#Pharmacovigilence Course
Text
Navigating Pharmacovigilance Training Fees: A Comprehensive Guide
In the dynamic landscape of healthcare, where advancements in medical sciences occur at a rapid pace, the role of pharmacovigilance has become increasingly vital. As pharmaceutical companies strive to ensure the safety of their products, professionals equipped with pharmacovigilance training play a crucial part in monitoring and assessing adverse drug reactions. In this article, we will delve into the intricacies of pharmacovigilance training fees, shedding light on the costs associated with this essential medical course.
Pharmacovigilance training fees can vary significantly based on the provider, course duration, and the comprehensiveness of the curriculum. Individuals seeking to embark on a career in pharmacovigilance often grapple with the question of affordability, and rightly so. It is paramount to choose a reputable institution that not only offers quality education but also ensures that the financial investment made translates into a rewarding career.
One reputable option for pharmacovigilance training is The White Board Institute, renowned for its commitment to delivering comprehensive courses in medical sciences. Prospective students keen on acquiring pharmacovigilance skills often inquire about the fees associated with The White Board Institute's training program. The institution understands the importance of transparency in this regard and provides a breakdown of the pharmacovigilance training fees on its official website.
The pharmacovigilance training fees at The White Board Institute cover a wide array of components, including tuition, study materials, and access to state-of-the-art facilities for practical training. The curriculum, designed in alignment with international standards, ensures that graduates are well-prepared to navigate the challenges of pharmacovigilance in the real world. The institute also offers flexible payment plans to accommodate the diverse financial situations of its students.
In the realm of pharmacovigilance, where precision and attention to detail are paramount, the importance of quality training cannot be overstated. The White Board Institute, having garnered a stellar reputation in the medical education sector, prioritizes the delivery of up-to-date and relevant content. The training program covers pharmacovigilance principles, regulatory frameworks, and the latest technological tools used in monitoring and reporting adverse drug reactions.
Prospective students contemplating pharmacovigilance training often express concerns about the return on investment. The pharmaceutical industry, however, has a consistently high demand for skilled pharmacovigilance professionals, making the investment in training a wise career move. Individuals completing their training at The White Baord Institute find themselves well-positioned for lucrative career opportunities, thanks to the institute's strong industry connections and emphasis on practical skills development.
It is crucial for individuals considering pharmacovigilance training to conduct thorough research and compare fees from different institutions. While The White Board Institute stands out for its commitment to quality education, there are other reputable options available as well. Prospective students are encouraged to explore various institutions, keeping in mind factors such as accreditation, industry reputation, and alumni success stories.
In conclusion, the journey towards a career in pharmacovigilance involves a thoughtful consideration of training fees, coupled with the assurance of receiving a high-quality education. Aspiring professionals must prioritize institutions like The white Board Institute that not only offer competitive pharmacovigilance training fees but also uphold the standards necessary for success in this dynamic field. By making an informed decision and investing in a reputable training program, individuals can embark on a fulfilling career in pharmacovigilance, contributing to the safety and efficacy of pharmaceuticals on a global scale.
0 notes
Text
Advanced Pharmacovigilance Training: Monitoring Drug Risks
This comprehensive course offers participants an in-depth understanding of pharmacovigilance, the science and practice of monitoring, assessing, and preventing adverse effects of medications.

0 notes
Text

Read about Steps to become a computer system validation specialist in the given infographic and get more details at: www.companysconnects.com
0 notes
Text

Post Graduate Diploma/Executive Diploma in Pharmacovigilance
The Post Graduate Diploma/Executive Diploma in Pharmacovigilance has been structured by experts from the industry themselves and thus comprehensive coverage and understanding of the industry and its functional areas is promised. The goal of the Post Graduate Diploma/Executive Diploma Programme is to familiarize the participant with the updated theoretical and practical aspects of Pharmacovigilance.
#Diploma in Pharmacovigilance#pharmacovigilance course#clinical Research#pharmacovigilance#Post Graduate Diploma in Pharmacovigilance#online Pharmacovigilance
0 notes
Text
Investigating Clinical Data Management and Pharmacovigilance Courses: A Way to Drug Greatness

Presentation
In the powerful scene of drugs, guaranteeing the wellbeing and adequacy of medications is principal. Clinical Data Management (CDM) and pharmacovigilance course (PV) assume essential parts in this space. As the interest for talented experts here develops, specific courses have arisen to address industry issues. How about we dive into what these courses involve and why they are fundamental for those trying to succeed in drugs.
