#inorganicchemistry
Text
Shapes of ammonia molecules

Shapes of ammonia molecules are trigonal pyramidal or distorted tetrahedral in shape. These three types of molecules contain one nitrogen atom and three hydrogen atoms. They never form flat, trigonal planar molecules. In this article, we will discuss the different shapes and polarities of ammonia molecules. Molecular shapes of ammonia molecules are important for their properties.
Trigonal pyramidal shape

Shapes of ammonia molecules has a tetrahedral molecular structure, with four Hydrogen atoms surrounding one Nitrogen atom. The nitrogen atom acts as the base and tip of the molecule, with its electronic configuration 1s2 2s2 or 2px1 2py1 2pz1. For instance, ammonia also has a sp3 hybridization. Therefore, it has one more electron than the other two atoms.
The NH3 molecular geometry illustrates the number of valence electrons and bond electron pairs in the molecule. The tetrahedral shape of ammonia is a result of electronic repulsion between the hydrogen and nitrogen atoms. The hydrogen-nitrogen-hydrogen atoms have a bond angle of 107 degrees, which is refer to as the tetrahedral geometry.
The Lewis structure of ammonia shows that the nitrogen atom has four electrons. Three of them are bonding pairs, while the four lone electrons are nonbonding. This configuration results in a pyramidal shape, with the most electrons on the apex of the pyramid. However, electron geometry considers all electrons, while molecular geometry only considers bonding atoms.
Ammonia is a colorless, pungent gas that contains two atoms of each element. The tetrahedral molecular geometry of ammonia is determine by drawing a Lewis structure of the compound. Ammonia contains three hydrogen atoms bound to one nitrogen atom. This molecule is also considered a conjugate acid/base and is soluble in chloroform, ether, and ethanol.
Ammonia molecule's bond polarities
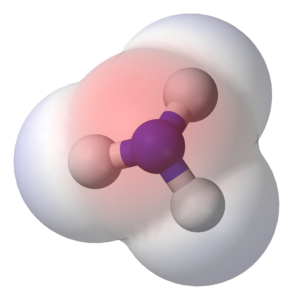
The dipole moment is the measure of the polarity of a molecule's bonds. The NH3 molecule's dipole moment is 1.46D, and the three N-H bonds have a total net dipole moment of 1.4D. Both hydrogen and nitrogen are polar, and their dipole moments are opposite. The polarity of a molecule is determine by its dipole moment, which is determine by the electronegativity of the molecule.
The shapes of ammonia molecule's three Hydrogen-Hydrogen bond dipoles are asymmetrical. The nitrogen atom is more electronegative than the hydrogen atom, so its lone pair will attract the partially positive hydrogen atom. The result is a tetrahedral geometrical structure. Its polarity is cause by the unequal distribution of electrons. Nonpolar molecules, on the other hand, arise when the electrons are evenly distributed in a diatomic molecule.
The asymmetrical structure of the NH3 molecule plays a role in the molecule's polarity. While the hydrogen and nitrogen atoms are non-reactive, the lone pair of the central nitrogen atom contributes to its polarity. It is also the nonreactive noble gas. It is not necessary to consider polarity when comparing NH3 to other chemical compounds, however.
The polarity of an NH3 molecule depends on its electronegativity. The lone pair of electrons on the nitrogen atom is polar. If the opposite happens, the hydrogen atoms will act as proton acceptors. Furthermore, the dipole moment in NH3 is 1.42D. This is a highly polar molecule with a net dipole moment of 1.42D.
NH3 molecule's electron geometry
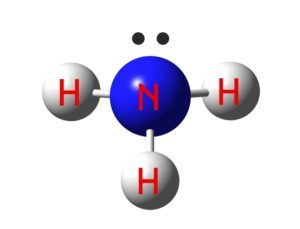
The trigonal pyramidal molecular geometry , the shapes of ammonia molecules is based on the VSEPR theory. The NH3 molecule has three atoms and one lone pair surrounding a central atom, and has eight valence electrons. The hydrogen atoms are always outside of the Lewis structure. If you have an atomic model of ammonia, you should be able to see how these two properties interact.
The sp3 hybridization of the NH3 molecule produces a T-shaped molecule. The nitrogen atom is bond to each hydrogen atom with one pair of electrons, and another pair is bond to the outer shell of the nitrogen atom. The tetrahedral angle is 109.5 degrees, but the NH3 molecule's tetrahedral geometry is reduced to 107 degrees.
The Lewis structure of a molecule can help you understand a molecule's electron geometry and polarity. A Lewis structure represents a molecule's valence electrons in a pictorial format. The electrons that form a bond are called a bonding pair, while those that do not form a bond are referred to as nonbonding electrons. A Lewis structure typically shows the valence electrons as dots and lines, while the nonbonding electrons are labeled with lines.
This molecular structure is explain by the Valence Shell Electron Pair Repulsion theory. This theory explains how a nitrogen atom's presence bends the entire structure of a molecule. This gives ammonia its trigonal pyramidal or deformed tetrahedral molecular geometry. This is due to the nitrogen atom's nonbonding lone electron pair which exerts repulsion on bonding orbitals.
NH3 molecule's lone pair

