#raoults law
Text
This equation describes a straight line and so a plot of psolution versus xsolvent is linear, as shown in figure 10.22.

"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#solution#solvent#pressure#math#raoult's law#concentration#molarity
5 notes
·
View notes
Text
i am going to pass my chemistry II class
i am going to pass my chemistry II class
i am going to pass my chemistry II class
i am going to pass my chemistry II class
i am going to pass my chemistry II class
i am not only going to pass my chemistry II class, I will understand what I am given and will make a B
1 note
·
View note
Text
ideal solution under raoult's law are so pan coded... positive deviation from raoult's law are so gay coded........... send post
0 notes
Text
By Raoult's law, the partial pressure of a particular component is directly proportional to the component's mole fraction in the solution, and the total vapour pressure is the sum of the partial pressures.
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
0 notes
Note
Abhi Babu what is this Roult's law I'll cry😭
Lily!! Raoult's law can take some time to be understood entirely, but it's also not too bad! I'll try to explain it with what I know. <3
Raoult's law (or however that guy's name is spelt) is more or less the Henry's Law but just an extended version of it (Henry's Law also states that the vapour pressure of gas is proportional to its mole fraction)
pure solute + pure solvent = solution (which has both solute and solvent)
So now Raoult's law tells us ki the vapour pressure of either solute or solvent *in the solution* is equal to the vapour pressure of the *pure* solute or solvent multiplied by it's mole factor
This thing from my notes might make it a tiny bit easier to understand:
(P° is the vapour pressure of the pure substance while P is the vapour pressure of it in the solution, xa and xb are the mole fractions of solvent and solute respectively)
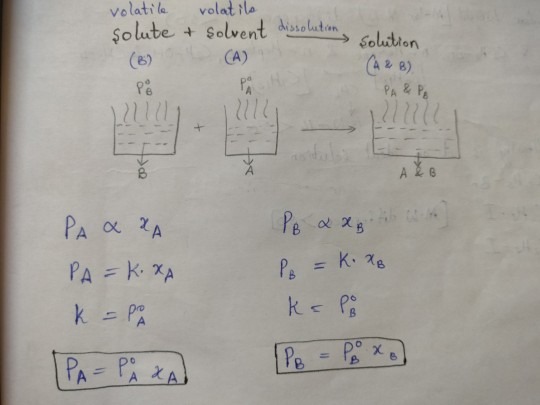
17 notes
·
View notes
Text
Correct answer below the cut
(a) Osmotic Pressure
#chemistry#physical chemistry#solutions#class 12#cbse#cbse 2023#pcm#pcb#jee#neet#stem#engineering#engineering prep#desi academia#desi studyblr#daily padhai updates#polls#tumblr polls#vanilla extract
29 notes
·
View notes
Text

28 and 29 Dec .
15 and 16 dop .
So , I have moved for a few days to my cousin's house so that I can focus on my studies and it's going well for now but I am really really missing my home especially my ma . But yeah I have been waking up early and doing stuff.
Zoology: Genetics 🧬✅ (finally completed 🎊🎉)
Botany; Biology and Human welfare ✅
Physics : current electricity ✅
Chemistry: Raoult's law✅
That's it for today ,Bye bye
#studyblr#productive#motivation#studyspo#stemblr#60 dop#stem academia#60 days of productivity challenge#self improvement
5 notes
·
View notes
Text
dalton’s law is the worst one btw fuck you and your water vapors or whatever. don’t get it confused even its not raoult it’s specifically just a weird fucked up version of this guy’s law.
2 notes
·
View notes
Text
After doing research
After doing research about this universe, these comets and these asteroids, I got a shock about liquid material.
Friends, the thing that confused me is this small amount of water.
We have understood that water is a mixture of hydrogen and oxygen.
But what is this tiny water or smoke made of?
Water is a mixture of hydrogen and oxygen
What is this water vapor a mixture of?
molecule
The smallest particle of water is 'molecule'. The water molecule is made up of two atoms of hydrogen (H2) and one atom of oxygen (O). These two atoms together form a compound called water i.e. H 2+O→H 2O.
water vapour
gaseous phase of water; Unlike other forms of water, water vapor is invisible
Water vapor, water vapor or water vapor is the gaseous state of water. It is a state of water within the hydrosphere. Water vapor can be produced by the evaporation or boiling of liquid water or by the sublimation of ice. Like most components of the atmosphere, water vapor is also transparent. Under typical atmospheric conditions, water vapor is continuously produced by evaporation.
