#Gilead Sciences Inc
Text

"THEY [BARDA, DOD] ARE NOT IMPLEMENTING THOSE [CRITICAL] CONTROLS"
EUA History & Anthrax Vaccine | Colonel Thomas Rempfer, USAF (Retired) (TPC #1,280)
Tommy's Podcast
CAPTAIN THOMAS L. REMPFER TESTIMONY OF MARCH 1998
See footnote 83, Testimony of Capt. Thomas Rempfer, NSVAIR anthrax hearing (I), pp. 40–41.
https://www.congress.gov/106/crpt/hrpt556/CRPT-106hrpt556.pdf
Or...
https://www.govinfo.gov/content/pkg/CHRG-106hhrg57559/html/CHRG-106hhrg57559.htm
"...We are not speaking out against a vaccine for public health issues. We take a lot of shots. We have always taken them. We are speaking out against vaccines against [used as] biological weapons."
"It is imperative to disobey unlawful orders"
Discussion surrounding DOD unlawful experimentation on military personnel, leading to the upholding of Constitutional law, informed consent;. Also, the highly unusual sequence of events during the Anthrax scare, and Oct 2001, who was holding the reins of power?
Robert S. Mueller, III, September 4, 2001- September 4, 2013, Donald H. Rumsfeld served as the 21st Secretary of Defense from January 2001 to December 2006. Before assuming this post, the former Navy pilot had also served as the 13th Secretary of Defense, White House Chief of Staff, U.S. Ambassador to NATO, U.S. Congressman and chief executive officer of two Fortune 500 companies [and Bilderberg participant]. Rumsfeld has served on the boards of pharmaceutical companies Gilead Sciences, Pfizer Inc., and Amylin Pharmaceuticals;. BARDA formed 2006;.
related;.
Bilderberg reconvenes in person after two-year pandemic gap | ... theguardian.com › world › 2022 › jun › 04 › bilderberg-reconvenes-in-person-after-two-year-pandemic-gap
June 8, 2022 - Bilderberg is back with a vengeance. After a pandemic gap of two years, the elite global summit is being rebooted in a security-drenched hotel in Washington DC, with a high-powered guest list that includes the heads of Nato, the CIA, GCHQ, the US national security council, two European prime ministers, a healthy sprinkle of tech billionaires, and Henry Kissinger.
Project Bioshield Act
BARDA grew from The Project Bioshield Act was an act passed by the United States Congress in 2004 calling for $5 billion for purchasing vaccines that would be used in the event of a bioterrorist attack.[1] This was a ten-year program to acquire medical countermeasures to biological, chemical, radiological, and nuclear agents for civilian use. A key element of the Act was to allow stockpiling and distribution of vaccines which had not been tested for safety or efficacy in humans, due to ethical concerns.
0 notes
Text
Trodelvy Injection: A Game-Changer in Cancer Care
Trodelvy contains the active pharmaceutical ingredients sacituzumab govitecan. The medicine is used for the treatment of patients with triple-negative breast cancer (TNBC), HR+/HER2- metastatic breast cancer, and advanced bladder cancer. If you are searching for a genuine Trodelvy supplier from India, then Indian Pharma Network (IPN) can be your most reliable source/platform.
IPN is WHO GDP & ISO 9001 2015 Certified Trodelvy Supplier, Wholesaler, Importer, and Exporter from India. Trodelvy which is Manufactured by Gilead Sciences, Inc, is available in a strength of 180 mg/mL. Trodelvy 180 mg is supplied for Tenders/ exports/ imports/ Named patient program/ RLD supplies/ Reference listed drugs/ Comparator Drug/ Bio-Similar/ Innovator samples for Clinical trials.
Indian Pharma Network is the legitimate source of Trodelvy (sacituzumab govitecan-hziy) for injection, all the customers can get in touch with us to buy/order/procure this pharmaceutical product in approved quantity.
Buy Trodelvy 180 mg Injection at Lowest Price from India
Trodelvy 180 mg for injection is designed to carry cancer-fighting drug to cells that have Trop-2 proteins. Certain tumor cells have high Trop-2 proteins. The medicine trodelvy 180 mg injection is approved for 3 different types of cancer:
Advanced Bladder Cancer
HR+/HER2- Metastatic Breast Cancer
Metastatic Triple Negative Breast cancer
Trodelvy injection 180 mg is a type of antibody-drug conjugate (ADC) treatment that is designed to work differently than traditional chemotherapy. It is designed to deliver promising anticancer drug directly into cells with Trop-2 proteins.