Figuring out Clinical Data Management (CDM)
1. What is CDM?
CDM includes the assortment, mix, and management of data from clinical preliminaries. It guarantees that data gathered during clinical exploration is precise, solid, and genuinely sound. Experts in CDM administer the whole data lifecycle, from assortment to examination.
2. For what reason is CDM Significant?
Precise data is significant for administrative entries and dynamic by drug organizations. CDM guarantees consistence with administrative guidelines and improves the believability of clinical preliminary outcomes. It additionally works with proficient joint effort between different partners engaged with drug advancement.
Investigating Pharmacovigilance (PV)
1. What is PV?
Pharmacovigilance is the science and exercises connected with the recognition, appraisal, understanding, and avoidance of unfavorable impacts or some other medication related issues. It includes observing the wellbeing of promoted sedates and evaluating the dangers and advantages related with their utilization.
2. For what reason is PV Significant?
PV adds to general wellbeing by recognizing and limiting dangers related with drug items. Opportune discovery and revealing of unfriendly medication responses (ADRs) are fundamental for guaranteeing patient wellbeing and keeping up with public confidence in the medical services framework. Moreover, PV assumes a urgent part in consistence with administrative prerequisites.
The Meaning of Specific Courses
1. Extensive Educational program
Courses in CDM and PV give top to bottom information on applicable guidelines, systems, and apparatuses utilized in clinical examination and medication security. Understudies gain viable abilities through active preparation in data management programming and unfavorable occasion announcing frameworks.
2. Industry-Significant Abilities
These courses are intended to meet the developing necessities of the drug business. Graduates are outfitted with the abilities expected to explore complex administrative systems, lead clinical preliminaries proficiently, and guarantee the wellbeing of drug items all through their lifecycle.
3. Vocation Open doors
Experts with aptitude in CDM and PV are sought after across drug organizations, contract research associations (CROs), administrative offices, and medical services foundations. Vocation open doors incorporate jobs, for example, clinical data director, pharmacovigilance researcher, drug wellbeing partner, and administrative undertakings trained professional.
End
In a time of quick headways in drugs, the significance of clinical data management and pharmacovigilance couldn't possibly be more significant. Particular courses in these fields furnish hopeful experts with the information and abilities expected to succeed in the business. By guaranteeing the trustworthiness of clinical data and the security of drug items, CDM and PV experts assume a crucial part in propelling general wellbeing and development in medication.
0 notes
Text
Unleashing Your Potential: Pharmacovigilance Certificate Course Online

It takes a combination of interpersonal and intrapersonal talents to keep ahead in the fast-paced pharmaceutical industry of today. An online course leading to a pharmacovigilance certificate is a crucial step towards career progression. This article is a thorough guide to help you grasp the nuances of pharmacovigilance certification and how it can advance your career. Read more: https://worldnewsfox.com/uncategorised/unleashing-your-potential-pharmacovigilance-certificate-course-online/
0 notes
Text
Why Should You Consider a Career in Medical Coding?
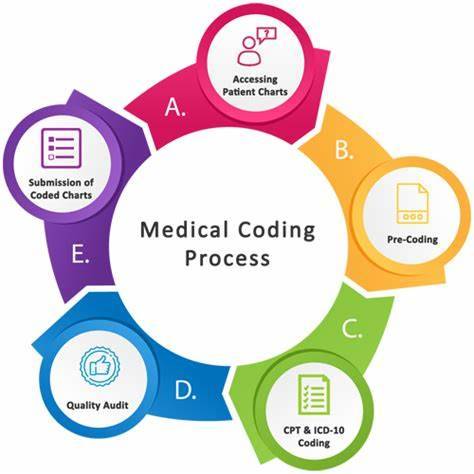
Introduction
Are you looking for a rewarding career in the healthcare industry that doesn't require years of medical school? If so, medical coding might be the perfect choice for you. In this blog, we'll explore the exciting world of medical coding, its importance in clinical research, and why you should seriously consider it as a career option. Let's dive in!
What is Medical Coding?
