Text
ASTM E2180 - Antimicrobial Test for Hydrophobic Materials
ASTM E2180 test is a quantitative method used for determining the antimicrobial effectiveness of the agent(s) incorporated in polymeric or hydrophobic surfaces.
ASTM E2180 Test Procedure
Agar slurry inoculated by test microorganisms is spread onto the test (treated) and control (untreated) sample of hydrophobic material.
After a contact period of 24 hrs, microorganisms from both control and test samples are recovered separately using a neutralizing solution.
Serial dilutions are made and plated on agar medium. These plates are further incubated for 48 hrs under specific conditions optimal for the growth of test organisms.
Bacteria colonies are counted and antimicrobial activity is calculated by comparing the reduction of bacterial colonies in treated samples with bacteria colonies present on untreated samples.
0 notes
Text
ASTM E2276 - Adult Fingerpad Test Method
Explore ASTM E2276 testing services for assessing the bacteria-eliminating effectiveness of handwash agents using fingerpads of adults. Contact us today!
ASTM E2276 Test Method
This test method is carried out on a group of adult subjects who have given informed consent and whose hand skin is free of any visible damage. The subjects are required to avoid the use of any products containing antimicrobial agents for one week before the test.
A specific amount of test suspension is applied on a demarcated area on each finger pad and then allowed to dry.
The contaminated fingertips are then exposed to the control ( hard water) and test ( product sample) for the specified contact time.
In the next step, surviving microorganisms from the fingerpads are eluted and the viable counts are determined.
Reductions in the numbers of viable bacteria from the control and product samples are calculated and analyzed for antibacterial efficacy.
0 notes
Text
ASTM E2755: Test for Healthcare Personnel Hand Rub Formulations
Learn about ASTM E2755 - a standard test method for hand sanitizer effectiveness. Conditions, method, importance, and lab role for manufacturers.
0 notes
Text
ISO 21150 - Cosmetics - Escherichia coli Test
ISO 21150 standard specifies guidelines for the detection and identification of the specified microorganism Escherichia Coli in cosmetic products.
ISO 21150 Test Method
Product samples are dispersed into the enrichment broth to make initial suspension. For water-miscible products, 1 ml of product sample is dispersed in 9 ml of enrichment broth. In the case of water-immiscible products, 1g of product sample is premixed with a dispersing agent (e.g. polysorbate 80) and then dispersed in 9 ml of enrichment broth. While dealing with filterable products, filtration is performed through the membrane and followed by initial suspension preparation.
0 notes
Text
ISO 18416 - Cosmetics - Candida Albicans Test
ISO 18416 standard specifies guidelines for the detection of the specified microorganism Candida albicans in cosmetic products.
ISO 18416 Test Method
The cosmetic test sample is diluted directly in an enrichment broth at a 1:10 ratio, given that the sample is homogeneous in water.
The cosmetic samples that are non-homogenous in water are mixed with a solubilising agent before adding to the enrichment broth.
The cosmetic samples containing any antimicrobial agent acting as a preservative, their enrichment broth should be supplemented with a suitable neutraliser for the antimicrobial agent.
The cosmetic sample is incubated in the enrichment broth for 24 to 72 hours at 32.5°C and then transferred to a selective agar medium.
The selective agar medium is incubated at 32.5°C and examined at 24 and 48 hours.
For detecting the presence or absence of Candida albicans in the cosmetic sample, the selective medium is observed for compatible colonies.
The Candida albicans microorganism is identified by performing phenotypic and morphological tests on the recovered colonies.
0 notes
Text
ISO 18415 - Cosmetics - Microbiology Testing
ISO 18415 standard specifies guidelines for the detection of specified & non-specified microorganisms in cosmetic products.
ISO 18415 Test Method
To perform the test, cosmetic product samples are diluted in enrichment broth to prepare initial suspension.
Water-miscible products – 1 ml of product sample is diluted in 9 ml of enrichment broth.
Water-immiscible products – Product sample is mixed with a dispersing agent (e.g. polysorbate 80) and then added to the enrichment broth.
Filterable products – Product sample is transferred onto the membrane in a filtration apparatus. After filtration, the membrane is washed using water or diluent. This membrane is transferred into enrichment broth to make initial suspension.
