#2-stage asymmetric phase shifting
Text
SolidGoldFX AURRAS Optical Vibraphase
The SolidGoldFX AURRAS Optical Vibraphase redefines the essence of sonic modulation, blending vintage allure with cutting-edge innovation. This next-gen effects pedal, inspired by the sound deity Aurras and building upon the legacy of the acclaimed Athena Vibraphase, offers a symphony of legendary Uni-Vibe textures and contemporary sonic artistry.
SolidGoldFX AURRAS
At the heart of the AURRAS…

View On WordPress
#2-stage asymmetric phase shifting#analog#Athena Vibraphase#AURRAS#Band of Gypsys#Canada#expression pedal#Hendrix#Jimi Hendrix#Montreal#Optical Vibraphase#pedal#phase#phase shifting#Pink Floyd#shifting#SolidGoldFX#SolidGoldFX AURRAS#SolidGoldFX AURRAS Optical Vibraphase#speed ramping#stompbox#Tap Tempo#The Dark Side of the Moon#true-bypass#Uni-Vibe#video#YouTube
0 notes
Text
(-)-Taddol CAS#: 93379-48-7
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product Name(-)-TaddolIUPAC Name-2,2-dimethyl-1,3-dioxolan-4-yl]-diphenylmethanolMolecular Structure
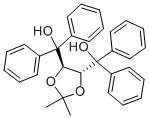
CAS Registry Number 93379-48-7Beilstein Registry Number3657855 Synonyms(R,R)-TADDOL, (4R,5R)-2,2-dimethyl-α,α,α′,α′-tetraphenyl-1,3-dioxolane-4,5-dimethanol, (4R,5R)-(-)-2,2-dimethyl-α,α,α',α'-tetraphenyl-1,3-dioxolane-4,5-dimethanol, (4R,5R)-α,α,α′,α′-tetraphenyl-1,3-dioxolane-4,5-dimethanol, (4R,5R)-2,2-Dimethyl-α,α,α',α'-tetraphenyl-1,3-dioxolane-4,5-dimethanol, α,α,α',α'-tetraphenyl-(2,2-dimethyl-1,3-dioxolane-4,5-diyl)-dimethanol, (R,R)-α,α,α',α'-tetraphenyl-2,2-dimethyl-1,3-dioxolane-4,5-dimethanol Molecular FormulaC31H30O4 Molecular Weight466.568 InChIInChI=1S/C31H30O4/c1-29(2)34-27(30(32,23-15-7-3-8-16-23)24-17-9-4-10-18-24)28(35-29)31(33,25-19-11-5-12-20-25)26-21-13-6-14-22-26/h3-22,27-28,32-33H,1-2H3/t27-,28-/m1/s1 InChI KeyOWVIRVJQDVCGQX-VSGBNLITSA-NCanonical SMILESCC1(OC(C(O1)C(C2=CC=CC=C2)(C3=CC=CC=C3)O)C(C4=CC=CC=C4)(C5=CC=CC=C5)O)CIsomeric SMILESCC1(O((O1)C(C2=CC=CC=C2)(C3=CC=CC=C3)O)C(C4=CC=CC=C4)(C5=CC=CC=C5)O)C
Patent InformationPatent IDTitlePublication DateCN104844654 A quaternary phosphonium salt compound and its preparation method (by machine translation) 2016US2009/30235 METHOD FOR FRACTIONATING STEREOISOMERIC COMPOUNDS 2009US6184404 Process for the selective alkylation of aldehydes by means of organozinc compounds 2001
Physical Data
AppearanceWhite solid
Melting Point, °C Solvent (Melting Point) 196 - 197 192 - 194 185211 - 212
Density, g·cm-3Measurement Temperature, °C1.213
Description (Association (MCS))Solvent (Association (MCS))Temperature (Association (MCS)), °C Partner (Association (MCS))NMR spectrum of the complex CDCl3 253,3'-dimethoxy-2,2'-bipyridine-N,N'-dioxide Association with compound 25(S)-1-phenylethanol Association with compound 2-oxo-2-phenyl-N,N-dipropylacetamide Association with compound N,N-diethyl-2-oxo-2-phenylacetamide NMR spectrum of the complex CDCl3 2-Amino-3-methyl-pentanoic acid methyl ester NMR spectrum of the complex CDCl3 (R)-isopropyl 2-aminopropanoate
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Frequency (NMR Spectroscopy), MHzChemical shifts1Hchloroform-d1 200Chemical shifts 13Cchloroform-d1 100Chemical shifts13Cchloroform-d1 75Chemical shifts 1Hchloroform-d1 400Spectrum 1H CDCl3 400.13 Spectrum 13C CDCl3 100.613 Chemical shifts 1H CDCl3 200Chemical shifts 1H CDCl3 300
(-)-Taddol CAS 93379-48-7 NMR

Description (IR Spectroscopy)Solvent (IR Spectroscopy)Comment (IR Spectroscopy) ATR (attenuated total reflectance), Bands Bands, Spectrumneat (no solvent, solid phase) Spectrum CH2Cl2 BandsKBr Bands 3434 - 3206 cm**(-1) Bands3600 - 3400 cm**(-1) Bands CHCl3 3590 - 1815 cm**(-1)
(-)-Taddol CAS 93379-48-7 Raman

Description (UV/VIS Spectroscopy)nmSolvent (UV/VIS Spectroscopy)Ext./Abs. Coefficient, l·mol-1cm-1 193 134000
Route of Synthesis (ROS)

Route of Synthesis (ROS) of (-)-Taddol CAS# 93379-48-7
ConditionsYieldStage #1: bromobenzene With n-butyllithium In diethyl ether; hexane at 20℃; for 2h;
Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In diethyl ether; hexane at 20℃;92%Stage #1: bromobenzene With magnesium In tetrahydrofuran Cooling with ice; Inert atmosphere;
Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In tetrahydrofuran for 1.5h; Reflux;91% Stage #1: bromobenzene With iodine; magnesium In tetrahydrofuran Inert atmosphere; Reflux;
Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In tetrahydrofuran Inert atmosphere; Cooling with ice;
Stage #3: In tetrahydrofuran for 1.5h; Inert atmosphere; Reflux;88%Stage #1: bromobenzene With iodine; magnesium In tetrahydrofuran for 1h; Reflux;
Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In tetrahydrofuran for 1.5h; Reflux;
Experimental Procedure
Under argon atmosphere, a reflux vessel was installed on a three-necked flask,A drip funnel with a pressure balance valve, and a thermometer; then add fresh magnesium bars 4.1 g, 579 mmol, 1.02 equiv.) And a small piece of iodine as the initiator. Then, bromobenzene (86.5 g, 551 mmol) was added to the dropping funnel,In tetrahydrofuran (386 mL) was added dropwise slowly until the reaction started. Constantly dropping to the end,The reaction was continued by reflux for one hour and then cooled to room temperature.To the above-mentioned format reagent, dimethyl tartrate 2 (24.1 g, 124 mmol) was added, slowly added,To ensure that the temperature does not exceed 20 degrees, after the drop is completed,The reaction system was heated to reflux for 1.5 hours,And then cooled to room temperature.Slowly adding saturated ammonium chloride solution quenching reaction,Extracted three times with ethyl acetate (40 mL X3)Then dried over anhydrous magnesium sulfate; filtered, dried and dried in vacuo to give a slightly yellow foamy solid;Recrystallization from methylene chloride and methanol gave white solid 4 (50.8 g, 88percent yield).88%Stage #1: bromobenzene With iodine; magnesium In tetrahydrofuran Inert atmosphere;
Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In tetrahydrofuran for 8h; Inert atmosphere;
Experimental Procedure
