#Intra-cranial Pressure
Text
Delayed onset of intracerebral tension pneumocephalus 2 years after an anterior skull base fracture: Case report by Sokchan Sim in Journal of Clinical Case Reports Medical Images and Health Sciences
ABSTRACT
Pneumocephalus, the presence of air within the cranial cavity, is most commonly caused by trauma, tumor, infection and fistulation into the intracranial cavity or secondary to neurosurgery. We describe an unusually delayed neurological deficit from intracerebral tension pneumocephalus, 2 years following a head trauma with anterior skull base fracture. A 22-year-old man presented to our neurosurgical consultation with recurrent seizures and progressive right hemiparesis. The brain CT scan without iv contrast revealed an intracerebral tension pneumocephalus in the left frontal lobe, and a persistent hole in the left anterior frontal skull base connecting to pneumocephalus. We performed a left frontal craniotomy, and dura-plasty using galea flap to cover the skull-base bone defect. The patient has recovered gradually from his motor deficit after this surgery, finally to the level that he could play his favorite guitar. This is a rare case of a delayed development neurological deficit due to pneumocephalus from a “ball-valve” effect secondary to an old anterior skull base fracture.
Key words: Pneumocephalus, hemiparesis, craniotomy, dura-plasty
INTRODUCTION
Pneumocephalus is an air entrapment in the cranial cavity. It is commonly seen after head and facial trauma, ear infections, and tumors of the skull base or neurosurgical interventions. In some extremely rare cases, it happens spontaneously. Pneumocephalus is a complication of head injury in 3.9–9.7% of the cases. The accumulation of intracranial air can be acute (<72 h) or delayed (≥72 h). In tension pneumocephalus, the continuous accumulation of intracranial air is thought to be caused by a “ball-valve” mechanism. In turn, this may lead to a mass effect on the brain, with subsequent neurological deterioration and signs of herniation. Delayed tension pneumocephalus is extremely rare and requires proper neurosurgical attention. Surgical treatment involves aspiration of air into a syringe and closure of the dura defect through a cranial surgery.
CASE REPORT
A 22-year-old male presented to our neurosurgical consultation with chronic headaches, progressive right-sided weakness and occasional seizures. Two years prior to this visit, he suffered a severe traumatic brain injury by motorcycle accident. He had lost his consciousness for three days, and hospitalized in a provincial hospital for two weeks without any surgical intervention. He was then discharged home with persistent rhinorrhea for 10 months before it ceased spontaneously. 18 months after his injury, this patient began having progressive weakness on his right side of the body, and some episodes of seizures. He also reported occasional headaches. He was otherwise healthy before this accident. On examination, the young man had full consciousness, was alert and oriented. He had grade 3 out of 5 hemiparesis on his right side. A brain CT scan without iv contrast was obtained revealing a large pneumocephalus in the left frontal lobe. We noted a continuity of the air and the anterior skull base defect. (Figure.1)
CSF examination and culture were negative for infection, as well as the nasal swab.
Figure 1: A. Axial view of the CT scan showing hypodensity area in the left frontal lobe, pneumocephalus. B. Sagittal view presenting the large air space with its connection to the frontal skull base. C. Coronal view showing the bony defect of the anterior skull base.
We decided to perform the surgery by doing bi-coronal approach for a left frontal craniotomy and repair of the dura defect on the frontal skull base using the pedunculated galea flap. (Figure.2)
Figure 2 :A. Bi-coronal incision with preservation of large frontal galea. B. Galea still attached to the frontal base is lifted up.
The surgery went well without any complication. The post-operative course was without any significant event. No sign of infection was noticed. The patient recovered gradually from his motor deficit on his right side. The post-operative CT scan showed complete resorption of the intracerebral pneumocephalus. (Figure.3). Intravenous prophylactic antibiotics were used to prevent meningitis.
Figure 3: Post-operative CT scan showing no hypodensity area in the left frontal lobe, complete disappearance of the pneumocephalus A. Axial view B. Sagittal view C. Coronal view. Noted the small bone defect from craniotomy site.
At one-month follow-up, his motor function on the right body became normal that he could play his favorite guitar again. At three-month follow up, he had an episode of new seizures, we controlled his seizures with anti-epileptic drugs for two years afterward.
DISCUSSION
The term “pneumocephalus” was first coined more than one century ago by Luckett and Wolff independently. The term “tension pneumocephalus” was proposed by Ectors, Kessler, and Stern in 1962. Pneumocephalus or also known as pneumatocele or intracranial aerocele is defined as the presence of air in the epidural, subdural, or subarachnoid space, within the brain parenchyma or ventricular cavities. It is a complication of head injury in 3.9 – 9.7% cases. It also appears after supratentorial craniotomy surgery. The accumulation of intracranial air can be acute, less than 72 hours, or delayed, more than 72 hours.
