#Point of Care (PoC) Diagnostics Market demand
Text

As per Business Intelligence Insights (BII) study, the global Point of Care [POC] Diagnostics Market attained revenue growth of USD 45.80 billion in 2021 and it is projected to reach around USD 67.15 billion by 2030, growing at a 4.90% CAGR.
#Point of Care [POC] Diagnostics Market#Point of Care [POC] Diagnostics Market Trends#Point of Care [POC] Diagnostics Market Share#Point of Care [POC] Diagnostics Market Size#Point of Care [POC] Diagnostics Market Analysis#Point of Care [POC] Diagnostics Market Demand#Point of Care [POC] Diagnostics Market Growth 2023#Point of Care [POC] Diagnostics Market Industry
0 notes
Text
Middle East IVD Market - Opportunity Analysis and Industry Forecast (2024-2031)
Meticulous Research®, a leading global market research company, published a research report titled, ‘Middle East IVD Market by Offering (Kits, Software), Technology (Immunoassay, Molecular Diagnostics [PCR, NGS, Microarray], Rapid Tests, Biochemistry), Application (Infectious Diseases, Oncology), Diagnostic Approach (Lab) - Forecast to 2031.’
According to this latest publication from Meticulous Research®, the Middle East In Vitro Diagnostics (IVD) market is expected to reach $2.15 billion by 2031 at a CAGR of 4.1% from 2024 to 2031. The growth of the Middle East IVD market is attributed to the rising prevalence of chronic and infectious diseases, the growing demand for point-of-care (PoC) and rapid diagnostics, rising healthcare expenditures, growth in the aging population, and rising awareness about healthcare. However, high prices for IVD tests and the variance in test results observed in rapid IVD tests restrain the growth of the IVD market.
The rising awareness regarding early disease diagnosis and increasing inclination toward personalized medicine in the UAE and Saudi Arabia are expected to create growth opportunities for the players operating in this market. However, the concerns pertaining to false positive results in immunoassays and POC are a major challenge for market growth.
Key Players
The key players operating in the Middle East IVD market are Abbott Laboratories (U.S.), Becton, Dickinson and Company (U.S.), BioMérieux S.A. (France), Danaher Corporation (U.S.), F. Hoffmann-La Roche (Switzerland), QIAGEN N.V. (Netherlands), Siemens Healthineers AG (Germany), Thermo Fisher Scientific Inc. (U.S.), Bio-Rad Laboratories, Inc. (U.S.), Illumina, Inc. (U.S.), QuidelOrtho Corporation. (U.S.), Agilent Technologies, Inc. (U.S.), and DiaSorin S.p.A. (Italy).
Middle East IVD Market: Future Outlook
The Middle East IVD market is segmented by Offering (Kits & Reagents, Instruments, and Software & Services), Technology (Immunoassay/Immunochemistry, Biochemistry/Clinical Chemistry, Molecular Diagnostics, Point of Care (POC) Diagnostics, Whole Blood Glucose Monitoring, Microbiology, Hematology, Coagulation/Hemostasis, Urinalysis, and Other Technologies), Application (Infectious Diseases, Oncology, Diabetes, Cardiology, Nephrology, Autoimmune Disorders, and Other Applications), Diagnostic Approach (Lab Testing, OTC/Self-testing, and Point of Care Testing), and End User (Diagnostic Laboratories, Hospitals & Clinics, Home Healthcare, and Other End Users), and Countries. The study also evaluates industry competitors and analyzes their market share at the country level.
Among all offerings studied in this report, the kits & reagents segment is expected to register the highest CAGR over the forecast period. The highest CAGR of this segment is attributed to the frequent use of reagents and kits in the detection of various chronic and infectious diseases, the wide commercial availability of a diverse range of reagents and consumables for the diagnosis of various diseases, the increase in the volume of testing for infectious diseases such as HIV and influenza, and the growing awareness about self-testing among the general population. According to UNAIDS, global HIV prevalence is decreasing, but the Middle East and North Africa are one of the few regions where new HIV infections are increasing at a rapid rate. In 2022, only 67% of people living with HIV knew their HIV-positive status.
Among all technologies studied in this report, in 2024, the molecular diagnostic segment is expected to account for the largest share of the Middle East IVD market. The large market share of this segment is attributed to the high prevalence of communicable and non-communicable diseases requiring molecular diagnostics (molecular diagnostic tests give more access to high-volume testing in less time and better specificity) and increasing the adoption of molecular diagnostic techniques in hospitals and laboratories.
Among all applications studied in this report, in 2024, the infectious disease segment is expected to account for the largest share of the Middle East IVD market. Infectious diseases are further categorized into respiratory infections (excluding influenza), hepatitis, HIV, HAIs, tropical diseases, influenza, sexually transmitted diseases (STDs), and other infectious diseases. The increasing prevalence of seasonal influenza and sexually transmitted diseases (STDs) is increasing the demand for IVD testing. For instance, people aged over 65 years account for approximately 50-70% of hospitalizations & 90% of influenza-related deaths.
Among all diagnostic approaches studied in this report, the point-of-care testing segment is expected to grow at the highest CAGR during the forecast period. The growth of the POC testing segment is attributed to the increasing prevalence of chronic diseases, the launch of PoC tests by market players, and the lack of skilled technicians for performing lab tests. Additionally, the launch of PoC testing in the region is contributing to the growth of this segment.
Among all end users studied in this report, in 2024, the hospitals & clinics segment is expected to account for the largest share of the Middle East IVD market. Hospitals and clinics perform a wide range of tests to diagnose various medical conditions, including communicable and non-communicable diseases. Hospitals in urban and rural areas provide large populations with access to diagnosis. Also, there was a significant increase in hospital admissions due to a rise in the rate of infectious diseases, which boosted the demand for diagnostic products.
Geographic Review
This research report comprehensively analyzes major countries: Saudi Arabia, UAE, Qatar, Kuwait, Oman, Israel, and the rest of the Middle East. In 2024, Saudi Arabia is expected to account for the largest share of the Middle East IVD market. The largest share of the country is attributed to the growing aging, rising chronic and infectious diseases, and the increasing adoption of self-testing. Additionally, rising healthcare expenditure is increasing the demand for IVD. The healthcare expenditure in Saudi Arabia is rising. The Government of Saudi Arabia has implemented various reforms under the “Vision 2030” strategy to improve the business environment and sectors, including healthcare. Saudi Arabia spent an estimated 6.6% of its GDP on healthcare expenditure in 2021, which is 3.7% higher than the healthcare expenditure a decade ago. Healthcare expenditure in Saudi Arabia is expected to increase at an annual average of 3.0% from 2022 to 2025 to meet “Vision 2030” goals.
Download Sample Report Here @ https://www.meticulousresearch.com/download-sample-report/cp_id=5764
Key questions answered in the report:
Which are the high-growth market segments in terms of the offering, technology, application, diagnostic approach, end user, and country?
What was the historical market for IVD testing in the Middle East?
What are the market forecasts and estimates for the period 2024–2031?
What are the major drivers, restraints, challenges, and opportunities in the Middle East IVD market?
Who are the major players in the Middle East IVD market?
Contact Us:
Meticulous Research®
Email- [email protected]
Contact Sales- +1-646-781-8004
Connect with us on LinkedIn- https://www.linkedin.com/company/meticulous-research
#MiddleEastInVitroDiagnosticsMarket#MiddleEastIVDMarket#RapidDiagnostics#InVitroDiagnostics#IVD#Immunodiagnostics#PointofCareDiagnostics#MolecularDiagnostics
0 notes
Text
Analyzing the Procalcitonin Tests Market by Test Type, End User, and Region
Analyzing the procalcitonin tests market by test type, end user, and region provides valuable insights into the factors driving demand and the dynamics of this market.

Buy the Full Report for More Insights on the Procalcitonin Tests Market Forecast,
Download a Free Sample Report
Here's an analysis of each category:
Test Type:a. Procalcitonin Assay Kits: Procalcitonin assay kits are used in clinical laboratories and healthcare facilities to measure procalcitonin levels in patient blood samples. These kits typically utilize immunoassay techniques such as enzyme-linked immunosorbent assay (ELISA) or chemiluminescent immunoassay (CLIA) to quantitatively detect procalcitonin levels. Assay kits may be available in various formats, including manual assays and automated assay systems.b. Point-of-Care (POC) Tests: Point-of-care procalcitonin tests are rapid diagnostic tests designed for use at the bedside or in outpatient settings. POC tests offer the advantages of fast turnaround times, enabling timely decision-making regarding antibiotic therapy in patients with suspected sepsis or bacterial infections. These tests are typically based on lateral flow immunoassay or other rapid immunoassay technologies.
