#quinolinium
Text
0 notes
Text
1-Benzylquinolinium chlorideCAS#:15619-48-4

IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product Name1-Benzylquinolinium chlorideIUPAC Name1-benzylquinolin-1-ium;chloride Molecular StructureCAS Registry Number 15619-48-4EINECS Number239-695-2MDL NumberMFCD03939541Beilstein Registry NumberSynonyms1-Benzylquinolinium chloride15619-48-41-benzylquinolin-1-ium chlorideBenzylquinolinium chlorideN-Benzylquinolinium chlorideQuinolinium, 1-(phenylmethyl)-, chlorideQuinolinium, 1-(phenylmethyl)-, chloride (1:1)1-(Benzyl)quinolinium chloride1-benzylquinolin-1-ium;chlorideQuinolinium, 1-benzyl-, chlorideQuinoline-N-benzyl chloride quaternaryAI3-51333DTXSID8044593NSC-1903761-benzylquinolin-1-ium,chlorideEINECS 239-695-2NSC 1903761-benzylquinoliniumchloride1-benzylquinolin-1-iumchlorideSCHEMBL1071121CHEMBL3184319DTXCID60245939P729Y93KATox21_302628NSC190376AKOS016373521SB70509NCGC00256768-01LS-14470CAS-15619-48-4FT-0607413W-1104131-Benzylquinolinium chloride15619-48-41-benzylquinolin-1-ium chlorideBenzylquinolinium chlorideN-Benzylquinolinium chlorideQuinolinium, 1-(phenylmethyl)-, chlorideQuinolinium, 1-(phenylmethyl)-, chloride (1:1)1-(Benzyl)quinolinium chloride1-benzylquinolin-1-ium;chlorideQuinolinium, 1-benzyl-, chlorideQuinoline-N-benzyl chloride quaternaryAI3-51333DTXSID8044593NSC-1903761-benzylquinolin-1-ium,chlorideEINECS 239-695-2NSC 1903761-benzylquinoliniumchloride1-benzylquinolin-1-iumchlorideSCHEMBL1071121CHEMBL3184319DTXCID60245939P729Y93KATox21_302628NSC190376AKOS016373521SB70509NCGC00256768-01LS-14470CAS-15619-48-4FT-0607413W-110413Molecular FormulaC16H14ClNMolecular Weight255.74InChIInChI=1S/C16H14N.ClH/c1-2-7-14(8-3-1)13-17-12-6-10-15-9-4-5-11-16(15)17;/h1-12H,13H2;1H/q+1;/p-1 InChI KeyGFEJZIULYONCQB-UHFFFAOYSA-M Canonical SMILESC1=CC=C(C=C1)C2=CC=CC3=CC=CC=C32.
Physical Data
AppearancePink and brown powder
Melting Point, °C Solvent (Melting Point) 17065H2O
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Temperature (NMR Spectroscopy), °C Frequency (NMR Spectroscopy), MHzChemical shifts1Hwater-d2400Chemical shifts13Cchloroform-d1101
Route of Synthesis (ROS)
Route of Synthesis (ROS) of 1-benzylquinolinium-chloridecas-15619-48-4
ConditionsYieldWith sodium hydroxide In ethyl acetate at 80℃70 %Experimental Procedure General procedure: Halogenated quinolinium/pyridinium salts 1 (0.20 mmol, 1 equiv), sulfonyl azides 2 (0.24 mmol, 1.2 equiv), and NaOH (0.6 mmol, 3 equiv) were added to a 35-mL tube in EA (2 mL), and then the mixture was stirred at 80 °C for 7 h under air atmosphere. After complete conversion of the substrate (monitored by TLC), 20 mL of H2O was added, and the reaction mixture was extracted with EA (20 + 10 mL), washed with water (30 mL) and brine (30 mL), dried over Na2SO4 and concentrated under reduced pressure. Then the solution was filtered by flash chromatography (petroleum ether/ethyl acetate 10:1). The filtrate was evaporated by rotary evaporator, and the residue was purified by silica gel column chromatography to give the desired product 3.