Two mechanisms have been proposed to explain pneumocephalus. In the first mechanism, the pathophysiologic process starts with Cerebro-Spinal Fluid (CSF) leak in the presence of associated discontinuity of the cranium and leptomeningeal disruption. Subsequent development of relative negative Intra-cranial Pressure (ICP) results in a sufficient “vacuum effect” to cause additional accumulation of air within the cranial cavity. This air is generally distributed in the subarachnoid space. The second mechanism is based on the presence of a “one-way valve” at the site of the leptomeningeal tear. In this case, we found on the CT scan images a bone and dura defect in the left anterior skull base, in connection with intracerebral air collection. The air went in, and was trapped inside the frontal cerebral parenchyma. Slowly it became larger and more significant, putting mass effect into the brain tissue of the patient’s frontal lobe. The patient had experienced rhinorrhea (CSF leak through the nose) after the head trauma but disappeared spontaneously after 10 months. He then developed right hemiparesis and experienced episodes of seizures. Recurrent headaches were also a main complaint. These signs and symptoms were described in previous reports about tension pneumocephalus.
The diagnostic imaging for pneumocephalus is CT scan. “Mount Fuji sign” is described when there are bilateral hypoattenuation collections, causing compression and separation of the frontal lobes on CT scan. In our case, an intraparenchymal air-filled long cavity was seen in the left frontal lobe, with its tip connecting to the frontal skull base.
Most cases of pneumocephalus tend to resolve spontaneously with conservative management. Nonoperative management involves oxygen therapy, maintaining the patient supine or in Trendelenburg position, prophylactic antimicrobial therapy (especially in posttraumatic cases), adequate analgesia, frequent neurologic checks, and repeated CT scans. The use of continuous high concentration inspired oxygen as a treatment modality for traumatic pneumocephalus may have certain theoretical benefits. Prompt decompression of intracranial air is the initial treatment of symptomatic pneumocephalus. The principles of subsequent treatment parallel those for a CSF leak. It is important to identify the site where the communication between the air cavity and the external environment occurs. If the site can be identified, the passage should be sealed off, thereby decreasing the possibility of worsening or recurrent pneumocephalus. Effective therapy of tension pneumocephalus through a controlled decompression using a closed water-seal drainage system has also been described. In our case, we performed a full scale left frontal craniotomy to evacuate air from the intraparenchymal cavity, closure of the skull base defect by using pedunculated galea flap, re-enforced by bio-glue as a sealing material.
CONCLUSION
Tension pneumocephalus is a life-threatening neurosurgical case. Although the development of this massive intracerebral air trap was delayed in this case, it caused significant neurological deficit. The patients who suffer from head trauma, with CSF leak should be subject for long term follow up.
Disclosure: Nothing to disclose, and there was no conflict of interest among the authors.
Research ethics: Informed consent has been obtained from the patient.
For more information: https://jmedcasereportsimages.org/about-us/
For more submission : https://jmedcasereportsimages.org/
#Pneumocephalus#hemiparesis#craniotomy#dura-plasty#CT#neurosurgical#headaches#Cerebro-Spinal Fluid#ICP#Intra-cranial Pressure#CSF#Sokchan Sim#jcrmhs
0 notes
Text
https://dmbd.space/read-blog/336183
Intra-Cranial Pressure Monitoring Market Size Projection, Regional Insights, Growth, Share Estimation 2030
0 notes
Text
Intra Cranial Pressure Monitoring Market Share, Focuses on SWOT analysis, Synopsis, Development Plans 2022 to 2030
Intra Cranial Pressure Monitoring Market Share, Focuses on SWOT analysis, Synopsis, Development Plans 2022 to 2030
0 notes
Text
Intentional vs Pathological Cranial Vault Modifications

Danielle Kurin, Ph.D. is a bioarchaeologist and former assistant professor and later tenured associate professor in the Department of Anthropology at the University of California at Santa Barbara. She analyzes skeletal remains and mortuary practices in order to better understand the ancient impact of diseases, migration and conflict. She also examines cultural practices like cranial modification and medical treatment like trepanation--or ancient brain surgery..
Kurin's research work in Peru indicates very clearly how various prehistoric communities manipulated the shape of their children's skulls,
Evidence of artificial cranial vault modification, also known as head shaping, reaches far back in the development of human society and is found in virtually all regions of the ancient world.. Anthropologists have posed various motivations for cranial vault modification--ranging from marking ethnic or social identity, to signifying ritual or sacred status, from being regarded as a standard rearing practice to a measure for the avoidance or treatment of disease..
Other forms of cranial modification include medical or surgical treatment. Trepanation, the drilling of holes in the skull has been found in the ancient old and new worlds--indicative of parallel invention. It was likely used as a means of relieving intra-cranial pressure caused either by accidental falls or as a result of violent injuries in battle assaults.
