#actinium 225 cancer treatment
Link
With over 20 years of experience, Dr. Ishita B Sen is currently the Director and Head of the Department of Nuclear Medicine & Molecular Imaging at Fortis Memorial Research Institute (FMRI), Gurgaon.
#177lu#177lu-psma-617#actinium 225 cancer treatment#lu 177 cancer treatment#mibg therapy neuroblastoma#nuclear medicine in treatment#nuclear medicine therapy#Dr. Ishita B. Sen#Nuclear Medicine Therapy in India#Nuclear Medicine Expert in India
0 notes
Photo

Researchers streamline production of purified actinium-225 isotope, for potential cancer treatments
Thanks to a recent upgrade to the medical isotope facilities at the U.S. Department of Energy's (DOE) Brookhaven National Laboratory, actinium-225 (Ac-225), an isotope that shows great promise for treating cancer, can now be produced, purified, and shipped ready for use directly from the Lab. The first shipment left Brookhaven in mid-March.
"This upgrade will streamline the overall production and distribution of Ac-225 to research centers by eliminating the need to ship material off site for final processing," said Cathy Cutler, Director of Medical Isotope Research & Production (MIRP) at Brookhaven Lab.
The promise of Ac-225 stems from the way this radioactive isotope decays. It emits alpha particles, which can deliver a lethal punch to cancerous cells over a short distance. Attaching Ac-225 to molecules that target tumors could potentially kill cancer cells without harming surrounding tissue. Ac-225 can also be used to generate bismuth-213, another alpha-emitter.
Read more.
16 notes
·
View notes
Text
How is radioligand therapy developed?
In the changing dynamics of medical science, innovations are the hope for people fighting life-threatening illnesses. Radioligand treatment, commonly denoted to as targeted radionuclide treatment, is one such pioneering method that has been generating waves in the field of oncology. While the name may sound multifaceted, the concept is quite attractive, providing a promising avenue for cancer treatment.
The radioligand therapy market is experiencing growth and is projected to reach USD 13,073.9 million by 2030.

Understanding the Basics
Radioligand treatment includes the utilization of dedicated molecules, called radioligands, which are made to target specific receptors on cancer cells. Such radioligands are fortified with a radioactive isotope, like actinium-225 or lutetium-177. When directed into the patient's body, such radioligands seek out and assign themselves to the receptors on cancer cells, providing a powerful dose of radiation straight to the tumor.
Exactness and Minimalized Side Effects
One of the major benefits of radioligand treatment is its accuracy. Not like conservative radiation treatment, which can affect healthy tissues covering the tumor, radioligand treatment selectively targets cancer cells. This targeted method not only increases the efficiency of treatment but also reduces the harm to healthy tissue, decreasing the danger of side effects.
Prostate Cancer Treatment
Prostate cancer is one of the zones where radioligand treatment has revealed marvelous potential. The FDA sanction of treatments such as Lutathera® for neuroendocrine Xofigo® and tumors for metastatic castration-resistant prostate cancer establishes the rising recognition of radioligand therapy's potential.
The Patient Experience
For patients, radioligand treatment can be the only hope in a complex diagnosis. The treatment is generally managed as an outpatient process, permitting patients to back to their everyday lives comparatively rapidly. Although there might be some side effects, like nausea or fatigue, they are typically wieldy and temporary.
Ongoing Research and Growth
The arena of radioligand treatment is constantly changing. Investigators are discovering new radioligands and radioisotopes, directing to increase the variety of cancers that can be efficiently treated using this method. Clinical trials are ongoing, providing patients the chance to access pioneering treatments.
Challenges
While radioligand treatment has great potential, it is not without challenges. Obtainability and price can be fences for some patients. Moreover, the logistics of managing radioactive materials need particular amenities and expertise.
The U.S. Is Principal Contributor to Market
The market for radioligand therapy in the U.S. will witness a high growth rate during the forecast period. The growth is attributed to the government initiatives for improving chronic disease care and the rising number of specialized cancer hospitals and research centers. For instance, in February 2022, the U.S. president reinvigorated the Cancer Moonshot Program with new goals, to reduce the death rate from cancer by at least 50% over the next 25 years. Thus, the rising number of government initiatives for advancing oncologic practices augments the market for radioligand therapy in the U.S.
0 notes
Text
Research team develops new system for imaging and treating tumors
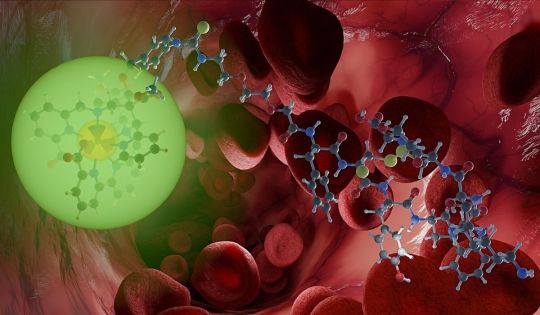
Research team develops a new system for imaging and treating tumors.