Medical coding is like the language of healthcare. It involves transforming medical information such as diagnoses, procedures, and treatments into universal codes. These codes are used for various purposes, including billing, insurance claims, and clinical research. Essentially, medical coders are responsible for ensuring that the healthcare system runs smoothly by accurately documenting patient records.
Why Choose a Career in Medical Coding?
1. In-Demand Career: The healthcare industry is constantly growing, and with it, the demand for skilled medical coders. Hospitals, clinics, insurance companies, and research institutions are always in need of qualified professionals to handle their coding needs.
2. Short Training Period: Unlike many other healthcare careers that require years of education, you can become a medical coder relatively quickly. Numerous institutes offer courses and training programs in medical coding that can be completed in a matter of months.
3. Diverse Opportunities: Medical coding isn't limited to just one type of job. You can find opportunities in various settings, including hospitals, private practices, pharmaceutical companies, and research organizations. If you want to explore related fields, you can also transition into areas like pharmacovigilance, drug regulatory affairs, or clinical data management.
4. Stability and Job Security: The healthcare industry is known for its stability, and medical coding is no exception. As long as there are healthcare services, there will be a need for medical coders. This translates to job security and peace of mind in your career.
5. Work-Life Balance: Many medical coding jobs offer excellent work-life balance. You'll typically work regular hours in a comfortable office setting, allowing you to maintain a healthy work-life balance.
6. Good Earning Potential: While salaries can vary based on location and experience, medical coders generally earn a competitive wage. With experience and additional certifications, you can increase your earning potential even further.
7. Contributing to Healthcare: By ensuring accurate coding, you help maintain the integrity of patient records, improve patient care, and support clinical research, pharmacovigilance, drug regulatory affairs, and clinical data management.
The Role of Medical Coding in Clinical Research
Now, let's delve into the connection between medical coding and clinical research. Clinical research plays a vital role in advancing healthcare treatments and therapies. It involves testing new drugs, medical devices, and treatment protocols to ensure their safety and effectiveness. Medical coding is an essential part of this process for several reasons:
1. Data Accuracy: Accurate coding ensures that the data collected during clinical trials is reliable. Researchers rely on this data to make informed decisions about the safety and efficacy of new treatments.
2. Regulatory Compliance: Regulatory agencies, such as the FDA, require precise documentation of clinical trial data. Medical coding helps maintain compliance with these regulations, which is critical for getting new drugs and treatments approved.
3. Patient Safety: Proper coding helps identify any adverse events or side effects experienced by patients during clinical trials. This information is crucial for patient safety and determining the risks and benefits of a new treatment.
4. Data Analysis: Medical coding simplifies the process of data analysis by categorizing information into standardized codes. This makes it easier for researchers to identify trends and draw conclusions from the data.
How to Start Your Career in Medical Coding?
1. Take a course: Look for reputable institutes or online courses that offer medical coding training. These courses cover topics like anatomy, medical terminology, and coding systems such as ICD-10 and CPT.
2. Get Certified: While certification isn’t always required, it can significantly boost your job prospects. Consider obtaining certifications like Certified Professional Coder (CPC) or Certified Coding Specialist (CCS) through recognized organizations.
3. Gain Experience: Entry-level positions may require some on-the-job experience. Look for internships or entry-level coding jobs to build your skills and resume.
4. Stay Updated: Medical coding guidelines and regulations can change, so it’s essential to stay current. Attend workshops, and seminars, and continue your education to remain competitive in the field.
5. Network: Join professional organizations such as the American Health Information Management Association (AHIMA) or the American Academy of Professional Coders (AAPC) to connect with other professionals in the industry.
Conclusion
In conclusion, a career in medical coding offers stability, good earning potential, and diverse opportunities within the healthcare industry. Moreover, it plays a crucial role in clinical research, contributing to the development of new and better treatments for various medical conditions. If you're interested in healthcare, have an eye for detail, and enjoy working in a structured environment, medical coding could be the perfect career choice for you. Consider enrolling in a reputable training program or course to kickstart your journey into this rewarding field. Your future as a medical coder awaits!
