#Laboratory Information Systems (LIS) industry
Link
#market research future#laboratory information systems#lis market size#laboratory systems market size#laboratory systems industry
0 notes
Text

Let's Explore a Metal-Rich Asteroid 🤘
Between Mars and Jupiter, there lies a unique, metal-rich asteroid named Psyche. Psyche’s special because it looks like it is part or all of the metallic interior of a planetesimal—an early planetary building block of our solar system. For the first time, we have the chance to visit a planetary core and possibly learn more about the turbulent history that created terrestrial planets.
Here are six things to know about the mission that’s a journey into the past: Psyche.

1. Psyche could help us learn more about the origins of our solar system.
After studying data from Earth-based radar and optical telescopes, scientists believe that Psyche collided with other large bodies in space and lost its outer rocky shell. This leads scientists to think that Psyche could have a metal-rich interior, which is a building block of a rocky planet. Since we can’t pierce the core of rocky planets like Mercury, Venus, Mars, and our home planet, Earth, Psyche offers us a window into how other planets are formed.

2. Psyche might be different than other objects in the solar system.
Rocks on Mars, Mercury, Venus, and Earth contain iron oxides. From afar, Psyche doesn’t seem to feature these chemical compounds, so it might have a different history of formation than other planets.
If the Psyche asteroid is leftover material from a planetary formation, scientists are excited to learn about the similarities and differences from other rocky planets. The asteroid might instead prove to be a never-before-seen solar system object. Either way, we’re prepared for the possibility of the unexpected!
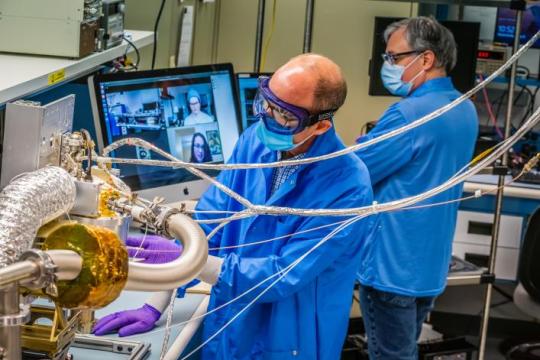
3. Three science instruments and a gravity science investigation will be aboard the spacecraft.
The three instruments aboard will be a magnetometer, a gamma-ray and neutron spectrometer, and a multispectral imager. Here’s what each of them will do:
Magnetometer: Detect evidence of a magnetic field, which will tell us whether the asteroid formed from a planetary body
Gamma-ray and neutron spectrometer: Help us figure out what chemical elements Psyche is made of, and how it was formed
Multispectral imager: Gather and share information about the topography and mineral composition of Psyche
The gravity science investigation will allow scientists to determine the asteroid’s rotation, mass, and gravity field and to gain insight into the interior by analyzing the radio waves it communicates with. Then, scientists can measure how Psyche affects the spacecraft’s orbit.

4. The Psyche spacecraft will use a super-efficient propulsion system.
Psyche’s solar electric propulsion system harnesses energy from large solar arrays that convert sunlight into electricity, creating thrust. For the first time ever, we will be using Hall-effect thrusters in deep space.

5. This mission runs on collaboration.
To make this mission happen, we work together with universities, and industry and NASA to draw in resources and expertise.
NASA’s Jet Propulsion Laboratory manages the mission and is responsible for system engineering, integration, and mission operations, while NASA’s Kennedy Space Center’s Launch Services Program manages launch operations and procured the SpaceX Falcon Heavy rocket.
Working with Arizona State University (ASU) offers opportunities for students to train as future instrument or mission leads. Mission leader and Principal Investigator Lindy Elkins-Tanton is also based at ASU.
Finally, Maxar Technologies is a key commercial participant and delivered the main body of the spacecraft, as well as most of its engineering hardware systems.

6. You can be a part of the journey.
Everyone can find activities to get involved on the mission’s webpage. There's an annual internship to interpret the mission, capstone courses for undergraduate projects, and age-appropriate lessons, craft projects, and videos.
You can join us for a virtual launch experience, and, of course, you can watch the launch with us on Oct. 12, 2023, at 10:16 a.m. EDT!
For official news on the mission, follow us on social media and check out NASA’s and ASU’s Psyche websites.
Make sure to follow us on Tumblr for your regular dose of space!
#Psyche#Mission to Psyche#asteroid#NASA#exploration#technology#tech#spaceblr#solar system#space#not exactly#metalcore#but close?
2K notes
·
View notes
Text
Two MIT teams selected for NSF sustainable materials grants
New Post has been published on https://thedigitalinsider.com/two-mit-teams-selected-for-nsf-sustainable-materials-grants/
Two MIT teams selected for NSF sustainable materials grants

Two teams led by MIT researchers were selected in December 2023 by the U.S. National Science Foundation (NSF) Convergence Accelerator, a part of the TIP Directorate, to receive awards of $5 million each over three years, to pursue research aimed at helping to bring cutting-edge new sustainable materials and processes from the lab into practical, full-scale industrial production. The selection was made after 16 teams from around the country were chosen last year for one-year grants to develop detailed plans for further research aimed at solving problems of sustainability and scalability for advanced electronic products.
Of the two MIT-led teams chosen for this current round of funding, one team, Topological Electric, is led by Mingda Li, an associate professor in the Department of Nuclear Science and Engineering. This team will be finding pathways to scale up sustainable topological materials, which have the potential to revolutionize next-generation microelectronics by showing superior electronic performance, such as dissipationless states or high-frequency response. The other team, led by Anuradha Agarwal, a principal research scientist at MIT’s Materials Research Laboratory, will be focusing on developing new materials, devices, and manufacturing processes for microchips that minimize energy consumption using electronic-photonic integration, and that detect and avoid the toxic or scarce materials used in today’s production methods.
Scaling the use of topological materials
Li explains that some materials based on quantum effects have achieved successful transitions from lab curiosities to successful mass production, such as blue-light LEDs, and giant magnetorestance (GMR) devices used for magnetic data storage. But he says there are a variety of equally promising materials that have shown promise but have yet to make it into real-world applications.
“What we really wanted to achieve is to bring newer-generation quantum materials into technology and mass production, for the benefit of broader society,” he says. In particular, he says, “topological materials are really promising to do many different things.”
Topological materials are ones whose electronic properties are fundamentally protected against disturbance. For example, Li points to the fact that just in the last two years, it has been shown that some topological materials are even better electrical conductors than copper, which are typically used for the wires interconnecting electronic components. But unlike the blue-light LEDs or the GMR devices, which have been widely produced and deployed, when it comes to topological materials, “there’s no company, no startup, there’s really no business out there,” adds Tomas Palacios, the Clarence J. Lebel Professor in Electrical Engineering at MIT and co-principal investigator on Li’s team. Part of the reason is that many versions of such materials are studied “with a focus on fundamental exotic physical properties with little or no consideration on the sustainability aspects,” says Liang Fu, an MIT professor of physics and also a co-PI. Their team will be looking for alternative formulations that are more amenable to mass production.
One possible application of these topological materials is for detecting terahertz radiation, explains Keith Nelson, an MIT professor of chemistry and co-PI. This extremely high-frequency electronics can carry far more information than conventional radio or microwaves, but at present there are no mature electronic devices available that are scalable at this frequency range. “There’s a whole range of possibilities for topological materials” that could work at these frequencies, he says. In addition, he says, “we hope to demonstrate an entire prototype system like this in a single, very compact solid-state platform.”
Li says that among the many possible applications of topological devices for microelectronics devices of various kinds, “we don’t know which, exactly, will end up as a product, or will reach real industrial scaleup. That’s why this opportunity from NSF is like a bridge, which is precious, to allow us to dig deeper to unleash the true potential.”
In addition to Li, Palacios, Fu, and Nelson, the Topological Electric team includes Qiong Ma, assistant professor of physics in Boston College; Farnaz Niroui, assistant professor of electrical engineering and computer science at MIT; Susanne Stemmer, professor of materials at the University of California at Santa Barbara; Judy Cha, professor of materials science and engineering at Cornell University; industrial partners including IBM, Analog Devices, and Raytheon; and professional consultants. “We are taking this opportunity seriously,” Li says. “We really want to see if the topological materials are as good as we show in the lab when being scaled up, and how far we can push to broadly industrialize them.”
