#Point of Care (PoC) Diagnostics Market forecast
Text
Latin America Molecular Diagnostics Market - Opportunity Analysis and Industry Forecasts (2024-2031)
Meticulous Research®—a leading market research company, published a research report titled, 'Latin America Molecular Diagnostics Market by Offering (Reagents, Kits, Systems, Software, Services), Test Type (Lab, PoC), Technology (PCR, ISH, INAAT, Sequencing, Microarray), Application (HIV, Influenza, HPV, Oncology), End User - Forecast to 2031’
According to this latest publication from Meticulous Research®, the Latin America molecular diagnostics market is projected to reach $2.50 billion by 2031, at a CAGR of 6.3% from 2024 to 2031. The growth of the Latin America molecular diagnostics market is driven by several factors, including the rising global geriatric population, increasing prevalence of communicable & noncommunicable diseases, technological advancements in molecular diagnostics, and rising healthcare expenditures. Factors such as growing scope in emerging economies, increasing focus on companion diagnostics, and the rising popularity of direct-to-consumer (DTC) testing are a few opportunities that would help grow the market in the future. A shortage of skilled professionals could be considered a challenge for the Latin America molecular diagnostics market. However, unfavorable regulatory frameworks and high costs of molecular diagnostic tests restrain the growth of the market.
Key Players:
The key players profiled in the Latin America molecular diagnostics market report are Bio-Manguinhos (Brazil), F. Hoffmann-La Roche Ltd. (Switzerland), Thermo Fisher Scientific Inc. (U.S.), Hologic, Inc. (U.S.), Illumina, Inc. (U.S.), OmicronLab (Mexico), QIAGEN N.V. (Netherlands), Danaher Corporation (U.S.), Abbott Laboratories (U.S), Agilent Technologies, Inc. (U.S.).
The Latin America molecular diagnostics market is segmented by Offering (Kits & Reagents, Instruments, Software & Services), Test Type (Laboratory Tests and Point-of-Care (POC) Tests), Technology (Polymerase Chain Reaction (PCR), In Situ Hybridization (ISH), Isothermal Nucleic Acid Amplification Technology (INAAT), Microarrays, Sequencing, Mass Spectrometry, and Other Technologies), Application (Infectious Diseases [Respiratory Diseases, Hepatitis, HIV, Chlamydia Trachomatis/Neisseria Gonorrhoeae, Human Papillomavirus (HPV), Healthcare-Associated Infections (HAIs), and Other Infectious Diseases], Oncology [Breast Cancer, Colorectal Cancer, Lung Cancer, Prostate Cancer, Lymphoma, Leukemia, Cervical Cancer, and Other Cancer Types], Genetic Testing, Neurological Diseases, Cardiovascular Diseases, and Other Applications), End User (Hospitals & Clinics, Diagnostic Laboratories, Academic & Research Institutes, and Other End Users). The study also evaluates industry competitors and analyzes the regional and country-level markets.
Among offerings, in 2024, the kits and reagents segment is expected to account for the largest share of the market. The large share of the segment is attributed to the commercial availability of a diverse range of diagnostic reagents & consumables, the availability of diseases-specific test kits & assays, and the growing awareness regarding early disease diagnosis.
Among test types, in 2024, the laboratory test segment is expected to account for the largest share of the market. The large share of the segment is attributed to the large number of laboratory tests available in hospitals, laboratories, and academic & research institutes and also preferred tests by the patients. Additionally, the majority of developments are being done using laboratory tests.
Among technologies, in 2024, the polymerase chain reaction (PCR) segment is expected to account for the largest share of the market. The large share of the segment is attributed to benefits, such as the ability to test multi-drug resistance, its use in several laboratories and clinical techniques, including DNA fingerprinting, detection of bacteria or viruses (particularly AIDS), and diagnosis of genetic disorders.
Among applications, in 2024, the infectious diseases segment is expected to account for the largest share of the market. The large share of the segment is attributed to the rising prevalence of infectious diseases, the increase in funding for the development of new infectious disease diagnostic tools, and the emergence of the COVID-19 pandemic.
Among end users, the hospitals & clinics segment is expected to account for the largest share of the market in 2024. The large share of the segment is attributed to the increased number of hospitalizations due to various diseases requiring molecular diagnosis and the proliferation of hospitals and clinics in emerging countries like Brazil, Chile, Colombia, and Mexico, among others, leading to the growth in the utilization of molecular diagnostic products.
Download Sample of Report @ https://www.meticulousresearch.com/download-sample-report/cp_id=5759
Key questions answered in the report:
Which are the high-growth market segments in terms of molecular diagnostics by offering, test type, technology, application, end user, and geography?
What was the historical market for molecular diagnostics across Latin America?
What are the market forecasts and estimates for the period 2024–2031?
What are the major drivers, restraints, opportunities, and challenges in the Latin America molecular diagnostics market?
Who are the major players in the Latin America molecular diagnostics market?
What is the competitive landscape, and who are the market leaders in the Latin America molecular diagnostics market?
What are the recent developments in the Latin America molecular diagnostics market?
What are the different strategies adopted by the major players in the Latin America molecular diagnostics market?
What are the geographical trends and high-growth regions/countries?
Contact Us:
Meticulous Research®
Email- [email protected]
Contact Sales- +1-646-781-8004
Connect with us on LinkedIn- https://www.linkedin.com/company/meticulous-research
#LatinAmerica#MolecularDiagnosticsMarket#MolecularDiagnostics#MolecularTest#MolecularLaboratory#PCR#MolecularTesting#MDx
0 notes
Text
Southeast Asia Molecular Diagnostics Market Outlook: Global Opportunity Analysis and Industry Forecast (2024-2031)
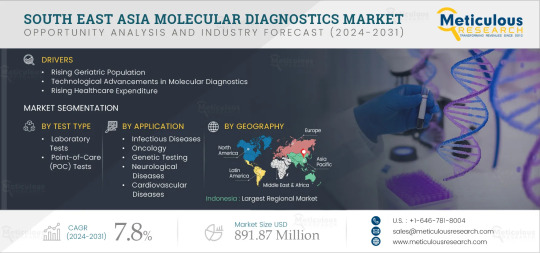
Meticulous Research®—a leading market research company, published a research report titled, ‘South East Asia Molecular Diagnostics Market by Offering (Reagents, Kits, Systems, Software), Test Type (Lab, PoC), Technology (PCR, ISH, INAAT, Sequencing, Microarray), Application (HIV, Influenza, HPV, Oncology, Gene Testing), End User - Forecast to 2031’
Download Free Sample Report@ https://www.meticulousresearch.com/download-sample-report/cp_id=5775?utm_source=pdf&utm_medium=social&utm_campaign=product&utm_content=25-04-2024
According to this latest publication from Meticulous Research®, the South East Asia molecular diagnostics market is projected to reach $891.87 million by 2031, at a CAGR of 7.8% from 2024 to 2031. The growth of the South East Asia molecular diagnostics market is driven by several factors, including the rising geriatric population, increasing prevalence of communicable & non-communicable diseases, technological advancements in molecular diagnostics, and rising healthcare expenditure. Factors such as emerging medical tourism in South East Asian countries and increasing focus on companion diagnostics are a few opportunities that would help grow the market in the future. A shortage of skilled professionals could be considered a challenge for the South East Asia molecular diagnostics market. Moreover, the lack of harmonization of the medical device regulations across South East Asian countries and the high costs of molecular diagnostic tests restrain the growth of the market.
Key Players
The key players profiled in the South East Asia molecular diagnostics market report are Sansure Biotech, Inc. (China), F. Hoffmann-La Roche Ltd. (Switzerland), Thermo Fisher Scientific Inc. (U.S.), Hologic, Inc. (U.S.), Illumina, Inc. (U.S.), Xiamen Zeesan Biotech Co., Ltd (China), QIAGEN N.V. (Netherlands), Danaher Corporation (U.S.), Abbott Laboratories (U.S), Agilent Technologies, Inc. (U.S.).