Buy Trodelvy in India I Sacituzumab Govitecan 180 MG Vial
Trodelvy (sacituzumab govitecan) is a type of medicinal product known as antibody-drug conjugate, or ADC for short. Unlike typical (traditional) chemotherapy, ADCs consist of three parts: an antibody, an anticancer medicine, and a linker. If you want to buy trodelvy in India for triple-negative breast cancer, HR+/HER2- metastatic breast cancer, and advanced bladder cancer, then Indian Pharma Network (IPN) can be your one-stop solution.
We are famed and esteemed in the pharmaceutical industry for our best quality service, vast industry experience, market credentials, and timely delivery. Our all the tie-ups and sourcing from the reputed brands allow us to offer the best price for Trodelvy 180 mg vial.
Trodelvy (sacituzumab govitecan 180 mg vial) is an intravenous (IV) infusion (10mg/kg). Proposed doses are administered once weekly for two weeks (Day 1 and 8) of 21-day treatment cycles. Each treatment cycle is 21 days (3 weeks).
Sacituzumab Govitecan- Trodelvy Price for 180 MG Vial in India
Trodelvy is made of two different drugs joined together: a monoclonal antibody drug (which attaches to Trop -2 receptors, present on the outside of some cancer cells, and a chemotherapy drug (which stops all cells including unhealthy cells from growing and dividing). The monoclonal antibody drug attaches to the unhealthy cells and then releases the chemotherapy medicine directly into the cell. All pharmaceutical products – comparator drugs, adjunctive therapies, RLDs, co-meds and rescue meds, and Exports/Imports drugs – are transported in standard temperature-controlled conditions with active monitoring in order to ensure the integrity of products. Contact us today to get/buy your hard-to-access prescription medicine at the lowest price from India.

#trodelvy in India#trodelvy price in India#trodelvy suppliers in noida#importers of trodelvy from india#innovator drug suppliers
2 notes
·
View notes
Link
A federal judge in Texas ruled that the Affordable Care Act’s mandate for free coverage of groundbreaking HIV prevention drugs made by Gilead Sciences Inc. “substantially burdens” the religious freedom of a Christian-owned company.
US District Judge Reed O’Connor in Fort Worth on Wednesday granted summary judgment to Braidwood Management Inc. in its challenge to coverage of Gilead’s Truvada and Descovy. The two pre-exposure prophylactic drugs, commonly known as PrEP, are taken daily by hundreds of thousands of Americans, particularly men who have sex with men.
The suit is being led by attorney Jonathan Mitchell, the Republican former solicitor general of Texas known for his efforts to restrict abortion access in the state. Mitchell argues that mandatory PrEP coverage forces Christians to subsidize “homosexual behavior”
O’Connor, a George W. Bush appointee, said the government failed to demonstrate a state interest in providing coverage of the drugs that overcame the plaintiffs’ religious objections.
“The government defendants in the suit “outline a generalized policy to combat the spread of HIV, but they provide no evidence connecting that policy to employers such as Braidwood,” the judge wrote. “Thus, defendants have not carried their burden to show that the PrEP mandate furthers a compelling governmental interest.”
The ruling is the latest win for conservatives who support a broad interpretation of the Religious Freedom Restoration Act, a 1993 law that has been used to challenge access to abortion and contraception and to block equal protection for the LGBTQ community.
“This ruling is about imposing extreme religious beliefs -- not, as it purports, about protecting religious freedom,” said Ivy Hill, community health program director of the Campaign for Southern Equality. “Far-right extremist judges are attacking privacy and access to health care.”
US Department of Health and Human Services spokeswoman Rachel Seeger declined to comment or say whether the government might appeal the ruling. HHS “continues to work to ensure that people can access health care, free from discrimination,” she said in a statement.
The judge reserved deciding the “appropriate remedy” for resolving the claim, and it’s unclear what impact the ruling will have beyond the plaintiff company, which employs about 70 people. He said there was no evidence that the government couldn’t assume the cost of providing PrEP drugs to people who are unable to obtain them from religious employers.
PrEP drugs can cost as much as $20,000 a year, according to the ruling.
In another win for the plaintiffs, O’Connor ruled the US Preventive Services Task Force, or PSTF, which makes recommendations for what qualifies as a covered preventive measure under the ACA, is unconstitutional because it “wields a power to compel private action that resembles legislative authority.”
“Because PSTF members are principal officers, they must be appointed by the President and confirmed by the Senate,” O’Connor said. “The PSTF members indisputably fail that constitutional requirement.”
The judge also reserved ruling on the appropriate remedy for dealing with that alleged violation.
O’Connor dismissed a claim that challenged the preventive mandate itself, though it’s unclear how the provision might be affected by the rest of the ruling.
Mitchell seeks to block the entire preventive services mandate under the ACA, arguing it’s invalid because the government employees who manage the list have too much power to not be confirmed by the Senate. Depending on O’Connor’s remedy in the case, that could have a far-reaching impact nationwide, putting at risk requirements for no-cost access to other services, like care for pregnant women and infants.
