcells#redbloodcell#fungus
0 notes
Text
High-Fat Diet May Fuel Spread of Prostate Cancer
High-Fat Diet May Fuel Spread of Prostate Cancer
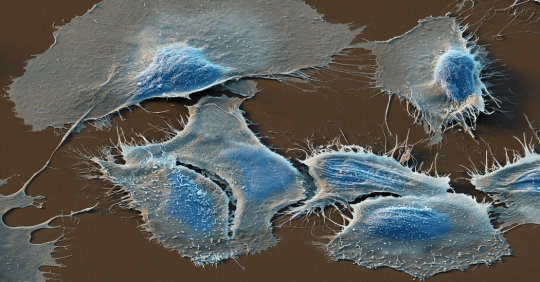
Health|High-Fat Diet May Fuel Spread of Prostate Cancer
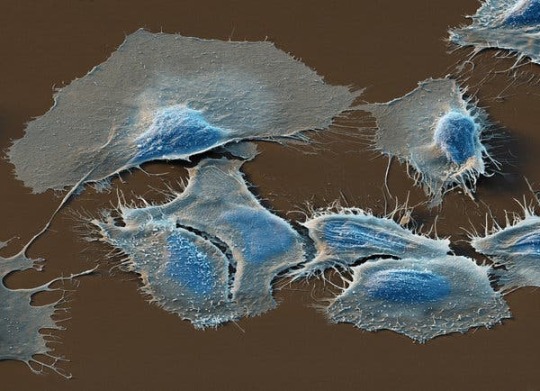
A color-enhanced scanning electron microscope image of prostate cancer cells. A new study suggests that dietary fat may feed prostate tumors and help them spread.Credit…Eye of Science/Science Source
Obesity is linked to prostate cancer, scientists know, but it’s not clear why. On Monday, researchers reported a surprising connection.
When…
View On WordPress
0 notes
Text
Alpelisib throughout Coronavirus Illness 2019 (COVID-19) therapy: an assessment of data
henryi and also T. rosthornii are generally strongly related, and that we propose their particular category in to #Link# subsect. Leucolirion 6a. Our own final results assist Comber's subdivision associated with sect. Leucolirion, which was dependent in light color. Chinese varieties were separated into 5 sections: sect. Martagon, sect. Archelirion, sect. Leucolirion, sect. Sinomartagon, as well as sect. Lophophorum. These bits of information help with our understanding of the particular phylogeny, beginning, and group associated with Lilium.This research looks into the consequences of all-natural growing older as well as cold operating just before man-made aging in microstructures along with physical qualities regarding Al-4.6Cu-0.5Mg-0.5Ag alloy. Hardware attributes when compared with microstructure variants have been elucidated with the findings from the to prevent microscope (OM), differential scanning calorimeter (DSC), power conductivity gauge (% IACS), and indication electron microscopy (TEM). The final results indicated that normal aging treatment method offers minor obvious gain around the level of precipitation conditioning periods and mechanical attributes, nevertheless it enhances the rain conditioning rate in the preliminary phase involving synthetic growing older. Frosty functioning brings more lattice flaws which in turn curb Al-Cu (GP zone) as well as Mg-Ag clustering, and so the rain involving Omega period lessens. Additionally, a lot more dislocations are formed, ultimately causing precipitate the greater heterogeneous nucleation regarding theta' cycle. Your above-mentioned rain phenomena and tension hardening influence tend to be more obvious together with increased degrees of cool operating.Parathyroid carcinoma is an rare malignancy along with a unusual reason for principal hyperparathyroidism. Even though this tumour can do #Link# metastasis, metastatic ailment is very unusual intracranially, with only several cases noted in the literature. Whenever intracranial metastases take place, they typically current several weeks for you to decades following the diagnosis of the principal #Link# growth along with hypercalcemia refractory to be able to healthcare traditional treatment method. Aggressive operative resection of all metastases is essential regarding charge of the illness. Many of us record an instance of metastatic parathyroid carcinoma with a couple of intracranial metastatic foci (within the left front lobe and left cerebellar hemisphere) recognized before the key cancer prognosis in the patient who presented with pointing to hypercalcemia.Rhabdoid tumours (RTs) tend to be uncommon, very aggressive tumours regarding undetermined beginning in human beings, and they are sub-classified because renal/extrarenal RTs depending on place. The particular origins of extrarenal rhabdoid tumours are generally an enigma and also neoplasms get hardly ever been recently reported inside non-primate types. A good 11-year-old man Maltese canine has been given the submandibular bulk. Histologically, the actual muscle size has been composed of bedding regarding very pleomorphic "rhabdoid" tissue, more recognized through the existence of huge epithelioid tissues along with globular/fibrillar paranuclear inclusions. Additional immunohistochemical analysis said the actual neoplastic tissues had been good pertaining to vimentin along with desmin just like individual tumours. Additionally, ultrastructural examination showed that your intracytoplasmic inclusions were mainly composed of whorled lots of intermediate filaments. Each of our benefits suggest a valuable analytical method of cutaneous, extrarenal rhabdoid tumours inside canines as well as identify their qualities.
