#Open access Journals
Text
We're taking a little break from posting about Frankenstein to remind you that, in addition to offering the ability to read 100 articles online for free every month, JSTOR also has TONS of OA and free downloadable content, and we even have this little not-so-secret search bar you can use to find it.
471 notes
·
View notes
Text

through all the breaking and crisis she is the constant
#jonny bolduc#original poetry#image poem#image macro#public domain#smithstonian#open access journals
88 notes
·
View notes
Text
Open Access Journals
Open Access (OA) is a set of principles and practices surrounding the free and open sharing of research and other materials.
In academia, there is a growing push for academic journals to be open access for the sake of easy to share and compare of information.
The links below are two resources that exist to try to connect researchers to reputable journals.
Think. Check. Submit
https://thinkchecksubmit.org/
A resource that is meant to try to help researchers judge if a journal or publisher is reputable.
Directory of OA Journals
https://doaj.org/
A directory of many Open Access Journals. Journals have to request to be added to the directory and the directory have to apply to various standards to be included beyond simply being Open Access. Each journal has a page linking to a journal’s individual standards and more.
25 notes
·
View notes
Text

Full text of the news article below:
U.S. to require free access to papers on all
research it funds
The plan, to start at the end of 2025, is a blow to journal paywalls, but its impact on publishing is unclear.
By Jeffrey Brainard and Jocelyn Kaiser
[link]
A decadeslong battle over how best to provide public access to the fruits of research funded by the U.S. government has taken a major turn.
President Joe Biden’s administration announced yesterday that, by the end of 2025, federal agencies must make papers that describe taxpayer-funded work freely available to the public as soon as the final peer-reviewed manuscript is published. Data underlying those publications must also be made freely available “without delay.”
Many details of the new policy, including exactly how the government will fund immediate public access, remain to be decided. But it significantly reshapes and expands existing—and fiercely contested—U.S. access rules that have been in place since 2013. Most notably, the White House has substantially weakened, but not formally eliminated, the ability of journals to keep final versions of federally funded papers behind a subscription paywall for up to 1 year.
Many commercial publishers and nonprofit scientific societies have long fought to maintain that 1-year embargo, saying it is critical to protecting subscription revenues that cover editing and production costs and fund society activities. But critics of paywalls argue that they obstruct the free flow of information, have enabled price gouging by some publishers, and force U.S. taxpayers to “pay twice”—once to fund the research and again to see the results. Since the late 1990s, the critics have lobbied Congress and the White House to require free and immediate “open access” to government-funded research.
The Biden administration has heeded those pleas, although the new policy does not expressly embrace the term open access—it uses the words “public access.” It is “de facto an open-access mandate,” says Stefano Bertuzzi, CEO of the American Society for Microbiology (ASM), which publishes 16 journals. And many open-access advocates are applauding it.
“This is an enormous leap forward,” says Heather Joseph, executive director of the Scholarly Publishing and Academic Resources Coalition, one of the oldest open-access advocacy groups in the United States. “Getting rid of that embargo is huge.”
The embargo and related policies “were pure sellouts of the public interest,” tweeted molecular biologist Michael Eisen of the University of California, Berkeley, a prominent critic of U.S. access policies and co-founder of the PLOS journals, which have helped pioneer an open-access business model in which authors pay a fee to make their papers immediately free to all. “The best thing I can say about this new policy is that publishers will hate it.”
Many publishers say they support a transition to immediate public access but criticized the new U.S. policy. “We would have preferred to chart our own course to open access without a government mandate,” Bertuzzi says. Six of ASM’s journals are already fully open access, with the rest to follow by 2027.
The Association of American Publishers, a leading trade group, complained in a statement that the policy arrived “without formal, meaningful consultation or public input … on a decision that will have sweeping ramifications, including serious economic impact.” (White House officials say they met with large and small publishers over the past year to discuss the change.)
Others took a wait-and-see approach. Sudip Parikh, CEO of AAAS, which publishes the Science family of journals, says “it is too soon to tell if this guidance will impact our journals.” (AAAS publishes a fully open-access journal, Science Advances, and in 2021 its paywalled Science journals began to allow authors to deposit the peer-reviewed, almost-final version of manuscripts in institutional repositories on publication.)
The impact of the new requirement could vary depending on which of the more than 20 U.S. funding agencies underwrite the author’s research. Each agency must finalize its policy by the end of 2024 and implement it by the end of 2025.
The policy is not intended to mandate any particular business model for publishing, said Alondra Nelson, acting director of the White House Office of Science and Technology Policy (OSTP), in an interview with ScienceInsider. For example, it will not require federally funded researchers to publish only in pay-to-publish open-access journals. Researchers who publish in subscription journals might be able to satisfy the rule by depositing the almost-final, peer-reviewed, and accepted version into a public depository or other agency-approved outlet. Journals will still be able to keep their final, published version of a paper behind a paywall. (But some researchers say only the final published version is adequate for scholarly purposes. The not-quite-final, “author-accepted” versions might lack final editing, typesetting, and formatted data tables.)
Nelson says OSTP is acutely aware of concerns about who will pay the costs associated with the new policy, especially if publishing in a pay-to-publish journal becomes a widespread practice. Some fear the U.S. policy—combined with similar policies adopted in Europe and elsewhere—could accelerate the rise of such journals, ultimately making publishing more difficult for authors with modest or no grant funding, especially ones who work in underresourced institutions and in developing countries.
OSTP says in a blog post it wants “to ensure that public access policies are accompanied by support for more vulnerable members of the research ecosystem.” Agencies could, for example, allow researchers to use grant funds to cover open-access publishing costs—as some do already—or could fund the expansion of public repositories, Nelson says. “We’re not naïve about the challenges we face,” she says. “Implementation on any new policy is key.”
The new policy reflects the profound changes that have rocked academic publishing since the U.S. public access debate began in earnest more than 25 years ago. Then, subscription-based print journals were the primary means of disseminating research results, and publishers fiercely resisted any policy change that threatened an often highly profitable business model. But pressure from university libraries tired of paying rising subscription fees, and patient groups angry about having to pay to read taxpayer-funded biomedical studies, helped catalyze serious discussion of policy change. At the same time, the rise of the internet fueled publishing experiments, such as open-access journals and the posting of freely accessible “preprints” that have not been peer reviewed.
In Washington, D.C., these shifts prompted both Republicans and Democrats to urge the federal government to revise its access policies. In 2013, then-President Barack Obama attempted to strike a compromise—via the 1-year embargo rule—between publishers and open-access advocates.
But many—including Biden, then Obama’s vice president—were not happy with that deal. In a 2016 speech, for example, Biden noted, “The taxpayers fund $5 billion a year in cancer research, but once it’s published, nearly all of that sits behind [pay]walls. Tell me how this is moving the [scientific] process along more rapidly.”
The administration of former President Donald Trump also considered requiring immediate public access. And several developments in recent years increased the pressure for a revamp. In 2019, the U.S. National Cancer Institute’s “Cancer Moonshot” research program, which Biden helped create under Obama, required grantees to make papers developed with its funding free to read. In 2018, a group of European science funders called Coalition S unveiled a similar policy, which takes full effect in January 2025. (Coalition S imposes an additional requirement that publishers give up copyright; the existing and new U.S. policies do not.) And in 2020, publishers agreed to make all papers relevant to COVID-19 open access, at least temporarily.
Now, the new U.S. rules will apply to a substantial share of the world’s academic literature—and hundreds of thousands of new scholarly papers will become freely available to all with no delay. In 2020, OSTP estimates federal research funds produced 195,000 to 263,000 published articles, or some 7% to 9% of the 2.9 million papers published worldwide that year. And because the policy now applies to any federal agency that funds research—and not just those that spend $100 million or more annually—the free material could also include work funded by the national endowments for the arts and humanities. OSTP says agencies also could decide that the rule covers other materials, such as book chapters and conference proceedings, that are peer reviewed.
How the change will ultimately affect the finances of specific journals, publishers, and researchers is hard to predict, analysts say. In some journals, for example, just a small fraction of papers might be the product of U.S. funding. And university libraries might still be willing to pay subscription fees, even if their faculty can read the same papers elsewhere for free, if publishers offer a better interface, search functions, or other services.
Bertuzzi, however, says the new policy is likely to have a global impact that will be hard to ignore, because “the U.S. government is the 800-pound gorilla in the room.”
#at least its a step in the right direction#science#academia#open access journals#current events#science blogging
8 notes
·
View notes
Text
INNSpub | An Open Access Research Journal Publisher
INNSpub | An Open Access Research Journal Publisher
INNSpub stands for the International Network For Natural Sciences, INNSpub is an open-access scholarly research journal publisher. INNSpub is dedicated to publishing scholarly research Journals and Books in the English Language and believes in sharing new scientific knowledge in the field of Natural sciences, Biology, Agriculture, Biomedicine, Microbiology, and Genetics all over the world. All…

View On WordPress
#Agriculture#Biology#biomedicine#Genetics#innspub#Microbiology#Natural sciences#open access journals#Research Journal Publisher
5 notes
·
View notes
Text
Lupine Publishers | Palauamine and Olympiadane Nano Molecules Incorporation into the Nano Polymeric Matrix (NPM) by Immersion of the Nano Polymeric Modified Electrode (NPME) as Molecular Enzymes and Drug Targets for Human Cancer Cells, Tissues and Tumors Treatment under Synchrotron and Synchrocyclotron Radiations

Editorial
In the current editorial, we study Palau’amine and Olympiadane Nano molecules (Figures 1 & 2) incorporation into the Nano Polymeric Matrix (NPM) by immersion of the Nano Polymeric Modified Electrode (NPME) as molecular enzymes and drug targets for human cancer cells, tissues and tumors treatment under synchrotron and synchrocyclotron radiations. In this regard, the development of Chemical Modified Electrodes (CEMs) is at present an area of great interest. CEMs can be divided broadly into two main categories; namely, surface modified and bulk modified electrodes. Methods of surface modification include adsorption, covalent bonding, attachment of polymer Nano films, etc. Polymer Nano film coated electrodes can be differentiated from other modification methods such as adsorption and covalent bonding in that they usually involve multilayer as opposed to monolayer frequently encountered for the latter methods. The thicker Nano films imply more active sites which lead to larger analytical signals. This advantage coupled with other, their versatility and wide applicability, makes polymer Nano film modified electrodes particularly suitable for analytical applications [1–27].
Electrochemical polymerization offers the advantage of reproducible deposition in terms of Nano film thickness and loading, making the immobilization procedure of a metal–based electro catalyst very simple and reliable for Palau’ amine and Olympiadane Nano molecules–encapsulating Carbon nanotubes incorporation into the Nano Polymeric Matrix (NPM) by immersion of the Nano Polymeric Modified Electrode (NPME) as molecular enzymes and drug targets for human cancer cells, tissues and tumors treatment under synchrotron and synchrocyclotron radiations. Also, it must be notice that the nature of working electrode substrate in electro preparation of polymeric Nano film is very important, because properties of polymeric Nano films depend on the working electrode anti–cancer Nano materials. The ease and fast preparation and of obtaining a new reproducible surface, the low residual current, porous surface and low cost of Multi–Walled Carbon Nanotubes (MWCNTs) paste are some advantages of Carbon Paste Electrode (CPE) over all other solid electrodes [28–92].
On the other hand, it has been shown that, macrocyclic complexes of Palau’amine and Olympiadane Nano molecules– encapsulating Carbon nanotubes are interest as modifying agents because in basic media Palau’amine and Olympiadane Nano molecules–encapsulating Carbon nanotubes redox centers show high catalytic activity towards the oxidation of small organic anti-cancer Nano compounds. The high–valence species of Palau’amine and Olympiadane Nano molecules–encapsulating Carbon nanotubes seem to act as strong oxidizing agents for low-electroactivity organic substrates. 1,2–Dioxetane (1,2– Dioxacyclobutane), 1,3–Dioxetane (1,3– Dioxacyclobutane), DMDM Hydantoin and Sulphobe as the anti–cancer organic intermediate products of methanol oxidation as well as formic acid, is important to investigate its electrochemical oxidation behavior in Palau’ amine and Olympiadane Nano molecules-encapsulating Carbon nanotubes incorporation into the Nano Polymeric Matrix (NPM) by immersion of the Nano Polymeric Modified Electrode (NPME) as molecular enzymes and drug targets for human cancer cells, tissues and tumors treatment under synchrotron and synchrocyclotron radiations [93–110].
In this editorial, we decided to combine the above mentioned advantageous features for the aim of Palau’ amine and Olympiadane Nano molecules–encapsulating Carbon nanotubes incorporation into the Nano Polymeric Matrix (NPM) by immersion of the Nano Polymeric Modified Electrode (NPME) as molecular enzymes and drug targets for human cancer cells, tissues and tumors treatment under synchrotron and synchrocyclotron radiations. Furthermore, in this editorial, we prepared poly Nano films by electropolymerization at the surface of Multi-Walled Carbon Nanotubes (MWCNTs) paste electrode. Then, Palau’amine and Olympiadane Nano molecules–encapsulating Carbon nanotubes were incorporated into the Nano Polymeric Matrix (NPM) by immersion of the Nano Polymeric Modified Electrode (NPME) in a solution. The modifier layer of Palau’amine and Olympiadane Nano molecules–encapsulating Carbon nanotubes at the electrode surface acts as a Nano catalyst for the treatment of human cancer cells, tissues and tumors under synchrotron and synchrocyclotron radiations. Suitability of this Palau’amine and Olympiadane Nano molecules–encapsulating Carbon nanotubes–modified polymeric Multi–Walled Carbon Nano tubes (MWCNTs) paste electrode toward the electrocatalytic treatment of human cancer cells, tissues and tumors under synchrotron and synchrocyclotron radiations in alkaline medium at ambient temperature was investigated [111– 153].