0 notes
Text
0 notes
Link
0 notes
Text
An Uncommon Case of Streptococcus Pyogenes Endocarditis Causing Intracranial Mycotic Aneurysms by Ameur A
Abstract
Intra-Cranial Mycotic Aneurysms [ICMA] or infection aneurysms are rare and represent less than 10% of the neurological complication of infective endocarditis. The most common causative organisms are alpha-hemolytic streptococcus of the viridans group and staphyl ococcusaureus, respectively responsible for 50%and10%ofICMA.
We report a case of group A beta-hemolytic streptococcus –streptococcus pyogenes- native mitral valve endocarditis complicated with intracranial mycotic aneurysms. A review of the existing literature revealed that acute IE caused by streptococcus pyogenes has rarely been reported, only 40 cases since 1940, of which no case was complicated with intracranial mycotic aneurysm.
Keywords: Infective endocarditis; Streptococcus pyogenes; Intracranial mycotic aneurys
Introduction
Infective endocarditis is an uncommon infectious disease with an annual incidence that varies from 3 to 7 per 100 000 person-years. Although relatively rare, IE continues to be characterized by increased morbidity and mortality through its complications [1].Neurologic complications related to IE are diverse (ischemic, hemorragic and infective), and are the most frequent extracardiac complications, occurring in 17 to 82% of patients with left-sided IE [2].
Mycotic aneurysm are rare inflammatory neurovascular lesions, comprising 0.7 – 5.4% of all intracranial aneurysms. S.viridans and S.aureus are the most common organisms that cause IE. Therefore, they are the two most frequently associated pathogens with CMAs in the course of IE[3]. Streptococcus pyogenes, whilst known to infect immunocompromised patients, is a rare cause of endocarditis and there are no published reports of this organism causing intracerebral mycotic aneurysms. We report a case of streptococcus pyogenes endocarditis complicated within tracranial haemorrhage in the setting of cerebral mycoticaneurysm.
Case Presentation
An 18-year-old female was admitted with a one-month history of persistent fever, associated with shortness of breath and precordialgia. She had previously received some unspecified treatment with no apparent amelioration of symptomatology. She reported a history of dental procedure, undergone one year earlier. Physical examination, showed a temperature 38.5°C, (Blood pressure: 120/70, Heart rate= 100 beats/minute, respiratory rate=20 breaths per minute). Cardiovascular examination revealed an intensive mitral holosystolic heart murmur. Osler nodes were observed on fingers andtoes.Biological test revealed anaemia of chronic inflammation, blood cell count= 10500/μL, platelet count= 360000/μL, prothrombin time-international normalized ratio=1.23, C- reactive protein=98mg/dL, creatinine=6mg/L and estimated glomerular filtration rateof138.5 mL/mn. The urine analysis was negative for infection. Electrocardiograson demonstrated sinus tachycardia, and Chest x-ray demonstrated cardiomegaly with features of left atrialenlargement.
Blood cultures were found to be positive for streptococcus group A (Streptococcus pyogenes).
A transthoracic echocardiogram, along with a Trans oesophageal echocardiography, documented a severe mitral regurgitation and a 16×6mm vegetation, attached to the anterior mitral leaflet and 8 mm vegetation implanted on the tricuspid valve was also noted (Figure 1and 2) Pan-computed tomography (CT) scans revealed features of splenic infarction, with no other sign of secondary lesions elsewhere.
She met two major and three minor modified Duke Criteria and was diagnosed with definite endocarditis. The neurological examination was steadily normal, and ECG was undergone daily, during antibiotic therapy.
A Trans oesophageal echocardiography was repeated 9thday of antibiotic therapy, it showed three calcified masses implanted on the two leaflets of the mitral valve, with the following dimensions (14mm, 13mm, 6mm), and suspected a perforation of the anterior mitral leaflet. A decision of cardiac surgery wastaken.
16thdayofantibiotictherapy,she reports headache and drowsiness, a CranialCT-scanshoed an intracerebral haemorrhage located in the left frontal and insular lobes with manifest signs of cerebral herniation (Figure3).
The patient was admited in intensive care unit, where a decompressive craniectomy was conducted.
Thereafter, a cerebral CT angiography was done, giving evidence of two aneurysms arising from the ending of the left middle cerebral artery (Figure 4).Given the poor prognosis, endovascular therapy was precluded. She died after a rapidly progressive decline in her condition.
