#Electronic Medical Records Market Industry
Text
#Electronic Medical Records Market#Electronic Medical Records Market Trends#Electronic Medical Records Market Growth#Electronic Medical Records Market Industry#Electronic Medical Records Market Research#Electronic Medical Records Market Report
0 notes
Text
Consumer-driven healthcare
For Branding Lab, we're supporting a team that is designing a solution to help the go-getter with an unpredictable schedule build a habit of taking their daily pills. Among the prescriptions filled in the US, roughly 50% aren't taken correctly (CDC). The team needs to build a brand that sparks joy and encourages users to build a new habit.
Something we're thinking through are the cognitive associations tied with taking prescription medications vs. vitamins/ supplements. The healthcare system often has a reputation for being less consumer-friendly compared to the "wellness/ self-care" industry.
However, new entrants into traditional healthcare industries are leveraging "consumer-friendly" branding and CX as a way to differentiate themselves. For example, in the electronic health record (EHR) market, one of the largest incumbents (Epic) has an infamously poor UX. Just looking at their website, it's not clear what problems they solve for their user--it looks like a school newsletter:
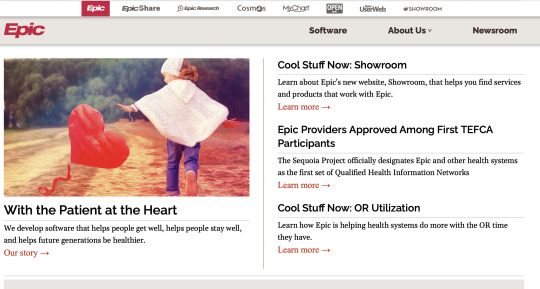
Meanwhile, new entrants like Abridge are playing with what it means to be a B2B brand selling software to hospital systems, with playful font and clear, physician-centered messaging:

I'm curious to see how this "consumer-forward" play into a traditional B2B industry plays out, and whether we can leverage a similar approach to bring better CX to medication taking.
2 notes
·
View notes
Text
Dealing with challenges in Quality Evidence Generation with a Real-Time Analytical Framework that makes Clinical Sense for Innovators
Evidence linking interventions with health outcomes is vital for healthcare decision-making. Making sound choices about healthcare requires the best possible and quality evidence from clinical research. However, some of the decisions currently made during the drug development process are not supported by high-quality evidence. As such, making informed decisions for allocating adequate resources to guide clinical Research development becomes challenging. At mid-stage clinical development, the challenge entails in determining the specific indication, if there are multiple potential indications. Moreover, evidence that is complete for some individuals or groups may be incomplete for others, leading to inefficiencies in decision-making.
Evidence generation strategies are especially important at Phase III and Phase IV trials to allow for effective navigation through competitive and regulatory hurdles, while it may be difficult to effectively communicate potentially attractive product attributes to the stakeholders, especially when it has no clear advantage over comparators. Stakeholders also lack the evidence needed to make real-world decisions on approval, coverage and use of treatments as most current processes used in evidence generation focus narrowly on the safety and efficacy of treatment.
Datasets to inform real-time decision making
The traditional demarcation between pre- and post-approval phases does not fit many medical products, as regulatory decisions could be informed by the same evidence that informs the use and coverage decisions, though the criteria for decisions should not be the same for both cases. Validated tools, based on large datasets to help inform real-time decision making are invaluable, yet they are currently limited. When new treatments are approved, healthcare payers and those who participate in shared savings base coverage determination on their value which is calculated by the evidence of benefit and net costs. The incorporation of real-world data (RWD) and patient-reported outcomes (PRO) into the evidence generation process could assist in making coverage determinations by rendering clinical evidence and research more immediately translatable to the beneficiary population.

Real-world data (RWD) and real-world evidence (RWE)
Additionally, large differences usually exist between the evidence required for initial adopters, such as surveys and studies, and that required for most prospective randomized control trials (RCTs). While the healthcare community uses RWD and RWE to develop decision support tools for use in clinical practices, medical product developers use these data to support clinical trial designs and observational studies to generate innovative treatment approaches. FDA uses RWE and RWD to monitor adverse events, post-market safety of the drug, and to make regulatory decisions. While RWD can be collected from various sources such as electronic health records (EHRs) and product and disease registries, RWE can be generated by different study designs including observational studies and randomized trials.
Aligning stakeholders for evidence generation
Aligning stakeholders is another big challenge of evidence generation as different stakeholders will have their own perspectives on uncertainties throughout the drug development lifecycle. Regulators may have different views as to what is acceptable to that of the patient. As such, it remains an industry-wide challenge to provide credible evidence for clinical research to innovators and investigators. Challenges exist for healthcare innovators to keep up to date with compliance and regulations about evidence generation as regulatory space evolves fast.
Because pharmaceutical companies tend to delegate evidence generation to individual departments that are often siloes, the process occurs sequentially, resulting in delays in crucial milestones such as getting regulatory approval before initiating an outcomes-based study.
https://www.futuremedicine.com/doi/10.2217/cer-2017-0073

An analytical framework model that makes clinical sense
There is a pressing need for high-quality evidence generation as regulators and payers seek more long-term data on product safety and effectiveness. As such, more efficient methodologies for generating evidence are required for decision-making, and to enhance clinical evidence collection and interpretation. An analytical framework model makes clinical sense as an evidentiary pathway, however, the challenge for investigators in evidence gathering is to fill out the framework. If the study design is weak, then the link in the evidence chain is also weak. Studies need to be carefully and prospectively designed, and opportunities exist to add well-designed studies into current practices. Study teams and researchers should consider how to most effectively translate diagnostic tests into practice needs within clinical settings.
Quality clinical evidence of safety and efficacy
The Jeeva™ eClinical Cloud platform provides clinical decision-makers with a modular and integrated approach to evidence planning and generation. From a single dashboard, study leaders can monitor data in real time to track safety and efficacy in representative patient populations across vast distances. The Jeeva™ eClinical Cloud is designed for efficient, remote long-term follow-up, natural history and other observational studies as well as interventional clinical trials regardless of therapeutic area. Jeeva™ enables quality clinical evidence generation to evaluate treatment safety and efficacy and tracks patients’ adherence to medications, in compliance with regulatory agencies such as Institutional Review Boards, EMA, FDA, and GDPR.
Digital-first approach to evidence generation
Study teams, innovators, drug developers, biopharmaceutical sponsors, clinical researchers, hospital sites and contract research organizations (CROs) face challenges to overcome the “no evidence, no implementation—no implementation, no evidence” paradox. Jeeva™ provides a new, digital-first, patient-centric approach to evidence generation that considers patients as partners for clinical trials, not merely subjects.
The Jeeva™ eClinical Cloud is user-designed software-as-a-service (SaaS) platform that allows volunteers to conveniently complete clinical trials wherever they are. The flexible and modular bring-your-own-device (BYOD) solution works on any browser-enabled mobile device and cuts out 70% of logistical burdens for study teams and patients. The modular and flexible Software as a Service (SaaS) subscription-based model is enriched with many features such as automated enrollment workflows, electronic patient-reported outcomes, 2-way email and SMS communication, uploading of lab reports, and more that are designed to encourage innovators to undertake research activities, rather than be intimidated by the complexity, logistical burdens, duration and costs of the traditional evidence generation approaches.
Quickly setup clinical studies of any scale or duration
Jeeva™ applies an innovative approach to remote screening, eConsent, patient registries and natural history studies can enable the generation of higher-quality, low-cost and more timely evidence generation for clinical trials. Jeeva™ offers a cost-effective solution to quickly set up and conduct clinical studies, of any scale or duration, with or without patient travel involved (e.g. hybrid or fully decentralized clinical trial protocols). Jeeva™ provides a more effective clinical trial design in terms of evidence generation, accelerating patient recruitment, site feasibility and endpoints that bring unmatched efficiencies in terms of the quality of evidence, time, and costs.
#clinical trial protocol software#clinical trials recruitment and retention#patient retention in clinical trials#software used in clinical trials#clinical solutions research platforms#clinical research software#software for clinical trials#software used in clinical research#clinical trials software#clinical study software#decentralized clinical trials software#clinical trial recruitment challenges#mobile eclinical#clinical trial cloud software#eclinical platform#eclinical cloud
2 notes
·
View notes
Text
Global Market for Oncology Informatics - Current Business Trends
According to a new analysis from Grand View Research, the worldwide oncology informatics market is anticipated to expand at a CAGR of 5.7% between 2016 and 2030. This expansion is driven by the increase in cancer incidence rates, the rising expense of cancer treatment, and the trend toward fewer medical errors and hospital readmissions.