#medical coding institute#medical coding training#medical coding course#Clinical research training#Clinical Research Institute#Clinical research course#Pharmacovigilance course#Pharmacovigilance training institute#Pharmacovigilance jobs#Clinical data management course#Clinical data management training institute#Clinical research management
0 notes
Text

#Clinical Research Associate Training#Advanced Clinical Research Associate Certification#Clinical Research Trainng - Best Clinical Research Training Institute - Clinical Research Advance certification#Gratisol Labs is a leading Clinical Research Institute Offering Clinical Research Course#Pharmacovigilance Course#Clinical data Management Course#SAS Course#Medical Writing Course and Regulatory Affairs Course.. We offer Clinical Research Training program. This Advance Certification Clinical Res#Clinical Data Management training#SAS Training. Medical Writing training and Regulatory Affairs course. We offer clinical research Certification Program also offers Clinical#have B.Pharmacy#M.Pharmacy#Life Sciences#Microbiology#Biotechnology#Chemistry. BPT#BDS and BHMS Also eligible for Clinical Research training program. For B.Pharmacy and M.Pharmacy the pharmacovigilance course is best suita#Biotechnology and Medical Device industries to both experienced professionals and entry level candidates.#The Clincal Data Manamgent Training offered fall into the broad categories of Recruitment Solutions#Temporary Staffing#Outsourcing#Consulting#Application Training in Clinical Data Management course (Oracle Clinical#Oracle Inform/Central Designer)#Pharmacovigilance (Oracle Argus Safety Database#Oracle AERS)#CDISC SDTM & SAS to aspirants of Clinical Research Industry. We successfully help some of the top Pharmaceutical#Biotechnology and Medical Device organizations from around the world to recruit high caliber experienced & trained professionals.#The Pharmaceutical & Clinical Research Industry#worldwide is facing a huge shortage of trained manpower resources. With a View to fill this gap between the demand & supply of trained manp
0 notes
Text
Unveiling the Future of Learning: The Benefits of a Pharmacovigilance Course Online
Introduction:
In an era where digital transformation is reshaping education and professional development, the accessibility and convenience of online courses have become pivotal. The field of pharmacovigilance is no exception, with professionals seeking to enhance their skills and stay abreast of evolving industry practices turning to online courses. This article delves into the advantages and key considerations of opting for a pharmacovigilance course online, exploring how this mode of learning is shaping the future of education in the pharmaceutical and healthcare sectors.
Accessible Anytime, Anywhere: One of the primary advantages of an online pharmacovigilance course is the flexibility it offers. Professionals, often juggling demanding schedules, can access course materials and lectures at their convenience. This flexibility eliminates geographical constraints and time barriers, allowing participants to learn at their own pace from anywhere in the world.
Self-Paced Learning: Online pharmacovigilance courses often adopt a self-paced learning model, enabling participants to progress through the material at a speed that suits their individual learning styles. This accommodates diverse learning preferences and ensures that individuals can delve deeper into complex topics or move more quickly through familiar concepts.
Cost-Effective Learning: Traditional classroom-based courses can incur substantial costs, including travel, accommodation, and venue expenses. In contrast, online courses eliminate these overheads, making education more cost-effective. Participants can often access quality pharmacovigilance training without the financial burden associated with attending in-person classes.
Global Expertise and Networking: Online pharmacovigilance courses frequently attract a diverse cohort of participants from various parts of the world. This diversity enriches the learning experience by bringing in different perspectives and insights. Additionally, the virtual nature of these courses facilitates networking opportunities, allowing professionals to connect with peers, industry experts, and instructors globally.
Industry-Relevant Content: Online pharmacovigilance courses are designed to align with current industry trends and regulations. The digital format allows for swift updates to course content, ensuring that participants receive the most recent and relevant information. This is particularly crucial in the dynamic field of pharmacovigilance, where regulatory requirements and best practices evolve.
Interactive Learning Tools: To enhance engagement and understanding, online pharmacovigilance courses often incorporate interactive learning tools. These may include quizzes, case studies, discussion forums, and virtual simulations. Such tools not only reinforce theoretical knowledge but also provide practical insights into the real-world application of pharmacovigilance principles.
Expert-Led Instruction: Quality online pharmacovigilance courses are typically led by industry experts and experienced instructors. Participants benefit from the expertise of professionals who have firsthand experience in pharmacovigilance practices, regulatory compliance, and risk management. This ensures that the learning is not only theoretical but also grounded in practical, industry-relevant knowledge.
