#melting point and intermolecular forces
Text
Best Chemistry Classes for 9th in Dwarka - Adhyayanam Academy
Chemistry classes for 9th graders mark the beginning of a fascinating journey into the world of atoms and molecules. One of the fundamental concepts that lay the groundwork for understanding various chemical phenomena is chemical bonding.
Chemical bonding is the glue that holds atoms together to form compounds, and understanding it opens the door to comprehending the diversity of matter around us. Atoms, the building blocks of matter, interact with each other through chemical bonds to attain stability, typically by achieving a full outer electron shell.
In these chemistry classes for 9th graders, students delve into three primary types of chemical bonds: ionic, covalent, and metallic. Ionic bonds form between atoms when one atom donates an electron to another, resulting in the formation of positively and negatively charged ions that attract each other. Covalent bonds, on the other hand, occur when atoms share electrons to achieve stability, forming molecules. Meanwhile, metallic bonds involve the delocalization of electrons within a metal lattice, giving metals their unique properties.
Understanding the concept of chemical bonding is crucial as it explains various properties of substances, including their reactivity, solubility, and conductivity. For instance, substances with ionic bonds tend to dissolve in water and conduct electricity when dissolved or molten due to the mobility of ions. Covalent compounds, on the other hand, often have lower melting and boiling points compared to ionic compounds, as they consist of discrete molecules held together by weaker intermolecular forces.
Moreover, chemical bonding plays a vital role in the formation of complex structures such as polymers and biological macromolecules like proteins and DNA. These structures rely on specific types of chemical bonds to maintain their integrity and function.
In conclusion, grasping the fundamentals of chemical bonding is essential for chemistry classes for 9th students, as it provides a solid foundation for understanding the behavior of matter at the atomic and molecular levels. Through exploration and experimentation in their chemistry classes for 9th grade, students embark on a journey of discovery that unveils the intricate world of chemical bonding.
know more:
Maths Coaching for 9th
Maths Coaching for 10th
Maths Classes in Dwaraka for 11th
Maths Coaching for 11th
Cuet Coaching in delhi
CA Coaching in delhi
CS Coaching in delhi
best ca foundation coaching in delhi
ca foundation coaching in delhi
0 notes
Text
Does Hot Glue Work on Plastic? Discover the Ultimate Bonding Power!

Hot glue is effective for bonding plastic materials together. It forms a strong and durable bond.
The Science Behind Hot Glue On Plastic
If you've ever wondered whether hot glue actually works on plastic, you're not alone. Understanding the science behind this adhesive mechanism can help you make an informed decision about whether hot glue is the right solution for your plastic projects. In this article, we'll explore the adhesion mechanism of hot glue on plastic and the crucial step of plastic surface preparation.
Adhesion Mechanism
Hot glue is a versatile adhesive that is commonly used in various crafting and DIY projects. When it comes to adhering plastic, hot glue utilizes a unique mechanism to bond the two materials together.
Hot glue, also known as thermoplastic adhesive, is composed of polymer-based thermoplastic components. These components melt when exposed to heat and solidify upon cooling, creating a strong bond. The adhesion occurs due to the intermolecular forces, specifically Vander Waals forces, between the solidified hot glue and the plastic surface.
This adhesive mechanism allows hot glue to effectively stick to a wide range of plastics, such as polyethylene, polypropylene, PVC, and ABS. However, it may not be suitable for all types of plastic, particularly those with low melting points or that are highly flexible.
Plastic Surface Preparation
To maximize the adhesion of hot glue on plastic, proper surface preparation is essential. Before applying hot glue, it's crucial to clean the plastic surface thoroughly to remove any dust, oils, or contaminants that may interfere with adhesion.
Here are some steps you can follow for effective plastic surface preparation:
- Begin by wiping the plastic surface with a clean cloth or paper towel to remove any loose debris.
- If the plastic surface is particularly dirty or greasy, consider using a mild detergent or plastic-friendly cleaning solution to clean it.
- After cleaning, rinse the plastic surface with water and allow it to dry completely.
- If necessary, you can use sandpaper or a soft abrasive pad to lightly roughen the surface. This can enhance the bonding ability of hot glue by providing a rougher surface for the adhesive to grip onto.
Remember to always wear safety goggles and gloves when working with hot glue and follow the manufacturer's instructions for proper usage and handling.
By understanding the adhesion mechanism of hot glue on plastic and following the necessary steps for plastic surface preparation, you can ensure a strong and durable bond for your projects. So, next time you're wondering if hot glue works on plastic, you can confidently grab that glue gun and get crafting!

Credit: www.gluegun.com
Types Of Hot Glue For Plastic
If you're wondering whether hot glue works on plastic, the answer is a resounding yes! Hot glue is a versatile adhesive that bonds well with a wide range of materials, including plastic. However, it's important to choose the right type of hot glue for your plastic project to ensure a strong and durable hold. In this article, we'll explore two main types of hot glue for plastic: low-temperature hot glue and high-temperature hot glue.Low-temperature Hot GlueLow-temperature hot glue, also known as cool melt glue, is a popular choice for bonding plastic materials. As the name suggests, this type of hot glue operates at a lower temperature than its high-temperature counterpart. The lower temperature reduces the risk of damaging heat-sensitive plastics during the bonding process.One of the key advantages of low-temperature hot glue is its quick-drying nature. It sets rapidly, allowing you to work efficiently on your projects without the need for extended waiting times. This is especially useful when working with delicate or intricate plastic components that require precise placement and alignment.While low-temperature hot glue offers a strong bond, it may not be suitable for heavy-duty applications. Its lower temperature can result in a less robust hold compared to high-temperature hot glue. Therefore, it's important to consider the specific requirements of your plastic project before opting for this type of hot glue.High-temperature Hot GlueHigh-temperature hot glue, on the other hand, is ideal for projects that demand a stronger and more durable bond. This type of hot glue operates at a higher temperature, allowing it to provide a secure hold on various types of plastics. Whether you're working with polypropylene, PVC, ABS, or other common plastic materials, high-temperature hot glue can deliver reliable results.With its high melting point, this hot glue type boasts better resistance to heat and humidity. It can withstand challenging environmental conditions, making it suitable for both indoor and outdoor applications. Whether you're designing crafts, repairing household items, or creating prototypes, high-temperature hot glue is a reliable choice for plastic bonding.It's worth noting that high-temperature hot glue requires a glue gun with a higher wattage and temperature control settings. This ensures that the adhesive reaches the optimal temperature for proper bonding. Always refer to the manufacturer's instructions and recommended temperatures to ensure the best results with this type of hot glue.In ConclusionWhen it comes to bonding plastic materials, selecting the right type of hot glue is crucial. Both low-temperature and high-temperature hot glues have their unique advantages and considerations. Assess the specific needs of your plastic project, such as the material type, required bond strength, and environmental conditions, to determine the most suitable hot glue option. With the right choice, you can achieve a strong, lasting bond that withstands the test of time.
Tips For Using Hot Glue On Plastic
When using hot glue on plastic, it’s important to understand the proper application techniques and considerations for different types of plastic. By following these tips, you can ensure a successful and long-lasting bond between the hot glue and the plastic material.Proper Application TechniquesWhen applying hot glue to plastic, it's essential to ensure that the surface is clean and free of any dust or debris. This can be achieved by wiping the plastic with a cloth and some rubbing alcohol. Additionally, preheating the plastic surface with a hairdryer can help the hot glue adhere more effectively. When applying the hot glue, it’s important to work quickly and efficiently to prevent it from hardening before the plastic pieces are properly aligned.Consideration Of Plastic TypeIt’s important to consider the type of plastic you are working with when using hot glue. Some plastics, such as polypropylene and polyethylene, may require specialized adhesives rather than hot glue due to their low surface energy. However, hot glue can be effective on plastics such as PVC, ABS, and styrene. Before applying hot glue, it’s crucial to identify the type of plastic and ensure that it is compatible with hot glue adhesion.