North America dominated the global market for oncology-related information in 2015. The market is driven by the increasing frequency of cancer in the region, advances in medical technology, and growing healthcare costs. Nonetheless, the industry faces a variety of restrictions. These disadvantages restrict the market's expansion.
The research evaluates current trends and forecasts future market growth. In addition, it provides information regarding the competitive landscape and critical product positioning. The research also examines the growth potential of various nations. For example, between 2020 and 2030, North America is anticipated to develop at the quickest rate, with a CAGR of XX%.
This market's expansion is hampered by several reasons, including a lack of skilled workers and ethical and legal restraints. Nonetheless, the incorporation of NGS technology into other research initiatives and the rise in cancer patients are anticipated to stimulate market growth.
By end-users, regions, and applications, the market is segmented. Each segment contains a market overview for the predicted period. The increasing incidence of cancer and the aim to reduce medical errors and readmission rates are driving the expansion of the Oncology informatics industry. However, various obstacles may impede the market's expansion. These obstacles include a lack of medical oncologists, the high cost of implementing devices, and data security concerns.
The market for Oncology informatics is moderately fragmented, with several regional competitors vying for market share. As a result, corporations have devised a range of corporate expansion tactics. Among these methods include investing in product development and maintaining competitive pricing. A significant example is a collaboration between Tempus and Precision Health Informatics to improve oncology precision medicine. These advancements are anticipated to support market expansion and generate profitable prospects for industry participants.
The COVID-19 pandemic has had multiple effects on the oncology informatics sector. It has affected the supply chain in numerous nations and slowed numerous procedures. Although supply was quickly restored, the pandemic presented cancer patients with distinct obstacles. For instance, patients were unable to access checkups and cancer medications. In addition, breast and colorectal cancer screening rates have dropped significantly.
The epidemic of COVID-19 has increased the financial strain on healthcare organizations. This has caused a change in how vendors interact with them. This new dynamic has given rise to intense competition among industry participants. Small start-ups are expanding their product lines and emerging as a competitive threat.
From 2020 to 2027, the Global Oncology Informatics Market is anticipated to expand at a moderate rate. This expansion results from rising healthcare expenses, cancer patients, and the increasing adoption of EHRs. The report comprehensively analyzes the market's key segments, drivers, and competitive landscape.
The oncology informatics market is booming due to increased cancer treatment costs, a growing patient population, and the increasing adoption of oncology-specific electronic health records (EHRs). However, concerns about data security and ineffective data integration are the industry's most significant obstacles. With sophisticated cancer informatics solutions, however, it is anticipated that these concerns will be reduced.
The primary segments of the Oncology Informatics Market are surgical oncology and radiation oncology. By 2021, the latter is anticipated to hold the highest market share. Increased electronic health record systems adoption will improve clinicians' work-life balance and increase productivity.
Between 2017 and 2023, the global oncology informatics market is anticipated to expand at a CAGR of 7.2%. This segment's growth is anticipated to be driven by new technologies and test kits. In addition, new market entrants may alter the competitive landscape. To obtain a competitive advantage, new players must concentrate on creating and integrating new technologies and decreasing operational complexity and total costs. In contrast, existing businesses concentrate on product releases and talk with competitors to increase their market share. In such a circumstance, they may not receive the rewards of a successful product launch or financial gain.
In addition, the growing number of cancer patients and the aging of the global population are anticipated to fuel the expansion of this market. Additionally, the adoption of electronic health records and rising investments in life sciences R&D activities are anticipated to contribute to the expansion of this market.
5 notes
·
View notes
Text
Absolute EMS Inc.: Leading the Charge in High-Reliability Manufacturing for Next-Generation Satellites
The aerospace industry is characterized by continuous development. Miniaturization of satellites combined with increasing demand for highly functional and stable satellite systems necessitate a focus on manufacturing solutions. Absolute EMS Inc. is an established EMS manufacturing company that plays a crucial role in enriching the development of next-generation satellites due to its focus on high-reliability manufacturing and serving its customers with exceptional Electronic Manufacturing Services (EMS).
Introduction to High-Reliability Manufacturing
High-reliability manufacturing is a concept of manufacturing that can be described as a process that uses various strategies for the intended purpose of getting the parts in electronics to have a longer life span that conforms to their use and delivers the intended service. We, at Absolute EMS, strongly hold the belief that our clients are entitled to the best and most reliable EMS services in the industry.Concerning quality assurance, it is clear that our company holds itself to high standards that are reflected in each step of the manufacturing processes that involve the selection of the components and established testing techniques.
What is EMS Manufacturing?
Electronic Manufacturing Services (EMS) may refer to a wide variety of services delivered by one company to another within the electronics industry.
Absolute EMS is functioning as a full-service EMS manufacturing company, focused on providing high-quality Made in USA solutions. By extending its core competency to target more sustainable clients, namely the medical industry, Absolute EMS established itself as a leader in niche services.
At Absolute EMS, it is our goal to strive as the perfect EMS partner where we provide prototype to full turn-key, miniaturization & mid to high volume assembly services.
These services typically include:
Printed Circuit Board (PCB) Assembly
Box Build Assembly
Testing and Inspection
Supply Chain Management
Programming and Customization:
Absolute EMS and High-Reliability Manufacturing
High-reliability manufacturing is a specialized subset of EMS focusing on producing electronic devices with minimal failure rates. These devices are often mission-critical, meaning their failure could have severe consequences. They are typically used in industries like:
Medical Devices: Medical device manufacturing
Military and Defense: Avionics, communication equipment, and weapons systems.
Aerospace: Control systems, navigation equipment, and communication devices.
Industrial Automation: Control systems, Robotics, Networking, and Semiconductor for critical infrastructure and manufacturing processes.
Absolute EMS caters to these demanding industries by prioritizing several key aspects of their manufacturing processes:
Advanced Manufacturing Techniques: 4.0 touchless manufacturing line with built-in automated optical inspection stations throughout the process which increases the quality of work produced.
Process Control and Repeatability: The key elements of effective manufacturing processes include following manufacturing procedure manuals. It enables the exact repetition of admired production runs and also reduces the possibility of making some errors in the process.
Material Traceability: Preservation of records of all such components that were used in each of the devices so that in case of any failure in any of the components, the faulty component can be replaced.
Highly Skilled Workforce: Hiring technical staff that has adequate experience and relevant qualifications to the job and putting them to task with high production standards.
Continuous Improvement and Innovation
Absolute EMS has been driven by the principles of ongoing improvement and reinvention in the field of high-reliability manufacturing. We are committed to working hand in hand with our clientele and other stakeholders in the market to meet or even go a step further in adapting to innovations in technology or higher reliability requirements. This creates the systems to develop new materials and processes, as well as test methods, that when combined help us produce products that achieve the highest reliability standards.
Quality Assurance: The Bedrock of High-Reliability Manufacturing
Absolute EMS has made a name for itself as a leading provider of EMS manufacturing services by rigorously focusing on sustaining consistent quality throughout its manufacturing processes. They have established magnificent quality control measures and are certified to meet international standards including the ISO 9001 and AS 9100 for use in aerospace companies. This means that there is a careful examination of the product for defects and this is done throughout the manufacturing process with the help of tools such as the AOI or the X-ray checker. In addition, another strength that Absolute EMS highlights is the application of Statistical Process Control, which enables the firm to perpetually refine its manufacturing procedures while ensuring that the satellites being churned out are high quality, dependable, and can withstand rigorous use and often harsh environments commonly associated with space science and telecommunications.
The Final Say:
In conclusion, Absolute EMS is committed to being a company that provides EMS solutions that adhere to the highest level of reliability in manufacturing electronic products. Through the client-oriented approach and employing state-of-the-art materials, innovative design techniques, extensive testing procedures, and constant improvement in the company, we guarantee that customers will get the best quality and reliable products.
Come to Absolute EMS for high-reliability manufacturing services and let us show you just how much better business can be when your service provider serves as an extension of your team.
0 notes
Text
Transforming Healthcare: The Rise of the Healthcare Automation Market
Healthcare automation is rapidly transforming the medical landscape, streamlining operations, and enhancing patient care. This dynamic market is driven by technological advancements, increasing demand for efficient healthcare services, and the need to reduce costs while improving outcomes. As healthcare systems worldwide grapple with the challenges of rising patient numbers and limited resources, automation presents a viable solution to optimize processes and ensure high-quality care.