Toward sustainable microchip production and use
The microchips behind everything from smartphones to medical imaging are associated with a significant percentage of greenhouse gas emissions today, and every year the world produces more than 50 million metric tons of electronic waste, the equivalent of about 5,000 Eiffel Towers. Further, the data centers necessary for complex computations and huge amount of data transfer — think AI and on-demand video — are growing and will require 10 percent of the world’s electricity by 2030.
“The current microchip manufacturing supply chain, which includes production, distribution, and use, is neither scalable nor sustainable, and cannot continue. We must innovate our way out of this crisis,” says Agarwal.
The name of Agarwal’s team, FUTUR-IC, is a reference to the future of the integrated circuits, or chips, through a global alliance for sustainable microchip manufacturing. Says Agarwal, “We bring together stakeholders from industry, academia, and government to co-optimize across three dimensions: technology, ecology, and workforce. These were identified as key interrelated areas by some 140 stakeholders. With FUTUR-IC we aim to cut waste and CO2-equivalent emissions associated with electronics by 50 percent every 10 years.”
The market for microelectronics in the next decade is predicted to be on the order of a trillion dollars, but most of the manufacturing for the industry occurs only in limited geographical pockets around the world. FUTUR-IC aims to diversify and strengthen the supply chain for manufacturing and packaging of electronics. The alliance has 26 collaborators and is growing. Current external collaborators include the International Electronics Manufacturing Initiative (iNEMI), Tyndall National Institute, SEMI, Hewlett Packard Enterprise, Intel, and the Rochester Institute of Technology.
Agarwal leads FUTUR-IC in close collaboration with others, including, from MIT, Lionel Kimerling, the Thomas Lord Professor of Materials Science and Engineering; Elsa Olivetti, the Jerry McAfee Professor in Engineering; Randolph Kirchain, principal research scientist in the Materials Research Laboratory; and Greg Norris, director of MIT’s Sustainability and Health Initiative for NetPositive Enterprise (SHINE). All are affiliated with the Materials Research Laboratory. They are joined by Samuel Serna, an MIT visiting professor and assistant professor of physics at Bridgewater State University. Other key personnel include Sajan Saini, education director for the Initiative for Knowledge and Innovation in Manufacturing in MIT’s Department of Materials Science and Engineering; Peter O’Brien, a professor from Tyndall National Institute; and Shekhar Chandrashekhar, CEO of iNEMI.
“We expect the integration of electronics and photonics to revolutionize microchip manufacturing, enhancing efficiency, reducing energy consumption, and paving the way for unprecedented advancements in computing speed and data-processing capabilities,” says Serna, who is the co-lead on the project’s technology “vector.”
Common metrics for these efforts are needed, says Norris, co-lead for the ecology vector, adding, “The microchip industry must have transparent and open Life Cycle Assessment (LCA) models and data, which are being developed by FUTUR-IC.” This is especially important given that microelectronics production transcends industries. “Given the scale and scope of microelectronics, it is critical for the industry to lead in the transition to sustainable manufacture and use,” says Kirchain, another co-lead and the co-director of the Concrete Sustainability Hub at MIT. To bring about this cross-fertilization, co-lead Olivetti, also co-director of the MIT Climate and Sustainability Consortium (MCSC), will collaborate with FUTUR-IC to enhance the benefits from microchip recycling, leveraging the learning across industries.
Saini, the co-lead for the workforce vector, stresses the need for agility. “With a workforce that adapts to a practice of continuous upskilling, we can help increase the robustness of the chip-manufacturing supply chain, and validate a new design for a sustainability curriculum,” he says.
“We have become accustomed to the benefits forged by the exponential growth of microelectronic technology performance and market size,” says Kimerling, who is also director of MIT’s Materials Research Laboratory and co-director of the MIT Microphotonics Center. “The ecological impact of this growth in terms of materials use, energy consumption and end-of-life disposal has begun to push back against this progress. We believe that concurrently engineered solutions for these three dimensions will build a common learning curve to power the next 40 years of progress in the semiconductor industry.”
The MIT teams are two of six that received awards addressing sustainable materials for global challenges through phase two of the NSF Convergence Accelerator program. Launched in 2019, the program targets solutions to especially compelling challenges at an accelerated pace by incorporating a multidisciplinary research approach.
#000#2023#ai#analog#applications#approach#assessment#Blue#bridge#Business#CEO#chemistry#chips#Cleaner industry#climate#climate change#CO2#collaborate#Collaboration#college#computer#Computer Science#computing#concrete#Concrete Sustainability Hub#conductors#continuous#cutting#data#Data Centers
2 notes
·
View notes
Text
Empowering Developers: Building Solutions with Healthcare API
Healthcare API (Application Programming Interface) is a pivotal component in the modernization of the healthcare industry, offering a bridge between disparate systems and facilitating the seamless exchange of information. At its core, a healthcare API is a set of protocols, tools, and definitions that allow different software applications to communicate with each other, enabling the sharing and utilization of data across various platforms and devices securely.
One of the primary benefits of healthcare APIs lies in their ability to enhance interoperability among different healthcare systems and applications. In an increasingly digital healthcare landscape where patient data resides in numerous electronic health record (EHR) systems, laboratory databases, medical imaging systems, and more, APIs provide a standardized method for these systems to communicate and share information. This interoperability streamlines workflows, reduces manual data entry errors, and ultimately improves the quality and continuity of patient care.
Moreover, healthcare APIs empower developers and healthcare organizations to create innovative solutions that address specific needs and challenges within the industry. Developers can leverage APIs to build custom applications, integrate third-party services, or develop new features within existing healthcare software. For example, APIs can be used to develop patient portals, mobile health applications, telemedicine platforms, and population health management tools, among others. This flexibility encourages innovation and allows healthcare organizations to tailor solutions to meet the unique requirements of their patient populations.
In addition to improving interoperability and fostering innovation, healthcare APIs play a crucial role in data security and privacy. By utilizing secure authentication and authorization mechanisms, APIs ensure that sensitive patient information is protected during data exchange processes. Compliance with healthcare regulations, such as the Health Insurance Portability and Accountability Act (HIPAA) in the United States, is also facilitated through API-driven solutions, as they provide the necessary controls and safeguards to maintain compliance with regulatory requirements.
Overall, healthcare APIs are instrumental in driving digital transformation within the healthcare industry. By enabling seamless data exchange, fostering innovation, and ensuring data security and privacy, APIs play a vital role in improving patient care, enhancing operational efficiency, and ultimately advancing the delivery of healthcare services. As technology continues to evolve, healthcare APIs will remain at the forefront of efforts to modernize and optimize healthcare delivery worldwide.
#Healthcare API Market Demand#Healthcare API Market Share#Healthcare API Market Trend#Healthcare API Market Size
0 notes
Text
Why delve into an insightful overview of HL7 Integration?
In the intricate realm of healthcare systems, the seamless exchange of data is paramount. Health Level Seven (HL7) integration emerges as a key player in achieving this goal, revolutionizing how healthcare systems interact and share vital information.
What is HL7 Integration and Why Does It Matter?
HL7 integration is the backbone of modern healthcare, enabling seamless data exchange among different systems. This standardized approach ensures efficient communication, facilitating the swift sharing of patient information.
Its significance lies in promoting interoperability, reducing errors, and enhancing overall healthcare effectiveness. In essence, HL7 integration is crucial for creating a connected and responsive healthcare ecosystem.

What Sets HL7 Integration Apart?
HL7 integrations are designed to facilitate communication and data exchange between different healthcare systems. Whether it's hospitals, laboratories, or other healthcare entities, the ability to seamlessly share information is paramount for effective and coordinated patient care.
How Does HL7 Interface Improve Healthcare Interoperability?
An HL7 interface serves as the bridge between disparate healthcare applications. But what does it mean for healthcare interoperability? In essence, it allows diverse systems to speak the same language, ensuring that critical patient data flows seamlessly across various platforms.
The Role of HL7 Integration Engines
HL7 integration engines play a pivotal role in making data exchange possible. These engines act as the orchestrators, ensuring that information adheres to HL7 standards and can be understood by both sending and receiving systems.
Breaking Down HL7 Interface Engines
HL7 interface engines, like the gears in a well-oiled machine, enable the smooth flow of data. They not only interpret data but also transform it into a format that can be comprehended by different systems.
HL7 Integration Challenges:
Implementing and maintaining robust HL7 integration solutions pose common hurdles in healthcare systems. Overcoming these challenges is crucial for ensuring a seamless flow of information within healthcare ecosystems.
Why Choose HL7 Integration?