For more information about Archive of Organic and Inorganic Chemical Sciences please click https://lupinepublishers.com/chemistry-journal/
For more Lupine Publishers please click on below link
https://lupinepublishers.com/index.php
#lupine#lupine publishers#archive of organic and inorganic chemical sciences#organic compounds#biomolecules#polymer chemistry#cluster compounds#chemical biology#mechanism of action#xenobiotic metabolism#lupine publishers llc#open access journals
4 notes
·
View notes
Text



what is your favorite noninvasive introduced species? mine is this lil guy, Apis mellifera, or the western honey bee. honey bees are so helpful for the environment, but they are such an interesting species of insect. western honey bees have evolved to have a highly complex social caste system that biologically determines the particular social niche an individual bee will fulfill.
interested in learning more? you may want to check out this open-access academic article:
Molecular determinants of caste differentiation in the highly eusocial honeybee Apis mellifera by Barchuk et al. 2007.
#florida#nature#naturalists#photography#florida wildlife#adventure#hippie#nature photography#bugblr#cool bugs#bugs#apis mellifera#western honeybee#entomology#entomologists#insects#open access journals#learning#environmental science#science#honeybee#knowledge#biology
3 notes
·
View notes
Text
Lupine Publishers | The Early Treatment of a Bodybuilder with Symptomatic Chronic Renal Failure with Intestinal Dialysis: A New Recommendation for Intestinal Dialysis Enhancement
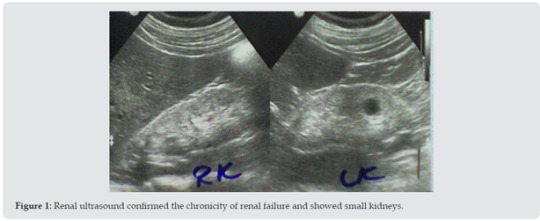
Abstract
Background: Dietary therapy aiming primarily at reducing the generation and accumulation of urea through protein restriction is the most important non-dialytic therapeutic intervention in the management of chronic renal failure. The use of a urea lowering agent “acacia gum” with protein restriction has been increasingly used as a new form of dietary dialysis which has been increasingly known as intestinal dialysis. Just like in other forms of dialysis, the use of conservative dietary and pharmacological measures is also necessary in intestinal dialysis.
Patients and methods: The early treatment of a bodybuilder with symptomatic chronic renal failure with intestinal dialysis is described, and the relevant literatures were reviewed with the primary of identifying the evidence that can contribute to enhancing intestinal dialysis.
Results: At about the age of 50 years (March, 2022), a professional bodybuilder who presented with progressive symptomatic uremia associated with nausea, vomiting, pruritus, and mild anemia. His weight was about 100 Kg, and before his current illness he reported that his bench press single maximum repetition was 140Kg. On the 19th of March, blood urea level was 162 mg /dL and serum creatinine was 6.2 mg /dL. Renal ultrasound confirmed the chronicity of renal failure and showed small kidneys. The conservative dietary (Acacia gum supplementation plus very low protein diet) and pharmacological managements were prescribed according to the latest published intestinal dialysis guidelines and included oral iron and folic acid capsule, and calcium carbonate. After two weeks, the patient was asymptomatic and blood urea was lowered to 126.4 mg/dL, and the hemoglobin was increased to 11g/d.
Conclusion: This is just another case to demonstrate that intestinal dialysis is effective in lowering blood urea level and improving symptoms in symptomatic chronic renal failure. There is a convincing evidence to support that the addition of essential amino acids and ketoanalogues in the management of chronic renal failure with intestinal dialysis can contribute to its enhancement.
Keywords: Symptomatic uremia; Intestinal dialysis; Ketoanalogues of essential amino acids.
Introduction
Dietary therapy aiming primarily at reducing the generation and accumulation of urea through protein restriction is the most important non-dialytic therapeutic intervention in the management of chronic renal failure. The use of a urea lowering agent “acacia gum” with protein restriction has been increasingly used as a new form of dietary dialysis which has been increasingly known as intestinal dialysis. Just like in other forms of dialysis, the use of conservative dietary and pharmacological measures is also necessary in intestinal dialysis [1-14].
Patients and methods
The early treatment of a bodybuilder with symptomatic chronic renal failure with intestinal dialysis is described, and the relevant literatures were reviewed with the primary of identifying the evidence that can contribute to enhancing intestinal dialysis.
Results
At about the age of 50 years (March, 2022), a professional bodybuilder who presented with progressive symptomatic uremia associated with nausea, vomiting, pruritus, and mild anemia. He was not havening reduction in urine output, edema or hypertension. His weight was about 100 Kg, and before his current illness he reported that his bench press single maximum repetition was 140Kg. On the 19th of March, blood urea level was 162 mg / dL and serum creatinine was 6.2 mg /dL. Urinalysis showed 2 plus albuminuria and one plus amorphous urate. Blood calcium and serum electrolytes were within normal ranges, but he had mild hyperphosphatemia with serum phosphorus of 4.9 mg/dL (Normal range 2.4-4.4mg/dL). Hemoglobin was 10.7 mg/dL (Normal ranges: 11.5-16.5 g/dL). Liver function tests were normal (Total serum bilirubin 0.8 mg/dL, Aspartate aminotransferase (SGOT) 25 iu/L, alanine aminotransferase (SPOT) 21 iu/L, alkaline phosphatase 284 iu/L).
He reported history of episodes of hyperglycemia that in the case of bodybuilders is generally attributed to growth hormone administration in excessive doses. However, the patient was reluctant to provide details about the performance enhancing medications such as anabolic steroids and growth hormone, and he was not confirming or denying the use of such agent. He was simply saying that he was taking protein supplements. Renal ultrasound (Figure 1) confirmed the chronicity of renal failure and showed small kidneys (RK: 8 x 4, cortex 6 mm, LK: 8.2 x 4, cortex 6 mm). The kidneys had hyper-echoic texture with reduced cortical thickness and loss of the cortico-medullary differentiation. There were small cysts on both kidneys, not more than 1.5 cm in diameter. Abdominal ultrasound also showed small polyp in the gall bladder and mild enlargement of the prostate with a volume of 27 cm3 (Normally up to 25). The patient initially required oral prochorperazine 5 mg for two days control the nausea and vomiting, and oral antihistamine plus topical crotamiton 10% to control pruritus. The conservative dietary (Acacia gum supplementation plus very low protein diet) and pharmacological managements were prescribed according to the latest published intestinal dialysis guidelines and included oral iron and folic acid capsule, and calcium carbonate. He also received oral finasteride 5 mg daily for the prostatic enlargement. After two weeks, the patient was asymptomatic and blood urea was lowered to 126.4 mg/dL and the hemoglobin was increased to 11g/d. Ultrasound showed normal prostate size of 20 cm3. Literature review suggested that the addition of essential amino acids and ketoanalogues in the management of chronic renal failure with intestinal dialysis can contribute to its enhancement. Therefore, Ketosteril (Fresenius), was prescribed in a low initial dose of three tablets, and was ordered to be brought to the patient from Turkey.
Discussion
Until now, there is no evidence to support that high protein diet per se can cause chronic renal failure. However, nephrocalcinosis caused exogenous vitamin D intoxication was reported to cause renal failure in a bodybuilder athlete [15]. Therefore, an accurate causation of the chronic renal failure cannot be determined. Carrero et al (2020) emphasized the importance and benefits of fruits and vegetables in patients with chronic renal failure. The intake of fruits and vegetables is associated with a higher fiber intake which can cause a shift in the gut microbiota towards reduced production of uremic toxins. The intake of fruits and vegetables is also associated with lower intake phosphorus, and thus help in controlling hyperphosphataemia [16]. However, the latest published intestinal dialysis guidelines have already suggested intake of fruits and vegetables [17]. The use of Keto-analogues of essential amino acids in the management of chronic renal failure has been reported as early as the 1970s (Walser, 1978; Bauerdick and colleagues, 1978, Giovannetti et al, 1980) [18]. Bauerdick and colleagues (1978) reported the use of nitrogen-free hydroxy and keto precursers of amino acids in the treatment of patients with chronic renal failure with essential amino acid and a low-protein diet was associated with a more positive nitrogen balance [19]. In 1980, Giovannetti et al treated twenty patients with advanced chronic renal failure with a low protein diet (0.2 g/kg/day hour vegetable proteins) and essential aminoacids and ketoanalogues. They reported that treatment was associated with a favorable outcome [20]. In 1981, Barsotti et al emphasized that treatment of chronic renal failure a very low protein diet plus essential amino acids and ketoanalogues is not associated with reduction of creatinine clearance, while treatment with hemodialysis and free protein intake is associated with reduction of creatinine clearance. They treated thirty-one patients with a conventional low-protein diet, and treatment was associated with a linear reduction of creatinine clearance. A thirteen patient treated with hemodialysis experienced significantly accelerated decline of creatinine clearance. However, only one of a twelve patients treated with a very low protein diet supplemented plus essential amino acids and ketoanalogues, experienced continued a continued reduction in creatinine clearance [21].
Mitch and colleagues (1982) described the treatment of 9 patients who severe chronic renal failure (mean glomerular filtration rate 4.8 ml/min; mean serum creatinine 11.3 mg/dl). They were treated with protein restriction (22.5 g daily of mixed quality protein) plus essential amino acids and keto-analogues of essential amino acids including tyrosine, ornithine, and a high proportion of branched-chain ketoacids, and very little methionine. Phenylalanine and tryptophan were not provided. One month of treatment was associated with significant lowering of serum urea nitrogen. Hyperphosphatemia which was observed in three patients, improved. Treatment was not associated with side effects. The treatment precluded the need for dialysis in patients with severe chronic renal failure who would otherwise need dialysis [22]. In 1983, Barsotti et al described the treatment of 48 patients with chronic uraemia for a maximum of 36 months with low protein diet plus essential amino acids and keto-analogues. Ten patients experienced reduction of renal function and required dialysis.
Eight patients experienced difficulties in complying with treatment and also required dialysis. Three died for causes that are not directly related to renal failure. 27 patients continued with treatment without important reduction in renal function, and enjoyed satisfactory subjective and objective states without evidence of protein malnutrition or unwanted effects [23]. In 1985, Barsotti et al reported that the treatment of men who had uremia with a low protein diet plus essential amino acids and ketoanalogues was associated with restoration of testosterone levels in blood [24]. In 1985, Ciardella et al described the treatment of eighty-five patients with chronic renal failure with a vegetarian low-protein, low-phosphorus diet plus essential amino acids and ketoanalogues. Treatment was associated with marked reduction of serum triglycerides in the 61 men, but the reduction was not significant in woman. When the patients were later treated by maintenance hemodialysis without dietary restrictions, the experienced elevations in serum triglycerides levels which was attributed to the loss of the effect of the dietary restriction on uremic male hypogonadism [25].
Conclusion
This is just another case to demonstrate that intestinal dialysis is effective in lowering blood urea level and improving symptoms in symptomatic chronic renal failure. There is a convincing evidence to support that the addition of essential amino acids and ketoanalogues in the management of chronic renal failure with intestinal dialysis can contribute to its enhancement.
Conflict of interest
None.
For more Lupine Journals please click here: https://lupinepublishers.com/index.php
For more Journal of Urology & Nephrology Studies articles please click here: https://lupinepublishers.com/urology-nephrology-journal/index.php
#lupine publishers#articles#urology#submission#lupine journals#journal of urology & nephrology studies#open access journals#nephritis#nephrology#juns
0 notes
Text
Enteritis: Still a Problem in Dairy Calves

Abstract
The neonatal phase of calves is a phase that needs extra care due to newborns’ vulnerability. Enteritis - an inflammation of the intestinal mucosa, resulting mainly in diarrhea - stands out among the conditions that affect animals in this period. Enteritis are responsible for huge losses in cattle breeding, especially in the early stages of rearing. Besides the losses caused by mortality, there are also expenses with veterinarians, treatments and decreased performance of the animal throughout its productive life. The present study aimed to perform a review of diarrhea in newborn calves.
Keywords: Neonatal diarrhea; Infectious agents; Dairy cattle
Abbrevations: ETEC: E. coli enterotoxigenic; EHEC: E. coli enterohemorragic; BVDV: Bovine Viral Diarrhea Virus
Introduction
The neonatal period in cattle - that goes from birth to 28 days of age - is especially important from a health point of view, since approximately 75% of losses in young calves occur in this phase [1], and the first week of life is considered the most critical phase, with 50% of losses. Therefore, maintaining the health of calves is highly related to the hygiene of the place where they live, as they are extremely sensitive to environmental pathogens [2]. Lorenz [3] report that there are several measures to maintain calf health from birth to weaning, including the provision of good quality colostrum in adequate quantity in the first hours after birth and the need to emphasize the prevention of diseases of the gastrointestinal tract and respiratory system. Among the main conditions that cause loss in the early stages of calves development are pneumonia, malformations, central nervous system diseases, and enteritis [4]. Enteritis is clinically mainly manifested by diarrhea and stands out due to its high mortality rate [2,3,5,6], since it is commonly difficult to recover because it is almost always accompanied by malnutrition [7].
Diarrhea is a complex multifactorial disease involving animal, environmental, nutritional, and infectious agents and it is a major cause of mortality, morbidity, and economic loss in cattle worldwide [8], because the treatment of affected calves is slow and impacts on growth, weight gain to weaning and loss of genetic potential of recovered animals [9]. Due its clinical and economic importance and due the preventive measures are often neglected, it is necessary an approach on this subject, to broaden the knowledge and to promote a better conduct regarding the prevention, diagnosis and treatment of the affected animals. Therefore, the present study aimed to review diarrhea in newborn calves.