The lone pair of electrons on the nitrogen atom in the NH3 molecule is responsible for its distorted molecular geometry. It exerts repulsive forces on other bonding pairs. The four groups of electrons surrounding the nitrogen atom form a tetrahedral structure. The arrangement of the electrons in the NH3 molecule is similar to that of the hydrogen atom.
The tetrahedral structure of a molecule can be calculated using the AXN technique. In this method, the central nitrogen atom is designated with the letter A, and the electrons that are bound to the atom's core are designated with the letters X and N. This is done to indicate that the NH3 molecule has three bound pairs of electrons and one lone pair of electrons.
Because the nitrogen central atom has no formal charge, it is best to study the lone pair of electrons in a molecule using the Lewis dot structure. This structure is the most stable and appropriate for nature. The lone pair influences the overall shape of the molecule, and can also be use to determine its steric number. When you're analyzing a molecule, you can use this information to analyze the chemical properties of a given molecule.
The three single bonds in the NH3 molecule's structure give it two nonbonding electrons and one bonding electron on the central nitrogen atom. This is the reason why the lone pair is so important. It allows you to draw the Lewis structure of the NH3 molecule and identify the various parts that make up its structure. You'll also find out how to draw the lone pair in an NH3 molecule.
NH3 molecule's electronic structure

The NH3 molecule's electronic structure is determined by the fact that its three hydrogen atoms and one nitrogen atom are covalently bound to one another. The presence of this hydrogen bonding is responsible for the elevated normal boiling point of ammonia. The lone pair of electrons on the nitrogen atom acts as a repulsive force on the bonding orbitals. This arrangement gives the molecule a tetrahedral electron geometry.
The tetrahedral electron geometry of the NH3 molecule makes it a stable binary hydride. The NH3 molecule have four electron pairs. Three are attach to the core nitrogen atom, and one lone pair of electrons on the outermost nitrogen atom. These two features help explain the NH3 molecule's hexagonal molecular shape.
The Lewis structure helps us understand molecular geometry, electron polarity, and electron valence pairs. This pictorial representation helps us understand what the NH3 molecule's valence electrons look like. The valence electrons are in the form of dots. The electrons on the outside of the Lewis structure are hydrogen atoms. Consequently, there are eight valence electrons on the NH3 molecule.
Using a molecular geometry simulator, students can construct their own NH3 molecule and study the electron-pair geometry of the molecule. By clicking on the NH3 molecule, students can click on the lone pair or double bond to reveal its full geometry. Next, they can name each atom's electron group geometry and predict the bond angle. The NH3 molecule is easy to draw.
NH3 molecule's sp3 hybridization