Discussion
Beta haemolytic streptococci, which include serogroups A, B, C and G, cause a wide variety of infections including cellulitis, necrotising fasciitis, bacteremia and infective endocarditis [4]. Although BHS endocarditis is relatively uncommon, it is very aggressive, with a high rate of cardiac valve destruction, cardiac abscess formation and systemic embolization [4].Of the different subgroups, group B streptococcus (S.agalactiae) is the most common cause of BHS endocarditis. BHS Endocarditis due to groups C and G are less common, but presents similarly to that caused by group B BHS [4].It is noted that Group A β-hemolytic Streptococcus pyogenes is the least common cause of BHS endocarditis [4].Forty cases of endocarditis caused by Streptococcus pyogenes in children and adults have been reported since 1940, with a median age of 32 years, ages ranging from four months to 80 years. The mortality rate was 24% (62.5% before 1990 and 15.4% after 1990) and was mainly due to cardiac failure and/or septic shock; most patients recovered after antibiotic therapy [50].In our case, the outcome was dramatic, due to a neurologic complication, namely, intracranial haemorrhage. Intracranial Mycotic aneurysm, which results from the septic embolism of vegetation in the cerebral circulation, has been reported secondary to infective endocarditis, with an estimated range between 2% and 10%, The most common causative organisms being Streptococcus viridans, Streptococcus group D and Staphyl ococcusaureus species [6].The clinical presentation of unruptured ICMAs is variable and lacks specificity: Fever, headache, convulsions and focal deficit. Ruptured ICMAs are responsible of a clinical profile of cerebral or subarachnoid haemorrhage: headache, loss of consciousness, intracranial hypertension and focal deficit[7].The diagnosis of ICMAs is possible on brain CT scan that visualises the indirect signs. Cerebral angiography, cerebral MRI and/or cerebral angio MRI have a 95% sensitivity in diagnosing ICMAs greater than 5mm. CT angiography may not be sufficient to detect small aneurysms at the base of the skull[7].Brain angiography remains the benchmark examination, in particular for the diagnosis of small ICMA[7].In our case, cerebral CT angiography gave evidence of two aneurysms.Mortality associated with rupture of intracranial mycotic aneurysm, leading to subarachnoid haemorrhage or intracerebral haemorrhage, is reported to be as high as 80%[6].There are, however, no published reports of S.Pyogenes causing intracerebral mycotic aneurysms.Treatment of intracranial mycotic aneurysms is challenging and to date, there are no standard guidelines. Antibiotic therapy is an essential component of the treatment and studies have reported variable rates of resolution in response to antimicrobial therapy [6]. Taking into consideration the variable response to antibiotics and the high rate of mortality associated with rupture of intracranial mycotic aneurysms, some authors have advocated more aggressive treatment, using endovascular or open surgical treatment, to permit securing of aneurysms and timely cardiac valve replacement [6]. Furthermore, timing of cardiac surgery is difficult to determine, owing to the cerebral damage thatmaybeamplifiedbyheparinizationandhypotensionduringthe cardiopulmonary bypass or postoperative anticoagulant therapy. Therecent European society of cardiology guidelines recommend, that after ICH, surgery should be postponed for more than one month [3].In our case, the clinical presentation has deteriorated rapidly, even after the decompressive craniectomy.
Conclusion
Group A β-hemolytic Streptococcus pyogenes IE is a rare condition, however, it can be lethal through extra cardiac complications, which is approved through our case which confirms its virulent character. There are currently no guidelines in treatment of Intracranial mycotic aneurysms in the setting of IE, rendering its management extremely challenging.
For more information about Article : https://ijclinmedcasereports.com/
https://ijclinmedcasereports.com/ijcmcr-cr-id-00137/
https://ijclinmedcasereports.com/pdf/IJCMCR-CR-00137.pdf
#Infective endocarditis#Streptococcus pyogenes#Intracranial mycotic aneurys#Ameur A#IJCMCR#clinical studies
0 notes
Photo
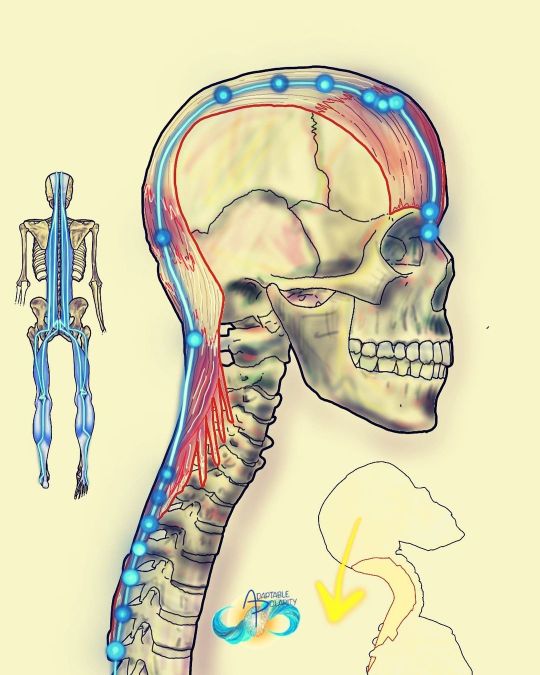
Movement, Muscles & Meridians: head and neck superficial back line and urinary bladder channel It’s fascinating to me that from the top down to initiate extension, we start with our eyebrows. It explains or makes some sense why frontal bone concussions can affect this entire line down to altering intra abdominal pressure. In Applied Kinesiology, there’s even a cranial fault under the galea aponeurotica (fascia on top your head) and the sagittal suture related to this line of fascia and back pain. Conversely, or polar reversely, I’ve heard stories of ancient Chinese cutting the foot fascia of peasants so they could never lift their head again. What an intense punishment of superficial back line fascial understanding!?! Movement: head and neck extension Muscles: neck extensor and occipitofrontalis #muscles #movement #meridians #fascia #energychannels #myofascia #myofascial #cranials #cranialbones #cranialsacraltherapy #cranialsacral #craniosacraltherapy #craniosacral #neckextension #bladdermeridian #ubchannel #myofascialmeridians #energyhealers #bodymechanics #adaptablepolarity https://www.instagram.com/p/Cfef1amlbc1/?igshid=NGJjMDIxMWI=
#muscles#movement#meridians#fascia#energychannels#myofascia#myofascial#cranials#cranialbones#cranialsacraltherapy#cranialsacral#craniosacraltherapy#craniosacral#neckextension#bladdermeridian#ubchannel#myofascialmeridians#energyhealers#bodymechanics#adaptablepolarity
0 notes
Link
“Intra-cranial pressure (ICP) monitoring Market ” report analyse the market by countries, by type and, by application giving Industry Insights & Opportunity Evaluation.