Diarrhea in Newborn Ruminants
Newborn calf diarrhea is a disease of great impact on the economic viability of cattle herds worldwide [10] (Table 1). The economic impact caused by this condition is significant, although many new intervention strategies, such as vaccine development drug development and herd management, have been developed and implemented to minimize it [2]. In this sense, the veterinarian needs to assess the status of immunoglobulins in calves, feeding, shelter, environmental disinfection, hygiene and sanitary management, to prevent neonatal deaths caused by the disease [11]. The processes involved in the pathophysiology of diarrhea are related to intestinal secretion/ hypersecretion, nutrient bad absorption and digestion, osmolarity, abnormal intestinal motility, increased hydrostatic pressure, and gastrointestinal inflammation [12-21], which may occur singly or, more commonly, by the combination of two or more factors of these mechanisms [22,23].
Secretory diarrheas occur due to abnormal stimuli to the intestinal mucosa crypts that may be caused by the action of enterotoxins and/ or the action of inflammation mediators such as prostaglandins, causing an imbalance in physiological processes, like secretion and intestinal resorption, with consequent diarrhea [24]. Diarrhea is typically profuse without blood or effort, and signs in affected calves include depression, weakness, and sometimes shock and death secondary to hypovolemia and mild acidemia [25]. The difference in osmolarity with increased concentration of solutes within the intestinal lumen, promotes greater absorption of water by the lumen, thus resulting in dehydration of the animal. Osmotic particles include poorly digested disaccharides and increased levels of D-lactate from bacterial fermentation of unabsorbed nutrients entering the colon. Reduced intestinal transit time can lead to poor digestion and malabsorption due to inadequate time for digestion and absorption of ingested food, impaired fluid resorption has a major impact on fluid balance [23].
When a calf has diarrhea, there is a huge loss of fluids and electrolytes from its body. Thus, the consequent dehydration and the appearance of metabolic acidosis are the main causes of death of these animals [26]. This happens partly because the evaluation of the animal is generally based only on clinical examination, and a more detailed approach to assessing the degree of electrolyte disturbance and acidosis through blood gas analysis is lacking or not [27]. Although this condition being common in rural properties, treatment is usually inadequate and / or insufficient, because the administration of antibiotics and anti-inflammatory drugs do not correct the hydroelectrolytic disorders and acid-base [28]. Therefore, in order for the recovering of the animal, these parameters must be measured and corrected quickly, enabling the return to homeostasis. The high frequency and persistence of calf neonatal diarrhea has attracted the interest of many researchers. The multifactorial etiology (bacteria, viruses and protozoa) influenced by nutritional and environmental factors, as well as difficulties in the precise diagnosis of the agent and the failure of treatment has required the adoption of prophylactic measures, such as cow hygiene, management and vaccination [8].
Diarrhea Infectious Agents
Diarrhea is a condition of complex multifactorial etiology, influenced by infectious, nutritional and environmental factors, as well as improper management practices. Causes include toxins, bacteria, protozoa, viruses, and management / environmental factors such as overfeeding, low temperature, poor hygiene, colostrum deprivation, and individual susceptibility of the animal [8]. Numerous infectious agents have been implicated in diarrhea of calves, such as Escherichia coli, Salmonella spp., Cryptosporidium spp., Rotavirus and coronavirus. Coinfection is commonly seen in diarrheal calves, although a single primary pathogen may be the cause in some cases. The non-infectious causes of origin are related to improper management and poor hygiene of the environment in which the animals are placed. The incidence of the disease may vary according to the geographical location of the farms, farm management practices and herd size [2]. Rotaviruses, coronaviruses and cryptosporides, the most commonly recognized enteric pathogens of calves, all produce intestinal villi atrophy, intestinal bacterial overgrowth, malabsorption, and osmotic diarrhea [25].
In general, infections caused by viruses and protozoans tend to damage the intestinal mucosa promoting alteration in intestinal absorption due to damage to intestinal cells, compromising the normal absorption of nutrients, fluids and electrolytes, without alteration in intestinal secretion [22]. Rotaviruses are the most common cause of diarrhea in newborn calves and are often involved in co-infections with other agents [11,23,25]. Clinical signs usually appear 1 to 3 days after infection lasting 5 to 9 days [23]. High environmental contamination, herds with high numbers of animals and management that favors the transmission of the agent, associated with an inexpressive immunization rate, provide favorable conditions for the spread of rotavirus in dairy herds in Brazil, justifying the prevalence and difficulty to control the infection and the spread of the virus [28]. The incidence of many etiological agents varies with the calf’s age (Table 2) and this is useful for establishing the probability of a particular agent being involved and it is generally impossible to establish a definitive field diagnosis [11].
Diarrhea may result from hypersecretion or decreased absorption. Enteropathogenic strains of E. coli are occasionally causing diarrhea in calves [29]. Enterotoxigenic E. coli, Salmonella spp, Campylobacter spp. and rotavirus cause diarrhea by secreting enterotoxins that stimulate increased intestinal secretions, while protozoa and enteric viruses cause epithelial destruction of the absorptive cell villi. Enterotoxigenic E. coli produces profuse watery diarrhea, mainly in calves older than 4 days of age and occasionally in older calves. The F5 antigen may produce a mild clinical syndrome characterized by diarrhea, dehydration and weakness in calves from 1 to 4 days of age with rapid course and may progress from healthy to decubitus and death from 6 to 12 hours [11]. Salmonella spp. is an important causative agent of diarrhea and septicemia in dairy calves and the depression caused in the animal is probably due in part to endotoxemia, not just dehydration and acidosis. Campylobacter jejuni and Campylobacter fecalis are believed to be of minor importance in calves and lambs [11].
Cryptosporidium is cited as the main agent of diarrhea in calves, not only as an opportunistic agent, but also as a primary agent. Preventive measures should be taken related to the management of cows at the time of giving birth, avoiding the agglomeration of animals and environmental contamination to reduce economic losses, and to avoid the risks to public health arising from infection [24]. The recognition of enteropathogens guides the adoption of effective prevention and control measures, besides alerting to public health reflexes, due to the zoonotic potential of several of these enteric pathogens [29,30].
Treatment
Physical examination of the diarrheal calf comprises the first step in establishing the therapeutic approach, requiring the determination of the presence of any intercurrent disease. Treatment of simple cases depends on the estimative of dehydration (Table 3), severity of acidosis, likelihood of concomitant infection, presence or absence of hypothermia and hypoglycemia [11]. The most common causes of death are dehydration and acidosis. Blood gas analysis will accurately determine the degree of metabolic acidosis [29] (Table 4). Therefore, the immediate goal in treating depressed calves is to restore them to physiological systemic status. The estimated severity of dehydration can be combined with estimates of diarrhea loss and maintenance of essential functions to manage total daily fluid requirement [11,29].
Abbreviations: pCO2, carbon dioxide pressure; pO2, oxygen pressure; HCO3-, plasma bicarbonate concentration; TCO2, total carbon dioxide in plasma; BE, base excess in the blood; StB, standard bicarbonate blood concentration; SatO2, blood oxygen saturation. Fonte: Lisbôa et al. [31]. Replacement may be administered intravenously or orally, reminding that for the latter one should be increased by 60 to 80% for partial fluid absorption [11,29]. If performed early in the disease, oral replacement can be highly effective and inexpensive. In animals with severely impaired intestinal motility, the intravenous way may be more effective in correcting hydroelectrolytic imbalances than oral administration [23]. Success of therapy is monitored based on clinical signs of calf and restoration of urination [11]. Another point to consider in chronically diarrheal calf is the need for nutritional support. When a samll quantity of milk or solid food is ingested, energyrich oral electrolytes may be used to maintain the body condition of the animal. Stop giving milk can reduce the severity of diarrhea and depression in severe diarrhea, because malabsorption exacerbates diarrhea by the osmotic effect of unabsorbed milk nutrients and also promotes bacterial proliferation and possibly poor fermentation generating organic acids. However, stop giving milk reduces weight gain [11].
Antibiotic use is frequent in the treatment of diarrhea, although few agents respond to antimicrobials, viral and parasitic agents are not directly sensitive to antibiotics. Their indiscriminate use promotes the selection of resistant strains and complicates future therapeutic efforts. However, they can attenuate clinical disease, decrease the release of pathogens to the environment and animal mortality [11,29]. Some treatment protocols include the use of anti-inflammatory drugs to help reduce the secretory effects of some agents [11]. The use of non-steroidal anti-inflammatory drugs (NSAIDs) should be restricted in dehydrated animals and administered only when the patient is sufficiently hydrated [23]. The use of probiotics, oligosaccharides and intestinal protectors is also cited, and the use of gastrointestinal motility modifiers is contraindicated, as the reduction in motility will lead to the accumulation of bacteria and pathogenic toxins [29].
Prevention
The principles of prevention are based on ensuring adequate colostral intake, specific help and nonspecific immunity, reduction of the possibility of introduction / dissemination of infectious agents [11]. Colostrum is important in preventing morbidity and mortality of diarrheal calves. Colostral antibody is responsible for the low incidence of rotavirus infections in calves under 4 days of age. Vaccination of pregnant cows is important to increase colostral immunity. Colostrum privation, lack of maternal instinct, and early separation of cow and calf are major causes of failure to transfer immunity in dairy calves [11]. Prophylactic measures include separating calves from each other with enough space to prevent contact and infection through contaminated feces and urine. All feeding facilities and equipment (buckets and bottles) must be maintained with strict hygiene conditions. There is not much difference between the patterns of disease development and the prevention of calf diarrhea according to each etiological agent. Knowledge of the causal pathogen (s) is important to accurately avaliate the current status of the affected property and to develop new interventions [2].
To Know More About Journal of Dairy & Veterinary sciences
Please click on: https://juniperpublishers.com/jdvs/index.php
For more Open Access Journals in Juniper Publishers
please click on: https://juniperpublishers.com/index.php
#wildlife management#wild life rehabilitation#wildlife diseases#dairy microbiology#Juniper Publishers#open access journals
0 notes
Text
Influence of oligochitosans and highly molecular chitosan on Lactobacillus bulgaricus cultivation

Abstract
It was established that decrease of oligochitosans with molecular masses 7.0, 25.4, 45.3 kDa concentration in the process of Lactobacillus bulgaricus cultivation leads to fermented dairy product pH reduction and titratable acidity increase. Further increase in titratable acidity and decrease of lactic acid microorganisms’ amount was determined during the fermented dairy product storage process. Oligochitosans with molecular masses 7.0, 25.4, 45.3 kDa in concentrations interval from 0.0025 to 0.01 per cent did not exhibit prebiotic properties. Active acidity elevation and titratable acidity depression was observed at the chitosan with molecular mass 350 kDa concentration rises. Also increase of highly molecular chitosan concentration leads to elevation of lactic acid microorganisms’ total amount, which was more than three degree as many as total count of lactic acid bacteria in control sample.
Keywords: Chitosan; Oligochitosan; Lactic acidbacteria; Lactose, Lactic acid fermentation; Lactic acid
Introduction
Starters of the Lactobacillus bulgaricus species pure cultures are widely used for manufacturing of functional fermented dairy products with dietary and health-promoting properties. The prospective way of fermented milks production technological development is enrichment with chitosan [1-3]. Chitosan is a biogenic heteropolymer consists of N-acetylglucosaminaine and glucosaminresidues [2,4]. Chitosan has high molecular mass and soluble in organic acids [5,6]. Low-molecular derivatives of chitosan are represented byolygochitosans with a molecular mass from 2 to 50 kDa, which are well soluble in water. Chitosan and olygochitosans are able to interact with Lactobacillus bulgaricus cells by a different mechanism depending of their molecular mass [7-9]. Teichoic acid negatively charged molecules of lactic acidbacteria cells are capable to multi-point ion binding with positively charged high-molecular chitosan, whereas their cytoplasmic membrane proteins interact with oligochitosans [4,9]. The consequence of this process may be a change in metabolic processes in lactic acid bacteria cells. The goal of research was to study the effect of different concentrations of high-molecular chitosan and oligochitosans with varying molecular mass on lactic acid fermentation process driven by Lactobacillus bulgaricus.
Materials and Methods
Targets of research were skim milk, starter culture of lactic acid bacteria Lactobacillus bulgaricus (producer: Dairy Plant “Stavropolsky”, Russia), chitosan with a molecular mass of 350 kDa and a 95 per cent degree of deacetylation (manufacturer: “Bioprogress LLC”, Russia). Oligochitosans with molecular masses of 7.0, 25.4, 45.3 kDa and 96 per cent degree of deacetyration was prepared by the previously described technique [5]. Dry skim milk was reconstituted to a dry mass concentration of (10 ± 0.2) % by dissolving in distilled water at temperature 30 to 35 °C. Reconstituted skim milk after recombination was characterized by the following parameters: mass concentration of fat 0.15 per cent, mass concentration of protein 3.2 per cent,mass concentration of lactose 5 per cent. The solution of chitosan with molecular mass 350kDa in 2 per cent concentration lactic acid aqueous solution with mass concentration 1 per cent was added into skim milk experimental samples for preparation of mixture with final concentration of chitosan 0.0025, 0.005, 0.0075 and 0.01 per cent respectively. Similar experiments were carried out using oligohitosans with molecular masses of 7.0, 25.4, 45.3 kDa in above mentioned concentrations. The starter culture of Lactobacillus bulgaricus was inoculated in the amount of 3 per cent of the total samples volume after pasteurization of the mixture and cooling to the fermentation temperature of (43 - 45) °C. The end of the fermentation process was determined by organoleptic curd density, as well as by titratable and active acidity. Experimental and control samples were stored during 17 days at 4 ± 2 °С after completion of fermentation process. Following parameters were tested in triplicate during storage of control and experimental samples: pH by potentiometry, titratable acidity by titrimetric analysis and total count of lactic acid bacteria (CFU per gram).