Geographic Review
The South East Asia molecular diagnostics market is segmented by Offering (Kits & Reagents, Instruments, Software & Services), Test Type (Laboratory Tests and Point-of-Care (POC) Tests), Technology (Polymerase Chain Reaction (PCR), In Situ Hybridization (ISH), Isothermal Nucleic Acid Amplification Technology (INAAT), Microarrays, Sequencing, Mass Spectrometry, and Other Technologies), Application (Infectious Diseases [Respiratory Diseases, Hepatitis, HIV, Chlamydia Trachomatis/Neisseria Gonorrhoeae, Human Papillomavirus (HPV), Healthcare-Associated Infections (HAIs), and Other Infectious Diseases], Oncology [Breast Cancer, Colorectal Cancer, Lung Cancer, Prostate Cancer, Lymphoma, Leukemia, Cervical Cancer, and Other Cancer Types], Genetic Testing, Neurological Diseases, Cardiovascular Diseases, and Other Applications), End User (Hospitals & Clinics, Diagnostic Laboratories, Academic & Research Institutes, and Other End Users). The study also evaluates industry competitors and analyzes the regional and country-level markets.
Among offerings, in 2024, the kits and reagents segment is expected to account for the largest share of the market. The large share of the segment is attributed to the commercial availability of a diverse range of diagnostic reagents & consumables, the availability of diseases-specific test kits & assays, and the growing awareness regarding early disease diagnosis.
Request Sample@ https://www.meticulousresearch.com/request-sample-report/cp_id=5775?utm_source=pdf&utm_medium=social&utm_campaign=product&utm_content=25-04-2024
Among test types, in 2024, the laboratory test segment is expected to account for the largest share of the market. The large share of the segment is attributed to the large number of laboratory tests available in hospitals and laboratories, and the high preference for laboratory tests owing to the accuracy of the test results supports the largest share of the market.
Among technologies, in 2024, the polymerase chain reaction (PCR) segment is expected to account for the largest share of the market. The large share of the segment is attributed to benefits, such as the ability to test multi-drug resistance, its use in several laboratories and clinical techniques, including DNA fingerprinting, detection of bacteria or viruses (particularly AIDS), and diagnosis of genetic disorders. Improved PCR testing capabilities in the laboratories during the COVID-19 pandemic to manage the growing burden also supported the segment’s largest share.
Among applications, in 2024, the infectious diseases segment is expected to account for the largest share of the market. The large share of the segment is attributed to the rising prevalence of infectious diseases, the increase in funding for the development of new infectious disease diagnostic tools, and the emergence of the COVID-19 pandemic. According to the World Health Organization (WHO), in 2022, the largest number of tuberculosis (TB) cases occurred in the South-East Asian region, with a prevalence of 46%. The increasing prevalence of such infections and diseases leads to the increased demand for rapid, easy-to-use, and affordable infectious disease testing tools, therefore driving the growth of the segment.
Among end users, the hospitals & clinics segment is expected to account for the largest share of the market in 2024. The large share of the segment is attributed to the increased number of hospitalizations due to various diseases requiring molecular diagnosis and the proliferation of hospitals and clinics in emerging countries, leading to the growth in the utilization of molecular diagnostic products. The emergence of new hospitals in South East Asian countries is leading to the growing demand for molecular diagnostic tests. For instance, in March 2023, Adventist Hospital was launched in Palangka Raya, Central Kalimantan, Indonesia, which is a 51-bed, state-of-the-art hospital that offers general medicine, surgery, obstetrics and gynecology, pediatrics, and critical care services to the inhabitants of Palangka Raya and nearby communities. This not only increases the demand for molecular tests but also provides these advanced technologies to people living in rural areas.
Among geographies, in 2024, Indonesia is expected to account for the largest share of the market. The large share of the segment is attributed to the increasing prevalence of communicable & non-communicable diseases, growing adoption of molecular diagnostics, increasing prevalence of cancer and musculoskeletal diseases, rising disposable income, improvements in the healthcare infrastructure, and the growing healthcare expenditure. The increasing prevalence of diseases is driving the need for better healthcare options among patients. In such cases, increasing healthcare expenditure can help increase people’s access to healthcare and improve the quality of care. For instance, the healthcare expenditure in Indonesia increased from USD 8.07 billion in 2019 to USD 17.62 billion in 2022.
Buy Now@ https://www.meticulousresearch.com/Checkout/49985136?utm_source=pdf&utm_medium=social&utm_campaign=product&utm_content=25-04-2024
About Meticulous Research®:
Meticulous Research® is a leading global market research company that helps businesses make better decisions through insightful market intelligence and comprehensive industry analysis. With a repository of over [Number] reports spanning various industries, Meticulous Research® offers unparalleled expertise and actionable insights to empower organizations to navigate complex market challenges and achieve sustainable growth.
Contact Us:
Meticulous Research®
Email- [email protected]
Contact Sales- +1-646-781-8004
Connect with us on LinkedIn- https://www.linkedin.com/company/meticulous-research
0 notes
Text
Analyzing the Procalcitonin Tests Market by Test Type, End User, and Region
Analyzing the procalcitonin tests market by test type, end user, and region provides valuable insights into the factors driving demand and the dynamics of this market.

Buy the Full Report for More Insights on the Procalcitonin Tests Market Forecast,
Download a Free Sample Report
Here's an analysis of each category:
Test Type:a. Procalcitonin Assay Kits: Procalcitonin assay kits are used in clinical laboratories and healthcare facilities to measure procalcitonin levels in patient blood samples. These kits typically utilize immunoassay techniques such as enzyme-linked immunosorbent assay (ELISA) or chemiluminescent immunoassay (CLIA) to quantitatively detect procalcitonin levels. Assay kits may be available in various formats, including manual assays and automated assay systems.b. Point-of-Care (POC) Tests: Point-of-care procalcitonin tests are rapid diagnostic tests designed for use at the bedside or in outpatient settings. POC tests offer the advantages of fast turnaround times, enabling timely decision-making regarding antibiotic therapy in patients with suspected sepsis or bacterial infections. These tests are typically based on lateral flow immunoassay or other rapid immunoassay technologies.
End User:a. Hospitals and Clinics: Hospitals and clinics represent the largest end-user segment for procalcitonin tests, accounting for a significant portion of test volumes. Procalcitonin testing is commonly performed in hospital laboratories and clinical settings to aid in the diagnosis and management of sepsis, bacterial infections, and other inflammatory conditions. Hospitals may utilize both laboratory-based assays and point-of-care tests depending on their testing requirements and infrastructure.b. Diagnostic Laboratories: Independent diagnostic laboratories and reference laboratories also play a key role in the procalcitonin tests market. These laboratories offer testing services to healthcare providers, including hospitals, clinics, and physician offices, and may perform procalcitonin testing as part of routine clinical laboratory services or specialized infectious disease testing panels.c. Ambulatory Care Centers: Ambulatory care centers, including urgent care centers and outpatient clinics, may perform procalcitonin testing to aid in the diagnosis and management of infectious diseases in ambulatory patients. Point-of-care procalcitonin tests are particularly well-suited for use in ambulatory care settings due to their rapid turnaround times and ease of use.
Region:a. North America: North America is a significant market for procalcitonin tests, driven by factors such as the high prevalence of sepsis and bacterial infections, well-established healthcare infrastructure, and increasing adoption of advanced diagnostic technologies. The region is characterized by a strong presence of key market players, extensive research and development activities, and stringent regulatory standards governing diagnostic testing.b. Europe: Europe is another major market for procalcitonin tests, with countries such as Germany, France, and the United Kingdom leading in terms of market share. The region benefits from a robust healthcare system, growing awareness of sepsis management protocols, and favorable reimbursement policies for diagnostic testing. Increasing efforts to combat antimicrobial resistance also drive demand for procalcitonin testing in Europe.c. Asia-Pacific: The Asia-Pacific region represents a rapidly growing market for procalcitonin tests, fueled by factors such as the rising incidence of infectious diseases, expanding healthcare infrastructure, and increasing healthcare expenditure. Countries such as China, India, and Japan are witnessing significant market growth, driven by investments in healthcare modernization, improving access to diagnostic services, and growing awareness of sepsis management strategies.d. Latin America, Middle East, and Africa: These regions also contribute to the global procalcitonin tests market, albeit to a lesser extent compared to North America, Europe, and Asia-Pacific. Market growth in these regions is influenced by factors such as the burden of infectious diseases, healthcare infrastructure development, and efforts to enhance diagnostic capabilities in underserved areas.
Analyzing the procalcitonin tests market by test type, end user, and region enables stakeholders to identify key market segments, understand regional variations in demand, and formulate targeted strategies to address the needs of different market segments. Factors such as healthcare infrastructure, regulatory landscape, reimbursement policies, and disease epidemiology play a crucial role in shaping market dynamics and market growth opportunities across different regions.