End User:a. Hospitals and Clinics: Hospitals and clinics represent the largest end-user segment for procalcitonin tests, accounting for a significant portion of test volumes. Procalcitonin testing is commonly performed in hospital laboratories and clinical settings to aid in the diagnosis and management of sepsis, bacterial infections, and other inflammatory conditions. Hospitals may utilize both laboratory-based assays and point-of-care tests depending on their testing requirements and infrastructure.b. Diagnostic Laboratories: Independent diagnostic laboratories and reference laboratories also play a key role in the procalcitonin tests market. These laboratories offer testing services to healthcare providers, including hospitals, clinics, and physician offices, and may perform procalcitonin testing as part of routine clinical laboratory services or specialized infectious disease testing panels.c. Ambulatory Care Centers: Ambulatory care centers, including urgent care centers and outpatient clinics, may perform procalcitonin testing to aid in the diagnosis and management of infectious diseases in ambulatory patients. Point-of-care procalcitonin tests are particularly well-suited for use in ambulatory care settings due to their rapid turnaround times and ease of use.
Region:a. North America: North America is a significant market for procalcitonin tests, driven by factors such as the high prevalence of sepsis and bacterial infections, well-established healthcare infrastructure, and increasing adoption of advanced diagnostic technologies. The region is characterized by a strong presence of key market players, extensive research and development activities, and stringent regulatory standards governing diagnostic testing.b. Europe: Europe is another major market for procalcitonin tests, with countries such as Germany, France, and the United Kingdom leading in terms of market share. The region benefits from a robust healthcare system, growing awareness of sepsis management protocols, and favorable reimbursement policies for diagnostic testing. Increasing efforts to combat antimicrobial resistance also drive demand for procalcitonin testing in Europe.c. Asia-Pacific: The Asia-Pacific region represents a rapidly growing market for procalcitonin tests, fueled by factors such as the rising incidence of infectious diseases, expanding healthcare infrastructure, and increasing healthcare expenditure. Countries such as China, India, and Japan are witnessing significant market growth, driven by investments in healthcare modernization, improving access to diagnostic services, and growing awareness of sepsis management strategies.d. Latin America, Middle East, and Africa: These regions also contribute to the global procalcitonin tests market, albeit to a lesser extent compared to North America, Europe, and Asia-Pacific. Market growth in these regions is influenced by factors such as the burden of infectious diseases, healthcare infrastructure development, and efforts to enhance diagnostic capabilities in underserved areas.
Analyzing the procalcitonin tests market by test type, end user, and region enables stakeholders to identify key market segments, understand regional variations in demand, and formulate targeted strategies to address the needs of different market segments. Factors such as healthcare infrastructure, regulatory landscape, reimbursement policies, and disease epidemiology play a crucial role in shaping market dynamics and market growth opportunities across different regions.
0 notes
Text
Point of Care (PoC) Lipid Test Market Size, Share, Trends, Demand, Growth and Competitive Analysis
Data Bridge Market research has recently released expansive research titled Global Point of Care (PoC) Lipid Test Market guarantees you will remain better informed than your competition.
Point of Care (PoC) Lipid Test Market report gives explanation about the different segments of the market analysis which is demanded by today’s businesses. Key players are taking actions such as developments, product launches, acquisitions, mergers, joint ventures and competitive analysis in the industry. All the market aspects are estimated and analysed by a team of innovative, enthusiastic and motivated researchers and analysts so that nothing lefts uncovered in the report. Global Point of Care (PoC) Lipid Test Market research report, it becomes easy to figure out brand awareness and insight about the brand and product among potential customers.
Data Bridge Market Research analyses that the point of care (PoC) lipid test market which is USD 2.86 million in 2022, is expected to reach USD 4.17 million by 2030, at a CAGR of 4.84% during the forecast period 2023 to 2030.
Access Full 350 Pages PDF Report @
https://www.databridgemarketresearch.com/reports/global-point-of-care-poc-lipid-test-market
Points of care (POC) tests are medical tests that aid in the diagnosis of a problem in the human body at a specific point. The point of care tests aid in providing real-time results in a matter of minutes. As a result, a point of care (POC) lipid test is performed to assess the function of the lipid in the cells. When cholesterol levels rise, it disrupts the functioning of lipids in the body, leading to serious illnesses such as heart attack and coronary artery disease.
Some of the major players operating in the point of care (PoC) lipid test market are:
Abbott (U.S.)
F. Hoffmann-La Roche Ltd (Switzerland)
BD (U.S.)
Siemens Healthcare Private Limited (Germany)
Bio-Rad Laboratories, Inc. (U.S.)
Danaher (U.S.)
Sekisui Diagnostics (U.S.)
Trinity Biotech (Ireland)
BIOMÉRIEUX (France)
EKF Diagnostics (Germany)
AccuBioTech Co., Ltd. (China)
Instrumentation Laboratory (U.S.)
Beckman Coulter, Inc. (U.S.)
PTS Diagnostics (U.S.)
Nova Biomedical (U.S.)
Chembio Diagnostics, Inc. (U.S.)
Quidel Corporation (U.S.)
Sienco, Inc (U.S.)
Core Objective of Point of Care (PoC) Lipid Test Market:
Every firm in the Point of Care (PoC) Lipid Test Market has objectives but this market research report focus on the crucial objectives, so you can analysis about competition, future market, new products, and informative data that can raise your sales volume exponentially.
Size of the Point of Care (PoC) Lipid Test Market and growth rate factors.
Important changes in the future Point of Care (PoC) Lipid Test Market.
Top worldwide competitors of the Market.
Scope and product outlook of Point of Care (PoC) Lipid Test Market.
Developing regions with potential growth in the future.
Tough Challenges and risk faced in Market.
Global Point of Care (PoC) Lipid Test top manufacturers profile and sales statistics.
Key takeaways from the Point of Care (PoC) Lipid Test Market report:
Detailed considerate of Point of Care (PoC) Lipid Test Market-particular drivers, Trends, constraints, Restraints, Opportunities and major micro markets.
Comprehensive valuation of all prospects and threat in the
In depth study of industry strategies for growth of the Point of Care (PoC) Lipid Test Market-leading players.
Point of Care (PoC) Lipid Test Market latest innovations and major procedures.
Favorable dip inside Vigorous high-tech and market latest trends remarkable the Market.
Conclusive study about the growth conspiracy of Point of Care (PoC) Lipid Test Market for forthcoming years.
Frequently Asked Questions
What is the Future Market Value for Point of Care (PoC) Lipid Test Market?
What is the Growth Rate of the Point of Care (PoC) Lipid Test Market?
What are the Major Companies Operating in the Point of Care (PoC) Lipid Test Market?
Which Countries Data is covered in the Point of Care (PoC) Lipid Test Market?
What are the Main Data Pointers Covered in Point of Care (PoC) Lipid Test Market Report?
Browse Trending Reports:
https://www.databridgemarketresearch.com/reports/global-whole-genome-bisulfite-sequencing-wgbs-market
https://www.databridgemarketresearch.com/reports/global-behavioral-health-market
https://www.databridgemarketresearch.com/reports/global-medical-second-opinion-market
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Contact Us:
Data Bridge Market Research
US: +1 888 387 2818
UK: +44 208 089 1725
Hong Kong: +852 8192 7475
Email: [email protected]
0 notes
Text
Magnetic Beads Market Size by Research Nester Reveals the Market to Grow with a CAGR of ~11% During 2023-2036
Research Nester’s recent market research analysis on “Magnetic Beads Market: Global Demand Analysis & Opportunity Outlook 2036” delivers a detailed competitors analysis and a detailed overview of the global magnetic beads market in terms of market segmentation by product, type, application, end-user and by region.
Request Report Sample@
Rising Application in Molecular Biology Application to Promote Global Market Share of Magnetic Beads Market
The global magnetic beads market is estimated to grow majorly on account of the increased usage in medical applications, DNA & RNA extraction methods, bioassays, and life sciences applications. Other than this, rising incidences of tumors and cancer are also propelling the demand for magnetic beads, especially for leukemia. It has been estimated that in the United States, a total of 184,721 people were diagnosed with leukemia. The magnetic beads are considered to help separate the cells and remove cell tumors from the bone marrow and isolate the lymphoid cells from the blood film. On the back of this, the global magnetic beads market is projected to flourish in the forecasted period. Other than this, research related to the microbiomes has become prominent in recent years. The increase in investment in microbiological and genetic research over a prolonged period is projected to boost the demand for the product remarkably. The usage of these beads will accelerate more research-based investigations will be conducted throughout, to discover new therapeutic methods worldwide. Furthermore, the demand for magnetic beads market is projected to expand on the back of the rising production of healthcare products. The rise in the trend of POC which is the point of care in the laboratories as well as at the door of patients is projected to raise the usage of magnetic beads.