Technological Innovations Driving Growth
One of the main drivers of the healthcare automation market is the relentless pace of technological innovation. Advanced technologies such as artificial intelligence (AI), machine learning (ML), robotic process automation (RPA), and the Internet of Things (IoT) are revolutionizing healthcare delivery. These technologies enable healthcare providers to automate routine tasks, reduce administrative burdens, and enhance clinical decision-making.
For instance, AI-powered diagnostic tools can analyze medical images with remarkable accuracy, often surpassing human performance. Machine learning algorithms can predict patient outcomes and recommend personalized treatment plans, improving the precision of care. Robotic systems are being used for surgeries, providing unparalleled precision and reducing recovery times. Additionally, IoT devices facilitate remote patient monitoring, allowing healthcare providers to track vital signs and manage chronic conditions more effectively.
For a comprehensive analysis of the market drivers, visit https://univdatos.com/report/healthcare-automation-market/
Efficiency and Cost Reduction
The integration of automation in healthcare systems significantly improves operational efficiency. By automating repetitive tasks such as appointment scheduling, billing, and patient record management, healthcare providers can free up valuable time for medical staff to focus on patient care. Electronic health records (EHRs) and automated data entry systems reduce the likelihood of errors, ensuring more accurate and reliable patient information.
Moreover, automation helps in reducing healthcare costs. Automated systems streamline workflows, minimize waste, and optimize resource allocation. For example, inventory management systems use real-time data to track medical supplies, preventing overstocking and reducing waste. In laboratories, automated equipment can process large volumes of tests quickly and accurately, lowering labor costs and increasing throughput.
Enhanced Patient Care and Experience
Healthcare automation not only benefits providers but also significantly enhances patient care and experience. Automated systems facilitate seamless communication between patients and healthcare providers, improving accessibility and engagement. Telemedicine platforms, for instance, enable patients to consult with doctors remotely, eliminating the need for travel and reducing waiting times.
Automation also plays a crucial role in patient monitoring and management. Wearable devices and remote monitoring tools collect real-time health data, allowing for continuous monitoring of patients with chronic conditions. This real-time data enables early detection of potential health issues and timely interventions, ultimately improving patient outcomes.
Furthermore, automated systems ensure better medication management. Automated dispensing systems and smart medication management platforms reduce the risk of errors in prescribing and administering medications, enhancing patient safety. These systems also provide reminders and alerts to patients, ensuring adherence to treatment plans.
Regulatory Compliance and Data Security
The healthcare industry is highly regulated, and compliance with various standards and regulations is crucial. Automation helps healthcare providers adhere to regulatory requirements more effectively. Automated systems can track and document compliance-related activities, generate necessary reports, and ensure that all processes align with regulatory guidelines.
Data security is another critical aspect of healthcare automation. With the increasing digitization of patient records and health data, ensuring data privacy and security is paramount. Advanced encryption techniques, secure data storage solutions, and automated access control systems protect sensitive patient information from unauthorized access and cyber threats.
Challenges and Future Outlook
Despite the numerous benefits, the healthcare automation market faces several challenges. High implementation costs, especially for small and medium-sized healthcare providers, can be a significant barrier. Additionally, there is a need for comprehensive training programs to ensure that healthcare staff can effectively use automated systems.
Interoperability is another challenge, as different automation systems and devices need to seamlessly integrate with existing healthcare infrastructure. Standardization of technologies and protocols is essential to address this issue.
For a sample report, visit https://univdatos.com/get-a-free-sample-form-php/?product_id=22641
Looking ahead, the healthcare automation market is poised for continued growth. Ongoing advancements in AI, machine learning, and other technologies will further enhance the capabilities of automated systems. As healthcare providers increasingly recognize the value of automation in improving efficiency, reducing costs, and enhancing patient care, the adoption of automation technologies is expected to accelerate.
In conclusion, healthcare automation is revolutionizing the medical industry, offering numerous benefits to providers and patients alike. With continued innovation and increased adoption, the healthcare automation market is set to play a pivotal role in shaping the future of healthcare, ensuring more efficient, cost-effective, and high-quality care for all.
Contact Us:
UnivDatos Market Insights
Email - [email protected]
Contact Number - +1 9782263411x
Website -www.univdatos.com
#Healthcare Automation Market#Healthcare Automation Market Size#Healthcare Automation Market Growth#Healthcare Automation Market Forecast
0 notes
Text
Medication Management Market Worldwide Industry Analysis, Future Demand and Forecast till 2032

The Medication Management Market Size is expected to grow from USD 5.23 billion in 2023 to USD 11.45 billion by 2032, at a CAGR of 9.1% during the forecast period (2024-2032).
The market for medication management includes a wide range of services and technologies intended to guarantee that patients receive their medications in a secure and efficient manner. Medication administration records (MARs), automated dispensing systems, clinical decision support systems (CDSS), computerized physician order entry (CPOE), electronic health records (EHRs), and pharmacy information systems are all included in this sector. Reducing prescription errors, improving patient safety, and improving overall health outcomes are the main objectives.
By 2024, the market will have grown significantly due to a number of important factors. The aging of the world's population, the rising incidence of chronic illnesses, and the ensuing increase in the quantity of prescription drugs written are all important factors. The industry has been further driven by technological breakthroughs like the use of machine learning (ML) and artificial intelligence (AI) in medication management systems. These technologies allow for more accurate and customized drug regimens, better operational efficiencies for healthcare professionals, and predictive analytics for possible adverse drug events. The government's efforts and regulatory frameworks to enhance patient safety and healthcare quality are also propelling market expansion.
Get Full PDF Sample Copy of Report: (Including Full TOC, List of Tables & Figures, Chart) @
https://introspectivemarketresearch.com/request/9799
Updated Version 2024 is available our Sample Report May Includes the:
Scope For 2024
Brief Introduction to the research report.
Table of Contents (Scope covered as a part of the study)
Top players in the market
Research framework (structure of the report)
Research methodology adopted by Worldwide Market Reports
Leading players involved in the Medication Management Market include:
Omnicell (USA), Cerner Corporation (USA), McKesson Corporation (USA), BD (Becton, Dickinson and Company) (USA), Swisslog Healthcare (Switzerland), Parata Systems (USA), ScriptPro (USA), Capsa Healthcare (USA), MedMinder Systems (USA), Talyst (USA), CareFusion (USA), Aethon (USA), Tabula Rasa HealthCare (USA)
Moreover, the report includes significant chapters such as Patent Analysis, Regulatory Framework, Technology Roadmap, BCG Matrix, Heat Map Analysis, Price Trend Analysis, and Investment Analysis which help to understand the market direction and movement in the current and upcoming years.
If You Have Any Query Medication Management Market Report, Visit:
https://introspectivemarketresearch.com/inquiry/9799
Segmentation of Medication Management Market:
By Type
Computerized provider order entry (CPOE)
Clinical decision support systems (CDSS)
Electronic Medication Administration Record
Automated Dispensing
Others
By Delivery Mode
On-premise
Cloud-based
By End-User
Hospitals
Pharmacies
Others
An in-depth study of the Medication Management industry for the years 2024–2032 is provided in the latest research. North America, Europe, Asia-Pacific, South America, the Middle East, and Africa are only some of the regions included in the report's segmented and regional analyses. The research also includes key insights including market trends and potential opportunities based on these major insights. All these quantitative data, such as market size and revenue forecasts, and qualitative data, such as customers' values, needs, and buying inclinations, are integral parts of any thorough market analysis.
Market Segment by Regions: -
North America (US, Canada, Mexico)
Eastern Europe (Bulgaria, The Czech Republic, Hungary, Poland, Romania, Rest of Eastern Europe)
Western Europe (Germany, UK, France, Netherlands, Italy, Russia, Spain, Rest of Western Europe)
Asia Pacific (China, India, Japan, South Korea, Malaysia, Thailand, Vietnam, The Philippines, Australia, New Zealand, Rest of APAC)
Middle East & Africa (Turkey, Bahrain, Kuwait, Saudi Arabia, Qatar, UAE, Israel, South Africa)
South America (Brazil, Argentina, Rest of SA)
Key Benefits of Medication Management Market Research:
Research Report covers the Industry drivers, restraints, opportunities and challenges
Competitive landscape & strategies of leading key players
Potential & niche segments and regional analysis exhibiting promising growth covered in the study
Recent industry trends and market developments
Research provides historical, current, and projected market size & share, in terms of value
Market intelligence to enable effective decision making
Growth opportunities and trend analysis
Covid-19 Impact analysis and analysis to Medication Management market
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
Acquire This Reports: -
https://introspectivemarketresearch.com/checkout/?user=1&_sid=9799
About us:
Introspective Market Research (introspectivemarketresearch.com) is a visionary research consulting firm dedicated to assist our clients grow and have a successful impact on the market. Our team at IMR is ready to assist our clients flourish their business by offering strategies to gain success and monopoly in their respective fields. We are a global market research company, specialized in using big data and advanced analytics to show the bigger picture of the market trends. We help our clients to think differently and build better tomorrow for all of us. We are a technology-driven research company, we analyze extremely large sets of data to discover deeper insights and provide conclusive consulting. We not only provide intelligence solutions, but we help our clients in how they can achieve their goals.