The NH3 molecule has four sp3 hybrid orbitals in its center, and these are tetrahedrally oriented. One of the sp3 orbitals contains a lone pair of electrons. Three of the N atom's half-filled sp3 hybrid orbitals overlap axially with the three hydrogen atoms' half-filled 1s orbitals. The result is a molecule with three bonding pairs and one lone electron.
The NH3 molecule's st3 hybridization occurs on the nitrogen atom in the central molecule. This is a chemical process in which unstable elements like nitrogen bond with other elements. The process of hybridization occurs by sharing, gaining, or losing electrons. In this process, the unstable elements give up one or more of their valence electrons to join with another element. The valence electrons of the atom are far from the nucleus and therefore easily bond with other elements.
The NH3 molecule's sc3 hybridization is an important process in studying the structure of the NH3 molecule. It is a three-step process in which you sketch out the molecular geometry of the molecule, calculate its sp3 hybridization, and then give the molecular geometry of NH3 in perfect notation. This process teaches you how to understand sp3 hybridization and how to use it in real-life applications.
https://www.youtube.com/embed/9MLOgywe84k
Read the full article
10 notes
·
View notes
Photo

Check link in bio for more..... Do follow @cgchemistrysolutions . . . . . . . . #organicchemistry #organicsynthesis #studychemistry #chemistryclass #chemistryeducation #clickyourlab #chemiker #academiclife #inorganicchemistry #chemistryclass #chemistry chemistrylove #chemistry2 #chemistrymemes #chemistrynotes #chemistrymeme #chemistrylovers #chemistryteacher #chemistryteachers #chemistryisfun #chemistry2 #chemistry #chemistrynotes #chemistrystudent #chemistryeducation #chemistrymajor #chemistry #chemistrylife #chemistrylab #chemistryboys #chemistrymemes #organicchemistry #chemistrylover #organicchemistry #chemistrycafe #alevelchemistry #chemistryclass #trending #chemistrymemes #chemistrycafe #chemistrymeme #viralpost #chemistryteacher #chemistryteachers #viral (at Aligarh, UP) https://www.instagram.com/p/CpyjF7YhiuR/?igshid=NGJjMDIxMWI=
#organicchemistry#organicsynthesis#studychemistry#chemistryclass#chemistryeducation#clickyourlab#chemiker#academiclife#inorganicchemistry#chemistry#chemistry2#chemistrymemes#chemistrynotes#chemistrymeme#chemistrylovers#chemistryteacher#chemistryteachers#chemistryisfun#chemistrystudent#chemistrymajor#chemistrylife#chemistrylab#chemistryboys#chemistrylover#chemistrycafe#alevelchemistry#trending#viralpost#viral
1 note
·
View note
Text
Global Third Party Chemical Distribution Market Report 2023
In an exclusive report by Decipher Market Research, the Third Party Chemical Distribution is projected to grow at a CAGR of 5.1% during the forecast period (2023-2030)
View Report :
** Key Players **
Azelis, Barentz, Brenntag North America Inc., ICC Chemicals Inc., IMCD N.V., Jebsen & Jessen Group, Petrochem, Protea Chemicals, REDA Chemicals, Univar Solutions