Some of the major growth factors and challenges that are associated with the growth of the global magnetic beads market are:
Growth Drivers:
Rising Research Spending is Augmenting the Demand
Increased Usage of Magnetic Beads in Medicated Wearables
Challenges:
Factors such as a decrease in magnetic force with the rise in distance, exorbitant price of magnetic beads, and less research in the development of magnetic beads are some of the major factors anticipated to hamper the global market size of the global magnetic beads market.
By application, the global magnetic beads market is segmented into cell separation & cell expansion, exosome analysis, protein sample prep and protein isolation, IVD assay development, and nucleic acid isolation. Out of these, the IVD assay development segment will rise the most during the forecast period and is projected to garner revenue of almost 50%.
By region, the Europe magnetic beads market is to generate the highest revenue by the end of 2036. The market is projected to flourish because of the rising demand for diagnostic and therapeutic solutions.
Access our detailed report at:
Research Nester is a leading service provider for strategic market research and consulting. We aim to provide unbiased, unparalleled market insights and industry analysis to help industries, conglomerates and executives to take wise decisions for their future marketing strategy, expansion and investment etc. We believe every business can expand to its new horizon, provided a right guidance at a right time is available through strategic minds. Our out of box thinking helps our clients to take wise decision in order to avoid future uncertainties.
Contact for more Info:
AJ Daniel
0 notes
Text
Breaking Barriers: Exploring the Point of Care (PoC) Molecular Diagnostics Market 🌐🔬
Hey there, health advocates and science enthusiasts! Today, let's embark on an exhilarating journey through the cutting-edge realm of point of care (PoC) molecular diagnostics – where innovation meets accessibility to revolutionize healthcare on a global scale. 💡💊
So, what exactly is PoC molecular diagnostics? Picture this: a paradigm-shifting approach that brings the power of molecular testing out of the lab and into the hands of healthcare providers and patients, right at the point of care. From rapid DNA and RNA analysis to on-the-spot pathogen detection, PoC molecular diagnostics enable real-time, accurate diagnosis and treatment decisions with unprecedented speed and precision. It's like putting the future of healthcare in the palm of your hand! 🚀🖐️
Now, let's dive into the market trends. The PoC molecular diagnostics market is experiencing explosive growth, driven by the increasing demand for rapid, accurate diagnostic solutions that can be deployed anytime, anywhere. With the rise of infectious diseases, antimicrobial resistance, and chronic conditions, the need for on-demand testing capabilities has never been greater. PoC molecular diagnostics are not just tools; they're game-changers that are democratizing access to healthcare and leveling the playing field for communities around the world. It's time to break down barriers and build bridges to better health for all! 🌍🔓
But what sets PoC molecular diagnostics apart from traditional testing methods? Get ready to geek out, because we're about to dive into the science behind the revolution! By leveraging advanced nucleic acid amplification techniques – like PCR and isothermal amplification – PoC molecular diagnostics can detect the presence of specific genetic sequences with unparalleled sensitivity and specificity, even in low-resource settings. Add to that the portability and user-friendliness of handheld devices and disposable cartridges, and you've got a recipe for transformative impact that transcends borders and boundaries. It's like empowering healthcare providers with the superpowers of molecular analysis, right at their fingertips! 💪🔍
And let's not overlook the societal impact of PoC molecular diagnostics. From rapid screening and early detection to personalized treatment and surveillance, these technologies are not just tools; they're catalysts for change that are redefining the way we approach healthcare delivery and disease management. Whether you're a clinician in a remote village, a researcher in a bustling metropolis, or a patient navigating a complex healthcare system, PoC molecular diagnostics offer hope, empowerment, and peace of mind in the face of uncertainty. It's time to embrace the future of healthcare with courage, curiosity, and unwavering optimism! 🌟💉
But amidst the excitement, let's not forget the challenges. Regulatory hurdles, infrastructure limitations, and cost considerations remain barriers to the widespread adoption of PoC molecular diagnostics, particularly in resource-limited settings. As the market continues to evolve, it's imperative that stakeholders across the healthcare ecosystem – from policymakers and industry leaders to healthcare providers and patients – work together to address these challenges and unlock the full potential of PoC molecular diagnostics for the benefit of all. Together, we can bridge the gap between innovation and impact, and pave the way to a future where healthcare is truly accessible, equitable, and empowering for everyone, everywhere. 🤝🌈
So there you have it, health advocates and science enthusiasts – a glimpse into the transformative power of PoC molecular diagnostics. From its humble beginnings to its monumental impact on global health, PoC molecular diagnostics are a testament to the resilience of the human spirit and the boundless potential of innovation and collaboration. Here's to the visionary minds driving progress in healthcare and the countless lives yet to be transformed by the power of molecular analysis at the point of care. Keep shining, keep innovating, and remember – together, we can change the world, one test at a time! ✨🔬🌐
0 notes
Text
The Transformative Impact of Point-of-Care Infectious Disease Diagnostics: Enabling Rapid and Accurate Diagnosis, Improved Patient Outcomes, and Reduced Healthcare Costs
Point-of-Care Infectious Disease Diagnostics Market: A Comprehensive Analysis
Overview
The point-of-care (POC) infectious disease diagnostics market is a rapidly growing segment of the healthcare industry. POC tests are performed outside of a traditional clinical laboratory setting, such as in a doctor's office, clinic, or even at home. This makes them ideal for diagnosing and monitoring infectious diseases, as they can provide rapid and accurate results without the need to send samples to a central laboratory.
The POC infectious disease diagnostics market is driven by a number of factors, including:
The increasing prevalence of infectious diseases
The need for rapid and accurate diagnosis
The growing demand for patient-centered care
The development of new technologies
The increasing adoption of POC testing in developing countries and home care settings
Key Trends
A number of key trends are shaping the POC infectious disease diagnostics market, including:
The development of new technologies: New technologies, such as microfluidics and molecular diagnostics, are enabling the development of more accurate and sensitive POC tests.
The increasing adoption of POC testing in developing countries: POC testing is becoming increasingly popular in developing countries, where access to laboratory testing is limited.
The growing use of POC testing in home care: POC testing is increasingly being used in home care settings, which can help to improve patient outcomes and reduce healthcare costs.
Market Share
The POC infectious disease diagnostics market is a fragmented market with a number of key players. The top five players in the market accounted for approximately 30% of the global market share in 2021. The major players in the market include Abbott Laboratories, Becton, Dickinson and Company (BD), Chembio Diagnostics, Danaher Corporation, and F. Hoffmann-La Roche Ltd.
Market Segments
The POC infectious disease diagnostics market can be segmented by product, application, and end user.
By product: The market can be segmented into rapid diagnostic tests, molecular diagnostic tests, and other tests. Rapid diagnostic tests are the largest segment of the market, accounting for approximately 60% of the global market share in 2021.
By application: The market can be segmented into respiratory infections, sexually transmitted infections, and other infections. Respiratory infections are the largest application segment of the market, accounting for approximately 30% of the global market share in 2021.
By end user: The market can be segmented into hospitals, clinics, home care settings, and other settings. Hospitals are the largest end user segment of the market, accounting for approximately 40% of the global market share in 2021.
Challenges
The POC infectious disease diagnostics market faces a number of challenges, including:
The high cost of development and commercialization of new POC tests
The regulatory hurdles involved in bringing new POC tests to market
The lack of reimbursement for POC tests in some countries
Conclusion
The POC infectious disease diagnostics market is a rapidly growing market with a number of opportunities for growth. The market is driven by a number of factors, including the increasing prevalence of infectious diseases, the need for rapid and accurate diagnosis, and the growing demand for patient-centered care. The development of new technologies and the increasing adoption of POC testing in developing countries and home care settings are also driving the growth of the market.
Image Enhancement
The image of a rapid diagnostic test for COVID-19 enhances the content of this article by providing a visual representation of the type of POC test that is being discussed. The image also helps to illustrate the increasing adoption of POC testing for the diagnosis of infectious diseases.
Overall, the POC infectious disease diagnostics market is a promising market with a number of opportunities for growth. The market is driven by a number of factors, including the increasing prevalence of infectious diseases, the need for rapid and accurate diagnosis, and the growing demand for patient-centered care. The development of new technologies and the increasing adoption of POC testing in developing countries and home care settings are also driving the growth of the market.