In healthcare, HL7 integrations stand out among various options due to their unique suitability for industry demands. HL7 interface preferred status is attributed to meeting specific healthcare standards, ensuring effective integration within healthcare systems.
The Critical Role of HL7 Integration in Patient Care:
HL7 integration significantly contributes to improving patient outcomes by providing accurate and timely information. Its impact on the overall quality of healthcare services is pivotal, ensuring better-informed decision-making and enhanced patient care.
Enhancing Workflow Efficiency with HL7 Integration:
In the dynamic healthcare environment, HL7 integrations streamline workflow, reducing manual interventions, and minimizing the risk of errors. The tangible benefits extend to healthcare professionals and patients alike, ensuring a more efficient and error-resistant system.
HL7 Integration Engine Selection: Key Considerations:
When selecting an HL7 integration engine, healthcare organizations should consider factors that distinguish an effective engine from others. Features and capabilities play a vital role in ensuring the chosen engine aligns with the specific needs of the organization.
The Future of HL7 Integration: What Lies Ahead?
As technology evolves, the future of HL7 integration holds promise. Emerging trends and innovations are expected to further enhance their role in shaping the future of healthcare data exchange, ensuring continued relevance and effectiveness.
Conclusion:
HL7 integrations stand as a linchpin in the modern healthcare ecosystem. From ensuring interoperability to enhancing workflow efficiency, the impact of HL7 integration is undeniable. As healthcare systems continue to evolve, embracing robust HL7 integration solutions becomes not just a choice but a necessity for delivering optimal patient care.
0 notes
Text
Introduction to the Revolutionary CFR® F5 Cetane Rating Unit

In the ever-evolving landscape of diesel fuel testing, few innovations have left a mark as significant as the CFR® F5 Cetane Rating Unit. Since its debut in 1938, this ground-breaking tool has continually redefined how we evaluate and certify diesel fuels' ignition quality. The CFR F5 remains a pivotal element in maintaining the integrity of the fuel supply chain from refinery to pump.
Setting the Gold Standard in Fuel Testing
At the heart of this revolution lies the CFR F5 Cetane Rating Unit, meticulously engineered to not only meet but exceed globally recognized standards such as ASTM D613, IP 41, and EN ISO 5165. This unit ensures that diesel fuels consistently reach their highest potential in quality.
Seamless Integration for Testing Confidence
The comprehensive cetane fuel testing system provided by CFR Engines Inc. includes the F5 engine unit, XCP™ Technology, and a CFR® exhaust surge tank. These components work harmoniously to manage critical parameters, ensuring a seamless cetane test process. Users can trust this integrated system for heightened efficiency and reliability, whether they opt for a complete unit or upgrade/conversion kits.
Data Prowess with XCP Technology
The XCP Digital Control Panel is a transformative feature that automates data collection and presentation. By eliminating manual errors and integrating with Laboratory Information Management Systems (LIMS), it ensures precise data on handwheel positions, fuel flow rates, and environmental conditions are always available.
Reliability Woven into Design
Since 1929, CFR Engines Inc. products have built a legacy of reliability. The latest upgrades and enhancements to engine crankcases and cylinder heads ensure that these units withstand modern testing environments with minimal maintenance required.
Precision Unleashed Through Modern Instrument Control
Combining the CFR F5 with XCP Technology leverages digital instrumentation for recording and processing crucial system data. Features like on-board handwheel position recording, automatic cetane number calculation, and multi-pass data recording contribute significantly to achieving greater precision and accuracy in tests.
Cost-Efficiency Through One System Flexibility
Beyond precision improvements, the CFR F5 offers unmatched flexibility and cost savings. With push-button control and guided testing prompts, users benefit from more efficient resource utilization—leading to faster tests and reduced operator training requirements.
Additional Highlights: Elevating Safety and Convenience
Safety is paramount with integrated monitoring systems guarding against power loss, low oil pressure, overheating, and electrical overloads. A fuel flow safety shut-off solenoid prevents uncontrolled engine over-speed during power outages. User-friendly XCP Technology further simplifies operation via an easy-to-use touch-screen HMI panel featuring on-screen stability indicators for "Injection Advance" and "Ignition Delay." An electronic maintenance log records essential maintenance information for future reference.
Experience Tomorrow’s Diesel Fuel Testing Today
Embrace the future of diesel fuel testing with the CFR F5 Cetane Rating Unit—transforming your processes for superior results and peace of mind.
Frequently Asked Questions (FAQs) About the CFR® F5 Cetane Rating Unit
What is the CFR F5 Cetane Rating Unit?
The CFR F5 is essential equipment used to determine and certify diesel fuels' ignition quality—ensuring their suitability for use in automotive engines within petroleum industries.
How long has it been in use?
Introduced in 1938, the CFR F5 has proven its reliability over more than eight decades of service.
Which standards does it comply with?
The unit adheres to globally recognized standards including ASTM D613, IP 41, and EN ISO 5165—guaranteeing accurate cetane number measurements consistently.
What components are included in a complete system?
A complete system consists of an F5 engine unit paired with XCP™ Technology along with a CFR® exhaust surge tank—all working together seamlessly during cetane tests.
How does XCP Technology enhance data integrity?
XCP Technology automates capturing/presenting data while reducing manual errors; providing accurate details about handwheel positions/fuel flow/environmental conditions through LIMS integration.
Is it user-friendly? How easy is operator training?
Absolutely! Designed for ease-of-use—the intuitive interface makes operator proficiency achievable quickly—saving both time/resources during training sessions effectively!
How does it ensure reliability/longevity?
CFR Engines Inc.'s long-standing legacy dating back since 1929 assures enduring durability through well-planned upgrades/enhancements minimizing maintenance needs significantly!
Are there additional safety/monitoring features integrated within this unit?
Yes indeed! Various integrated safety measures protect against multiple factors ensuring operational equipment/operator safety throughout usage durations effectively!
Does it offer on-screen stability indicators too? Certainly! On-screen indicators eliminate manual averaging enhancing consistency among operators massively overall improving efficiency/productivity rates exponentially higher beyond expectations usually met beforehand traditionally speaking generally speaking categorically put forth clearly stated objectively noted herein specified concisely elaborated upon succinctly expressed precisely articulated definitively concluded.
0 notes
Text
Two MIT teams selected for NSF sustainable materials grants
New Post has been published on https://sunalei.org/news/two-mit-teams-selected-for-nsf-sustainable-materials-grants/
Two MIT teams selected for NSF sustainable materials grants
Two teams led by MIT researchers were selected in December 2023 by the U.S. National Science Foundation (NSF) Convergence Accelerator, a part of the TIP Directorate, to receive awards of $5 million each over three years, to pursue research aimed at helping to bring cutting-edge new sustainable materials and processes from the lab into practical, full-scale industrial production. The selection was made after 16 teams from around the country were chosen last year for one-year grants to develop detailed plans for further research aimed at solving problems of sustainability and scalability for advanced electronic products.
Of the two MIT-led teams chosen for this current round of funding, one team, Topological Electric, is led by Mingda Li, an associate professor in the Department of Nuclear Science and Engineering. This team will be finding pathways to scale up sustainable topological materials, which have the potential to revolutionize next-generation microelectronics by showing superior electronic performance, such as dissipationless states or high-frequency response. The other team, led by Anuradha Agarwal, a principal research scientist at MIT’s Materials Research Laboratory, will be focusing on developing new materials, devices, and manufacturing processes for microchips that minimize energy consumption using electronic-photonic integration, and that detect and avoid the toxic or scarce materials used in today’s production methods.
Scaling the use of topological materials
Li explains that some materials based on quantum effects have achieved successful transitions from lab curiosities to successful mass production, such as blue-light LEDs, and giant magnetorestance (GMR) devices used for magnetic data storage. But he says there are a variety of equally promising materials that have shown promise but have yet to make it into real-world applications.
“What we really wanted to achieve is to bring newer-generation quantum materials into technology and mass production, for the benefit of broader society,” he says. In particular, he says, “topological materials are really promising to do many different things.”
Topological materials are ones whose electronic properties are fundamentally protected against disturbance. For example, Li points to the fact that just in the last two years, it has been shown that some topological materials are even better electrical conductors than copper, which are typically used for the wires interconnecting electronic components. But unlike the blue-light LEDs or the GMR devices, which have been widely produced and deployed, when it comes to topological materials, “there’s no company, no startup, there’s really no business out there,” adds Tomas Palacios, the Clarence J. Lebel Professor in Electrical Engineering at MIT and co-principal investigator on Li’s team. Part of the reason is that many versions of such materials are studied “with a focus on fundamental exotic physical properties with little or no consideration on the sustainability aspects,” says Liang Fu, an MIT professor of physics and also a co-PI. Their team will be looking for alternative formulations that are more amenable to mass production.