705 g (290 mmol) of metal magnesium was ground and ground, and then poured into 90 mL of dry treated anhydrous tetrahydrofasmonan,A small pellet was added and 42.06 g (267.9 mmol) of bromobenzene was dissolved in 120 mL of anhydrous tetrahydrofenamyl,In the N2 protection, the first small amount of drop into the magnesium iodine mixture, to be yellow solution faded, there are bubbles emerge, and then continue to drop the remaining bromobenzene tetrahydrofuran solution, such as magnesium dissolved disappear, the reaction was gray-green, Then 9.77 g (44.8 mmol) of the ketal-protected dimethyl tartrate was dissolved in 90 mL of anhydrous tetrahydrofuran and added dropwise to the format reagent under an oil bath for about 8 h,The reaction was quenched with saturated aqueous ammonium chloride solution, The organic phase was separated and the aqueous phase was extracted three times with ethyl acetate. The combined organic phases were washed twice with saturated brine, dried over anhydrous magnesium sulfate and recrystallized from methanol to give the product as a white solid Α, α, α ', α-tetraphenyl-1,3-dioxolane-4,5-dimethanol 16 · 28 g, yield 78percent.78%Stage #1: bromobenzene With iodine; magnesium In tetrahydrofuran at 20℃; Inert atmosphere; Reflux;
Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In tetrahydrofuran at 0℃; for 1.5h; Inert atmosphere; Reflux;62%
Safety and Hazards
GHS Hazard StatementsNot Classified
Other Data
TransportationNot dangerous goodsUnder the room temperature and away from lightHS Code294200StorageUnder the room temperature and away from lightShelf Life2 yearsMarket PriceUSD
Use Pattern(-)-Taddol CAS#: 93379-48-7 is used as a chiral ligand for enantioselective oxidative coupling of 3-phenylacetyl-2-oxazolidinone to afford dimer with good enantioselectivity phase transfer catalyst for Schiff's base alkylation Chiral agent for the asymmetric allylation of alhehydes with allyl bromide in the presence of CrCl2Use as asymmetric induction Zr catalyst ligand in kinetically controlled Meerwein-Ponndorf-Verley reductions Efficiant catalyst for enantioselective addition of primary alkyl Grignard reagents to aldehydes
Read the full article
0 notes
Text
Bayer Leverkusen vs Bayern Munich
New Post has been published on https://bestfreebettingtips.com/bayer-leverkusen-vs-bayern-munich/
Bayer Leverkusen vs Bayern Munich
Artwork by @chapulana
In the Bundesliga, Bayern Munich lost their encounter with Bayer Leverkusen. Leverkusen dominated in the early stages of the clash but the guests took control of the game and revealed some issues of the home side. Kovac’s man won the first half with a goal by Leon Goretzka, but Leverkusen made a comeback in the second half with a great free kick and two counter-attack goals. This tactical analysis will explore these crucial factors of the game.
Team news
Bayern opted for their usual 4-2-3-1 formation which transformed into a 4-4-1-1 or 4-5-1 in defence with Thomas Muller and Kingsley Coman dropping to the midfield line. On the other hand, the recently appointed former Borussia Dortumund coach Peter Bosz employed his trademark 4-3-3 with the same intense manner. The expectation towards Leverkusen and Bosz’s system is quite high since the fans hope he will utilise the potential of their young talents, especially Kai Havertz.
Early exchanges
Leverkusen started with a high intensity that appeared in high pressing and aggressive counter-pressing. This can be an effective weapon to score early on since this aims to force to opponent back and not let them escape the pressure to breathe. Therefore, when they lost possession, Leverkusen counter-pressed with a man-oriented fashion to quickly win the ball back.
One player pressing the opponent on the ball while two cover the nearby options.
Even at goal kicks they stayed high. On these occasions, full-backs Wendell and Mitchell Weiser pushed forward to pin the opposite full-backs. This meant Jonathan Tah could be left in a one-on-one situation with Coman if Bayern bypassed the press. This will be important later.
Leon Bailey (#9) and Karim Bellarabi (#38) occupied the two wide centre-backs, while Kevin Volland (#31) stayed central to mark Joshua Kimmich (#32). Julian Brandt (#10) and Kai Havertz (#29) stuck to Leon Goretzka (#18) and James (#11).
In pressing, Leverkusen used a 4-3-3 formation where the basic idea of Volland’s role was to cut off the passing route between the Bayern centre-backs. Meanwhile Bellarabi and Bailey positioned themselves around the half-spaces to get quick access to the opposite full-backs.
One of the patterns in Leverkusen’s pressing scheme was to trap the opponent. The setup was designed to apply pressure from two directions on the centre-back in possession and invite the pass towards Kimmich who was left open. The instant the pass was made, one player rushed on to separate from the ball and set up a dangerous counter-attack.
Volland cuts off the passing option to the far centre-back. Bellarabi presses diagonally to block the route towards the full-back. Havertz rushes on Kimmich.
Again, Volland presses with a curved run to block the route between the centre-backs as Bailey prevents access to the full-back. Thus the only option was Kimmich. Now, Aranguiz presses Kimmich.
Bayern Munich take control of the game
The change started in the sixth minute when the guests exploited the space behind Weiser with a through ball towards Coman. This led to a corner which allowed Bayern to breathe a little bit. Now they could use the press to disrupt the opponent’s play. In the ensuing minutes, Bayern caused serious issues for the home side by building from deep.
By the time of 11th minutes, the whole game had changed. Bayern were able to set up their pressing system, and by implementing their counter-press they become more stable in the game. At this point, Leverkusen struggled to reach the opponent’s final third.
In pressing, Bayern used the 4-2-3-1 shape with an interesting tweak to it. The concept was to using man-marking in the middle while the wingers were responsible for the opposite full-backs.
Goretzka and Kimmich often marked Havertz and Brandt while James stuck to Aranguiz.
Moreover, the tweak was asymmetric within the formation since Coman’s positioning was based on the position of Weiser. Therefore, we could often see Coman in deeper, whilst Muller occupied higher positions due to his task. He stayed somewhere between Bender and Wendell. Firstly, he pinned the full-back, but when the situation required he ran diagonally to press while blocking the way to Wendell.
Positioning of Bayern’s front four players.
In some cases, Wendell provided the outlet for Leverkusen. However, by the time the ball had travelled Muller was able to shift, close him down and force play backwards.
Aerial ball towards Wendell to escape Bayern’s press.
Positional play and late goal for Bayern
Bosz’s system is famous for the good connection between its players. The major element of this is the spatial awareness that allows the players to create such a good connection. In addition, there is a pattern in the system that aims to create space for the full-backs who advance down the sidelines.