Continuous Learning and Updates: The pharmaceutical industry is dynamic, with regulatory landscapes and pharmacovigilance practices continually evolving. Online courses often offer avenues for continuous learning and updates, allowing participants to stay informed about the latest developments in the field. This commitment to ongoing education is vital for professionals aiming to maintain relevance in their roles.
Considerations When Opting for an Online Pharmacovigilance Course:
Accreditation and Certification: Ensure that the online pharmacovigilance course is accredited by relevant industry bodies and provides a recognized certification upon completion. This adds credibility to the acquired knowledge and enhances its applicability in professional settings.
Technological Requirements: Before enrolling in an online course, verify that you have the necessary technological resources. This includes a reliable internet connection, compatible devices, and any software or platforms required for the course. Familiarity with online learning platforms is also beneficial.
Support and Interaction: Look for courses that offer adequate support and opportunities for interaction. This can include access to instructors for clarification of doubts, discussion forums for peer interaction, and support services to address technical issues.
Course Structure and Curriculum: Review the course structure and curriculum to ensure that it aligns with your learning objectives. A well-structured course should cover essential pharmacovigilance topics, offer a balanced blend of theory and practical applications, and provide comprehensive insights into the regulatory landscape.
Reviews and Testimonials: Explore reviews and testimonials from previous participants to gauge the effectiveness of the course. Insights from individuals who have completed the program can provide valuable perspectives on the quality of instruction, course content, and overall learning experience.
Conclusion:
The advent of online education has transformed the way professionals acquire knowledge and skills, and the field of pharmacovigilance is no exception. Opting for a pharmacovigilance course online offers a myriad of advantages, including flexibility, cost-effectiveness, and access to global expertise. As the pharmaceutical and healthcare industries continue to evolve, embracing online learning becomes instrumental in staying abreast of industry trends, regulations, and best practices. By leveraging the benefits of online education, professionals in pharmacovigilance pave the way for a future where learning is accessible, dynamic, and tailored to the demands of a rapidly changing industry.
0 notes
Text

The ICRI Online Learning Certificate in Pharmacovigilance provides a comprehensive understanding of drug safety monitoring and regulatory compliance. Through specialized coursework, students delve into adverse event reporting, risk management, and global pharmacovigilance practices, preparing them for pivotal roles in ensuring the safety of pharmaceutical products worldwide.
#Certificate in Pharmacovigilance#pharmacovigilance certificate course online#pharmacovigilance online courses with certificate
0 notes
Text
Advance Your Career in Drug Safety: Premier Pharmacovigilance Courses in India.

Dive into the top Pharmacovigilance courses in India designed for healthcare professionals. Increase your drug safety and post-marketing surveillance skills with our 2024 curated guide. Start your career transformation today!
#medical#pharmacy#pharmacovigilance#courses#healthcare#training course#skill development course#soft skills
1 note
·
View note
Text
MES System
Explore our comprehensive MES system, designed to streamline manufacturing processes and enhance production efficiency. Our MES courses cover key aspects of this essential Manufacturing Execution System, providing in-depth knowledge and practical skills for a competitive edge in your industry. Enroll today to master MES.
Get more details at: www.companysconnects.com/manufaturing-execution-system-mes
#pharmacovigilance courses#drug regulatory affairs certification#CSV Certification#Manufacturing Execution System
0 notes
Text
Navigating the Future of Drug Safety: A Comprehensive Guide to Pharmacovigilance Courses

Introduction
In the dynamic field of healthcare, ensuring the safety and efficacy of pharmaceutical products is of paramount importance. Pharmacovigilance, the science of monitoring and assessing the safety of drugs, plays a crucial role in safeguarding public health. This blog explores the significance of pharmacovigilance courses, shedding light on the key components that contribute to a successful career in drug safety
Understanding Pharmacovigilance
Definition and Scope
Pharmacovigilance is the science and activities related to the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problems. A pharmacovigilance course equips individuals with the knowledge and skills necessary to contribute to the safe use of medications.
Regulatory Landscape
A comprehensive pharmacovigilance course covers the regulatory frameworks governing drug safety at both national and international levels. Understanding these regulations is essential for professionals working in the pharmaceutical industry to ensure compliance with safety standards.
Role in Public Health
Emphasizing the broader impact of pharmacovigilance courses highlight its role in protecting public health. Professionals trained in pharmacovigilance contribute to the identification and mitigation of potential risks associated with drug use.