0 notes
Text
Decoding U.S. Cardiac Troponin Market: Insights, Trends, and Patient Impact
The report presents an in-depth assessment of the ‘U.S. Cardiac Troponin Market’. This includes enabling technologies, key trends, market drivers, challenges, standardization, regulatory landscape, deployment models, competitive analysis, operator case studies, opportunities, future trends, value chains, ecosystem player profiles, and strategies included. The report also presents a SWOT analysis and forecast for U.S. Cardiac Troponin investments from 2024 to 2033.
Click the link to get a sample copy of the report: https://wemarketresearch.com/reports/request-free-sample-pdf/us-cardiac-troponin-market/1434
Top Companies in the U.S. Cardiac Troponin Market:
Hoffmann-La Roche Ltd.
bioMérieux SA
Abbott
Siemens Healthineers AG
PerkinElmer Inc.
Beckman Coulter, Inc.
LifeSign LLC.
Global U.S. Cardiac Troponin Market Segments:
By Indication
Myocardial Infarction
Congestive Heart Failure
Acute Coronary Syndrome
Others
By Setting
Laboratory Testing
Point-of-Care (POC) Testing
By End-User
Hospitals & Clinics
Diagnostic Laboratories
Homecare Settings
Others
Regional Analysis for U.S. Cardiac Troponin Market:
For a comprehensive understanding of market dynamics, the global U.S. Cardiac Troponin market is analysed across key geographies namely North America, Europe, China, Japan, Southeast Asia, India, Central & South America. Each of these regions is analyzed based on market research findings for the key countries in the region for a macro-level understanding of the market.
Important sections of the TOC
Economic Impact Variables on U.S. Cardiac Troponin Market: Illuminates the consequences of environmental, political and economic fluctuations, and explains changes in customer and consumer requirements. We also provide a detailed report of U.S. Cardiac Troponin on the technology risks and advancements in the global market.
Forecasts based on macro- and micro-economy: ensuring price, revenue and volume EV charging service forecasts for the market. It also includes, in addition to forecasting growth, revenue and import volume for the region, with revenue forecasting for the U.S. Cardiac Troponin application, along with revenue forecasting by cost, revenue and type.
Marketing Strategy Analysis: In this section, U.S. Cardiac Troponin analysis aims at niche positioning and provides information regarding target audience, new strategies and pricing strategies. We provide a comprehensive U.S. Cardiac Troponin marketing station analysis that investigates the problem. Marketing channel development trends, direct marketing as well as indirect marketing.
Business Intelligence: The U.S. Cardiac Troponin companies studied in this section are also assessed by key business, gross margin, price, sales, revenue, product category, applications and specifications, U.S. Cardiac Troponin competitors, and manufacturing base.
Directly Buy a Copy of this U.S. Cardiac Troponin Market research report at@ https://wemarketresearch.com/purchase/us-cardiac-troponin-market/1434?license=single
Why to buy this Report?
The report provides valuable insights into market trends, growth opportunities, and competitive landscapes. By reading a technology report, businesses and investors can gain a better understanding of the market they are operating in or considering entering, and make more informed decisions based on data and analysis.
The report reports provide detailed information on competitors' strengths, weaknesses, and strategies, which can help businesses identify potential threats and opportunities in the market.
The report provides insights into emerging technologies and trends, which can help businesses stay up-to-date with the latest developments and make informed decisions about where to invest their resources.
The report can be used by investors and acquirers as part of their due diligence process when considering investing in or acquiring a technology company. These reports can provide valuable information on the company's financials, technology, market position, and other key factors.
About We Market Research:
WE MARKET RESEARCH is an established market analytics and research firm with a domain experience sprawling across different industries. We have been working on multi-county market studies right from our inception. Over the time, from our existence, we have gained laurels for our deep rooted market studies and insightful analysis of different markets.
Our strategic market analysis and capability to comprehend deep cultural, conceptual and social aspects of various tangled markets has helped us make a mark for ourselves in the industry. WE MARKET RESEARCH is a frontrunner in helping numerous companies; both regional and international to successfully achieve their business goals based on our in-depth market analysis. Moreover, we are also capable of devising market strategies that ensure guaranteed customer bases for our clients.
Contact Us:
Mr. Robbin Joseph
Corporate Sales, USA
We Market Research
USA: +1-724-618-3925
Websites: https://wemarketresearch.com/
Email: [email protected]
0 notes
Text
Point of Care (PoC) Lipid Test Market Size, Share, Trends, Demand, Growth and Competitive Analysis
Data Bridge Market research has recently released expansive research titled Global Point of Care (PoC) Lipid Test Market guarantees you will remain better informed than your competition.
Point of Care (PoC) Lipid Test Market report gives explanation about the different segments of the market analysis which is demanded by today’s businesses. Key players are taking actions such as developments, product launches, acquisitions, mergers, joint ventures and competitive analysis in the industry. All the market aspects are estimated and analysed by a team of innovative, enthusiastic and motivated researchers and analysts so that nothing lefts uncovered in the report. Global Point of Care (PoC) Lipid Test Market research report, it becomes easy to figure out brand awareness and insight about the brand and product among potential customers.
Data Bridge Market Research analyses that the point of care (PoC) lipid test market which is USD 2.86 million in 2022, is expected to reach USD 4.17 million by 2030, at a CAGR of 4.84% during the forecast period 2023 to 2030.
Access Full 350 Pages PDF Report @
https://www.databridgemarketresearch.com/reports/global-point-of-care-poc-lipid-test-market
Points of care (POC) tests are medical tests that aid in the diagnosis of a problem in the human body at a specific point. The point of care tests aid in providing real-time results in a matter of minutes. As a result, a point of care (POC) lipid test is performed to assess the function of the lipid in the cells. When cholesterol levels rise, it disrupts the functioning of lipids in the body, leading to serious illnesses such as heart attack and coronary artery disease.
Some of the major players operating in the point of care (PoC) lipid test market are:
Abbott (U.S.)
F. Hoffmann-La Roche Ltd (Switzerland)
BD (U.S.)
Siemens Healthcare Private Limited (Germany)
Bio-Rad Laboratories, Inc. (U.S.)
Danaher (U.S.)
Sekisui Diagnostics (U.S.)
Trinity Biotech (Ireland)
BIOMÉRIEUX (France)
EKF Diagnostics (Germany)
AccuBioTech Co., Ltd. (China)
Instrumentation Laboratory (U.S.)
Beckman Coulter, Inc. (U.S.)
PTS Diagnostics (U.S.)
Nova Biomedical (U.S.)
Chembio Diagnostics, Inc. (U.S.)
Quidel Corporation (U.S.)
Sienco, Inc (U.S.)
Core Objective of Point of Care (PoC) Lipid Test Market:
Every firm in the Point of Care (PoC) Lipid Test Market has objectives but this market research report focus on the crucial objectives, so you can analysis about competition, future market, new products, and informative data that can raise your sales volume exponentially.
Size of the Point of Care (PoC) Lipid Test Market and growth rate factors.
Important changes in the future Point of Care (PoC) Lipid Test Market.
Top worldwide competitors of the Market.
Scope and product outlook of Point of Care (PoC) Lipid Test Market.
Developing regions with potential growth in the future.
Tough Challenges and risk faced in Market.
Global Point of Care (PoC) Lipid Test top manufacturers profile and sales statistics.
Key takeaways from the Point of Care (PoC) Lipid Test Market report:
Detailed considerate of Point of Care (PoC) Lipid Test Market-particular drivers, Trends, constraints, Restraints, Opportunities and major micro markets.
Comprehensive valuation of all prospects and threat in the
In depth study of industry strategies for growth of the Point of Care (PoC) Lipid Test Market-leading players.
Point of Care (PoC) Lipid Test Market latest innovations and major procedures.
Favorable dip inside Vigorous high-tech and market latest trends remarkable the Market.
Conclusive study about the growth conspiracy of Point of Care (PoC) Lipid Test Market for forthcoming years.
Frequently Asked Questions
What is the Future Market Value for Point of Care (PoC) Lipid Test Market?
What is the Growth Rate of the Point of Care (PoC) Lipid Test Market?
What are the Major Companies Operating in the Point of Care (PoC) Lipid Test Market?
Which Countries Data is covered in the Point of Care (PoC) Lipid Test Market?
What are the Main Data Pointers Covered in Point of Care (PoC) Lipid Test Market Report?