Safety and Hazards
No data available
Other Data
TransportationUnder the room temperature;sealed.HS CodeStorageUnder the room temperature;sealed.Shelf Life1 yearMarket Price
DruglikenessLipinski rules componentMolecular Weight255.747logP5.807HBA0HBD0Matching Lipinski Rules3Veber rules componentPolar Surface Area (PSA)3.88Rotatable Bond (RotB)2Matching Veber Rules2
Quantitative Results1 of 3Comment (Pharmacological Data)Bioactivities presentReferenceThe Oxidation of 1-Alkyl(aryl)quinolinium Chlorides with Rabbit Liver Aldehyde Oxidase2 of 3Comment (Pharmacological Data)Bioactivities presentReferenceStructure of a novel Benzyl Quinolinium Chloride derivative and its effective corrosion inhibition in 15 wt.% hydrochloric acid3 of 3Comment (Pharmacological Data)physiological behaviour discussedReferenceIndolizine quaternary ammonium salt inhibitors, part III: Insights into the highly effective low-toxicity acid corrosion inhibitor-synthesis and protection performance
Use Pattern1-Benzylquinolinium chlorideCAS#:15619-48-4 is an intermediate.
Read the full article
0 notes
Text
Comprehending Affected individual Individuality inside Medical Care: Five-Factor Model.
vaginalis insensitive in order to metronidazole concentrations of up to One mM and also avoided the development involving metronidazole adducts with protein. Thioredoxin reductase activity was lacking from DPI-treated tissues along with Flavin reductase exercise has been sharply lowered. Additionally, DPI-treated tissues furthermore upregulated the actual phrase read more of antioxidant digestive enzymes, i.at the. thioredoxin peroxidases and also superoxide dismutases, and exhibited the fundamentally transformed metabolic rate a result of inactivation of pyruvate:ferredoxin oxidoreductase (PFOR) as well as concomitant upregulation regarding lactate dehydrogenase (LDH) task. As a result, the dysfunction in the cellular flavin metabolic rate by DPI mediated metabolism actions which can be similar to that regarding cells with metronidazole weight induced within vitro. Lastly, we found primary evidence that the increased expression regarding antioxidising digestive support enzymes can be dispensable with regard to getting effectiveness against metronidazole. (H) The year of 2010 Elsevier W.Versus. Almost all protection under the law earmarked.Color-tunable luminescent ionic liquid deposits have been developed like a brand-new compilation of luminescent resources. To realize tuning regarding exhaust shades, intramolecular cost exchange (ICT) character continues to be utilized in tripodal elements. A number of the actual compounds offers 3 chromophores in each compound, added with the two electron-donating moieties such as alkylaminobenzene and also alkoxybenzene, as well as electron-accepting moieties for example pyridinium, pyrimidinium, and also quinolinium elements. These C-3-symmetrical compounds self-assemble directly into liquid-crystalline (LC) columnar (Col) houses around extensive temperature by means of nanosegregation involving ionic moieties and nonionic aliphatic restaurants. Photoluminescent (PL) emissions of such tripodal elements tend to be seen in the actual obvious place in your self-assembled compacted states along with remedies. For example, any pyrimidinium sodium using didodecylaminobenzene moieties demonstrates yellowish or golden-tinged lemon exhaust (lambda(em) Is equal to 586 nm within a skinny film). Multicolor PL pollutants tend to be successfully achieved through easy adjusting of changing electron-donating and also electron-accepting moieties with the ingredients, in the noticeable location via blue-green for you to red-colored. It has been said ICT functions in the excited says and fragile intermolecular friendships perform crucial roles in the resolution of the actual PL components from the supplies, simply by sizes regarding UV-vis absorption and also emission spectra, fluorescence lives, along with PL massive produces.The actual predictive worth of the extra estrogen receptor (Emergeny room) W for treatment selections within breast cancer remains not clear. Retrospective scientific studies using preoperative endemic therapy (PST) revealed that radiation treatment and also hormonal treatment can lead to adjustments to phrase degrees of Im or her alpha dog as well as progesterone receptor (Public relations). The principle reason for these studies ended up being to examine Emergeny room beta appearance amounts before neoadjuvant chemotherapy or endocrine treatments and to discover a possible predictive valuation on ER experiment with. Coordinating 'baseline' biopsies along with post-therapy surgical examples involving Sixty nine breast cancers patients given neoadjuvant anthra-cycline- or even taxane-based radiation or even along with aromatase inhibitors had been examined for appearance numbers of ER leader, Public realtions, complete Im experiment with (Im or her experiment with to) Im or her try out 1, ER beta Only two and also the proliferation-related antigen Ki-67 employing immunohistochemistry. A marked appearance associated with Im or her experiment with big t considerably correlates along with low proliferation costs right after PST (p=0.0013) with reply to the idea.