O’Connor didn’t entirely side with Mitchell. The judge ruled that two other bodies involved in ACA preventive services, the Health Resources and Services Administration and the Advisory Committee on Immunization Practices, were properly empowered under the constitution. The judge also ruled that the Preventive Services Task Force doesn’t violate a separate provision of the constitution.
The case is Braidwood Management Inc. v. Becerra, 4:20-cv-00283, US District Court, Northern District of Texas (Fort Worth).
(Updates with statement from HHS.)
#'pro life'#thou shalt not kill#'family values'#reed o'connor#jonathan mitchell#religious freedom restoration act
6 notes
·
View notes
Text
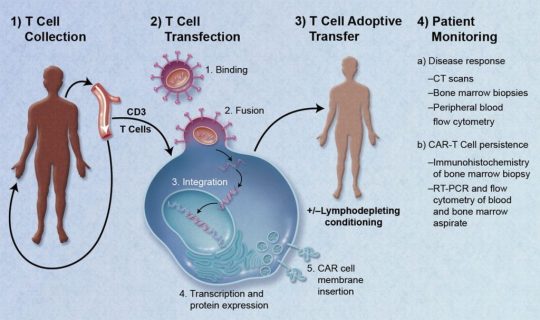
The global CAR T-Cell Therapy Market is projected to reach more than USD 22.2 billion by 2032 from USD 2.1 billion in 2023, growing at a CAGR of 30% from 2024-2032.
Key Players in Global CAR T-Cell Therapy Market
Novartis AG
Bluebird Bio, Inc.
Cellectis
Bristol-Myers Squibb
Merck & Co., Inc.
Juno Therapeutics, Inc.
Celyad Oncology
Celgene Corporation
Sorrento Therapeutics, Inc.
Miltenyi Biotech
Intellia Therapeutics
Pfizer, Inc.
Autolus Therapeutics
Gilead Sciences, Inc. (Kite Pharma Inc.)
Cartesian Therapeutics, Inc.
Caribou Biosciences, Inc.
Other Prominent Players
0 notes
Text
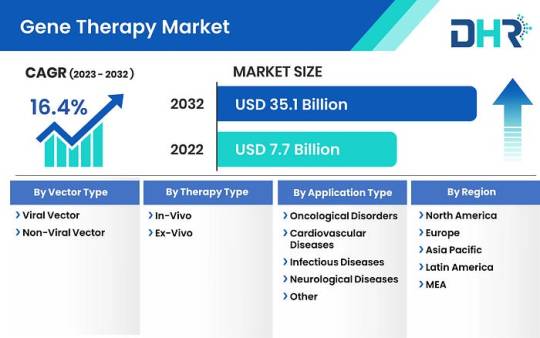
Gene Therapy Market size was USD 7.7 Billion in 2022 and is projected to reach USD 35.1 Billion by 2032
The gene therapy market size was USD 7.7 Billion in 2022 and is projected to reach USD 35.1 Billion by 2032 with a CAGR of 16.4%.
Gene therapy is a rapidly evolving field in biotechnology that holds immense promise for treating a wide array of genetic disorders by replacing, inactivating, or introducing genes into a patient’s cells. This approach aims to address the root cause of diseases rather than just managing their symptoms, offering potentially curative solutions.
In recent years, the gene therapy market has witnessed significant growth driven by advancements in molecular biology, gene editing technologies like CRISPR-Cas9, and improved delivery mechanisms such as viral vectors. Additionally, increasing investment from both public and private sectors, along with growing partnerships between pharmaceutical companies and research institutions, has accelerated the development and commercialization of gene therapies.
Recent developments in the gene therapy market:
FDA Approvals: The U.S. Food and Drug Administration (FDA) has granted approvals for several gene therapies targeting various diseases, including rare genetic disorders, certain types of cancer, and inherited retinal diseases.
Advancements in Gene Editing Technologies: Continuous improvements in gene editing technologies, particularly CRISPR-Cas9, have facilitated more precise and efficient gene modifications, opening up new possibilities for therapeutic interventions.
Expanded Clinical Trials:These trials not only evaluate the safety and efficacy of new gene therapy approaches but also contribute to our understanding of disease mechanisms and potential therapeutic targets.
Emergence of Non-Viral Delivery Systems: While viral vectors have been traditionally used as delivery vehicles for gene therapies, there is growing interest in developing non-viral delivery systems due to concerns over immunogenicity and manufacturing scalability associated with viral vectors.
Investment and Partnerships: The gene therapy market continues to attract significant investment from pharmaceutical companies, venture capitalists, and government agencies. Moreover, strategic partnerships and collaborations between industry players and academic institutions have accelerated research and development efforts.