May 26, 2023 - Thanks to the radiation they emit, radioactive compounds are suited both to imaging and treating cancers. By appropriately combining them in the novel, so-called radionuclide theranostics, both applications can be dovetailed. A radiopharmacy team at Helmholtz-Zentrum Dresden-Rossendorf (HZDR) and Heidelberg University has now presented such a system in the Journal of the American Chemical Society (DOI: 10.1021/jacs.2c08438) that successfully solves one of the biggest problems to date: it works at physiologically relevant temperatures.
“Basically, we can think of it as functioning like a smart key that we use to control our automobiles. We use so-called radionuclides, i.e., unstable atomic nuclei, that spontaneously emit ionized radiation when they decay. We track down the tumor with a diagnostic radionuclide. The targeted internal irradiation close to the diseased tissue is then taken on by a different, therapeutic radionuclide,”
says Dr. Manja Kubeil of HZDR’s Institute of Radiopharmaceutical Cancer Research, describing her theranostic approach.
Her team in the Department of Radionuclide Theragnostics develops exactly these types of substances to track and destroy tumors. Thus, the researchers employ matched pairs of radionuclides which, due to their decomposition characteristics, can be used both for imaging and for tumor therapy on the same target molecule.
The relevant radionuclide is stably bound in what is known as a chelator and linked to a biomolecule by a kind of chemical bridge.
“The word chelator comes from the Latin; its stem relates to being encircled by the claws of a crayfish. We prefer the image of a molecular cage that firmly encloses the radionuclide so that it can’t spread in the body uncontrollably. The target-seeking biomolecule for its part must fit perfectly with the docking site on the cancer cells, just like a key in a lock. The radionuclide then accumulates on the tumor tissue and exclusively develops its destructive impact there – that’s the idea,”
says Kubeil
Stable bonds at practicable temperatures
Lutetium-177, for instance, is particularly suitable as a beta emitter for releasing electrons to treat various tumors as well as a source of gamma rays for imaging. Actinium-225, an alpha emitter that can be used for efficient treatment, is even more effective in destroying tumors and is also very tightly bound by the chelator. Neither radionuclide occurs naturally on Earth. Appropriate methods have to be used to produce them artificially.
Alpha emitters release particles composed of two protons and two neutrons. They are used in cancer therapy because their range in the tissue is very small, but they nonetheless attack and kill cancer cells very effectively thanks to their high energy. Their half-life of seven days in the case of Lutetium-177 and ten in the case of Actinium-225 is ideal for the purpose: it is long enough to enable effective treatment.
New chelator with advantages
So far, there has only been one complexing agent on the market that binds both radionuclides equally well: DOTA. The most frequently used chelator in nuclear medicine is known for its very stable metal complexes. But DOTA has one big disadvantage: only at what are very high temperatures for biochemical conditions, beyond 80 degrees Celsius, is it possible to bond theranostic radionuclides completely.
“If you are working with protein derivatives, these temperatures are way too high because even at 40 degrees Celsius denaturation kicks in: they are destroyed. Our new chelator system functions reliably at these lower temperatures,”
Kubeil is pleased to report.
Moreover, under these milder conditions, it achieves faster radiolabeling than the known chelators. Another advantage is that the new system efficiently attaches to various bioconjugates. This means an increase in the choice of docking sites on diseased tissue. The newly developed chelator could thus form the basis for new modular and personalized pharmaceutical systems that could be geared towards different fields for imaging and therapy by simply exchanging partial chemical structures.
Publication:
P. Cieslik, M. Kubeil, K. Zarschler, M. Ullrich, F. Brandt, K. Anger, H. Wadepohl, K. Kopka, M. Bachmann, J. Pietzsch, H. Stephan, P. Comba, Toward Personalized Medicine: One Chelator for Imaging and Therapy with Lutetium-177 and Actinium-225, Journal of the American Chemical Society, 2022 (DOI: 10.1021/jacs.2c08438)
Read the full article
1 note
·
View note
Text
Chemists develop faster way to purify elements
Washington (UPI) Jun 4, 2019
Scientists at the Lawrence Berkeley National Laboratory have developed a faster way to purify elements. The breakthrough could help researchers discover new elements, reprocess nuclear fuel more efficiently and isolate actinium-225, an isotope with promise as a cancer treatment. The involved chemists described their new process this week in the Nature Communications. "Our propose
Full article
26 notes
·
View notes
Text
SpectronRx Receives U.S. Nuclear Regulatory Commission Materials License for New Facility

Indiana-based radiopharmaceutical Contract Development and Manufacturing Organization expands nuclear pharmacy capabilities with second location
SpectronRx, a leading radiopharmaceutical contract development and manufacturing organization (CDMO), announced today that the U.S. Nuclear Regulatory Commission (NRC) has issued a Materials License for its new Indianapolis, IN headquarters. The license also expands SpectronRx’s roster of authorized nuclear pharmacists and authorized users. The news comes as SpectronRx continues to scale its early-stage development and commercialization services for leading pharmaceutical companies working to develop and deploy radiopharmaceutical compounds for the treatment and detection of certain cancers and other diseases.