Credit: www.gluegun.com
Benefits And Limitations
Hot glue can work effectively on certain types of plastics, providing a fast and strong bond. However, it may not be suitable for all plastic materials, particularly those with low surface energy or flexibility. It's essential to consider the specific type of plastic and its characteristics before using hot glue as an adhesive.
Benefits Of Hot Glue On Plastic
When it comes to crafting and DIY projects, hot glue is a versatile and popular adhesive choice. It is known for its quick-drying time and strong bond, making it suitable for various materials, including plastic. Using hot glue on plastic offers several advantages that make it a go-to option for many crafters and DIY enthusiasts.
1. Quick and Easy Application: Hot glue guns are user-friendly, allowing for precise application on plastic surfaces. The glue melts quickly and adheres to the plastic almost instantly, saving you valuable time and effort.
2. Strong Bond: Hot glue forms a sturdy bond, creating a lasting connection between plastic pieces. This is particularly useful when you want to secure different parts or create 3D structures that require extra support.
3. Versatility: Whether you are working with hard plastic, flexible plastic, or even smooth surfaces like acrylic or PVC, hot glue can effectively stick to a wide range of plastic materials. It is a versatile option that can be used for various projects, such as repairing broken plastic items, assembling plastic pieces, or decorating plastic surfaces.
4. No Specialized Equipment Required: Hot glue guns are inexpensive and readily available, making them accessible to DIYers of all levels. Unlike other adhesives that require mixing or specific tools, hot glue can be applied using a standard glue gun, often found in craft stores or online.
5. Temporary Bonding: Hot glue allows for temporary bonding on plastic surfaces as it can be easily removed or repositioned. If you make a mistake or need to make adjustments, the glue can be softened with heat (e.g., a hairdryer) and peeled off without damaging the plastic surface.
Challenges And Solutions
While hot glue is a convenient adhesive for plastic, it does have its limitations. Understanding these challenges and their solutions can help you achieve the desired results in your projects.
1. Heat Sensitivity: Some plastic materials are sensitive to high temperatures, which can cause them to warp or become damaged when exposed to hot glue. To mitigate this issue, use a low-temperature hot glue gun or test a small, inconspicuous area of the plastic before applying the glue to ensure compatibility.
2. Limited Strength on Certain Plastics: While hot glue provides a strong bond on most plastics, certain types, such as polyethylene or polypropylene, pose challenges due to their low surface energy. In such cases, consider roughening the plastic surface or using specialized adhesives designed for bonding these specific plastics.
3. Longevity: Hot glue may not provide a permanent bond on plastic and can sometimes lose its grip over time, especially when exposed to extreme temperatures, moisture, or frequent stress. If you need a more durable and long-lasting bond, exploring other adhesive options specific to your project can be beneficial.
4. Visible Glue Lines: Hot glue can create visible glue lines on plastic surfaces, which may not be desirable depending on the project. To minimize this effect, apply the glue sparingly and evenly distribute it to ensure a seamless appearance.
By understanding the benefits hot glue offers when working with plastic, along with the challenges it may present, you can make informed decisions and achieve successful results in your crafting and DIY endeavors.
Comparing Hot Glue With Other Plastic Bonding Methods
When it comes to bonding plastic, there are several methods to choose from. Two commonly used options are hot glue and epoxy, as well as hot glue and super glue. In this section, we will compare these plastic bonding methods to help you determine which one is right for your project.Hot Glue Vs. EpoxyHot glue and epoxy are both effective options for bonding plastic. However, they have some significant differences that you should consider before making a choice.
Hot Glue
Epoxy
Quick and easy to use
Requires mixing of resin and hardener
Provides a strong bond
Offers an exceptionally strong bond
May not be suitable for high-temperature applications
Can withstand high temperatures
Can be easily removed and repositioned
Difficult to remove once fully cured
Hot glue is an excellent choice for quick and easy plastic bonding. It provides a strong bond and can be used for a wide range of applications. However, it may not be the best option for projects that require exposure to high temperatures, as hot glue can melt.Epoxy, on the other hand, offers an exceptionally strong bond that can withstand high temperatures. It requires a bit more effort to use, as you need to mix the resin and hardener before applying it. Once fully cured, epoxy becomes difficult to remove or reposition.Hot Glue Vs. Super GlueAnother popular plastic bonding method to consider is super glue. Let's take a look at how hot glue and super glue compare:
Hot Glue
Super Glue
Quick and easy to use
Quick and easy to use
Provides a strong bond
Provides an incredibly strong bond
May not be suitable for high-temperature applications
Not suitable for high-temperature applications
Can be easily removed and repositioned
Difficult to remove once fully cured
Hot glue and super glue share many similarities when it comes to plastic bonding. Both are quick and easy to use, providing strong bonds. However, they both have limitations when it comes to high-temperature applications. Additionally, like epoxy, super glue becomes difficult to remove once fully cured.In conclusion, when deciding on a plastic bonding method, consider the specific needs of your project. Hot glue is a versatile option that is quick and easy to use, while epoxy and super glue offer even stronger bonds. Choose the method that best suits the requirements of your application to ensure a successful and lasting bond.