Top Companies are:
· Novartis AG,
· GlaxoSmithKline plc,
· Biogen,
· Spark Therapeutics,
· Gilead Sciences, Inc.,
· Amgen, Inc.,
· Jazz Pharmaceuticals
· Sarepta Therapeutics
· Orchard Therapeutics
· Intellia Therapeutics
· Merck and Company Inc.
Market Segmentations:
By Vector Type (2023–2032)-
Viral Vector
Non-viral Vector
By Therapy Type (2023–2032)-
In-vivo
Ex-vivo
By Application (2023–2032)-
Oncological Disorders
Cardiovascular Diseases
Infectious Diseases
Neurological Diseases
Other
Regional Analysis:
The gene therapy market is predominantly led by North America on a global scale, owing to several key factors. Government support, coupled with the approval of vector-based gene therapies, has significantly bolstered the region’s gene therapy landscape. Initiatives aimed at advancing healthcare, such as Pfizer’s establishment of its first site in Phase 3 trials for investigational gene treatment targeting Duchenne Muscular Dystrophy patients, underscore North America’s commitment to innovation in genetic medicine.
Moreover, collaborative research endeavors between pharmaceutical companies and cancer research institutes have propelled advancements in gene therapy, enhancing treatment options for various diseases. The region’s heightened awareness about chronic disorders, coupled with easily accessible medical facilities, further contributes to the robust growth of the gene therapy market in North America.
Key highlights of the report include:
1. The report delivers thorough Market analysis, furnishing valuable insights to guide strategic decision-making.
2. The comprehensive research outlined in the study enhances the depth of your presentations and marketing strategies.
3. By offering crucial insights into key market competitors, the study empowers businesses with a strategic edge.
4. It delivers a precise assessment of evolving market dynamics, ensuring readers stay abreast of the latest industry trends.
5. With meticulous breakdowns of various market niches, the report facilitates informed decision-making processes.
0 notes
Text
Anti-infective Agents Market Set for Explosive Growth

Advance Market Analytics added research publication document on Worldwide Anti-infective Agents Market breaking major business segments and highlighting wider level geographies to get deep dive analysis on market data. The study is a perfect balance bridging both qualitative and quantitative information of Worldwide Anti-infective Agents market. The study provides valuable market size data for historical (Volume** & Value) from 2018 to 2022 which is estimated and forecasted till 2028*. Some are the key & emerging players that are part of coverage and have being profiled are Pfizer Inc. (United States), Johnson & Johnson (United States), Abbott Laboratories (United States), Gilead Sciences, Inc. (United States), Bristol-Myers Squibb Co. (United States), Merck & Co., Inc. (United States), Bayer Healthcare AG (Germany), AstraZeneca Plc. (United Kingdom), Boehringer Ingelheim (Germany), Novartis AG (Switzerland), Astellas Pharma, Inc. (Japan), GlaxoSmithKline Plc. (United Kingdom).
Get free access to Sample Report in PDF Version along with Graphs and Figures @ https://www.advancemarketanalytics.com/sample-report/1828-global-anti-infective-agents-market
Anti-infectives are drugs that are used to act on the body against invading foreign organisms, especially those that can cause infection. Anti-infectives act on invading organisms in a number of ways. Over time, invading pathogens develop resistance to anti-infectives. Resistance is the ability to adapt to an anti-infectious drug over time and produce cells that are no longer affected by a particular drug. Anti-infectives like metronidazole, clindamycin, tigecycline, linezolid, and vancomycin work against many types of bacteria that have become resistant to other antibiotics.
Keep yourself up-to-date with latest market trends and changing dynamics due to COVID Impact and Economic Slowdown globally. Maintain a competitive edge by sizing up with available business opportunity in Anti-infective Agents Market various segments and emerging territory.