One possible application of these topological materials is for detecting terahertz radiation, explains Keith Nelson, an MIT professor of chemistry and co-PI. This extremely high-frequency electronics can carry far more information than conventional radio or microwaves, but at present there are no mature electronic devices available that are scalable at this frequency range. “There’s a whole range of possibilities for topological materials” that could work at these frequencies, he says. In addition, he says, “we hope to demonstrate an entire prototype system like this in a single, very compact solid-state platform.”
Li says that among the many possible applications of topological devices for microelectronics devices of various kinds, “we don’t know which, exactly, will end up as a product, or will reach real industrial scaleup. That’s why this opportunity from NSF is like a bridge, which is precious, to allow us to dig deeper to unleash the true potential.”
In addition to Li, Palacios, Fu, and Nelson, the Topological Electric team includes Qiong Ma, assistant professor of physics in Boston College; Farnaz Niroui, assistant professor of electrical engineering and computer science at MIT; Susanne Stemmer, professor of materials at the University of California at Santa Barbara; Judy Cha, professor of materials science and engineering at Cornell University; industrial partners including IBM, Analog Devices, and Raytheon; and professional consultants. “We are taking this opportunity seriously,” Li says. “We really want to see if the topological materials are as good as we show in the lab when being scaled up, and how far we can push to broadly industrialize them.”
Toward sustainable microchip production and use
The microchips behind everything from smartphones to medical imaging are associated with a significant percentage of greenhouse gas emissions today, and every year the world produces more than 50 million metric tons of electronic waste, the equivalent of about 5,000 Eiffel Towers. Further, the data centers necessary for complex computations and huge amount of data transfer — think AI and on-demand video — are growing and will require 10 percent of the world’s electricity by 2030.
“The current microchip manufacturing supply chain, which includes production, distribution, and use, is neither scalable nor sustainable, and cannot continue. We must innovate our way out of this crisis,” says Agarwal.
The name of Agarwal’s team, FUTUR-IC, is a reference to the future of the integrated circuits, or chips, through a global alliance for sustainable microchip manufacturing. Says Agarwal, “We bring together stakeholders from industry, academia, and government to co-optimize across three dimensions: technology, ecology, and workforce. These were identified as key interrelated areas by some 140 stakeholders. With FUTUR-IC we aim to cut waste and CO2-equivalent emissions associated with electronics by 50 percent every 10 years.”
The market for microelectronics in the next decade is predicted to be on the order of a trillion dollars, but most of the manufacturing for the industry occurs only in limited geographical pockets around the world. FUTUR-IC aims to diversify and strengthen the supply chain for manufacturing and packaging of electronics. The alliance has 26 collaborators and is growing. Current external collaborators include the International Electronics Manufacturing Initiative (iNEMI), Tyndall National Institute, SEMI, Hewlett Packard Enterprise, Intel, and the Rochester Institute of Technology.
Agarwal leads FUTUR-IC in close collaboration with others, including, from MIT, Lionel Kimerling, the Thomas Lord Professor of Materials Science and Engineering; Elsa Olivetti, the Jerry McAfee Professor in Engineering; Randolph Kirchain, principal research scientist in the Materials Research Laboratory; and Greg Norris, director of MIT’s Sustainability and Health Initiative for NetPositive Enterprise (SHINE). All are affiliated with the Materials Research Laboratory. They are joined by Samuel Serna, an MIT visiting professor and assistant professor of physics at Bridgewater State University. Other key personnel include Sajan Saini, education director for the Initiative for Knowledge and Innovation in Manufacturing in MIT’s Department of Materials Science and Engineering; Peter O’Brien, a professor from Tyndall National Institute; and Shekhar Chandrashekhar, CEO of iNEMI.
“We expect the integration of electronics and photonics to revolutionize microchip manufacturing, enhancing efficiency, reducing energy consumption, and paving the way for unprecedented advancements in computing speed and data-processing capabilities,” says Serna, who is the co-lead on the project’s technology “vector.”
Common metrics for these efforts are needed, says Norris, co-lead for the ecology vector, adding, “The microchip industry must have transparent and open Life Cycle Assessment (LCA) models and data, which are being developed by FUTUR-IC.” This is especially important given that microelectronics production transcends industries. “Given the scale and scope of microelectronics, it is critical for the industry to lead in the transition to sustainable manufacture and use,” says Kirchain, another co-lead and the co-director of the Concrete Sustainability Hub at MIT. To bring about this cross-fertilization, co-lead Olivetti, also co-director of the MIT Climate and Sustainability Consortium (MCSC), will collaborate with FUTUR-IC to enhance the benefits from microchip recycling, leveraging the learning across industries.
Saini, the co-lead for the workforce vector, stresses the need for agility. “With a workforce that adapts to a practice of continuous upskilling, we can help increase the robustness of the chip-manufacturing supply chain, and validate a new design for a sustainability curriculum,” he says.
“We have become accustomed to the benefits forged by the exponential growth of microelectronic technology performance and market size,” says Kimerling, who is also director of MIT’s Materials Research Laboratory and co-director of the MIT Microphotonics Center. “The ecological impact of this growth in terms of materials use, energy consumption and end-of-life disposal has begun to push back against this progress. We believe that concurrently engineered solutions for these three dimensions will build a common learning curve to power the next 40 years of progress in the semiconductor industry.”
The MIT teams are two of six that received awards addressing sustainable materials for global challenges through phase two of the NSF Convergence Accelerator program. Launched in 2019, the program targets solutions to especially compelling challenges at an accelerated pace by incorporating a multidisciplinary research approach.
0 notes
Text
Unveiling Excellence: The Craftsmanship of Biotin Manufacturing
Biotin, also known as vitamin B7 or vitamin H, is a vital nutrient that plays a crucial role in supporting healthy hair, skin, nails, metabolism, and overall well-being. Behind every biotin supplement lies a meticulous manufacturing process that ensures the product's purity, potency, and effectiveness. In this blog, we'll explore the intricate world of biotin manufacturing, shedding light on the key steps involved and the commitment to quality exhibited by leading manufacturers in the industry.
Sourcing High-Quality Raw Materials: The journey of biotin manufacturing begins with the careful selection of raw materials. Biotin supplements are typically synthesized through chemical processes using specialized laboratories and manufacturing facilities. Leading manufacturers prioritize sourcing high-quality raw materials that meet strict quality and safety standards. These raw materials may include precursors such as d-pantothenic acid or other organic compounds that serve as starting materials for biotin synthesis.
Synthesis and Formulation: Once the raw materials are procured, the synthesis of biotin begins. Chemical processes are employed to convert the precursor compounds into biotin through various reactions and purification steps. Formulation experts work to create stable and bioavailable biotin formulations that can be easily absorbed and utilized by the body. This may involve experimenting with different formulations, dosage forms, and delivery systems to optimize the product's efficacy and consumer experience.
Quality Control and Assurance: Quality control and assurance are paramount in biotin manufacturing. Rigorous testing and analysis are performed at every stage of the production process to ensure the purity, potency, and safety of the finished product. Advanced analytical techniques, such as high-performance liquid chromatography (HPLC) and mass spectrometry, are utilized to verify the identity and concentration of biotin and detect any impurities or contaminants. Stringent quality control protocols are followed to uphold product integrity and compliance with regulatory standards.
Packaging and Labeling: Once the biotin is synthesized and tested, it is packaged into capsules, tablets, softgels, or powders and labeled with essential product information, including dosage instructions, nutritional facts, and storage recommendations. Packaging materials are chosen to preserve product freshness and stability while ensuring consumer safety and convenience. Clear and accurate labeling is essential to provide consumers with transparent information about the product's ingredients, benefits, and usage guidelines.
Distribution and Consumer Education: Efficient distribution channels are utilized to deliver biotin supplements to retailers, online platforms, and consumers worldwide. Manufacturers may also engage in consumer education initiatives to raise awareness about the benefits of biotin supplementation and provide guidance on proper usage and dosage. This may include educational content, such as blog posts, articles, and social media campaigns, to empower consumers to make informed choices about their health and wellness.