#Intra-cranial pressure (ICP) monitoring Market end users#Intra-cranial pressure (ICP) monitoring Market key players#Intra-cranial pressure (ICP) monitoring Market major geographies
0 notes
Link
0 notes
Link
“Intra-Cranial Pressure Monitoring” gives detailed outlook by Type, by Application, by Segmentation and Regional Forecasts.
#Intra-Cranial Pressure Monitoring statistics#Intra-Cranial Pressure Monitoring development#Intra-Cranial Pressure Monitoring growth
0 notes
Text
https://mlmzone.in/blogs/93065/Intra-Cranial-Pressure-Monitoring-Market-Growth-Outlook-Future-Trends-Share
Intra-Cranial Pressure Monitoring Market Size Projection, Regional Insights, Growth, Share Estimation 2030
0 notes
Text
Intra Cranial Pressure Monitoring Market Players, Size, Share, Growth, Newest Industry Data, Future Trends and Forecast
Intra Cranial Pressure Monitoring Market Players, Size, Share, Growth, Newest Industry Data, Future Trends and Forecast
0 notes
Text
Anaesthetic Problems and How to Fix ‘Em
Anaesthesia can be fraught with morbidity and mortality due to the sheer number of things that can go wrong in a drug-induced sleep without correct monitoring and trouble-shooting. The animal loses their ability to thermoregulate, their cardiovascular and pulmonary systems becomes depressed, and they lose many reflexes that are vital for controlling blood pressure, peripheral vascular resistance, and more.
So, let’s begin. I’ll put a read-more option because this post is fairly long.
AIRWAY OBSTRUCTION
How to recognise it --> Reduced tidal volume, chest doesn’t rise with intermittent positive pressure ventilation (IPPV), hypoxia and hypercapnia, inadequate depth, rebreathing or flat line capnograph (partial vs total), whistling sound, apnoea
What causes it --> Intubated patients: kinked ET tube, blocked ET tube with secretions, faulty cuff, laryngospasm. Non-intubated: material blocking the pharynx, laryngospasm, anatomical defects (e.g. elongated soft palate)
How to fix it --> If intubated, suction the ET tube, extend the head and neck, give oxygen, and replace the ET tube if necessary. Otherwise, PREVENTION! Pack the pharynx for oral procedures, gentle intubation, appropriate timing of extubation, proper patient positioning
REBREATHING (breathing in CO2)
How to recognise it --> Hypercapnia; ETCO2 >55 mmHg or inspirational CO2 >0 mmHg, brick red mucous membranes, hypertension, tachypnoea/increased tidal volume, apparent increased anaesthetic depth
What causes it --> Faulty one-way valves in rebreathing circuits, inadequate fresh gas flow in non-rebreathing circuits, increased dead space (leaks, long ET tube), or exhausted CO2 absorber.
How to fix it --> Maintain/replace equipment, ensure gas flow rates are adequate (300-500 ml/kg in a non-rebreathing circuit), minimise dead space with correct ET tube size, change CO2 absorber, supplement oxygen and IPPV,
LEAKING (of gas within the anaesthetic circuit)
How to recognise it --> failed pressure checks prior to anaesthesia (leaking >200 ml/min at 20 mmHg pressure), signs of rebreathing and hypoxia, inadequate depth control, air pollution (smell, hissing), chest not rising with IPPV
What causes it --> Faulty equipment machine or breathing circuit, ET tube cuff not adequately inflated.