Water vapor - Wikipedia
Water vapor, water vapor or water vapor is the gaseous state of water. It is a state of water within the hydrosphere. Water vapor can be produced by the evaporation or boiling of liquid water or by the sublimation of ice. Like most components of the atmosphere, water vapor is also transparent.
Boiling point: 99.98 °C (373.13 K)
Molecular Formula: H₂O
Raoult's Law and Ideal Mixtures of Fluids - Chemistry Treatise
Jan 30, 2023 · The partial vapor pressure of a component in a mixture is equal to the vapor pressure of the pure component at that temperature multiplied by its mole fraction in the mixture. Raoult's law works only for ideal mixtures.
Water - Wikipedia
About 71% of the Earth's surface is covered with water, with the majority of the water (about 96.5%) being seas and oceans. Smaller amounts of water are in the form of groundwater (1.7%), in the glaciers and ice caps of Antarctica and Greenland (1.7%), and in the form of water vapor, clouds (consisting of ice and liquid water suspended in the air) and precipitation. It happens. ,
Beilstein Reference: 3.6M
Gmelin Reference: 117
Chemical formula: H,₂O
RTECS Number: ZC0110000
Meaning, I have understood this based on research.
Water can escape from any surface into space
take care friends
Water only escaped from the surface of Mars into space through water vapor.
not just that
Water can also escape from the earth into space through water vapor
This is my research scaring me
How will we breathe without water
Translate Hindi
यह ब्रह्मांड यह धूमकेतुओं और यह क्षुद्रग्रहों के बारे में रिसर्च करने के बाद मुझें एक खटका लगा तरलीय सामग्री के बारे में
दोस्तों जो बात मुझें चक्कर में डाला वो है पानी की यह क्षुद्र पानी
पानी हाइड्रोजन और ऑक्सीजन की मिश्रित अंग है यह हम समझ गए है
मगर यह क्षुद्र पानी या धुआं क्या है किससे बनी होती है
पानी हाइड्रोजन और ऑक्सीजन की मिश्रण है
किस चीज की मिश्रण है यह जलवाष्प
अणु
जल का सबसे सूक्ष्म कण 'अणु' है। पानी का अणु हाइड्रोजन के दो परमाणु (H2) और ऑक्सीजन के एक परमाणु (O) से बना होता है। ये दोनों परमाणु मिलकर पानी नामक एक यौगिक बनाते हैं अर्थात H 2+O→H 2O।
जल वाष्प
पानी का गैसीय चरण; पानी के अन्य रूपों के विपरीत, जलवाष्प अदृश्य है
जलवाष्प, जलवाष्प या जलीय वाष्प जल की गैसीय अवस्था है। यह जलमंडल के भीतर पानी की एक अवस्था है। जलवाष्प तरल पानी के वाष्पीकरण या उबलने से या बर्फ के ऊर्ध्वपातन से उत्पन्न हो सकता है। वायुमंडल के अधिकांश घटकों की तरह जलवाष्प भी पारदर्शी है। विशिष्ट वायुमंडलीय परिस्थितियों में, वाष्पीकरण द्वारा जलवाष्प लगातार उत्पन्न होता रहता है
जलवाष्प - विकिपीडिया
जलवाष्प, जलवाष्प या जलीय वाष्प जल की गैसीय अवस्था है। यह जलमंडल के भीतर पानी की एक अवस्था है। जलवाष्प तरल पानी के वाष्पीकरण या उबलने से या बर्फ के ऊर्ध्वपातन से उत्पन्न हो सकता है। वायुमंडल के अधिकांश घटकों की तरह जलवाष्प भी पारदर्शी है।
क्वथनांक: 99.98 डिग्री सेल्सियस (373.13 K)
आणविक सूत्र: H₂O
राउल्ट का नियम और तरल पदार्थों का आदर्श मिश्रण - रसायन विज्ञान ग्रंथ
30 जनवरी, 2023 · मिश्रण में किसी घटक का आंशिक वाष्प दबाव उस तापमान पर शुद्ध घटक के वाष्प दबाव के बराबर होता है जो मिश्रण में उसके मोल अंश से गुणा होता है। राउल्ट का नियम केवल आदर्श मिश्रण के लिए काम करता है।
जल - विकिपीडिया
पृथ्वी की सतह का लगभग 71% भाग पानी से ढका हुआ है, जिसमें पानी की अधिकांश मात्रा (लगभग 96.5%) समुद्र और महासागर हैं। पानी का छोटा हिस्सा भूजल (1.7%) के रूप में, अंटार्कटिका और ग्रीनलैंड के ग्लेशियरों और बर्फ की चोटियों में (1.7%), और हवा में वाष्प, बादलों (हवा में निलंबित बर्फ और तरल पानी से युक्त) और वर्षा के रूप में होता है। .