Browse Trending Reports:
https://www.databridgemarketresearch.com/reports/global-whole-genome-bisulfite-sequencing-wgbs-market
https://www.databridgemarketresearch.com/reports/global-behavioral-health-market
https://www.databridgemarketresearch.com/reports/global-medical-second-opinion-market
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Contact Us:
Data Bridge Market Research
US: +1 888 387 2818
UK: +44 208 089 1725
Hong Kong: +852 8192 7475
Email: [email protected]
0 notes
Text
Magnetic Beads Market Size by Research Nester Reveals the Market to Grow with a CAGR of ~11% During 2023-2036
Research Nester’s recent market research analysis on “Magnetic Beads Market: Global Demand Analysis & Opportunity Outlook 2036” delivers a detailed competitors analysis and a detailed overview of the global magnetic beads market in terms of market segmentation by product, type, application, end-user and by region.
Request Report Sample@
Rising Application in Molecular Biology Application to Promote Global Market Share of Magnetic Beads Market
The global magnetic beads market is estimated to grow majorly on account of the increased usage in medical applications, DNA & RNA extraction methods, bioassays, and life sciences applications. Other than this, rising incidences of tumors and cancer are also propelling the demand for magnetic beads, especially for leukemia. It has been estimated that in the United States, a total of 184,721 people were diagnosed with leukemia. The magnetic beads are considered to help separate the cells and remove cell tumors from the bone marrow and isolate the lymphoid cells from the blood film. On the back of this, the global magnetic beads market is projected to flourish in the forecasted period. Other than this, research related to the microbiomes has become prominent in recent years. The increase in investment in microbiological and genetic research over a prolonged period is projected to boost the demand for the product remarkably. The usage of these beads will accelerate more research-based investigations will be conducted throughout, to discover new therapeutic methods worldwide. Furthermore, the demand for magnetic beads market is projected to expand on the back of the rising production of healthcare products. The rise in the trend of POC which is the point of care in the laboratories as well as at the door of patients is projected to raise the usage of magnetic beads.
Some of the major growth factors and challenges that are associated with the growth of the global magnetic beads market are:
Growth Drivers:
Rising Research Spending is Augmenting the Demand
Increased Usage of Magnetic Beads in Medicated Wearables
Challenges:
Factors such as a decrease in magnetic force with the rise in distance, exorbitant price of magnetic beads, and less research in the development of magnetic beads are some of the major factors anticipated to hamper the global market size of the global magnetic beads market.
By application, the global magnetic beads market is segmented into cell separation & cell expansion, exosome analysis, protein sample prep and protein isolation, IVD assay development, and nucleic acid isolation. Out of these, the IVD assay development segment will rise the most during the forecast period and is projected to garner revenue of almost 50%.
By region, the Europe magnetic beads market is to generate the highest revenue by the end of 2036. The market is projected to flourish because of the rising demand for diagnostic and therapeutic solutions.
Access our detailed report at:
Research Nester is a leading service provider for strategic market research and consulting. We aim to provide unbiased, unparalleled market insights and industry analysis to help industries, conglomerates and executives to take wise decisions for their future marketing strategy, expansion and investment etc. We believe every business can expand to its new horizon, provided a right guidance at a right time is available through strategic minds. Our out of box thinking helps our clients to take wise decision in order to avoid future uncertainties.
Contact for more Info:
AJ Daniel
0 notes
Text
0 notes
Text
Infectious Disease Diagnostics Market Size Worth USD 45,090.5 Million in 2030
The global infectious disease diagnostics market size was USD 24,879.8 Million in 2021 and is expected to register a steady revenue CAGR of 6.8% during the forecast period, according to latest analysis by Emergen Research. Rising adoption of Point of Care (POC) devices, technological advancement for infectious diseases, increasing prevalence of infectious diseases especially Hospital Acquired Infections (HAIs), and number of diagnostic laboratories, and regulatory approvals and government guidelines related to infectious diagnostics are major factors driving market revenue growth.
0 notes
Text
Latin America PoC Diagnostics Market by Product (Blood glucose monitoring, Coagulation monitoring, Hematology testing, Cholesterol testing), Platform (Microfluidics, Molecular diagnostics), Mode (OTC Testing), End User- Forecast to 2027
#Latin America PoC Diagnostics Market#PoC Diagnostics Market#PoC Diagnostics#point of care diagnostics#point of care testing#rapid test#healthcare#medical equipment#medical devices
0 notes
Text
Middle East IVD Market - Opportunity Analysis and Industry Forecast (2024-2031)
Meticulous Research®, a leading global market research company, published a research report titled, ‘Middle East IVD Market by Offering (Kits, Software), Technology (Immunoassay, Molecular Diagnostics [PCR, NGS, Microarray], Rapid Tests, Biochemistry), Application (Infectious Diseases, Oncology), Diagnostic Approach (Lab) - Forecast to 2031.’
According to this latest publication from Meticulous Research®, the Middle East In Vitro Diagnostics (IVD) market is expected to reach $2.15 billion by 2031 at a CAGR of 4.1% from 2024 to 2031. The growth of the Middle East IVD market is attributed to the rising prevalence of chronic and infectious diseases, the growing demand for point-of-care (PoC) and rapid diagnostics, rising healthcare expenditures, growth in the aging population, and rising awareness about healthcare. However, high prices for IVD tests and the variance in test results observed in rapid IVD tests restrain the growth of the IVD market.
The rising awareness regarding early disease diagnosis and increasing inclination toward personalized medicine in the UAE and Saudi Arabia are expected to create growth opportunities for the players operating in this market. However, the concerns pertaining to false positive results in immunoassays and POC are a major challenge for market growth.
Key Players
The key players operating in the Middle East IVD market are Abbott Laboratories (U.S.), Becton, Dickinson and Company (U.S.), BioMérieux S.A. (France), Danaher Corporation (U.S.), F. Hoffmann-La Roche (Switzerland), QIAGEN N.V. (Netherlands), Siemens Healthineers AG (Germany), Thermo Fisher Scientific Inc. (U.S.), Bio-Rad Laboratories, Inc. (U.S.), Illumina, Inc. (U.S.), QuidelOrtho Corporation. (U.S.), Agilent Technologies, Inc. (U.S.), and DiaSorin S.p.A. (Italy).
Middle East IVD Market: Future Outlook
The Middle East IVD market is segmented by Offering (Kits & Reagents, Instruments, and Software & Services), Technology (Immunoassay/Immunochemistry, Biochemistry/Clinical Chemistry, Molecular Diagnostics, Point of Care (POC) Diagnostics, Whole Blood Glucose Monitoring, Microbiology, Hematology, Coagulation/Hemostasis, Urinalysis, and Other Technologies), Application (Infectious Diseases, Oncology, Diabetes, Cardiology, Nephrology, Autoimmune Disorders, and Other Applications), Diagnostic Approach (Lab Testing, OTC/Self-testing, and Point of Care Testing), and End User (Diagnostic Laboratories, Hospitals & Clinics, Home Healthcare, and Other End Users), and Countries. The study also evaluates industry competitors and analyzes their market share at the country level.
Among all offerings studied in this report, the kits & reagents segment is expected to register the highest CAGR over the forecast period. The highest CAGR of this segment is attributed to the frequent use of reagents and kits in the detection of various chronic and infectious diseases, the wide commercial availability of a diverse range of reagents and consumables for the diagnosis of various diseases, the increase in the volume of testing for infectious diseases such as HIV and influenza, and the growing awareness about self-testing among the general population. According to UNAIDS, global HIV prevalence is decreasing, but the Middle East and North Africa are one of the few regions where new HIV infections are increasing at a rapid rate. In 2022, only 67% of people living with HIV knew their HIV-positive status.
Among all technologies studied in this report, in 2024, the molecular diagnostic segment is expected to account for the largest share of the Middle East IVD market. The large market share of this segment is attributed to the high prevalence of communicable and non-communicable diseases requiring molecular diagnostics (molecular diagnostic tests give more access to high-volume testing in less time and better specificity) and increasing the adoption of molecular diagnostic techniques in hospitals and laboratories.
Among all applications studied in this report, in 2024, the infectious disease segment is expected to account for the largest share of the Middle East IVD market. Infectious diseases are further categorized into respiratory infections (excluding influenza), hepatitis, HIV, HAIs, tropical diseases, influenza, sexually transmitted diseases (STDs), and other infectious diseases. The increasing prevalence of seasonal influenza and sexually transmitted diseases (STDs) is increasing the demand for IVD testing. For instance, people aged over 65 years account for approximately 50-70% of hospitalizations & 90% of influenza-related deaths.