How to fix it --> Maintain equipment properly, inflate cuff sufficiently (no leaking heard from trachea when reservoir bag squeezed up to 20 cm H2O)
BAROTRAUMA (pressure trauma to the lungs)
How to recognise it --> Pressure >45 cmH2O in healthy or 14-16 cm H2O in compromised patients, distended reservoir bag, apnoea or dyspnoea, tachycardia, hypotension,cyanosis, loss of lung sounds, SC emphysema, cardiac arrest
What causes it --> Prolonged closing of the pop-off valve, flushing the circuit with patient connected, IPPV with too high pressure, lung pathology
How to fix it --> Use positive end expiratory pressure (PEEP) ventilation, oxygen, fluids and cardiovascular support, ensure the ET cuff leaks >20 cm H2O, keep pop-off valve open
TACHYCARDIA (higher than normal HR)
How to recognise it --> HR >140-160 bpm in a dog, or >180 bpm in a cat; using a stethoscope, doppler, pulse oximetry, or ECG
What causes it --> Anaesthetic plane that is too light, painful or strong stimuli, hypovolaemia, hypotension, anaemia, hypercapnia, hypoxaemia hyperthermia, heart disease, drugs such as atropine and ketamine
How to fix it --> Deepen anaesthesia, give analgesia, IV fluid therapy, oxygen and IPPV, active cooling, positive ionotropic agents such as dopamine/ dobutamine/ ephedrine for hypotension, fluids, wait for drugs to wear off
BRADYCARDIA (lower than normal HR)
How to recognise it --> HR <60 bpm in dogs, or <100 bpm in cats
What causes it --> Anaesthetic plane that is too deep, hypertension, vagal stimulus, drugs such as propofol, opioids, or alpha2-agonist sedatives, hypothermia, hyperkalaemia, increased intra-cranial pressure (ICP), decompensated hypoxaemia
How to fix it --> Lighten anaesthesia, give analgesia, warm the patient, give oxygen and IPPV, treat for increased ICP if also hypotensive with mannitol/ furosemide, fluid therapy with calcium/dextrose +/- insulin for hyperkalaemia, atropine if not hypothermic cause
HYPOTHERMIA (lower than normal temperature)
How to recognise it --> Body temperature <37 degrees Celsius, critical temperature 32 degrees Celsius
What causes it --> Anaesthesia!
How to fix it --> Provide passive warming (towels, blankets) and active warming (bair huggers, hot mats, warm water bags).
ARRHYTHMIA (abnormal heart rhythm)
How to recognise it --> ECG, auscultation, pulse deficits present
What causes it --> Inappropriate anaesthetic plane, hypercapnia, hypoxaemia, hypotension, hypothermia, medetomidine, electrolyte or acid-base abnormalities, cardiac disease
How to fix it --> Maintain adequate anaesthetic depth, atropine for sinus arrhythmia (bradycardia) only if BP affected too, oxygen and IPPV, stop drug administration, lignocaine bolus or CRI, fluids, warming
HYPOTENSION (decreased blood pressure)
How to recognise it --> Systolic arterial pressure (SAP) <90 mmHg, Mean arterial pressure (MAP) <60-70 mmHg, arrhythmias, tachycardia
What causes it --> anaesthetic drugs, too deep anaesthesia, hypovolaemia, heart disease, arrhythmias, organ manipulation, IPPV capillary occlusion
How to fix it --> Adjust anaesthetic depth + use MAC/dose-sparing adjunctive drugs, fluids, atropine if bradycardia, dopamine CRI or ephedrine bolus (positive ionotropy)
HYPERTENSION (increased blood pressure)
How to recognise it --> SAP >160 mmHg, MAP >140 mmHg
What causes it --> Too light anaesthesia, painful stimulus, drugs such as ketamine, hypoxaemia, cardiac disease, renal insufficiency, increased ICP (Cushing’s reflex - increases MAP)
How to fix it --> Deepen anaesthetic plane or provide analgesia, IPPV/PEEP and oxygen, wait for hypertensive drugs to wear off, mannitol or furosmide if bradycardia too (increased ICP)
HYPOVOLAEMIA (relative or absolute decreased blood volume)
How to recognise it --> monitoring blood loss during surgery, pale mucous membranes and increased capillary refill time (CRT), tachycardia, hypotension, increased apparent depth due to reduced cardiac output to lungs
What causes it --> Relative (vasodilation) and absolute (blood loss, dehydration)
How to fix it --> Minimise inhalant use with MAC-sparing agents such as opioids as well as close depth monitoring, fluids, positive ionotropy (dopamine e.g.), calcium, keeping the patient warm
APNOEA (not breathing)
How to recognise it --> patient not breathing, flat line capnograph
What causes it --> Post-induction (light plane anaesthesia), drugs (propofol e.g.), too deep plane of anaesthesia, lung pathology, paralysis
How to fix it --> IPPV and oxygenation, analgesia, manage anaesthetic depth closely
TACHYPNOEA/ HYPERVENTILATION (breathing too fast)
How to recognise it --> Respiratory rate >20 breaths/min in cats and 30 breaths/min in dogs
What causes it --> Light plane anaesthesia, painful or strong stimulus, hypercapnia, hypoxaemia, hyperthermia, weak respiratory muscles, abdominal enlargement