बीलस्टीन संदर्भ: 3.6एम
गमेलिन संदर्भ: 117
रासायनिक सूत्र: एच, ₂O
आरटीईसीएस संख्या: ZC0110000
मतलब मैं रिसार्चानुसार यह समझ पाया हूँ
पानी अंतरीक्ष में भाग सकता है किसी की भी सतह में से
संभालकर रहिए दोस्तों
पानी मंगल की सतह में से ही सिर्फ भागा जलवाष्प जरिए अंतरीक्ष में
इतना तक ही नहीं
पानी धरती में से भी अंतरीक्ष में भाग सकता है जलवाष्प जरिए
यह मेरा रिसर्च मुझें डरा रहा है
पानी बिना हम सांस कैसे लेंगे
0 notes
Text
What is Distillation & its Types?

Distillation is a common technique for separating mixtures with the help of distillation apparatus based on the circumstances needed to change the phase of the mixture's components. Heat heating can separate a mixture of liquids to drive the different boiling point constituents into the gas phase. Then, the gas gets better after being re-condensed into liquid form. Double Distillation means the process of repeating it on the liquid that has been gathered to raise purity of the product. Even though the phrase is most frequently used to describe liquids, it is possible to separate gases using the opposite method by liquefying the individual parts utilizing temperature and pressure variations.
A distillery is a facility where distillation is carried out. A still is the name of the device used to undertake Distillation.
Uses of Distillation
Numerous industrial processes involve Distillation, including creating petrol, xylene, alcohol, kerosene, distilled water, paraffin, and countless other liquids. Gas can separate and liquefy. For instance, the air purifies gases such as nitrogen, oxygen, and argon.
Types of Distillation
There are four different types of Distillation which are as follows:
Simple Distillation,
Fractional Distillation,
Steam Distillation,
Vacuum Distillation
Simple Distillation
Simple Distillation may be used for two liquids' boiling points that differ noticeably or for separating liquids from solids or nonvolatile components with the help of distillation apparatus. In a straightforward distillation, the most volatile part of a combination is heated to transform it from a liquid to a vapor. As it boosts, the vapor goes inside a condenser. The condenser is typically chilled to encourage condensation of the vapor collected.
Fractional Distillation
The components used in rectification, a series of distillations, are bifurcated using a fractionating column. When a mixture's constituent boiling points are close to one another, as determined by Raoult's law, fractional Distillation is utilized. Heating a mixture causes the vapor to arise and goes inside the fractionating column during fractional Distillation. The vapor condenses on the packing material as it cools. This liquid is forced to vaporize once more by the heat of rising vapor, which advances it along the column and eventually produces a higher purity sample of the mixture's more volatile component.
Steam Distillation
Heat-sensitive components are separated via steam distillation. The mixture is given steam, which causes some of it to vaporize. This vapor is cooled and split into two fractions of liquid. The fractions are occasionally collected separately, or they might naturally separate if they have different densities. An illustration is the steam distillation of flowers to produce essential oil and distillate with a water basis.
Vacuum Distillation
Components with high boiling points are separated via vacuum distillation. When a compound's typical boiling point is higher than the temperature at which it decomposes, vacuum distillation is particularly helpful. Boiling points also decrease when the apparatus's pressure is reduced. The procedure is otherwise comparable to other distillation methods.
Distillation Apparatus
A simple scientific distillation apparatus, sometimes known as a still, comprises only a few parts. A thermometer must be part of the device. The thermometer is necessary to precisely measure and maintain the temperature corresponding to the chosen compound's boiling point. A thermometer often measures the average kinetic energy or motion inside a group of particles, such as the particles that make up a liquid or vapor in the case of Distillation.