Key Components of Pharmacovigilance Courses
Drug Safety Surveillance
Courses delve into the methodologies and tools used for monitoring and surveillance of adverse drug reactions. This includes the analysis of real-world data, clinical trials, and post-marketing surveillance.
Signal Detection and Management
Participants learn how to identify signals that may indicate potential safety concerns. Additionally, courses cover the management of identified signals, including risk assessment and communication strategies.
Pharmacovigilance Systems and Databases
Understanding the infrastructure supporting pharmacovigilance is essential. Courses explore the design and implementation of pharmacovigilance systems, as well as the utilization of databases for efficient data collection and analysis.
Risk Management and Communication
An integral part of pharmacovigilance education is learning how to develop and implement risk management plans. Communication strategies for disseminating safety information to healthcare professionals and the public are also emphasized.
Ethics and Compliance
Ethics in pharmacovigilance is a critical aspect covered in courses. Professionals must adhere to ethical standards while reporting and managing safety data. Compliance with regulations and guidelines is essential to maintaining the integrity of drug safety practices.
Career Opportunities in Pharmacovigilance
Pharmacovigilance Officer
Monitoring and reporting adverse drug reactions, pharmacovigilance officers play a crucial role in ensuring drug safety compliance.
Drug Safety Specialist
Specialists focus on analyzing safety data, conducting risk assessments, and implementing risk management plans.
Regulatory Affairs Manager
Professionals in regulatory affairs ensure that pharmaceutical products comply with safety regulations and manage interactions with regulatory authorities.
Conclusion
Enrolling in a pharmacovigilance course is a strategic step towards a fulfilling and impactful career in drug safety. As the pharmaceutical industry continues to evolve, the demand for skilled pharmacovigilance professionals remains high. With the right education and training, individuals can contribute to a safer and more secure healthcare landscape, making a positive impact on public health globally
#Pharmacovigilance course#clinical Research#Medical Writing programmes#Clinical DataManagement Course#online Pharmacovigilance course
0 notes
Text
Navigating the Landscape of Pharmacovigilance: Online Courses and Clinical Data Management
Introduction
In the dynamic realm of pharmaceuticals, the significance of pharmacovigilance courses online and proficient clinical data management cannot be overstated. As the healthcare industry continues to evolve, the demand for skilled professionals in pharmacovigilance and clinical research data management escalates. In this article, we delve into the pivotal role of these disciplines, explore the avenues of online education, and shed light on the importance of adeptly managing clinical data.
The Foundation of Pharmacovigilance
Pharmacovigilance, often dubbed as the watchdog of the pharmaceutical world, encompasses the systematic detection, assessment, understanding, and prevention of adverse effects or any other drug-related problems. In the wake of increasing complexities in drug development and stringent regulatory standards, pharmacovigilance plays a central role in ensuring drug safety and efficacy throughout its lifecycle.
Unveiling Online Pharmacovigilance Courses
With the surge in demand for skilled pharmacovigilance professionals, the accessibility and flexibility of online courses have become paramount. Online pharmacovigilance courses offer a comprehensive curriculum tailored to meet industry standards and regulatory requirements. These courses provide aspiring professionals with a thorough understanding of pharmacovigilance principles, regulatory frameworks, risk management strategies, and signal detection methodologies.
The Crux of Clinical Research Data Management
Clinical data management serves as the backbone of clinical research, encompassing the collection, organization, validation, and analysis of data obtained from clinical trials. In an era driven by data-centric decision-making, efficient clinical data management is indispensable for ensuring the integrity, accuracy, and reliability of clinical trial data.
Navigating the Landscape of Clinical Data Management
In today's evolving healthcare landscape, the significance of adept clinical data management cannot be overstated. As the volume and complexity of clinical trial data continue to escalate, there is a growing demand for skilled professionals proficient in clinical data management. Online courses in clinical data management offer a structured curriculum covering essential aspects such as database design, data cleaning, quality control, and regulatory compliance.
The Intersection: Integrating Pharmacovigilance and Clinical Data Management
The convergence of pharmacovigilance and clinical data management heralds a new era of synergy and collaboration within the pharmaceutical industry. By seamlessly integrating pharmacovigilance principles into clinical data management processes, organizations can enhance their ability to detect, assess, and mitigate potential risks associated with investigational drugs.