Credit: www.amazon.com
Frequently Asked Questions Of Does Hot Glue Work On Plastic
Will Hot Glue Peel Off Plastic?
Yes, hot glue can peel off plastic surfaces.
What Is The Best Glue For Plastic?
The best glue for plastic is generally considered to be cyanoacrylate glue, also known as super glue. It provides strong, fast bonds on plastic surfaces and is widely available in various brands. Make sure to follow the instructions and use it in a well-ventilated area.
What Material Does Hot Glue Not Stick To?
Hot glue does not stick well to certain materials such as silicone, polyethylene, and Teflon. These materials have low surface energy, making it difficult for hot glue to adhere properly. Additionally, hot glue may not adhere to powdery surfaces or surfaces that are too smooth.
Can You Fix Plastic With Hot Glue Gun?
Yes, a hot glue gun can fix plastic. The heat melts the glue, which bonds to the plastic, creating a strong adhesive.
Conclusion
Hot glue can effectively bond with plastic materials for various DIY projects. Its versatility and quick adhesion make it a popular choice for crafters and hobbyists. When using hot glue on plastic, it’s important to consider the type of plastic and ensure proper surface preparation for optimal bonding.
Whether for repairs or creative endeavors, hot glue is a reliable solution for plastic adhesion.
Read the full article
0 notes
Text
Liquid State
Intermolecular forces in liquids are collectively called van der Waals forces. These forces are essentially electrical in nature and result from the attraction of charges of opposite sign.
The principal kinds of intermolecular attractions are: dipole-dipole attractions, London forces, and hydrogen bonding.
Dipole-dipole attractions exist between molecules that are polar. This requires the presence of polar bonds and an unsymmetrical molecule. These molecules have a permanent separation of positive and negative charge. For example HCl, H atom is always slightly positive and the Cl atom is always slightly negative, the H atom in one molecule is attracted to the Cl in a neighbor.
London forces exist in nonpolar molecules. They result from temporary charge imbalances. Temporary charges exist because the electrons in a molecule or ion move randomly in the structure. The nucleus of one atom attracts electrons form the neighboring atom. At the same time, the electrons in one particle repel the electrons in the neighbor and create a short lived charge imbalance.
Hydrogen bonding is unique and requires two conditions: Covalent bonds between an H atom and either F, O, or N atom, and interaction of the H atom in this kind of polar bond with a lone pair of electrons on a nearby atom with F, O, or N. Hydrogen bonding can increase boiling point(ehh) and is responsible for the expansion of water when it freezes.
Structural differences between solids, liquids and gases:
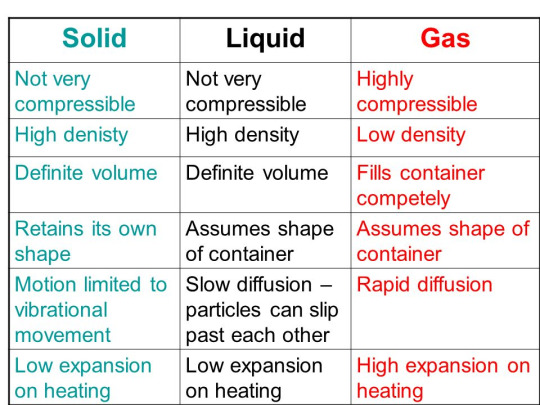
Liquid crystals are an intermediate stage between solids and liquids. Some organic solids having long rod-like molecules which do not melt to give the liquid substance directly, they pass through the liquid crystal state first.
In a liquid, the molecules have a random arrangement and are able to move past each other. In a solid crystal, molecules have an ordered arrangement and are in fixed positions. In a liquid crystals,
however, molecules are arranged parallel to each other and can flow like a liquid. Thus the liquid crystals have the fluidity of a liquid and the optical properties of a solid.
Liquid crystals are classified into three types: Nematic, Smectic, and Chloesteric.
Nematic liquid crystals have molecules parallel to each other, but they are free to slide or roll individually.
Smectic liquid crystals also have molecules parallel to each other, but they are arranged in compact layers. The layers can slide past each other.
Chloesteric: molecules are again parallel and arranged in layers, but molecules in successive layers are slightly rotated with respect to the layers above and below so as to form a spiral structure.
Applications of LCDs:
Aircraft cockpit displays
Calculator screens
Displays images in digital cameras
Television
Computer Monitors
Phone screens
Digital watches
Number Displays
0 notes
Text
Table 7.1 contrasts the forces and energies associated with these four types of solids. (...) The cause of the great difference in the melting points of these two elements is evident from table 7.1. (...) The average Si-Si bond energy is 225 kJ/mol, whereas attractive energies due to molecule forces in P4 are much lower (as shown in table 7.1, intermolecular forces are generally less than 50 kJ/mol) so it takes a much higher temperature to melt silicon than white phosphorus.

"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#force#energy#atom#molecule#network#ion#dispersion#dipolar#hydrogen bond#delocalized#bonding#covalent#electrostatic#arsenic#hydrogen chloride#water#copper#silicon dioxide#sodium chloride#melting point#phosphorus#white phosphorus#silicon
0 notes
Text
What Temperature Does Ice Melt: Safe Thaw's Efficiency Explained.