How to fix it --> Deepen anaesthetic plane, provide analgesia, oxygenation +/- IPPV, active cooling, optimal patient positioning
HYPOVENTILATION (breathing too slow/shallow)
How to recognise it --> ETCO2 >55 mmHg, apnoea/bradypnoea/shallow tachypnoea, brick red mucous membranes, tachycardia, increased blood pressure, tidal volume <10 ml/kg
What causes it --> Too deep in anaesthesia, drugs (e.g. inhalants, propofol), poor positioning, large abdomen, thoracic pathology, paralysis, rebreathing, open thoracic surgery
How to fix it --> Oxygenation, IPPV, and reducing depth
HYPOXIA (low tissue oxygenation)
How to recognise it --> SpO2 (pulse oximetry) <90-95% or PaO2 <60-80 mmHg, bradycardia, cyanosis, cardiac arrest
What causes it --> Too deep anaesthesia, rebreathing, inadequate oxygen flow rate, airway obstruction, V/Q mismatch (pulmonary shunt), hypovolaemia, cardiac failure, hypoxaemia, shock, cyanide toxicity, anaemia
How to fix it --> Assess airway, oxygenation, lighten anaesthesia, IPPV, positive ionotropy (dopamine e.g.) if cardiac failure, fluids, check your anaesthesia set up
PATIENT TOO LIGHT
How to recognise it --> positive withdrawal reflex and palpebral reflexes +/- blinking, tachycardia, hypertension, tachypnoea, movement with stimulus, swallowing/ chewing/ salivation, central dilated pupils,
What causes it --> Low vaporiser output, low flow rate, inadequate ventilation, rebreathing, disconnected circuit, ET tube too long (only one lung gassed down), leaks
How to fix it --> Check the anaesthetic circuit for disconnection, excessive dead space, or leaks (should be done prior to every anaesthesia), icrease vaporiser output, increase flow rate, change ET tube, IPPV
PATIENT TOO DEEP
How to recognise it --> weak jaw tone, central dilated pupils, reduced or absent palpebral reflexes, withdrawal reflexes, and ear flick (cats), bradypnoea and reduced tidal volume, hypotension, pale mucous membranes + increased CRT, no response to strong stimulus
What causes it --> Low cardiac output, high vaporiser output, hypothermia, poor depth control/complication troubleshooting
How to fix it --> Disconnect the patient, turn off the vaporiser, and flush the system through with oxygen via squeezing and refilling the rebreathing bag, connect to the patient with pure oxygen, warm the patient.
30 notes
·
View notes
Text
Brain Hemorrhage Treatment In Gurgaon
The facts:
A brain bleed or brain hemorrhage as it is called is bleeding in or around the brain. It can occur spontaneously as a large hemorrhage, with sudden onset catastrophic symptoms or it can occur gradually where it bleeds slowly over a period of time, with no apparent symptoms at all. It is said that almost 13% of strokes are caused because of hemorrhagic causes. It can be Traumatic or Spontaneous.
Why do they have different names?
These hemorrhages are given different names based on the exact location inside the skull where the bleeding occurs. Bleeding anywhere inside the cranial cavity is known as intracranial hemorrhage. Whereas bleeding into and within the brain tissue itself is called an intra-cerebral hemorrhage. But between the skull and the brain matter, you have what is the lining of the brain, made of three layers called the Dura mater, which is closest to the skull, Arachnoid mater, which lies in the middle and Pia mater, which is closest to the brain tissue. Bleeding can occur anywhere in between these layers as well.
Broadly they can be Traumatic or Non Traumatic.
These different types of hemorrhages are:
Extradural/epidural hemorrhage – which is bleeding between the skull and the Dura mater, which is commonly caused by trauma due to acceleration-deceleration forces and rupture of the meningeal vessels.
Subdural hemorrhage – which is bleeding between the Dura mater and Arachnoid mater. These hemorrhages are also again caused by trauma, but it is the subdural hemorrhages that most often tend to be chronic and innocuous as well.
Subarachnoid hemorrhage – is bleeding between the Arachnoid mater and the Pia mater. This type of bleeding is commonly caused by the rupture of an aneurysm.
NonTraumatic Basal Ganglia ICH (Most Common ICH encountered in Clinical practice), Thalamic ICH, Spontaneous Cerebellar Hematomas.
What are the signs to look out for to suspect an ICH?
The common symptoms which patients experience when they develop and ICH include:
Sudden onset severe headache
Any changes which follow trauma to the head should warn you about the development of an ICH
Neck stiffness
Nausea and vomiting
Altered levels of consciousness/loss of consciousness
Development of seizures
Neurological deficits such as numbness, weakness and impairment of vision and speech
In children you must suspect and ICH when the child presents with vomiting, seizures, loss of consciousness, swelling in the head or retinal hemorrhages. On most occasions the trauma which causes these types of injuries in children is due to child abuse.