The wingers become narrow, attract the opposition full-back to in turn provide space for their own full-back.
In this period of the game, both sides often sat back in their defensive structures while the opponents tried to break it. Bayern attempted to overload the oppositional last line with James occupying space in there besides the narrow wingers and Lewandowski.
Leverkusen’s 4-5-1 defence. Goretzka had the freedom to move higher in between the lines. The full-backs provided width.
As mentioned earlier, Bosz often ask his full-backs to occupy high positions. However, without proper pressure this could be a very dangerous situation. Bayern were aware of this flaw and from the beginning aimed to utilise Coman’s speed in behind Weiser. In the 40th minute, Leverkusen failed to applying proper pressure on the ball which allowed Bayern to target Coman with a long ball. This led to the opening goal.
Goretzka arrived into the box from deep to score.
Second half adjustment
For the second 45 minutes Bosz had to change things, so he replaced Havertz with Baumgartlinger. In turn, this led to a formation change. Leverkusen switched to a 4-2-3-1 where Baumgartlinger and Aranguiz paired up as a double pivot.
Thus in the second half, the home side’s formation mostly fluctuated between 4-2-3-1 and 4-4-1-1 depending on the situation.
Leverkusen’s 4-4-1-1 shape in defence.
Leverkusen’s comeback
Leverkusen started better in the second half since Bayern felt able to sit back due to their lead. However, they were not able to create quality chances, so it was out of blue when Leverkusen equalised with a great free kick. After that, Bayern become more active in the attacking phases which resulted in riskier play from the Bavarians. As they pushed forwards, Leverkusen gained space which allowed them to score two more goals.
Buildup before the second goal for Leverkusen. Notice the huge space in between Bayern’s lines and numerically superior situations on the flanks for Leverkusen. This allowed them to progress through combinations.
Summary
Despite the wonderful result for the home side, Bosz’s system has some flaws which will be a difficult task to deal with. However, considering the short period since the Dutchman was appointed, this is really good so far. The squad and the system fit together, therefore it holds lots of potential that can give a second chance for Bosz, and also Leverkusen’s talents, to shine.
Meanwhile, Kovac cannot be as happy since with this loss RB Leipzig edged closer to them. In the first half, they showed great quality in gaining control of the game but lacked something in their finishing. Fortunately they were able to score, but in the second half they had too much risk in their performance.
If you love tactical analysis, then you’ll love the digital magazines from totalfootballanalysis.com – a guaranteed 100+ pages of pure tactical analysis covering topics from the Premier League, Serie A, La Liga, Bundesliga and many, many more. Buy your copy of the January issue for just ₤4.99 here, or even better sign up for a ₤50 annual membership (12 monthly issues plus the annual review) right here.
0 notes
Link
The tie can be an equipment that will decorate a smart -casual wardrobe even more. You will be taken by it up a step in the style section whether it is worn by you using a complete suit or perhaps a clothing. http://google.com Some careers will demand you to use a wrap, and this might be what sets people off the notion of carrying one in relaxed environments, nonetheless it comes with an increased consequence when used casually, since so few of us do this. There are numerous unique types of casual ties which are great for sporting on the night out or on the first-date. The Four-in-Hand Knot First up may be the Four-in-Hand knot, because it’s easy and simple to understand. It’s a small, slightly asymmetrical knot that is best suited for collars that are narrow. Here is because it doesn’t seem like you invested too much time before the mirror carefully tying it, the knot that is best suited for relaxed scenarios. It seems like you merely quickly put on a link before you went the entranceway, virtually being an afterthought. Also, if on the go, this is the knot. By crossing the vast end within the narrow end, begin. Fold the extensive end within the end that is thin. Complete the end that is large horizontally within the narrow stop again. Consider the broad end up and through the trap around your neck. Consider the large end through the knot in the front. Proceed to tighten the knot and take on it up to your collar. Half Windsor The Half Windsor is an easier model of the Full Windsor knot that is original. It will be described as a piece of cake understanding the latter once you've become experienced in this one. One might state it’s a little of the steppingstone to the real deal, but it is disqualified by that doesn’t being a fantastic knot in a unique right. Therefore I don’t although it’s more simple than it’s counterpart that is full , elegant counsel you to wear it gently. It’s definitely better suited for any office environment. By crossing the wide end within the narrow end begin. Fold the large end underneath the end that is thin. Take the wide end up. Take the extensive end back through the loop. Shift the end that is broad horizontally within the end that is thin. Consider the vast end-up through the loop. Draw on the broad stop through the knot in front. Proceed to tighten the knot and pull it up to your collar. Full Windsor Tie Knot The Full Windsor tie knot is better useful for proper events. You shouldn’t wear this one gently. It’s a dense, vast knot shaped. It’s suitable for shirts having a wide spread collar. That one must be reserved for displays, marriages and critical business conferences. Begin by crossing the large end on the thin end. Take the extensive finish back through the trap around your neck. Consider the vast end-over the narrow end in the identical route you entered it at phase 1. Collapse the broad end within the end that is narrow. Consider the end up… that is large And back through the loop inside the same direction as stage 4. Fold the large end horizontally on the end that is thin. Provide the