“Securing Nuclear Regulatory Commission materials licensing for our new headquarters and additional staff is a significant milestone for the growth of SpectronRx,” said John Zehner, CEO of SpectronRx. “We now have the necessary approvals to scale our newly opened 60,000 square foot Indianapolis facility. This is great news for both patients and the State of Indiana alike, as it means a bigger pipeline for life-saving therapies and more jobs for medical professionals specializing in radiopharmaceuticals.”
SpectronRx now has two Indiana locations; a 6,300 Sq. Ft. facility located at 17490 Dugdale Dr. in South Bend, and a new 60,000 Sq. Ft. facility at 9550 Zionsville Rd. in Indianapolis. SpectronRx opened its South Bend facility in 2016, and has now relocated its headquarters to the new Indianapolis location. SpectronRx also has plans to expand into the European Union.
“In response to increased demand for early phase therapeutic and diagnostic development services, SpectronRx has experienced significant growth as an early-stage contract developer for global and domestic life sciences companies,” added Anwer Rizvi, President of SpectronRx. “The scope of our new location and materials license gives us the ability to operate as a large-scale developer, manufacturer and nuclear pharmacy.”
The NRC materials license authorizes that SpectronRx can receive, acquire, possess and transfer byproduct, source, and special nuclear material in chemical and/or physical form. This includes any byproduct material with Atomic Numbers 1 through 83 with a half-life less than or equal to 120 days, with some exceptions. The license lists more than 25 different isotopes, ranging from Lutetium-177, Actinium-225, Iodine-131,and Iodine-123. Authorized uses include the preparation and distribution of radioactive drugs and radiochemicals for medical use to authorized recipients.
To learn more, visit SpectronRx.com.
The post SpectronRx Receives U.S. Nuclear Regulatory Commission Materials License for New Facility appeared first on .
from https://pharmaphorum.com/partner-content/spectronrx-receives-u-s-nuclear-regulatory-commission-materials-license-for-new-facility/
0 notes
Text
Alpha Emitter Market to Gain a Stronghold During COVID-19 Pandemic Period | 2020-2026
New York, Oct 2020: Axiom Market Research & Consulting™ added a report on global alpha emitter market which includes study on radionuclide type, medical application, end user across various countries of key regions across the globe. The global alpha emitter market was projected to grow at a CAGR of 23.44% for the forecast period 2020 to 2026. The global market is estimated and forecasted in terms of revenue (USD Million) generated by the alpha emitter market.
The report analyses the global alpha emitters market based on radionuclide type, medical application, end user and geography. Various radionuclide type studied are segmented into Actinium (Ac-225), Astatine (At-211), Bismuth (Bi -213), Bismuth (Bi-212), Lead (Pb-212), Radium (Ra-223) and Terbium (Tb-149). The medical application segment is categorized into prostate cancer, bone metastases, ovarian cancer, pancreatic cancer, endocrine tumors and others. Furthermore, the major end users considered in the study are hospitals, clinics and research laboratories. The global market is studied for the key regions such as, North America, Europe, Asia Pacific and Rest of the World.
The major players operating in the alpha emitter market include Actinium Pharmaceuticals (United States), Alpha Tau Medical (Israel), Bayer AG (Germany), Fusion Pharmaceuticals Inc. (Canada), IBA RadioPharma Solutions (Belgium), Orano Med (France), Plexus Corp. (Columbia), RadioMedix, Inc. (United States) and Telix Pharmaceuticals Ltd (Australia).
Get Sample Of This Research Report@ https://www.axiommrc.com/request-for-sample/report?=1717
Alpha Emitter Market Regional Trends
The North America accounted for the largest market share in 2019 in terms of revenue followed by Europe. Owing to the fact that the increase in oncology and neurological chronic diseases is the major driving factor for the market growth in the NA region. Moreover, the Asia Pacific alpha emitters market is anticipated to grow at a fastest growth rate during the forecast period. Over the forecast period, the phenomenon anticipated to boost market expansion, is the rise in number of healthcare facilities and improvement in care provision.
Alpha Emitter Market Segmental Highlights
· The Radium (Ra-223) segment dominated the global alpha emitters market in 2019
· Increase in awareness of the potential benefits of targeted alpha emitter and high number of patients with various types of cancers such as ovarian cancer, pancreatic cancer, lymphoma, and melanoma are likely to augment market growth
· The ovarian cancer segment dominated the global alpha emitters market, by medical application in 2019
· Use of targeted anticancer or alpha therapy (TAT) in the treatment of cancer and alpha particles to boost growth
· In addition, increasing use of targeted anticancer or alpha therapy (TAT) in the treatment of cancer and alpha particles has an advantage in targeted therapy because of their exceptionally high cell-killing ability. Ovarian cancer uses radio immunotherapy as locally injected adjuvant therapy.