Among all diagnostic approaches studied in this report, the point-of-care testing segment is expected to grow at the highest CAGR during the forecast period. The growth of the POC testing segment is attributed to the increasing prevalence of chronic diseases, the launch of PoC tests by market players, and the lack of skilled technicians for performing lab tests. Additionally, the launch of PoC testing in the region is contributing to the growth of this segment.
Among all end users studied in this report, in 2024, the hospitals & clinics segment is expected to account for the largest share of the Middle East IVD market. Hospitals and clinics perform a wide range of tests to diagnose various medical conditions, including communicable and non-communicable diseases. Hospitals in urban and rural areas provide large populations with access to diagnosis. Also, there was a significant increase in hospital admissions due to a rise in the rate of infectious diseases, which boosted the demand for diagnostic products.
Geographic Review
This research report comprehensively analyzes major countries: Saudi Arabia, UAE, Qatar, Kuwait, Oman, Israel, and the rest of the Middle East. In 2024, Saudi Arabia is expected to account for the largest share of the Middle East IVD market. The largest share of the country is attributed to the growing aging, rising chronic and infectious diseases, and the increasing adoption of self-testing. Additionally, rising healthcare expenditure is increasing the demand for IVD. The healthcare expenditure in Saudi Arabia is rising. The Government of Saudi Arabia has implemented various reforms under the “Vision 2030” strategy to improve the business environment and sectors, including healthcare. Saudi Arabia spent an estimated 6.6% of its GDP on healthcare expenditure in 2021, which is 3.7% higher than the healthcare expenditure a decade ago. Healthcare expenditure in Saudi Arabia is expected to increase at an annual average of 3.0% from 2022 to 2025 to meet “Vision 2030” goals.
Download Sample Report Here @ https://www.meticulousresearch.com/download-sample-report/cp_id=5764
Key questions answered in the report:
Which are the high-growth market segments in terms of the offering, technology, application, diagnostic approach, end user, and country?
What was the historical market for IVD testing in the Middle East?
What are the market forecasts and estimates for the period 2024–2031?
What are the major drivers, restraints, challenges, and opportunities in the Middle East IVD market?
Who are the major players in the Middle East IVD market?
Contact Us:
Meticulous Research®
Email- [email protected]
Contact Sales- +1-646-781-8004
Connect with us on LinkedIn- https://www.linkedin.com/company/meticulous-research
#MiddleEastInVitroDiagnosticsMarket#MiddleEastIVDMarket#RapidDiagnostics#InVitroDiagnostics#IVD#Immunodiagnostics#PointofCareDiagnostics#MolecularDiagnostics
0 notes
Text
MarketPoint of Care POC Devices Market Growth, Trend, and Prospects from 2023–2030

Point of Care POC Devices Market Growth,
The Point of Care POC Devices Market is expected to grow from USD 1.70 Billion in 2022 to USD 3.10 Billion by 2030, at a CAGR of 7.80% during the forecast period.
Get the Sample Report:https://www.reportprime.com/enquiry/sample-report/10621
Point of Care POC Devices Market Size
Point of Care (POC) devices are portable diagnostic tools designed to provide rapid and accurate test results at the patient’s bedside. These devices are applicable for diagnosing various medical conditions such as blood glucose diagnostic, infectious diseases diagnostic, pregnancy diagnostic, urinalysis diagnostic, and others. The market for POC devices is segmented based on type, application, and region. The major players in this market include Roche, Abbott, Quidel, Siemens Healthcare, Danaher, Bio-Rad Laboratories, BioMerieux, ARKRAY, and Nova Biomedical. The market is dominated by North America, followed by Europe, Asia Pacific, the Middle East, Africa, and Australia. Regulatory and legal factors specific to this market include the implementation of strict regulations and guidelines by various regulatory bodies concerning the safety and efficacy of POC devices. Additionally, the increasing prevalence of chronic diseases such as diabetes and cardiovascular diseases has led to a rise in demand for POC devices.
Point of Care POC Devices Market Key Players
Mazor Key Player is listed in the Point of Care POC Devices
Roche
Abbott
Quidel
Siemens Healthcare
Danaher
Buy Now & Get Exclusive Discount on this:https://www.reportprime.com/enquiry/request-discount/10621
Point of Care POC Devices Market Segment Analysis
The Point of Care (POC) Devices market has witnessed significant growth in recent years, and it is expected to continue its growth trajectory in the future. The POC Devices market comprises diagnostic testing devices that can be used at the point where care is delivered, that is, at the patient’s bedside or in the immediate vicinity. The target market for POC devices includes hospitals, clinics, ambulatory care centers, nursing homes, and other healthcare facilities. The major factors driving revenue growth in this market include the increasing prevalence of chronic diseases, the rising demand for decentralized testing, and the need for rapid test results.
One of the latest trends in the POC Devices market is the integration of digital technologies. POC devices are being developed with advanced features such as connectivity, cloud computing, and artificial intelligence, which facilitate seamless data sharing and faster diagnostic decisions. The integration of digital technologies in POC devices is expected to enhance the accuracy and reliability of test results, while also improving workflow efficiency.
However, the POC Devices market also faces various challenges, such as the high costs associated with POC devices and the lack of adequate funding for their development and implementation. In addition, the lack of regulatory clarity and standardization creates uncertainty and hinders market growth. Manufacturers of POC devices also face intense competition from established players as well as new entrants.
The main findings of the report on POC Devices market indicate that the market is poised for significant growth in the coming years, driven primarily by the increasing demand for rapid testing and decentralized diagnostics. The market is also ripe for technological advancements, particularly in digital connectivity and artificial intelligence. To realize the market’s potential, manufacturers must focus on developing cost-effective and user-friendly devices while navigating regulatory hurdles.
The report recommends that manufacturers collaborate with healthcare institutions and providers to better understand their needs and preferences. Additionally, investment in research and development, as well as strategic partnerships with digital technology companies, can help accelerate innovation and market penetration. Ultimately, the successful adoption and implementation of POC devices will require the cooperation of stakeholders across the healthcare industry.
This report covers impact on COVID-19 and Russia-Ukraine wars in detail.
Purchase This Report:https://www.reportprime.com/checkout?id=10621&price=3590
Point of Care POC Devices (by Application)
Clinics
Hospitals
Laboratory
Others
Information is sourced from www.reportprime.com
0 notes
Text
Heart Failure POC And LOC Devices Market Growth, Trend, and Prospects from 2023–2030

Heart Failure POC And LOC Devices Market Growth
The Heart Failure POC And LOC Devices Market is expected to grow from USD 1.80 Billion in 2022 to USD 3.80 Billion by 2030, at a CAGR of 10.10% during the forecast period.
Get the Sample Report:https://www.reportprime.com/checkout?id=11300&price=3590
Heart Failure POC And LOC Devices Market Size
Heart Failure POC and LOC Devices are medical devices designed for the diagnosis and management of heart failure. The global market for these devices is segmented by type, application, and region. Types of devices include Microfluidics, Array-based Systems, and Others. Applications for these devices include Clinics, Hospitals, Home, Laboratory, and Others. The market players in this industry include Abbott, Danaher Corporation, Siemens Healthineers, F. Hoffmann-La Roche Ltd, Quidel Corporation, bioMérieux, Trinity Biotech, Abaxis, Inc, Instrumentation Laboratory, and Jant Pharmacal Corporation. The regulatory and legal factors specific to market conditions include government regulations, such as the FDA in the United States, and patent laws that protect intellectual property. Overall, the Heart Failure POC and LOC Devices market is expected to see significant growth in the coming years due to a rise in the prevalence of heart failure and an increasing demand for point-of-care diagnostic tools.
Heart Failure POC And LOC Devices Market Key Players
Mazor Key Player is listed in the Heart Failure POC And LOC Devices
Abbott
Danaher Corporation
Siemens Healthineers
F. Hoffmann-La Roche Ltd
Quidel Corporation
Buy Now & Get Exclusive Discount on this:https://www.reportprime.com/enquiry/request-discount/11300
Heart Failure POC And LOC Devices Market Segment Analysis
The global Heart Failure POC and LOC Devices market has experienced significant revenue growth in recent years due to the rising incidence of heart failure and increasing demand for point-of-care devices. The target market for Heart Failure POC and LOC Devices includes patients with heart failure who require regular monitoring of their condition, healthcare facilities, and home healthcare settings.