Results and Discussions
The effect of highly molecular chitosan and oligohitosans with a molecular weight of 7.0, 25.4, 45.3 kDa various concentrations on fermented dairy products physical and chemical properties during the cultivation of Lactobacillus bulgaricus and long-term storage process was studied.
As shown in Tables 1 &2, decrease in the concentration of oligochitosans leads to significant decrease in pH and increase of titratable acidity after 20 hours of cultivation.
This is explained by the fact that oligohitosans with molecular masses of 7.0, 25.4, 45.3 kDa in concentrations of 0.0025 and 0.005 percent effectively interact with the proteins of the lactic acid bacteria cytoplasmic membrane. This interaction induces bacterial stress [10]. Consequently, lactose enzymatic hydrolysis and lactic acid production are accelerated resulting in titratable acidity increase. The elevation of oligohitosans concentration leads to promotion of their interaction with bacterial cells teichoic acid molecules. This type of interaction influences on lactic acid bacteria cells cytoplasmic membrane permeability and as a result inhibit rate of lactose metabolism. Highly molecular chitosan concentration variation did not lead to significant changes of pH and titratable acidity of fermented skim milk in comparison with control samples. Chitosan with a molecular mass of 350 kDa puts into effective multi-point ion binding with negatively charged teichoic acid molecules of Lactobacillus bulgaricus cells. This is due to the presence into highly molecular chitosan structure of about 1850 amino groups. Lactose assimilation and lactic acid formation rates are changed depending on highly molecular chitosan concentration.
Physical and chemical properties of fermented dairy products during long-term storage at 4 ± 2 °С were studied after the completion of the Lactobacillus bulgaricus cultivation process. It was established that optimal organoleptic attributes (taste and odor) of fermented product control sample are achieved after 5 days of storage at pH 4.2 - 4.5 and titratable acidity 70 - 140 °T. Organoleptic attributes of this product deteriorated during the further storage.
As shown in Table 3, optimal titratable acidity of fermented milks experimental samples containing oligochitosans at a concentration of 0.01 per cent persisted for up to 17 days. Further increase of titratable acidity of experimental samples containing oligochitosans at a concentration 0.0025, 0.005 and 0.0075 per cent was observed during the storage after the completion of the fermentation process.
Decrease in titratable acidity of fermented dairy product experimental samples was detected when concentration of chitosan with molecular mass 350 kDa increased in interval from 0.0025 to 0.01 percent. Therefore high-molecular chitosan concentration elevation reduces the intensity of lactic acid fermentation in experimental samples. The most powerful process of lactose homo fermentative fermentation inhibition occurred in a sample containing high-molecular chitosan in concentration of 0.01 per cent. The decrease of lactose assimilation intensity by Lactobacillus bulgaricus cells may be propelled by two reasons. The interaction between chitosan molecules and lactic acid bacteria cells cytoplasmic membrane leads to disturbance of membrane permeability for β-galactosidase enzyme, which catalases the reaction of lactose into glucose and galactose hydrolysis. At the same time structural changes in cell cytoplasmic membrane cause retardation of lactose hydrolysis products active transport into bacterial cells.
Thus, there is an inhibition of lactic acid formation in the process of fermented dairy product containing high-molecular chitosan storage, which stimulates the preservation of a large number of lactic acid bacteria. This is confirmed by the data of lactic acid microorganisms ‘quantitative accounting in control and experimental samples after 17 days of storage, as shown in Table 4.
The data presented in Table 4 indicates that oligohitosans with molecular masses of 7.0, 25.4, 45.3 kDa did not affect significantly on Lactobacillus bulgaricus grows rates during fermented dairy products storage process. Addition of highly molecular chitosan in concentrations of 0.0075 and 0.01 per cent in fermented milks increased the content of lactic acid microorganisms,which was more than three degree as many as total count of lactic acid bacteria in control sample.
Thus, tested samples ofoligohitosans with varying degrees of polymerization did not exhibit prebiotic properties and did not prolong the shelf life of fermented dairy products. High-molecular chitosan in a concentration of 0.01 per cent can be recommended as a prebiotic, prolonging the shelf life of fermented milks, manufactured with application of Lactobacillus bulgaricus starter cultures.
To Know More About Nutrition and Food Science International Journal
Please click on: https://juniperpublishers.com/nfsij/index.php
For more Open Access Journals in Juniper Publishers
please click on: https://juniperpublishers.com/index.php
#diabetes nutrition#food biotechnology#food toxicology#Mass spectrometry in food technology#Juniper Publishers#open access journals
0 notes
Text
Omega-3 Polyunsaturated Fatty Acids, Metabolic Syndrome and Diabetes Mellitus

Authored by Victoria Serhiyenko
Abstract
Omega-3 polyunsaturated fatty acids (ω-3 PUFAs) are increasingly being used to prevent cardiovascular diseases (CVD), and cardiac societies recommend the intake of 1g/day of the two ω-3 PUFAs eicosapentaenoic and docosahexaenoic acid for primary and secondary prevention of CVD. Clinical trials clearly suggest beneficial effects of ω-PUFAs consumption on lipid metabolism profile, their anti-inflammatory actions; on endothelial activation, which are likely to improve vascular function; antithrombotic and antiatherosclerotic properties. Experimental studies demonstrate direct antiarrhythmic effects, which have been challenging to document in humans. By targeting arterial stiffness and endothelial dysfunction administration of ω-3 PUFAs may prevent atherosclerosis and CVD development. A synergistic interplay showed by ω-3 PUFAs prescription suggest the potential to beneficially impact on fundamental steps involved in the development of preclinical atherosclerosis. We reviewed available evidence of the benefits of ω-PUFAs administration, especially to patients with CVD, metabolic syndrome and type 2 diabetes mellitus, including their effects on potential molecular pathways, effects on glucose and lipids metabolism parameters, thrombocyte aggregation parameters and haemostasis, endothelial function, antioxidant/anti-inflammation and antiarrhythmic properties.
Keywords: Omega-3 polyunsaturated fatty acids; Coronary heart disease, atherosclerosis; Diabetes mellitus; Glucose, lipids; Inflammation; Platelets; Haemostasis; Endothelium; Heart rate variability; Arrhythmias; Arterial stiffness
Abbrevations: ω-3 and ω-6 PUFAs: Ω-3 and ω-6 Polyunsaturated Fatty Acids; MetS: Metabolic Syndrome; T2DM: Type 2 Diabetes Mellitus; CVD: Cardiovascular Diseases; DLP: Dyslipoproteinemia; OS: Oxidative Stress
Go to
Introduction
Numerous studies report salutary effects of ω-3 polyunsaturated fatty acids (ω-PUFAs), i.e. eicosapentaenoic (EPA) and docosahexaenoic acid (DHA) on cardiovascular diseases (CVD) risk factors. These effects include lowering of serum triglyceride (TG) by reducing of hepatic TG production; lowering of blood pressure (BP) by improving of endothelial cell functution; decreasing of platelet aggregation by reducing of prothrombotic prostanoids; decreasing inflammation via reduction in 4-series leukotrienes (LT) production; protection from arrhythmias by modulation of electrophysiological properties of cardiac myocytes. Systematic meta analysis suggests that high doses of ω-3 PUFAs (~3g/day) produce a small, but significant decrease in systolic blood pressure (SBP) in older and hypertensive subjects [1,2]. The aim of this study was to review the latest evidence about the ω-PUFAs, metabolic syndrome (MetS) and type 2 diabetes mellitus (T2DM).
Go to
Discussion
Ω-3 and ω-6 PUFAs are essential fatty acids, as they cannot be synthesized de novo in humans. There are limited data available regarding the exact amount of dietary ω-3 PUFAs consumed by the general population. It is reported that the total daily intake of dietary ω-3 PUFAs in the US is approximately 1.6g. Of this α-linolenic acid (α-LLA) accounts for approximately 1.4g/q.d, and only 0.1–0.2g/q.d. comes from EPA and DHA. The conversion rate from α-LLA to EPA and DHA is variable (0.2-15%). Therefore, in general, the total amount of EPA and DHA available to the body from current dietary patterns is well below the recommended amounts. EPA and DHA didn’t show a significant negative effect on glucose metabolism [3].
Several experimental studies have shown that long-chain ω-PUFAs inhibit the absorption of cholesterol in the intestine and its synthesis in the liver, lead to increased clearance of lipoproteins in the blood, prevent the development of insulin resistance (IR) in experimental diabetes, increase the level of glucose transporter 4 in skeletal muscles, have a positive effect on age related decrease of blood flow in the brain and improve glucose utilization under stress; there isn’t any influence on the development of hypertension (HT) and MetS. Ω-3 PUFAs decrease level of BP, dose-dependent prevent the development of T2DM, IR, contribute to positive changes of blood coagulation parameters; enhance endothelial cell migration and inhibits the proliferation of smooth muscle cells [4]. A meta-analysis of 18 studies found a significant effect of fish oil to lower TG concentrations and increase high-density lipoprotein cholesterol (HDL-C) in the blood; while there were no statistically significant changes in preprandial glucose, glycated hemoglobin A1c, total cholesterol, low density-lipoprotein cholesterol levels. Ω-3 PUFAs may affect the IR and glucose homeostasis by inhibition of IR in the muscle tissue >adipose tissue >>liver, inhibition of insulin secretion, which defer the development of T2DM; and on the state of lipid metabolism (in particular, reduce the concentration of TG, very low density-lipoprotein cholesterol (VLDL-C), increase of HDL-C, improve lipid profile by mixed hyperlipidaemia (HLP), slightly decrease BP, improve endothelial function, have an positive impact on the antioxidant status and inflammatory reactions [5]. Ω-3 PUFAs decrease VLDL assembly and secretion, resulting in diminished TG production, through a decreased sterol receptor element binding protein-1c activity [6,5].
The highly concentrated pharmaceutical preparation Omacor™ (Pronova Biocare, Lysaker, Norway), known as Lovaza™ (Glaxo Smith Kline, St Petersberg, FL, US) in North America is approved by the FDA as an adjunct to diet to reduce very high TG levels (≥500 mg•dL-1) in adults. Each 1-g capsule of ω-3-acid ethyl esters contains ethyl esters of EPA (0.465 g) and DHA (0.375g). Patients take a q.d. dose of 4-g or two 2-g doses (two capsules b.i.d.) [7]. Clinical trials have shown that administration of 4 g•day-1 of Lovaza™ results in a decrease in TG levels of 30-50%; does not affect the efficacy of statins [8,5]. In patients with combined HLP, co-administration of Lovaza™ with statins was a safe and effective means of lowering serum TG, despite the persistent high TG levels when the patients received statins alone [9,5].
The anti-inflammatory actions of marine ω-3 PUFAs are [10]: reduced leucocyte chemotaxis (via decreased production of some chemoattractants (e.g. leukotriene B4 down-regulated expression of receptors for chemoatttactants); reduced adhesion molecule expression and decreased leucocyte-endothelium interaction (via down-regulated expression of adhesion molecule genes [via the nuclear factor kappa B (NF-kB) (i.e. peroxisome proliferator-activated receptor-ɣ (PPAR-ɣ) etc.); decreased production of eicosanoids from arachidonic acid (AA) (via lowered membrane content of AA; inhibition of AA metabolism); decreased production of AA containing endocannabinoids (via lowered membrane content of AA); increased production of ‘weak’ eicosanoids from EPA (via increased membrane content of EPA); increased production of anti-inflammatory EPA and DHA containing endocannabinoids (via increased membrane content of EPA and DHA); increased production of pro-resolution resolvins and protectins (via increased membrane content of EPA and DHA); decreased production of inflammatory cytokines (via down-regulated expression of inflammatory cytokine genes (via NF-kB, i.e. PPAR-ɣ etc.); decreased T cell reactivity (via disruption of membrane rafts (via increased content of EPA and DHA in specific membrane regions).
Ω-3 PUFAs may decrease the risk of atherothrombosis by affecting platelet aggregation and haemostasis. The antithrombotic properties of EPA and DHA have been attributed to the incorporation into platelet phospholipids at the expense of the ω-6 PUFAs, such as AA. An important set of pathways clearly influenced by changes in the ω-3/ω-6 ratio are those for synthesis of eicosanoids. These include the cyclooxygenase (COX), lipoxygenase and cytochrome P450 epoxygenase pathways, for which EPA and DHA compete with AA as a substrate, inhibiting the production of the proaggregatory thromboxane A2 (TXA2) originating from AA. Indeed, the production of TXA2 from platelets stimulated by a variety of agonists decreased by between 60% and 80% after fatty acid supplementation both in vitro and in vivo [11,5]. The mechanism by which ω-3 PUFAs influence endothelial function is mediated by their incorporation into biological membrane phospholipids; this allows modulation of membrane composition and fluidity. The reason lies in the fact that endothelial cell membrane houses caveolae and lipid rafts where several receptors and signaling molecules crucial for cell function are concentrated [12]. Caveolae-associated receptormediated cellular signal transduction includes important pathways such as the, the nitric oxide (NO)/cyclic guanosine monophosphate signaling pathway, the nicotinamide adenine dinucleotide phosphate oxidase and tumor necrosis factor-α/ NF-kB induced COX-2 and prostaglandin E2 activation pathway. By modulating the composition of caveolae, as described for other classes of lipids ω-3 PUFAs may exert their beneficial effects, which include increased NO production and reduced production of proinflammatory mediators [13,12]. In addition to increasing NO production, ω-3 PUFAs decrease oxidative stress.