0 notes
Text
HOW A PHOTOCOPY Machine Functions
A photocopier (also acknowledged as a copier or copy equipment) is a machine that makes copies of files and other visual photos on to paper or plastic movie rapidly and cheaply. Most contemporary photocopiers use a technologies called xerography, a dry procedure that utilizes electrostatic fees on a mild-sensitive photoreceptor to very first appeal to and then transfer toner particles (a powder) onto paper in the type of an image. Warmth, force or a combination of equally is then employed to fuse the toner on to the paper. Copiers can also use other technologies this sort of as ink jet, but xerography is standard for workplace copying. Previously variations provided the Gestetner stencil duplicator, invented by David Gestetner in 1881.
Business xerographic office photocopying was released by Xerox in 1959,[one][two] and it slowly changed copies made by Verifax, Photostat, carbon paper, mimeograph equipment, and other duplicating devices.
Photocopying is commonly employed in the organization, education, and government sectors. ΚΑΙΝΟΥΡΙΑ ΦΩΤΟΤΥΠΙΚΑ there have been predictions that photocopiers will eventually turn out to be obsolete as details workers increase their use of digital document development, storage and distribution, and depend significantly less on distributing genuine items of paper, as of 2015, photocopiers continue to be widely utilized. In the 2010s, there is a convergence in some substantial-stop equipment between the roles of a photocopier, a fax equipment, a scanner, and a computer network-connected printer into a multi-operate printer. Reduced-stop equipment that can duplicate and print in coloration have ever more dominated the property-office market place as their costs fell steadily by means of 2017. Increased-conclude shade photocopiers capable of handling large obligation cycles and huge-structure printing stay a costlier specialty for print and layout shops.
Contents
1 History
one.one Shade photocopiers
1.two Electronic technological innovation
2 How it performs (making use of xerography)
three Copyright troubles
4 Counterfeiting
5 Wellness issues
six Forensic identification
seven See also
8 References
9 More looking through

Heritage
Chester Carlson, the inventor of photocopying, was originally a patent legal professional, as properly as a element-time researcher and inventor. His task at the patent place of work in New York essential him to make a large amount of copies of critical papers. Carlson, who was arthritic, located this to be a unpleasant and cumbersome procedure. This inspired him to perform experiments with picture conductivity. Carlson used his kitchen for his "electrophotography" experiments, and, in 1938, he used for a patent for the approach. He made the initial photocopy employing a zinc plate coated with sulfur. The phrases "10-22-38 Astoria" had been written on a microscope slide, which was put on best of more sulfur and below a bright light. After the slide was taken off, a mirror impression of the words and phrases remained. Carlson attempted to offer his creation to some businesses, but failed since the procedure was nonetheless underdeveloped. At the time, a number of copies had been most commonly made at the level of doc origination, using carbon paper or handbook duplicating equipment, and individuals did not see the require for an digital machine. In between 1939 and 1944, Carlson was turned down by more than twenty organizations, which includes IBM and General Electric—neither of which considered there was a important industry for copiers.
In 1944, the Battelle Memorial Institute, a non-profit firm in Columbus, Ohio, contracted with Carlson to refine his new approach. In excess of the subsequent five many years, the institute conducted experiments to enhance the method of electrophotography. In 1947, Haloid Corporation (a little New York-based mostly manufacturer and vendor of photographic paper) approached Battelle to receive a license to produce and industry a copying device based on this engineering.[two]
Haloid felt that the phrase "electrophotography" was also challenging and did not have very good remember worth. Following consulting a professor of classical language at Ohio State College, Haloid and Carlson altered the identify of the process to "xerography," which was derived from Greek terms that intended "dry creating." Haloid named the new copier equipment "Xerox Devices" and, in 1948, the word "Xerox" was trademarked. Haloid ultimately modified its name to Xerox Company.