This initial suspension is incubated at 32.5 °C for at least 20 h.
After incubation, an aliquot of the incubated enrichment broth is streaked onto the surface of non-selective agar medium in petri dishes.
Petri dishes are inverted and incubated at 32.5 °C for 48 h to 72 h.
Identification of microorganisms is done using an identification kit. This kit provides instructions for inoculation, incubation, and reading. Final results of the identification methods are compared with the database for final analysis.
0 notes
Text
ISO 20743 - Determine Antibacterial Activity of Textile Clothes
ISO 20743 is a quantitative test used to determine the antibacterial activity of finished textile products. ISO 20743 testing is applicable to products such as cloths, wadding, thread, etc.
Products to be Tested with ISO 20743
ISO 20743 test method is applicable to all textile products regardless of the type of antibacterial agents used for method of application. It includes –
cloth,
wadding,
thread,
bedclothes,
home furnishings,
and miscellaneous goods.
0 notes
Text
ASTM G22 - Determines Resistance of Plastic to Bacteria
ASTM G22 test is used to determine if antimicrobial plastic material is capable of killing the growth of microorganisms under specific conditions.
Plastic materials that can be tested using ASTM G22 include,
fabricated articles,
tubes,
rods,
Sheets,
coatings,
and film materials.
0 notes
Text
ISO 22196 - Antibacterial Plastic Test - Microbe Investigations
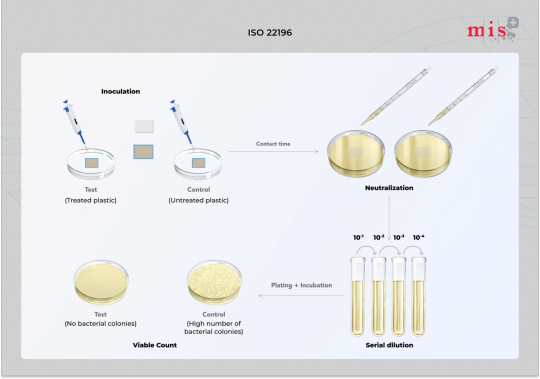
ISO 22196 test method is used to measure the antibacterial activity on plastics & other non-porous surfaces . Get A Quote today!
0 notes
Text
Conduct EPA Protocol #01-01A Test to evaluate the residual sanitizing efficacy
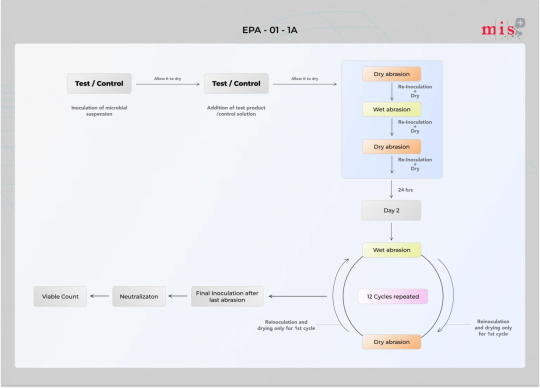
EPA Protocol #01 01A is a test Protocol for Residual Self-Sanitizing Activity of Dried Chemical Residues on Hard, Non-Porous Surfaces
0 notes
Text
ASTM E2799 - Assess Pseudomonas Aeruginosa biofilm disinfection
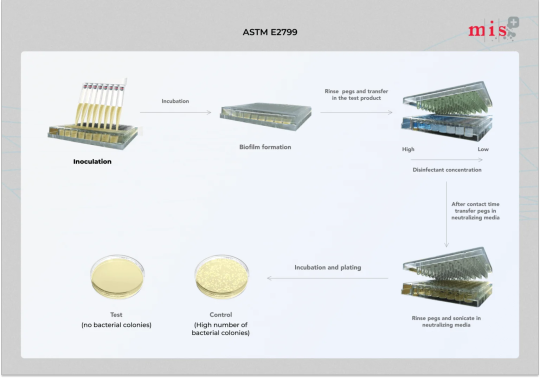
ASTM E2799 is a test method that measures disinfectant efficacy against Pseudomonas aeruginosa biofilm using the MBEC Assay.