Contact us:
Introspective Market Research
3001 S King Drive,
Chicago, Illinois
60616 USA
Ph no: +1 773 382 1049
Email: [email protected]
#Medication Management#Medication Management Market#Medication Management Market Size#Medication Management Market Share#Medication Management Market Growth#Medication Management Market Trend#Medication Management Market segment#Medication Management Market Opportunity#Medication Management Market Analysis 2024
0 notes
Text
Revolutionizing Healthcare: The Impact of Blockchain Technology in Digital Healthcare Market

Introduction:
In the ever-evolving landscape of healthcare, technological advancements continue to reshape the industry, offering innovative solutions to age-old challenges. Among these advancements, blockchain technology stands out as a transformative force, promising enhanced security, transparency, and efficiency across the healthcare ecosystem. This article delves into the burgeoning field of blockchain technology in healthcare, exploring its applications, benefits, and recent developments shaping the digital healthcare market.
According to Next Move Strategy Consulting, the global Digital Healthcare Market is predicted to reach USD 1043.39 billion by 2030 with a CAGR of 23.3% from 2024-2030.
Download FREE Sample: https://www.nextmsc.com/digital-healthcare-market/request-sample
Understanding Blockchain Technology: At its core, blockchain is a decentralized, distributed ledger technology that enables secure and transparent transactions without the need for intermediaries. Each transaction, or "block," is securely linked to the previous one, forming a chronological chain of data. This immutable and transparent nature of blockchain makes it an ideal solution for various healthcare applications, including medical records management, supply chain integrity, and patient data security.
Inquire before buying: https://www.nextmsc.com/digital-healthcare-market/inquire-before-buying
Applications in Healthcare:
Medical Records Management: Blockchain technology offers a decentralized and secure platform for managing electronic health records (EHRs). By storing patient data on a distributed ledger, healthcare providers can ensure data integrity, interoperability, and accessibility while maintaining patient privacy and security. This streamlined approach to medical records management reduces administrative burdens, minimizes errors, and improves patient outcomes.
Supply Chain Integrity: With the rise of counterfeit drugs and medical devices, ensuring the integrity of the healthcare supply chain is paramount. Blockchain enables end-to-end visibility and traceability of pharmaceuticals, medical devices, and other healthcare products, from manufacturing to delivery. By recording each transaction and verifying the authenticity of products, blockchain mitigates the risk of counterfeit goods, enhances regulatory compliance, and safeguards patient safety.
Patient Data Security: Protecting sensitive patient data from unauthorized access and breaches is a top priority for healthcare organizations. Blockchain technology employs cryptographic techniques and consensus algorithms to secure patient data, ensuring confidentiality, integrity, and availability. By decentralizing data storage and utilizing smart contracts, blockchain minimizes the risk of data breaches and empowers patients to control access to their health information.
Streamlined Claims Processing: Blockchain technology streamlines the cumbersome process of insurance claims processing in healthcare. By automating claim verification, adjudication, and payment through smart contracts, blockchain reduces administrative overhead, minimizes fraud, and accelerates reimbursement cycles. This efficiency not only lowers costs for payers and providers but also enhances the overall patient experience by expediting claims settlement.
Clinical Trials and Research: The integration of blockchain technology in clinical trials and research holds immense potential for advancing medical discoveries and treatments. Blockchain facilitates secure and transparent sharing of clinical trial data among researchers, sponsors, and regulatory bodies, ensuring data integrity and compliance. Additionally, blockchain-based platforms enable patients to contribute their health data to research initiatives securely, fostering collaboration and accelerating the pace of medical innovation.
Prescription Drug Monitoring: Blockchain enhances the monitoring and tracking of prescription drugs, particularly controlled substances prone to misuse and diversion. By recording prescription transactions on a tamper-proof ledger, blockchain helps healthcare providers and regulatory agencies detect and prevent opioid abuse, counterfeit medications, and unauthorized dispensing. This real-time visibility into prescription drug movements improves patient safety and regulatory compliance while combating the opioid epidemic.
Identity Management and Verification: Identity management is a critical aspect of healthcare, encompassing patient registration, authentication, and consent management. Blockchain-based identity solutions offer a decentralized and secure method for managing patient identities, reducing identity theft, and ensuring data accuracy. Through digital identity verification and authentication, blockchain enhances patient trust, facilitates seamless interoperability, and enables frictionless access to healthcare services across providers and systems.
Telemedicine and Remote Patient Monitoring: The advent of telemedicine and remote patient monitoring has transformed healthcare delivery, particularly in remote or underserved areas. Blockchain technology enhances the security and privacy of telemedicine platforms by encrypting patient communications, maintaining audit trails of medical consultations, and securing IoT devices used for remote monitoring. This secure infrastructure not only protects patient data but also fosters the widespread adoption of telehealth solutions, improving access to quality care and reducing healthcare disparities.Top of Form
Recent Developments: The adoption of blockchain technology in healthcare is gaining momentum, with recent developments signaling its growing significance in the digital healthcare market:
Collaboration and Partnerships: Major healthcare organizations, technology firms, and government agencies are collaborating to harness the potential of blockchain technology. For instance, IBM Watson Health partnered with the FDA to explore blockchain solutions for secure sharing of patient data. Similarly, pharmaceutical giants like Pfizer and Merck are collaborating with blockchain startups to enhance supply chain transparency and drug traceability.
Regulatory Initiatives: Regulatory bodies worldwide are recognizing the importance of blockchain technology in healthcare and taking steps to foster its adoption. In the United States, the Health Information Technology for Economic and Clinical Health (HITECH) Act encourages the adoption of electronic health records, paving the way for blockchain-based solutions. Additionally, the European Union's General Data Protection Regulation (GDPR) emphasizes the importance of data protection and privacy, aligning with blockchain's principles of transparency and data control.
Pilot Projects and Implementations: Healthcare providers and institutions are piloting blockchain-based solutions to address specific challenges within the industry. For example, MedRec, a blockchain platform developed by researchers at MIT, aims to improve the interoperability and security of electronic medical records. Similarly, the Estonian e-Health Foundation implemented blockchain technology to secure health records and facilitate data exchange between healthcare providers and patients.
Conclusion:
As the digital healthcare market continues to evolve, blockchain technology emerges as a disruptive force, offering transformative solutions to longstanding issues in healthcare. From enhancing data security and interoperability to improving supply chain integrity and patient outcomes, blockchain holds immense promise for the future of healthcare. With ongoing research, collaboration, and regulatory support, blockchain technology is poised to revolutionize the way healthcare is delivered, ensuring a safer, more efficient, and patient-centric ecosystem.
0 notes
Text
Unlocking Innovation: Transformative Solutions in the Life Sciences Industry 🌟🔬
The life sciences industry, encompassing pharmaceuticals, biotechnology, medical devices, and more, is at the forefront of addressing global health challenges. With the increasing complexity of diseases and the demand for personalized medicine, innovative solutions are essential to propel the industry forward. These solutions range from advancements in technology and data analytics to novel research methodologies and regulatory strategies.
1. Leveraging Advanced Technology 🤖💊
One of the most significant drivers of innovation in the life sciences industry is the integration of advanced technology. Artificial intelligence (AI) and machine learning (ML) are revolutionizing drug discovery and development. By analyzing vast datasets, AI can identify potential drug candidates more efficiently than traditional methods. For instance, AI algorithms can predict how different compounds will interact with biological targets, significantly reducing the time and cost involved in bringing new drugs to market.

Moreover, the rise of digital health tools, such as wearable devices and mobile health apps, is transforming patient care. These tools enable real-time monitoring of patients' health conditions, facilitating early diagnosis and personalized treatment plans. This shift towards digital health not only improves patient outcomes but also enhances the efficiency of healthcare systems.