wide find yourself through the hook yet again, as you did in step 2. Pull the broad end through the knot. Pratt knot This is actually the proper; one that’s suitable for any special occasion, casual everyday. The Pratt knot is neither as wide while the Windsor or as slim while the Four-inhand and therefore pairs nicely with many dress-shirts. It’s a lot looser to wear compared to the Windsor, although It’s a knot. This multi-purpose knot is very good to use to function, and you can loosen up it to get a more casual glance if you head out to get a beverage along with your peers. As that one begins somewhat diverse from the last two, see the instructions nicely. Start with crossing the end that is wide underneath the stop that is thin, while the wrap hangs inside-out around your neck. Move the broad end up over the end that is narrow. Take down the stop that is extensive through the trap and tighten the-knot. Shift the end that is wide horizontally over the narrow end. Draw the finish that is wide backup through the cycle. Move the vast end through the-knot in the front. Go to tighten the-knot and move on it up to your collar http://google.com Follow us on Twitter: https://twitter.com/ayamalau Follow us on Pinterest: http://ift.tt/2bGM77l Follow us on Instagram: http://ift.tt/2bYPSmw Follow us on Wordpress: http://ift.tt/1HstLQq Follow us on Facebook: http://ift.tt/2bHpbWE Follow us on Google Plus: http://ift.tt/2bHmHmt Follow us on Medium: http://ift.tt/2bHmC6Z Follow us on Tumbler: http://ift.tt/1lx3AeT Follow us on Reddit: http://ift.tt/2bHmbVF SUBSCRIBE TO OUR CHANNEL: https://www.youtube.com/channel/UC-9Yzrk3qjF8BULAvYo3NvQ
0 notes
Text
(-)-Taddol CAS#: 93379-48-7
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product Name(-)-TaddolIUPAC Name-2,2-dimethyl-1,3-dioxolan-4-yl]-diphenylmethanolMolecular Structure

CAS Registry Number 93379-48-7Beilstein Registry Number3657855 Synonyms(R,R)-TADDOL, (4R,5R)-2,2-dimethyl-α,α,α′,α′-tetraphenyl-1,3-dioxolane-4,5-dimethanol, (4R,5R)-(-)-2,2-dimethyl-α,α,α',α'-tetraphenyl-1,3-dioxolane-4,5-dimethanol, (4R,5R)-α,α,α′,α′-tetraphenyl-1,3-dioxolane-4,5-dimethanol, (4R,5R)-2,2-Dimethyl-α,α,α',α'-tetraphenyl-1,3-dioxolane-4,5-dimethanol, α,α,α',α'-tetraphenyl-(2,2-dimethyl-1,3-dioxolane-4,5-diyl)-dimethanol, (R,R)-α,α,α',α'-tetraphenyl-2,2-dimethyl-1,3-dioxolane-4,5-dimethanol Molecular FormulaC31H30O4 Molecular Weight466.568 InChIInChI=1S/C31H30O4/c1-29(2)34-27(30(32,23-15-7-3-8-16-23)24-17-9-4-10-18-24)28(35-29)31(33,25-19-11-5-12-20-25)26-21-13-6-14-22-26/h3-22,27-28,32-33H,1-2H3/t27-,28-/m1/s1 InChI KeyOWVIRVJQDVCGQX-VSGBNLITSA-NCanonical SMILESCC1(OC(C(O1)C(C2=CC=CC=C2)(C3=CC=CC=C3)O)C(C4=CC=CC=C4)(C5=CC=CC=C5)O)CIsomeric SMILESCC1(O((O1)C(C2=CC=CC=C2)(C3=CC=CC=C3)O)C(C4=CC=CC=C4)(C5=CC=CC=C5)O)C
Patent InformationPatent IDTitlePublication DateCN104844654 A quaternary phosphonium salt compound and its preparation method (by machine translation) 2016US2009/30235 METHOD FOR FRACTIONATING STEREOISOMERIC COMPOUNDS 2009US6184404 Process for the selective alkylation of aldehydes by means of organozinc compounds 2001
Physical Data
AppearanceWhite solid
Melting Point, °C Solvent (Melting Point) 196 - 197 192 - 194 185211 - 212
Density, g·cm-3Measurement Temperature, °C1.213
Description (Association (MCS))Solvent (Association (MCS))Temperature (Association (MCS)), °C Partner (Association (MCS))NMR spectrum of the complex CDCl3 253,3'-dimethoxy-2,2'-bipyridine-N,N'-dioxide Association with compound 25(S)-1-phenylethanol Association with compound 2-oxo-2-phenyl-N,N-dipropylacetamide Association with compound N,N-diethyl-2-oxo-2-phenylacetamide NMR spectrum of the complex CDCl3 2-Amino-3-methyl-pentanoic acid methyl ester NMR spectrum of the complex CDCl3 (R)-isopropyl 2-aminopropanoate
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Frequency (NMR Spectroscopy), MHzChemical shifts1Hchloroform-d1 200Chemical shifts 13Cchloroform-d1 100Chemical shifts13Cchloroform-d1 75Chemical shifts 1Hchloroform-d1 400Spectrum 1H CDCl3 400.13 Spectrum 13C CDCl3 100.613 Chemical shifts 1H CDCl3 200Chemical shifts 1H CDCl3 300
(-)-Taddol CAS 93379-48-7 NMR

Description (IR Spectroscopy)Solvent (IR Spectroscopy)Comment (IR Spectroscopy) ATR (attenuated total reflectance), Bands Bands, Spectrumneat (no solvent, solid phase) Spectrum CH2Cl2 BandsKBr Bands 3434 - 3206 cm**(-1) Bands3600 - 3400 cm**(-1) Bands CHCl3 3590 - 1815 cm**(-1)
(-)-Taddol CAS 93379-48-7 Raman

Description (UV/VIS Spectroscopy)nmSolvent (UV/VIS Spectroscopy)Ext./Abs. Coefficient, l·mol-1cm-1 193 134000
Route of Synthesis (ROS)
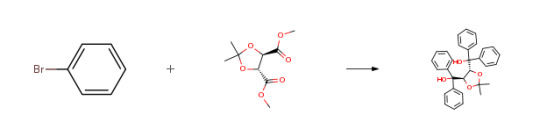
Route of Synthesis (ROS) of (-)-Taddol CAS# 93379-48-7
ConditionsYieldStage #1: bromobenzene With n-butyllithium In diethyl ether; hexane at 20℃; for 2h;
Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In diethyl ether; hexane at 20℃;92%Stage #1: bromobenzene With magnesium In tetrahydrofuran Cooling with ice; Inert atmosphere;
Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In tetrahydrofuran for 1.5h; Reflux;91% Stage #1: bromobenzene With iodine; magnesium In tetrahydrofuran Inert atmosphere; Reflux;
Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In tetrahydrofuran Inert atmosphere; Cooling with ice;
Stage #3: In tetrahydrofuran for 1.5h; Inert atmosphere; Reflux;88%Stage #1: bromobenzene With iodine; magnesium In tetrahydrofuran for 1h; Reflux;
Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In tetrahydrofuran for 1.5h; Reflux;
Experimental Procedure
Under argon atmosphere, a reflux vessel was installed on a three-necked flask,A drip funnel with a pressure balance valve, and a thermometer; then add fresh magnesium bars 4.1 g, 579 mmol, 1.02 equiv.) And a small piece of iodine as the initiator. Then, bromobenzene (86.5 g, 551 mmol) was added to the dropping funnel,In tetrahydrofuran (386 mL) was added dropwise slowly until the reaction started. Constantly dropping to the end,The reaction was continued by reflux for one hour and then cooled to room temperature.To the above-mentioned format reagent, dimethyl tartrate 2 (24.1 g, 124 mmol) was added, slowly added,To ensure that the temperature does not exceed 20 degrees, after the drop is completed,The reaction system was heated to reflux for 1.5 hours,And then cooled to room temperature.Slowly adding saturated ammonium chloride solution quenching reaction,Extracted three times with ethyl acetate (40 mL X3)Then dried over anhydrous magnesium sulfate; filtered, dried and dried in vacuo to give a slightly yellow foamy solid;Recrystallization from methylene chloride and methanol gave white solid 4 (50.8 g, 88percent yield).88%Stage #1: bromobenzene With iodine; magnesium In tetrahydrofuran Inert atmosphere;
Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In tetrahydrofuran for 8h; Inert atmosphere;
Experimental Procedure
705 g (290 mmol) of metal magnesium was ground and ground, and then poured into 90 mL of dry treated anhydrous tetrahydrofasmonan,A small pellet was added and 42.06 g (267.9 mmol) of bromobenzene was dissolved in 120 mL of anhydrous tetrahydrofenamyl,In the N2 protection, the first small amount of drop into the magnesium iodine mixture, to be yellow solution faded, there are bubbles emerge, and then continue to drop the remaining bromobenzene tetrahydrofuran solution, such as magnesium dissolved disappear, the reaction was gray-green, Then 9.77 g (44.8 mmol) of the ketal-protected dimethyl tartrate was dissolved in 90 mL of anhydrous tetrahydrofuran and added dropwise to the format reagent under an oil bath for about 8 h,The reaction was quenched with saturated aqueous ammonium chloride solution, The organic phase was separated and the aqueous phase was extracted three times with ethyl acetate. The combined organic phases were washed twice with saturated brine, dried over anhydrous magnesium sulfate and recrystallized from methanol to give the product as a white solid Α, α, α ', α-tetraphenyl-1,3-dioxolane-4,5-dimethanol 16 · 28 g, yield 78percent.78%Stage #1: bromobenzene With iodine; magnesium In tetrahydrofuran at 20℃; Inert atmosphere; Reflux;
Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In tetrahydrofuran at 0℃; for 1.5h; Inert atmosphere; Reflux;62%
Safety and Hazards
GHS Hazard StatementsNot Classified
Other Data
TransportationNot dangerous goodsUnder the room temperature and away from lightHS Code294200StorageUnder the room temperature and away from lightShelf Life2 yearsMarket PriceUSD
Use Pattern(-)-Taddol CAS#: 93379-48-7 is used as a chiral ligand for enantioselective oxidative coupling of 3-phenylacetyl-2-oxazolidinone to afford dimer with good enantioselectivity phase transfer catalyst for Schiff's base alkylation Chiral agent for the asymmetric allylation of alhehydes with allyl bromide in the presence of CrCl2Use as asymmetric induction Zr catalyst ligand in kinetically controlled Meerwein-Ponndorf-Verley reductions Efficiant catalyst for enantioselective addition of primary alkyl Grignard reagents to aldehydes
Read the full article
0 notes
Text
(-)-Taddol CAS#: 93379-48-7
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product Name(-)-TaddolIUPAC Name-2,2-dimethyl-1,3-dioxolan-4-yl]-diphenylmethanolMolecular Structure

CAS Registry Number 93379-48-7Beilstein Registry Number3657855 Synonyms(R,R)-TADDOL, (4R,5R)-2,2-dimethyl-α,α,α′,α′-tetraphenyl-1,3-dioxolane-4,5-dimethanol, (4R,5R)-(-)-2,2-dimethyl-α,α,α',α'-tetraphenyl-1,3-dioxolane-4,5-dimethanol, (4R,5R)-α,α,α′,α′-tetraphenyl-1,3-dioxolane-4,5-dimethanol, (4R,5R)-2,2-Dimethyl-α,α,α',α'-tetraphenyl-1,3-dioxolane-4,5-dimethanol, α,α,α',α'-tetraphenyl-(2,2-dimethyl-1,3-dioxolane-4,5-diyl)-dimethanol, (R,R)-α,α,α',α'-tetraphenyl-2,2-dimethyl-1,3-dioxolane-4,5-dimethanol Molecular FormulaC31H30O4 Molecular Weight466.568 InChIInChI=1S/C31H30O4/c1-29(2)34-27(30(32,23-15-7-3-8-16-23)24-17-9-4-10-18-24)28(35-29)31(33,25-19-11-5-12-20-25)26-21-13-6-14-22-26/h3-22,27-28,32-33H,1-2H3/t27-,28-/m1/s1 InChI KeyOWVIRVJQDVCGQX-VSGBNLITSA-NCanonical SMILESCC1(OC(C(O1)C(C2=CC=CC=C2)(C3=CC=CC=C3)O)C(C4=CC=CC=C4)(C5=CC=CC=C5)O)CIsomeric SMILESCC1(O((O1)C(C2=CC=CC=C2)(C3=CC=CC=C3)O)C(C4=CC=CC=C4)(C5=CC=CC=C5)O)C
Patent InformationPatent IDTitlePublication DateCN104844654 A quaternary phosphonium salt compound and its preparation method (by machine translation) 2016US2009/30235 METHOD FOR FRACTIONATING STEREOISOMERIC COMPOUNDS 2009US6184404 Process for the selective alkylation of aldehydes by means of organozinc compounds 2001
Physical Data
AppearanceWhite solid
Melting Point, °C Solvent (Melting Point) 196 - 197 192 - 194 185211 - 212
Density, g·cm-3Measurement Temperature, °C1.213
Description (Association (MCS))Solvent (Association (MCS))Temperature (Association (MCS)), °C Partner (Association (MCS))NMR spectrum of the complex CDCl3 253,3'-dimethoxy-2,2'-bipyridine-N,N'-dioxide Association with compound 25(S)-1-phenylethanol Association with compound 2-oxo-2-phenyl-N,N-dipropylacetamide Association with compound N,N-diethyl-2-oxo-2-phenylacetamide NMR spectrum of the complex CDCl3 2-Amino-3-methyl-pentanoic acid methyl ester NMR spectrum of the complex CDCl3 (R)-isopropyl 2-aminopropanoate
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Frequency (NMR Spectroscopy), MHzChemical shifts1Hchloroform-d1 200Chemical shifts 13Cchloroform-d1 100Chemical shifts13Cchloroform-d1 75Chemical shifts 1Hchloroform-d1 400Spectrum 1H CDCl3 400.13 Spectrum 13C CDCl3 100.613 Chemical shifts 1H CDCl3 200Chemical shifts 1H CDCl3 300
(-)-Taddol CAS 93379-48-7 NMR
Description (IR Spectroscopy)Solvent (IR Spectroscopy)Comment (IR Spectroscopy) ATR (attenuated total reflectance), Bands Bands, Spectrumneat (no solvent, solid phase) Spectrum CH2Cl2 BandsKBr Bands 3434 - 3206 cm**(-1) Bands3600 - 3400 cm**(-1) Bands CHCl3 3590 - 1815 cm**(-1)
(-)-Taddol CAS 93379-48-7 Raman
Description (UV/VIS Spectroscopy)nmSolvent (UV/VIS Spectroscopy)Ext./Abs. Coefficient, l·mol-1cm-1 193 134000
Route of Synthesis (ROS)

Route of Synthesis (ROS) of (-)-Taddol CAS# 93379-48-7
ConditionsYieldStage #1: bromobenzene With n-butyllithium In diethyl ether; hexane at 20℃; for 2h;
Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In diethyl ether; hexane at 20℃;92%Stage #1: bromobenzene With magnesium In tetrahydrofuran Cooling with ice; Inert atmosphere;
Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In tetrahydrofuran for 1.5h; Reflux;91% Stage #1: bromobenzene With iodine; magnesium In tetrahydrofuran Inert atmosphere; Reflux;
Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In tetrahydrofuran Inert atmosphere; Cooling with ice;
Stage #3: In tetrahydrofuran for 1.5h; Inert atmosphere; Reflux;88%Stage #1: bromobenzene With iodine; magnesium In tetrahydrofuran for 1h; Reflux;
Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In tetrahydrofuran for 1.5h; Reflux;
Experimental Procedure
Under argon atmosphere, a reflux vessel was installed on a three-necked flask,A drip funnel with a pressure balance valve, and a thermometer; then add fresh magnesium bars 4.1 g, 579 mmol, 1.02 equiv.) And a small piece of iodine as the initiator. Then, bromobenzene (86.5 g, 551 mmol) was added to the dropping funnel,In tetrahydrofuran (386 mL) was added dropwise slowly until the reaction started. Constantly dropping to the end,The reaction was continued by reflux for one hour and then cooled to room temperature.To the above-mentioned format reagent, dimethyl tartrate 2 (24.1 g, 124 mmol) was added, slowly added,To ensure that the temperature does not exceed 20 degrees, after the drop is completed,The reaction system was heated to reflux for 1.5 hours,And then cooled to room temperature.Slowly adding saturated ammonium chloride solution quenching reaction,Extracted three times with ethyl acetate (40 mL X3)Then dried over anhydrous magnesium sulfate; filtered, dried and dried in vacuo to give a slightly yellow foamy solid;Recrystallization from methylene chloride and methanol gave white solid 4 (50.8 g, 88percent yield).88%Stage #1: bromobenzene With iodine; magnesium In tetrahydrofuran Inert atmosphere;
Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In tetrahydrofuran for 8h; Inert atmosphere;
Experimental Procedure
705 g (290 mmol) of metal magnesium was ground and ground, and then poured into 90 mL of dry treated anhydrous tetrahydrofasmonan,A small pellet was added and 42.06 g (267.9 mmol) of bromobenzene was dissolved in 120 mL of anhydrous tetrahydrofenamyl,In the N2 protection, the first small amount of drop into the magnesium iodine mixture, to be yellow solution faded, there are bubbles emerge, and then continue to drop the remaining bromobenzene tetrahydrofuran solution, such as magnesium dissolved disappear, the reaction was gray-green, Then 9.77 g (44.8 mmol) of the ketal-protected dimethyl tartrate was dissolved in 90 mL of anhydrous tetrahydrofuran and added dropwise to the format reagent under an oil bath for about 8 h,The reaction was quenched with saturated aqueous ammonium chloride solution, The organic phase was separated and the aqueous phase was extracted three times with ethyl acetate. The combined organic phases were washed twice with saturated brine, dried over anhydrous magnesium sulfate and recrystallized from methanol to give the product as a white solid Α, α, α ', α-tetraphenyl-1,3-dioxolane-4,5-dimethanol 16 · 28 g, yield 78percent.78%Stage #1: bromobenzene With iodine; magnesium In tetrahydrofuran at 20℃; Inert atmosphere; Reflux;
Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In tetrahydrofuran at 0℃; for 1.5h; Inert atmosphere; Reflux;62%
More: Inquiry all available synthetic routes with detailed experimental procedures
Safety and Hazards
GHS Hazard StatementsNot Classified
Other Data
TransportationNot dangerous goodsUnder the room temperature and away from lightHS Code294200StorageUnder the room temperature and away from lightShelf Life2 yearsMarket PriceUSD
Use Pattern(-)-Taddol CAS#: 93379-48-7 is used as a chiral ligand for enantioselective oxidative coupling of 3-phenylacetyl-2-oxazolidinone to afford dimer with good enantioselectivity phase transfer catalyst for Schiff's base alkylation Chiral agent for the asymmetric allylation of alhehydes with allyl bromide in the presence of CrCl2Use as asymmetric induction Zr catalyst ligand in kinetically controlled Meerwein-Ponndorf-Verley reductions Efficiant catalyst for enantioselective addition of primary alkyl Grignard reagents to aldehydes
Read the full article
0 notes
Text
(-)-Taddol CAS#: 93379-48-7
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product Name(-)-TaddolIUPAC Name-2,2-dimethyl-1,3-dioxolan-4-yl]-diphenylmethanolMolecular Structure

CAS Registry Number 93379-48-7Beilstein Registry Number3657855 Synonyms(R,R)-TADDOL, (4R,5R)-2,2-dimethyl-α,α,α′,α′-tetraphenyl-1,3-dioxolane-4,5-dimethanol, (4R,5R)-(-)-2,2-dimethyl-α,α,α',α'-tetraphenyl-1,3-dioxolane-4,5-dimethanol, (4R,5R)-α,α,α′,α′-tetraphenyl-1,3-dioxolane-4,5-dimethanol, (4R,5R)-2,2-Dimethyl-α,α,α',α'-tetraphenyl-1,3-dioxolane-4,5-dimethanol, α,α,α',α'-tetraphenyl-(2,2-dimethyl-1,3-dioxolane-4,5-diyl)-dimethanol, (R,R)-α,α,α',α'-tetraphenyl-2,2-dimethyl-1,3-dioxolane-4,5-dimethanol Molecular FormulaC31H30O4 Molecular Weight466.568 InChIInChI=1S/C31H30O4/c1-29(2)34-27(30(32,23-15-7-3-8-16-23)24-17-9-4-10-18-24)28(35-29)31(33,25-19-11-5-12-20-25)26-21-13-6-14-22-26/h3-22,27-28,32-33H,1-2H3/t27-,28-/m1/s1 InChI KeyOWVIRVJQDVCGQX-VSGBNLITSA-NCanonical SMILESCC1(OC(C(O1)C(C2=CC=CC=C2)(C3=CC=CC=C3)O)C(C4=CC=CC=C4)(C5=CC=CC=C5)O)CIsomeric SMILESCC1(O((O1)C(C2=CC=CC=C2)(C3=CC=CC=C3)O)C(C4=CC=CC=C4)(C5=CC=CC=C5)O)C
Patent InformationPatent IDTitlePublication DateCN104844654 A quaternary phosphonium salt compound and its preparation method (by machine translation) 2016US2009/30235 METHOD FOR FRACTIONATING STEREOISOMERIC COMPOUNDS 2009US6184404 Process for the selective alkylation of aldehydes by means of organozinc compounds 2001
Physical Data
AppearanceWhite solid
Melting Point, °C Solvent (Melting Point) 196 - 197 192 - 194 185211 - 212
Density, g·cm-3Measurement Temperature, °C1.213
Description (Association (MCS))Solvent (Association (MCS))Temperature (Association (MCS)), °C Partner (Association (MCS))NMR spectrum of the complex CDCl3 253,3'-dimethoxy-2,2'-bipyridine-N,N'-dioxide Association with compound 25(S)-1-phenylethanol Association with compound 2-oxo-2-phenyl-N,N-dipropylacetamide Association with compound N,N-diethyl-2-oxo-2-phenylacetamide NMR spectrum of the complex CDCl3 2-Amino-3-methyl-pentanoic acid methyl ester NMR spectrum of the complex CDCl3 (R)-isopropyl 2-aminopropanoate