Influencing Market Trend
Spreading Awareness amongst People Regarding the Fatal Implications of Infectious Diseases and the Importance of Early Treatment
Increased Prescription and Accessibility of Antibacterial Due To Their Over-The-Counter Status
Development of Combination Dr
Market Drivers
Rising Prevalence of Infectious Diseases Such As HIV, H1N1, and Ebola Virus
Increasing Awareness among Healthcare Professionals and Patients
Increased Potency and Efficacy and the Commercialization of Pipeline Products
Opportunities:
New Product Development and Presence of Strong Product Pipeline
An Increase in Funding From the Government for the Research Activities Pertaining To Discovery of the Drugs
Spread Awareness and To Enhance the Treatment of Communicable Diseases in Develop
Challenges:
Lack of Awareness in Underdeveloped Regions
Have Any Questions Regarding Global Anti-infective Agents Market Report, Ask Our Experts@ https://www.advancemarketanalytics.com/enquiry-before-buy/1828-global-anti-infective-agents-market
Analysis by Type (Antibacterial Drugs, Antifungal Drugs, Antiviral Drugs), Application (Hospital Use, Clinic Use, Household, Other), Drug Type (Penicillins, Sulfonamides, Antimycobacterial, Trimethoprim-Sulfamethoxazole, Aminoglycosides, Macrolides, Chloramphenicol, Others), Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online, Others), End-User (Children, Adults), Side Effects (Kidney Damage, GI Toxicity, Neurotoxicity, Hypersensitivity Reactions, Superinfections)
Competitive landscape highlighting important parameters that players are gaining along with the Market Development/evolution
• % Market Share, Segment Revenue, Swot Analysis for each profiled company [Pfizer Inc. (United States), Johnson & Johnson (United States), Abbott Laboratories (United States), Gilead Sciences, Inc. (United States), Bristol-Myers Squibb Co. (United States), Merck & Co., Inc. (United States), Bayer Healthcare AG (Germany), AstraZeneca Plc. (United Kingdom), Boehringer Ingelheim (Germany), Novartis AG (Switzerland), Astellas Pharma, Inc. (Japan), GlaxoSmithKline Plc. (United Kingdom)]
• Business overview and Product/Service classification
• Product/Service Matrix [Players by Product/Service comparative analysis]
• Recent Developments (Technology advancement, Product Launch or Expansion plan, Manufacturing and R&D etc)
• Consumption, Capacity & Production by Players
The regional analysis of Global Anti-infective Agents Market is considered for the key regions such as Asia Pacific, North America, Europe, Latin America and Rest of the World. North America is the leading region across the world. Whereas, owing to rising no. of research activities in countries such as China, India, and Japan, Asia Pacific region is also expected to exhibit higher growth rate the forecast period 2023-2028.
The World Health Organization has identified antimicrobial resistance as one of the three greatest threats to human health. Unfortunately, many large pharmaceutical companies have discontinued their antibiotic discovery and development programs. With a marked decline in the discovery of new antimicrobials, the world is now facing an enormous and growing threat from the emergence of bacteria that are resistant to almost all available antibiotics. As stated by the Infectious Diseases Society of America in their 'Bad Bugs, No Drugs' paper: "As antibiotic discovery stagnates, a public health crisis brews." There is therefore an urgent need to discover novel antimicrobials and develop innovative strategies for better use of available antibiotics to combat the global antimicrobial resistance crisis.
Table of Content
Chapter One: Industry Overview
Chapter Two: Major Segmentation (Classification, Application and etc.) Analysis
Chapter Three: Production Market Analysis
Chapter Four: Sales Market Analysis
Chapter Five: Consumption Market Analysis
Chapter Six: Production, Sales and Consumption Market Comparison Analysis
Chapter Seven: Major Manufacturers Production and Sales Market Comparison Analysis
Chapter Eight: Competition Analysis by Players
Chapter Nine: Marketing Channel Analysis
Chapter Ten: New Project Investment Feasibility Analysis
Chapter Eleven: Manufacturing Cost Analysis
Chapter Twelve: Industrial Chain, Sourcing Strategy and Downstream Buyers
Read Executive Summary and Detailed Index of full Research Study @ https://www.advancemarketanalytics.com/reports/1828-global-anti-infective-agents-market
Highlights of the Report
• The future prospects of the global Anti-infective Agents market during the forecast period 2023-2028 are given in the report.
• The major developmental strategies integrated by the leading players to sustain a competitive market position in the market are included in the report.
• The emerging technologies that are driving the growth of the market are highlighted in the report.
• The market value of the segments that are leading the market and the sub-segments are mentioned in the report.
• The report studies the leading manufacturers and other players entering the global Anti-infective Agents market. Thanks for reading this article; you can also get individual chapter wise section or region wise report version like North America, Middle East, Africa, Europe or LATAM, Southeast Asia.
Contact US :
Craig Francis (PR & Marketing Manager)
AMA Research & Media LLP
Unit No. 429, Parsonage Road Edison, NJ
New Jersey USA – 08837
Phone: +1 201 565 3262, +44 161 818 8166
[email protected]
#Global Anti-infective Agents Market#Anti-infective Agents Market Demand#Anti-infective Agents Market Trends#Anti-infective Agents Market Analysis#Anti-infective Agents Market Growth#Anti-infective Agents Market Share#Anti-infective Agents Market Forecast#Anti-infective Agents Market Challenges
0 notes
Link
Gilead Sciences, Inc. is a biopharmaceutical company that has pursued and achieved breakthroughs in medicine for more than three decades
0 notes
Text
Beyond Diagnosis: Exploring Global drug formulation market
The demand for global drug formulation market size is anticipated to expand at a 5.6% compound annual growth rate (CAGR). The market for pharmaceutical formulations was estimated to be worth US$ 1.58 trillion in 2022 and is projected to reach US$ 2.87 trillion by 2032. The study projects that over the projection period, oral formulations will develop exponentially, with a compound annual growth rate (CAGR) of 6.0%.