2. Data-Driven Insights 📊🔍
The explosion of big data in life sciences offers unprecedented opportunities to gain deeper insights into disease mechanisms and treatment responses. Advanced analytics and bioinformatics tools are crucial in interpreting complex biological data. For example, genomic sequencing generates massive amounts of data that require sophisticated algorithms to decode. These insights can lead to the development of targeted therapies tailored to individual genetic profiles, marking a significant step towards precision medicine.
Clinical trials, a critical phase in drug development, also benefit from data-driven approaches. Utilizing electronic health records (EHRs) and real-world evidence (RWE), researchers can design more effective and inclusive clinical trials. This approach not only accelerates the trial process but also ensures that new treatments are safe and effective for diverse patient populations.

3. Innovative Research Methodologies 🧪🤝
Collaborative research models are emerging as a powerful solution to accelerate innovation in life sciences. Public-private partnerships, such as those seen during the COVID-19 pandemic, exemplify how collaboration can lead to rapid development and distribution of vaccines. These partnerships leverage the strengths of various stakeholders, including academic institutions, government agencies, and private companies, to drive breakthrough innovations.
Additionally, the adoption of agile research methodologies allows for more flexible and adaptive research processes. Agile methodologies enable researchers to quickly pivot and respond to new findings or challenges, ensuring continuous progress in scientific discovery.
4. Navigating Regulatory Landscapes 🛤️✔️
Navigating the complex regulatory landscape is crucial for bringing new life sciences products to market. Innovative regulatory strategies, such as adaptive licensing and accelerated approval pathways, are designed to expedite the approval process for promising therapies. These strategies involve close collaboration with regulatory authorities to ensure that new treatments meet safety and efficacy standards while reaching patients faster.

Conclusion 🌍✨
The life sciences industry is experiencing a transformative era driven by technological advancements, data analytics, collaborative research, and innovative regulatory strategies. These solutions not only enhance the development and delivery of new treatments but also pave the way for a future where personalized medicine and digital health are integral to patient care. As the industry continues to evolve, embracing these innovations will be key to addressing the ever-growing healthcare challenges and improving global health outcomes.
#life science solutions#laboratory relocation services#global life sciences industry#life science laboratory services
1 note
·
View note
Text
DrCloudEHR: Revolutionizing Healthcare in Idaho with Cutting-Edge EHR Solutions
In the rapidly evolving landscape of healthcare, the adoption of advanced electronic medical record systems in Idaho is transforming how medical professionals manage patient information and deliver care. At the forefront of this transformation is DrCloudEHR, a leading provider dedicated to implementing state of the art electronic patient record in Idaho solutions. This article delves into the pivotal role DrCloudEHR plays in enhancing healthcare through an innovative electronic health records platform in Idaho. Their comprehensive approach ensures that digital health records in Idaho are seamlessly integrated across various medical practices, improving efficiency and patient outcomes.
DrCloudEHR's commitment to mental health solutions in Idaho is evident in their specialized modules that cater to mental health professionals, facilitating better patient management. Moreover, the electronic health records in hospitals in Idaho offer robust functionalities for large-scale medical facilities. Addressing the needs of addiction treatment centers, DrCloudEHR provides tailored substance abuse EHR in Idaho, ensuring comprehensive care for individuals struggling with substance abuse. By leading the charge in the implementation of a modern medical electronic record system in Idaho, DrCloudEHR is setting new standards in the healthcare industry.
.
The Rise of Digital Health Records in Idaho
With the increasing need for efficient and accurate patient data management, digital health records in Idaho have become a critical component of modern healthcare systems. DrCloudEHR's comprehensive solutions ensure that healthcare providers can easily access and update patient records, leading to improved patient outcomes and streamlined operations. The electronic health records in hospitals in Idaho supported by DrCloudEHR offer seamless integration across various departments, fostering a cohesive healthcare environment.
DrCloudEHR excels in providing solutions for substance abuse EHR in Idaho, ensuring that specialized care is both effective and well-documented. Their electronic health records platform and mental health solutions in Idaho are designed to meet the diverse needs of healthcare providers, offering flexibility and scalability. Additionally, DrCloudEHR’s medical EMR systems in Idaho enhance data accuracy and accessibility. As a leading electronic health record vendor in Idaho, DrCloudEHR ensures a smooth transition to digital systems.
The company’s role as an electronic medical record vendor in Idaho is pivotal in advancing healthcare technology. By adopting DrCloudEHR’s electronic health record in Idaho, healthcare providers can deliver superior patient care. The electronic health record platforms in Idaho offered by DrCloudEHR are robust and user-friendly, supporting various healthcare settings. Their EHR platform in Idaho facilitates better clinical decision-making and improved patient care.
Addressing Mental Health and Substance Abuse
DrCloudEHR supports substance abuse EHR in Idaho through its comprehensive medical electronic record system in Idaho. The medical EMR systems in Idaho provided by DrCloudEHR are integrated seamlessly, ensuring that both mental health and substance abuse treatments are managed effectively. As a leading electronic health record vendor in Idaho, DrCloudEHR ensures robust support and innovative solutions. Their reputation as a top electronic medical record vendor in Idaho further solidifies their position in the market. The electronic health record in Idaho solutions offered by DrCloudEHR are part of their extensive electronic health record platforms in Idaho, designed to cater to diverse healthcare needs. These ehr solutions are essential for advancing mental health and substance abuse care in the region.
These electronic medical record systems in Idaho are designed to support the specific requirements of substance abuse treatment centers, ensuring comprehensive documentation and improved patient management. By leveraging these specialized electronic patient records in Idaho, Idaho’s healthcare providers can deliver more effective and personalized care to individuals struggling with substance abuse. The electronic patient record in Idaho managed by these systems ensures that all relevant data is accurately recorded and easily accessible.
Additionally, the integration of digital health records in Idaho facilitates the seamless sharing of information among healthcare professionals, enhancing the overall quality of care. These systems are also part of electronic health records in hospitals in Idaho, ensuring that substance abuse treatment is well-coordinated with other medical services. DrCloudEHR's approach to mental health solutions in Idaho further supports comprehensive care for patients with co-occurring mental health and substance abuse issues.
Comprehensive EHR Platforms
DrCloudEHR's electronic health records platform in Idaho is designed to cater to a wide array of medical specialties and practices. These platforms offer a flexible and scalable solution, allowing healthcare providers to customize their systems according to their specific needs. Whether it’s a small clinic or a large hospital, DrCloudEHR's electronic health record platforms in Idaho provide the necessary tools to enhance operational efficiency and patient care.
Choosing the Right EHR Vendor
Selecting the right electronic health record vendor in Idaho is crucial for the successful implementation and utilization of quality management system and EHR systems. DrCloudEHR stands out as a trusted partner for healthcare providers, offering comprehensive support and seamless integration. The company’s expertise as an electronic medical record vendor in Idaho ensures that healthcare facilities can transition smoothly to digital systems with minimal disruption to their operations.
Enhancing Medical Electronic Record Systems
The medical electronic record system in the Idaho landscape is continually evolving, and DrCloudEHR is at the forefront of these advancements. Their solutions are designed to meet the stringent requirements of modern healthcare, providing robust security features to protect patient data. By adopting DrCloudEHR’s medical EMR systems in Idaho, healthcare providers can ensure compliance with regulatory standards while benefiting from improved data accuracy and accessibility.
Benefits of EHR Adoption
The adoption of electronic health record in Idaho brings numerous benefits to both healthcare providers and patients. For providers, the shift to digital records means reduced paperwork, easier access to patient information, and enhanced coordination of care. Patients, on the other hand, benefit from more accurate diagnoses, timely treatments, and better overall healthcare experiences.
Conclusion
As the healthcare industry in Idaho continues to embrace digital transformation, DrCloudEHR remains a pivotal player in this journey. Their advanced electronic medical record systems in Idaho and specialized solutions for mental health and substance abuse are setting new standards in patient care. By providing a flexible and scalable electronic health records platform in Idaho, DrCloudEHR empowers healthcare providers to deliver superior care while enhancing operational efficiency. The company’s role as a leading electronic health record vendor in Idaho underscores its dedication to improving healthcare outcomes through cutting-edge technology and comprehensive support.
For healthcare providers looking to transition to or upgrade their electronic health record in Idaho systems, DrCloudEHR offers a reliable and effective solution. With a focus on improving patient care and optimizing healthcare operations, DrCloudEHR is transforming the landscape of electronic health record management and medical electronic record system in Idaho, making it a cornerstone of modern healthcare in the region.