0 notes
Text
Harnessing the Power of Technology: Portable Vascular Doppler Devices Revolutionize Vascular Imaging By 2023 to 2030

The portable vascular doppler market is expected to grow at a CAGR of 7.5% from 2023 to 2030, reaching a value of US$ 2.2 billion by 2030, The growth of this market is being driven by several factors, including the increasing prevalence of cardiovascular diseases, the growing demand for minimally invasive procedures, and the rising adoption of portable vascular doppler devices in home healthcare settings.
Portable vascular doppler devices are used to assess blood flow and circulation in the body. These devices are non-invasive and easy to use, making them a popular choice for both medical professionals and home healthcare users. The devices are available in a variety of sizes and configurations, and can be used to assess blood flow in a variety of locations, including the arms, legs, neck, and abdomen.
Get your Sample Report with Latest Market Information! https://absolutemarketresearch.com/Global-Portable-Vascular-Doppler-Market/1700/request-sample
The increasing prevalence of cardiovascular diseases, such as coronary artery disease (CAD), peripheral artery disease (PAD), and venous thromboembolism (VTE), is the primary factor driving growth of the portable vascular doppler market. These diseases are major causes of morbidity and mortality worldwide, and portable vascular dopplers are essential diagnostic tools for their early detection and management.
In addition, the rising adoption of minimally invasive procedures, such as endovascular therapies, is also contributing to the growth of the portable vascular doppler market. These procedures require real-time visualization of blood flow, which can be effectively provided by portable vascular dopplers.
Furthermore, the increasing demand for point-of-care (POC) testing is also driving the growth of the portable vascular doppler market. POC testing allows healthcare providers to make rapid diagnoses and treatment decisions, which can improve patient outcomes.
North America is the largest market for portable vascular dopplers, followed by Europe and Asia Pacific. The growth of the North American market is being driven by the high prevalence of cardiovascular diseases and the increasing adoption of minimally invasive procedures. The European market is also growing steadily, due to the increasing demand for POC testing and the rising prevalence of cardiovascular diseases. The Asia Pacific market is the fastest growing market, due to the increasing economic development and the rising prevalence of cardiovascular diseases.
Market Drivers:
There are several factors driving the growth of the portable vascular doppler market, including:
Rising prevalence of PAD: PAD is a condition that affects over 200 million people worldwide. It is a major cause of cardiovascular disease and death. The increasing prevalence of PAD is driving the demand for portable vascular doppler devices, which can be used to diagnose and monitor PAD.
Growing aging population: The global aging population is expected to reach 2 billion by 2050. Older adults are more likely to develop PAD, which is driving the demand for portable vascular doppler devices.
Increasing demand for minimally invasive procedures: Minimally invasive procedures are becoming increasingly popular, as they are associated with fewer complications and shorter recovery times. Portable vascular doppler devices are often used in minimally invasive procedures, which is driving the demand for these devices.
Key Takeaways:
The Portable Vascular Doppler Market is expected to grow at a CAGR of 7.5% from 2023 to 2030, reaching a value of US$ 2.2 billion by 2030.
The market is driven by the increasing prevalence of vascular diseases, the growing aging population, and the rising demand for minimally invasive procedures.
North America is expected to be the largest market for portable vascular Dopplers, followed by Europe and Asia Pacific.
Regional Outlook:
North America is expected to hold the largest market share of the global portable vascular Doppler market, owing to the high prevalence of vascular diseases, the presence of leading market players, and the well-established healthcare infrastructure in the region.
Europe is expected to be the second-largest market for portable vascular Dopplers, driven by the increasing demand for minimally invasive procedures and the growing awareness of vascular diseases in the region.
Asia Pacific is expected to be the fastest-growing market for portable vascular Dopplers, owing to the rising prevalence of vascular diseases, the growing aging population, and the increasing adoption of minimally invasive procedures in the region.
Key Players:
GE Healthcare
Siemens Healthineers
Koninklijke Philips N.V.
Hitachi Ltd.
Esaote SpA
Samsung Medison Co., Ltd
Mindray Medical International Limited
Analogic Corporation
FUJIFILM Sonosite Inc.
Huntleigh Medical
Spengler Holtec Medical GmbH
doppler ultrasound systems
Bionano Genomics
VTI Medical Technology
Segmentation:
By Portability:
Handheld Doppler Ultrasound System
Trolley-based Doppler Ultrasound System
By Application:
Obstetrics and Gynecology
Radiology
Cardiology
Others
By End User:
Hospitals
Clinics
Diagnostic Centers
Others
0 notes
Text
MarketPoint of Care POC Devices Market Growth, Trend, and Prospects from 2023–2030

Point of Care POC Devices Market Growth,
The Point of Care POC Devices Market is expected to grow from USD 1.70 Billion in 2022 to USD 3.10 Billion by 2030, at a CAGR of 7.80% during the forecast period.
Get the Sample Report:https://www.reportprime.com/enquiry/sample-report/10621
Point of Care POC Devices Market Size
Point of Care (POC) devices are portable diagnostic tools designed to provide rapid and accurate test results at the patient’s bedside. These devices are applicable for diagnosing various medical conditions such as blood glucose diagnostic, infectious diseases diagnostic, pregnancy diagnostic, urinalysis diagnostic, and others. The market for POC devices is segmented based on type, application, and region. The major players in this market include Roche, Abbott, Quidel, Siemens Healthcare, Danaher, Bio-Rad Laboratories, BioMerieux, ARKRAY, and Nova Biomedical. The market is dominated by North America, followed by Europe, Asia Pacific, the Middle East, Africa, and Australia. Regulatory and legal factors specific to this market include the implementation of strict regulations and guidelines by various regulatory bodies concerning the safety and efficacy of POC devices. Additionally, the increasing prevalence of chronic diseases such as diabetes and cardiovascular diseases has led to a rise in demand for POC devices.
Point of Care POC Devices Market Key Players
Mazor Key Player is listed in the Point of Care POC Devices
Roche
Abbott
Quidel
Siemens Healthcare
Danaher
Buy Now & Get Exclusive Discount on this:https://www.reportprime.com/enquiry/request-discount/10621
Point of Care POC Devices Market Segment Analysis
The Point of Care (POC) Devices market has witnessed significant growth in recent years, and it is expected to continue its growth trajectory in the future. The POC Devices market comprises diagnostic testing devices that can be used at the point where care is delivered, that is, at the patient’s bedside or in the immediate vicinity. The target market for POC devices includes hospitals, clinics, ambulatory care centers, nursing homes, and other healthcare facilities. The major factors driving revenue growth in this market include the increasing prevalence of chronic diseases, the rising demand for decentralized testing, and the need for rapid test results.
One of the latest trends in the POC Devices market is the integration of digital technologies. POC devices are being developed with advanced features such as connectivity, cloud computing, and artificial intelligence, which facilitate seamless data sharing and faster diagnostic decisions. The integration of digital technologies in POC devices is expected to enhance the accuracy and reliability of test results, while also improving workflow efficiency.
However, the POC Devices market also faces various challenges, such as the high costs associated with POC devices and the lack of adequate funding for their development and implementation. In addition, the lack of regulatory clarity and standardization creates uncertainty and hinders market growth. Manufacturers of POC devices also face intense competition from established players as well as new entrants.
The main findings of the report on POC Devices market indicate that the market is poised for significant growth in the coming years, driven primarily by the increasing demand for rapid testing and decentralized diagnostics. The market is also ripe for technological advancements, particularly in digital connectivity and artificial intelligence. To realize the market’s potential, manufacturers must focus on developing cost-effective and user-friendly devices while navigating regulatory hurdles.
The report recommends that manufacturers collaborate with healthcare institutions and providers to better understand their needs and preferences. Additionally, investment in research and development, as well as strategic partnerships with digital technology companies, can help accelerate innovation and market penetration. Ultimately, the successful adoption and implementation of POC devices will require the cooperation of stakeholders across the healthcare industry.
This report covers impact on COVID-19 and Russia-Ukraine wars in detail.