0 notes
Text
Biochemistry Career Scope
Biochemistry, as a field, offers a fascinating exploration into the chemical processes and molecules that underpin life itself. Over the years, advancements in technology and research have broadened the scope of biochemistry, making it a highly dynamic and rewarding career path. In this article, we will delve into the diverse array of career opportunities available in biochemistry, ranging from research and academia to industry and healthcare.

Additionally, we will address common questions that individuals may have about pursuing a career in this exciting field, providing insights and guidance for those considering a future in biochemistry. Whether you're passionate about unraveling the mysteries of cellular pathways, developing innovative biotechnologies, or improving human health through biochemical research, there are numerous avenues to explore and opportunities to make a meaningful impact in the field of biochemistry.
Why Choose Biochemistry?
Understanding Life at a Molecular Level: Biochemistry serves as a gateway to comprehending life processes at their most fundamental level. It delves into the intricate mechanisms that govern biological systems, shedding light on the molecular underpinnings of various phenomena. Through the study of biochemistry, individuals gain profound insights into the structure, function, and interactions of biomolecules. From the complex folding patterns of proteins to the genetic information encoded in nucleic acids, biochemistry unravels the mysteries of life's building blocks with precision and clarity.
Exploring Career Opportunities: One of the most compelling reasons to pursue biochemistry lies in its vast array of career opportunities across multiple sectors. Graduates with a background in biochemistry are well-equipped to embark on diverse career paths, spanning industries such as research, healthcare, pharmaceuticals, biotechnology, agriculture, forensics, and environmental science. Within these fields, individuals can find roles as research scientists, clinical laboratory technologists, pharmaceutical analysts, biotechnologists, forensic scientists, and more. The versatility of a biochemistry degree allows professionals to apply their expertise to address pressing challenges in areas ranging from drug discovery and development to sustainable agriculture and environmental conservation.
Contributing to Scientific Advancements: Biochemistry plays a pivotal role in driving scientific advancements and innovation. By pursuing a career in biochemistry, individuals have the opportunity to contribute to groundbreaking research projects that have the potential to transform healthcare, agriculture, and industry. Whether it's developing novel therapeutics for treating diseases, engineering enzymes for industrial applications, or deciphering the molecular mechanisms of cellular processes, biochemists are at the forefront of innovation, pushing the boundaries of knowledge and discovery.
Meeting Global Challenges: In an increasingly interconnected world facing complex challenges such as infectious diseases, climate change, and food security, biochemistry offers valuable insights and solutions. Through interdisciplinary collaboration and cutting-edge research, biochemists work towards addressing global health crises, developing sustainable agricultural practices, and mitigating environmental degradation. By leveraging their expertise in molecular biology, bioinformatics, and biotechnology, biochemists play a vital role in shaping a healthier, more sustainable future for humanity and the planet.
Fostering Personal and Professional Growth: Beyond its practical applications and societal impact, biochemistry fosters personal and professional growth. The discipline encourages critical thinking, problem-solving, and creativity, nurturing individuals to become innovative thinkers and lifelong learners. Whether working in a laboratory, clinical setting, or corporate environment, biochemists continuously expand their knowledge and skills, adapting to new technologies and emerging trends in science and medicine. Moreover, the collaborative nature of biochemistry fosters teamwork, communication, and leadership abilities, preparing individuals to excel in diverse professional settings and make meaningful contributions to society.
Career Paths in Biochemistry:
Research Scientist: Research scientists in biochemistry play a crucial role in advancing scientific knowledge and innovation. They design and conduct experiments, analyze data, and contribute to groundbreaking discoveries in fields such as molecular biology, genetics, drug development, and protein engineering. Working in diverse settings including academic institutions, government agencies, pharmaceutical companies, biotechnology firms, and research laboratories, research scientists drive forward the frontiers of scientific understanding and technological progress.
Clinical Laboratory Technologist: Clinical laboratory technologists specializing in biochemistry are integral members of healthcare teams, performing diagnostic tests on patient samples to identify and analyze biochemical markers associated with diseases and medical conditions. Operating in hospitals, clinics, diagnostic laboratories, and research institutions, clinical laboratory technologists collaborate with healthcare professionals to facilitate accurate diagnosis, treatment planning, and patient care.
Pharmaceutical Analyst: Pharmaceutical analysts in biochemistry ensure the quality, safety, and efficacy of pharmaceutical products through rigorous testing and analysis. They conduct assays, stability studies, and validation tests to assess the composition, purity, and performance of drugs and pharmaceutical formulations. Employed in pharmaceutical companies, regulatory agencies, and quality control laboratories, pharmaceutical analysts play a critical role in maintaining the integrity and compliance of the pharmaceutical industry.
Biotechnologist: Biotechnologists leverage principles of biochemistry and molecular biology to develop innovative products, processes, and technologies with applications in healthcare, agriculture, and industrial biotechnology. Engaged in research and development activities, biotechnologists work in biotechnology companies, research institutions, government agencies, and academic settings. Their work spans diverse areas such as genetic engineering, bioprocessing, and bioinformatics, driving progress in fields ranging from medicine to renewable energy.
Forensic Scientist: Forensic scientists specializing in biochemistry apply their expertise to analyze biological evidence and provide crucial insights in criminal investigations and legal proceedings. By examining blood, saliva, DNA, and other biological samples, forensic scientists assist law enforcement agencies and government forensic departments in identifying perpetrators, establishing links between suspects and crime scenes, and presenting evidence in court. Working in forensic laboratories and law enforcement agencies, forensic scientists uphold the integrity and reliability of biochemical analysis in the criminal justice system.
Academic Researcher: Academic researchers in biochemistry pursue fundamental scientific inquiries and scholarly endeavors in educational institutions such as universities and research centers. They conduct independent research, publish scientific papers, and secure grants to support their investigations into diverse areas of biochemistry. Academic researchers also mentor graduate students and contribute to the training of the next generation of scientists, fostering a vibrant intellectual environment and advancing the frontiers of knowledge in biochemistry and related disciplines.
Quality Control Specialist: Quality control specialists in biochemistry are responsible for ensuring that products, processes, and systems meet established standards of quality, safety, and compliance. They develop and implement quality control protocols, perform inspections and audits, and recommend corrective actions to address deficiencies. Quality control specialists work in various industries, including pharmaceuticals, biotechnology, food and beverage, and healthcare, safeguarding the integrity and reliability of products and services delivered to consumers.
Biochemistry Jobs:
Quality Assurance/Quality Control Inspector: Quality assurance/quality control inspectors play a crucial role in ensuring the quality and safety of products, materials, and equipment. They examine, test, and sample materials or products for flaws, compare them against specifications, and provide necessary corrective actions. This role involves collaboration with various stakeholders to maintain quality standards and compliance with regulations.
Research Analyst: Research analysts utilize a variety of sources to gather and analyze data on specific topics. They conduct quantitative analysis, interpret findings, and present results in written and oral formats. Research analysts often collaborate with clients or team members to gather relevant information and provide insights for decision-making.
Senior Research Associate: Senior research associates lead and oversee data collection activities, serving as the point of contact and representing the organization's expertise in research endeavors. They develop quality assurance protocols, analyze findings, and contribute to the development of research strategies. Senior research associates play a key role in advancing scientific knowledge and innovation within their respective fields.
Research Scientist: Research scientists are skilled researchers who primarily work with data and conduct research activities to advance scientific knowledge. They design and execute experiments, analyze data, and contribute to research publications and presentations. Research scientists play a vital role in driving forward the frontiers of knowledge and innovation in biochemistry and related disciplines.
Quality Assurance Engineer: Quality assurance engineers develop and execute tests to identify any issues with software products before they are released to customers. They analyze and document bugs found during testing, work collaboratively with development teams to address issues, and ensure that products meet quality standards and user requirements.
Technical Consultant: Technical consultants engage with clients to understand their needs and provide technical expertise and support. They install, upgrade, maintain, and evaluate products and services, troubleshoot technical and equipment issues, and provide training to clients. Technical consultants play a key role in ensuring the successful implementation and operation of technology solutions.
Quality Manager: Quality managers collaborate with line managers to establish and achieve quality standards across production facilities. They monitor and report on quality and safety metrics, implement quality assurance processes, and drive continuous improvement initiatives to enhance product quality and customer satisfaction. Quality managers are responsible for ensuring compliance with regulatory requirements and fostering a culture of quality and excellence within the organization.