Picture this: a world blanketed in a crystalline coat of ice, a captivating yet treacherous sight. Amidst the cold embrace of winter, an age-old question emerges: at what temperature does ice surrender to its liquid form? As the frosty tendrils of curiosity beckon, we delve into the intricacies of this phenomenon, aiming to decode the melting point of ice and the game-changing efficiency of Safe Thaw.
The Dance Of Molecules: Unraveling The Melting Process
Ice, that solid reservoir of frozen water molecules, holds its structure together thanks to intermolecular forces. But as temperatures rise, a molecular transformation commences, and the bonds weaken. This heralds the realm of the melting point, that precise temperature at which the delicate equilibrium between solid and liquid shifts.
The Magical Number: Discovering The Melting Point
So, what temperature does ice melt? And, how long does it take salt to melt ice? With precision, it's a dance between 0°C (32°F) and 0.01°C (32.018°F). It's as if nature orchestrates this delicate balance, allowing the transformation to occur within a narrow range. As heat energy infuses the ice, its molecules regain mobility, slipping away from their crystalline structure.
Navigating The Real World: Practical Implications
In a world where practicality reigns supreme, understanding the melting point of ice has significant implications. It's a dance of safety, the difference between icy sidewalks and secure pathways. Traditionally, municipalities have turned to salt as a remedy. However, while salt does indeed lower the freezing point of water, it's not the most efficient solution.
The Salt Predicament: Beyond The Freezing Point
Salt's effectiveness hinges on its ability to depress the freezing point of water. But there's a catch—it doesn't work indefinitely. At extremely low temperatures, salt's power wanes, leaving surfaces susceptible to black ice. Moreover, the corrosive nature of salt can wreak havoc on concrete and vehicles, a testament to the intricate web of consequences that simple actions can trigger.
Safe Thaw's Symphony: A Solution That Transcends
Amidst this icy puzzle emerges Safe Thaw, a dynamic solution that transcends conventional boundaries. Beyond the question of what temperature does ice melt, Safe Thaw introduces a paradigm shift. With an advanced formulation designed to tackle extreme cold, it's a catalyst for transformation even at temperatures as low as -2°F.
A Symphony Of Safety: From Idea To Reality
Imagine a world where pathways remain surefooted and vehicles glide effortlessly. Safe Thaw realizes this vision, melting ice with precision and swiftness. In a symphony of safety, it goes beyond traditional deicers, addressing the concerns of both efficiency and environmental impact. It's the harmony of innovation and nature working in tandem.
Conclusion: Paving The Path To Safety
So, what temperature does ice melt? It's not just a scientific curiosity; it's a pivotal question that shapes our daily lives, especially during the icy embrace of winter. As we navigate the delicate balance between safety and environment, solutions like Safe Thaw stand out, offering a nuanced answer to a seemingly simple question. In the ever-evolving realm of winter maintenance, understanding the science behind ice melting brings us closer to building a safer, more secure world for all.
Read the full article
0 notes
Text
Melting point range of adipic acid
Adipic acid is an organic compound with the formula (CH2)4(COOH)2. It is a white crystalline powder that is mainly used as a precursor for the production of nylon. Adipic acid is also used as a food additive, a plasticizer, and a component of some polyurethanes. In this article, we will explore the melting point range of adipic acid and its implications for its applications.
What is the Melting Point of Adipic Acid?
The melting point of a substance is the temperature at which it changes from a solid to a liquid state. The melting point range is the interval between the onset and the completion of melting. The melting point range can indicate the purity and stability of a substance, as well as its intermolecular forces.
According to EU Method A.1 (Melting / Freezing Temperature), the melting point of adipic acid is 150.85°C. This means that adipic acid starts to melt at this temperature and becomes completely liquid when heated further. The melting point range of adipic acid is not very wide, which suggests that it is a relatively pure and stable substance.
How Does the Melting Point Affect the Properties of Adipic Acid?
The melting point of adipic acid affects its physical and chemical properties, as well as its suitability for different applications. For example, adipic acid has a high solubility in water, which increases with temperature. At 25°C, adipic acid dissolves in water at 24 g/L, while at 100°C, it dissolves at 1600 g/L. This means that adipic acid can be easily dissolved and purified by recrystallization from water.
Another example is that adipic acid has a low vapor pressure, which decreases with temperature. At 18.5°C, adipic acid has a vapor pressure of 0.097 hPa, while at 25°C, it has a vapor pressure of 0.073 mmHg. This means that adipic acid does not evaporate easily and has a low volatility. This makes adipic acid suitable for applications that require high thermal stability, such as nylon production.
What are Some Applications of Adipic Acid?
Adipic acid is mainly used as a raw material for the production of nylon 6,6, which is a synthetic polymer with high strength, elasticity, and resistance to abrasion and chemicals. Nylon 6,6 is widely used in textiles, carpets, clothing, ropes, and industrial materials.
Adipic acid is also used as a food additive (E355) to provide acidity and flavor to some beverages and foods. It can also act as a leavening agent in baked goods and a gelling agent in some desserts.
Additionally, adipic acid is used as a plasticizer to improve the flexibility and durability of some plastics, such as polyvinyl chloride (PVC). It can also be used as a component of some polyurethanes, which are versatile polymers that can form foams, coatings, adhesives, and elastomers.
Conclusion
Adipic acid is an important dicarboxylic acid that has a melting point of 150.85°C. The melting point range of adipic acid affects its solubility, volatility, and thermal stability, which in turn influence its properties and applications. Adipic acid is mainly used for nylon production, but it also has other uses in food, plastics, and polyurethanes.
If you are looking for a reliable Adipic Acid supplier(CAS124-04-9), you can visit our website www.qiboch.com. We supply high quality Adipic Acid with competitive price and fast delivery. We also provide technical support and customer service to ensure your satisfaction. Whether you need adipic acid for industrial or laboratory use, we have the right product for you, contact us today!
0 notes
Text
mmmmmmmmm chemistry mmmmm hydrocarbons mmmm polymer chains mmm intermolecular forces, boiling points, melting points, double bonding, chain length, fatty acids mmmmmmm love this shit
#i mean hey at least im fucking understanding all this shit#im gonna take like a 20 min power nap cause omfg im exhauste#im gonna fall asleep listening to the lecture and hope i absorb information via osmosis#sleep with the textbook over my face or smth
1 note
·
View note
Text
Ion bonding facts

These substances are not soluble in water. Of energy leading to high melting and boiling points. These bonds have to be broken by large amounts These compounds are solid at room temperature, because all of the atoms in a giant covalent structure are held together by strong covalent bonds. Graphite and diamond are examples of giant covalent structures. Some solids can turn straight into a gas, skipping the liquid phase this is called sublimation. Lowring the amounf of energy particles have. Gases can be turned back into liquids by condensation, Liquids can turn into gases by evaporation or boiling (again by increasing the energy of the particles). Lowering the amount of energy particles have. Liquids can be turned back into solids by freezing, This involves increasing the energy of the particles normally by heating. Solids can turn into liquids through the process of melting. Changing from one state to another is a physical change as you end up with the same chemical as you began with (whereas a chemical change would Some of the forces need to be broken during melting, whereas all of theįorces must be broken during evaporating/boiling. The amount of energy required to change state depends on the strength of the intermolecular forces between the particles of each substance. The particles gain energy, which is used to break or overcome the intermolecular forces of attraction.įluids (liquids and gases) are able to take the shape of their containers, however, only gases can be compressed as their particles are far apart and have space to move into. To get from a solid to a liquid, or a gas, energy has to be supplied - usually through heating. During these changes Particles in solids have less energy than liquids, which in turn have less energy than gases. In these diagrams, particles are represented by solid spheres. Solids (s), liquids (l) and gases (g) can be represented using particle diagrams.

1 note
·
View note
Photo
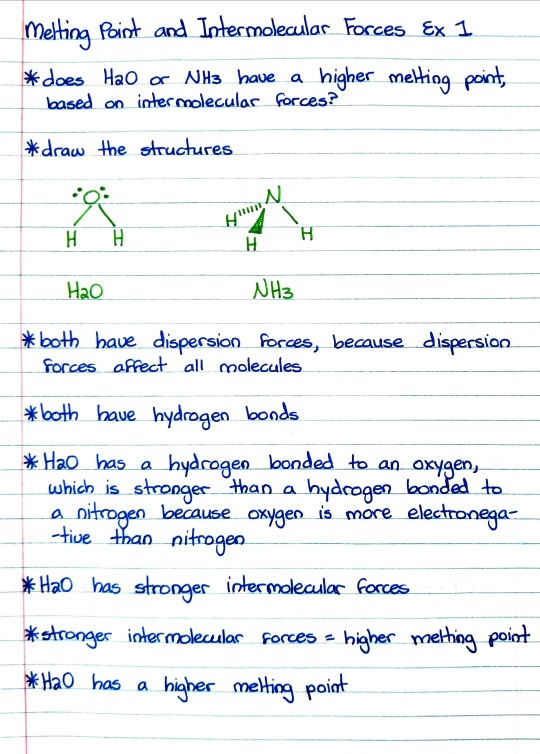
Patreon | Ko-fi
#studyblr#notes#chemistry#chem#chem notes#chemistry notes#medblr#medical notes#med notes#intermolecular forces#melting point#melting point and intermolecular forces#h2o#water#hydrogen bonding#dispersion forces#london dispersion forces#oxygen#nitrogen#electronegativity
12 notes
·
View notes
Text
You know you're a chem student when you start fangirling about how cool water is
#water is so cool#I'm fangirling about water send help#it can form up to 4 hudrogen bonds#which somehow push the molecules away from each other as a solid so it becomes less dense and thats why ice floats#and it has higher melting and boiling points because hydrogen bond are stronger than the other intermolecular forces#and it is very neutral because it can be broken down to a hydrogen ion and a hydroxide ion#oh and water has strong surface tension because hydrogen bonds are so strong#chemistry#chemistry student#a level chemistry
11 notes
·
View notes
Text
Uni Study Diary 16th Entry: 1st Weekend Plan for Finals