The incorporation of ω-3 PUFAs in synaptic membranes could potentially influence the autonomic control of the heart. Both nervous tissue and heart tissue have a high content of ω-3 PUFAs (especially DHA) and this may be consistent with the finding that this marine ω-3 PUFAs may modulate cardiac autonomic function as assessed by heart rate variability (HRV) [14]. Thus, ω-3 PUFAs may modulate HRV both at the level of the autonomic nervous system and the heart. Most of the data support that ω-3 PUFAs beneficially modulates cardiac autonomic control thereby possibly reducing the risk of arrhythmias. Accumulating evidence from in vivo and in vitro experiments has demonstrated that ω-3 PUFAs exert antiarrhythmic effects through modulation of myocyte electrophysiology. Ω-3 PUFAs reduce the activity of membrane Na+ channels in cardiomyocytes, thus increasing the threshold for membrane potential depolarization. EPA and DHA also modulate the activity of L-type Ca2+ channels, leading to a reduction in free cytosolic Ca2+ ion, which stabilizes myocyte electrical excitability to prevent fatal arrhythmia. EPA blocks the Na+/Ca2+ channel; however, a single amino-acid point mutation in this channel attenuated the inhibitory effect of EPA. These findings suggested that the cardioprotective effect of ω-3 PUFAs is mediated by direct interaction with membrane ion channels [15].
Ω-3 PUFAs intake has shown to reduce BP especially in HT by interacting with several mechanisms of BP regulation: reduction of stroke volume and heart rate; improvement of left ventricular (LV) diastolic filling; reduction of peripheral vascular resistances; improvement of endothelial-dependent and endothelial-independent vasodilation (stimulation of NO production; reduction of the asymmetric di-methyl-arginine; reduction of endothelin-1; relaxation of vascular smooth muscle cells; metabolic effects on perivascular adipocytes; endothelial regeneration. Mechanisms of HT-related organ damage protection: anti-inflammatory, antioxidant, and antithrombotic effects; reduction of arterial stiffness; experimental effects on LV hypertrophy and abnormal gene expression; effects on atherosclerotic plaque progression and stability [7]. Ω-3 PUFAs offer a scientifically supported means of reducing arterial stiffness and this may account for some of the purported cardioprotective effects of ω-3 PUFAs [16,17].
Go to
Conclusion
The antiarrhythmic effects of ω-3 PUFAs, which occur by blocking various ion channels, are encouraging. So, cardiovascular benefits of ω-3 PUFAs [7,18] are: antidysrhythmic effects (reduced sudden death; possible prevention of atrial fibrillation; possible protection against pathologic ventricular arrhythmias; improvement in HRV; antiatherogenic effects (reduction in non- HDL-C levels; reduction in TG and VLDL-C levels; reduction in chylomicrons; reduction in VLDL and chylomicron remnants; increase in HDL-C levels; plaque stabilization; antithrombotic effects (decreased platelet aggregation; improved blood rheologic flow); anti-inflammatory and endothelial protective effects (reduced endothelial adhesion molecules and decreased leukocyte adhesion receptor expression; reduction in proinflammatory eicosanoids and LT’s; vasodilation); decreased SBP and diastolic BP. Thus, further research to understand the mechanism of action and confirm the beneficially effect of ω-3 PUFAs on BP profile, artery stiffness and HRV parameters in patiens with MetS, T2DM is needed.
To Know More About Current Research in Diabetes & Obesity Journal Please click on:
https://juniperpublishers.com/crdoj/index.php
To Know More About Open Access Journals Please click on: https://juniperpublishers.com/index.php
0 notes
Text

Leah Perry presents a feminist history of Riot Grrrl and Kathleen Hanna to explore the hope and the limits of an individualist revolution in the 1990s. Perry concludes that shamelessness might remain a promising space for an urgent anti-racist, feminist politics if it can work to destabilize power and center women from oppressed groups.
Open access article — free to everyone, no login required.
#riot grrrl#kathleen hanna#bikini kill#punk rock#feminism#1990s music#open access journals#research#academic research#jstor
233 notes
·
View notes
Text
Expect the Unexpected with Erector Spinae Plane Block in Spine Surgery - Plan for the Worst and Hope for the Best: An Anesthesiologist Perspective-Juniper Publishers
Abstract
Spine surgery is associated with multiple postoperative complications, ranging from simple nausea and vomiting to devastating complications leading to postoperative morbidity or mortality. The postoperative neurological impairment, especially in the neurologically intact patient, is a dreadful event that makes it difficult for the surgeon to perform technically challenging or high-risk spine surgeries. Preoperative or intraoperative factors that can influence the postoperative neurological status include nature and the severity of the pathology, comorbid conditions of the patient, preexisting neurological symptoms, multiple levels involved, complex surgery or instrumentation, surgical blood loss, neurological monitoring, hemodynamic parameters, polypharmacy, and total duration of the surgery.
In addition to several known contributing factors (fixation failure, epidural hematoma, spinal cord edema, and ischemia-reperfusion injury), the role of the erector spinae plane block (ESPB) has recently been cited as a potential cause of postoperative transient paralysis after spine surgery. ESPB is considered a simple and safe regional anesthesia technique that may have an advantage in success rate and analgesic efficacy when used as an adjunct to general anesthesia in spine surgeries. Despite varied patterns of the drug spread, ESPB has been showing promising results due to consistent involvement of dorsal rami that supply all pain generators of the spine surgeries.
The potential role of ESPB in causing postoperative transient neurological complications is a diagnosis of exclusion that requires thorough clinical assessment and step-by-step evaluation using imaging modalities. Before administering ESPB in spine surgery, essential knowledge includes anatomical and technical considerations, drug distribution patterns, safe and effective volumes/types of local anesthetics, and possible associated complications. This review article describes the possible roles of all factors that lead to postoperative neurological impairment and suggests some tips and tricks for using ESPB in spine surgeries to prevent or manage such serious complications appropriately.
Keywords: Transient paraplegia; Erector spinae plane block; ESP block complications; ESP block in spine surgery; Paraplegia due to RA
Keywords: RA: Regional anesthesia; GA: General anesthesia; ESPB: Erector spinae plane block; ERAS: Enhanced recovery after surgery; LA: Local anesthetics; CT: Computed tomography; MRI: Magnetic resonance imaging; ESM: Erector spinae muscles; TP: Transverse process; SMPB: Sacral multifidus plane block; RLB: Retrolaminar block
Introduction
The occurrence of perioperative complications may be inevitable, but their prevention and management are always a shared responsibility of all team members involved. Thorough evaluation of such complications will help develop strategies to prevent and manage the same in the future. A systematic and stepwise approach is warranted before categorizing it as a surgical or anesthetic complication. Several interventions have been introduced in the surgical and anesthetic techniques to improve patient safety and satisfaction. Application of regional anesthesia (RA) alone or as an adjunct to general anesthesia (GA) is one such advance that helps reduce many polypharmacy-related side effects or complications. If a particular complication-reduction modality is inherently causing complications, it requires a comprehensive understanding of the situation and its contributing factors.
An erector spinae plane block (ESPB), a safe and simple RA technique, has shown promising results as an adjunct to multimodal analgesia in various orthopedic, general, thoracic, abdominal, obstetrics, and spine surgeries. In addition to its superior postoperative analgesic profile in spine surgeries at various levels, ESPB reduces hospitalization costs and the possible side effects of extensive anesthetic use. Since opioids have been linked to tumor recurrence [1,2], ESPB also reduces the risk of spine tumor recurrences by significantly reducing its consumption. ESPB meets all criteria suitable for enhanced recovery after surgery (ERAS) protocol [3] by facilitating early discharge and mobilization of patients. Being a novel RA technique, not many complications have been reported so far except for some anecdotal reports of bilateral quadriceps weakness, transient apathy or aphasia, minor neurological complications due to inadvertent intravascular injection of local anesthetics (LA) [4].
Recently, it has been described as a potential cause of transient paralysis after spine surgeries [5]. Therefore, it is essential to understand the differential diagnoses of postoperative neurological impairment, follow the step-by-step approach to rule them out one by one, determine the possible role of ESPB in their development, and learn the tricks for safely administering ESPB during spine surgery. This review article elaborates the essential background knowledge required before and after the administration of ESPB in spine surgeries.
Discussion
Postoperative neurological impairment after spine surgery in a neurologically intact patient is always daunting for the operating surgeon and the patient. Several common theories on neurological deterioration after decompressive spine surgeries include vascular compromise, hypotension, ischemia, direct trauma, or stretching of the neural elements. The major contributing factors of acute paralysis following spine surgery include fixation failure, epidural hematoma, spinal cord edema, and ischemia‑reperfusion injury [6].
Contributory factors
Neurons in the spinal cord are susceptible to ischemia and hypoxia. The mechanisms of spinal cord ischemia are multi-factorial and multi-channel. The pathogenesis of spinal cord lesions after spine surgeries is usually mechanical (pressure) damage via extensive hematoma or edema, resulting in pressure on the spinal cord leading to ischemic damage [7]. An altered cerebrospinal fluid flow dynamic may also cause cord compression [8]. In either case, the ultimate pathogenic cause is a secondary cellular injury due to the disruption of ionic homeostasis, development of free radicals, lipid oxidation, and degeneration of the cytoskeleton [7]. White cord syndrome, an imaging feature of spinal cord ischemia [9], is diagnosed as high intramedullary signal changes on sagittal T2 weighted MRI scans and is often seen in surgeries on the cervical spine.
The spinal infarct is one of the leading causes of paraplegia or quadriplegia in patients with preexisting vascular pathologies (thrombosis) or embolic events during surgery [10]. The anterior spinal cord has a higher risk of ischemia due to fewer anterior spinal artery feeding vessels [10] than the highly vascular posterior spinal cord due to anastomotic pial vessels. The sparing of the posterior column leads to unchanged intraoperative somatosensory evoked potentials [11]. The ischemia-reperfusion injury occurs upon restoring the blood flow to previously ischemic tissues and organs. Increased inflammatory cytokines such as TNF α and IL 1β may be considered vital indicators for evaluating decompression-associated spinal cord ischemia-reperfusion injury [12,13]. Its reported incidence is 2-5.7% following cervical and 14.5% following posterior thoracic decompression surgeries [14, 15].
Transient paralysis is one such complication that manifests itself as a temporary (up to 72 hours) loss of sensations, movements, anal reflexes, and sphincter function below the affected spinal segments [16]. It can occur after vertebroplasty, laminectomy, or thoracic decompressive procedures [17,18]. The longer duration of symptoms, multiple compression sites, and the high degree of preoperative stenosis are considered poor prognostic factors [18].
Who is the culprit?
The exact cause of the postsurgical neurological impairment is a diagnosis of exclusion requiring thorough clinical evaluation and imaging guidance to rule out each contributing factor (Table 1) in a step-by-step manner. Postoperative radiographic studies like computed tomography (CT) scan and magnetic resonance imaging (MRI) can help detect changes suggestive of misplaced implants, hematomas, edema, compressive lesions, white cord syndrome, or direct trauma to the spinal cord. Symptoms due to spinal cord edema typically occur at 48-72 hours post-surgery and may be relieved by anti-edema measures like fluid restriction [19].
The occurrence and severity of ischemia-reperfusion injury correlate with tissue ischemia time, the extent of ischemic tissue, and the oxygen requirement of the affected tissue [20]. The presence of deep tendon and superficial reflexes may rule out the possibility of hysterical paraplegia [18]. After excluding all contributing factors that may cause postoperative neurological impairment, the possible role of ESPB and LA can be considered and further evaluated. It requires an understanding of the anatomical and technical aspects, mechanism of drug spread, factors favoring neuraxial spread, and measures to avoid such incidents in the future [21].
Role of ESPB
ESPB involves depositing the local anesthetic solution between the erector spinae muscles (ESM) and the transverse process (TP) under ultrasound guidance. The ESM consists of three muscles: iliocostalis, longissimus, and spinalis. They arise from and insert into various bony components of the vertebral column [22] and form a paraspinal column that extends from the sacrum to the base of the skull. It gradually tapers upwards in the paravertebral groove on either side of the spinous processes. The retinaculum (thoracolumbar fascia in the lumbar region) that envelops this muscular column also facilitates the LA spread to several thoracic and lumbosacral levels [23]. The diverse multilayered fascial arrangement deep to the ESM may cause the inconsistent LA spread, resulting in multisegmented sensory block mainly involving dorsal rami with sometimes ventral rami.
This Para neuraxial block, when given bilaterally in spine surgery, can be advantageous in success rate and analgesic efficacy [24]. The absence of risks such as hypotension, vascular spread, or pneumothorax makes ESPB relatively safer than epidural anesthesia or paravertebral block. Bilateral ESPB offers effective perioperative analgesia without influencing the hemodynamic parameters. It significantly reduces the perioperative opioid requirements in spine surgeries at various levels (cervical, thoracic, and lumbar, and sacral) [25-32]. Its outcome depends on the volume and concentration of LA used, drug spread, and the anesthesiologist’s experience in selecting and locating the correct level of the TP.
The exact mechanism of action of the ESP block and pattern of the drug spread is still unclear. It has been suggested to anesthetize the spinal nerves by passing through the costotransverse foramen of Cruveilhier, accompanying the dorsal ramus and artery to the paravertebral space [33]. The deposited drug can spread in any direction, such as craniocaudal, anterior-posterior, and lateral-medial planes to reach the paravertebral space, neural foramina, epidural space, or sympathetic chain [34-38]. Fluoroscopic, CT, and MR imaging in living subjects have similarly confirmed the injectate tracking to the paravertebral area, intervertebral foramina, and epidural space following lumbar ESPB [39-42]. There is also a possibility of LA diffusion through the microscopic gaps in the mostly acellular architecture of interlinked collagen fibers of the fascia covering the erector spinae muscle [43].