Research Objectives of Alpha Emitter Market Report:
· Analyzing the outlook of the market with the recent trends and Porter’s five forces analysis.
· Market dynamics, which essentially consider the factors that are impelling the present market scenario, along with growth prospects of the market over the forecast period.
· Market segmentation analysis, including qualitative and quantitative research, incorporating the impact of economic and non-economic aspects.
· Country-level analysis, integrating the demand and supply forces that are influencing the growth of the Alpha Emitter Market.
· Competitive landscape involving the market share of major players, along with the key strategies adopted for development over the past five years.
· Comprehensive company profiles, covering the product offerings, key financial information, recent developments and strategies employed by the major market players
Request To Buy This Research Report@ https://www.axiommrc.com/buy_now/1717-alpha-emitters-market-report
About Us:
Axiom Market Research & Consulting™ is a full-service market research and data analytics company providing key market intelligence to global companies to take informed business decisions pertaining to their marketing strategy, investments, new product launches, market competition, consumer or end users, social media trends etc.
Axiom Market Research & Consulting™ offers market research services such as syndicated market research, custom market research, business consulting, and consumer/end user surveys. Under Business to Consumer (B2C) market research offerings, Axiom MRC assists its clients in finding quantitative information/preferences of its brands and services such as, awareness, usages, satisfaction, tracking, ethnicity etc. Axiom MRC offers data collection services through online surveys, social media, data processing and interpretation.
Axiom MRC with its experienced team of research and data analysts, has delivered more than 700+ Market Research Projects, 2100+ Data Analytics Projects, 260+ Business Support Projects and has a 400+ Global Client Base. Axiom Market Research & Consulting™ aims to become the preferred market research and data Analytics Company by providing key market intelligence solutions for client’s business growth.
For more information, visit Axiom Market Research & Consulting™ at www.axiommrc.com
Contact Us:
Axiom Market Research & Consulting
United States
3 Germay Dr.Ste 4 - 4666
Wilmington DE 19804
U.S.:- + 1 (845) 875-9786
U.K.:- + 44 (0) 20 3286 9707
Email: [email protected]
Website: https://www.axiommrc.com
0 notes
Text
Separation anxiety no more: A faster technique to purify elements
Researchers have developed a new chemical separation method that is vastly more efficient than conventional processes, opening the door to faster discovery of new elements, easier nuclear fuel reprocessing, and, most tantalizing, a better way to attain actinium-225, a promising therapeutic isotope for cancer treatment.
Separation anxiety no more: A faster technique to purify elements syndicated from https://triviaqaweb.blogspot.com/
0 notes
Link
Best PRRT Therapy Centre for Neuroendocrine Tumours in India. To know more about what is PRRT, how it`s performed, benefits & its side-effects visit nuclearmedicinetherapy.in.
#actinium 225 cancer treatment#actinium therapy#peptide receptor radionuclide therapy#prrt#prrt in neuroendocrine tumors#prrt therapy side effects#prrt treatment#prrt treatment cost in india#prrt treatment for neuroendocrine tumors#Alpha PRRT in India#Ac 225 Therapy in India#Actinium Ac 225 Alpha PRRT#Actinium Therapy in India#Actinium 225 Therapy in India#PRRT in India#PRRT Treatment in India#PRRT Therapy
0 notes
Text
Separation anxiety no more: A faster technique to purify elements
Researchers have developed a new chemical separation method that is vastly more efficient than conventional processes, opening the door to faster discovery of new elements, easier nuclear fuel reprocessing, and, most tantalizing, a better way to attain actinium-225, a promising therapeutic isotope for cancer treatment.