Research Methods
The final exam will be a recall and application-based multiple choice test with no chance of explanation, so I will cleaning up and combining ANKI decks for this exam, as well as adding in more examples of the different terms I need to know. I'm not too worried for the multiple choice test, since most of the grade relies on the final assignment. I will be writing up a draft of the final assignment today; I am detailing a setup for an independent research project on resilience in students. After I write up this draft, I will go over any questions I might have with an email to the teacher, and if needed, I will go to her office hours on Monday. This is due Tuesday night.
Biopsychology
Based on the previous exams, this class rarely uses application-based multiple choice questions, so I just have to be familiar with the key concept and terms for recall. I will also be combining ANKI decks and drawing diagrams of major processes in the nervous system that we have covered. On Friday, my TA has an hour open for questions for the final, and I will create a question bank of things I still don't understand to ask.
Organic Chemistry
The trickiest subject to navigate out of all classes. Organic Chemistry tests require fluency in mechanisms, resonance, and drawing lots of molecules, as well as a deep understanding of the major relationships in polarity, stability, intermolecular/intramolecular forces, boiling/melting points, energy, and so much more. Additionally, this alone will not guarantee success on an exam; exams have "alternate universe" problems (ex. manipulation of chirality rules) and situations that concern the subjects in Organic Chemistry 2, connecting our current knowledge with what we will learn in the next quarter (ex. alkyne mechanisms). The application aspect always trips me up, but I will not let that happen to me this time, since I have my hands on past practice exams for the final, and I understand what kinds of problems the professor throws at us. I will be looking at unit summaries to write down a list of concepts I need to understand and go over my old exam questions, worksheet questions, and homework questions. I will not be passively looking at my previous work; I will be using something called the "Feynman Technique" to diagnose my weaknesses. I will be writing down everything I know about a certain topic on a piece of paper without my notes, and I will write it in a way as if I were teaching a child. Aside from explaining the content, I will go from the opposite direction and bring in topics from the next course to see what connections exist between what I know now and future topics. If applicable, I will think in "alternate universe" rules in explaining some of the chapters. After that, I will be filling in the blanks with my notes and old problems. At the same time, I will create a list of questions to email my TA, and I will go to my TA's office hours on Wednesday. The office hours for my professor will be packed this Thursday, but I will still pop in to hear from what others may be struggling on. In the upcoming week, based on my proficiency in the topics, I will do more problems that match up the ones that I still need help with, and I will continue to email for help along the way.
Python Course
I will be marathoning the Codeacademy Python 3 course this Sunday and all of the extra problems given by my professor for exam review. I do need to review some of the basics, which includes dictionaries and nested loops and doing this activity for the weekend will help be diagnose other problem areas.
#study diary#vstudies diary#vstudies#v's study diary#studyblr#studyspo#finals#student#studygram#study plan#planning#planner#exam
2 notes
·
View notes
Text
Best Chemistry Classes for 9th in Dwarka - Adhyayanam Academy
Chemistry classes for 9th graders mark the beginning of a fascinating journey into the world of atoms and molecules. One of the fundamental concepts that lay the groundwork for understanding various chemical phenomena is chemical bonding.
Chemical bonding is the glue that holds atoms together to form compounds, and understanding it opens the door to comprehending the diversity of matter around us. Atoms, the building blocks of matter, interact with each other through chemical bonds to attain stability, typically by achieving a full outer electron shell.
In these chemistry classes for 9th graders, students delve into three primary types of chemical bonds: ionic, covalent, and metallic. Ionic bonds form between atoms when one atom donates an electron to another, resulting in the formation of positively and negatively charged ions that attract each other. Covalent bonds, on the other hand, occur when atoms share electrons to achieve stability, forming molecules. Meanwhile, metallic bonds involve the delocalization of electrons within a metal lattice, giving metals their unique properties.
Understanding the concept of chemical bonding is crucial as it explains various properties of substances, including their reactivity, solubility, and conductivity. For instance, substances with ionic bonds tend to dissolve in water and conduct electricity when dissolved or molten due to the mobility of ions. Covalent compounds, on the other hand, often have lower melting and boiling points compared to ionic compounds, as they consist of discrete molecules held together by weaker intermolecular forces.
Moreover, chemical bonding plays a vital role in the formation of complex structures such as polymers and biological macromolecules like proteins and DNA. These structures rely on specific types of chemical bonds to maintain their integrity and function.
In conclusion, grasping the fundamentals of chemical bonding is essential for chemistry classes for 9th students, as it provides a solid foundation for understanding the behavior of matter at the atomic and molecular levels. Through exploration and experimentation in their chemistry classes for 9th grade, students embark on a journey of discovery that unveils the intricate world of chemical bonding.
know more:
Maths Coaching for 9th
Maths Coaching for 10th
Maths Classes in Dwaraka for 11th
Maths Coaching for 11th
Cuet Coaching in delhi
CA Coaching in delhi
CS Coaching in delhi
best ca foundation coaching in delhi
ca foundation coaching in delhi
0 notes
Text
AQA CHEMISTRY PAPER 1 (Higher Tier, Triple Science) - KEY POINTS FOR GCSE 2018
(Forgot to specify tier etc on the last post, sorry!)
So like I did for Biology Paper 1, here’s a list of things for Chemistry Paper 1 which I find a good idea to remember/tend to forget! I hope it’s useful!
How Fuel Cells work:

Moles = mass/Mr
Avogadro’s constant = 6.02 x 10^23
1 dm3 = 1000 cm3
Concentration in mol/dm3 = moles/volume
Concentration in g/dm3 = mass/volume
Remember to convert volume in cm3 to dm3 (divide by 1000!!) if it’s given in cm3
Moles of a gas = volume (dm3)/24
Periodic table development went: tiny spheres arranged by atomic weights (Dalton), triads where middle element was avg. mass (Dobereiner), period similarities noticed but ignored due to lack of symbols, law of octaves but left no space for discoveries (Newlands), elements placed by mass and properties with spaces (Mendeleev), order of atomic numbers after discovery of nucleus (Moseley)
The size of an atom is approximately 1 x 10^-10
The size of the nucleus is approximately 1 x 10^-14
If percentage yield is more than 100%, there may have been a greater mass of the reactant, or the sample was not dry
If the percentage yield is less than 100%, it may be because the reaction was incomplete, other unexpected reactions took place, or some of the product was lost
History of the atom goes as: Thomson discovered electrons so developed plum pudding model, Geiger & Marsden’s experiment showed a nucleus (because some of the alpha particles were deflected rather than going straight through meaning mass was concentrated in the centre) which had a positive charge, Bohr’s experiments showed electrons orbited at a distance or they would spiral inwards, Chadwick provided evidence of neutrons, later experiments then showed the positive charge could be split up into protons
Metallic bonding is a lattice is positive ions held by electrostatic attraction to negative delocalised electrons
Ionic bonding is strong electrostatic forces of attraction between non-metal negative ion and metal positive ion
Conduct when liquid as ions are not fixed and can therefore move around
Simple covalent structures have weak intermolecular forces between individual molecules
Fullerenes are good lubricants because they are round and therefore roll
Course particles are between 1 x 10^-5 m and 2.5 x 10^-6 m
Fine particles are between 1 x 10^-6 m and 2.5 x 10^-7 m
One nanometre is 0.000000001 (billionth) of a metre as is written as 1 x 10^-9 m
Nanoparticles have a diameter between 1nm and 100nm
Boiling points of noble gases increase as you go down the group
Alkali metals have lower melting/boiling points, are softer, less dense, and are weaker than transition metals
A more reactive halogen will displace a less reactive halogen from a solution of its salt
This is the reactivity series:

A more reactive metal will displace a less reactive metal from a solution of its salt
The easier it is for a metal to lose an electron, the more reactive it is
In the electrolysis of a molten compound, metals go form at the cathode and non-metals form/are released at the anode
In the electrolysis of an aqueous solution, hydrogen is released from the cathode if the metal is MORE reactive than it, and oxygen is released from the anode UNLESS the solution contains halide ions (in which case the halide ions are released as halogens)
For bonds to be broken, reacting particles must collide with sufficient energy
This is what Energy profile diagrams look like (remember the products of an exothermic reaction will have LESS energy):
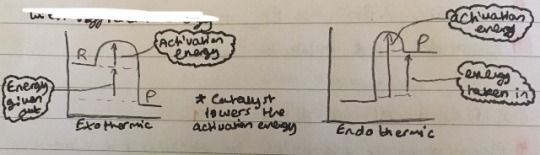
Overall energy change = bonds broken - bonds made
A negative energy change is exothermic because the products had MORE energy, as it was released
Bases are insoluble alkalis and can include metal carbonates, metal oxides, and metal hydroxides
Filtration is used to separate a soluble solid from an insoluble solid
Crystallisation is used to obtain a soluble solid from a solution
Simple distillation is used to obtain a solvent from a solution
Fractional distillation is used to separate mixtures in which the components have different boiling points
Chromatography is used to separate the different soluble, coloured compounds in a mixture
GOOD LUCK ON YOUR EXAM TOMORROW EVERYONE!! Get plenty of sleep, especially since it’s an AM exam!
548 notes
·
View notes
Text
What Is HDPE, and What Are Its Features?
HDPE, or high-density polyethylene, is a thermoplastic polymer made from petroleum and one of the best plastic materials available. HDPE is used in various sectors, and it has a wide range of applications. But the HDPE plastic bottle is one of the major products manufactured out of this material. HDPE is used to make milk jugs, Bleach bottles, shampoo bottles, and piping ad well. HDPE is known for its high strength, and it offers high-impact and melting points. Due to this, this material is used in various sectors. Besides these applications, the use of HDPE is also found in
Wood-plastic composites
Shoe lasts
Plastic surgery and facial reconstruction
3-D printing filament
Snowboards
Food & beverage containers

What are the features of HDPE?
HDPE is designed to be low-maintenance, long-lasting, and safe. They offer a good grip on the handle and surface to hold food safely. They also have:
Easily moldable and meltable
One of the major benefits of HDPE is that it can be melted easily. At the same time, it has a high melting point, which makes it useful for various applications. But once it reaches the melting point, then it can be easily Molded for various applications, including detergent bottles, cutting boards, food storage containers, milk jugs, plastic lumber, and so on. HDPE plastic bottles can be easily recycled and turned into various products.
Corrosion resistance
HDPE resists rotting, mould growth, and mildew which is ideal for underground piping to supply water. HDPE is also weather resistant and can be sterilized by boiling. Due to this, HDPE is an ideal option for food and beverage containers. HDPE can also withstand strong bases and acids and has resistance against the chemicals. Furthermore, it doesn’t react with chemicals, cleaning fluids, acids, and detergents.
Strength to density ratio
HDPE comes with a density of 0.93-0.97 g. The density of HDPE is only higher than the LDPE. HDPE has little branching that gives it strong intermolecular strength and forces than LDPE. That’s why it is used in various applications, such as carrying gallons of water or liquid, which is very heavy.
Recyclable
This is one of the main benefits of using HDPE material. HDPE plastic bottles and other HDPE products can be recycled. From an environmental point of view, HDPE is very healthy. Over the years, with increasing environmental concerns, consumers and manufacturers have been looking for environmentally friendly options. Due to this, HDPE has gained massive popularity. If you are also looking for cost-effective and environmentally friendly options, then HDPE would be best for you.

Why Use HDPE?
As you can see, there are so many benefits of using HDPE material. It is lightweight that makes it easier for transportation, and reduces the cost. HDPE can also replace heavier materials that can help companies and individuals to achieve affordable and sustainable manufacturing project goals. HDPE is the perfect combination of cost-efficiency, strength, and environmental friendliness.
HDPE vs. PP
There are many similarities between HDPE and PP plastic; that’s why many people get confused between these two materials. But if you are searching for the right product for your project, then make sure to use the best among these. Both of these materials offer similar benefits as well. They have similar strengths, and both of them are considered to be heat-resistant, and they are not toxic. That’s why they can be used in the food and beverage sector. So do proper research before making a final decision.
Quality Blow Moulders offers a wide range of HDPE packaging products at an affordable price. They have an HDPE bottle designed for every industry requirement.
Read more blog links here
Things to Know Before Buying Plastic Bottles From Wholesalers
Plastic Bottle Utility Guide to Protect Environment
0 notes
Text
Chapter 5 Physical States of Matter 9th Chemistry Notes