Purchase This Report:https://www.reportprime.com/checkout?id=10621&price=3590
Point of Care POC Devices (by Application)
Clinics
Hospitals
Laboratory
Others
Information is sourced from www.reportprime.com
0 notes
Text
IVD Contract Manufacturing Services Market - Global Opportunity Analysis and Industry Forecast (2024-2031)
Meticulous Research®—a leading market research company, published a research report titled, 'IVD Contract Manufacturing Services Market by Type (Assay Development, Manufacturing), Category (Reagents, Systems), Technology (Immunoassay, Molecular Diagnostics, Clinical Chemistry, Hematology, Microbiology, Urinalysis) – Global Forecast to 2031’
According to this latest publication from Meticulous Research®, the IVD contract manufacturing services market is projected to reach $25.80 billion by 2030, at a CAGR of 7.8% from 2024 to 2031. The growth of the IVD contract manufacturing services market is driven by several factors, including the rising prevalence of infectious diseases, the shift in focus from centralized laboratories to point-of-care testing services, regulatory complexities faced by IVD companies, and the need for cost-effective manufacturing of IVD tests. Factors such as high economic growth and increased outsourcing to emerging countries serve as an opportunity that would help grow the market in the future.
Factors such as the lack of skilled professionals could be considered as a challenge for the IVD contract manufacturing services market. However, maintaining product quality and protection of proprietary information restrains the growth of the market.
Key Players
The key players profiled in the IVD contract manufacturing services market report are Cenogenics Corporation (U.S.), In-Vitro Diagnostic Developers, Inc. (IDxDI) (U.S.), Savyon Diagnostics (Israel), KMS Systems, Inc. (U.S.), Nova Biomedical (U.S.), LRE Medical (Germany), Cone Bioproducts (U.S.), Invetech, Inc. (Australia), Avioq, Inc. (U.S.), TCS Biosciences Ltd. (U.K.), Affinity Life Sciences, Inc. (U.S.), Coris BioConcept (Belgium), Meridian Bioscience, Inc. (U.S.), Affinity Biologicals, Inc. (Canada), Biokit S.A. (Spain), Merck KgaA (Germany), Thermo Fisher Scientific, Inc. (U.S.), and Maxim Biomedical, Inc. (U.S.).
The IVD contract manufacturing services market is segmented by Type (Assay Development Services, Manufacturing Services, and Other Services), Category (Reagents & Consumables and Instruments & Systems), Technology (Immunoassay, Molecular Diagnostics, Clinical Chemistry, Hematology, Microbiology, and Urinalysis), and Geography. The study also evaluates industry competitors and analyzes the regional and country-level markets.
Based on type, in 2024, the manufacturing services segment is expected to account for the largest share of the market. Manufacturing services are services that include ISO-certified manufacturing facilities that allow them to carry out full project and production management, quality and regulatory support, and flexibility in scale and process, which results in increased demand for manufacturing services.
Based on category, in 2024, the reagents & consumables segment is expected to account for the largest share of the market. Reagents & consumables are products that are used for the in-vitro examination of specimens derived from the human body during disease diagnosis, treatment monitoring, prognosis, and evaluation of the patient’s health status. The large market share of the segment is attributed to factors such as increasing virulence of infectious diseases, growth of companion diagnostics, and rapid growth in molecular testing, which leads to an increased demand for POC diagnostic kits, therefore increasing the use of reagents & consumables used in the tests.
Based on technology, in 2024, the immunoassay segment is expected to account for the largest share of the market. The large share of the segment is attributed to factors such as a higher preference for immunodiagnostics owing to its inherent specificity, high throughput, and the emergence of advanced diagnostic immunoassay formats. Further, the increasing use of immunoassays in Point of Care (PoC) infectious disease testing, the need for developing novel tests, increasing usage of miniaturized devices, rising demand attributed to an aging population, and the rising trend of automation in immunoassay instruments are driving the growth of this segment.
This research report analyzes major geographies and provides a comprehensive analysis of North America (U.S., Canada), Europe (Germany, France, U.K., Italy, Spain, and the Rest of Europe), Asia-Pacific (China, Japan, India, and Rest of Asia-Pacific), Latin America and the Middle East & Africa. In 2024, North America is expected to account for the largest share of the IVD contract manufacturing services market, followed by Europe and Asia-Pacific. North America’s major market share is attributed to the rising prevalence of various infectious diseases, the growing healthcare sector, increasing awareness regarding early disease diagnosis, growing adoption of advanced innovative products, and increasing funding activities coupled with novel advanced diagnostic technologies.
Download Sample Report Here @ https://www.meticulousresearch.com/download-sample-report/cp_id=5226
Key questions answered in the report:
Which are the high-growth market segments in terms of IVD contract manufacturing services type, category, technology, and geography?
What was the historical market for IVD contract manufacturing services across the globe?
What are the market forecasts and estimates for the period 2024–2031?
What are the major drivers, restraints, opportunities, and challenges in the IVD contract manufacturing services market?
Who are the major players in the IVD contract manufacturing services market?
What is the competitive landscape, and who are the market leaders in the IVD contract manufacturing services market?
What are the recent developments in the global IVD contract manufacturing services market?
What are the different strategies adopted by the major players in the global IVD contract manufacturing services market?
What are the geographical trends and high-growth regions/countries?
Contact Us:
Meticulous Research®
Email- [email protected]
Contact Sales- +1-646-781-8004
Connect with us on LinkedIn- https://www.linkedin.com/company/meticulous-research
#IVD Contract Manufacturing Services Market#Contract Manufacturing Services#IVD Manufacturing#In-vitro Diagnostics Contract Manufacturing Services#Immunoassays#Assay Development
0 notes
Text
Heart Failure POC And LOC Devices Market Growth, Trend, and Prospects from 2023–2030

Heart Failure POC And LOC Devices Market Growth
The Heart Failure POC And LOC Devices Market is expected to grow from USD 1.80 Billion in 2022 to USD 3.80 Billion by 2030, at a CAGR of 10.10% during the forecast period.
Get the Sample Report:https://www.reportprime.com/checkout?id=11300&price=3590
Heart Failure POC And LOC Devices Market Size
Heart Failure POC and LOC Devices are medical devices designed for the diagnosis and management of heart failure. The global market for these devices is segmented by type, application, and region. Types of devices include Microfluidics, Array-based Systems, and Others. Applications for these devices include Clinics, Hospitals, Home, Laboratory, and Others. The market players in this industry include Abbott, Danaher Corporation, Siemens Healthineers, F. Hoffmann-La Roche Ltd, Quidel Corporation, bioMérieux, Trinity Biotech, Abaxis, Inc, Instrumentation Laboratory, and Jant Pharmacal Corporation. The regulatory and legal factors specific to market conditions include government regulations, such as the FDA in the United States, and patent laws that protect intellectual property. Overall, the Heart Failure POC and LOC Devices market is expected to see significant growth in the coming years due to a rise in the prevalence of heart failure and an increasing demand for point-of-care diagnostic tools.
Heart Failure POC And LOC Devices Market Key Players
Mazor Key Player is listed in the Heart Failure POC And LOC Devices
Abbott
Danaher Corporation
Siemens Healthineers
F. Hoffmann-La Roche Ltd
Quidel Corporation
Buy Now & Get Exclusive Discount on this:https://www.reportprime.com/enquiry/request-discount/11300
Heart Failure POC And LOC Devices Market Segment Analysis
The global Heart Failure POC and LOC Devices market has experienced significant revenue growth in recent years due to the rising incidence of heart failure and increasing demand for point-of-care devices. The target market for Heart Failure POC and LOC Devices includes patients with heart failure who require regular monitoring of their condition, healthcare facilities, and home healthcare settings.
Factors driving the revenue growth of the Heart Failure POC and LOC Devices market include the increasing prevalence of cardiovascular disease, the rising demand for efficient and cost-effective healthcare solutions, and the development of innovative and advanced technologies. The adoption of these devices in home healthcare settings has also contributed significantly to the revenue growth of the market.
The latest trends observed in the Heart Failure POC and LOC Devices market include the emergence of wearable devices and implantable devices, increasing adoption of remote patient monitoring, and the integration of artificial intelligence and machine learning in monitoring devices.
The major challenges faced by the Heart Failure POC and LOC Devices market include the presence of regulatory hurdles, limited reimbursement policies, and high costs of these devices. The lack of awareness among patients and healthcare professionals also poses a challenge to the growth of the market.
The report’s main findings suggest that the Heart Failure POC and LOC Devices market will continue to experience significant growth in the coming years. The increasing prevalence of heart failure and the growing demand for point-of-care devices are the primary drivers of the market. The report recommends that companies focus on developing innovative devices that can improve patient outcomes and reduce healthcare costs.
In conclusion, the Heart Failure POC and LOC Devices market has significant potential for growth due to the increasing prevalence of cardiovascular disease and the rising demand for efficient and cost-effective healthcare solutions. However, companies must overcome regulatory hurdles and address reimbursement policies to fully realize the market’s potential. Developing innovative and advanced technologies will be crucial to future growth in the Heart Failure POC and LOC Devices market.