Conclusion:
Biochemistry offers a dynamic and rewarding career path with diverse opportunities for research, innovation, and impact. Choosing to pursue a career in biochemistry at Top Medical College in Meerut, offers individuals can contribute to scientific advancements, improve healthcare outcomes, and address global challenges in areas such as health, food security, and environmental sustainability.
With its renowned faculty, state-of-the-art facilities, research opportunities, and networking support, GS Medical College and Hospital provides an ideal environment for students interested in pursuing careers in biochemistry. Whether aspiring to become research scientists, clinical laboratory technologists, pharmaceutical analysts, biotechnologists, or forensic scientists, students at Best Medical colleges in Uttar Pradesh at GS Medical College and Hospital can embark on a fulfilling journey in the field of biochemistry with confidence and competence.
If you have any further questions about pursuing a career in biochemistry or the resources available at the Best MBBS college in UP at GS Medical College and Hospital, feel free to reach out to the college administration or visit the college website for more information. Your journey toward a successful and impactful career in biochemistry begins here at GS Medical College and Hospital.
#Biochemistry#CareerScope#CareerOpportunities#Healthcare#Academia#Industry#DelhiNCR#Ghaziabad#UttarPradesh#India
0 notes
Text
Enhancing Healthcare Efficiency: Laboratory Interfaces at Life Point
Introduction:
In today's rapidly evolving healthcare landscape, the integration of laboratory interfaces plays a pivotal role in optimizing patient care and operational efficiency. At Life Point, we recognize the importance of seamless connectivity between laboratory systems and other healthcare platforms. In this blog post, we'll explore the significance of laboratory interfaces and how they contribute to the success of healthcare organizations like Life Point.
Understanding Laboratory Interfaces:
Laboratory interfaces serve as the bridge between laboratory instruments and the electronic systems used to manage patient data, such as Electronic Medical Records (EMRs) and Laboratory Information Systems (LIS). These interfaces facilitate the secure and real-time exchange of critical information, including test orders, results, and patient demographics.
Enhanced Data Accessibility:
By implementing robust laboratory interfaces, Life Point ensures that healthcare providers have instant access to accurate and up-to-date laboratory data. This accessibility enables timely decision-making, resulting in improved patient outcomes and satisfaction. Whether it's retrieving test results or monitoring patient progress, our integrated systems empower healthcare professionals to deliver high-quality care.
Streamlined Workflows:
Efficiency is paramount in healthcare settings, and laboratory interfaces play a key role in streamlining workflows. At Life Point, our interfaces automate various tasks, such as order entry and result reporting, reducing the burden on laboratory staff and minimizing the risk of errors. With smoother workflows, we can deliver faster turnaround times and enhance overall operational efficiency.
Enhanced Collaboration:
Effective communication and collaboration are essential for delivering coordinated patient care. Laboratory interfaces facilitate seamless communication between different departments and healthcare facilities, enabling interdisciplinary teams to work together seamlessly. Whether it's sharing test results or consulting with specialists, our integrated systems foster collaboration and support comprehensive patient care.
Ensuring Compliance and Security:
In the healthcare industry, compliance with regulatory standards and data security are top priorities. At Life Point, we adhere to stringent regulations and implement robust security measures to protect patient information. Our laboratory interfaces are designed to meet HIPAA requirements and other industry standards, ensuring the confidentiality and integrity of sensitive data.
Looking Ahead:
As technology continues to advance, so too will the capabilities of laboratory interfaces. At Life Point, we remain committed to leveraging the latest innovations to enhance patient care and operational efficiency. By investing in cutting-edge solutions and fostering partnerships with industry leaders, we aim to set new standards for excellence in healthcare delivery.
Conclusion:
Laboratory interfaces are indispensable tools in modern healthcare, enabling seamless connectivity and enhancing patient care. At Life Point, we recognize the importance of these interfaces and continuously strive to optimize their functionality to meet the evolving needs of healthcare providers and patients alike. Through innovation, collaboration, and a steadfast commitment to excellence, we are transforming the future of healthcare, one interface at a time.
0 notes
Text
Safeguarding Our Nuclear Future: Argonne's Visionary Approach to Climate-Resilient Energy Infrastructure in Washington State

In an era defined by the urgency of climate change, the nexus of environmental sustainability and energy production emerges as a critical battleground. Nowhere is this more pronounced than in the picturesque landscapes of Washington state, where the verdant Pacific Northwest meets the arid expanses east of the Cascade Mountain Range. Amidst this backdrop of natural diversity and ecological fragility, Argonne National Laboratory stands as a beacon of innovation, spearheading efforts to fortify the region's nuclear power infrastructure against the existential threats posed by a changing climate.
At the heart of this endeavor lies a collaborative partnership between Argonne and Washington's Energy Northwest, fueled by the visionary support of the U.S. Department of Energy's Gateway for Accelerated Innovation in Nuclear (GAIN) program. Together, these trailblazing institutions are charting a course toward a future where nuclear power remains a steadfast pillar of clean, reliable energy, resilient in the face of environmental upheaval.
Leading this charge are Rick Vilim and Rao Kotamarthi, esteemed scientists whose expertise spans the spectrum of nuclear engineering, climate science, and environmental resilience. Drawing upon decades of collective experience, Vilim, Kotamarthi, and their interdisciplinary team embark on a journey of exploration and innovation, seeking to reimagine the very foundations of nuclear power plant design in a world increasingly defined by uncertainty.
Central to their mission is the development of climate-resilient cooling systems, a cornerstone of nuclear reactor safety and efficiency. Traditionally, these plants have relied on "wet" cooling methods, harnessing the cooling properties of local waterways such as the mighty Columbia River. However, with climate models projecting shifts in precipitation patterns and river flows, the viability of these conventional cooling mechanisms hangs in the balance.
In response, Vilim, Kotamarthi, and their colleagues are pioneering the adoption of "dry" cooling systems, which utilize ambient air to dissipate heat from the reactor. While inherently less efficient than their wet counterparts, dry cooling systems offer a viable alternative in regions where water scarcity or environmental constraints preclude the use of traditional cooling methods. Through rigorous experimentation, advanced computational modeling, and real-world validation, the team aims to elucidate the trade-offs and implications of each cooling approach, empowering stakeholders to make informed decisions in a rapidly changing world.
Yet the scope of Argonne's ambition extends far beyond mere technological innovation. Recognizing the interconnectedness of energy infrastructure and environmental resilience, Vilim, Kotamarthi, and their colleagues are trailblazers in the emerging field of climate-adaptive infrastructure planning. Leveraging cutting-edge climate modeling capabilities and advanced computational resources, they are developing holistic strategies to future-proof nuclear power plants against a spectrum of climate-related risks, from heatwaves and droughts to extreme precipitation events.
Moreover, Argonne's commitment to public service and stakeholder engagement underscores the institution's ethos of inclusivity and collaboration. By fostering partnerships with industry stakeholders, government agencies, and academic institutions, Argonne is catalyzing a paradigm shift in how we approach energy resilience in the age of climate change. Through knowledge sharing, capacity building, and community empowerment, they are paving the way toward a more sustainable and equitable future for generations to come.
If you want to learn more about nuclear energy, check out Nuclear Power Energy Fundamentals.
Explore the resurgent world of nuclear energy in this comprehensive video course. From core concepts to industry applications, gain essential knowledge and an advantage in the nuclear industry. Whether you're a seasoned pro or just starting out, this course is your key to a fulfilling career in a zero-carbon future. Start your journey today and mark a pivotal moment in your phenomenal career!
As we stand on the cusp of a new era in energy and environmental stewardship, the importance of Argonne's work cannot be overstated. By investing in innovation, collaboration, and foresight, we can navigate the turbulent waters of climate change with confidence and resilience. Join us on this transformative journey as we chart a course toward a brighter, more sustainable future for Washington state and beyond. Together, we can safeguard our nuclear legacy and forge a path toward a world where clean, reliable energy powers prosperity for all.
#Climate-resilient infrastructure#Nuclear power plant resilience#Climate change adaptation#Energy sustainability#Environmental resilience
0 notes
Text
Healthcare IT Outsourcing Market Share, Trends, Top Companies, Forecast 2024-2032
IMARC Group, a leading market research company, has recently releases report titled “Healthcare IT Outsourcing Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2024-2032” offers a comprehensive analysis of the industry, which comprises insights on the healthcare IT outsourcing market share. The global market size reached US$ 50.3 Billion in 2023. Looking forward, IMARC Group expects the market to reach US$ 100.6 Billion by 2032, exhibiting a growth rate (CAGR) of 7.9% during 2024-2032.