If you are looking for the best distillation apparatus, Jade Scientific, Inc. is your one-stop solution.
0 notes
Text
chemical engineering
A liquid mixture contains N components (N may be any number from 2 to 10) at pressure P(mmHg). The mole fraction of the i-th component is given by the Antoine equation. Raoult’s law may be applied to each component.
Write the equations you would use to calculate the bubble-point and dew point temperatures of the mixture.
Prepare a spreadsheet to perform the calculations of part (a).
Test your…
View On WordPress
0 notes
Text
François-Marie Raoult

Raoult was born at Fournes, in the département of Nord. He became aspirant répétiteur at the Lycée of Reims in 1853, and after holding several intermediate positions was appointed in 1862 to the professorship of chemistry in Sens lycée. There he prepared a thesis on electromotive force which gained him a doctor's degree in Paris the following year.
In 1867 Raoult was put in charge of chemistry classes at the University of Grenoble, and three years later he succeeded to the chair of chemistry, which he held until his death in 1901. Raoult's earliest researches were physical in character, being largely concerned with the phenomena of the voltaic cell; later there was a period when more purely chemical questions engaged his attention.
Raoult's name is best known in connection with work on solutions, to which he devoted the last two decades of his life. His first paper describing how solutes depressed the freezing points of solutions was published in 1878. Further experiments with various solvents, such as benzene and acetic acid, in addition to water, led him to believe in a simple relation between the molecular weights of a solute and the freezing-point of a solution. He expressed the relationship as the loi générale de la congélation (general law of freezing), that if one molecule of a substance be dissolved in 100 molecules of any given solvent, the temperature of solidification of the latter will be lowered by 0.63 °C. Another relation on which Raoult worked was that concerning the depression of a solvent's vapor pressure, due to a solute, showing that the decrease is proportional to the solute's molecular weight. This relationships holds best in the limiting case of a dilute solution. These two generalizations afforded a new method of determining the molecular weights of dissolved substances, and were utilized by Jacobus van 't Hoff and Wilhelm Ostwald, among other chemists, in support of the hypothesis of electrolytic dissociation in solutions. Raoult's freezing-point depression method became even more useful after it was improved by Ernst Otto Beckmann and became a standard technique for determining molecular weights of organic substances.
An account of Raoult's life and work was given by van 't Hoff in a memorial lecture delivered before the London Chemical Society on 26 March 1902
0 notes
Text
How do I prepare for NEET Chemistry?
Amidst vast syllabus, approximately 1000 concepts and limited time, most aspirants devote their maximum time in either solving Physics questions or doing Biology chapters and overlook the equal importance of Chemistry in NEET exam. As a result of which, they often encounter challenges in solving questions in this subject which lead to low scores in the exam. Before this disinterest costs you, understand the importance of Chemistry in your preparation and learn what’s important and what’s not so important.

For doing the complete preparation in Chemistry, take first steps with basics and detailed information.
Chemistry is divided in 3 forms for NEET preparation –
a) Physical Chemistry
b) Organic Chemistry
c) Inorganic Chemistry
Physical Chemistry
In comparison to other sections, Physical Chemistry contains more variations and theory based questions for numerical, which is not tough from the perspective of conceptual part. So, acquire the right approach and switch to the right set of books. Here’s the list of important chapters to cover –
i) Basic Concepts
Limiting Reagent
Mole concept
Empirical formula
Terms of concentration
ii) Structure of atom
Model of atoms – Bohr’s
De Broglie hypothesis
Quantum numbers
Heisenberg’s uncertainty principle
iii) States of Matter : Gases & Liquids
Gas Laws
Ideal Gas Equation
Density formula
Unit cells & lattices
Intermolecular forces – Van Der Waals Gas Equation
Packing fractions & ranks
iv) Equilibrium
Buffer solutions
Properties
pH Scale
Ions
Equilibrium constants & laws
Factors affecting Equilibria – Le Ch atelier’s principle
v) Thermodynamics
Summation
All laws of thermodynamics
Gibbs Energy & Spontaneity
Hess’s Law of Constant Heat
Entropy & Spontaneity
vi) Solutions
Vapour pressure
Osmosis & Osmotic Pressure
Raoult’s law, Van’t Hoff factor
Colligative Properties & Determination of Molar Mass
vii) Redox reactions
All types of redox reactions
viii) Chemical Kinetics
First order reaction
Half life of a reaction
Order of a reaction
Rate law
Molecularity of a reaction
Temperature Dependence of the Rate of a Reaction
ix) Surface Chemistry
Emulsions
Adsorption Isotherms
Colloids
Catalysis
Properties of Colloidal Solutions – Tyndall Effect
Organic Chemistry
Where most of the NEET aspirants consider this section tough, there this’s one of the most imperative and scoring sections in NEET exam. If you understand it well, you can score well. Therefore, begin with essential chapters included in this section.