0 notes
Text
HIPAA Innovators: Unleashing Healthcare's Interoperable Revolution
The Health Insurance Portability and Accountability Act (HIPAA) was enacted in 1996 to safeguard patient data and ensure the privacy and security of health information. Over the years, HIPAA has significantly influenced the healthcare industry, shaping how organizations manage patient information, enhance security measures, and adopt new technologies. This article explores HIPAA's impact on the healthcare industry, providing relevant statistics and insights from various sources.
Strengthening Patient Privacy and Security
HIPAA's primary objective is to protect patient information from unauthorized access and breaches. According to the HIPAA Journal, since the implementation of HIPAA, healthcare organizations have improved their data protection measures, reducing the risk of data breaches. A report from the Department of Health and Human Services (HHS) reveals that in 2020 alone, there were 642 healthcare data breaches affecting over 29.3 million individuals. This highlights the critical need for stringent data security protocols mandated by HIPAA.
Enhancing Trust and Patient Confidence
The enactment of HIPAA has fostered greater trust between patients and healthcare providers. Patients are more confident that their personal and medical information is handled with the utmost care and confidentiality. According to a survey by the American Medical Association (AMA), 87% of patients believe that their health information is protected due to HIPAA regulations. This increased trust encourages patients to share sensitive information with their healthcare providers, leading to better diagnosis and treatment outcomes.
Impact on Healthcare Technology and Telemedicine
HIPAA has also significantly impacted the adoption and development of healthcare technology, particularly in the realm of telemedicine. Telemedicine apps for doctors must comply with HIPAA regulations to ensure patient data privacy and security. The growth of telemedicine, accelerated by the COVID-19 pandemic, has been supported by HIPAA-compliant solutions, allowing healthcare providers to offer remote consultations and services.
A report by Mobisoft Infotech highlights that the demand for custom telemedicine app development solutions has surged, with an estimated market growth rate of 23.5% from 2020 to 2027. HIPAA compliance is a critical factor in this growth, as it reassures patients and providers that their interactions and data are secure.
Compliance and Administrative Burden
While HIPAA has brought numerous benefits, it has also introduced compliance challenges for healthcare organizations. Ensuring compliance with HIPAA regulations requires significant administrative effort and resources. Healthcare providers must implement comprehensive training programs, regular audits, and robust security measures to remain compliant.
A study by Secureframe found that healthcare organizations spend an average of $8.3 million annually on compliance activities. Despite the financial and administrative burden, these efforts are essential to protect patient information and avoid hefty fines for non-compliance. The HHS has issued fines totaling over $130 million since 2003 for HIPAA violations, emphasizing the importance of adherence to these regulations.
Benefits of HIPAA for the Healthcare Industry
HIPAA has played a crucial role in transforming the healthcare industry by introducing standards that enhance efficiency, combat fraud, and ensure continuity of health insurance coverage for individuals between jobs. The legislation has provided a clear set of rules for healthcare professionals to follow regarding the protection of patient data, leading to improved care delivery and patient outcomes. Additionally, HIPAA has facilitated the transition from paper-based medical records to Electronic Health Records (EHRs), enabling secure access to patient information, enhancing care coordination, and reducing medical errors.
Promoting Innovation and Interoperability
HIPAA has also driven innovation in healthcare technology by promoting the adoption of interoperable systems. The need to comply with HIPAA standards has led to the development of secure EHR systems and other digital health solutions. These innovations facilitate the seamless exchange of information between healthcare providers, enhancing the quality of care and patient outcomes.
The HHS Office of the National Coordinator for Health Information Technology (ONC) reports that 86% of office-based physicians and 96% of hospitals use EHR systems. The interoperability of these systems, driven by HIPAA standards, allows for better coordination and continuity of care, especially in complex cases requiring multiple healthcare providers.
Challenges and Criticisms
Despite its benefits, HIPAA has faced criticism for its complexity and the burden it places on healthcare organizations. Critics argue that the stringent requirements can be overwhelming, particularly for smaller practices with limited resources. The complexity of HIPAA regulations can also lead to inadvertent violations, resulting in penalties that could financially strain healthcare providers.
Moreover, the rapid advancement of technology presents ongoing challenges for HIPAA compliance. As new technologies emerge, healthcare organizations must continuously update their systems and protocols to remain compliant. This dynamic landscape requires ongoing education and adaptation, which can be resource-intensive.
Future Outlook
As the healthcare industry continues to evolve, HIPAA will remain a cornerstone of patient privacy and data security. The rise of telemedicine, driven by the need for remote healthcare services, will further emphasize the importance of HIPAA-compliant telemedicine app development services. Healthcare providers and technology developers must collaborate to create innovative solutions that meet HIPAA standards while addressing the growing demand for digital health services.
In the future, HIPAA regulations may need to adapt to address new challenges posed by emerging technologies such as artificial intelligence (AI) and the Internet of Things (IoT). These technologies have the potential to revolutionize healthcare but also introduce new risks to patient data privacy and security. Policymakers must balance innovation with robust data protection measures to ensure that the benefits of these technologies are realized without compromising patient trust.
Conclusion
HIPAA has profoundly impacted the healthcare industry by enhancing patient privacy, promoting trust, and driving the adoption of secure healthcare and life science technologies. While it presents compliance challenges, the benefits of HIPAA in protecting patient information and fostering innovation are undeniable. As the industry continues to embrace digital health solutions, HIPAA will play a crucial role in ensuring that patient data remains secure in an increasingly interconnected world. The ongoing evolution of HIPAA regulations will be essential to address new technological advancements and maintain the integrity of patient information.
#telehealth#hire developers#hire app developer#mobile app development#hire mobile app developers#telemedicine
0 notes
Text
Product Design and Development Companies in Chennai: Innovating for the Future
Chennai, known for its robust industrial base and thriving technology sector, is home to numerous product design and development companies. These firms play a pivotal role in bringing innovative products to market, leveraging advanced technology, creative design, and efficient development processes. Whether you're a startup looking to develop a new product or an established business seeking to innovate, Chennai's product design and development companies in Chennai offer a range of services to meet your needs.
Key Services Offered
Concept Development:

Market Research: Understanding market needs and trends to identify opportunities for new products.
Ideation: Generating innovative ideas through brainstorming sessions and creative techniques.
Feasibility Studies: Assessing the technical, financial, and market feasibility of new product ideas.
Design and Prototyping:
Industrial Design: Creating aesthetically pleasing and functional designs that meet user needs and preferences.
CAD Modeling: Using Computer-Aided Design (CAD) software to create detailed 3D models of the product.
Prototyping: Developing physical prototypes to test and refine product concepts before full-scale production.
Engineering and Development:
Mechanical Engineering: Designing and developing the mechanical components of the product.
Electrical Engineering: Integrating electronic systems and ensuring product functionality.
Software Development: Creating embedded software or applications required for product operation.
Testing and Validation:
Quality Assurance: Conducting rigorous testing to ensure the product meets all quality and performance standards.
Regulatory Compliance: Ensuring the product complies with relevant industry regulations and standards.
User Testing: Gathering feedback from potential users to refine and improve the product.
Manufacturing and Production Support:
Manufacturing Planning: Developing plans for efficient and cost-effective production.
Supplier Management: Coordinating with suppliers to source high-quality materials and components.
Production Oversight: Supervising the manufacturing process to ensure product quality and consistency.
Leading Companies in Chennai
Tata Elxsi:
A well-established name in the field, Tata Elxsi offers comprehensive product design and development services, including industrial design, engineering, and prototyping. They serve various industries, including automotive, healthcare, and consumer electronics.
HCL Technologies:
Known for its innovation and technological expertise, HCL Technologies provides end-to-end product development solutions. Their services encompass concept development, design, engineering, and digital transformation.
Mistral Solutions:
Specializing in embedded design and development, Mistral Solutions offers services ranging from concept development to product realization. Their expertise includes defense, aerospace, and medical devices.
SrinSoft Technologies:
SrinSoft Technologies provides a range of services, including CAD/CAM/CAE solutions, product design, and development. They focus on delivering high-quality, cost-effective solutions tailored to client needs.
Scope T&M:
Scope T&M offers product design and development services with a focus on innovation and technology. Their offerings include hardware design, software development, and testing services.
Choosing the Right Company
Expertise and Experience: Look for companies with a proven track record in your industry and extensive experience in product design and development.
Comprehensive Services: Choose a firm that offers a full range of services, from concept development to production support, ensuring a seamless development process.