Chapter 5 Physical States of Matter 9th Chemistry Notes Notes. Easy notes that contain questions, exercises,s and key points of the chapter.
Short Questions Chapter 5 Physical States of Matter 9th Chemistry Notes
Can you give a reason why it takes a longer time to cook at high altitudes?Boiling points depend upon the external pressure over the surface of the liquid. At sea level external pressure is 1 atm, the boiling point of water is 100oC. As we go at higher altitudes, the external pressure decreases, and the water boils at lower temperatures. Because the water is boiling at lower temperatures and less heat is being transferred, more time is required to cook the same amount of food.
Q.2) Glass softens over a wide range of temperatures. Ice melts at a specific temperature. Explain the reason for this difference.
Answer:
Glass is an amorphous solid in which the particles are not regularly arranged. Because of this, the intermolecular forces among its particles vary in strength within a sample. Melting thus occurs at different temperatures for different portions of the same sample as the intermolecular forces are overcome. Therefore, glass does not exhibit sharp melting point but softens over a wide range of temperature.
Ice contains regularly repeating arrangements of particles. The intermolecular forces among particles are same everywhere within a sample. Therefore, it melts at a specific temperature.
Q.3) Explain why it happens that on a hot summer day when there is sweat on the body of a person, one feels cool under fast moving fan?
Answer:
For evaporation to occur, the molecules need energy which they get from the body by taking away heat and body gets cooler by losing heat. If there is no wind, the evaporating molecules keep hovering near the surface of the body and impede the evaporation of more molecules and even push some back to the body. A fast moving fan blows air around, which speeds the evaporation by taking the vapour away. Since evaporation is a cooling process, more evaporation causes more cooling due to fan air.
Q.4) Why are the densities of gases lower than that of liquids?
Answer:
Gases are light in mass and their molecules occupy more volume than liquids. Moreover, molecules of gases are farther apart. As the liquid molecules have strong intermolecular forces, they are closely packed and the spaces between them are negligible. Therefore, densities of gases are lower than that of liquids.
Read more: Structure of Atoms Cha 2 Chemistry 9 class notes
Q.5) What is the relationship between the atmospheric pressure and boiling point of a liquid?
Answer:
Boiling point of a liquid depens on the atmospheric pressure. With the increase in atmospheric pressure, the boiling point also increases. Similarly, the decrease in atmospheric pressure causes decrease in boiling point.
For example, at sea level atmoshperic pressure is 1 atm, the boiling point of water is 100oC. As we go at higher altitude, the atmospheric pressure decreases, and the water boils at lower temperatures.
Q.6) Why gas is compressible but a solid is not compressible? Give reason(s).
Answer:
Gas can be easily compressed because the molecules are far away from each other and very weak intermolecular forces are present between them.
Solid is incompressible because the particles are closely packed in a fixed pattern and there are strong forces of attraction between the molecules.
Long Questions Chemistry 9 class notes
Q.1) Define Boyle's law and verify it experimentally.
Answer:
Boyle’s Law
Boyle’s law states that the pressure and volume of a gas have an inverse relationship, when temperature is held constant.
It means as pressure increases, the volume of the gas decreases in proportion. Similarly, as pressure decreases, the volume of the gas increases.
Mathematically it can be written as:
P ∝ 1 / V or PV = k
where ‘P’ is the pressure of the gas, ‘V’ is the volume of the gas, and ‘k’ is a constant.
The equation above states that product of pressure and volume is constant for a given mass of confined gas at constant temperature.
If P1V1 = k then P2V2 =k
Where P1 = initial pressure V1 = initial volume
P2 = final pressure V2 = final volume
As both equations have same constant, their variable have same value.
Thus P1V1 = P2V2
Experimental verification of Boyle’s law
Suppose there’s a gas confined in a cylinder with a piston at the top. The initial state of the gas has a volume equal to 8.0 dm3 and the pressure is 1.0 atm. Temperature and number of moles is constant. Weights are slowly added to the top of the piston to increase the pressure. When the pressure is 2 atm the volume decreases to 4.0 dm3. The product of pressure and volume remains a constant i.e 8.
P1V1 = P2V2
1 x 8 = 2 x 4
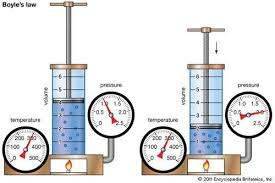
Q.2) Differentiate between:
a. Evaporation and Boiling Point
b. Effusion and Diffusion of gases
c. Condensation and Evaporation
Answer:
a) Difference between evaporation and boiling
Read more: Class 9 Chemistry Cha 2 Periodic Table and Periodicity of Properties
EvaporationBoilingEvaporation is a process in which a substance changes its state from liquid state to gaseous state without boiling.Boiling is a process in which a substance changes its state from liquid state to gaseous state with boiling.It is a slow process.It is a rapid process.No bubbles form in evaporation because vapour pressure is less than atmospheric pressure.Bubbles form in boiling because vapour pressure is equal to the atmospheric pressure.It takes place at all temperatures.It occurs at a particular temperature.Evaporation takes place from the exposed surface.Boiling occurs throughout the liquid.Some particles move fast and some move slowly in evaporation.All the particles move very rapidly in the process of boiling.read
b) Difference between diffusion and effusion of gases
Read more: Chapter 4 Structure of Molecules Chemistry Class 9 Notes
DiffusionEffusionThis process of mixing of gases by random motion of molecules is called diffusion.The escape of molecules in the gaseous state one by one without collision through a hole of molecular dimension is called effusion.It is the ability of a gas to travel through a small opening.It is the ability of gases to mix with each other usually in the absence of a barrier.Diffusion occurs due to difference in concentrations.Effusion is facilitated by a difference of pressures.The rate at which diffusion occurs is limited by the size and kinetic energy of the other particles.Effusion typically transports particles more quickly.
c) Difference between condensation and evaporation
CondensationEvaporationCondensation is the change from a vapor to a condensed state (solid or liquid).Evaporation is the change of a liquid to a gas.Condensation occurs at a constant temperature.Evaporation does not occur at a constant temperature.Condensation is a phase change regardless of the temperature.Evaporation occurs before a liquid reaches its boiling point.Condensation happens mainly at higher altitudes.Evaporation usually takes place in low altitudes.In the process of condensation, energy is released.In the process of evaporation, energy is consumed.
Q.3) Define the term allotropy with examples. Explain the three allotropic forms of carbon in detail.
Answer:
Allotropy
Allotropy is the property of elements to exist in two or more different crystalline forms, in the same physical state. They are known as the allotropes of these elements.
Examples:
Allotropes of oxygen are oxygen (O2) and ozone (O3).
Allotropes of sulphur are orthorhombic and monoclinic.
Allotropes of tin are white tin and grey tin.
Allotropes of Carbon
Carbon exists in crystalline allotropic form as well as non-crystalline or amorphous allotropic form.
Carbon exists in three crystalline allotropic form namely diamond, graphite and bucky balls.
Carbon also exists in non- crystalline or amorphous allotropic form such as coal,coke, charcoal, lamp black etc.
Crystalline Allotropic Form of Carbon
i) Diamond
In diamond each carbon atom is covalently bonded to four others, creating a rigid compact array. This makes diamond the hardest known substance. Diamonds are used for cutting and polishing hard surface. It is bad conductor of electricity because the valence electrons are tightly held by covalent bonds.