#GPCR Compound Library#Androgen Receptor Antagonist#Vinorelbine#Hydrocortisone#Melatonin#Fluoxetine#Favipiravir#Dabigatran#Apixaban#Nitazoxanide#PMA#Pexidartinib#MG132#Rapamycin#Navitoclax#Telaglenastat#Mirdametinib#PEG300#Entinostat#Galunisertib#CL 59806#TKI-258#SHR-1258#NMS-P937#PLB-1001#NP031112#ABL001#IMI 28#SC 58635#NSC 113928
0 notes
Link
#Quinolinium#Dibromide '#Quinolinium Dibromide#Quinolinium Dibromide Market#Dibromide Market#Quinolinium Market
0 notes
Text
1-Benzylquinolinium chlorideCAS#:15619-48-4
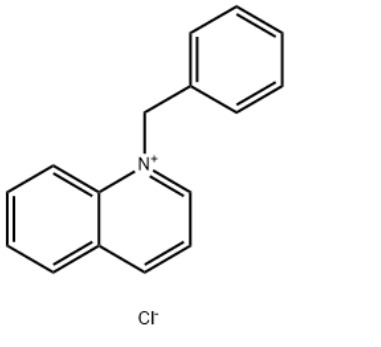
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product Name1-Benzylquinolinium chlorideIUPAC Name1-benzylquinolin-1-ium;chloride Molecular StructureCAS Registry Number 15619-48-4EINECS Number239-695-2MDL NumberMFCD03939541Beilstein Registry NumberSynonyms1-Benzylquinolinium chloride15619-48-41-benzylquinolin-1-ium chlorideBenzylquinolinium chlorideN-Benzylquinolinium chlorideQuinolinium, 1-(phenylmethyl)-, chlorideQuinolinium, 1-(phenylmethyl)-, chloride (1:1)1-(Benzyl)quinolinium chloride1-benzylquinolin-1-ium;chlorideQuinolinium, 1-benzyl-, chlorideQuinoline-N-benzyl chloride quaternaryAI3-51333DTXSID8044593NSC-1903761-benzylquinolin-1-ium,chlorideEINECS 239-695-2NSC 1903761-benzylquinoliniumchloride1-benzylquinolin-1-iumchlorideSCHEMBL1071121CHEMBL3184319DTXCID60245939P729Y93KATox21_302628NSC190376AKOS016373521SB70509NCGC00256768-01LS-14470CAS-15619-48-4FT-0607413W-1104131-Benzylquinolinium chloride15619-48-41-benzylquinolin-1-ium chlorideBenzylquinolinium chlorideN-Benzylquinolinium chlorideQuinolinium, 1-(phenylmethyl)-, chlorideQuinolinium, 1-(phenylmethyl)-, chloride (1:1)1-(Benzyl)quinolinium chloride1-benzylquinolin-1-ium;chlorideQuinolinium, 1-benzyl-, chlorideQuinoline-N-benzyl chloride quaternaryAI3-51333DTXSID8044593NSC-1903761-benzylquinolin-1-ium,chlorideEINECS 239-695-2NSC 1903761-benzylquinoliniumchloride1-benzylquinolin-1-iumchlorideSCHEMBL1071121CHEMBL3184319DTXCID60245939P729Y93KATox21_302628NSC190376AKOS016373521SB70509NCGC00256768-01LS-14470CAS-15619-48-4FT-0607413W-110413Molecular FormulaC16H14ClNMolecular Weight255.74InChIInChI=1S/C16H14N.ClH/c1-2-7-14(8-3-1)13-17-12-6-10-15-9-4-5-11-16(15)17;/h1-12H,13H2;1H/q+1;/p-1 InChI KeyGFEJZIULYONCQB-UHFFFAOYSA-M Canonical SMILESC1=CC=C(C=C1)C2=CC=CC3=CC=CC=C32.