Latest Science News -- ScienceDaily https://www.sciencedaily.com/releases/2019/06/190604084907.htm
0 notes
Text
Separation nervousness no extra: A quicker approach to purify parts
http://tinyurl.com/y5u5ehbn
IMAGE: (From left) Rebecca Abergel, Abel Ricano, and Gauthier Deblonde of Berkeley Lab’s Chemical Sciences Division have pioneered a quicker technique of purifying parts. view more Credit score: Marilyn Chung/Berkeley Lab The actinides – these chemical parts on the underside row of the periodic desk – are utilized in purposes starting from medical remedies to area exploration to nuclear power manufacturing. However purifying the goal ingredient so it may be used, by separating out contaminants and different parts, might be troublesome and time-consuming. Now researchers on the Division of Power’s Lawrence Berkeley Nationwide Laboratory (Berkeley Lab) have developed a brand new separation technique that’s vastly extra environment friendly than typical processes, opening the door to quicker discovery of latest parts, simpler nuclear gas reprocessing, and, most tantalizing, a greater technique to attain actinium-225, a promising therapeutic isotope for most cancers therapy. The analysis, “Extremely-Selective Ligand-Pushed Separation of Strategic Actinides,” has been printed within the journal Nature Communications. The authors are Gauthier Deblonde, Abel Ricano, and Rebecca Abergel of Berkeley Lab’s Chemical Sciences Division. “The proposed method provides a paradigm change for the manufacturing of strategic parts,” the authors wrote. “Our proposed course of seems to be far more environment friendly than current processes, includes fewer steps, and might be performed in aqueous environments, and subsequently doesn’t require harsh chemical compounds,” mentioned Abergel, lead of Berkeley Lab’s Heavy Element Chemistry group. “I feel that is actually essential and will probably be helpful for a lot of purposes.” Berkeley Lab is certainly one of a handful of establishments around the globe finding out the nuclear and chemical properties of the heaviest parts. Most of them had been, the truth is, found at Berkeley Lab within the final century. Abergel’s group has beforehand printed discoveries on berkelium and plutonium and treatments for radioactive contamination. Abergel famous that the brand new separation technique achieves separation elements which might be many orders of magnitude increased than present state-of-the-art strategies. The separation issue is a measure of how effectively a component might be separated from a combination. “The upper the separation issue, the less contaminants there are,” she mentioned. “Normally whenever you purify a component you will undergo the cycle many instances to cut back contaminants.” With a better separation issue, fewer steps and fewer solvents are wanted, making the method quicker and cheaper. For instance, the scientists demonstrated for one of many three methods they purified that they might cut back the method from 25 steps to simply two steps. The Berkeley Lab researchers demonstrated their technique first on actinium-225, an isotope of actinium that has proven very promising radio-therapeutic purposes. It really works by killing most cancers cells however not wholesome cells, by focused supply. DOE’s Isotope Program is actively working on ramping up manufacturing of actinium-225 all through the advanced of nationwide laboratory-based accelerators. This new separation technique could possibly be an alternative choice to chemical processes at present below growth. “With any manufacturing course of, it’s worthwhile to purify the ultimate isotope,” Abergel mentioned. “Our technique could possibly be used proper after manufacturing, earlier than distribution.” The 2 different actinides purified on this research had been plutonium and berkelium. An isotope of plutonium, plutonium-238, is used for energy technology in robots being despatched to discover Mars. Plutonium isotopes are additionally current in waste generated at nuclear energy crops, the place they have to be separated out from the uranium with a view to recycle the uranium. Lastly, berkelium is essential for basic science analysis. One in all its makes use of is as a goal for discovery of latest parts. The method depends on the unprecedented means of artificial ligands – small molecules that bind steel atoms – to be extremely selective in binding to metallic cations (constructive ions) primarily based on the dimensions and cost of the steel. The subsequent step, mentioned Abergel, is to discover utilizing the method on different medical isotopes. “Based mostly on what we have seen, this new technique can actually be generalized, so long as we’ve got totally different prices on the metals we need to separate,” she mentioned. “Having an excellent purification course of out there may make every thing simpler by way of post-production processing and availability.” ### The research was funded by the DOE Workplace of Science. Ricano was beforehand a participant in DOE’s Science Undergraduate Laboratory Internship (SULI) program at Berkeley Lab. Based in 1931 on the idea that the largest scientific challenges are greatest addressed by groups, Lawrence Berkeley Nationwide Laboratory and its scientists have been acknowledged with 13 Nobel Prizes. In the present day, Berkeley Lab researchers develop sustainable power and environmental options, create helpful new supplies, advance the frontiers of computing, and probe the mysteries of life, matter, and the universe. Scientists from around the globe depend on the Lab’s services for their very own discovery science. Berkeley Lab is a multiprogram nationwide laboratory, managed by the College of California for the U.S. Division of Power’s Workplace of Science. DOE’s Workplace of Science is the one largest supporter of fundamental analysis within the bodily sciences in the US, and is working to deal with among the most urgent challenges of our time. For extra info, please go to science.energy.gov. Disclaimer: AAAS and EurekAlert! are usually not chargeable for the accuracy of reports releases posted to EurekAlert! by contributing establishments or for the usage of any info by the EurekAlert system. Source link
0 notes
Text
Exploring Targeted Alpha Therapy for Neuroendocrine Tumors
Increasing Precision in Cancer Cell Death to Prevent Metastasis
In housework and lawn work, it is important to have the right tool for the job. When you want to remove dust out of the corners of the ceiling, you use a crevice took, not a full-size vacuum cleaner head. Likewise, to cut the grass along a fence line, you reach for an edge trimer.
