#Medical Device First in Human Clinical Research Study in Colombia
Text
The Significance of Medical Device CROs in Costa Rica and Clinical Trials in Mexico
Within the ever-evolving realm of Medical Device CRO in Costa Rica emerges as a strategic nucleus for Clinical Research Organizations (CROs). These entities play a pivotal role in steering the intricate path of regulatory adherence and clinical trials, ensuring the triumph of medical device innovations. Costa Rica's pro-business ambiance, complemented by a resilient healthcare infrastructure, positions it as an optimal locale for Medical Device CROs.
#Medical Device Contract Research Organization in Latin America#Medical Device CRO in Mexico#Medical Device CRO in the Dominican Republic#Medical Device CRO in El Salvador#Medical Device CRO in Brazil#Medical Device CRO in Peru#Medical Device CRO in Panama#Medical Device CRO in Chile#Medical Device CRO in Costa Rica#Medical Device CRO in Honduras#Medical Device CRO in Argentina#Medical Device CRO in Ecuador#Medical Device First in Human Clinical Research Study in Latin America#Medical Device First in Human Clinical Research Study in Colombia
2 notes
·
View notes
Text
Research Report Provides In-Depth Industry Analysis on Laryngoscope Handles SegmentFrequently Asked QuestionsWhat is the USP of the report?What are the key content of the report?What are the value propositions and opportunities offered in this market research report?Related Reports
The global Laryngoscope Handle industry is segmented according to various types and uses. All segments are analyzed and predicted based on Sales and Volume in 2021 to 2021. Based on the above analysis, the global Laryngoscope Handle industry will experience five growing segments in the coming years:
The first one is the table global handle industry market. This sector projects a significant increase in sales driven by its unique applications. It is forecast that sales of this type of laryngoscope handle will increase in both the Asia and Europe. Driven by these unique applications, more medical professionals are moving to purchase this laryngoscope handle to use them for their applications. The five forces analysis predicts that this sector will experience a high degree of competition over the coming years.
The second segment is the Asia Pacific. The laryngoscope handle research industry overview reports that this region will experience a growth in both revenues and employment. This is due to the rising demand for laryngoscope devices in the regions of this region. In Asia Pacific, the top five players covered by the forecast include Korea Electric, Hanwha, Sanyo, Jomox and Laryngoscope. These companies are expected to expand their sales in Asia Pacific countries such as China, India and Malaysia.
The third market segment is the European market overview. The laryngoscope handle industry forecasts that this region will experience growth in both revenues and employment. The key market segments covered by the market analysis are Covid-RN, RN-augmentated, Naxcite, Medtronic, Omron and C-Store.
The fourth market segment is the United States. The laryngoscope handle market analysis reports that this region will experience strong growth in revenues and employment. The major players covered by this forecast include Apollo, Siemens, Scott, Littmann and Medtronic.
The fifth and last market segment we will cover is the Middle East. This region's laryngoscope handle industry competition is weak due to lower prices of laryngoscope devices. This is the main reason why this market segment is expected to experience strong growth only in revenues. The main five forces analysis of Middle East laryngoscope offers two estimates - the historical and the current case. From the historical case, it is estimated that this industry can expect revenues in both the high and low price points.
The six laryngoscope handle segments we will study in this study are the European countries, Asia, Latin America, Russia, Middle East and North America. The analysis shows that Latin America will experience strong revenues in the coming years. This is due to the large scale deployment of health care systems and the low cost of supplies. Latin America's in-depth business strategies also contribute to this regional laryngoscope handle market report. The business strategies of Brazil, Mexico and Argentina show strong potential and growth in the future.
The seventh laryngoscope handle industry sector we will study is the European Union. The European Union is one of the world's leading suppliers of equipment for the treatment of breathing disorders. It is a leading exporter of respiratory medical devices. The recent report of the European commission says that the six laryngoscope handle market competitors will experience tough competition. As a result, the manufacturers of respiratory medical device will be forced to increase their prices or else they might have to face the threat of having no clients and therefore no sales.
The eighth laryngoscope handle industry chain we will study is the Asia-Pacific region. This region has a number of large manufacturers and importers with major players such as Siemens, Phillips and Cetron. These manufacturers and importers have a clear strategy to target the growing demands of the Chinese market. The two most important features of the Chinese market, its massive disposable income and its massive human resource will be the main focus for these manufacturers. This overview of the laryngoscope handle market segment shows that China will emerge as the largest potential player in the future.
The ninth laryngoscope handle segment analysis we will study is the global market. There is a huge demand for laryngoscope units in the global market. This demand is caused by the need of patients to have access to quality sound equipment in order to cure their breathing disorders. This demand has been accelerated by the increase in the number of people who are reporting cases of hearing loss and by the introduction of the medical device industry into the global market.