0 notes
Text
ASTM E2149 - Antimicrobial Activity Test

ASTM E2149 test method is used to determine the antimicrobial activity of Immobilized Antimicrobial agents under dynamic contact conditions. Get a Quote today!
0 notes
Text
JIS Z 2801 - Test for Antibacterial Activity of Plastics

JIS Z 2801 test method is used to measure the antibacterial activity on plastics surfaces & textiles. Get A Quote today!
0 notes
Text
Conduct EPA MLB SOP MB-40 Test to Evaluate Antimicrobial Surface Coatings
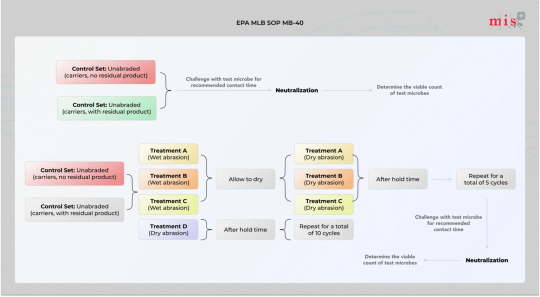
EPA MLB SOP MB-40 is a Test Method for Evaluating the Efficacy of Antimicrobial Surface Coatings
0 notes
Text
EPA MLB SOP MB-41 Test to Evaluate Antimicrobial Surface Coatings
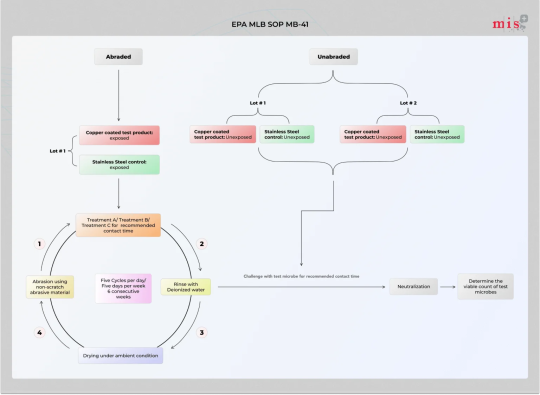
EPA MLB SOP MB-41 is a test Method for the Evaluation of Antimicrobial Activity of Hard, Non-porous Copper-Containing Surface Products
0 notes
Text
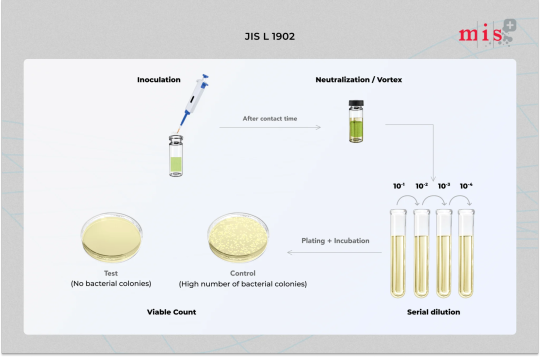
JIS L 1902 - Antibacterial Textile Test
JIS L 1902 is a certified testing method used to determine the antibacterial activity of finished textile products, including nonwovens.
0 notes
Text
ASTM E2871 - Evaluate disinfectant on CDC biofilm reactor-grown biofilm
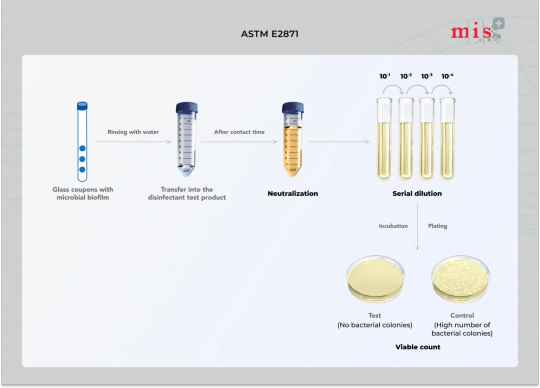
ASTM E2871 is a test method that evaluates the efficacy of disinfectants against biofilms grown in the CDC biofilm reactor, using the single tube method.
0 notes