Physical Data
AppearancePink and brown powder
Melting Point, °C Solvent (Melting Point) 17065H2O
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Temperature (NMR Spectroscopy), °C Frequency (NMR Spectroscopy), MHzChemical shifts1Hwater-d2400Chemical shifts13Cchloroform-d1101
Route of Synthesis (ROS)
Route of Synthesis (ROS) of 1-benzylquinolinium-chloridecas-15619-48-4
ConditionsYieldWith sodium hydroxide In ethyl acetate at 80℃70 %Experimental Procedure General procedure: Halogenated quinolinium/pyridinium salts 1 (0.20 mmol, 1 equiv), sulfonyl azides 2 (0.24 mmol, 1.2 equiv), and NaOH (0.6 mmol, 3 equiv) were added to a 35-mL tube in EA (2 mL), and then the mixture was stirred at 80 °C for 7 h under air atmosphere. After complete conversion of the substrate (monitored by TLC), 20 mL of H2O was added, and the reaction mixture was extracted with EA (20 + 10 mL), washed with water (30 mL) and brine (30 mL), dried over Na2SO4 and concentrated under reduced pressure. Then the solution was filtered by flash chromatography (petroleum ether/ethyl acetate 10:1). The filtrate was evaporated by rotary evaporator, and the residue was purified by silica gel column chromatography to give the desired product 3.
Safety and Hazards
No data available
Other Data
TransportationUnder the room temperature;sealed.HS CodeStorageUnder the room temperature;sealed.Shelf Life1 yearMarket Price
DruglikenessLipinski rules componentMolecular Weight255.747logP5.807HBA0HBD0Matching Lipinski Rules3Veber rules componentPolar Surface Area (PSA)3.88Rotatable Bond (RotB)2Matching Veber Rules2
Quantitative Results1 of 3Comment (Pharmacological Data)Bioactivities presentReferenceThe Oxidation of 1-Alkyl(aryl)quinolinium Chlorides with Rabbit Liver Aldehyde Oxidase2 of 3Comment (Pharmacological Data)Bioactivities presentReferenceStructure of a novel Benzyl Quinolinium Chloride derivative and its effective corrosion inhibition in 15 wt.% hydrochloric acid3 of 3Comment (Pharmacological Data)physiological behaviour discussedReferenceIndolizine quaternary ammonium salt inhibitors, part III: Insights into the highly effective low-toxicity acid corrosion inhibitor-synthesis and protection performance
Use Pattern1-Benzylquinolinium chlorideCAS#:15619-48-4 is an intermediate.
Read the full article
0 notes
Text
1-Benzylquinolinium chlorideCAS#:15619-48-4
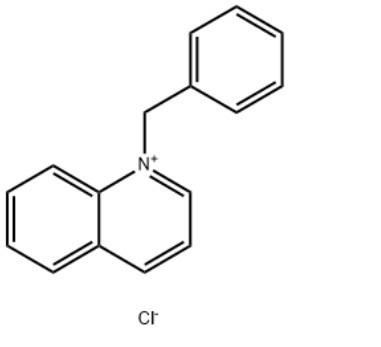
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product Name1-Benzylquinolinium chlorideIUPAC Name1-benzylquinolin-1-ium;chloride Molecular StructureCAS Registry Number 15619-48-4EINECS Number239-695-2MDL NumberMFCD03939541Beilstein Registry NumberSynonyms1-Benzylquinolinium chloride15619-48-41-benzylquinolin-1-ium chlorideBenzylquinolinium chlorideN-Benzylquinolinium chlorideQuinolinium, 1-(phenylmethyl)-, chlorideQuinolinium, 1-(phenylmethyl)-, chloride (1:1)1-(Benzyl)quinolinium chloride1-benzylquinolin-1-ium;chlorideQuinolinium, 1-benzyl-, chlorideQuinoline-N-benzyl chloride quaternaryAI3-51333DTXSID8044593NSC-1903761-benzylquinolin-1-ium,chlorideEINECS 239-695-2NSC 1903761-benzylquinoliniumchloride1-benzylquinolin-1-iumchlorideSCHEMBL1071121CHEMBL3184319DTXCID60245939P729Y93KATox21_302628NSC190376AKOS016373521SB70509NCGC00256768-01LS-14470CAS-15619-48-4FT-0607413W-1104131-Benzylquinolinium chloride15619-48-41-benzylquinolin-1-ium chlorideBenzylquinolinium chlorideN-Benzylquinolinium chlorideQuinolinium, 1-(phenylmethyl)-, chlorideQuinolinium, 1-(phenylmethyl)-, chloride (1:1)1-(Benzyl)quinolinium chloride1-benzylquinolin-1-ium;chlorideQuinolinium, 1-benzyl-, chlorideQuinoline-N-benzyl chloride quaternaryAI3-51333DTXSID8044593NSC-1903761-benzylquinolin-1-ium,chlorideEINECS 239-695-2NSC 1903761-benzylquinoliniumchloride1-benzylquinolin-1-iumchlorideSCHEMBL1071121CHEMBL3184319DTXCID60245939P729Y93KATox21_302628NSC190376AKOS016373521SB70509NCGC00256768-01LS-14470CAS-15619-48-4FT-0607413W-110413Molecular FormulaC16H14ClNMolecular Weight255.74InChIInChI=1S/C16H14N.ClH/c1-2-7-14(8-3-1)13-17-12-6-10-15-9-4-5-11-16(15)17;/h1-12H,13H2;1H/q+1;/p-1 InChI KeyGFEJZIULYONCQB-UHFFFAOYSA-M Canonical SMILESC1=CC=C(C=C1)C2=CC=CC3=CC=CC=C32.