Factors driving the revenue growth of the Heart Failure POC and LOC Devices market include the increasing prevalence of cardiovascular disease, the rising demand for efficient and cost-effective healthcare solutions, and the development of innovative and advanced technologies. The adoption of these devices in home healthcare settings has also contributed significantly to the revenue growth of the market.
The latest trends observed in the Heart Failure POC and LOC Devices market include the emergence of wearable devices and implantable devices, increasing adoption of remote patient monitoring, and the integration of artificial intelligence and machine learning in monitoring devices.
The major challenges faced by the Heart Failure POC and LOC Devices market include the presence of regulatory hurdles, limited reimbursement policies, and high costs of these devices. The lack of awareness among patients and healthcare professionals also poses a challenge to the growth of the market.
The report’s main findings suggest that the Heart Failure POC and LOC Devices market will continue to experience significant growth in the coming years. The increasing prevalence of heart failure and the growing demand for point-of-care devices are the primary drivers of the market. The report recommends that companies focus on developing innovative devices that can improve patient outcomes and reduce healthcare costs.
In conclusion, the Heart Failure POC and LOC Devices market has significant potential for growth due to the increasing prevalence of cardiovascular disease and the rising demand for efficient and cost-effective healthcare solutions. However, companies must overcome regulatory hurdles and address reimbursement policies to fully realize the market’s potential. Developing innovative and advanced technologies will be crucial to future growth in the Heart Failure POC and LOC Devices market.
This report covers impact on COVID-19 and Russia-Ukraine wars in detail.
Purchase This Report:https://www.reportprime.com/checkout?id=11300&price=3590
Market Segmentation (by Application)
Clinics
Hospitals
Home
Laboratory
Others
Information is sourced from www.reportprime.com
0 notes
Text
In Vitro Diagnostics Market Competitive Landscape: 2030
In Vitro Diagnostics Market By Product And Services (Reagents, Instruments, Software And Services), by Technique (Immunodiagnostics, Hematology, Molecular Diagnostics, Tissue Diagnostics, Clinical Chemistry, Others), by Application (Infectious Diseases, Cancer, Cardiac Diseases, Immune System Disorders, Nephrological Diseases, Gastrointestinal Diseases, Others), by End User (Standalone Laboratories, Hospitals, Academic And Medical Schools, Point Of Care, Others) and region (North America, Europe, Asia-Pacific, Middle East and Africa and South America).
Market Overview
The global In-Vitro Diagnostics market size was estimated at USD 120.7 billion in 2023 and is projected to reach USD 161.2 billion in 2030 at a CAGR of 4.2% during the forecast period 2023-2030.
In May 2021, the esteemed University of California accomplished a remarkable feat in the field of medical science by developing a highly sensitive molecular test. This particular test capitalizes on cutting-edge chip technology and is capable of detecting the presence of both influenza A and SARS-CoV-2 antigens.

It is currently undergoing further scrutiny and investigation for potential conversion into a Point-of-Care (POC) test. The industry dynamics have witnessed a significant shift, with an ever-growing number of players directing their focus towards the development and launch of tests for at-home use. Furthermore, in the year 2021, the Food and Drug Administration (FDA) has demonstrated an increased proclivity towards prioritizing home-based molecular diagnostic tests. Specifically in the month of March, the renowned BATM Advanced Communications Ltd. announced the launch of its revolutionary molecular diagnostics self-test kit, designed for the sole purpose of detecting COVID-19.
Request for the Sample Copy: https://www.delvens.com/get-free-sample/in-vitro-diagnostics-market-trends-forecast-till-2030
The ascension of the elderly demographic and the expansion of acumen concerning initial screening has brought about a notable escalation in the quantity of routine medical examinations, given that a majority of fatalities as a result of infections and persistent pathologies come to pass in the populace exceeding 75 years of age. Based on the Office for Budget Responsibility in the United Kingdom, healthcare expenses have undergone a sharp upturn, which could generate economic strain on nations that are aging rapidly. Nonetheless, it is anticipated that this disbursement will yield a favorable outcome for the in vitro diagnostics industry, propelling the advancement of the market.
The unprecedented and devastating COVID-19 pandemic has brought about a significant and much-needed emphasis on in vitro diagnostics (IVD) in light of the rapidly growing and exigent necessity for IVD kits and reagents geared towards the prompt and precise diagnosis of SARS-CoV2 virus infection among the world's populace. The outbreak of this highly contagious virus has had a salutary effect on the market, as in vitro diagnostics encompass a diverse range of tests on a multitude of biological samples, thereby facilitating the diagnosis of an array of infectious diseases.
Delvens Industry Expert's Standpoint
The expansion of the in-vitro diagnostics (IVD) market is predominantly propelled by the escalation in the number of occurrences of enduring and communicable infirmities. In the current commercial milieu, there has been a surge in the incidence of persistent ailments such as tuberculosis (TB), cancer, cardiovascular disorders, and diabetes. Moreover, there has been a momentous upsurge in the number of patients afflicted with communicable maladies, such as gastrointestinal, respiratory, and sexually transmitted diseases (STDs). The upswing in the prevalence of these diseases is expected to augment the requirement for diagnostic devices, thereby boosting the IVD market. Furthermore, the technological progressions such as rapid test kits for the IVD sector have proliferated at an astounding pace in recent years and are foreseen to persist in the forthcoming times. The application of in-vitro diagnostics is extensive in various aspects of healthcare. Additionally, there has been a growth in the utilization of in vitro diagnostic testing across the globe.
Make an Inquiry Before Buying: https://www.delvens.com/Inquire-before-buying/in-vitro-diagnostics-market-trends-forecast-till-2030
Key Findings
On the basis of product, the segment pertaining to reagents was found to have secured the most substantial market share in the in vitro diagnostic market in the year 2020, and it is anticipated that it shall continue to maintain its preeminent position during the projected period, all due to the irrefutable fact that reagents in this field are an indispensable and integral component of every diagnostic test conducted in vitro.
On the basis of Technique, the immunodiagnostics sector, which specializes in the identification of antibodies or antigens within a biological sample, was found to have the largest proportion of market share during the year 2020. Furthermore, it is anticipated to maintain its dominant status throughout the forecasted period due to its exceptional sensitivity analysis, superior throughput, minimal cost, and inherent specificity. This is a result of its ability to precisely identify and quantify the presence of specific proteins or other biomolecules, which are integral in the analysis of biological samples.
On the basis of application, The segment of infectious diseases, which pertains to the study of diseases caused by pathogenic microorganisms such as bacteria, viruses, parasites, and fungi, held the largest market share in the field of in vitro diagnostics (IVD) in the year 2020. Furthermore, it is expected to maintain its dominance in the market during the forecast period. This can be attributed to the notable increase in the prevalence of infectious diseases such as tuberculosis, human immunodeficiency viruses (HIV), and sexually transmitted diseases (STDs) among the population.
The market is also divided into various regions such as North America, Europe, Asia-Pacific, South America, and Middle East and Africa. The North American region, is experiencing noteworthy expansion in the market for In Vitro Diagnostics (IVDs), with projections indicating that it will sustain its preeminent position for a few additional years. In the future, this particular region is anticipated to augment its market share, thereby solidifying its position as a dominant player in the IVDs market.
Regional Analysis
North America to Dominate the Market
The market dominance of the United States in the North American region can be attributed to the escalation of healthcare expenditure and the expeditious implementation of point-of-care testing. This notion is supported by the Centers for Disease Control and Prevention, which has provided data on chronic diseases.
According to the American Cancer Society, in January 2022, cancer continues to be the second most common cause of death in the United States
Get the Direct Order for the Research Report: https://www.delvens.com/checkout/in-vitro-diagnostics-market-trends-forecast-till-2030
Competitive Landscape
Abbott
bioMérieux SA
Quidel Corp.
Siemens Healthineers
Bio-Rad Laboratories, Inc.
Qiagen
Sysmex Corp.
Charles River Laboratories
Quest Diagnostics
Agilent Technologies, Inc.
Danaher Corporation
Becton Dickinson and Company
F. Hoffmann-La Roche Ltd.
Thermo Fisher Scientific
Illumina
Hologic
Devyser
PerkinElmer
Chembio Diagnostics
Surmodics. Inc
Menarini Silicon
Recent Developments
In November 2022, Thermo Fisher Scientific, launched the rapid RT-PCR Accula Flu A/Flu B Test Designed to enable healthcare providers to detect and differentiate influenza A and B in about 30 minutes.