In 1949, Xerox Corporation introduced the first xerographic copier known as the Design A.[3] Xerox became so productive that, in North The united states, photocopying came to be popularly known as "xeroxing." Xerox has actively fought to prevent "Xerox" from becoming a genericized trademark. Whilst the phrase "Xerox" has appeared in some dictionaries as a synonym for photocopying, Xerox Corporation usually requests that this kind of entries be modified, and that people not use the time period "Xerox" in this way. Some languages consist of hybrid terms, such as the widely utilised Polish expression kserokopia ("xerocopy"), even though fairly couple of photocopiers are of the Xerox model.
In the early nineteen fifties, Radio Corporation of America (RCA) released a variation on the process known as Electrofax, whereby pictures are formed directly on specially coated paper and rendered with a toner dispersed in a liquid.
Throughout the 1960s and by way of the 1980s, Savin Corporation developed and sold a line of liquid-toner copiers that executed a technology dependent on patents held by the firm.
Prior to the common adoption of xerographic copiers, photograph-direct copies made by devices this sort of as Kodak's Verifax ended up employed. A major impediment connected with the pre-xerographic copying technologies was the large expense of provides: a Verifax print necessary supplies costing US$.fifteen in 1969, even though a Xerox print could be created for $.03 including paper and labor. The coin-operated Photostat equipment even now identified in some public libraries in the late sixties produced letter-dimension copies for $.twenty five each, at a time when the least wage for a US worker was $1.sixty five for each hour the Xerox devices that replaced them normally charged $.10.
Xerographic copier manufacturers took edge of a high perceived-price of the sixties and early 1970s, and promoted paper that was "specially made" for xerographic output. By the finish of the nineteen seventies, paper producers manufactured xerographic "runability" one of the needs for most of their place of work paper brand names.
DADF or Duplex Computerized Doc feeder - Canon IR6000
Some products sold as photocopiers have changed the drum-based mostly method with inkjet or transfer movie technology.
Among the key advantages of photocopiers over before copying systems are their ability:
to use simple (untreated) business office paper,
to apply duplex (or two-sided) printing,
Able to scan many pages instantly with an ADF, and
eventually, to sort and/or staple output.
Coloration photocopiers
Coloured toner grew to become accessible in the fifties, although full-colour copiers were not commercially available until finally 3M unveiled the Coloration-in-Color copier in 1968, which employed a dye sublimation approach rather than standard electrostatic engineering. The initial electrostatic coloration copier was introduced by Xerox (the 6500) in 1973. Colour photocopying is a problem to governments, as it facilitates counterfeiting forex and other paperwork: for a lot more information, see Counterfeiting area.
Digital engineering
There is an escalating trend for new photocopiers to adopt digital technologies, thus replacing the older analog engineering. With digital copying, the copier efficiently consists of an integrated scanner and laser printer. This design has several benefits, this kind of as computerized impression high quality enhancement and the potential to "construct employment" (that is, to scan page photographs independently of the procedure of printing them). Some electronic copiers can function as high-velocity scanners this sort of versions typically provide the capacity to send documents by means of e-mail or to make them obtainable on file servers.
A wonderful gain of electronic copier technology is "computerized digital collation." For instance, when copying a established of 20 pages twenty occasions, a electronic copier scans every website page only as soon as, then utilizes the stored details to create 20 sets. In an analog copier, both every website page is scanned 20 moments (a total of 400 scans), making a single established at a time, or 20 different output trays are used for the 20 sets.
Low-finish copiers also use electronic technologies, but have a tendency to consist of a common Pc scanner coupled to an inkjet or minimal-finish laser printer, each of which are considerably slower than their counterparts in large-conclude copiers. Nonetheless, low-stop scanner-inkjets can offer shade copying at a decrease purchase cost but with a much increased expense for every copy. The cost of electronics is such that blended scanner-printers occasionally have created-in fax machines. (See Multifunction printer.)
0 notes