The demand for innovative medication formulations that provide efficient clinical care and an economical manufacturing method is rising as medicines continue to enhance people's quality of life. Biologics have become a well-liked therapeutic alternative for managing chronic illnesses.
Get a Sample Copy of the Report:
https://www.futuremarketinsights.com/reports/sample/rep-gb-15755
The emergence of biosimilars has forced pharmaceutical companies to stay competitive by lowering their costs or coming up with new ideas, which has sparked innovation in the biologics industry. As of right now, the FDA has approved 37 biosimilars; nevertheless, in 2019, the number of biosimilars approved climbed by 65% while the total number of biosimilar medications available in the US expanded by 157%.
Nonetheless, as evidenced by the widespread use of biosimilars over the past 13 years in Europe, payers, providers, and manufacturers anticipate that biosimilar competition will lead to ongoing price reductions.
The amount of treatment alternatives available for a given disease or condition will incentivize manufacturers to lower the price of their products, which will propel the drug formulation market's rise in the upcoming years.
Key Takeaways:
The oral formulation is estimated to have a 44.6% market value share by the end of 2032 and to increase at a 6.0% CAGR over the forecast period.
Based on various indications, central nervous system diseases account for 14.1% of the market in 2021 and are expected to dominate throughout the projection period.
In terms of end-user, the big pharma corporations are predicted to grow at a 5.1% CAGR in the next years.
North America is expected to be the leading region by the conclusion of the forecast period, with a value share of 48.1%.
“Growing prevalence of chronic disorders and Rising needs for novel drug formulation in the pharmaceutical sectors is expected to witness the growth of Drug Formulation Market over the forecast period,”says an analyst of Future Market Insights.
Market Competition
Key Players:
AstraZeneca plc.
Bristol-Myers Squibb
Eli Lilly and Company
Gilead Sciences, Inc.
Merck & Co., Inc.
Novartis AG
Pfizer Inc.
AbbVie Inc.
Boehringer Ingelheim International GmbH
F. Hoffmann-La Roche AG
Johnson & Johnson
3M Company
Others
The drug formulation industry’s pharmaceutical and biotech businesses are concentrating on leveraging market potential by implementing mergers and acquisitions strategies. The trend continues as a result of the effective marketing campaign of novel and innovative drug formulation portfolios following strategic partnerships, acquiring their place in the market. The key techniques employed by the manufacturers to extend their business units and customer bases in both developed and emerging economies include strategic collaborations, mergers and acquisitions, production capacity expansions, and expanding product sales.
For instance,
The COVID-19 vaccine was developed by Pfizer with the assistance of Sanofi in 2021, and Bayer and CureVac formed a complex cooperation to distribute 160M doses of the vaccine by 2022.
The German mRNA CDMO AmpTec was acquired by Millipore Sigma, the life science division of Merck KGaA. This strengthened offerings across the mRNA value chain, especially for MilliporeSigma, which already supplies lipids to Pfizer-BioNTech for their COVID-19 vaccines. Lipids are the main mode of delivery for mRNA therapeutics.
More Insights Available:
North America is one of the largest pharmaceutical formulations market due to the high prevalence of chronic diseases, such as diabetes and cardiovascular diseases, and the presence of a large number of pharmaceutical companies. The United States is the largest market in the region, with a well-established healthcare system, high healthcare spending, and favorable regulatory policies.
Key Segments:
By Dosage Form:
Oral formulations
Tablets
Immediate Release
Modified Release
Chewable
Effervescent
Capsules
Hard Gelatin Capsules
Softgel Capsules
Others
Powders & Granules
Lozenges & Pastilles
Gummies
Others
Parenteral formulations
Solutions
Suspensions
Emulsions for injection or infusion
Powders for injection or infusion
Gels for injection implants
Topical formulations
Pastes
Ointments and oils
Creams, lotions, and foams.