In principle, a neuroendocrine tumor (NET) treatment specialist sometimes needs a precision tool to target lingering cancer cells in the tissue surrounding a neuroendocrine tumor.
The Neuroendocrine Tumor Research Foundation (NETRF) will fund a new grant to Tara Mastren, PhD, University of Utah, to study a novel theranostic agent that could increase precision in neuroendocrine tumor treatment. This 2019 Nuclear Medical Pilot Grant is offered in collaboration with the Education and Research Foundation for Nuclear Medicine and Imaging (ERF).
Eliminating all neuroendocrine cancer cells
Surgery removes neuroendocrine tumors as well as surrounding tissue that appears to be affected. Peptide Receptor Radionuclide Therapy (PRRT) with lutetium 177 dotatate (Lu-177) uses radioactive beta particles to target larger tumors. But beta particles are like a full-size vacuum cleaner head or a lawn mower; they carve a wide swath; they are not very precise. When a neuroendocrine tumor is in a vital organ like the lung or pancreas, it is important to preserve as much healthy tissue as possible. This requires pinpoint precision.
“One of the problems we are trying to solve with our funding is the prevention of metastasis,” said Elyse Gellerman, Chief Executive Office, Neuroendocrine Tumor Research Foundation. “We want to improve the long-term outlook for people with NETs by supporting research to destroy every last trace of neuroendocrine cancer to reduce the risk of recurrence.”
Exploring targeted alpha therapy in neuroendocrine tumors
The Neuroendocrine Tumor Research Foundation is exploring the role alpha particles could play as a precision cancer-cell-killing tool. “Targeted Alpha Therapy” (TAT) Developing the technology, however, requires research and funding.
Challenges of alpha-particle therapy in NETs
Mastren’s laboratory research project, Functionalized Silica Nanoparticles: Development of a Combined PET and TAT Theranostic Agent for Neuroendocrine Tumors, will try to resolve some of the inherent challenges of targeted alpha therapy.
When radionuclides decay via the emission of an alpha particle, they release energy that can harm healthy cells.
Alpha particles are hard to monitor using SPECT scans as they do not travel far in the body and typically have weak gamma emissions that are below the sensitivity of SPECT cameras. (Doctors use SPECT scans during treatment to plan, monitor, and refine doses.)
Radionuclides, such as Actinium-225 (Ac-225), when looked at for use in targeted alpha therapy, decays through a chain of short-lived intermediates before reaching a stable element. Keeping these intermediates near the cancer cells is imperative to maximize the dose to diseased cells while minimizing the dose to healthy cells.
It’s unclear whether this new theranostic agent will remain stable during use.
Targeted alpha therapy solutions to be studied
Using a methodological approach, Mastren and her colleagues at the University of Utah will try to overcome these challenges and develop a nanoparticle-based therapeutic agent that can be detected with PET scans by making some modifications.
Mastren will place an alpha-emitting radionuclide Ac-225 in a silica nanoparticle, which could help to contain radioactive daughters produced during decay, to reduce healthy cell exposure and increase cancer cell damage.
Mastren will insert a radionuclide (Zr-89) into the nanoparticle that can be detected by PET scan cameras to support treatment planning and monitoring.
The nanoparticle system will then be tested in laboratory models for stability.
Next steps
This theranostic agent (Ac-225/Zr-89-octreotate silica nanoparticles) is intended to be delivered using Peptide Receptor Radionuclide Therapy (PRRT). Promising results from this pilot study, which explores the feasibility of this PET/TAT agent, could pave the way for pre-clinical studies as a next step.
NETRF’s funding of neuroendocrine tumor research is made possible through generous donations from individuals and families committed to supporting a search for a cure to neuroendocrine cancer.
The Education and Research Foundation for Nuclear Medicine and Molecular Imaging (ERF) secures, invests and distributes funding to support rigorously vetted physicians, scientists and technologists in the pursuit of advanced academic studies or breakthrough discoveries in the fields of nuclear medicine and molecular imaging. ERF is the leading source of private philanthropic financial support focused exclusively on education and research programs in nuclear medicine and molecular imaging. To learn more, please visit us at http://www.mierf.org.
The post Exploring Targeted Alpha Therapy for Neuroendocrine Tumors appeared first on NETRF.
http://bit.ly/2PcQFnI
from WordPress https://netrf.wordpress.com/2019/04/16/exploring-targeted-alpha-therapy-for-neuroendocrine-tumors/
0 notes
Text
Motus GI taps Moran as CEO | Personnel Moves – September 25, 2018
Motus GI said today that it named Timothy Moran as its new chief executive officer, effective October 1, replacing former CEO Mark Pomeranz who will assume the role of president and COO.
Moran has previously served ConvaTec‘s (LON:CTEC) Americas president and helped lead the company through its initial public offering in 2016. Prior to ConvaTec, Moran spent 18 years with Covidien and joined Medtronic (NYSE:MDT) after it’s acquisition, taking on the role of patient care and safety division GM and VP.