Innovation and Technology: Select a company that leverages the latest technologies and innovative approaches to deliver cutting-edge solutions.
Client Testimonials: Check client reviews and testimonials to gauge the company's reliability, quality of service, and customer satisfaction.
Conclusion
Chennai's product design and development companies are at the forefront of innovation, providing businesses with the expertise and resources needed to bring new products to market successfully. By partnering with a reputable firm, you can ensure that your product development process is efficient, innovative, and aligned with market needs. Whether you are developing a new consumer gadget, a medical device, or an industrial tool, these companies offer the services and expertise to turn your vision into reality.
For more info. Visit us:
mobile app development companies in Chennaiiot product development companies in Chennai
#iot product development companies in Chennai#iot application development companies in Chennai#iot hardware development companies in Chennai
0 notes
Text
20 Facts about the Pharmaceutical Industry Post AI Revolution
Introduction:
The pharmaceutical industry has undergone a profound transformation with the advent of Artificial Intelligence (AI). AI-powered technologies have revolutionized drug discovery, clinical trials, personalized medicine, and many other aspects of healthcare. In this article, we will explore ten key facts about how AI is reshaping the pharmaceutical landscape.

Personalized Medicine: One of the most significant impacts of AI in the pharmaceutical industry is the advancement of personalized medicine. By leveraging AI algorithms to analyze vast amounts of patient data, including genomic information, lifestyle factors, and medical history, healthcare providers can tailor treatments to individual patients. This approach improves treatment efficacy, reduces adverse reactions, and enhances patient outcomes.
Drug Discovery Acceleration: AI has dramatically accelerated the drug discovery process. Traditional methods of drug discovery are time-consuming and costly, with high rates of failure. AI algorithms can analyze massive datasets to identify potential drug candidates, predict their efficacy, and optimize their chemical structures. This enables pharmaceutical companies to bring new drugs to market faster and more efficiently than ever before.
Precision Drug Manufacturing: AI is also revolutionizing drug manufacturing processes. By implementing AI-driven optimization techniques, pharmaceutical companies can improve the quality and consistency of their products while reducing production costs and minimizing waste. This enables them to deliver high-quality medications to patients at lower prices.
Enhanced Clinical Trials: AI-powered analytics are transforming the clinical trial process. These algorithms can analyze patient data to identify suitable candidates for clinical trials, optimize trial designs, and predict patient outcomes. By streamlining the clinical trial process, AI is helping pharmaceutical companies bring new drugs to market faster and more cost-effectively.
Drug Repurposing: AI algorithms are being used to identify existing drugs that have the potential to treat new diseases or conditions—a process known as drug repurposing. By analyzing molecular structures, biological pathways, and clinical data, AI can identify promising candidates for repurposing, accelerating the discovery of new treatments.
Real-time Disease Surveillance: AI is playing a critical role in disease surveillance and outbreak detection. By analyzing vast amounts of health data from various sources, including electronic health records, social media, and internet searches, AI algorithms can detect disease outbreaks in real-time. This enables public health officials to respond quickly and effectively to emerging health threats.
Predictive Analytics for Market Trends: AI-powered predictive analytics are helping pharmaceutical companies anticipate market trends and optimize their supply chain and distribution strategies. By analyzing sales data, patient demographics, and other factors, AI algorithms can predict market demand, identify emerging trends, and inform strategic decision-making.
Virtual Drug Screening: AI-powered virtual screening platforms are revolutionizing the drug discovery process. These platforms use machine learning algorithms to analyze millions of chemical compounds and predict their potential to interact with specific biological targets. By rapidly screening large chemical libraries, AI is accelerating the identification of promising drug candidates.
Drug Safety Prediction: AI algorithms can predict potential adverse drug reactions by analyzing patient data and drug characteristics. By identifying safety risks early in the drug development process, AI is helping pharmaceutical companies improve the safety profiles of their medications and reduce the risk of unexpected side effects.
Read More..
#pharmacy#pcd pharma franchise#pharmaceutical#third party manufacturing#health & fitness#medicine#medical#health
1 note
·
View note
Text
Integrating AI into Medical Research and Diagnostics

Artificial Intelligence (AI) is revolutionizing numerous sectors, and its integration into medical research and diagnostics is proving to be transformative. The adoption of AI-driven healthcare solutions is enhancing the accuracy, efficiency, and personalization of medical services, paving the way for a new era in healthcare. This article delves into the impact of AI in medical research and diagnostics, highlighting the role of AI consulting services in this evolution.
The Role of AI in Medical Research
AI is a powerful tool in medical research, enabling scientists to process and analyze vast datasets more efficiently than ever before. Traditionally, medical research has been a time-consuming process, often limited by the capabilities of human researchers. However, with AI-driven healthcare solutions, researchers can now analyze large volumes of data at unprecedented speeds, uncovering patterns and insights that were previously impossible to detect.
Key Applications of AI in Medical Research
Drug Discovery and Development: AI algorithms can predict how different drugs will interact with various biological targets, significantly accelerating the drug discovery process. This capability reduces the time and cost associated with bringing new medications to market.
Genomic Research: AI for personalized medicine leverages machine learning to analyze genetic data, helping researchers understand the genetic underpinnings of diseases. This knowledge is crucial for developing targeted therapies tailored to individual patients’ genetic profiles.
Clinical Trials: AI can optimize clinical trial designs, identify suitable candidates for trials, and monitor patient responses in real-time. This not only improves the efficiency of trials but also enhances patient safety and outcomes.
AI in Diagnostics
AI-driven diagnostics are transforming how diseases are detected and managed. Advanced AI algorithms can analyze medical images, lab results, and patient records to provide accurate and early diagnoses, often surpassing the capabilities of human doctors.
Enhancing Diagnostic Accuracy
AI in healthcare consulting is pivotal in developing diagnostic tools that can interpret complex medical data with high precision. For instance:
Medical Imaging: AI algorithms can detect anomalies in medical images, such as X-rays, MRIs, and CT scans, with remarkable accuracy. This helps radiologists identify diseases like cancer at an early stage, improving treatment outcomes.
Pathology: AI systems can analyze tissue samples to detect cellular abnormalities. This aids pathologists in diagnosing conditions such as cancer more accurately and efficiently.
Electronic Health Records (EHRs): AI can sift through vast amounts of patient data in EHRs to identify patterns that may indicate the early onset of diseases. This proactive approach allows for earlier intervention and better management of chronic conditions.
The Importance of AI Consulting Services
The integration of AI into healthcare is not without its challenges. Hospitals, research institutions, and healthcare providers often require specialized expertise to implement and utilize AI effectively. This is where AI consulting services play a crucial role.
Benefits of AI Consulting Services
Expert Guidance: AI consultants offer expert advice on the best AI technologies and methodologies to adopt, ensuring that healthcare organizations maximize the benefits of AI.
Customized Solutions: AI in healthcare consulting provides tailored solutions that meet the specific needs of different medical institutions, whether it’s for research, diagnostics, or patient care.
Implementation Support: Implementing AI systems requires significant technical knowledge and experience. AI consulting services help organizations navigate the complexities of integration, from selecting the right tools to training staff.
Regulatory Compliance: The healthcare industry is highly regulated, and compliance is critical. AI consultants ensure that AI solutions adhere to all relevant regulations and standards, mitigating legal and ethical risks.
Future Prospects
The future of AI in medical research and diagnostics looks promising, with continuous advancements expected to further revolutionize healthcare. AI-driven personalized medicine will become increasingly prevalent, offering treatments tailored to individual genetic profiles and improving patient outcomes. Moreover, ongoing developments in AI technologies will enhance the capabilities of diagnostic tools, making them more accurate and accessible.
Conclusion
Integrating AI into medical research and diagnostics is a game-changer for the healthcare industry. With the support of AI consulting services, healthcare providers can harness the full potential of AI, leading to more efficient research, early and accurate diagnoses, and ultimately, better patient care. As we continue to explore the possibilities of AI in healthcare, the prospects for a healthier future are brighter than ever.
0 notes
Text
Medication Management Market Worldwide Industry Analysis, Future Demand and Forecast till 2032
The Medication Management Market Size is expected to grow from USD 5.23 billion in 2023 to USD 11.45 billion by 2032, at a CAGR of 9.1% during the forecast period (2024-2032).
The market for medication management includes a wide range of services and technologies intended to guarantee that patients receive their medications in a secure and efficient manner. Medication administration records (MARs), automated dispensing systems, clinical decision support systems (CDSS), computerized physician order entry (CPOE), electronic health records (EHRs), and pharmacy information systems are all included in this sector. Reducing prescription errors, improving patient safety, and improving overall health outcomes are the main objectives.