The ten laryngoscope handle industry segments have been analyzed based on their growth rate and their potential in the future. The growth rates are presented in terms of percentage points by which their revenue is expected to double over five years. Each of these industry segments is presented with data on the current market situation and a forecast of their future market position. This market research report provides in-depth information on the trends observed across each industry.
The research team projects that the Laryngoscope Handle market size will grow from XXX in 2020 to XXX by 2027, at an estimated CAGR of XX. The base year considered for the study is 2020, and the market size is projected from 2020 to 2027.
The prime objective of this report is to help the user understand the market in terms of its definition, segmentation, market potential, influential trends, and the challenges that the market is facing with 10 major regions and 50 major countries. Deep researches and analysis were done during the preparation of the report. The readers will find this report very helpful in understanding the market in depth. The data and the information regarding the market are taken from reliable sources such as websites, annual reports of the companies, journals, and others and were checked and validated by the industry experts. The facts and data are represented in the report using diagrams, graphs, pie charts, and other pictorial representations. This enhances the visual representation and also helps in understanding the facts much better.
By Market Players:
Bound Tree Medical
Timesco
Teleflex
KARL STORZ GmbH
TRUPHATEK
By Type
Electronic Type
Others
By Application
Hospital
Clinic
Medical Center
Others
By Regions/Countries:
North America
United States
Canada
Mexico
East Asia
China
Japan
South Korea
Europe
Germany
United Kingdom
France
Italy
Russia
Spain
Netherlands
Switzerland
Poland
South Asia
India
Pakistan
Bangladesh
Southeast Asia
Indonesia
Thailand
Singapore
Malaysia
Philippines
Vietnam
Myanmar
Middle East
Turkey
Saudi Arabia
Iran
United Arab Emirates
Israel
Iraq
Qatar
Kuwait
Oman
Africa
Nigeria
South Africa
Egypt
Algeria
Morocoo
Oceania
Australia
New Zealand
South America
Brazil
Argentina
Colombia
Chile
Venezuela
Peru
Puerto Rico
Ecuador
Rest of the World
Kazakhstan
Points Covered in The Report
The points that are discussed within the report are the major market players that are involved in the market such as market players, raw material suppliers, equipment suppliers, end users, traders, distributors and etc.
The complete profile of the companies is mentioned. And the capacity, production, price, revenue, cost, gross, gross margin, sales volume, sales revenue, consumption, growth rate, import, export, supply, future strategies, and the technological developments that they are making are also included within the report. This report analyzed 12 years data history and forecast.
The growth factors of the market is discussed in detail wherein the different end users of the market are explained in detail.
Data and information by market player, by region, by type, by application and etc, and custom research can be added according to specific requirements.
The report contains the SWOT analysis of the market. Finally, the report contains the conclusion part where the opinions of the industrial experts are included.
Key Reasons to Purchase
To gain insightful analyses of the market and have comprehensive understanding of the global market and its commercial landscape.
Assess the production processes, major issues, and solutions to mitigate the development risk.
To understand the most affecting driving and restraining forces in the market and its impact in the global market.
Learn about the market strategies that are being adopted by leading respective organizations.
To understand the future outlook and prospects for the market.
Besides the standard structure reports, we also provide custom research according to specific requirements.
The report focuses on Global, Top 10 Regions and Top 50 Countries Market Size of Laryngoscope Handle 2016-2021, and development forecast 2022-2027 including industries, major players/suppliers worldwide and market share by regions, with company and product introduction, position in the market including their market status and development trend by types and applications which will provide its price and profit status, and marketing status & market growth drivers and challenges, with base year as 2020.
Key Indicators Analysed
Market Players & Competitor Analysis: The report covers the key players of the industry including Company Profile, Product Specifications, Production Capacity/Sales, Revenue, Price and Gross Margin 2016-2021 & Sales by Product Types.
Global and Regional Market Analysis: The report includes Global & Regional market status and outlook 2022-2027. Further the report provides break down details about each region & countries covered in the report. Identifying its production, consumption, import & export, sales volume & revenue forecast.
Market Analysis by Product Type: The report covers majority Product Types in the Laryngoscope Handle Industry, including its product specifcations by each key player, volume, sales by Volume and Value (M USD).
Markat Analysis by Application Type: Based on the Laryngoscope Handle Industry and its applications, the market is further sub-segmented into several major Application of its industry. It provides you with the market size, CAGR & forecast by each industry applications.
Market Trends: Market key trends which include Increased Competition and Continuous Innovations.