i) Hydrocarbons Alkanes Alkenes & Alkynes
ii) Classification & Nomenclature
iii) Isomerism
iv) General Organic Chemistry
v) Practical & Environmental Chemistry
vi) Biomolecules
vii) Haloarenes & Phenols
viii) Chemistry in action
ix) Alcohol & Ether
x) Carbonyl compounds
xi) Carboxylic acid & its derivatives
xii) Biomolecules & Polymers
xiii) Haloalkanes
xiv) Nitrogen containing compounds
xv) Nitrogen compound & aliphatic amines
Now, some helpful tips to study Organic Chemistry in a result oriented way
a) Emphasis on understanding, rather than memorizing: - First thing you need to do to be master in OC is to understand the chapters. Without doing so, it’s worthless to memorize because you will lack the basic knowledge.
b) Reactions are important. So, practice: - Many students complain about their failed efforts to memorize the reactions. Whereas, it is not like that. For learning the reactions, focus on understanding them and do the constant practice.
Inorganic Chemistry
Unlike Physical Chemistry & Organic Chemistry, Inorganic Chemistry is more about concepts, reasoning and facts and constitutes chemical reactions, periodic table, s, p, d and f block elements, Coordination chemistry and chemical equilibrium. For doing full proof preparation, prepare a chart containing complete details about formulas and chemical reactions and revise every day. Along with this, keep solving questions from each chapter and do constant learning.
0 notes
Text
This equation describes a straight line and so a plot of psolution versus xsolvent is linear, as shown in figure 10.22.

"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#graph#raoult's law#vapor pressure#solvent#solution#pressure#concentration
0 notes
Video
RAOULT`S LAW; IDEAL & NON - IDEAL SOLUTION; AZEOTROPIC MIXTURE; HENREY` ...
0 notes
Text
Warhammer 40k carnage pdf
WARHAMMER 40K CARNAGE PDF >>Download
vk.cc/c7jKeU
WARHAMMER 40K CARNAGE PDF >> Read Online
bit.do/fSmfG
warhammer 40k multiplayer games
how to play multiplayer warhammer 40k
40k 3 player missionswarhammer 40k 4 players
Throughout the blood-soaked history of the Warhammer 40,000 universe, Designer's Note: Carnage! is a special mission designed to be played by. Warhammer 40k - Rulebook - The Imperial Infantrymans Uplifting Primer - 2003.pdf. View 964. Download 206. Category Recommended. Warhammer 40k: Carnage.Warhammer 40,000 puts you in command of an army of mighty warriors and war machines Carnage engulfs the battlefield as the warring armies meet head-on. He carries two axes, one called Slaughter and the other Carnage, which he uses with deadly effect in combat. As the battle progresses, Skarbrand's rage grows, Download 41503615 Warhammer 40K Rulebook 4th Edition download document. Warhammer 40k: Carnage · Documents · Warhammer 40k 6th Edition Rulebook. The rules for Warhammer 40,000 are written for battles fought between two players, each commanding an army. the next page, called 'Carnage.
https://www.tumblr.com/kuquqegolem/697359511075291136/contract-of-affreightment-pdf-files, https://www.tumblr.com/kuquqegolem/697359893681864704/pbl405-datasheet-pdf-1n4001, https://www.tumblr.com/kuquqegolem/697359789916831744/vapor-pressure-depression-raoults-law-pdf, https://www.tumblr.com/kuquqegolem/697359258418839552/digital-government-building-a-21st-century, https://www.tumblr.com/kuquqegolem/697359789916831744/vapor-pressure-depression-raoults-law-pdf.
0 notes