ESPB at various spine levels
The anatomical differences at the various spine levels can cause varied drug spread and ultimately affect the outcomes of ESPB. Cervical ESPB is technically challenging due to the difficulty in identifying the tips of the cervical transverse processes due to their shorter length. It is mainly given at the C6 or C7 vertebral level. The probe needs to be kept anterolaterally rather than posteriorly to see the cervical TPs [44]. It may not be safe due to its proximity to the neuraxis (shorter transverse processes) and the possibility of bilateral phrenic nerve involvement [45-48].
Thoracic ESPB at the upper vertebral levels (T2 orT3) can be preferred in cervical spine surgery by inserting the needle from caudal-to-cranial direction to achieve the desired LA spread and avoid technical difficulties and complications associated with cervical ESPB. Thoracic ESPB can provide multilevel analgesia even with the small volumes of LA due to rigid boundaries of the thoracic paravertebral spaces that facilitate drug spread at several levels involving ventral and dorsal rami. Lower thoracic level ESPB is mainly performed for lumbar spine surgeries by inserting the needle from cranial-to-caudal direction to achieve the desired LA spread and avoid technical difficulties associated with lumbar ESPB [49,50].
The lumbar ESPB can also be technically challenging due to the increased thickness of the ESMs with their tendinous attachment to the TPs [51, 52] and increased corresponding depth of the intermuscular plane in the lumbar region. The psoas muscle is also closely adherent to the vertebral bodies and the anterior surface of the TPs. The anterior drug spread to include ventral rami may be compromised due to the lack of clear boundaries of lumbar paravertebral spaces [53]. There is a communication through the fat-filled plane between the ESM and TP with the fat-filled psoas compartment containing lumbar nerve roots and plexuses. The spread of LA to the epidural space is possible through this communication [54]. The compressed lamina and the ligaments of the lumbar spine favor LA spread more into the epidural space [55, 56]. Thus, the lumbar ESPB may result in either lumbar plexus block or epidural anesthesia. The resultant weakness in the quadriceps or lower extremity muscles depends on the LA concentration and volume used in ESPB.
Sacral ESPB is mainly described for gender reassignment surgery or perineal surgery [57-61]. Its application for lower lumbar or sacral spine surgery is yet to be determined. The sacral multifidus plane block (SMPB), one of the variants of the paraspinal block, involves the deposition of LA in the plane under the multifidus muscle and bony area between the median and intermediate crests of the sacrum. The possible mechanism of action of SMPB includes blocking the dorsal rami and medial cluneal nerves directly by LA deposition and ventral rami by anterior LA spread through dorsal and ventral sacral foramina. The SMPB may also block the pudendal nerve (S2–S4), lumbosacral plexus, and sciatic nerve via the anterior and cranial LA spread [61, 62].
The role of LA
The possible role of the LA used in ESPB in causing postoperative neurological compromise depends on its inadvertent spread into either the epidural or subarachnoid space. It can be determined based on the occurrence and recovery pattern of the neurological symptoms. Distal-to-proximal and motor-before-sensory recovery patterns are the hallmarks of the differential blockade of the LA [23]. Inadvertent spread of LA into the subarachnoid space can lead to severe hypotension and bradycardia, resulting in unstable intraoperative hemodynamics. The consequences of the epidural spread depend on the density of LA around the spinal nerves, which could be compromised in a subsequent surgical dissection affecting the potentiality of the epidural space.
The concentration of LA, which determines the mass of the drug, also affects the efficacy of any block. The deliberate use of LA in low concentrations can result in a preferred motor-sparing analgesic effect of such high-volume blocks [63, 64]. Bupivacaine and ropivacaine are the most commonly used LAs for bilateral ESPB. Both LA agents consistently display preferential blockade of C-fibres (slow pain) > A-delta fibers (fast pain) > A-beta fibers (touch/pressure) in both preclinical and clinical studies [64-66]. With the increasing concentration, these agents may result in loss of proprioception and loss of motor function. Lipid solubility and higher pKa of LA facilitate intraneural diffusion and ion channel blockade. Ropivacaine exhibits a relative motor-sparing effect due to its lower lipid solubility than bupivacaine [67]. Twenty milliliters of 0.375% ropivacaine is recommended for each side of the bilateral ESPB in adults [68, 69].
Technical aspects of ESPB
Unexpected outcomes like a neurological compromise can be correlated with possible technical errors while administrating ESPB. The first technical aspect is identifying the correct landmark under ultrasound depending on the surgical extent and the desired level of the block. It may further depend on the sonoanatomy quality and the experience of the anesthetist. Sometimes misidentifying the lamina as the tip of the TP can lead to the retrolaminar block (RLB), another variant of the paraspinal block. In RLB, the needle insertion is slightly medial, targeting the lamina of the vertebra instead of the tip of the TP. It works via diffusion of LA into the paravertebral space through the soft tissue gaps between adjacent vertebrae [70]. Both RLB and ESPB were consistently associated with the posterior spread of injectate to the back muscles and fascial layers [37].
Fluoroscopic-guided ESPB can lead to RLB due to the inability to see the tip of TP clearly like under ultrasound, resulting in deposition of the LA solution over the lamina. The proximity of the RLB to the neuraxis can lead to a high probability of epidural spread, which carries the risk of motor weakness. The second important aspect is the ergonomics associated with bilateral ESPB. Administering the bilateral ESPB by standing on only one side of the patient may result in deviation from the ideal needle trajectory on one side compared to the other. Therefore, technical considerations should focus on stabilizing the needle by one person, injecting LA by another person, and performing such bilateral blocks while standing on either side.
The third important aspect includes technical modifications such as keeping an ultrasound probe in a transverse view to help differentiate intramuscular drug spread from the effective linear drug spread between ESM and TP [71]. The fourth aspect is finding alternatives that involve dorsal rami consistently without causing drug spread to other unwanted areas. The thoracolumbar interfacial plane block is one such alternative that targets only the dorsal rami of the spinal nerve. Thus, it can provide more focused dermatomal coverage of the back required for thoracic and lumbar spine surgeries [72, 73]. However, its efficacy in spine surgeries is yet to be determined. We have suggested some tips and tricks for using ESPB in spine surgeries (Table 2), keeping all technical aspects in mind.
Conclusion
Postoperative neurological impairment following spine surgery is a serious concern for the operating surgeon and the patient. The role of ESPB in causing such complications is the diagnosis of exclusion made after a thorough evaluation of clinical symptoms and radiological studies. For that, understanding of various mechanisms involved in ESPB leading to neurological impairment is essential. It should encourage the anesthetists to take extreme precautions while administering this novel block, considering the anatomical differences at various spine levels. Surgeons should anticipate and explain the possibility of neurological deterioration while explaining the risks and benefits of the proposed surgical intervention. Intraoperatively, real-time neurophysiological monitoring is recommended as a useful tool to avoid further neurological deterioration, especially in extensive and multilevel surgeries or in high-risk and neurologically compromised patients.
After identifying or diagnosing such complications, intensive care and regular checking of spinal function are of great importance, along with simultaneous radiological workups to rule out various causative factors. Once paralysis occurs, early diagnosis and early intervention are essential in restoring spinal function. Despite the rare possibility of such complications, ESPB is still a promising option for ensuring effective perioperative analgesia in spine surgeries. It helps reduce postoperative morbidity by keeping the hemodynamic parameters stable and significantly reducing intraoperative blood loss. It can also avoid postoperative complications that lead to delay in mobility and discharge by significantly reducing the need for opioids and polypharmacy. However, further studies are needed to determine the safe concentration and volume of the LA solution used in ESPB, the exact surgery-specific vertebral level to cover desired surgical innervations, and the accurate LA deposition site to prevent spread to undesired areas.
To Know More About Journal of Head Neck & Spine Surgery Please click on:
https://juniperpublishers.com/jhnss/index.php
For more Open Access Journals in Juniper Publishers please click on:
https://juniperpublishers.com/index.php
0 notes
Text
Exosomal Consignment in Renal Allograft Injury

Abstract
Exosomes are small mobile endocytic vesicles (30-120nm), shredded by every cell to conduct trafficking of cell generated cargo. They are found in almost all body fluids (blood, csf, saliva, urine). These include proteins, lipids, DNA, mi(cro)RNAs etc. In multicellular organisms, they are packaged into numerous vesicles mainly in exosomes to conduct their transport for various cellular activities which can be exploited clinically. Presently the survival of renal allograft is monitored by mostly invasive methods (tissue biopsy, Creatinine, GFR) where curving the injury is quite difficult. Hence potency of molecular markers like proteins and then circulating miRNAs came to picture for early detection of renal injury (Acute Kidney Injury-AKI and Chronic Kidney Disease-CKD). However, due to lack of specificity of circulating miRNAs lose their feasibility and the discovery of these exosomal cargos in cellular communication has become an efficient tool for treatment of various complicated clinical condition including renal allograft injury.
Keywords: micro RNAs,exosome, Renal Allograft Injury
Go to
Introduction
Exosomal world: a prologue
Exosomes are membrane bound mobile vehicles that are found in almost all circulating body fluids like- blood, CSF, saliva, urine, etc. These are responsible for transport of respective cellular cargo to extracellular target sites [1]. Recent studies with exosomes have revealed that exosomal cargo delivery has many important biological, physiological and pathological significance thus, can be an effective diagnostic tool for various diseases [2]. Exosomes are small circulating units of 30-120 nm in diameter, generating from late endosomal compartments of cells by its cell membrane invagination or budding or released as shedding vesicles. Cellular cargos include proteins, lipids, DNAs, mRNAs, miRNAs, etc [1]. The exosomal cell membrane mainly constitute a limiting lipid bilayer, transmembrane proteins and a hydrophilic core containing proteins, mRNAs and microRNAs (miRNAs).
Exsosomes were first discovered by Pan and Johnstone in 1983 [3] when they found that the release of transferrin receptors into extracellular space during sheep reticulocyte maturation was released inside a type of small vesicles. In 1989 Johnstone regarded these mammalian cargo delivering vesicles as exosomes [1-5]. Valadi et al. in 2007 first described about the composition of exosomes that apart from proteins and lipids these also contains DNAs and RNAs [6] which are recorded in ExoCarta database [7,8] . The exosomal cargo delivery requires stimulation of target cell which may be direct by receptor mediated interactions or may aid in transport from cell of origin to various bioactive molecules e.g. membrane receptors, proteins, lipids, mRNAs, miRNAs, etc [7]. When exosomes deliver its contents into the respective target sites the property and behavior of these cells changes to a great extent [8]. It is also understood from various studies done in last couple of years that miRNA composition of parent cell and exosomal components vary a lot [8] and of all the components, miRNAs have drawn the attention due to their regulatory role in gene expression as these are protected against RNAase-dependent degradation [1-8]. Thus exosomal cell-to-cell communication influence both physiological as well as pathological environment of the body. These play important roles in immune reactions, tumorigenesis and in neurodegenerative disorders [1]. e.g. in prostate cancer, ovarian cancers, lymphoma glioblastoma, etc [1].
Biogenesis
Exosomes are formed from late endosomal compartments of cells through endosomal sorting complex required for transport (ESCRT-that recognizes ubiquitylated proteins) to deliver the cargo to target cell or to fuse with lysosomes to degrade the undesired contents [1]. Earlier these exosomes were only considered to be “garbage bags” as their diversified capabilities were unknown then. But now these are the most emerging field of research. The way of formation and secretion of these vesicles from mutlivesicular bodies (MVBs) are of two types [9]:
Microvesicles, which are directly shed from cell membrane.
Exosomes, which are released by exocytosis when MVBs fuse with plasma membrane.
Exosomes can be identified by transmission microscopy as a cup-shaped morphology with negative staining [10-12]. These can be concentrated in 1.10-1.21 g/ml section of a sucrose density gradient [10-12]. Exosomes can be identified by various protein markers e.g. tetraspanin proteins- CD63, CD9, CD81, HSP70 and HSP90, etc [1, 8]. ExoQuick (a one-step precipitation procedure for exosome extraction), Immuno affinity capture, Immunobead (EpCAM), combination of EpCAM and ultracentrifugation, size exclusion chromatography and EpCAM and followed by Quantitative PCR, Microarray techniques for extraction and quantification of exosomes [1,8,13].
Exosomes formation and secretion requires enzymes and ATP. First the cell membrane is internalized to produce endosomes. Subsequently, many small vesicles are formed within endosomes by invagination of its cell membranes [8, 14]. Such endosomes are called MVBs. Finally, the MVBs fuse with endosomal cell membranes to release intraluminal vesicles into extracellular space which become exosomes [14].
The secretion or cell-to-cell communication of exosomes requires certain regulatory factors which were first identified by Ostrowski et al. who observed that Rab27a and Rab27b were associated with exosomal secretion [8]. It was also found that effectors of Rab27- SYTL4 and EXPH5 could also inhibit exosomal secretion in HeLa cells [15]. Also Yu et al. discovered that tumor repressor protein p53 and its downstream effector TSAP6 were required for influencing exosome secretion [16]. Another working group, Baietti et al. observed the importance of syndecan-syntenin which directly interact with ALIX protein via Leu-Tyr-Pro-X(n)-Leu motif in secrection of exosomes by endosomal budding [17]. Koumangoye et al. found that disruption of lipid rafts in exosomal membranes could inhibit its internalization in breast cell carcinoma cell line [18]. Trafficking of exosomes to target sites occurs in following mechanisms:
The transmembrane proteins of exosomes directly interact with signaling receptors of target cell membranes [19].
The exosomal fusion with plasma membrane of recipient cells to deliver the cargo into their cytosol [20].
The exosomes internalization into recipient cells have two fates[21].
in one, some exosomes are engulfed by the cell and may merge with the cell’s endosome and undergo transcytosis
in other case, engulfed exosomes fuse with endosomes and mature into lysosomes for degradation.