Opportunities and Drivers: Identifying the Growing Demands and New Technology
Porters Five Force Analysis: The report will provide with the state of competition in industry depending on five basic forces: threat of new entrants, bargaining power of suppliers, bargaining power of buyers, threat of substitute products or services, and existing industry rivalry.
COVID-19 Impact
Report covers Impact of Coronavirus COVID-19: Since the COVID-19 virus outbreak in December 2019, the disease has spread to almost every country around the globe with the World Health Organization declaring it a public health emergency. The global impacts of the coronavirus disease 2019 (COVID-19) are already starting to be felt, and will significantly affect the Laryngoscope Handle market in 2021. The outbreak of COVID-19 has brought effects on many aspects, like flight cancellations; travel bans and quarantines; restaurants closed; all indoor/outdoor events restricted; over forty countries state of emergency declared; massive slowing of the supply chain; stock market volatility; falling business confidence, growing panic among the population, and uncertainty about future.
Laryngoscope Handle Market report offers great insights of the market and consumer data and their interpretation through various figures and graphs. Report has embedded global market and regional market deep analysis through various research methodologies. The report also offers great competitor analysis of the industries and highlights the key aspect of their business like success stories, market development and growth rate.
Mucus Suction Pump Market
Pediatric Nasal Aspirator Market
Pediatric Nasal Irrigator Market
Pediatric Rollator Market
Contact us: https://www.reportmines.com/contact-us.php
0 notes
Text
U.S. Bets On Small, Untested Company to Deliver COVID Vaccine
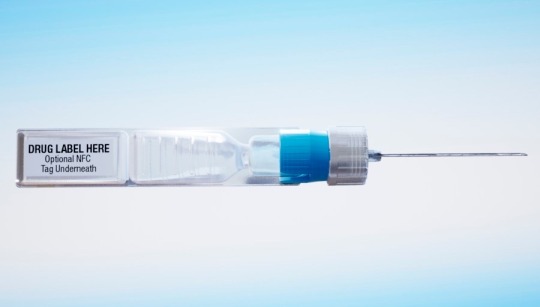
This undated image provided by ApiJect in July 2020 shows a prototype of their "BFS" prefilled syringe. (ApiJect file photo)
— AP | July 10, 2020 | By Martha Mendoza & Juliet Linderman
This story is part of an ongoing investigation by The Associated Press, FRONTLINE, and The Global Reporting Centre that examines the deadly consequences of the fragmented worldwide medical supply chain.
When precious vats of COVID-19 vaccine are finally ready, jabbing the lifesaving solution into the arms of Americans will require hundreds of millions of injections.
As part of its strategy to administer the vaccine as quickly as possible, the Trump administration has agreed to invest more than half a billion in tax dollars in ApiJect Systems America, a young company whose injector is not approved by federal health authorities and that hasn’t yet set up a factory to manufacture the devices.
The commitment to ApiJect dwarfs the other needle orders the government has placed with a major manufacturer and two other small companies.
“The fact of this matter is, it would be crazy for people to just rely on us. I would be the first to say it,” said ApiJect CEO Jay Walker. “We should be America’s backup at this point, but probably not its primary.”
Trump administration officials would not say why they are investing so heavily in ApiJect’s technology. The company has made only about 1,000 prototypes to date, and it’s not clear whether those devices can deliver the vaccines that are currently in development. So far, the leading candidates are using traditional vials to hold the vaccine, and needles and syringes in their clinical trials.
RELUCTANT SUPPLIER
ApiJect founder Marc Koska never intended to vaccinate the United States. For the past five years, he’s been working on his lifetime mission of creating an ultra low-cost prefilled syringe that would reduce the need to reuse needles in the developing world.
Instead, the company’s biggest customer has become the U.S. government.
ApiJect received a no-bid contract earlier this year from the Defense Department under an exception for “unusual and compelling urgency.” Authorities said the U.S. Department of Health and Human Services, tasked with buying the necessary supplies, “does not have the resources or capacity to conduct procurements necessary to respond to the COVID-19 pandemic,” according to a June 5 military document.
The government promised ApiJect $138 million to produce 100 million of its devices by the end of the year, which will require the company to retrofit new manufacturing lines in existing factories. And it’s offered another $456 million as part of a public-private partnership contract to bring online several new factories to make another 500 million devices to “contain the pandemic spread to minimize the loss of life and impact to the United States economy,” said the document.
These amounts are more than double the per-syringe cost the government is paying other companies for the work.
ApiJect first appeared on the U.S. government’s radar almost two years ago when the company piqued the interest of Admiral Brett P. Giroir, HHS’s assistant secretary for health, at the World Health Organization’s Global Conference on Primary Health Care in Astana, Kazakhstan.