In October 2022, Thermo Fisher Scientific entered a definitive areement to acquire the biding site group a global leader in specially diagnostics. The binding site provides specially diagnostics assays and instruments to improve the diagnosis and management of blood cancers and immune system disorders.
Reasons to Acquire
Increase your understanding of the market for identifying the most suitable strategies and decisions based on sales or revenue fluctuations in terms of volume and value, distribution chain analysis, market trends, and factors.
Gain authentic and granular data access for the In-Vitro Diagnostics Market to understand the trends and the factors involved in changing market situations.
Qualitative and quantitative data utilization to discover arrays of future growth from the market trends of leaders to market visionaries and then recognize the significant areas to compete in the future.
In-depth analysis of the changing trends of the market by visualizing the historic and forecast year growth patterns.
Browse for More Reports:
Report Scope
The In-Vitro Diagnostics Market is segmented into various segments such as Product And Services, Technique, Application, End-User, and region:
Based on Product And Services
Reagents
Instruments
Software and Services
Based on the Technique
Immunodiagnostics
Enzyme-linked Immunosorbent assay
Chemiluminescence Immunoassay
Fluoresence Immunoassay
Colorimetric Immunoassay
Rapid Tests
Enzyme-linked Immunospot
Radioimmunoassay
Western blot
Hematology
Molecular Diagnostics
Polymerize Chain Reaction
Isothermal Nucleic acid Amplification Technology
Hybridization
DNA diagnostics
Microarray
Tissue Diagnostics
Clinical Chemistry
Basic Metabolic Panel
Liver Panel
Renal Profile
Lipid Profile
Thyroid Function Panel
Electrolyte Panel
Specialty Chemicals
Based on the Application
Infectious Diseases
Cancer
Cardiac Diseases
Immune System Disorders
Nephrological Diseases
Gastrointestinal Diseases
Based on End-User
Standalone laboratories
Hospitals
Academic And Medical Schools
Point Of Care
About Us:
Delvens is a strategic advisory and consulting company headquartered in New Delhi, India. The company holds expertise in providing syndicated research reports, customized research reports and consulting services. Delvens qualitative and quantitative data is highly utilized by each level from niche to major markets, serving more than 1K prominent companies by assuring to provide the information on country, regional and global business environment. We have a database for more than 45 industries in more than 115+ major countries globally.
Delvens database assists the clients by providing in-depth information in crucial business decisions. Delvens offers significant facts and figures across various industries namely Healthcare, IT & Telecom, Chemicals & Materials, Semiconductor & Electronics, Energy, Pharmaceutical, Consumer Goods & Services, Food & Beverages. Our company provides an exhaustive and comprehensive understanding of the business environment.
Contact Us:
UNIT NO. 2126, TOWER B,
21ST FLOOR ALPHATHUM
SECTOR 90 NOIDA 201305, IN
+44-20-8638-5055
0 notes
Text
Global Rapid Diagnostics Market is Estimated to Witness High Growth Owing to Increased Adoption of Point-of-Care Testing and Rising Demand for Early and Accurate Disease Diagnosis
The global Rapid Diagnostics Market is estimated to be valued at US$ 33.4 Bn in 2022 and is expected to exhibit a CAGR of 9.8% over the forecast period 2023-2030, as highlighted in a new report published by Coherent Market Insights.
Market Overview:
Rapid diagnostics refer to the medical tests that provide quick results to diagnose various diseases and conditions. These tests are designed to detect the presence of specific biomarkers or antigens in a patient's body. Rapid diagnostics are commonly used in point-of-care settings to provide immediate results, allowing for timely diagnosis and treatment. The market includes a wide range of products such as rapid test kits, POC analyzers, and molecular diagnostic devices.
Market Dynamics:
The rapid diagnostics market is driven by two major factors: the increased adoption of point-of-care testing (POCT) and the rising demand for early and accurate disease diagnosis.
Firstly, the adoption of POCT has been rapidly increasing in recent years. POCT allows healthcare professionals to perform tests at the patient's bedside, eliminating the need for sending samples to a laboratory and waiting for results. This enables immediate decision-making and improves patient care. POCT is particularly useful in emergency departments, intensive care units, and remote healthcare settings where timely diagnosis is critical.
Secondly, the demand for early and accurate disease diagnosis is on the rise. Rapid diagnostics provide a quick and accurate diagnosis, enabling early detection and intervention. This is crucial for diseases such as infectious diseases, cardiac conditions, and cancer, where early diagnosis can significantly improve patient outcomes. Rapid diagnostic tests are also being increasingly used in screening programs and surveillance systems to detect diseases at an early stage.
SWOT Analysis:
Strength:
1. High demand for quick and accurate diagnostic solutions
2. Increasing adoption of rapid diagnostics in point-of-care settings
Weakness:
1. Limited sensitivity and specificity of some rapid diagnostic tests
2. Lack of awareness and accessibility in developing regions
Opportunity:
1. Growing application of rapid diagnostics in home healthcare settings
2. Advancements in technology leading to the development of innovative diagnostic products
Threats:
1. Stringent regulatory requirements for the approval of rapid diagnostic tests
2. Competition from traditional laboratory-based diagnostics
In conclusion, the global rapid diagnostics market is expected to experience significant growth in the coming years. The increasing adoption of point-of-care testing and the demand for early and accurate disease diagnosis are driving market growth. However, challenges such as regulatory requirements and competition from traditional diagnostics need to be addressed. Overall, the rapid diagnostics market holds immense potential for innovation and improved patient care.
#Rapid Diagnostics Market#Coherent Market Insights#Rapid Diagnostics Market Value#Rapid Diagnostics Market Size#Rapid Diagnostics Market Share#Rapid Diagnostics Market Growth#Rapid Diagnostics Market Analysis.
0 notes
Text
Point of Care Data Management Systems Market

The global Point of Care Data Management Systems Market is estimated to be valued at US$ 718.5 million in 2017 and is expected to exhibit a CAGR of 6.6% over the forecast period, according to a new report published by Coherent Market Insights.
A) Market Overview:
Point of Care (PoC) Data Management Systems are software platforms that enable real-time collection, documentation, and management of patient data at the point of care. These systems provide healthcare professionals with immediate access to patient information, helping them make faster and more informed decisions. PoC Data Management Systems have numerous advantages, including improved patient outcomes, reduced healthcare costs, enhanced workflow efficiency, and increased patient satisfaction. The need for these products is driven by the increasing demand for quality healthcare services, rising prevalence of chronic diseases, and the growing focus on patient-centered care.
B) Market key trends:
One key trend in the PoC Data Management Systems market is the increasing adoption of PoC testing. PoC testing refers to medical diagnostic testing performed at or near the site of patient care. It provides rapid and accurate results, allowing for immediate diagnosis and treatment decisions. PoC testing is gaining popularity due to its ability to improve patient outcomes by reducing turnaround time, eliminating the need for laboratory testing, and enabling timely intervention. For example, some PoC Data Management Systems can integrate with PoC testing devices such as glucose meters or cardiac markers to provide seamless data integration and analysis. This trend is expected to drive the growth of the PoC Data Management Systems market.
C) PEST Analysis:
Political: The political factors influencing the PoC Data Management Systems market include government regulations and policies regarding healthcare infrastructure, reimbursement policies for PoC testing, and data privacy and security regulations.
Economic: The economic factors impacting the market include healthcare expenditure, insurance coverage, and affordability of PoC Data Management Systems for healthcare facilities.
Social: The societal factors influencing the market include the aging population, increasing prevalence of chronic diseases, and the demand for improved healthcare services.
Technological: The technological factors impacting the market include advancements in connectivity and integration capabilities, development of cloud-based platforms, and increasing adoption of electronic health records.
D) Key Takeaways:
- The global Point of Care Data Management Systems Market is expected to witness high growth, exhibiting a CAGR of 6.6% over the forecast period, due to increasing adoption of PoC testing.
- North America is expected to dominate the market due to the presence of well-established healthcare infrastructure, high healthcare expenditure, and early adoption of PoC Data Management Systems.
- Key players operating in the global PoC Data Management Systems market include Abbott Laboratories, Danaher Corporation, F.Hoffman-La Roche Ltd., Orchard Software Corporation, Siemens Healthcare GmbH, Telcor, Inc., Thermo Fisher Scientific, Inc., Randox Laboratories Ltd., Hedera Biomedics Srl, and Seaward Electronic Ltd. These players are adopting various strategies such as mergers and acquisitions, partnerships, and product launches to strengthen their market position.