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Frequency (NMR Spectroscopy), MHzChemical shifts1Hchloroform-d1 200Chemical shifts 13Cchloroform-d1 100Chemical shifts13Cchloroform-d1 75Chemical shifts 1Hchloroform-d1 400Spectrum 1H CDCl3 400.13 Spectrum 13C CDCl3 100.613 Chemical shifts 1H CDCl3 200Chemical shifts 1H CDCl3 300
(-)-Taddol CAS 93379-48-7 NMR
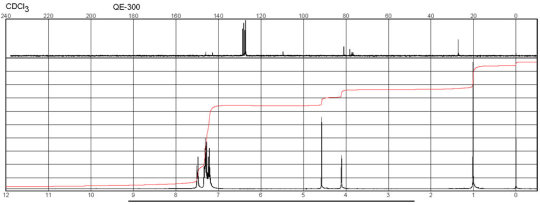
Description (IR Spectroscopy)Solvent (IR Spectroscopy)Comment (IR Spectroscopy) ATR (attenuated total reflectance), Bands Bands, Spectrumneat (no solvent, solid phase) Spectrum CH2Cl2 BandsKBr Bands 3434 - 3206 cm**(-1) Bands3600 - 3400 cm**(-1) Bands CHCl3 3590 - 1815 cm**(-1)
(-)-Taddol CAS 93379-48-7 Raman
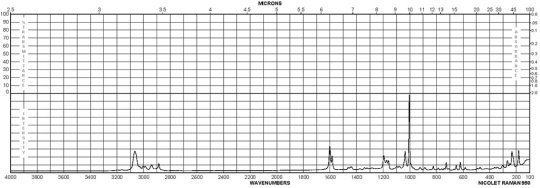
Description (UV/VIS Spectroscopy)nmSolvent (UV/VIS Spectroscopy)Ext./Abs. Coefficient, l·mol-1cm-1 193 134000
Route of Synthesis (ROS)
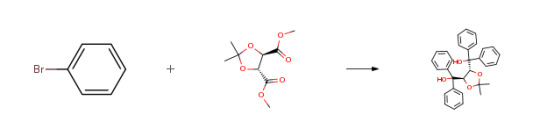
Route of Synthesis (ROS) of (-)-Taddol CAS# 93379-48-7
ConditionsYieldStage #1: bromobenzene With n-butyllithium In diethyl ether; hexane at 20℃; for 2h;
Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In diethyl ether; hexane at 20℃;92%Stage #1: bromobenzene With magnesium In tetrahydrofuran Cooling with ice; Inert atmosphere;
Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In tetrahydrofuran for 1.5h; Reflux;91% Stage #1: bromobenzene With iodine; magnesium In tetrahydrofuran Inert atmosphere; Reflux;
Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In tetrahydrofuran Inert atmosphere; Cooling with ice;
Stage #3: In tetrahydrofuran for 1.5h; Inert atmosphere; Reflux;88%Stage #1: bromobenzene With iodine; magnesium In tetrahydrofuran for 1h; Reflux;
Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In tetrahydrofuran for 1.5h; Reflux;
Experimental Procedure
Under argon atmosphere, a reflux vessel was installed on a three-necked flask,A drip funnel with a pressure balance valve, and a thermometer; then add fresh magnesium bars 4.1 g, 579 mmol, 1.02 equiv.) And a small piece of iodine as the initiator. Then, bromobenzene (86.5 g, 551 mmol) was added to the dropping funnel,In tetrahydrofuran (386 mL) was added dropwise slowly until the reaction started. Constantly dropping to the end,The reaction was continued by reflux for one hour and then cooled to room temperature.To the above-mentioned format reagent, dimethyl tartrate 2 (24.1 g, 124 mmol) was added, slowly added,To ensure that the temperature does not exceed 20 degrees, after the drop is completed,The reaction system was heated to reflux for 1.5 hours,And then cooled to room temperature.Slowly adding saturated ammonium chloride solution quenching reaction,Extracted three times with ethyl acetate (40 mL X3)Then dried over anhydrous magnesium sulfate; filtered, dried and dried in vacuo to give a slightly yellow foamy solid;Recrystallization from methylene chloride and methanol gave white solid 4 (50.8 g, 88percent yield).88%Stage #1: bromobenzene With iodine; magnesium In tetrahydrofuran Inert atmosphere;
Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In tetrahydrofuran for 8h; Inert atmosphere;
Experimental Procedure
705 g (290 mmol) of metal magnesium was ground and ground, and then poured into 90 mL of dry treated anhydrous tetrahydrofasmonan,A small pellet was added and 42.06 g (267.9 mmol) of bromobenzene was dissolved in 120 mL of anhydrous tetrahydrofenamyl,In the N2 protection, the first small amount of drop into the magnesium iodine mixture, to be yellow solution faded, there are bubbles emerge, and then continue to drop the remaining bromobenzene tetrahydrofuran solution, such as magnesium dissolved disappear, the reaction was gray-green, Then 9.77 g (44.8 mmol) of the ketal-protected dimethyl tartrate was dissolved in 90 mL of anhydrous tetrahydrofuran and added dropwise to the format reagent under an oil bath for about 8 h,The reaction was quenched with saturated aqueous ammonium chloride solution, The organic phase was separated and the aqueous phase was extracted three times with ethyl acetate. The combined organic phases were washed twice with saturated brine, dried over anhydrous magnesium sulfate and recrystallized from methanol to give the product as a white solid Α, α, α ', α-tetraphenyl-1,3-dioxolane-4,5-dimethanol 16 · 28 g, yield 78percent.78%Stage #1: bromobenzene With iodine; magnesium In tetrahydrofuran at 20℃; Inert atmosphere; Reflux;
Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In tetrahydrofuran at 0℃; for 1.5h; Inert atmosphere; Reflux;62%
More: Inquiry all available synthetic routes with detailed experimental procedures
Safety and Hazards
GHS Hazard StatementsNot Classified
Other Data
TransportationNot dangerous goodsUnder the room temperature and away from lightHS Code294200StorageUnder the room temperature and away from lightShelf Life2 yearsMarket PriceUSD
Use Pattern(-)-Taddol CAS#: 93379-48-7 is used as a chiral ligand for enantioselective oxidative coupling of 3-phenylacetyl-2-oxazolidinone to afford dimer with good enantioselectivity phase transfer catalyst for Schiff's base alkylation Chiral agent for the asymmetric allylation of alhehydes with allyl bromide in the presence of CrCl2Use as asymmetric induction Zr catalyst ligand in kinetically controlled Meerwein-Ponndorf-Verley reductions Efficiant catalyst for enantioselective addition of primary alkyl Grignard reagents to aldehydes
Read the full article
0 notes
Text
(-)-Taddol CAS#: 93379-48-7
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Other DataApproved Manufacturers
Identification
Product Name(-)-TaddolMolecular Structure

CAS Registry Number 93379-48-7Beilstein Registry Number3657855 Synonyms(R,R)-TADDOL, (4R,5R)-2,2-dimethyl-α,α,α′,α′-tetraphenyl-1,3-dioxolane-4,5-dimethanol, (4R,5R)-(-)-2,2-dimethyl-α,α,α',α'-tetraphenyl-1,3-dioxolane-4,5-dimethanol, (4R,5R)-α,α,α′,α′-tetraphenyl-1,3-dioxolane-4,5-dimethanol, (4R,5R)-2,2-Dimethyl-α,α,α',α'-tetraphenyl-1,3-dioxolane-4,5-dimethanol, α,α,α',α'-tetraphenyl-(2,2-dimethyl-1,3-dioxolane-4,5-diyl)-dimethanol, (R,R)-α,α,α',α'-tetraphenyl-2,2-dimethyl-1,3-dioxolane-4,5-dimethanol Molecular FormulaC31H30O4 Molecular Weight466.568 InChIInChI=1S/C31H30O4/c1-29(2)34-27(30(32,23-15-7-3-8-16-23)24-17-9-4-10-18-24)28(35-29)31(33,25-19-11-5-12-20-25)26-21-13-6-14-22-26/h3-22,27-28,32-33H,1-2H3/t27-,28-/m1/s1