Koska said Giroir was “blown away” by their technology and told them that if a pandemic hit, the strategic national stockpile was going to need a very fast way to get injections filled with vaccines or therapeutics and ready to deliver.
According to Walker, the CEO, ApiJect wasn’t interested in a federal contract — they were aiming to change the developing world with quick, inexpensive injection devices that could save millions of lives.
But at the conference, Walker found himself at a table with Giroir at a luncheon, just two seats apart. The admiral was fascinated by the low-cost injection technology, Walker said, and when Walker showed him the prototype that he always carries in his pocket, Giroir asked how they plan to do this in the U.S.
Walker said he told the admiral that the company wasn’t planning to operate in the U.S. but was struck by Giroir’s enthusiasm.
“He was the first person, if not the only person at the event, who understood the revolutionary nature of this platform,” Walker recalled in an interview with AP. “And he said, ‘Wow this is amazing. You need to do this in the U.S.’”
Walker continued to resist, he said, but Giroir — who is also a doctor specializing in pediatric critical care — “wasn’t big on taking no for an answer,” Walker said.
At Giroir’s urging they presented the prototype injector to U.S. officials. HHS declined to make agency officials available for interviews.
It wasn’t until later, when Walker was introduced by a friend to Col. Matthew Hepburn at the Defense Advanced Research Projects Agency, that a plan for ApiJect to work in the United States began to take shape, he said.
HHS Assistant Secretary for Preparedness and Response Robert Kadlec approved a $10 million contract for ApiJect for research and development in January 2020, according to a document in the federal procurement data system. The company was responsible for securing private investments to create new production lines where the devices would be made over three to five years.
When the pandemic emerged weeks later, officials sounded the alarm about a potential shortage of needles and syringes to deliver a vaccine if and when one became available.
The federal Strategic National Stockpile of medical supplies had only 15 million syringes, according to Dr. Rick Bright, who later left his position at Health and Human Services and filed a whistleblower complaint.
Bright warned White House trade adviser Peter Navarro and his HHS colleagues of a looming needle shortfall, according to a series of emails disclosed in his complaint.
“We are hearing rumblings about the US inventory of needles and syringes … heading to other countries,” wrote Bright. “There is limited inventory in the supply chain, it could take 2+ years to make enough to satisfy the U.S. vaccine needs.”
Navarro said the U.S. would need 850 million needles.
“We may find ourselves in a situation where we have enough vaccine but no way to deliver all of it,” he said in a February memo to the White House coronavirus task force.
He recommended the task force “direct HHS BARDA to initiate a program to identify all alternate vaccine delivery methods and ramp up production.“ BARDA is the Biomedical Advanced Research and Development Authority within HHS.
Suddenly ApiJect’s 5-year plan to mass produce its devices became a sprint measured in months with a new $138 million contract, announced in May, to produce 100 million devices by year’s end.
Jefferies Financial Group is acting as the leader of the public-private partnership with HHS and invested $10 million to help ApiJect build surge production facilities in March. The company said it would try to raise up to $1 billion more. There have been no additional announcements of funding.
Walker said due to nondisclosure agreements with both the government and investors, the company is unable to say what private funding they’ve secured so far.
OPERATION WARP SPEED
On a warm mid-May day in the White House Rose Garden, President Donald Trump introduced “a massive scientific, industrial and logistical endeavor” dubbed Operation Warp Speed.
The idea, he said, was to be ready to distribute a COVID-19 vaccine as soon as it was developed.
“We must not be caught short on our capacity to deliver emergency drugs to Americans in need,” said HHS Secretary Alex Azar.
An estimated 700 million injections may be needed to inoculate the nation — at least two shots for every person, according to the military document.
In early May, the government put in two orders, to Retractable Technologies in Little Elm, Texas, and Marathon Medical in Aurora, Colorado, totaling 320 million needles and syringes.
Later in May, the government announced plans for ApiJect to manufacture more than 500 million all-in-one devices that would come pre-loaded with the vaccine.
On Wednesday, the largest domestic manufacturer of needles and syringes, Becton Dickinson, announced the first U.S. order of $11.7 million for 50 million needles and syringes by the end of this year. It plans to ramp up manufacturing over the next year.
And earlier this month Retractable entered into a second contract with the government, this one for $53 million meant to boost domestic manufacturing.
Together that sounds like enough injection devices.
But Retractable, which was worried enough about its financial future that earlier this year it received a $1.36 million loan from the Paycheck Protection Program, has been doing about 80% of its manufacturing in China. And Marathon is a medical supply distributor, and there is no indication on its web site that it manufactures needles and syringes at all. The company did not respond to repeated requests for comment.