How is an ICH diagnosed?
The most common investigation used to confirm the diagnosis of an ICH is the CT scan, because it is widely available. But where facilities are available an MRI scan is also equally useful for confirmation. A Magnetic Resonance Angiogram, if available will help to get an idea about the underlying cause of the ICH.
What are the treatment options available for intracranial hemorrhages?
There are different ways in which intracranial hemorrhages are managed depending on the exact location of the bleeding and the amount of bleeding which has occurred. The symptoms which the patient presents with also plays a role in determining the treatment plan. The various methods available for the management of an intracranial hemorrhage include non-surgical methods and surgical methods.
Surgical methods are required if the bleeding is extensive, which is determined by imaging techniques such as CT and MRI, or if the patient is displaying symptoms which are life threatening such as development of seizures and altered level of consciousness, all which occur due to increased intracranial pressure.
Non-surgical methods are used when the bleeding is minimal and the patient is asymptomatic. Your doctor will continue to monitor you, looking out for signs of increased intracranial pressure, and in the meantime you will be prescribed some medication such as anti-anxiety medication as well as drugs to prevent the development of seizures, and even pain relief medication, because some patient may only complain of a mild headache. Medication to reduce the swelling of the brain such as steroids as well as medication to bring down your blood pressure may be helpful in the management of an ICH.
What is the role of urgent surgery in intracranial hemorrhages?
Urgent surgery is most often required in the case of ruptured aneurysms which can cause subarachnoid hemorrhages or intra-cerebral hemorrhages. When it comes to intracerebral hemorrhages more often than not, your doctor will opt for immediate surgery because the brain matter can be extensively damaged if not intervened at the earliest, because of the increased intracranial pressure, leading to poor prognosis. Therefore the goal of urgent surgery is to save as much of the brain tissue as possible. The surgical options available are:
Decompression surgery using one of the four methods mentioned below:
Craniotomy – where the surgeon will make and incision in the scalp, remove a part of the skull, and drain the hematoma which has formed and repair the ruptured blood vessel. This is high risk surgery and is only performed when the bleeding is extensive, or when higher functions have been impaired in the patient.
Burr holeaspiration – where a small hole is drilled into your skull, through which a needle is inserted, and the hematoma is drained out. But for this you need to be able to identify the exact location of the hemorrhage, and it might turn out that the surgeon cannot completely drain the hematoma as well.
Endoscopic evacuation – which is similar to aspiration, but it involves the use of an endoscope which has camera and special equipment fitted at one end, and helps the surgeon visualize the insides of the cranial cavity.
During these procedures as well as due to the hemorrhage itself there is an increased risk the brain swelling up (cerebral edema). Therefore when the surgeon removes a part of the skull during a craniotomy, they may decide to replace it after some time, to allow for the contents of the cranial cavity to expand, while the swelling is still present. And once the edema is settled the part of the skull is replaced. This is known as a craniectomy. There are other methods which been used in order to tackle this problem of cerebral edema due to hemorrhage and surgery, which includes:
Duraplasty – which is a procedure used during the Chiari decompression surgery, where an incision is made in the Dura mater, and an extra patch is sewn in place in order to expand the surface of the Dura, to allow for the edema.
Cisternostomy – is a procedure where the basal cisterns are opened up to atmospheric pressure, in order to relieve the increased intracranial pressure which occurs as a result of cerebral edema. It is a novel technique which has replaced the older procedure of decompressive hemicraniectomy.
Brain hemorrhages can be a life threatening condition if not managed appropriately at the right time. But with the advent of newer and technologically advanced surgical methods, the success rate of surgical management is on the rise.
What precautions can I take to prevent the development of an ICH?
There is no specific method which can ensure that you prevent the development of an ICH. The main factor is to prevent trauma to the head as much as possible even when accidents do happen. Therefore taking the below mentioned precautions may help:
Wearing helmets when traveling on bikes, motorbikes, scooters and skateboards
Wearing your seatbelt when travelling in vehicles
Trying to prevent falls in an elderly
Non Traumatic ICH can be prevented by control of Blood pressure, regular check up from Neurologist/ Neurosurgeon and Diagnostic Angiograms.
What outcome can I expect following an ICH?
As an ICH is life threatening condition, the sooner it is treated the better the prognosis. But the overall outcome is dependent on the site where the bleeding occurs and how severe the bleeding was. Some patients may recover completely following management of the ICH, while some others may need rehabilitation such as speech therapy, physiotherapy and occupational therapy to help them perform their daily activities, if they have to overcome remaining neurological deficits.
#Brain Hemorrhage Treatment In Gurgaon#Brain Hemorrhage Treatment In Sonipat#Neurosurgeon Doctor In Gurgaon#Best Neurologist In Gurgaon#Best Neurosurgeon In Rohtak#Neurosurgeon In Faridabad#Neurologist In Rewari
1 note
·
View note
Link
0 notes