Request For Sample Copy of Report For More Detailed Market insight: https://www.imarcgroup.com/healthcare-it-outsourcing-market/requestsample
Factors Affecting the Growth of the Healthcare IT Outsourcing Industry:
Cost Reduction and Efficiency Improvement:
The rising need for cost reduction and efficiency enhancement in healthcare operations represents one of the crucial factors impelling the market growth. Outsourcing information technology (IT) services enable healthcare providers to save substantially on operational costs, including expenses related to staffing, training, and maintaining an in-house IT team. Moreover, IT outsourcing firms often bring in advanced technologies and expertise that can streamline healthcare processes, leading to improved patient care and administrative efficiency. This dual benefit of cost savings and enhanced efficiency is particularly appealing in sectors where cost control is a constant challenge and where the efficient handling of data directly influences patient outcomes.
Core Healthcare Competencies:
Healthcare providers are recognizing the significance of focusing on their core competencies, including patient care and treatment, rather than dividing their attention with complex IT operations. Outsourcing IT tasks to external experts allows healthcare institutions to concentrate on their primary objectives without the distractions and complexities of managing IT infrastructure and services. This specialized focus is essential for enhancing the quality of patient care, as it ensures that medical professionals can devote their full attention and resources to clinical tasks. Healthcare providers can maintain a clear focus on delivering excellent patient care by entrusting IT operations to external experts.
Technological Advancements:
Technological advancements, such as electronic health records (EHR), telemedicine, artificial intelligence (AI), and data analytics, are positively influencing the market. The need to stay updated with these advancements is encouraging the adoption of outsourcing IT services to specialized vendors. These outsourcing services are adept at these technologies, which allows healthcare organizations to stay at the forefront of innovation without the need to invest heavily in new technologies and training. These vendors bring in expertise in the latest healthcare IT solutions, ensuring that healthcare providers benefit from cutting-edge technology, which is crucial in the industry to stay updated with technology for delivering high-quality care.
Competitive Landscape:
The competitive landscape of the market has been studied in the report with the detailed profiles of the key players operating in the market.
Accenture plc
Allscripts Healthcare Solutions Inc
Dell Technologies Inc.
Infosys Limited
International Business Machines
Koninklijke Philips N.V.
Optum Inc. (UnitedHealth Group Incorporated)
Siemens Healthineers AG
Tata Consultancy Services
Wipro Limited
Xerox Corporation
Healthcare IT Outsourcing Market Report Segmentation:
By Type:
Payers HCIT Outsourcing
Hospital Information System (HIS)
Laboratory Information System (LIS)
Radiology Information System (RIS)
Electronic Medical Records (EMR)
Others
Providers HCIT Outsourcing
Revenue Cycle Management (RCM) System
Healthcare Analytics
Payers HCIT outsourcing represents the largest segment attributed to the rising demand for IT outsourcing services among healthcare payers due to the need for cost-effective solutions and streamlined operations.
By End User:
Healthcare Provider System
Biopharmaceutical Industry
Clinical Research Organization
Others
Healthcare provider system accounts for the majority of the market share on account of the growing reliance of healthcare providers on IT outsourcing to enhance their patient care, administrative processes, and overall efficiency.
Regional Insights:
North America (United States, Canada)
Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, Others)
Europe (Germany, France, United Kingdom, Italy, Spain, Russia, Others)
Latin America (Brazil, Mexico, Others)
Middle East and Africa
North America dominates the market, which can be accredited to significant investments in healthcare IT, advanced infrastructure, and a robust healthcare ecosystem.
Global Healthcare IT Outsourcing Market Trends:
The increasing digitalization is making healthcare organizations more vulnerable to cyber threats, risking patient data and operational continuity. Outsourcing cybersecurity management to specialized IT service providers offers healthcare organizations access to advanced security measures, regular updates, and expert monitoring. These providers are adept at navigating the complex landscape of cyber threats and ensuring compliance with data protection regulations, thereby safeguarding sensitive patient information and reinforcing the overall trust in healthcare systems.
Besides this, the growing adoption of cloud computing in the healthcare sector is offering a favorable market outlook. Cloud-based solutions offer scalability, flexibility, and cost-effectiveness, enabling healthcare providers to manage large volumes of data efficiently and securely. Outsourcing IT services to cloud-based providers allows healthcare organizations to benefit from advanced data analytics, improved data sharing and collaboration, and reduced IT infrastructure costs.
Other Key Points Covered in the Report:
COVID-19 Impact
Porters Five Forces Analysis
Value Chain Analysis
Strategic Recommendations
About Us
IMARC Group is a leading market research company that offers management strategy and market research worldwide. We partner with clients in all sectors and regions to identify their highest-value opportunities, address their most critical challenges, and transform their businesses.
IMARC Group’s information products include major market, scientific, economic and technological developments for business leaders in pharmaceutical, industrial, and high technology organizations. Market forecasts and industry analysis for biotechnology, advanced materials, pharmaceuticals, food and beverage, travel and tourism, nanotechnology and novel processing methods are at the top of the company’s expertise.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: [email protected]
Tel No:(D) +91 120 433 0800
United States: +1-631-791-1145 | United Kingdom: +44-753-713-2163
0 notes
Text
How to Choose the Best HMS Software for Your Business

Selecting the right hospital management software (HMS software) is a critical decision for healthcare organizations, as it directly impacts patient care, operational efficiency, and overall success. With numerous options available on the market, choosing the best HMS software can be a daunting task. Here are some key factors to consider when evaluating and selecting the best HMS software for your business:
Define your requirements:
Before exploring HMS software options, it's essential to clearly define your organization's requirements, goals, and priorities. Consider factors such as the size of your organization, specialty areas, workflow complexities, regulatory compliance needs, and integration requirements with existing systems. Identifying specific features and functionalities that are essential for your business will help narrow down your options and ensure that the chosen HMS software aligns with your organization's objectives.
Assess Ease of Use and User Experience:
The best HMS software should be intuitive, user-friendly, and easy to navigate for healthcare professionals and staff members. Look for software solutions that offer a modern interface, customizable dashboards, and workflow automation features that streamline tasks and reduce manual effort. Consider conducting demos or trials of potential HMS software solutions to evaluate the user experience firsthand and assess how well the software aligns with your organization's workflow and user preferences.
Evaluate integration capabilities:
Integration with existing systems and technologies is crucial for seamless data exchange, interoperability, and workflow continuity. Evaluate the integration capabilities of HMS software solutions and ensure compatibility with your organization's electronic health records (EHR) systems, laboratory information systems (LIS), billing platforms, and other third-party applications. Choose HMS software that supports industry-standard protocols such as HL7 and FHIR and offers flexible integration options to meet your organization's unique needs.
Consider scalability and flexibility.
As your organization grows and evolves, your HMS software should be able to scale and adapt to changing needs and requirements. Assess the scalability and flexibility of potential HMS software solutions to accommodate future expansion, additional users, and new functionalities. Look for software vendors that offer modular solutions, customizable features, and flexible licensing options that can be tailored to your organization's size and growth trajectory.
Prioritize data security and compliance:
Data security and regulatory compliance are paramount in healthcare, and HMS software must adhere to stringent standards to protect patient information and ensure compliance with regulations such as HIPAA, GDPR, and HITECH. Evaluate the security measures and compliance features of HMS Software solutions, including data encryption, access controls, audit trails, and regular security updates. Choose HMS software vendors with a proven track record of prioritizing data security and compliance in their software development and implementation processes.
Assess vendor reputation and support:
Selecting a reputable HMS software vendor with a track record of success and a strong commitment to customer support is essential for a successful implementation and long-term partnership. Research vendor credentials, customer reviews, and case studies to gauge their reputation and reliability. Additionally, inquire about the level of customer support, training resources, and ongoing maintenance and support services offered by the vendor to ensure that your organization receives the necessary assistance and guidance throughout the implementation process and beyond.
In conclusion, choosing the best HMS software for your business requires careful consideration of your organization's requirements, user experience, integration capabilities, scalability, data security, vendor reputation, and support services. By assessing these key factors and conducting thorough research and evaluation, you can select an HMS software solution that aligns with your organization's objectives, enhances patient care delivery, and drives operational efficiency in the rapidly evolving healthcare landscape.