Despite the race to replenish the domestic needle and syringe supply, about 400 shipping containers of syringes have left the U.S. for countries including Germany, Colombia, Australia, Brazil and Italy this year, according to Panjiva Inc., a service that independently tracks global trade. That’s the same, on average, as syringe exports over the past five years.
Experts acknowledge that a mass vaccination campaign is going to be complicated.
“There are a lot of moving parts to this,” said Dr. Bruce Gellin, the Sabin Vaccine Institute’s president of global immunization.
Darin Zehrung, who studied medical devices at PATH, a nonprofit advocating for health equity, said it’s wise to invest in new injection technologies. But that only works if there are plenty of basic syringes and needles stocked up.
“Hedging bets is the best approach, but plan for the worst case scenario and hope for the best case scenario,” said Zehrung.
AWAITING APPROVAL
ApiJect’s devices are self-contained, with soft plastic blisters that are squeezed, like a nose spray or eye drop, to push the vaccine through an attached needle and into the patient.
The device includes a little computer chip — like the ones in credit cards — that can transmit information about the drug, dose, location and time of administration.
Other injection devices Koska designed have been used in the developing world, but this ApiJect technology has not.
The company said they have started discussions with the U.S. Food and Drug Administration to review the device on a priority basis while the company moves ahead fitting factories to make their injectors. The agency wouldn’t confirm this, citing its policy against discussing products involved in clinical trials.
Testing different vaccine candidates in the ApiJect devices will be critical before injecting the public.
Plastic could interact differently with the liquid than the glass vials currently used in trials, experts say. And there are strict temperature requirements. ApiJect’s planned process is to pour vaccine doses into the warm plastic blisters as they come off the production line, the company says. ApiJect says they can instantly cool the devices as they are made.
Walker, the ApiJect CEO, who founded the online travel agency Priceline, acknowledges that the government’s decision to rely on “an emergency plan of refitting established pharmaceutical manufacturing facilities is risky. But we feel good about it.”
NO COMMENT
The Associated Press asked the Health and Human Services department over many weeks to explain the government’s approach. The agency didn’t allow an official to speak on the record for this story.
A senior administration official, speaking on condition of anonymity because the agency declined to allow him to identified by name, told AP he wasn’t familiar with ApiJect or the contract. But he said the government was buying a range of devices to deliver the vaccine because they don’t know what they need. And, he said, the Trump administration is looking to boost domestic manufacturing.
When AP reached out directly to Trump’s vaccine czar, Moncef Slaoui, to discuss the new technology, a spokesperson said the query was inappropriate.
“If this continues, we will make no one else available either,” Natalie Baldassarre, a special assistant at HHS, wrote in an email.
Last week, HHS Assistant Secretary of Public Affairs Michael Caputo wrote that the agency has “lost interest in assisting your story” and offered no further comment.
— Mendoza reported from San Francisco. Linderman reported from Baltimore. Lauran Neergaard and Stephen Braun in Washington contributed.
0 notes
Text
Medical Stethoscopes Market Evolving Technology, Trends and Industry Analysis and Growth to 2026
Extensive evaluation of Global Medical Stethoscopes Market 2019-2026, underscoring product values, growing demand, considerable revenue, and escalating CAGR.
The global Medical Stethoscopes Market 2019 is comprehensively and Insightful information in the report, taking into consideration various factors such as competition, regional growth, segmentation, and Medical Stethoscopes Market size by value and volume. This is an excellent research study specially compiled to provide the latest insights into critical aspects of the Medical Stethoscopes market. The report includes different market forecasts related to market size, production, revenue, consumption, CAGR, gross margin, price, and other key factors. It is prepared with the use of industry-best primary and secondary research methodologies and tools.
In this report,global Medical Stethoscopes Market will reach 18.24 Million USD by the end of 2022 with a CAGR of 8.36%
The global Medical Stethoscopes market is valued at 12.21 Million USD in 2017 and will reach 18.24 Million USD by the end of 2022, growing at a CAGR of 8.36% during 2017-2022.
The Medical stethoscope is an acoustic medical device for auscultation, or listening to the internal sounds of an animal or human body. It typically has a small disc-shaped resonator that is placed against the chest, and two tubes connected to earpieces. It is often used to listen to lung and heart sounds.
Medical Stethoscopes can be divided into two categories--manual & mechanical stethoscopes type and digital stethoscopes type. In 2017, manual and mechanical stethoscopes account for 94.33% of the global sales market but only 47.89% of the revenue market, while digital stethoscopes account for 5.67% of the global sales market but 52.11% of the revenue market. This shows that the manual & mechanical stethoscope is very popular in the market, but the price is low. In 2017, manual stethoscopes sold for an average of $9.20, while digital stethoscopes sold for an average of $166.2, nearly 20 times the price of manual stethoscopes. And AMR predicts that prices will decline over the next five years in the global economy.