Patent InformationPatent IDTitlePublication DateCN104844654 A quaternary phosphonium salt compound and its preparation method (by machine translation) 2016US2009/30235 METHOD FOR FRACTIONATING STEREOISOMERIC COMPOUNDS 2009US6184404 Process for the selective alkylation of aldehydes by means of organozinc compounds 2001
Physical Data
AppearanceWhite solid
Melting Point, °C Solvent (Melting Point) 196 - 197 192 - 194 185211 - 212
Density, g·cm-3Measurement Temperature, °C1.213
Description (Association (MCS))Solvent (Association (MCS))Temperature (Association (MCS)), °C Partner (Association (MCS))NMR spectrum of the complex CDCl3 253,3'-dimethoxy-2,2'-bipyridine-N,N'-dioxide Association with compound 25(S)-1-phenylethanol Association with compound 2-oxo-2-phenyl-N,N-dipropylacetamide Association with compound N,N-diethyl-2-oxo-2-phenylacetamide NMR spectrum of the complex CDCl3 2-Amino-3-methyl-pentanoic acid methyl ester NMR spectrum of the complex CDCl3 (R)-isopropyl 2-aminopropanoate
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Frequency (NMR Spectroscopy), MHzChemical shifts1Hchloroform-d1 200Chemical shifts 13Cchloroform-d1 100Chemical shifts13Cchloroform-d1 75Chemical shifts 1Hchloroform-d1 400Spectrum 1H CDCl3 400.13 Spectrum 13C CDCl3 100.613 Chemical shifts 1H CDCl3 200Chemical shifts 1H CDCl3 300
Description (IR Spectroscopy)Solvent (IR Spectroscopy)Comment (IR Spectroscopy) ATR (attenuated total reflectance), Bands Bands, Spectrumneat (no solvent, solid phase) Spectrum CH2Cl2 BandsKBr Bands 3434 - 3206 cm**(-1) Bands3600 - 3400 cm**(-1) Bands CHCl3 3590 - 1815 cm**(-1)
Description (UV/VIS Spectroscopy)nmSolvent (UV/VIS Spectroscopy)Ext./Abs. Coefficient, l·mol-1cm-1 193 134000
Route of Synthesis (ROS)
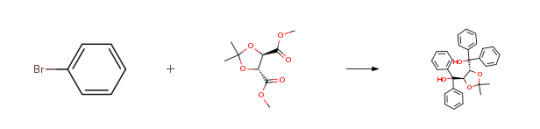
Route of Synthesis (ROS) of (-)-Taddol CAS# 93379-48-7
ConditionsYieldStage #1: bromobenzene With n-butyllithium In diethyl ether; hexane at 20℃; for 2h;
Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In diethyl ether; hexane at 20℃;92%Stage #1: bromobenzene With magnesium In tetrahydrofuran Cooling with ice; Inert atmosphere;
Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In tetrahydrofuran for 1.5h; Reflux;91% Stage #1: bromobenzene With iodine; magnesium In tetrahydrofuran Inert atmosphere; Reflux;
Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In tetrahydrofuran Inert atmosphere; Cooling with ice;
Stage #3: In tetrahydrofuran for 1.5h; Inert atmosphere; Reflux;88%Stage #1: bromobenzene With iodine; magnesium In tetrahydrofuran for 1h; Reflux;
Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In tetrahydrofuran for 1.5h; Reflux;
Experimental Procedure
Under argon atmosphere, a reflux vessel was installed on a three-necked flask,A drip funnel with a pressure balance valve, and a thermometer; then add fresh magnesium bars 4.1 g, 579 mmol, 1.02 equiv.) And a small piece of iodine as the initiator. Then, bromobenzene (86.5 g, 551 mmol) was added to the dropping funnel,In tetrahydrofuran (386 mL) was added dropwise slowly until the reaction started. Constantly dropping to the end,The reaction was continued by reflux for one hour and then cooled to room temperature.To the above-mentioned format reagent, dimethyl tartrate 2 (24.1 g, 124 mmol) was added, slowly added,To ensure that the temperature does not exceed 20 degrees, after the drop is completed,The reaction system was heated to reflux for 1.5 hours,And then cooled to room temperature.Slowly adding saturated ammonium chloride solution quenching reaction,Extracted three times with ethyl acetate (40 mL X3)Then dried over anhydrous magnesium sulfate; filtered, dried and dried in vacuo to give a slightly yellow foamy solid;Recrystallization from methylene chloride and methanol gave white solid 4 (50.8 g, 88percent yield).88%Stage #1: bromobenzene With iodine; magnesium In tetrahydrofuran Inert atmosphere;
Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In tetrahydrofuran for 8h; Inert atmosphere;
Experimental Procedure
705 g (290 mmol) of metal magnesium was ground and ground, and then poured into 90 mL of dry treated anhydrous tetrahydrofasmonan,A small pellet was added and 42.06 g (267.9 mmol) of bromobenzene was dissolved in 120 mL of anhydrous tetrahydrofenamyl,In the N2 protection, the first small amount of drop into the magnesium iodine mixture, to be yellow solution faded, there are bubbles emerge, and then continue to drop the remaining bromobenzene tetrahydrofuran solution, such as magnesium dissolved disappear, the reaction was gray-green, Then 9.77 g (44.8 mmol) of the ketal-protected dimethyl tartrate was dissolved in 90 mL of anhydrous tetrahydrofuran and added dropwise to the format reagent under an oil bath for about 8 h,The reaction was quenched with saturated aqueous ammonium chloride solution, The organic phase was separated and the aqueous phase was extracted three times with ethyl acetate. The combined organic phases were washed twice with saturated brine, dried over anhydrous magnesium sulfate and recrystallized from methanol to give the product as a white solid Α, α, α ', α-tetraphenyl-1,3-dioxolane-4,5-dimethanol 16 · 28 g, yield 78percent.78%Stage #1: bromobenzene With iodine; magnesium In tetrahydrofuran at 20℃; Inert atmosphere; Reflux;
Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In tetrahydrofuran at 0℃; for 1.5h; Inert atmosphere; Reflux;62%
More: Inquiry all available synthetic routes with detailed experimental procedures
Other Data
TransportationNot dangerous goodsUnder the room temperature and away from lightHS Code294200StorageUnder the room temperature and away from lightShelf Life2 yearsMarket PriceUSD
Use PatternAs chiral ligand for enantioselective oxidative coupling of 3-phenylacetyl-2-oxazolidinone to afford dimer with good enantioselectivity phase transfer catalyst for Schiff's base alkylation Chiral agent for the asymmetric allylation of alhehydes with allyl bromide in the presence of CrCl2Use as asymmetric induction Zr catalyst ligand in kinetically controlled Meerwein-Ponndorf-Verley reductions Efficiant catalyst for enantioselective addition of primary alkyl Grignard reagents to aldehydes
Read the full article
0 notes