By 2024, the market will have grown significantly due to a number of important factors. The aging of the world's population, the rising incidence of chronic illnesses, and the ensuing increase in the quantity of prescription drugs written are all important factors. The industry has been further driven by technological breakthroughs like the use of machine learning (ML) and artificial intelligence (AI) in medication management systems. These technologies allow for more accurate and customized drug regimens, better operational efficiencies for healthcare professionals, and predictive analytics for possible adverse drug events. The government's efforts and regulatory frameworks to enhance patient safety and healthcare quality are also propelling market expansion.
Get Full PDF Sample Copy of Report: (Including Full TOC, List of Tables & Figures, Chart) @
https://introspectivemarketresearch.com/request/9799
Updated Version 2024 is available our Sample Report May Includes the:
Scope For 2024
Brief Introduction to the research report.
Table of Contents (Scope covered as a part of the study)
Top players in the market
Research framework (structure of the report)
Research methodology adopted by Worldwide Market Reports
Leading players involved in the Medication Management Market include:
Omnicell (USA), Cerner Corporation (USA), McKesson Corporation (USA), BD (Becton, Dickinson and Company) (USA), Swisslog Healthcare (Switzerland), Parata Systems (USA), ScriptPro (USA), Capsa Healthcare (USA), MedMinder Systems (USA), Talyst (USA), CareFusion (USA), Aethon (USA), Tabula Rasa HealthCare (USA)
Moreover, the report includes significant chapters such as Patent Analysis, Regulatory Framework, Technology Roadmap, BCG Matrix, Heat Map Analysis, Price Trend Analysis, and Investment Analysis which help to understand the market direction and movement in the current and upcoming years.
If You Have Any Query Medication Management Market Report, Visit:
https://introspectivemarketresearch.com/inquiry/9799
Segmentation of Medication Management Market:
By Type
Computerized provider order entry (CPOE)
Clinical decision support systems (CDSS)
Electronic Medication Administration Record
Automated Dispensing
Others
By Delivery Mode
On-premise
Cloud-based
By End-User
Hospitals
Pharmacies
Others
An in-depth study of the Medication Management industry for the years 2024–2032 is provided in the latest research. North America, Europe, Asia-Pacific, South America, the Middle East, and Africa are only some of the regions included in the report's segmented and regional analyses. The research also includes key insights including market trends and potential opportunities based on these major insights. All these quantitative data, such as market size and revenue forecasts, and qualitative data, such as customers' values, needs, and buying inclinations, are integral parts of any thorough market analysis.
Market Segment by Regions: -
North America (US, Canada, Mexico)
Eastern Europe (Bulgaria, The Czech Republic, Hungary, Poland, Romania, Rest of Eastern Europe)
Western Europe (Germany, UK, France, Netherlands, Italy, Russia, Spain, Rest of Western Europe)
Asia Pacific (China, India, Japan, South Korea, Malaysia, Thailand, Vietnam, The Philippines, Australia, New Zealand, Rest of APAC)
Middle East & Africa (Turkey, Bahrain, Kuwait, Saudi Arabia, Qatar, UAE, Israel, South Africa)
South America (Brazil, Argentina, Rest of SA)
Key Benefits of Medication Management Market Research:
Research Report covers the Industry drivers, restraints, opportunities and challenges
Competitive landscape & strategies of leading key players
Potential & niche segments and regional analysis exhibiting promising growth covered in the study
Recent industry trends and market developments
Research provides historical, current, and projected market size & share, in terms of value
Market intelligence to enable effective decision making
Growth opportunities and trend analysis
Covid-19 Impact analysis and analysis to Medication Management market
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
Acquire This Reports: -
https://introspectivemarketresearch.com/checkout/?user=1&_sid=9799
About us:
Introspective Market Research (introspectivemarketresearch.com) is a visionary research consulting firm dedicated to assist our clients grow and have a successful impact on the market. Our team at IMR is ready to assist our clients flourish their business by offering strategies to gain success and monopoly in their respective fields. We are a global market research company, specialized in using big data and advanced analytics to show the bigger picture of the market trends. We help our clients to think differently and build better tomorrow for all of us. We are a technology-driven research company, we analyze extremely large sets of data to discover deeper insights and provide conclusive consulting. We not only provide intelligence solutions, but we help our clients in how they can achieve their goals.
Contact us:
Introspective Market Research
3001 S King Drive,
Chicago, Illinois
60616 USA
Ph no: +1 773 382 1049
Email: [email protected]
#Medication Management#Medication Management Market#Medication Management Market Size#Medication Management Market Share#Medication Management Market Growth#Medication Management Market Trend#Medication Management Market segment#Medication Management Market Opportunity#Medication Management Market Analysis 2024
0 notes
Text
Breaking Down the Process: What to Expect from Regulatory Certification

Navigating the maze of regulatory certification can be daunting for businesses, especially those venturing into new markets or industries. Regulatory certification is crucial as it ensures that products or services meet specific standards and comply with legal requirements, safeguarding consumers and ensuring fair competition. This blog will break down the process, highlighting what businesses can expect and how to streamline their path to compliance.
Understanding Regulatory Certification
Regulatory certification is a formal process through which a product, service, or company is evaluated to ensure it complies with the regulations and standards set by a governing body. These regulations can vary significantly depending on the industry, region, and specific product or service in question. Common sectors requiring stringent regulatory certification include pharmaceuticals, medical devices, automotive, electronics, and food and beverages.
Initial Assessment
The journey towards regulatory certification begins with an initial assessment. This stage involves thoroughly understanding the specific regulations and standards applicable to your product or service. It is crucial to identify the governing bodies relevant to your industry, such as the FDA (Food and Drug Administration) for medical devices in the United States or the CE (Conformité Européene) marking for products sold within the European Economic Area.
Key Steps in Initial Assessment:
Research Regulatory Requirements: Gather information on the regulatory affairs services requirements and standards relevant to your product or service. This may involve reviewing guidelines, standards documents, and industry publications.
Consult with Experts: Engage with regulatory consultants or industry experts to gain insights and clarify any ambiguities in the regulatory requirements.
Identify Compliance Gaps: Conduct a gap analysis to identify areas where your product or service may not meet regulatory standards. This will help in creating a roadmap for necessary modifications or improvements.
Documentation and Submission
Once the initial assessment is complete, the next step is to compile the required documentation. Proper documentation is critical as it provides evidence that your product or service meets the necessary standards and regulations. The documentation requirements can vary widely but generally include technical specifications, safety data, test reports, and quality management system documents.
Key Steps in Documentation:
Prepare Technical Files: Create detailed technical files that include product design, manufacturing processes, risk assessments, and compliance with relevant standards.
Conduct Testing: Perform necessary testing to demonstrate compliance with safety and performance standards. This may involve in-house testing or using accredited external laboratories.
Compile Quality Management System (QMS) Documents: If required, document your quality management system, including procedures, policies, and records that demonstrate your commitment to maintaining quality standards.
Submission and Review
With all documentation in place, the next step is to submit your application to the relevant regulatory body. This stage involves a thorough review process where the regulatory authority examines your documentation, conducts audits if necessary, and may request additional information or clarification.
Key Steps in Submission and Review:
Submit Application: Submit your complete application, including all required documentation and fees, to the relevant regulatory body.
Respond to Queries: Be prepared to respond promptly to any queries or requests for additional information from the regulatory authority.
Undergo Audits: In some cases, the regulatory body may conduct on-site audits or inspections to verify compliance with standards and regulations.
Certification and Maintenance
Upon successful review and approval, you will receive your regulatory certification. However, the process doesn’t end here. Maintaining compliance is an ongoing commitment, requiring regular audits, updates to documentation, and continuous monitoring of regulatory changes.
Key Steps in Certification and Maintenance:
Receive Certification: Obtain your regulatory certification, which may include certificates, marks, or official approval letters.
Conduct Regular Audits: Schedule and conduct regular internal and external audits to ensure ongoing compliance with regulatory standards.
Stay Informed: Keep abreast of changes in regulations and standards, and update your processes and documentation accordingly.
Conclusion
Regulatory certification is a critical step for businesses to ensure their products or services meet industry standards and legal requirements. By understanding the process, conducting thorough assessments, preparing detailed documentation, and maintaining ongoing compliance, businesses can navigate the regulatory landscape with confidence. This commitment not only ensures market access but also builds trust and credibility with consumers and stakeholders.
0 notes