“I am thrilled to welcome Tim, an industry leader with significant experience in the commercialization of medical devices for hospitals, to the Motus GI team. We have made great progress over the last year in positioning Motus GI for the next stage of commercial growth. Recognizing the scope of our market opportunity, it was clear to me that bringing in a leader with the right commercial scale-up expertise would have a positive impact on Motus GI’s ability to maximize our potential. In Tim Moran, we have secured a highly talented commercial leader who will enable me to focus on continuing to strengthen our operations, clinical execution and innovation pipeline. We believe Tim’s leadership style, business acumen, and commercial track-record from ConvaTec, Medtronic and Covidien made him the best candidate to lead the company. I really look forward to working alongside Tim and the rest of our management team to pursue our vision of establishing Pure-Vu as the new standard of care for in-hospital colonoscopy,” Pomeranz said in a press release.
“Motus GI has a very compelling new medical product opportunity. The Pure-Vu System has the potential to improve the colonoscopy process, particularly in the inpatient setting where it may improve and accelerate patient care while potentially saving hospitals thousands of dollars per episode of care. I am excited and honored to assume the role of chief executive officer alongside Mark and the team who have made great strides in establishing the foundation for future growth. I believe my experience in sales, marketing and general management of critical care product portfolios will help drive Motus GI forward and build a strong commercial organization focused on the successful launch of the Pure-Vu System. I look forward to working closely with the board of directors, Mark and the rest of the team to advance our efforts in establishing Motus GI as a preeminent medical technology company,” Moran said in a prepared release.
“The combination of Tim’s commercial leadership expertise with Mark’s operational track record give Motus GI a powerful leadership team that we are confident can position our company to take advantage of the commercial opportunity in front of us. Along with the recent appointment of Jeff Hutchison as our VP of U.S. sales and commercial Operations, Tim solidifies a dynamic leadership team with the skills and experience that we believe is capable to build and grow a market-leading medical technology company,” board chair David Hochman said in a press release.
MedX Health names Spearn as new CEO
MedX Health said yesterday it named current president Scott Spearn as its new chief executive officer, with former CEO Rob von der Porten taking on the role of board chair from a retiring Gary Van Nest.
Spearn has held senior executive roles at multinational medical device companies and has spent 30 years in the industry, Ontario-based MedX Health said.
“With the recent launch of our DermSecure telemedicine platform and anticipating a global roll-out of this leading product, the transition of Scott’s role to CEO represents a natural evolution at MedX. Scott’s experience in building sales organizations and developing international markets in the medical device field will help accelerate our growth from our SIAscopy technology and our therapeutic and dental laser products,” von der Porten said in a press release.
“On behalf of the board, we want to thank Rob von der Porten for his leadership during challenging times over the past few years and developing with the MedX team solid product roadmaps such as the delivery of DermSecure,” Van Nest said in a prepared release.
“I am excited to be taking on the CEO role, leading a dedicated team of hard-working people. MedX is a great Canadian company with innovative products and robust technology that is making a big difference in the health of people’s lives around the world,” Spearn said in prepared remarks.
Read more
NorthStar Medical Technologies appoints Merrick as prez, CEO
NorthStar Medical Technologies said earlier this month it appointed prez and COO Stephen Merrick as its president and CEO, replacing outgoing chair and CEO George Messina, who has been named chairman emeritus and president and CEO of wholly-owned subsidiary NorthStar Nuclear Therapies.
Prior to joining NorthStar, Merrick served as Baxter (NYSE:BAX) International’s hospital product biz international marketing VP, and also held positions with Mallinckrodt Pharma, Bristol-Myers Squibb and Eli Lilly.
NorthStar said that Hendricks Holding Co chair Diane Hendricks assumed the chairperson role for both companies.
“As founder, George has been the driving force from NorthStar’s inception through FDA approval of the RadioGenixSystem to the initial commercial sale of domestically produced Mo-99. We thank George for his successful leadership in NorthStar’s effort to become the first US-based producer of Mo-99. George’s passion and dedication were critical to building NorthStar into a global leader in the development of novel technologies for the production of medical isotopes for medical imaging. We are excited that George is assuming the leadership of NNT as president and chief executive officer. Targeted alpha therapy isotopes, including Actinium-225, produced by NNT have been identified as potential options in the treatment of certain cancers and infectious diseases. NNT’s therapeutic isotopes will offer more patients the potential for improved outcomes that were previously constrained by inadequate TAT isotope supply. We are looking to George to achieve similar success leading NNT as he has done with NorthStar,” Hendricks said in a prepared statement. “NorthStar is at a transformative point in its evolution from a development stage company to being the first domestic supplier of Mo-99, and we are excited that Steve has agreed to become NorthStar’s president and cEO. This company is strongly positioned for rapid growth in the US market, and there are attractive opportunities for NorthStar to grow outside the United States as well. Steve’s broad executive experience in the pharmaceutical industry at Bristol-Myers Squibb, Mallinckrodt and Baxter Healthcare, as well as his expertise in product development and driving domestic and global market demand are invaluable to NorthStar’s future success. The board of managers believes that this organizational realignment allows NorthStar to capitalize on both George and Steve’s strengths.”