#HMS#hmssoftware#hospitalsoftware#HealthcareManagementsystem#Healthcare#HospitalManagementSystem#HospitalManagement#BSA#GrapesHMS#MIS#EMR#GrapesIDMR#Grapes#HealthcareManagementSystemsoftware#Software#SoftwareSolution#Doctor#PatientRegistration#IPDManagement#OPDManagement#IDMR#Labmanagement#NursingStation#Dietmanagement#MRDManagement#BloodBank#Linenmanagement#HRManagement#Pharmacy#Radiology
0 notes
Text
Unveiling the CFR® F5 Cetane Rating Unit: The Future of Diesel Fuel Evaluation

Innovation in Diesel Fuel Testing
In the world of diesel fuel testing, the CFR® F5 Cetane Rating Unit stands out as a game-changer, revolutionizing the landscape since its inception in 1938. Spearheading the evaluation and certification of diesel fuels' ignition quality, the CFR F5 continues to make a significant impact on the automotive and petroleum industries while upholding the integrity of the fuel supply chain from refinery to pump.
Setting New Standards in Fuel Testing
At the core of this innovation lies the meticulously crafted CFR F5 Cetane Rating Unit, designed to meet and surpass global standards such as ASTM D613, IP 41, and EN ISO 5165. This powerhouse is dedicated to excellence, ensuring that diesel fuels consistently reach peak quality levels.
Seamless Integration for Enhanced Confidence in Testing
A complete CFR Engines Inc. cetane fuel testing system includes the F5 engine unit, XCP™ Technology, and a CFR® exhaust surge tank. These components seamlessly integrate to control critical parameters and guarantee a flawless cetane test. Whether opting for a complete unit, an upgrade/conversion kit, or genuine CFR service parts, users can rely on a system that enhances efficiency and reliability.
Harnessing Data Prowess with XCP Technology
The introduction of the XCP Digital Control Panel has transformed data collection and presentation. Say farewell to manual errors; the XCP ensures accuracy by integrating with a Laboratory Information Management System (LIMS) and providing crucial information such as handwheel positions, fuel flow rates, and environmental data.
Reliability Woven into Every Design Element
With a legacy dating back to 1929, CFR Engines Inc. products boast reliability at their core. Upgrades and enhancements to the engine crankcase and cylinder/head ensure longevity, enduring the demands of modern fuel testing environments with minimal maintenance.
Unleashing Precision Through Modern Instrument Control
The CFR F5 combined with XCP Technology utilizes digital instrumentation to record and process critical system data. On-board handwheel position recording, automatic cetane number calculation, and multi-pass data recording lead to heightened precision and accuracy.
Cost-Efficiency Through Versatile System Flexibility
The CFR F5 not only revolutionizes precision but also offers unparalleled flexibility and cost savings. With push-button control and guided testing prompts, users experience more efficient resource utilization, quicker tests, and reduced operator training.
Additional Highlights: Elevating Safety and Convenience
XCP integrated safety monitoring systems protect against power loss, low oil pressure, overheating, and electrical overload. A fuel flow safety shut-off solenoid prevents uncontrolled engine over-speed in case of electrical power loss. User-friendly XCP Technology reduces operator training time complemented by an easy-to-use touch-screen HMI panel. On-screen indicators inform when "Injection Advance" and "Ignition Delay" stability are achieved. An electronic maintenance log records vital maintenance information for future reference.
Experience Tomorrow’s Diesel Fuel Testing Today
Step into the future of diesel fuel testing with the CFR F5 Cetane Rating Unit - transforming your testing process for superior results and peace of mind.
Frequently Asked Questions (FAQs) About the CFR® F5 Cetane Rating Unit
What is the importance of the CFR F5 Cetane Rating Unit?
The CFR F5 is standard testing equipment crucial for determining and certifying the ignition quality of diesel fuels—ensuring their suitability for engines in automotive & petroleum industries.
How long has it been in use?
Since its introduction in 1938—the reliable platform boasts a rich history spanning over eight decades.
Which standards does it comply with?
It complies with globally recognized standards like ASTM D613—ensuring accurate & consistent cetane number measurements.
What's included in a complete system?
A complete system comprises an engine unit—XCP™ Technology—and an exhaust surge tank—all working together flawlessly.
How does XCP technology improve data integrity?
XCP automates data capture & presentation—reducing manual errors & providing accurate information on critical parameters.
Is it user-friendly? How easy is it to train operators?
Yes—it's designed to be user-friendly—making it easier for operators to become proficient quickly—to save time & resources.
How does it ensure reliability & longevity?
With upgrades dating back to 1929—it maintains reliability through well-designed system upgrades—ensuring durability with minimal maintenance.
Are there additional safety features integrated into it?
Yes—it includes integrated safety monitoring systems protecting against various factors—and ensuring both equipment & operator safety.
Does it offer on-screen indicators for monitoring stability?
Yes—the system includes on-screen indicators—enhancing operator consistency.
Can it handle different types of diesel fuels including biodiesel blends?
Absolutely—it's designed to assess a wide range including biodiesel blends—ensuring comprehensive testing across various formulations.
How does it contribute to environmental sustainability?
By incorporating environmentally friendly resistance temperature detectors (RTDs)—it manages critical temperature variables aligning with sustainability efforts.
0 notes
Text
The Importance of Customization in Hospital Software Solutions

In the ever-evolving landscape of healthcare, hospital software has become an indispensable tool for managing operations, improving patient care, and enhancing overall efficiency. One of the key factors that contributes to the success of hospital software solutions is customization. Let's delve into the importance of customization in hospital software solutions and how it can benefit healthcare organizations.
Tailored to unique needs:
Every healthcare organization has its own set of workflows, processes, and requirements. Off-the-shelf software solutions may not fully align with these unique needs, leading to inefficiencies and limitations in functionality. Customization allows hospital software solutions to be tailored specifically to the requirements of each organization, ensuring that they address specific pain points and facilitate seamless operations.
Enhanced User Experience:
Customized hospital software solutions prioritize the user experience by providing interfaces and functionalities that are intuitive and easy to use. By understanding the preferences and workflows of end-users, customization allows for the development of user-friendly interfaces that streamline tasks and minimize training requirements. This results in higher user adoption rates and increased productivity among healthcare staff.
Integration with Existing Systems:
Healthcare organizations often have existing systems and technologies in place, such as electronic health records (EHR) systems, laboratory information systems (LIS), and billing platforms. Customization enables hospital software solutions to integrate seamlessly with these existing systems, facilitating data exchange and interoperability. This integration eliminates silos and ensures that all systems work together harmoniously, maximizing efficiency and accuracy in data management.
Scalability and Flexibility:
Customized hospital software solutions are designed with scalability and flexibility in mind, allowing them to grow and evolve alongside the organization. As healthcare needs change and technology advances, customization ensures that the software can be easily adapted and expanded to accommodate new requirements and functionalities. This scalability enables healthcare organizations to future-proof their investments and stay ahead of emerging trends.
Addressing Regulatory Compliance:
The healthcare industry is highly regulated, with stringent requirements for data security, patient privacy, and regulatory compliance. Customized hospital software solutions can be developed with built-in features and controls to ensure compliance with industry standards and regulations, such as HIPAA and GDPR. By incorporating these compliance measures from the outset, customization helps healthcare organizations mitigate risks and avoid potential penalties.
Empowering Innovation:
Customization fosters a culture of innovation within healthcare organizations by empowering them to implement unique workflows and processes that drive efficiency and improve patient care. By working closely with software developers to customize solutions according to their specific needs, healthcare organizations can innovate and differentiate themselves in a competitive market, ultimately leading to better outcomes for patients.
In conclusion, customization plays a pivotal role in the success of hospital software solutions by tailoring them to the unique needs of healthcare organizations. From enhancing the user experience and integrating with existing systems to ensuring scalability and addressing regulatory compliance, customization enables hospitals to maximize the value of their software investments and deliver high-quality, patient-centered care. Embracing customization is essential for healthcare organizations looking to stay agile, innovative, and competitive in today's dynamic healthcare landscape.
#HMS#hmssoftware#hospitalsoftware#HealthcareManagementsystem#Healthcare#HospitalManagementSystem#HospitalManagement#BSA#GrapesHMS#MIS#EMR#GrapesIDMR#Grapes#HealthcareManagementSystemsoftware#Software#SoftwareSolution#Doctor#PatientRegistration#IPDManagement#OPDManagement#IDMR#Labmanagement#NursingStation#Dietmanagement#MRDManagement#BloodBank#Linenmanagement#HRManagement#Pharmacy#Radiology
0 notes