Physical Data
AppearancePink and brown powder
Melting Point, °C Solvent (Melting Point) 17065H2O
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Temperature (NMR Spectroscopy), °C Frequency (NMR Spectroscopy), MHzChemical shifts1Hwater-d2400Chemical shifts13Cchloroform-d1101
Route of Synthesis (ROS)
Route of Synthesis (ROS) of 1-benzylquinolinium-chloridecas-15619-48-4
ConditionsYieldWith sodium hydroxide In ethyl acetate at 80℃70 %Experimental Procedure General procedure: Halogenated quinolinium/pyridinium salts 1 (0.20 mmol, 1 equiv), sulfonyl azides 2 (0.24 mmol, 1.2 equiv), and NaOH (0.6 mmol, 3 equiv) were added to a 35-mL tube in EA (2 mL), and then the mixture was stirred at 80 °C for 7 h under air atmosphere. After complete conversion of the substrate (monitored by TLC), 20 mL of H2O was added, and the reaction mixture was extracted with EA (20 + 10 mL), washed with water (30 mL) and brine (30 mL), dried over Na2SO4 and concentrated under reduced pressure. Then the solution was filtered by flash chromatography (petroleum ether/ethyl acetate 10:1). The filtrate was evaporated by rotary evaporator, and the residue was purified by silica gel column chromatography to give the desired product 3.
Safety and Hazards
No data available
Other Data
TransportationUnder the room temperature;sealed.HS CodeStorageUnder the room temperature;sealed.Shelf Life1 yearMarket Price
DruglikenessLipinski rules componentMolecular Weight255.747logP5.807HBA0HBD0Matching Lipinski Rules3Veber rules componentPolar Surface Area (PSA)3.88Rotatable Bond (RotB)2Matching Veber Rules2
Quantitative Results1 of 3Comment (Pharmacological Data)Bioactivities presentReferenceThe Oxidation of 1-Alkyl(aryl)quinolinium Chlorides with Rabbit Liver Aldehyde Oxidase2 of 3Comment (Pharmacological Data)Bioactivities presentReferenceStructure of a novel Benzyl Quinolinium Chloride derivative and its effective corrosion inhibition in 15 wt.% hydrochloric acid3 of 3Comment (Pharmacological Data)physiological behaviour discussedReferenceIndolizine quaternary ammonium salt inhibitors, part III: Insights into the highly effective low-toxicity acid corrosion inhibitor-synthesis and protection performance
Use Pattern1-Benzylquinolinium chlorideCAS#:15619-48-4 is an intermediate.