Conclusion
In conclusion, the fields of pharmacovigilance and clinical data management stand as pillars of safety and efficacy in the pharmaceutical landscape. With the advent of online education, aspiring professionals now have unprecedented access to specialized courses that equip them with the knowledge and skills necessary to excel in these domains. As the healthcare industry continues to evolve, the demand for proficient pharmacovigilance and clinical data management professionals will only intensify, underscoring the importance of investing in education and training in these critical areas.
0 notes
Text
https://www.ssodl.edu.in/clinical-data-management-course.php
0 notes
Text
Balancing Benefit and Risk in Clinical Research
Introduction
The fields of medicine and healthcare are rapidly developing. Companies and research institutes play a vital role in advancing medical knowledge through clinical research. Clinical research surrounds various aspects, including medical coding, pharmacovigilance, drug regulatory affairs, and clinical data management. These fields are essential in ensuring the safety and effectiveness of new medical treatments. However, conducting clinical research comes with its own set of challenges, particularly when it comes to balancing the benefits and risks involved.

The Role of Clinical Research
Clinical research is the backbone of medical progress. It involves the systematic study of new drugs, medical devices, treatments, and procedures to determine their safety and efficacy. Companies and research institutes conduct clinical trials to gather data and evidence before these medical interventions are approved for widespread use.
Key Areas of Clinical Research
1. Medical Coding: Medical coding is like the language of healthcare. It involves translating medical records, diagnoses, and procedures into standardized codes. Accurate coding is crucial for proper billing and maintaining patient records.
2. Pharmacovigilance: This field focuses on monitoring the safety of drugs and vaccines post-approval. It helps identify and prevent adverse effects and ensures that patients receive safe medications.
3. Drug Regulatory Affairs: Drug regulatory affairs professionals work with regulatory agencies to ensure that new drugs meet safety and efficacy standards before they reach the market. They help companies navigate complex regulations.
4. Clinical Data Management: Managing clinical trial data is essential for maintaining the integrity of research. Data managers organize and validate information collected during trials.
Balancing Benefit and Risk
While clinical research is definitely important for medical progress, it also involves risks. Here are some ways in which companies and research institutes can strike a balance:
1. Ethical Considerations: Ethical guidelines and standards are the foundation of clinical research. Researchers must prioritize the well-being of participants and ensure that their rights and privacy are protected.
2. Informed Consent: Participants must provide informed consent before participating in a clinical trial. They should be fully aware of the potential risks and benefits, enabling them to make an informed decision.
3. Safety Monitoring: Continuous monitoring of participants' safety is essential. Any adverse events should be immediately reported and addressed.
4. Transparency: Transparency in reporting research findings is crucial. This includes disclosing both positive and negative results, helping to avoid biased information.
5. Regulatory Compliance: Companies and institutes must adhere to regulatory requirements in their respective fields, ensuring that the research meets high standards of safety and quality.
The Importance of Training
To ensure that clinical research is conducted responsibly, professionals in the field require acceptable training. Courses and training programs are available for medical coding, pharmacovigilance, drug regulatory affairs, and clinical data management. Proper training equips individuals with the knowledge and skills needed to conduct research while minimizing risks.
Job Placement in Clinical Research
For those interested in pursuing a career in clinical research, the job placement aspect is essential. Companies and institutes often offer placement opportunities for trained professionals, ensuring that they can apply their skills in real-world settings.
Conclusion
Balancing benefit and risk in clinical research is a complex but essential endeavor. Companies and research institutes play a critical role in advancing medical knowledge, but they must do so responsibly. Ethical considerations, informed consent, safety monitoring, transparency, and regulatory compliance are key factors in achieving this balance. Moreover, individuals interested in clinical research can benefit from training and job placement opportunities, enabling them to contribute to the field while ensuring the safety and well-being of patients. In this way, we can continue to make significant strides in healthcare while upholding the highest standards of ethics and safety.
#Medical billing and coding course#Pharmacovigilance jobs#Pharmacovigilance course#Pharmacovigilance training institute#Clinical data management course#Clinical data management training institute#Clinical research management#Clinical research training#Clinical Research Institute#Clinical research course#medical coding course#medical coding institute#medical coding training
0 notes