Gels, tinctures, and powders
Sprays and patches
Inhalation formulations
Pressurized Metered Dose Inhaler
Dry Powder Inhaler (DPI)
Nebulizer
By Indication:
Infectious Diseases
Cancer
Cardiovascular Diseases
Diabetes
Respiratory Diseases
Central Nervous System Disorders
Autoimmune Diseases
Gastrointestinal Diseases
Musculoskeletal Disorders
Dermatological Disorders
Other
By End User:
Big pharma
Small & Medium Size Pharma
Biotech Companies
0 notes
Text
Autologous Stem Cell and Non-Stem Cell-Based Therapies Market Size Advanced Technologies & Growth Opportunities in Global Industry by 2030
****Everything You Need to Know About Autologous Stem Cell and Non-Stem Cell-Based Therapies everything is Here....!
The Comprehensive study on Autologous Stem Cell and Non-Stem Cell-Based Therapies Market includes historical data as well as share, size, and projection information for the major players, geographies, applications, and product categories for the years 2024 to 2030. The Market study includes comprehensive insights on the competitive environment, description, broad product portfolio of key players, SWOT analysis, and significant business strategy implemented by rivals, revenue, Porters Five Forces Analysis, and sales projections. The report also features an impact analysis of the market dynamics, highlighting the factors currently driving and limiting market growth, and the impact they could have on the short, medium, and long-term outlook. The main goal of the paper is to further illustrate how the latest scenario, the economic slowdown, and war events affect the market for Autologous Stem Cell and Non-Stem Cell-Based Therapies.
Autologous Stem Cell and Non-Stem Cell-Based Therapies Market is growing at a +14.61% CAGR during the forecast period 2024-2030. The increasing interest of the individuals in this industry is that the major reason for the expansion of this market.
The Top Key Players profiled in the report:
BrainStorm Cell Limited, Cytori Therapeutics Inc., Dendreon Pharmaceuticals LLC., Fibrocell Science Inc., Opexa Therapeutics Inc., Orgenesis Inc, Regeneus Ltd., U.S. Stem Cell Inc., Castle Creek Biosciences Inc., Gilead Sciences Inc.
Click the link to get a free sample copy of the report :
(*If you have any special requirements, please let us know and we will provide you with the report as you wish.)
Autologous Stem Cell and Non-Stem Cell-Based Therapies Market Segmentation:
Autologous Stem Cell and Non-Stem Cell Based Therapies Market By Type, 2020-2029, (USD Billion).
Autologous Stem Cell
Autologous Non-Stem Cell
Autologous Stem Cell and Non-Stem Cell Based Therapies Market By Application, 2020-2029, (USD Billion).
Cancer
Neurodegenerative Disorders
Cardiovascular Disease
Orthopedic Diseases
Based on geography, the global market for Autologous Stem Cell and Non-Stem Cell-Based Therapies and Disruptions has been segmented as follows:
North America (United States, Canada, Mexico)
South America (Brazil, Argentina, Ecuador, Chile)
Asia Pacific (China, Japan, India, Korea)
Europe (Germany, UK, France, Italy)
Middle East Africa (Egypt, Turkey, Saudi Arabia, Iran) and more.
Strategic Points Covered in Autologous Stem Cell and Non-Stem Cell-Based Therapies Market Directory:
To study and analyze the global market size (value & volume) by company, key regions/countries, products and application, history data, and forecast to 2030.
To understand the structure of market by identifying its various sub segments.
To share detailed information about the key factors influencing the growth of the market (growth potential, opportunities, drivers, industry-specific challenges and risks).
Focuses on the key global manufacturers, to define, describe and analyze the sales volume, value, market share, market competition landscape, SWOT analysis and development plans in next few years.
To analyze the growth trends, future prospects, and their contribution to the total market.
To project the value and volume of submarkets, with respect to key regions (along with their respective key countries).
To analyze competitive developments such as expansions, agreements, new product launches, and acquisitions in the market.
To strategically profile the key players and comprehensively analyze their growth strategies.
The report provides insights on the following pointers:
Market Penetration: Comprehensive information on the product portfolios of the top players in the Autologous Stem Cell and Non-Stem Cell-Based Therapies
Product Development/Innovation: Detailed insights on the upcoming technologies, R&D activities, and product launches in the market.
Competitive Assessment: In-depth assessment of the Autologous Stem Cell and Non-Stem Cell-Based Therapies market strategies, geographic and business segments of the leading players in the market.
Market Development: Comprehensive information about emerging markets. This report analyzes the market for various segments across geographies.
Market Diversification: Exhaustive information about new products, untapped geographies, recent developments, and investments in the Autologous Stem Cell and Non-Stem Cell-Based Therapies
Take a look at the full report with detailed TOC here:
Some of the key questions scrutinized in the study are:
Which companies are expanding litanies of products with the aim to diversify product portfolio?
Which companies have drifted away from their core competencies and how have those impacted the strategic landscape of the Autologous Stem Cell and Non-Stem Cell-Based Therapies market?
Which companies have expanded their horizons by engaging in long-term societal considerations?
Which firms have bucked the pandemic trend and what frameworks they adopted to stay resilient?
What are the marketing programs for some of the recent product launches?
We offer customization on the Autologous Stem Cell and Non-Stem Cell-Based Therapies market report based on specific client requirements:
20% free customization.
Five Countries can be added as per your choice.