Read the full article
0 notes
Text
1-Benzylquinolinium chlorideCAS#:15619-48-4
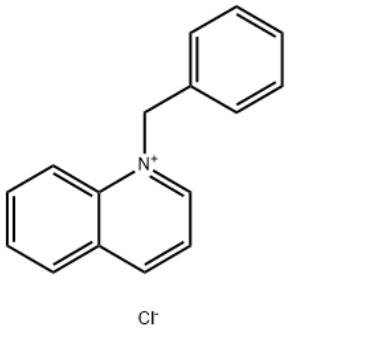
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product Name1-Benzylquinolinium chlorideIUPAC Name1-benzylquinolin-1-ium;chloride Molecular StructureCAS Registry Number 15619-48-4EINECS Number239-695-2MDL NumberMFCD03939541Beilstein Registry NumberSynonyms1-Benzylquinolinium chloride15619-48-41-benzylquinolin-1-ium chlorideBenzylquinolinium chlorideN-Benzylquinolinium chlorideQuinolinium, 1-(phenylmethyl)-, chlorideQuinolinium, 1-(phenylmethyl)-, chloride (1:1)1-(Benzyl)quinolinium chloride1-benzylquinolin-1-ium;chlorideQuinolinium, 1-benzyl-, chlorideQuinoline-N-benzyl chloride quaternaryAI3-51333DTXSID8044593NSC-1903761-benzylquinolin-1-ium,chlorideEINECS 239-695-2NSC 1903761-benzylquinoliniumchloride1-benzylquinolin-1-iumchlorideSCHEMBL1071121CHEMBL3184319DTXCID60245939P729Y93KATox21_302628NSC190376AKOS016373521SB70509NCGC00256768-01LS-14470CAS-15619-48-4FT-0607413W-1104131-Benzylquinolinium chloride15619-48-41-benzylquinolin-1-ium chlorideBenzylquinolinium chlorideN-Benzylquinolinium chlorideQuinolinium, 1-(phenylmethyl)-, chlorideQuinolinium, 1-(phenylmethyl)-, chloride (1:1)1-(Benzyl)quinolinium chloride1-benzylquinolin-1-ium;chlorideQuinolinium, 1-benzyl-, chlorideQuinoline-N-benzyl chloride quaternaryAI3-51333DTXSID8044593NSC-1903761-benzylquinolin-1-ium,chlorideEINECS 239-695-2NSC 1903761-benzylquinoliniumchloride1-benzylquinolin-1-iumchlorideSCHEMBL1071121CHEMBL3184319DTXCID60245939P729Y93KATox21_302628NSC190376AKOS016373521SB70509NCGC00256768-01LS-14470CAS-15619-48-4FT-0607413W-110413Molecular FormulaC16H14ClNMolecular Weight255.74InChIInChI=1S/C16H14N.ClH/c1-2-7-14(8-3-1)13-17-12-6-10-15-9-4-5-11-16(15)17;/h1-12H,13H2;1H/q+1;/p-1 InChI KeyGFEJZIULYONCQB-UHFFFAOYSA-M Canonical SMILESC1=CC=C(C=C1)C2=CC=CC3=CC=CC=C32.
Physical Data
AppearancePink and brown powder
Melting Point, °C Solvent (Melting Point) 17065H2O
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Temperature (NMR Spectroscopy), °C Frequency (NMR Spectroscopy), MHzChemical shifts1Hwater-d2400Chemical shifts13Cchloroform-d1101
Route of Synthesis (ROS)
Route of Synthesis (ROS) of 1-benzylquinolinium-chloridecas-15619-48-4
ConditionsYieldWith sodium hydroxide In ethyl acetate at 80℃70 %Experimental Procedure General procedure: Halogenated quinolinium/pyridinium salts 1 (0.20 mmol, 1 equiv), sulfonyl azides 2 (0.24 mmol, 1.2 equiv), and NaOH (0.6 mmol, 3 equiv) were added to a 35-mL tube in EA (2 mL), and then the mixture was stirred at 80 °C for 7 h under air atmosphere. After complete conversion of the substrate (monitored by TLC), 20 mL of H2O was added, and the reaction mixture was extracted with EA (20 + 10 mL), washed with water (30 mL) and brine (30 mL), dried over Na2SO4 and concentrated under reduced pressure. Then the solution was filtered by flash chromatography (petroleum ether/ethyl acetate 10:1). The filtrate was evaporated by rotary evaporator, and the residue was purified by silica gel column chromatography to give the desired product 3.
Safety and Hazards
No data available
Other Data
TransportationUnder the room temperature;sealed.HS CodeStorageUnder the room temperature;sealed.Shelf Life1 yearMarket Price
DruglikenessLipinski rules componentMolecular Weight255.747logP5.807HBA0HBD0Matching Lipinski Rules3Veber rules componentPolar Surface Area (PSA)3.88Rotatable Bond (RotB)2Matching Veber Rules2
Quantitative Results1 of 3Comment (Pharmacological Data)Bioactivities presentReferenceThe Oxidation of 1-Alkyl(aryl)quinolinium Chlorides with Rabbit Liver Aldehyde Oxidase2 of 3Comment (Pharmacological Data)Bioactivities presentReferenceStructure of a novel Benzyl Quinolinium Chloride derivative and its effective corrosion inhibition in 15 wt.% hydrochloric acid3 of 3Comment (Pharmacological Data)physiological behaviour discussedReferenceIndolizine quaternary ammonium salt inhibitors, part III: Insights into the highly effective low-toxicity acid corrosion inhibitor-synthesis and protection performance
Use Pattern1-Benzylquinolinium chlorideCAS#:15619-48-4 is an intermediate.
Read the full article
0 notes