As per ExoCarta database records, of all the components proteins and miRNAs have been exploited for various research to correlate some application with different diseased state that could render some remedy. Due to the regulatory role of miRNAs in gene expression these are used as recent area of research as diagnostic tool [8,22]. Goldie et al. demonstrated that among small RNAs, the percentage of miRNAs is higher in exosomes than in parent cells [23]. Studies done with exosomalmiRNAs shows there are specific sorting mechanisms for miRNAs into exosomes. These are:
The neural sphingomyelinase 2 (nSMase-2)-dependent pathway by Kosaka et al. [24].
The miRNA motif and sumoylated heterogeneous nuclear ribonucleoproteins (hnRNPs)-dependent pathway by Villarroya- Beltri et al. [25].
The 3’ end of the miRNA sequence-dependent pathway by Koppers-Lalic et al. [26].
The miRNA induced silencing complex (miRISC)-related pathway and human AGO2 protein [27].
In short there are specific sequence in miRNAs as well as enzymes and proteins that guide them for their sorting into exosomes [8]. Exosomes are shed by cells during both normal as well as pathological conditions. Thus several studies have been made with exosomes in diseased states.
A brief sketch on therapeutic exosomal cargos:
Exosomal miRNA: miRNAs are the recent findings in the field of clinical research which are thought to be crucial in depicting human health and diseases. These biomarkers can also be an indicator for rejection onset of transplanted allograft. miRNAs are a class of small 18-25 nucleotide (nt) long endogenous, noncoding RNAs which play an important role in regulating gene expression [28,29]. A single miRNA has the ability to regulate expression (mostly silencing) of hundreds of mRNAs and have been known to play important role in many cellular functions that include induction of post-translational repression, mRNA degradation/silencing and transcriptional inhibition, cell development, differentiation, proliferation and functional regulation of immune response among others [28-31].
The mystery behind the functional maturation of miRNAs has been solved by research in last couple of years. Similar to mRNAs, miRNAs are also initially transcribed in the nucleus [32]. miRNAs are at first transcribed in nucleus as primary transcript by RNA polymerase II called pri-miRNA [32-35]. This pri-miRNA has a hairpin stem-loop structure (~80nt length) that contains the mature miRNAs [36]. The pri-miRNA processing include recognition of the stem loop followed by its cleavage by DROSHA (a class 2 ribonuclease III) and DGCR8 (a miRNA-processing multiprotein complex) to release pre-miRNA [32-35]. Pre-miRNA is then recognized by Exportin-5 which allows its exports to cytosol for further maturation into 19-25 nucleotide strands by RNA endonuclease III called Dicer [32- 35, 37]. Cleavage of this pre-miRNA by Dicer result in double stranded (ds) RNA molecule of which one of the single strand with more unstable 5’ base pairing is selected and transferred to an Argonaute (AGO) protein and the other strand is degraded [35,38,39]. The selected strand induces silencing of mRNAs through RNA Induced Silencing Complex(RISC) thus affecting various cellular functions like cell differentiation, proliferation as well as development and functional regulation of immune system [32-35,40]. In normal tissues, RISC remain as a low molecular weight entity with reduced regulatory activity while under stressed or replicating conditions these become high molecular weight units with intensified regulatory activity when bound to mRNA [36]. Thus mRNA silencing by miRNAs results in lower protein levels in the body [36,41].
ExosomalProteins: Proteins are the building blocks of life in all living organisms. These are amino acid chains linked by peptide bonds. They are exquisite necessity in every cellular events, may it be the formation of new cells or cell repair. Thus, can be an important biomarker in depicting biological changes. Emerging research have exploited this idea and conducted various proteomic studies. A more burning concept is ofexosomal proteins. The work done and data obtained shows that besides miRNAs another important exosomal load isexosomal proteins. TrairakPisitkun et al had worked on urinary biomarkers and found that urinary exosomal proteins can also be an efficient protein biomarker in reporting renal tubulopathies and other renal disorders [42]. Exosomes normally found in urine accounts for around 3% of the total urinary protein contents and isolation of these exosomes can result in very large enrichment of urinary proteins derived from renal tubular epithelial cells [42]. The exosomal packaging occurs when the apical membrane proteins undergo endocytosis and packaged into MVBs. These MVBs undergo encapsulation of cytosolic proteins into small vesicles. Finally outer membrane of MVBs fuse with apical plasma membrane releasing exosomes containing both membrane and cytosolic proteins [42]. The proteomics study with LC-MS/MS coupled with upstream one dimensional SDS-PAGE separation experiments had disclosed a number of proteins associated with exosome biogenesis like class E vacuolar protein sorting (VPS), ALIX, Aquaporin 1, Aquaporin 2, ESCRT, etc [43]. A total of 295 proteins of urinary exosomeswere found to be associated with renal diseases and hypertension. These have been enlisted in Urinary Exosome Protein Database [42]. In another experiment where polypeptides were considered reflect that β2- microglobulin could be an indicator of damage of renal proximal tubule cells [42,44]. The techniques used to evaluate exosomal protein change is carried out by two dimensional difference in gel electrophoresis and change proteins are identified by mass spectroscopy and validated by Western Blotting [45]. Zhou et al worked with Fetuin-A, a protein of liver as an important exosomal protein that can indicate occurrence of AKI(Acute Kidney Injury) [45].
Go to
Early Molecular Biomarkers for Renal allograft status
Years of research with renal allograft injury for either Acute Kidney Injury (AKI) or Chronic Kidney Disease (CKD) suggest that instead of invasive detection of allograft status there are scopes for early and non-invasive detection of injury through molecular markers. The studies made at the molecular level have disclosed the fact that acute and chronic rejections to a transplanted graft at preliminary stage can be ascertained by alteration in levels as well as expressions of various molecular markers involved in signaling of graft injury. These can be measured from blood/urine samples of patients. In acute rejection the early pathological change is defined by Ischemia-Reperfusion Injury (IRI) where altered expression of various miRNAs [46] is observed 3-7 days post-injury [47]. At later stage when rejection is in progress changes in levels of miR-210,-10a and -10b as well as some proteins (like perforin, granzyme A andB mRNA, FAS Ligand, FOXP3, etc) are observed [48]. Chronic rejection in early graft injury is generally associated with Interstitial Fibrosis and Tubular Atrophy (IF/TA). Pathophysiology of IF/ TA is the deposition of Extracellular matrix (ECM) proteins and Epithelial-Mesenchymal Transition (EMT) which can be stimulated by Transforming Growth Factor beta (TGF-β)/Smad signaling cascades. Ample of literature suggest that TGF-β/ Smad signaling can cause up-regulation and down-regulation of various miRNAs (miR-21,-192 & miR-29 and -200 families under IF/TA conditions) [49,50]. Even though these biomarkers have provided fruitful information but they lack specificity and their cellular source is unknown since they circulate. So to get a much clearer picture of a particular injured cell research at molecular level have unrevealed the next generation biomarker –exosomalmiRNAs for early, specific and non-invasive detection. Moreover their cellular source is also defined so they can deliver exact status of a particular cell [1,8].
Go to
Urinary Exosomal proteins and miRNAs in renal allograft injury as Next Gen Molecular Biomarkers
Studies done with renal diseases is pretty less and still a burning area of research that reveals the fact that urinary exosomal proteins as well as miRNAs can be a potential therapeutic tool for kidney and associated diseases [1,8].
The urinary exosomal proteins can be easily attainable by noninvasive means for diagnosis of ESRD as well as Urinary Tract Infection (UTI) [1]. In 2006 Zhou et al. reported that urinary exosomal protein- fetuin A was found to be increased in AKI (Acute Kidney Injury) patients in ICU than AKI patients in normal care [1,41,45]. In 2008, same group discovered that activating transcription factor-3 (ATF-3) was found in exosomes isolated from AKI patients than CKD patients or control [41,45,51]. Thus suggesting urinary exosomal proteins could be a diagnostic tool. Moreover, a reduced level of urinary exosomal aquaporin-1 has been observed in ischemia-reperfusion injury in rats [7]. ExosomalmiRNAs demonstrate potential diagnostic biomarker for renal fibrosis [8]. MiR-29c and CD2APmRNA [52,53] were observed in urinary exosomes of renal fibrosis patients. The findings by Stefano Gatti1 et al. showed that bone marrow derived Mesenchymal Stem Cells (MSC) Microvesicles (MV) when administered immediately after IR injury can prevent AKI and CKD in rats [8,54] through their paracrine actions. Tara K Sigdel et al have described that in AKI patients with perturbation exosomal proteins like CLCA1, PROS1, KIAA0753 were observed. In addition to that exosomal ApoM is more than soluble ApoM [55]. M.W. Welker group found that in patients with chronic Hepatitis C serum soluble exosomal CD 81, a surface protein marker is elevated [56]..
Go to
Future Prospective and limitations
Lots of work have been done with circulating miRNAs but due to their less specificity with the exact status of injured tissues and accuracy in determining role of a miRNA and its cellular source, still more feasible molecular markers have been searched and scientists have found that the circulating vehicles of cells-circulating exosomes that carry respective cellular cargo to the target sites to conduct cell-to-cell communication can be an option. These can be more proficient in delivering the most specific information on the status of any cell, may it is normal or injured cells. The molecular composition of exosomes that has been found till date is being recorded in the ExoCarta database. By exploiting these data in different pathological diseases scientists have done lots of research with carcinomas. In renal diseases also these exosomal miRNAs are being used to find out a means for noninvasive early detection of graft rejection. The conclusion drawn from these studies that proteins like fetuin-A and activating transcription factor-3 (ATF-3) can be used as marker in acute kidney disease and miR-29c and CD2AP mRNA are identified from urinary exosomes in renal fibrosis patients.
Thus, the various convergent studies made in the field of transplantation have led to the discovery of potential therapeutic targets- non-invasive urinary exosomal miRNAs and proteins which can be used to investigate and confirm the injury of transplanted allograft at an early stage. Though the data obtained define exosomes as an appropriate marker when compared with mRNAs, still it has few limitations:
Diverse isolation procedures that can affect exosomal contents,
Exosomes containing large number of miRNAs that affect the signaling of the cell together but not itself alone and
TDifficultly in measuring the exact quantity of a particular miRNAs in a exosome when miRNA is in low concentration.
Go to
Conclusion
The exosome cell-to-cell communication mechanisms’ experiments are still at its infant stage. There is the need for development of more sophisticated techniques to detect the exact amount of specific functional exosomal proteins and miRNAs and their proper signaling pathways. Thus more investigation are still required to exploit exosomes in clinical fields as therapeutic targets.
#cell science#molecular biology#Stem Cells#cell biology#Journal of Cell Science#open access journals#Juniper Publishers
0 notes
Text

Huh…
1 note
·
View note
Text
Is US Patent Policy Strong Enough to Withstand the Winds of Change: A Study of the Need to Change United States Patent Policy

Author by Kent R Acheson
Abstract
The purpose of this case study was to learn how US patent policy requirements differ for the Software and Pharmaceutical Industries, specifically if United States Patent Policy adequately protects intellectual property rights [1] for two divergent industries while still encouraging research and development (R & D) investment sufficient to increase profits and innovation. Data for this study consisted of 38 witness testimonies delivered to US Congress between the years 2005 and 2010 by experts representing the two industries of interest: pharmaceutical and software. Key findings from the data analysis of these 38 testimonies revealed both within industry differences and between industry differences in patent law protection. Within industry differences showed variance based on size of the company and the degree to which they relied on their own R & D. Between industry differences reflected divergent ‘products’ with Pharmaceutical Industries needing long-term protection to recover R & D expenditures that include expenses for human trials research to proceed from patent application to market. Software industry, on the other hand, uses follow-on innovation of others to continue technological advancement by constantly improving upon existing software. The data show that these two industries use patent policy protection in different ways for different reasons. This information will enable Policymakers to develop another form of product protection in lieu of the current patent law to better meet the needs of these two industries rather than try to modify the existing law.
Introduction
Patent law was developed in parts, building on one another with a single purpose in mind of protecting all innovations in a society and this created the basis for patent laws imposed on the current and future generations. Bessen [2,3] stressed that patents may not be valuable in protecting innovation [4-6] but are used solely to diffuse new ideas in the public. Bessen and Maskin [7] had previously highlighted that little research and development (R&D) in the Software Industry is used to gain patent protections and the enforcement issue with patents is difficult, as many patents are issued for products that are not new. Levin [8] and others found much earlier that patents were rated weak at protecting the returns on innovation, far behind the protection gained through lead time and learning curve advantages.
Patent’s role in different industries
The purpose of this qualitative case study was to explore the different requirements for patent policy for the Software and Pharmaceutical Industries. All transcripts from testimonies from the spokespersons representing the two industries introduced to either House between the years 2005 to 2010 concerning the U.S. Patent Reform Bills were collected and analyzed to answer the research question in this case study. The findings could be useful in further adjusting patent policy to encourage innovation for diverse industries, or suggest the creation of another form of idea protection.
A similar problem may be in the type of intellectual property protection that a company chooses to obtain to avoid the constraints of getting a patent and extend the time frame for protection, such as copyright protection that extends protection from the 20 years for a patent to 120 years. Apple Inc. obtained a copyright protection for their popular iPhone [9], which recently lost in a suit against the Federal Government. The landmark decision helps to control copyright creep. Initially when buying an iPhone, Apple Inc. limited the service provider to AT&T and applications had to be bought from the Official Apple Store. Now, however, through a hack on the iPhone, users can choose a different service provider and load other, unofficial, applications not supported by Apple Inc., and hackers are not in violation of Copyright Law.
Policy Makers can use the findings of this study to explore new directions for the United States Patent Policy to optimize advancement of technology in the Software and Pharmaceutical Industries. Historically in the United States, there has been one patent policy. Scholars, academicians, and the United States Government still do not know the ideal amount of IPRs mainly because the objective has been to uphold one uniform policy. This study clarified if further changes were needed for patent policy through a Patent Reform Act, which enables Policy Makers to understand the needs of the Software Industry, or design another form of protection designed specifically for the Software Industry.