Five Companies can add as per your choice.
Free customization for up to 40 hours.
After-sales support for 1 year from the date of delivery.
Get More: https://exactitudeconsultancy.com/primary-research/
Thank you for your interest in the Autologous Stem Cell and Non-Stem Cell-Based Therapies Market research publications; you can also get individual chapters or regional/country report versions such as Germany, France, China, Latin America, GCC, North America, Europe or Asia……
Other Reports:
Bed Monitoring System & Baby Monitoring System market
Immunoprecipitation market
Laparoscopic and Open Hernia Mesh Repair Surgery market
Empty Capsules market
Neurodiagnostics market
About Us:
Exactitude Consultancy is a Market research & consulting services firm which helps its client to address their most pressing strategic and business challenges. Our professional team works hard to fetch the most authentic research reports backed with impeccable data figures which guarantee outstanding results every time for you. So, whether it is the latest report from the researchers or a custom requirement, our team is here to help you in the best possible way.
Contact: Exactitude Consultancy
PHONE NUMBER +1(704) 266-3234
EMAIL ADDRESS: [email protected]
#Autologous Stem Cell and Non-Stem Cell-Based Therapies Market#Autologous Stem Cell and Non-Stem Cell-Based Therapies Market growth#Autologous Stem Cell and Non-Stem Cell-Based Therapies Market size#Autologous Stem Cell and Non-Stem Cell-Based Therapies Market analysis 2024#Autologous Stem Cell and Non-Stem Cell-Based Therapies Market CAGR#Autologous Stem Cell and Non-Stem Cell-Based Therapies Growth#Autologous Stem Cell and Non-Stem Cell-Based Therapies Market 2024#Autologous Stem Cell and Non-Stem Cell-Based Therapies Market analysis by types#Autologous Stem Cell and Non-Stem Cell-Based Therapies Market data#Autologous Stem Cell and Non-Stem Cell-Based Therapies Market Outlook#Autologous Stem Cell and Non-Stem Cell-Based Therapies Market Size and Share#Autologous Stem Cell and Non-Stem Cell-Based Therapies Strategic Industry Latest News#Top Companies Analysis By Autologous Stem Cell and Non-Stem Cell-Based Therapies Market#Autologous Stem Cell and Non-Stem Cell-Based Therapies Market Trends#Autologous Stem Cell and Non-Stem Cell-Based Therapies Market Forecast#Autologous Stem Cell and Non-Stem Cell-Based Therapies Industry
0 notes
Text
The Global Cancer Gene Therapy Market was valued at USD 2.80 Billion in 2023 and is estimated to reach USD 15.8 Billion by 2032, growing at a CAGR of 20.45 % from 2023 to 2032.
Cancer is a group of diseases that grow abnormal cells in the human body and can grow in any part of the body. Gene Therapy is a technique to prevent diseases, where the mutated genes are replaced by the specific genes causing the disease or are inactive in this gene. Cancer gene therapy is receiving high importance due to its high success rate during pre-clinical and clinical trials. The research and development on this are trying to replace the use of surgeries and drugs with gene therapy to treat cancer. The ongoing investment in research and development, and acceptance of gene therapy in cancer treatment is propelling this cancer gene therapy market during the forecast period. Biotechnology firms are increasing novel gene therapy vectors that increase levels of protein production or gene expansion, and reducing immunogenicity. According to National Center for Health Statistics, in 2023 there are 19,58,310 cancer cases in the U.S.
Further, the growing geriatric population, advanced product technology, increase in per capita disposable income, and emerging economies are the factors that are creating lucrative opportunities for the growing cancer gene therapy market.
Economic Impact of Covid-19:
The analysis of the COVID-19 recovery trajectory provides an overview of the main strategies that industries are implementing to respond to and recover from the economic crisis. It also focuses on the post-pandemic and pre-pandemic era of the Global Cancer Gene Therapy Market through PEST analysis, SWOT, Quantitative and Qualitative analysis, Attractive analysis, and DROs.
Key Players:
Novartis AG
Pfizer Inc.
Amgen Inc
Johnson & Johnson
AstraZeneca
GlaxoSmithKline plc
Gilead Science, Inc.
Abeona Therapeutics
Altor Bioscience Inc.
Celgene Inc.
Introgen Therapeutics Inc.
BioCancell Inc.
Oncogenex
Shanghai Sunway Biotech
ZioPharm Oncology
Others
The above key players in the Global Cancer Gene Therapy Market can be changed according to the client’s requirements.
Moreover, the key players aim towards expansion, joint ventures, collaboration, mergers, and acquisitions to advance capabilities in gene therapy.
Know More- https://nexbindinsight.com/pharmaceutical-and-healthcare/the-global-cancer-gene-therapy-market-was-valued-at-usd
0 notes