The sales market share of global Medical Stethoscopes in hospital use, clinics use and other use have been stable year by year, at 57.15%, 42.85% and 28.12% respectively in 2017, and for several consecutive years, the amplitude was within one percent. This indicates that the segment of the Medical Stethoscopes in the global market tends to be fixed without great changes. Among them, the Medical Stethoscopes market has the most promising sales prospects in hospital use.
AMR center data shows that North America is the biggest contributor to the Medical Stethoscopes revenue market, accounted for 40.02% of the total global market with a revenue of 136.67 million USD in 2017, followed by Europe, 30.12% with a revenue of 102.88 million USD.
3M Littmann is the largest company in the global Medical Stethoscopes market, accounted for 35.35% of the revenue market share in 2017, followed by SUZUKEN and Welch Allyn, accounted for 17.31% and 7.68% of the revenue market share in 2017. Market competition is not intense and have been formed in the monopoly position in the industry. The top two producers account for above 50 % of the revenue market.
“ Connecting businesses through research Intel & everyday decisions to upping the economy We offer incisive analysis & encourage effective results.
This X’mas take the step towards powerful data and successful decisions!
Avail Flat 40% off on all our qualitative research reports. ”
Request Sample of Global Medical Stethoscopes Market @ https://www.acquiremarketresearch.com/sample-request/274256/
Top Companies Covered in the report: 3M Littmann, SUZUKEN, Welch Allyn, Yuwell, Omron, American Diagnostics, Rudolf Riester, Thinklabs, GF Health, Folee, MDF Instruments, Cardionics, EmsiG, HD Medical.
By the product type, the market is primarily split into: ManualandMechanicalStethoscopes, DigitalStethoscopes
By the end-users/application, this report covers the following segments: Hospitals, Clinics, Others
Buy 1 and get 1 report free on direct purchase Contact Us Avail the Offer
Be the Santa of your Happy Business!
The main sources are mainly industry experts in the core and related industries and manufacturers involved in all sectors of the industry supply chain. The bottom-up approach is used to plan the market size of Medical Stethoscopes based on the end-user industry and region in terms of value. With the help of data, we support the primary market through the three-dimensional survey procedure and the first interview and data verification through expert telephone, determine the individual market share and size and confirm with this study.
Read Table of Content of Medical Stethoscopes Market at @ https://www.acquiremarketresearch.com/industry-reports/medical-stethoscopes-market/274256/
Regions covered in the market report: North America (United States, Canada and Mexico), Europe (Germany, France, UK, Russia and Italy), Asia-Pacific (China, Japan, Korea, India and Southeast Asia), South America (Brazil, Argentina, Colombia etc.), Middle East and Africa (Saudi Arabia, UAE, Egypt, Nigeria and South Africa)
The Objective of the Study:
1)To study and forecast the market size of Medical Stethoscopes in Global
2)To analyze the global key players, SWOT analysis, value and Global Medical Stethoscopes Market share for top players.
3)To identify significant trends and factors driving or constraining the growth of the market.
4)To analyze competitive developments such as extensions, contracts, new product launches, mergers, and acquisitions in the market
5)To strategically analyze each sub-market in regards to the individual growth trends and their influence in the Medical Stethoscopes Market.
Some of the major questions are answered:
1)What are the different types of Medical Stethoscopes Market?
2)What are the market trends and major development patterns equipment’s and products?
3)Who are the key industry pioneers and what is their overall share in the global Medical Stethoscopes Market?
4)What are the multiple used case scenarios considered under various end-users and applications for the market?
5)What are the different sales, marketing, and distribution channels in the global industry?
Ask for discounts @ https://www.acquiremarketresearch.com/discount-request/274256/
Our experts and analysts evaluate the vendors in the Medical Stethoscopes market and provide understandings to articulate current and future market trends, innovation, customer expectations and competitive forces. The overviews, SWOT analysis and strategies of each vendor in the market provide understanding about the Medical Stethoscopes market forces and how those can be oppressed to create future opportunities.
0 notes
Text
Hancock Jaffe clears Colombian regulatory hurdle for VenoValve trial
Hancock Jaffe Laboratories (NSDQ:HJLI) said today that Colombia’s INVIMA, the country’s FDA equivalent, completed their review of the company’s application for a first-in-human trial of its VenoValve, clearing another hurdle towards launching the study.
The VenoValve is a porcine valve designed to be implanted into the femoral or popliteal vein to treat lower limb chronic venous insufficiency from damage to leg vein valves after deep vein thrombosis.