This report covers impact on COVID-19 and Russia-Ukraine wars in detail.
Purchase This Report:https://www.reportprime.com/checkout?id=11300&price=3590
Market Segmentation (by Application)
Clinics
Hospitals
Home
Laboratory
Others
Information is sourced from www.reportprime.com
0 notes
Text
Coronavirus Diagnostics Market to Scale New Heights as Market Players Focus on Innovations 2022 – 2027
Latest added Coronavirus Diagnostics Market research study by AMA Research offers detailed outlook and elaborates market review till 2027. The market Study is segmented by key regions that are accelerating the marketization. At present, the market players are strategizing and overcoming challenges of current scenario; some of the key players in the study are
F. Hoffmann-La Roche AG (Switzerland)
Bio-Rad Laboratories Inc. (United States)
Beckman Coulter Inc. (United States)
Becton, Dickinson, and Company (United States)
Lonza Group AG (Switzerland)
Hologic, Inc. (United States)
QIAGEN (Germany)
GlaxoSmithKline Biologicals SA (Belgium)
Abbott Laboratories (United States)
Perkin Elmer, Inc. (United States)
Neuberg Diagnostics (India)
1drop Inc. (Switzerland)
Veredus Laboratories (Hong Kong)
ADT Biotech (Malaysia)
Quest Diagnostics (United States)
LabCorp (United States)
altona Diagnostics GmbH (Germany)
bioMérieux SA (France)
Mylab Discovery Solutions Pvt Ltd. (India)
Covid-19 is an infectious disease caused by a newly discovered coronavirus. The name Covid-19 represents the virus's membership of the coronavirus family, with its first human interaction noted in 2019. Coronavirus is the term coined for the family of viruses that are transmitted between animals and humans. Hence they are also known as zoonotic viruses. In addition, the virus outbreak was confirmed on March 27, 2020, on six continents and in more than 197 countries. According to the World Health Organization (WHO), fever, fatigue and dry cough are common symptoms of Covid-19 infection. Other symptoms of the disease include shortness of breath, sore throat, pain, and diarrhea in some cases, as well as nausea or a runny nose. In addition, this infection can spread from one person to another through droplets of saliva, or it can leak out of the nose when an infected person coughs or sneezes. The time from a person's exposure to Covid-19 to the onset of symptoms is generally between two and fourteen days, with an average of five days. The current status of lead COVID testing is toward increasing capacity but mixed accuracy. For efficient and accurate COVID-19 diagnosis, doctors need a portable or on-site diagnostic test to be able to manage patients in real-time in the shortest possible time. This has encouraged the introduction of point-of-care (POC) tests for diagnosis, the primary aim of which is to cut test times from hours to minutes. The most common concern of the governments of all nations affected by Covid-19 is the intolerable need to screen and test large numbers of patients for possible Sars-Cov-2 infections. As a result, most of them face significant bottlenecks in the supply of diagnostic kits to test for the virus. Diagnostic virology companies have been under immense pressure so as to provide reliable test kits and also the demand for the in vitro or point-of-care testing capabilities in the laboratories in various countries is also increasing. However, with the introduction of automated SARS-CoV-2 test systems, the diagnosis increases its capacity to carry out such tests considerably. However, currently, only a few companies are focused on developing rapid immunoassay tests for Covid-19 diagnosis.
Influencing Trend: A Shift in Focus from Centralized Laboratories to Decentralized Point-Of-Care Testing
An Upsurge in Validating and Developing Rapid POC Immunodiagnostic Tests to Facilitate Decentralized Testing
Growing Trend of Incorporation of Artificial Intelligence (AI) in the COVID-19 Tests for Rapid and Efficient Diagnosis
Challenges: Shortage of Medical Professionals That Have Sufficient Knowledge Regarding the Use of Diagnostic Kits for COVID-19
Opportunities: Growth in Funding For Research on Coronavirus Diagnostics
Increasing Application of Molecular Diagnostic Technologies in Pharmacogenetics and Point-Of-Care Testing
The Rise in Integration of Novel Technologies and Software Solutions with COVID-19 Testing
Market Growth Drivers: The Increasing Global Prevalence of COVID-19
Unavailability of Specific Medicine or a Vaccine for the Coronavirus Disease
An Increase in the Need for Developing Diagnostic Tests Owing to the Global Conditions
The Increased Demand for Rapid Immunoassay Tests for Disease Diagnosis
The Global Coronavirus Diagnostics segments and Market Data Break Down by Type (Molecular, Serology, Antigen-Based), Product Used (Instruments, Reagents & Kits), Infection Type (HCoV-229E, HCoV-OC43, SARS-CoV, HKU1-CoV, MERS-CoV), End-Users (Hospitals, Public Health Labs, Private or Commercial Labs, Physician Labs, Diagnostic Centers and Clinics, Research Institutes, Others), Sample Type (Nasopharyngeal (NP) Swab, Oropharyngeal (OP) Swab, Nasal Swab), Mode of Diagnosis (Point-of-Care (POC), Non-Point-of-Care (Non-PoC))
Presented By
AMA Research & Media LLP
0 notes
Text
Global Rapid Diagnostics Market is Estimated to Witness High Growth Owing to Increased Adoption of Point-of-Care Testing and Rising Demand for Early and Accurate Disease Diagnosis
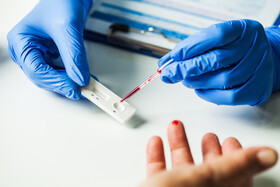
The global Rapid Diagnostics Market is estimated to be valued at US$ 33.4 Bn in 2022 and is expected to exhibit a CAGR of 9.8% over the forecast period 2023-2030, as highlighted in a new report published by Coherent Market Insights.
Market Overview:
Rapid diagnostics refer to the medical tests that provide quick results to diagnose various diseases and conditions. These tests are designed to detect the presence of specific biomarkers or antigens in a patient's body. Rapid diagnostics are commonly used in point-of-care settings to provide immediate results, allowing for timely diagnosis and treatment. The market includes a wide range of products such as rapid test kits, POC analyzers, and molecular diagnostic devices.
Market Dynamics:
The rapid diagnostics market is driven by two major factors: the increased adoption of point-of-care testing (POCT) and the rising demand for early and accurate disease diagnosis.
Firstly, the adoption of POCT has been rapidly increasing in recent years. POCT allows healthcare professionals to perform tests at the patient's bedside, eliminating the need for sending samples to a laboratory and waiting for results. This enables immediate decision-making and improves patient care. POCT is particularly useful in emergency departments, intensive care units, and remote healthcare settings where timely diagnosis is critical.
Secondly, the demand for early and accurate disease diagnosis is on the rise. Rapid diagnostics provide a quick and accurate diagnosis, enabling early detection and intervention. This is crucial for diseases such as infectious diseases, cardiac conditions, and cancer, where early diagnosis can significantly improve patient outcomes. Rapid diagnostic tests are also being increasingly used in screening programs and surveillance systems to detect diseases at an early stage.
SWOT Analysis:
Strength:
1. High demand for quick and accurate diagnostic solutions
2. Increasing adoption of rapid diagnostics in point-of-care settings
Weakness:
1. Limited sensitivity and specificity of some rapid diagnostic tests
2. Lack of awareness and accessibility in developing regions
Opportunity:
1. Growing application of rapid diagnostics in home healthcare settings
2. Advancements in technology leading to the development of innovative diagnostic products
Threats:
1. Stringent regulatory requirements for the approval of rapid diagnostic tests
2. Competition from traditional laboratory-based diagnostics
In conclusion, the global rapid diagnostics market is expected to experience significant growth in the coming years. The increasing adoption of point-of-care testing and the demand for early and accurate disease diagnosis are driving market growth. However, challenges such as regulatory requirements and competition from traditional diagnostics need to be addressed. Overall, the rapid diagnostics market holds immense potential for innovation and improved patient care.
#Rapid Diagnostics Market#Coherent Market Insights#Rapid Diagnostics Market Value#Rapid Diagnostics Market Size#Rapid Diagnostics Market Share#Rapid Diagnostics Market Growth#Rapid Diagnostics Market Analysis.
0 notes
Text
Point of Care Data Management Systems Market

The global Point of Care Data Management Systems Market is estimated to be valued at US$ 718.5 million in 2017 and is expected to exhibit a CAGR of 6.6% over the forecast period, according to a new report published by Coherent Market Insights.