#party#chemicalengineering#distro#hardwell#chemicalindustry#third#teamfollowback#chemical#chemistryisfun#inorganicchemistry#thirdpartychemicaldistributionreport2023
1 note
·
View note
Text
youtube
#basicconceptsofchemistry#chemistryconceptsetoosindia#gunjanmamchemistry#gunjanmametoosindia#importanceofchemistryinourdailylife#fertilizersacids and bases#soapsanddetergents#somebasicconceptsofchemistry#natureofmatterandelements#inorganicchemistry#useofchemistryinyourdailylifeanditsimportance#Youtube
0 notes
Link
Chemistry is more than mere science in a lab. Uncover the most common chemical reactions that demonstrate chemistry in action found in everyday life.
#baking#chemicalreactions#fruit#inorganicchemistry#moravek#organicchemistry#photosynthesis#ripeningoffruit
1 note
·
View note
Video
Bromine CAS: 7726-95-6 Chemical formula: Br2 The main uses are in the manufacture of bromine compounds with chemical and biological activity, high density or flame retardant and fire extinguishing properties. Bromine products occupy an important position in gasoline additives, flame retardants, agricultural chemicals, drilling fluids, photographic chemicals, disinfectants, dyes, pharmaceuticals and other applications. Bromine - uses 1. Manufacturing of organic and inorganic chemicals, such as fuel additives, flame retardants, pesticides, oil well drilling fluids, pharmaceuticals, and dyes. brominating agent. Disinfect in water; used as bleach, surface disinfectant. 2. Chemical intermediates of bromoethyl, methyl bromide, ethylene dibromide, other bromine CMPD and salts; in bleaches and disinfectants 3. Used in the manufacture of sedatives, anesthetics, antispasmodics, hydraulic oil, refrigerants and dehumidifiers, and volatile preparations 4. Used for bleaching, disinfection, and manufacturing of dibromoethane and other organic chemical products (fire extinguishers, analytical photography reagents, flame-retardant plastics, dyes, medicines); Used to disinfect water and bleach textiles, swimming pool disinfectant. Used as holographic bleach in photography 5. Medications ------ Sinoland Chemical, we are suppliers in China. Company/ Brand Name: QINGDAO SINOLAND INTERNATIONAL TRADE CO.,LTD Services:Chemical,import and export — Our website: www.sinolandinfo.com/index.php — Description: We very much hope to cooperate with your company and look forward to hearing from you. We can provide you with the chemical raw materials you need We can provide you with the most competitive market prices We can negotiate to provide you with samples. We also support customized outer packaging. — Best regards! Leo-Liu Sinoland-Foreign Trade Specialist QINGDAO SINOLAND INTERNATIONAL TRADE CO.,LTD whatsapp: (+86) 15963011049 Email Address: [email protected] [email protected] Vk:https://vk.com/sinoland_leo Facebook: https://www.facebook.com/profile.php?... Linkedin:https://www.linkedin.com/in/Sinoland-... Youtube: / @sinoland_chem_leo Tiktok:https://www.tiktok.com/@leoliu_wanl Instagram:https://www.instagram.com/liunuo_95 Twitter:https://twitter.com/HelloWorld_Leo #qingdao #china #chemical #supplier #samples #Rawmaterials #Sinoland #managementconsulting #businessconsulting #businessmanagement #organic chemicals #inorganicchemistry chemicals #fuel #flameretardant #pesticides #oilpasteldrawing#drug #dye #brominating#waterdispenser #bleach #disinfectant #sedative #anesthetic #antispasmodic #hydraulic #refrigerantes #dehumidifier #volatile preparations #fire #extinguisher #analytical#flame #retardant #plastic #disinfection #marketshare #marketsize #markettrends #marketgrowth #marketanalysis #marketresearch #research #instagram #facebook #twitter #ins #linkin #tiktok #youtube #vk #ok
0 notes
Text
How to get admission in Engineering / medical Coaching? See Process Here
Let's Start Medical & Engineering Journey.

𝐆𝐞𝐭 𝐀𝐝𝐦𝐢𝐬𝐬𝐢𝐨𝐧 𝐈𝐧 𝐅𝐨𝐮𝐧𝐝𝐚𝐭𝐢𝐨𝐧/𝐈𝐈𝐓 𝐉𝐄𝐄/𝐍𝐄𝐄𝐓. Contact Us
𝐈𝐂𝐎𝐍𝐈𝐂 𝐂𝐋𝐀𝐒𝐒𝐄𝐒
Ph: +91 7903993958 & 8651259660
Email id: [email protected]
#IITJEE#medical#foundation#NEET#11th#12th#physics#physicsteacher#maths#mathematics#chemistry#biology#biologclass#MatheClass#target#TargetBatch#crashcourse#newadmission2022#NewAdmission#medicalcourse#iitjeecourse#organic#organicchemistry#inorganicchemistry #2023 #happynewyear#newyear2023
0 notes
Text

International Conference on Organic Chemistry
#science #sciencefather #shorts #technology #conference #awards #research #engineering #inorganicchemistry #analyticphilosophy #spectroscopy
https://organic-chemistry-conferences.sciencefather.com
0 notes
Photo