Read more
Lumicell adds former ReWalk exec Hershberger as CFO
Lumicell said last week it added former ReWalk Robotics (NSDQ:RWLK) CFO Kevin Hershberger as its new chief financial officer.
The Wellesley, Mass.-based company said that Hershberger has spent more than 25 years in global finance management, and has held finance VP, controller and chief accounting officer positions with NxStage Medical (NSDQ:NXTM).
“In addition to his broad financial acumen and decades-long career in manufacturing and life sciences companies, Kevin has an excellent reputation for building strong teams and structuring successful companies. At Lumicell, we know that great companies start with great technology – but great technology doesn’t come to fruition without great people implementing a strong strategy. Kevin will join our leadership team and help pave the way for the future success of our organization,” CEO Kelly Londy said in a press release.
“It’s an exciting time to be joining the team at Lumicell. The company is on the precipice of significant innovations and making far-reaching strides in bringing its drug and device therapy to the market where it can help physicians and patients. But what I’m most excited about is Lumicell’s mission. Cancer, infection, wound care—battling these tough diseases is personal. And I look forward to supporting this mission, helping raise the profile of Lumicell’s technology, and improving people’s lives,” Hershberger said in a prepared statement.
Read more
Natus Medical taps Davies as CFO
Natus Medical (NSDQ:BABY) said last week it appointed Drew Davies as its new chief financial officer and exec VP, effective October 1, replacing interim Sharon Villaverde who will continue as finance VP.
Prior to joining Natus Medical, Davies served as CFO and exec VP of Extreme Networks, and as VP and corporate controller at Marvell Semiconductor before that.
“We are pleased to add a leader of Drew’s caliber to the Natus team. Drew brings a wealth of experience in financial and operational management across the technology industry, where innovation, cost management and constant change are critical to success. I am confident that Drew will complement the strengths of Natus’ executive team and play a key role as we progress forward. On behalf of everyone at Natus, I would also like to thank Sharon for serving as the company’s Interim CFO for the past 2 months. We are grateful that Sharon assumed this role at such an important time, and we look forward to continuing to benefit from her expertise,” prez & CEO Jonathan Kennedy said in a press release.
“I am excited to join Natus during a transformative time in the company’s history. Natus has a demonstrated track record of growth and earnings expansion and I look forward to working with Jonathan and the rest of the management team to drive further success,” Davies said in a prepared release.
Read more
NIH taps Tromberg as Nat’l Institute of Biomedical Imaging and Bioengineering head
Integra Lifesciences GC & corp VP Gorelick to retire
BioMedGPS names Tracy as SmartTrak business intelligence CCO
Myomo taps Frijters as EU biz dev manager
Pursuit Vascular adds Walpole as NA sales lead
Allurion Tech expands exec team
The post Motus GI taps Moran as CEO | Personnel Moves – September 25, 2018 appeared first on MassDevice.
from MassDevice https://ift.tt/2Q6gYev
0 notes
Text
There are several ways to turn the immune system against cancer. Checkpoint inhibitor therapy is one of them. But it doesn't work in all patients, especially children, whose cancers generally do not have the vast numbers of mutations needed to attract the attention of a newly brake-free immune system. So far, the beneficiaries of immune checkpoint therapy appear to be those with cancer that develops after repeated genetic mutations—metastatic melanoma, non-small-cell lung cancer, and bladder cancer, for example.
But of course, there's a problem: Now that the reactor program and the Cold War are both over, no one is making uranium-233 in the U.S. (or anywhere). And because it takes more than forty years for uranium-233 to turn into enough thorium-229 to be useful, it wouldn't matter much even if they did. There are currently only about fifteen hundred to seventeen hundred millicuries of actinium-225 anywhere in the world, which would just treat one hundred to two hundred patients a year.
Before long, two separate researchers had shown that you could change a mouse's response to immune checkpoint inhibitor therapy by giving him certain kinds of bacteria. Wargo added the microbiome to her slate of experiments. Along with her team, she collected gut microbiome samples from more than three hundred cancer patients who then went on to receive checkpoint inhibitors as treatment. The results were, Wargo says, "night and day." People who had a higher diversity of gut bacteria had a stronger response to checkpoint inhibitor therapy.
Now, Wargo is transplanting stool samples from patients into germ-free mice with melanoma, to see if she can predict whether the mice will mimic the treatment responses of the people whose bacteria they received.
http://www.popularmechanics.com/science/health/a26290/we-will-beat-cancer/
0 notes