The Irvine, Calif.-based company said that the next step is to present an official application for approval of the trial from INVIMA’s Medical Device and Other Technologies Committee at a meeting next month.
“Dr. Marc Glickman, our senior vice president and chief medical officer, will travel to Bogota at the beginning of December to begin site initiation, and surgical training for implantation of the VenoValve. Dr. Glickman has overseen many successful clinical trials, and will work closely with our Colombian partners to attend to all details leading up to the VenoValve implantations,” CEO Robert Berman said in a press release.
Upon approval, Hancock Jaffe said that it will begin patient enrollment immediately for the first-in-human trial and will make arrangements to export the VenoValve devices, which have already been manufactured and inspected, to Colombia. The company plans to announced the dates for the first implantations in the trial shortly.
In August, Hancock Jeffe said that it won approval from Colombia’s Medical Research Committee at Fundación Santa Fe de Bogotá to launch the trial.
The post Hancock Jaffe clears Colombian regulatory hurdle for VenoValve trial appeared first on MassDevice.
from MassDevice https://ift.tt/2Touflf
0 notes
Text
Bioaccess provides the best medical device CRO services in Costa Rica! With extensive experience and a proven track record, we offer comprehensive support throughout the product development lifecycle. From regulatory consulting to clinical trial management, our team ensures efficient and successful outcomes for your medical device. Trust us as your partner in innovation and accelerate your path to market approval. Contact us today to learn more about our tailored CRO services and propel your project forward! www.bioaccessla.com
#Medical Device Contract Research Organization in Latin America#Medical Device CRO in Mexico#Medical Device CRO in the Dominican Republic#Medical Device CRO in El Salvador#Medical Device CRO in Brazil#Medical Device CRO in Peru#Medical Device CRO in Panama#Medical Device CRO in Chile#Medical Device CRO in Costa Rica#Medical Device CRO in Honduras#Medical Device CRO in Argentina#Medical Device CRO in Ecuador#Medical Device First in Human Clinical Research Study in Latin America#Medical Device First in Human Clinical Research Study in Colombia
1 note
·
View note
Text
Experience excellence in medical device CRO services with Bioaccess. Our dedicated team specializes in providing comprehensive solutions tailored to your unique research needs. Trust us to streamline your path to success from regulatory consulting to clinical trial management. With proven expertise and unwavering commitment to quality, Bioaccess is your trusted partner in advancing medical device modification. Choose excellence, choose us for your next clinical research endeavor. For more details about our services, call +1 (407) 205-8930 or visit our website now. www.bioaccessla.com
#Medical Device Contract Research Organization in Latin America#Medical Device CRO in Mexico#Medical Device CRO in the Dominican Republic#Medical Device CRO in El Salvador#Medical Device CRO in Brazil#Medical Device CRO in Peru#Medical Device CRO in Panama#Medical Device CRO in Chile#Medical Device CRO in Costa Rica#Medical Device CRO in Honduras#Medical Device CRO in Argentina#Medical Device CRO in Ecuador#Medical Device First in Human Clinical Research Study in Latin America#Medical Device First in Human Clinical Research Study in Colombia
1 note
·
View note
Text
Medical Device Clinical Research Services in Latin America

Upgrade your knowledge in medical device research with Bioaccess in Latin America. Our comprehensive clinical research services encompass every stage of development, ensuring rigorous testing and regulatory compliance. From feasibility studies to post-market surveillance, trust us to navigate the complexities of medical device research. Choose Bioaccess for excellence in clinical trials and drive forward advancements in healthcare technology across Latin America. For more details about our services, you can call +1 (407) 205-8930 or visit our website now.
#Medical Device Contract Research Organization in Latin America#Medical Device CRO in Mexico#Medical Device CRO in the Dominican Republic#Medical Device CRO in El Salvador#Medical Device CRO in Brazil#Medical Device CRO in Peru#Medical Device CRO in Panama#Medical Device CRO in Chile#Medical Device CRO in Costa Rica#Medical Device CRO in Honduras#Medical Device CRO in Argentina#Medical Device CRO in Ecuador#Medical Device First in Human Clinical Research Study in Latin America#Medical Device First in Human Clinical Research Study in Colombia
1 note
·
View note
Text
Medical Device CRO in Mexico
Boost your medical device research with Bioaccess, your trusted CRO partner in Mexico. Our comprehensive services streamline clinical trials, ensuring precision and compliance. From regulatory support to data management, we pave the way for successful medical advancements. Choose Bioaccess for CRO services that redefine healthcare innovation. For more details about our services, you can call +1 (407) 205-8930.