A) Market Overview:
Point of Care (PoC) Data Management Systems are software platforms that enable real-time collection, documentation, and management of patient data at the point of care. These systems provide healthcare professionals with immediate access to patient information, helping them make faster and more informed decisions. PoC Data Management Systems have numerous advantages, including improved patient outcomes, reduced healthcare costs, enhanced workflow efficiency, and increased patient satisfaction. The need for these products is driven by the increasing demand for quality healthcare services, rising prevalence of chronic diseases, and the growing focus on patient-centered care.
B) Market key trends:
One key trend in the PoC Data Management Systems market is the increasing adoption of PoC testing. PoC testing refers to medical diagnostic testing performed at or near the site of patient care. It provides rapid and accurate results, allowing for immediate diagnosis and treatment decisions. PoC testing is gaining popularity due to its ability to improve patient outcomes by reducing turnaround time, eliminating the need for laboratory testing, and enabling timely intervention. For example, some PoC Data Management Systems can integrate with PoC testing devices such as glucose meters or cardiac markers to provide seamless data integration and analysis. This trend is expected to drive the growth of the PoC Data Management Systems market.
C) PEST Analysis:
Political: The political factors influencing the PoC Data Management Systems market include government regulations and policies regarding healthcare infrastructure, reimbursement policies for PoC testing, and data privacy and security regulations.
Economic: The economic factors impacting the market include healthcare expenditure, insurance coverage, and affordability of PoC Data Management Systems for healthcare facilities.
Social: The societal factors influencing the market include the aging population, increasing prevalence of chronic diseases, and the demand for improved healthcare services.
Technological: The technological factors impacting the market include advancements in connectivity and integration capabilities, development of cloud-based platforms, and increasing adoption of electronic health records.
D) Key Takeaways:
- The global Point of Care Data Management Systems Market is expected to witness high growth, exhibiting a CAGR of 6.6% over the forecast period, due to increasing adoption of PoC testing.
- North America is expected to dominate the market due to the presence of well-established healthcare infrastructure, high healthcare expenditure, and early adoption of PoC Data Management Systems.
- Key players operating in the global PoC Data Management Systems market include Abbott Laboratories, Danaher Corporation, F.Hoffman-La Roche Ltd., Orchard Software Corporation, Siemens Healthcare GmbH, Telcor, Inc., Thermo Fisher Scientific, Inc., Randox Laboratories Ltd., Hedera Biomedics Srl, and Seaward Electronic Ltd. These players are adopting various strategies such as mergers and acquisitions, partnerships, and product launches to strengthen their market position.
In conclusion, the global PoC Data Management Systems market is poised for significant growth due to increasing adoption of PoC testing. These systems enable healthcare professionals to access patient information in real-time, leading to improved patient outcomes and enhanced workflow efficiency. Factors such as government regulations, economic conditions, social factors, and technological advancements will continue to influence the market. North America is expected to be the fastest-growing and dominating region in the market. Key players in the market are ramping up their efforts to stay competitive and expand their market share.
0 notes
Text
Africa IVD Market to be Worth $1.78 Billion by 2029
According to a new market research report titled, ‘Africa In Vitro Diagnostics Market by Product & Services, Technology (Immunoassay, Point of Care, Molecular Diagnostics, Coagulation), Application (Infectious Diseases, Diabetes, Oncology), Diagnostic Approach (Lab, OTC, PoC), and Customer Type - Forecast to 2029,’ published by Meticulous Research®, the Africa in vitro diagnostics market is expected to reach $1.78 billion by 2029, at a CAGR of 4.8% from 2022 to 2029.
Download Free Sample Report Now @ https://www.meticulousresearch.com/download-sample-report/cp_id=5415
In vitro diagnostics (IVD) are tests performed on samples of blood or tissue taken from the human body to detect a wide range of diseases. These tests help monitor overall health to prevent and treat various diseases. IVD involves the use of various reagents, assays, and kits based on technologies such as immunoassay, whole blood glucose monitoring, molecular diagnostics, point of care, clinical chemistry, hematology, coagulation & hemostasis, critical care, and urinalysis.
High Prevalence of Infectious Diseases Driving the Growth of the Africa IVD Market
Diagnostic tests play a crucial role in the medical care of patients with infections. The demand for IVD kits used to diagnose infectious diseases is high in Africa due to the increasing awareness regarding emerging and re-emerging infectious diseases and the adoption of advanced molecular testing and lab-on-chip technology. The burden of diseases such as HIV, tuberculosis, malaria, viral hepatitis, and neglected tropical diseases (NTDs) is large in Africa. For example, according to WHO, in 2020, 25.6 million people were living with HIV in the African Region. The annual number of new HIV infections also increased in the Middle East and North Africa (source: UNAIDS data 2021).
In addition, there is a frequent outbreak of endemic diseases in Africa. For instance, Lassa fever is endemic in Nigeria and continues to be reported throughout the country. According to Nigeria Centre for Disease Control and Prevention (NCDC). In January 2022, 981 suspected and 211 confirmed cases of Lassa fever were reported in Nigeria. There were 40 deaths reported among confirmed cases, indicating a case fatality rate of 19%. Thus, the high burden of infectious diseases increases the demand for IVD tests in Africa.
Speak to our Analysts to Understand the Impact of COVID-19 on Your Business: https://www.meticulousresearch.com/speak-to-analyst/cp_id=5415
Africa IVD Market: Future Outlook
The Africa IVD market is segmented based on Product Category (Reagents, Assays, and Kits, Instruments, and Software & Services), Technology (Immunoassay, Whole Blood Glucose Monitoring, Point-of-Care Diagnostics, Clinical Chemistry, Molecular Diagnostics, Hematology, Coagulation & Hemostasis, Critical Care, Urinalysis, and Other IVD Technologies), Application (Infectious Diseases, Oncology, Diabetes, Endocrinology, Cardiology, and Other Applications), Diagnostic Approach (Laboratory Testing, Point-of-Care Testing, and OTC/Self Testing), and Customer Type (Hospital Labs, Private Labs, Home Care/Self Testing, Government, and Other Customer Types), and Geography (South Africa, Egypt, Nigeria, Kenya, Algeria, Tanzania, Morocco, Tunisia, and the rest of Africa). The study also evaluates industry competitors and analyzes their market shares.
Based on product category, the reagents, assays, and kits segment is slated to register the fastest growth rate during the forecast period. In Africa, infectious diseases cause chronic illnesses, premature deaths, and loss of productivity, affecting the region's economic growth. Thus, the emerging threats of infectious diseases drive the demand for IVD in Africa. The frequent use of reagents for diagnosing diseases and the easy availability & accessibility of these tests are the major factors driving the growth of this segment.
Based on technology, the point of care segment is slated to register the fastest growth rate during the forecast period. The rising geriatric population, the growing prevalence of chronic diseases, and the rising incidence of infectious diseases are driving the growth of this segment. The increasing burden of chronic diseases and pathogen outbreaks has significantly increased the demand for PoC diagnostics in Africa. The increased need for early and accurate diagnosis, precise confirmation of clinical findings, and immediate & informed decision-making on treating diseases contributes to the growth of the PoC segment.
Based on application, the oncology segment is slated to register the fastest growth rate during the forecast period. Cancer involves a complex set of genetic alternations and subsequent changes in gene expression, leading to the uncontrolled proliferation of cells. In cancer diagnostics, early diagnosis and knowing the exact nature of the tumor are of prime importance. Early cancer detection and access to effective anti-cancer treatment can result in higher rates of survival and a better quality of life. According to GLOBOCAN 2020, 1.1 million new cases and 711,429 deaths were recorded in Africa. The growing demand for personalized medicines in cancer therapy, the increasing prevalence of cancer, and the rising demand for rapid cancer diagnosis tests drive the growth of the IVD market for oncology applications.
Based on diagnostic approach, the OTC/self-testing segment is slated to register the fastest growth rate during the forecast period. In Africa, over-the-counter (OTC)/self-testing is promoted for diseases, such as HIV and COVID-19, due to the high prevalence of infectious diseases. Self-testing reduces the burden on healthcare workers and the system. The OTC/self-testing kits are diagnostic tests that can be purchased from any pharmacy store and used by the patient. These tests can give rapid results and can be taken from anywhere.
Quick Buy – Africa IVD Market Research Report: https://www.meticulousresearch.com/Checkout/47708335
Based on customer type, the home care/self-testing segment is slated to register the fastest growth rate during the forecast period. The growth of this segment is attributed to the increasing need to monitor and manage chronic diseases, the growing awareness about home healthcare testing products, and the reduced waiting time and healthcare costs offered by self-testing kits.