In conclusion, the global PoC Data Management Systems market is poised for significant growth due to increasing adoption of PoC testing. These systems enable healthcare professionals to access patient information in real-time, leading to improved patient outcomes and enhanced workflow efficiency. Factors such as government regulations, economic conditions, social factors, and technological advancements will continue to influence the market. North America is expected to be the fastest-growing and dominating region in the market. Key players in the market are ramping up their efforts to stay competitive and expand their market share.
0 notes
Text
Africa IVD Market to be Worth $1.78 Billion by 2029
According to a new market research report titled, ‘Africa In Vitro Diagnostics Market by Product & Services, Technology (Immunoassay, Point of Care, Molecular Diagnostics, Coagulation), Application (Infectious Diseases, Diabetes, Oncology), Diagnostic Approach (Lab, OTC, PoC), and Customer Type - Forecast to 2029,’ published by Meticulous Research®, the Africa in vitro diagnostics market is expected to reach $1.78 billion by 2029, at a CAGR of 4.8% from 2022 to 2029.
Download Free Sample Report Now @ https://www.meticulousresearch.com/download-sample-report/cp_id=5415
In vitro diagnostics (IVD) are tests performed on samples of blood or tissue taken from the human body to detect a wide range of diseases. These tests help monitor overall health to prevent and treat various diseases. IVD involves the use of various reagents, assays, and kits based on technologies such as immunoassay, whole blood glucose monitoring, molecular diagnostics, point of care, clinical chemistry, hematology, coagulation & hemostasis, critical care, and urinalysis.
High Prevalence of Infectious Diseases Driving the Growth of the Africa IVD Market
Diagnostic tests play a crucial role in the medical care of patients with infections. The demand for IVD kits used to diagnose infectious diseases is high in Africa due to the increasing awareness regarding emerging and re-emerging infectious diseases and the adoption of advanced molecular testing and lab-on-chip technology. The burden of diseases such as HIV, tuberculosis, malaria, viral hepatitis, and neglected tropical diseases (NTDs) is large in Africa. For example, according to WHO, in 2020, 25.6 million people were living with HIV in the African Region. The annual number of new HIV infections also increased in the Middle East and North Africa (source: UNAIDS data 2021).
In addition, there is a frequent outbreak of endemic diseases in Africa. For instance, Lassa fever is endemic in Nigeria and continues to be reported throughout the country. According to Nigeria Centre for Disease Control and Prevention (NCDC). In January 2022, 981 suspected and 211 confirmed cases of Lassa fever were reported in Nigeria. There were 40 deaths reported among confirmed cases, indicating a case fatality rate of 19%. Thus, the high burden of infectious diseases increases the demand for IVD tests in Africa.
Speak to our Analysts to Understand the Impact of COVID-19 on Your Business: https://www.meticulousresearch.com/speak-to-analyst/cp_id=5415
Africa IVD Market: Future Outlook
The Africa IVD market is segmented based on Product Category (Reagents, Assays, and Kits, Instruments, and Software & Services), Technology (Immunoassay, Whole Blood Glucose Monitoring, Point-of-Care Diagnostics, Clinical Chemistry, Molecular Diagnostics, Hematology, Coagulation & Hemostasis, Critical Care, Urinalysis, and Other IVD Technologies), Application (Infectious Diseases, Oncology, Diabetes, Endocrinology, Cardiology, and Other Applications), Diagnostic Approach (Laboratory Testing, Point-of-Care Testing, and OTC/Self Testing), and Customer Type (Hospital Labs, Private Labs, Home Care/Self Testing, Government, and Other Customer Types), and Geography (South Africa, Egypt, Nigeria, Kenya, Algeria, Tanzania, Morocco, Tunisia, and the rest of Africa). The study also evaluates industry competitors and analyzes their market shares.
Based on product category, the reagents, assays, and kits segment is slated to register the fastest growth rate during the forecast period. In Africa, infectious diseases cause chronic illnesses, premature deaths, and loss of productivity, affecting the region's economic growth. Thus, the emerging threats of infectious diseases drive the demand for IVD in Africa. The frequent use of reagents for diagnosing diseases and the easy availability & accessibility of these tests are the major factors driving the growth of this segment.
Based on technology, the point of care segment is slated to register the fastest growth rate during the forecast period. The rising geriatric population, the growing prevalence of chronic diseases, and the rising incidence of infectious diseases are driving the growth of this segment. The increasing burden of chronic diseases and pathogen outbreaks has significantly increased the demand for PoC diagnostics in Africa. The increased need for early and accurate diagnosis, precise confirmation of clinical findings, and immediate & informed decision-making on treating diseases contributes to the growth of the PoC segment.
Based on application, the oncology segment is slated to register the fastest growth rate during the forecast period. Cancer involves a complex set of genetic alternations and subsequent changes in gene expression, leading to the uncontrolled proliferation of cells. In cancer diagnostics, early diagnosis and knowing the exact nature of the tumor are of prime importance. Early cancer detection and access to effective anti-cancer treatment can result in higher rates of survival and a better quality of life. According to GLOBOCAN 2020, 1.1 million new cases and 711,429 deaths were recorded in Africa. The growing demand for personalized medicines in cancer therapy, the increasing prevalence of cancer, and the rising demand for rapid cancer diagnosis tests drive the growth of the IVD market for oncology applications.
Based on diagnostic approach, the OTC/self-testing segment is slated to register the fastest growth rate during the forecast period. In Africa, over-the-counter (OTC)/self-testing is promoted for diseases, such as HIV and COVID-19, due to the high prevalence of infectious diseases. Self-testing reduces the burden on healthcare workers and the system. The OTC/self-testing kits are diagnostic tests that can be purchased from any pharmacy store and used by the patient. These tests can give rapid results and can be taken from anywhere.
Quick Buy – Africa IVD Market Research Report: https://www.meticulousresearch.com/Checkout/47708335
Based on customer type, the home care/self-testing segment is slated to register the fastest growth rate during the forecast period. The growth of this segment is attributed to the increasing need to monitor and manage chronic diseases, the growing awareness about home healthcare testing products, and the reduced waiting time and healthcare costs offered by self-testing kits.
This research report analyzes the market across major African countries and provides a comprehensive analysis of South Africa, Algeria, Nigeria, Kenya, Morocco, Tunisia, Tanzania, Egypt, and the rest of Africa.
Key companies operating in the Africa IVD market are Abbott Laboratories (U.S.), Becton, Dickinson and Company (U.S.), bioMérieux SA (France), Danaher Corporation (U.S.), F. Hoffmann-La Roche Ltd. (Switzerland), QIAGEN N.V. (Netherlands), Siemens Healthineers AG (Germany), Thermo Fisher Scientific Inc. (U.S.), Bio-Rad Laboratories, Inc. (U.S.), Illumina, Inc. (U.S.), and Shenzhen Mindray Bio-Medical Electronics Co., Ltd (China).
To gain more insights into the market with a detailed table of content and figures, click here: https://www.meticulousresearch.com/product/africa-ivd-market-5415
Scope of the Report
Africa IVD Market, by Product Category
Reagents, Assays, and Kits
Instruments
Software & Services
Africa IVD Market, by Technology
Immunoassay
Whole Blood Glucose Monitoring
Point-of-Care Diagnostics
Clinical Chemistry
Molecular Diagnostics
Hematology
Coagulation & Hemostasis
Critical Care
Urinalysis
Other IVD Technologies
Note: Other technologies include microscopy, hybridization, and loop-mediated amplification.
Africa IVD Market, by Application
Infectious Diseases
Oncology
Diabetes
Endocrinology
Cardiology
Other Applications
Note: Other applications include nephrology, toxicology, gastroenterology, neonatal, genetic, and neurological disorders.
Africa IVD Market, by Diagnostic Approach
Laboratory Testing
Point-of-Care Testing
OTC/Self-testing
Africa IVD Market, by Customer Type
Hospital Labs
Private Labs
Home Care/Self Testing
Government
Other Customer Types
Note: Other customer types include long-term care facilities, academic & research institutes, ambulatory care centers, and transfusion laboratories.
Africa IVD Market, by Country
South Africa
Egypt
Nigeria
Kenya
Algeria
Tanzania
Morocco
Tunisia
Rest of Africa
Download Free Sample Report Now @ https://www.meticulousresearch.com/download-sample-report/cp_id=5415
In Vitro Diagnostic (IVD) Reagents Market - https://www.meticulousresearch.com/product/in-vitro-diagnostic-reagents-market-5110
0 notes