#Medical Device Contract Research Organization in Latin America#Medical Device CRO in Mexico#Medical Device CRO in the Dominican Republic#Medical Device CRO in El Salvador#Medical Device CRO in Brazil#Medical Device CRO in Peru#Medical Device CRO in Panama#Medical Device CRO in Chile#Medical Device CRO in Costa Rica#Medical Device CRO in Honduras#Medical Device CRO in Argentina#Medical Device CRO in Ecuador#Medical Device First in Human Clinical Research Study in Latin America#Medical Device First in Human Clinical Research Study in Colombia
1 note
·
View note
Text
Medical Device CRO in Brazil

Start your journey on the path to success in Brazil with Bioaccess, your trusted Medical Device CRO. We specialize in regulatory compliance, clinical trials, and market access strategies, ensuring a streamlined journey from development to market entry. Partner with us for comprehensive support and expert guidance in the dynamic landscape of medical device innovation in Brazil. Choose Bioaccess for excellence in CRO services. For more details about our services, you can call +1 (407) 205-8930 or visit our website now.
#Medical Device Contract Research Organization in Latin America#Medical Device CRO in Mexico#Medical Device CRO in the Dominican Republic#Medical Device CRO in El Salvador#Medical Device CRO in Brazil#Medical Device CRO in Peru#Medical Device CRO in Panama#Medical Device CRO in Chile#Medical Device CRO in Costa Rica#Medical Device CRO in Honduras#Medical Device CRO in Argentina#Medical Device CRO in Ecuador#Medical Device First in Human Clinical Research Study in Latin America#Medical Device First in Human Clinical Research Study in Colombia
1 note
·
View note
Text
Revolutionize your medical device development with Bioaccess Clinical Trials. Our comprehensive services encompass regulatory strategy, patient recruitment, and efficient trial management. Access global markets through our expertise in diverse regulatory landscapes. Trust Bioaccess for a streamlined path to market success in medical device innovation. Explore our tailored solutions for groundbreaking clinical trials. For more details about our services, you can call +1 (407) 205-8930 or visit our website now. www.bioaccessla.com/solutions
#Medical Device Contract Research Organization in Latin America#Medical Device CRO in Mexico#Medical Device CRO in the Dominican Republic#Medical Device CRO in El Salvador#Medical Device CRO in Brazil#Medical Device CRO in Peru#Medical Device CRO in Panama#Medical Device CRO in Chile#Medical Device CRO in Costa Rica#Medical Device CRO in Honduras#Medical Device CRO in Argentina#Medical Device CRO in Ecuador#Medical Device First in Human Clinical Research Study in Latin America#Medical Device First in Human Clinical Research Study in Colombia
1 note
·
View note
Text
Medical Device Clinical Study Services in Latin America

Accelerate your medical device development with Bioaccess Clinical Study Services in Latin America. Our expertise navigates regulatory pathways, offering seamless clinical trials. Access diverse patient populations and world-class facilities. Trust Bioaccess for comprehensive support, ensuring compliance and efficiency throughout your clinical study journey in Latin America. For more details about our services, you can call +1 (407) 205-8930 or visit our website now.
#Medical Device Contract Research Organization in Latin America#Medical Device CRO in Mexico#Medical Device CRO in the Dominican Republic#Medical Device CRO in El Salvador#Medical Device CRO in Brazil#Medical Device CRO in Peru#Medical Device CRO in Panama#Medical Device CRO in Chile#Medical Device CRO in Costa Rica#Medical Device CRO in Honduras#Medical Device CRO in Argentina#Medical Device CRO in Ecuador#Medical Device First in Human Clinical Research Study in Latin America#Medical Device First in Human Clinical Research Study in Colombia
1 note
·
View note
Text
Find the best medical device CRO

At Bioaccess, our medical device CRO services provide comprehensive solutions for regulatory compliance and product development. We specialize in bioassay testing to ensure accurate and reliable results. Our experienced team provides end-to-end support from study design to data analysis, helping you navigate the complex landscape of medical device development. Count on us to streamline your bioassay needs.
#Medical Device Contract Research Organization in Latin America#Medical Device CRO in Mexico#Medical Device CRO in the Dominican Republic#Medical Device CRO in El Salvador#Medical Device CRO in Brazil#Medical Device CRO in Peru#Medical Device CRO in Panama#Medical Device CRO in Chile#Medical Device CRO in Costa Rica#Medical Device CRO in Honduras#Medical Device CRO in Argentina#Medical Device CRO in Ecuador#Medical Device First in Human Clinical Research Study in Latin America#Medical Device First in Human Clinical Research Study in Colombia
1 note
·
View note