18.11.2020 || Endless study days
#masters#chemistry#laboratory#inorganicchemistry#study#studyblr#studying#mestrado#rotinadeestudos#inorgânica
17 notes
·
View notes
Photo

Do check out CG's Chemistry Solutions. Link in bio #chemistry #organicchemistry #organic #inorganicchemistry #physicalchemistry #class12chemistry #class11 #class12 #class11chemistry #class12th #chemistrynotes #chemistryteachers #onlineclasses #onlinecoaching #jeemains #jeeadvanced #jeemain #jee2023 #jee2023aspirants #neet2023 #neet2023aspirants #neetaspirants #jeeadvanced2023 https://www.instagram.com/p/CjYDEIapW6m/?igshid=NGJjMDIxMWI=
#chemistry#organicchemistry#organic#inorganicchemistry#physicalchemistry#class12chemistry#class11#class12#class11chemistry#class12th#chemistrynotes#chemistryteachers#onlineclasses#onlinecoaching#jeemains#jeeadvanced#jeemain#jee2023#jee2023aspirants#neet2023#neet2023aspirants#neetaspirants#jeeadvanced2023
0 notes
Text
Macato Murayama- Inorganic Flora
✳ Digital flora designed by the dissection of natural flower's ovaries.





1 note
·
View note
Text









Few colorful metallic compounds sintetized during inorganic lab👩🏻🔬
15 notes
·
View notes
Photo




Back in the Chemistry lab. These photos were from my 4th year Advanced Inorganic Chemistry lab. The experiment today was mainly about boiling ferrocene with aluminum, aluminium trichloride, Mesitylene and water in a Nitrogen environment. The rest of the photos are of purified ferrocene which was done by sublimation, precipitating orange crystals from a cruder ferrocene powder. The lab is a long one and won’t end for a few weeks so might post more photos. #chemistry #inorganicchemistry #chemistryclass #chemmajor #science #stem (at Thompson Rivers University - TRU World) https://www.instagram.com/p/BstpGGVHLSB/?utm_source=ig_tumblr_share&igshid=1j6n5ntd27j0h
328 notes
·
View notes
Video
What is Cancer ?
Cancer is a group of diseases characterized by the uncontrolled growth and spread of abnormal cells. If left untreated, these cells can invade nearby tissues and organs and can spread throughout the body through the bloodstream and lymphatic system. Cancer can develop in almost any part of the body and can have many different forms, with different causes and treatments.
#chemistryonline #scienceandtechnology #biochemistry #biochemistrystudent #chemistry #organicchemistry #inorganicchemistry #science #sciencevideos #alcohol #scienceclass9 #cancer #cancertreatment #cancersurvivor #cancerawareness #scicomm #scienceblogs #chemistryvideos #chemistryonline #scienceandtechnology #chemistryvideos #chemistrynotes #sciencefacts #sciencenotes #explore #youtubeshorts #ype #vlog #sciencevideos #sciencevlogs #publichealth #medical
0 notes
Text
How to find best competitive coaching in patna
⟣°•°⟢ 𝐁𝐞 𝐎𝐝𝐝 𝐓𝐨 𝐁𝐞 𝐍𝐨. 𝟏 ⟣°•°⟢
𝐆𝐞𝐭 𝐀𝐝𝐦𝐢𝐬𝐬𝐢𝐨𝐧 𝐈𝐧 𝐅𝐨𝐮𝐧𝐝𝐚𝐭𝐢𝐨𝐧/𝐈𝐈𝐓 𝐉𝐄𝐄/𝐍𝐄𝐄𝐓.

𝐈𝐂𝐎𝐍𝐈𝐂 𝐂𝐋𝐀𝐒𝐒𝐄𝐒
Ph: +91 7903993958 & 8651259660
Email id: [email protected]
#IITJEE#medical#foundation#NEET#11th#12th#physics#physicsteacher#maths#mathematics#chemistry#biology#biologclass#MatheClass#target#TargetBatch#crashcourse#newadmission2022#NewAdmission#medicalcourse#iitjeecourse#organic#organicchemistry#inorganicchemistry #2023 #happynewyear#newyear2023
0 notes