This research report analyzes the market across major African countries and provides a comprehensive analysis of South Africa, Algeria, Nigeria, Kenya, Morocco, Tunisia, Tanzania, Egypt, and the rest of Africa.
Key companies operating in the Africa IVD market are Abbott Laboratories (U.S.), Becton, Dickinson and Company (U.S.), bioMérieux SA (France), Danaher Corporation (U.S.), F. Hoffmann-La Roche Ltd. (Switzerland), QIAGEN N.V. (Netherlands), Siemens Healthineers AG (Germany), Thermo Fisher Scientific Inc. (U.S.), Bio-Rad Laboratories, Inc. (U.S.), Illumina, Inc. (U.S.), and Shenzhen Mindray Bio-Medical Electronics Co., Ltd (China).
To gain more insights into the market with a detailed table of content and figures, click here: https://www.meticulousresearch.com/product/africa-ivd-market-5415
Scope of the Report
Africa IVD Market, by Product Category
Reagents, Assays, and Kits
Instruments
Software & Services
Africa IVD Market, by Technology
Immunoassay
Whole Blood Glucose Monitoring
Point-of-Care Diagnostics
Clinical Chemistry
Molecular Diagnostics
Hematology
Coagulation & Hemostasis
Critical Care
Urinalysis
Other IVD Technologies
Note: Other technologies include microscopy, hybridization, and loop-mediated amplification.
Africa IVD Market, by Application
Infectious Diseases
Oncology
Diabetes
Endocrinology
Cardiology
Other Applications
Note: Other applications include nephrology, toxicology, gastroenterology, neonatal, genetic, and neurological disorders.
Africa IVD Market, by Diagnostic Approach
Laboratory Testing
Point-of-Care Testing
OTC/Self-testing
Africa IVD Market, by Customer Type
Hospital Labs
Private Labs
Home Care/Self Testing
Government
Other Customer Types
Note: Other customer types include long-term care facilities, academic & research institutes, ambulatory care centers, and transfusion laboratories.
Africa IVD Market, by Country
South Africa
Egypt
Nigeria
Kenya
Algeria
Tanzania
Morocco
Tunisia
Rest of Africa
Download Free Sample Report Now @ https://www.meticulousresearch.com/download-sample-report/cp_id=5415
In Vitro Diagnostic (IVD) Reagents Market - https://www.meticulousresearch.com/product/in-vitro-diagnostic-reagents-market-5110
0 notes
Text
POC Molecular Diagnostics Market 2022 | Growth Strategies, Opportunity, Challenges, Rising Trends and Revenue Analysis 2030
The latest market report published by Credence Research, Inc. “Global POC Molecular Diagnostics Market: Growth, Future Prospects, and Competitive Analysis, 2022 – 2030. The global demand for POC molecular diagnostics was valued at USD 6.9 Billion in 2022 and is expected to reach USD 8.6 Billion in 2030, growing at a CAGR of 3.3% between 2023 and 2030.
Point-of-Care (POC) Molecular Diagnostic solutions have become pivotal. These tools bridge the gap between rapid diagnostics and precision medicine, offering clinicians timely, actionable insights. This article provides a holistic perspective on the POC molecular diagnostic market, its current dynamics, and its potential trajectory.
POC Molecular Diagnostics Market Dynamics refer to the ever-evolving landscape of point-of-care molecular diagnostics, a field that combines the power of molecular biology with rapid testing at the patient's bedside. This market is driven by several key factors, including advancements in technology and growing demand for personalized medicine. The integration of portable devices and miniaturized laboratory equipment has revolutionized healthcare delivery, allowing for quick and accurate diagnosis of diseases like HIV, tuberculosis, influenza, and genetic disorders. With POC molecular diagnostics paving the way towards precision medicine, patients can receive targeted treatments based on their unique genetic makeup. This emerging market also benefits from increased accessibility as it eliminates the need for time-consuming laboratory processes and enables remote testing in resource-limited settings.
Point-of-Care (POC) Molecular Diagnostics market encompasses the sector of medical testing that employs molecular techniques to detect and diagnose conditions at or near the location of patient care. These diagnostics are pivotal for quick turn-around results, often enabling physicians to make immediate and informed decisions about patient care. The convenience and speed of POC molecular diagnostics are propelling their adoption, especially in settings like urgent care clinics, hospitals, and even remote field clinics. As technology has advanced, these tests have become more accurate, portable, and user-friendly, further driving their demand.
The market's growth is also influenced by the rising prevalence of infectious diseases, increased awareness for early detection, and the constant need for decentralized diagnostic solutions. Continuous research and development have led to the introduction of advanced products, leveraging technologies like PCR (polymerase chain reaction) and next-generation sequencing, adapted for POC settings. Given the current trajectory and the emphasis on personalized medicine and rapid diagnostics, the POC Molecular Diagnostics market is poised for significant growth in the foreseeable future.
Key Players in the Market
Roche: Recognized for their cutting-edge platforms like the Cobas Liat.
Abbott: Renowned for their ID NOW series.
Biomerieux: Delivering comprehensive solutions through platforms like the BioFire FilmArray.
Applications: Beyond Infectious Diseases
Though infectious diseases remain the most common application, the utility of POC molecular diagnostics extends to:
Oncology: Identifying tumor markers for precise cancer treatment.
Pharmacogenomics: Guiding therapeutic decisions based on patient genetics.
Browse 228 pages report POC Molecular Diagnostics Market By Technology (PCR-based, Genetic Sequencing-based, Hybridization-based, Microarray-based) By Application Type (Infectious Diseases, HIV POC, Clostridium difficile POC, HBV POC, Pneumonia or Streptococcus associated infections, Respiratory syncytial virus (RSV) POC, HPV POC) - Growth, Future Prospects & Competitive Analysis, 2016 – 2030)- https://www.credenceresearch.com/report/poc-molecular-diagnostics-market
Growth Drivers & Challenges:
Outbreak of new diseases: Recent pandemics have underlined the need for rapid diagnostic tools.
Technological advancements: Innovations, especially in PCR and isothermal amplification, have transformed diagnostics.
Government initiatives: Increasing funding and support for swift and accurate diagnostics.
Anticipated Market Hurdles:
High costs: The advanced technology can be expensive, potentially limiting widespread adoption.
Requirement for skilled personnel: Though designed to be user-friendly, some equipment demands specialized training.
Future Directions & Potential
The future of POC molecular diagnostics looks promising with:
Integration of AI: Predictive analytics can further enhance accuracy and speed.
Wearable diagnostics: The next frontier might be wearables that continually monitor and diagnose in real-time.
Conclusion
The Point-of-Care Molecular Diagnostic market is poised for significant growth, driven by technological advancements, rising disease outbreaks, and increasing healthcare demands globally. As the healthcare paradigm shifts towards precision and rapid diagnostics, POC molecular tools are set to play a more prominent role in shaping global health outcomes.
Why to Buy This Report-
The report provides a qualitative as well as quantitative analysis of the global POC Molecular Diagnostics Market by segments, current trends, drivers, restraints, opportunities, challenges, and market dynamics with the historical period from 2016-2020, the base year- 2021, and the projection period 2022-2028.
The report includes information on the competitive landscape, such as how the market's top competitors operate at the global, regional, and country levels.
Major nations in each region with their import/export statistics
The global POC Molecular Diagnostics Market report also includes the analysis of the market at a global, regional, and country-level along with key market trends, major players analysis, market growth strategies, and key application areas.
Browse Full Report: https://www.credenceresearch.com/report/poc-molecular-diagnostics-market
Visit: https://www.credenceresearch.com/
Related Report: https://www.credenceresearch.com/report/automatic-veterinary-biochemistry-analyzer-market
Related Report: https://www.credenceresearch.com/report/automated-and-rapid-microbiological-tests-market
Browse Our Blog: https://www.linkedin.com/pulse/poc-molecular-diagnostics-market-size-worth-usd-86-billion-singh
Browse Our Blog: https://tealfeed.com/poc-molecular-diagnostics-market-size-worth-gywps
About Us -
Credence Research is a viable intelligence and market research platform that provides quantitative B2B research to more than 10,000 clients worldwide and is built on the Give principle. The company is a market research and consulting firm serving governments, non-legislative associations, non-profit organizations, and various organizations worldwide. We help our clients improve their execution in a lasting way and understand their most imperative objectives. For nearly a century, we’ve built a company well-prepared for this task.
Contact Us:
Office No 3 Second Floor, Abhilasha Bhawan, Pinto Park, Gwalior [M.P] 474005 India
0 notes