ii) Graphite
In diamond each carbon atom is covalently bonded to three other carbon atoms rather than to four atoms asin diamond.
In graphite carbon atoms are arranged in layers of hexagonal arrays. Weak bonds exist between the layers that allow them to slide over one another. This makes graphite soft. Graphite is used as electrode, lubricant in machines, and black pigment.

iii) Bucky Ball
In bucky ball, 40 to 100 carbon atoms are arranged in a hollow cage like structure. Carbon atoms are arranged in pentagons (five member ring) and hexagons (six member ring) just like a soccer ball. In bucky ball, the carbon atoms joined together making pentagonal, hexagonal structures.
Bucky balls are used as semiconductors, superconductors and lubricants.
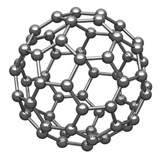
Q.4) What are solids? Differentiate between amorphous and crystalline solids.
Answer:
In solid state of matter the particles are closely packed in a fixed pattern. In solids there occurs a strong force of attraction between the solid particles, which hold them firmly together, so that they cannot leave their position. Solid particles possess only vibrational motion. Hence, solid cannot diffuse like gases and liquids.
Difference between amorphous and crystalline solids
Crystalline solidsAmorphous solidsA crystalline solid is a solid that is composed of orderly, repeating three-dimensional arrangement of particles.Amorphous solid does not have a well-defined arrangement of its particles.Crystalline solids have sharp melting points.Amorphous solids do not melt at a definite temperature but gradually soften when heated.Crystalline solids give clean cleavage.Amorphous solids give irregular cut.Crystalline solids are anisotropic i.e. the properties like electrical conductance, refractive index, thermal expansion, etc., have different values in different directions.Amorphous solids are isotropic i.e. the properties like electrical conductance, refractive index, thermal expansion, etc., have the same values in all directions.Examples:
Diamond, sodium chloride etcExamples:
Glass, plastic, rubber etc
Q.5) Define Charle's Law and verify it graphically and diagrammatically.
Answer:
Charles’s Law
Charles’s law states that , the volume is directly proportional to the absolute temperature at constant pressure. As the temperature of the gas increases, the volume increases.
Mathematically it can be written as:
V ∝ T
V = kcT
or V / T = k
where ‘V’ is the volume of the gas, ‘T’ is the temperature of the gas, and ‘kc’ is a constant.
The equation above states that ratio of volume to temperature of a given mass of a gas is constant at constant pressure.
If V1 / T1 = k then V2 / T2 = k
Where V1 = initial volume T1 = initial temperature
V2 = final volume T2 = final temperature
As both equations have same constant, their variable have same value.
Thus, V1 / T1 = V2 / T2
Graphic representation
If the values of volume 'V' is plotted against temperature 'T', a straight line is obtained, which shows that the volume is directly proportional to the absolute temperature.
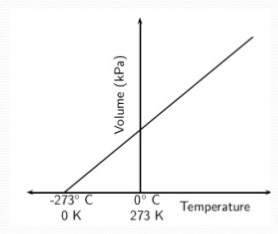
Diagrammatic representation
The Charles's law can be diagrammatically represented as,
Numericals Chemistry 9 class chapter 5
Q.1) Calculate the final pressure of a sample of a gas that is changed at constant temperature to 14.3 dm3 from 7.55 dm3 at 828 torr.
Answer:
Given that
P1 = 828 torr V1 = 7.55 dm3
P2 =? V2 = 14.3 dm3
Formula applied
P1V1 = P1V2
828 x 7.55 = P2 x 14.3
P2 = 6251.4 / 14.3
= 437.160 torr
Q.2) Calculate the final volume at 302 K of a 5.41 dm3 sample of a gas originally at 353 K if the pressure does not change.
Answer:
Given that
V1 = 5.41 dm3 T1 = 353 K
V2 =? T2 = 302 K
Formula applied
V1 / T1 = V2 / T2
5.41 / 353 = V2 / 302
V2 = 0.0153 x 302
= 4.628 dm3
Q.3) Calculate the initial volume at 0oC of a sample of gas that is changed to 731 cm3 by cooling to -14oC at constant pressure.
Answer:
Given that
V1 =? T1 = 00C = 0 + 273 = 273 K
V2 = 731 cm3 T2 = -14oC = -14 + 273 = 259 K
Formula applied
V1 / T1 = V2 / T2
V1 / 273 = 731 / 259
V1 = 2.822 x 273
= 770.406 cm3
Q.4) A sample of a gas at room temperature occupies 0.80 dm3 at 1.5 atm. What will be its volume when the pressure of the gas is raised to 2.1 atm?
Answer:
Given that
P1 = 1.5 atm V1 = 0.80 dm3
P2 = 2.1 atm V2 =?
Formula applied
P1V1 = P2V2
1.5 x 0.80 = 2.1 x V2
V2 = 1.2 / 2.1
= 0.571 dm3
Q.5) Calculate the final volume of 319oC of a sample of gas original 5.13 dm3 at 171oC, if the pressure does not change.
Answer:
Given that
V1 = 5.13 dm3 T1 = 319oC = 319 + 273 = 592 K
V2 =? T2 = 1710C = 171 + 273 = 444 K
Formula applied
V1 / T1 = V2 / T2
5.13 / 592 = V2 / 444
V2 = 0.0086 x 444
= 3.847 dm3 ≈ 4 dm3
Read the full article
0 notes
Text
How do plasticizers work
Generally, the higher the water content of concrete, the better its fluidity and workability. But when concrete has enough moisture, the strength of concrete after solidification is inversely proportional to the moisture content. Therefore, if the concrete is to have high strength, the water content of the concrete cannot be too much, and the workability at this time will become poor. The plasticizer can reduce the water content of the concrete without affecting the workability of the concrete (therefore it is called a water reducing agent). ), it also improves the strength of concrete. This method is often used to increase the strength when producing high-strength concrete or fiber reinforced concrete.

Plasticizers are usually polar or partly polar in structure, and are liquids or low-melting solids with high boiling point, hard to volatilize and good miscibility with polymers. The plasticizer is distributed between the macromolecular chains, which can reduce the intermolecular force, reduce the viscosity of the polymer and increase the flexibility. Plasticizers are divided into two categories: primary plasticizers and secondary plasticizers. The main plasticizers are compatible with resins, have low permeability and low volatility, and can improve plasticization efficiency.
Different types of plasticizers have different effects on the performance of products. In actual production, one plasticizer is rarely used alone, and several plasticizers are often mixed with complementary performances to achieve a good plasticizing effect.
0 notes