Crowe [10] and others stated that a case study design is most appropriate when little is known of a phenomenon in its natural context. A case study is “used to generate an in-depth, multifaceted understanding of a complex issue in its real-life context” (p. 1). The Pharmaceutical Industry has a profitable track record using the existing Patent Law to protect their R&D investments. The Software Industry is comparatively new and therefore their issues are only just now becoming obvious. Case law is outside the boundaries of this study.
The multiple dimensions of the phenomenon of the nature of protecting intellectual property rights in the Software Industry property and the Pharmaceutical Industry are worthy of study to allow all voices to be heard, including large corporations from both software and pharmaceutical companies, generic drug companies, and smaller software startups. After carefully examining all relevant transcripts, these diverse opinions can be given venue to state their needs.
Methodology and main results
The research question addressed in this study was: How do the patent policy requirements differ for the Software and Pharmaceutical Industries? From the Software and Pharmaceutical Industries’ objectives and needs for the United States Patent Policy to address, the questions spotlighted the sufficiency and effectiveness of the United States Patent Policy.
The focus of this study has two parts, they are:
1. What is the evidence United States Patent Policy adequately protects Intellectual Property Rights (IPRs) for both the Software and Pharmaceutical Industries?
2. How does the United States Patent System encourage companies to make R&D investment in the Software and the Pharmaceutical Industry?
The first research question dealt with the effectiveness of the United States Patent Policy in protecting IPRs in two industries: software and pharmaceutical. The second research question related to how companies invest in R&D with support of the United States Patent Policy. The study explored the ability of the United States Patent Policy to foster innovation with satisfactory IPR protection to encourage R&D spending focusing on two specific industries. The Software Industry experiences a sequential and complementary nature of innovations, building on previous discoveries; and may not use the patent policy in effect in the United States. If patent policy does not consider the different requirements within the Pharmaceutical Industry and is too lax then enough R&D spending will not be invested and technological advancements, including new medications, may come to the market slower or not at all.
The scope of the study is to understand how the Software and Pharmaceutical Industries use the patent system and how better to adjust the patent system to optimize technological advancement. As discussed in assumptions, because of the nature of the source of data and the possible bias that was not fully known, the assumptions may or may not have had a credible or dependable basis and may therefore have biased the findings. Qualitative designs such as the case study have inherent limitations that may threaten validity, they may lack rigor and they may not be generalizable. These limitations may be mitigated with transparency in data analysis and reporting. Crowe 5 and others explains on page 8 “seeking potential, alternative explanations, and being explicit about how interpretations and conclusions were reached, helped readers to judge the trustworthiness of the case study report.
Evidence from various sources highlight the United States Patent system does not work as intended and needs a solution to continue or increase innovative activity. The principal problem deals with innovative activity that is sequential in nature and innovative activity that involves much R&D investment to bring a product to market. Sequential inventions build on previous breakthroughs and do not require much R&D investment. Secrecy would hinder follow-on discoveries of sequential innovative products.
This study used a content analysis of witness [11] testimonies to Congress on the Software and Pharmaceutical Industries from the years 2005 to 2010, and the possibility to develop more than one patent policy to accommodate different sectors of the economy. The study concentrated on software and pharmaceutical companies, as these two industries are most at odds with each other, and have prevented the passage of the Patent Reform Act of 2005 through 2010. The Patent Reform Act of 2010 [12,13] is the result of non-passage of the 2009 Act, as was each successive year from 2005. The stance of the Software and Pharmaceutical Industries remained relatively unchanged in their requirements, but the patent reform acts changed to incorporate the majority opinion of industry. The most important recommendations of the Federal Trade Commission (FTC 11) and National Academies of Sciences (NAS) studies that were first introduced in 2005 by Senator [14] Lamar Smith were considered.
The purpose of this descriptive analysis was to examine the current United States Patent Policy and the proposed changes to United States Patent Policy, and answer the research question – How do the patent policy requirements differ for the Software and Pharmaceutical Industries? This study will help decide if the Software and Pharmaceutical Industries effectively use the U.S. Patent Policy through protecting Intellectual Property Rights (IPRs) and encouraged investment research and development (R&D). The qualitative case study was the most suitable approach to study the issues and answer the research questions because it explored real-life experiences of industries looking to patent Intellectual Property (IP).
Data and Sample Statistics
Data were collected and analysis began using the Content Analysis Guide developed for this study. The testimonies of the BSA representatives, other computer software witnesses, Computing Technology Industry Association, PhRMA representatives, other generic pharmaceutical representatives, and the Generic Pharmaceutical Association, Biotechnology Industry Association (BIO), Intellectual Property Owners Association (IPO) [15-18], and venture capital organizations were included in this study. The IPO was included because IPO members represent 30% of patent applications at the USPTO and include members from the Software and Pharmaceutical Industries, among others. The study included Venture capitalists because some members of BSA [19] and other smaller software companies began with venture capital dollars. Each data point was examined for inclusion of any reference to R&D, including duration and support for R&D, the need for patent protections [20,21], and future needs for patent policy.
The 38 documents submitted to the congressional hearings were analyzed. Documents relating to software and pharmaceutical companies reviewed were not ambiguous but very clear and straight forward following a consistent format, so that anyone conducting another study would reach the same conclusions. They all stated who authored the document, who the document represented, who presented opinion to Congress, their position on the patent reform act, and agreements and disagreements with specific points of the patent reform act. No ambiguity existed and no information required subjective judgments to interpret the information reported. The nature of the data supported the reliability of the findings.


Cisco, Hewlett-Packard, and other big high-tech companies began pushing for reform legislation to limit the number of patent infringement lawsuits and therefore the amounts paid in damages. The United States Patent and Trademark Office’s (USPTO) proceedings’ transcript from the public hearings showed the patent policy needs for BSA’s principle member and founder Microsoft. The public hearing titled Use of the Patent System to Protect Software Related Inventions took place in 1994 at the San Jose Convention Center, California, and at the Crystal Forum in Arlington, Virginia. A brief summary of Microsoft’s speech follows. Microsoft (BSA) recommended that patent protection allow an accused infringer to identify readily the activity forbidden under the claim. The success of a particular claim in meeting these objectives may depend less on the form and more on claim substance and the supporting details.
BSA represents a large base of computer software and hardware companies in the United States. Phelps (2005) from Microsoft Corporation stated that BSA does not want the patent holder to have automatically injunctive relief. Injunctive relief occurs when the courts rule an infringement occurred and automatically issue a ruling to stop the infringer from continuing operations. From the congressional hearing in 2005 on harmonization and other matters, Phelps for BSA supports publication in 18 months. Phelps [39] expressed support for establishing a post grant opposition procedure and supported third-party opportunity to alert USPTO to questionable patents during review. Phelps also supported allowing third parties the opportunity to suggest relevant prior art to examiner during review, supported a limit on damages for willful infringement to include only egregious behavior, and supported limiting damages to only the contributing, patented piece of the invention and not the market value of the whole product, as it is now.
In a congressional hearing in 2005, Simon [40] from Intel , a BSA representative, stated the patent system is difficult to maneuver because of many pieces that comprise computers and software contain “potentially hundreds of patents [that] may be relevant to a particular computer or software technology” [40]. The primary way to challenge a patent under current law is through costly litigation. Intel suggests Congress create a balanced post grant opposition enabling third parties to challenge issued patents that includes a post grant opposition of 2 years from patent grant or 1 year from receiving notice of patent infringement. Simon also encouraged Congress to create a second window to make the post grant review meaningful. Simon suggested a limit on patent application continuations and for the court not to issue a continuation on any claim broader than the broadest claim previously published or issued. BSA suggested a stay on the lower court’s decision in interlocutory appeals before final determination by the Federal Circuit Court of Appeals. Micron Technology, Inc., a non-BSA member, suggested the same patent law reforms as BSA.
In a congressional hearing in 2006, Chandler [41] of Cisco (BSA) suggested a second window triggered by receipt of an infringement complaint. During the first window, the patent issues with thousands or millions of parts making the effectiveness of the patent examination questionable. Chandler (2006) encouraged Congress to make changes to remove venue shopping, and prevent suits from worldwide damages in United States Courts like the Microsoft and AT&T case. The only patent policy need described on the BSA website dated 1994 had no updates, which is understandable because United States Patent Policy has not changed significantly for more than 50 years and the proposed changes have not made it into law. The agreement with the Patent Reform Act was from the most influential voice for the Software Industry; nevertheless, there were disagreements within the Software Industry mainly arising from smaller companies and individual inventors. Software companies wanted patent reform by Congress but differences remained among large software companies and smaller organizations. An overhaul of the patent system and other measures to promote tech development efforts are top priorities of the Business Software Alliance, Cisco, Hewlett-Packard, and other big high-tech companies . BSA members began pushing for reform legislation to limit the number of patent infringement lawsuits, and therefore, the amounts paid in damages.
In an article in PC World dated March 9, 2008, patent reform leads a list of five legislative priorities expressed by BSA in 2008. The opinion article stated that BSA members want Congress to approve the Patent Reform Act but the legislation stalled in the United States Senate because of objections from inventors, pharmaceutical companies and some small tech (computer software) firms. In addition the article proclaimed, more than 170 California businesses and organizations oppose the Patent Reform Act in its current form. They mention that research to stay competitive is both expensive and risky, but strong protections from patent policy attract the necessary investments to commercialize a new product. This is especially the case for the hundreds of smaller, venture capital-backed firms in the state, of which many spun from California’s world-class research universities and private research institutes. According to GlaxoSmithKline, California Wireless and Mi5 Networks in paragraph eight on page one of Gross [42] (2008), the Patent Reform Act “would increase costs to obtain and maintain patents, undermine patent certainty, incentivize infringement, and weaken the enforceability of patent rights and intellectual property protections.”
Dr. Myhrovold [43-45] started Dynamical Systems, a software company, in 1984 that Microsoft bought in 1986. He worked with Microsoft from 1986 to 2000 (14 years). Myhrovold retired from Microsoft in 2000 to start another company, Intellectual Ventures, which files more than 300 patents a year making it the 25th largest inventing organization in America. Dr. Myhrovold stated “[Software is] a complex topic…and it’s all about company culture and how companies use patents” (Perspectives on Patents [46,47]. Continuing Dr. Myhrovold stated “…for most tech companies patents have never been important; they have never been a way to make money” (p. 76, para. 2), and “…patents are, at best, a distraction and most tech companies have made a deliberate decision to ignore the patent system” (p. 76, para. 5). Many other non-BSA members agreed with Myhrovold.
Defensive patenting by software companies explains if a company holds enough patents then this company can steal another product company’s ideas with impunity, but the problem enters when the other entity does not create a product to attack (Myhrovold, 2006, p. 77, para 3). These are the battle lines in the patent reform debate with universities, small inventors and pharmaceutical companies whose lifeblood is the patent system on one side, and companies who decide to infringe or at least do not care about infringing on the other side. Dr. Myhrovold is a witness from the vantage point of a Microsoft senior executive in the 1990s who discussed this role with other firms in the earlier rounds of patent reform debate.
Technology companies exaggerate the problem when over the last 20 years patents have remained in last place of lawsuits for the three forms of idea protection: trademark, copyright, and patents. A study of four high-tech companies that are active in the patent reform debate paid out $3.7 billion in patent lawsuit settlement from 1993 to 2005, but those same four companies earned $1.4 trillion in revenue over the same period making the sums for infringement only 0.26% of revenues on average. The company with the highest number of lawsuits experienced sums for infringement at only 0.51% of revenues. “Patent trolls” are companies that do not market a product but only the idea for a product. Companies that do not produce a product comprise only 2% of the patent infringement lawsuits. Software companies like to blame an innocuous group of patent troll companies when they themselves perform the same litigious practices blamed on trolls. Myhrovold stated the need to embrace the trend to make the alternate resolutions more like a court trial by creating a separate Patent Court, much like the Tax Court, Bankruptcy Court, or Divorce Court to try only specific cases.
Inter Digital is a technology and software company that disagrees with BSA’s proposed changes to patent law. Inter Digital’s Bernstein summarized the differences in the Software industry on page 220 last paragraph at the 2007 congressional hearings: “…the IT industry is absolutely not united in its support for mandatory apportionment, post grant opposition, expansive USPTO rulemaking authority, and interlocutory appeals fall outside the realm of patent ‘reform’.” Bernstein continues by expressing how such an action would degrade patent rights and increase litigation for smaller innovators. The weakening of legitimate patents would protect a few corporate giants and increase the number of lawsuits Bernstein (2007), [48,49].
An article by Mc Dougall [50] and Chabrow (2006), [51,52] in InformationWeek explains the problems as they perceive them with the Patent Reform Act from other software and computer companies. Hans Hxu, founder of online gift registry Felicite.com, says the industry’s large players want the appearance of IP openness but do not practice it. “IBM patents almost everything they do, and then they sit on it, which does not encourage innovation” (Microsoft Agenda, para. 3) says Hxu, a McKinsey consultant although other critics suggest the sellers’ moves cement their advantages when they face rising [53] competition from startups. In an August 2005 essay, Harvard Law School professor and tech entrepreneur James Moore argued the collaborative patent review proposed by IBM, Microsoft, and others would result in fewer patents issued because it would give examiners more ammunition to shoot down patent applications. “If fewer patents are issued, but existing patents are not revoked, IBM and Microsoft win because they already possess vast existing portfolios,” Moore writes (Microsoft Agenda, para. 4). Some Web 2.0 companies dismiss IBM’s argument that business-method patents protect obvious ideas. “Everything is obvious after someone has done it,” says a spokesperson for online movie renter Netflix (Microsoft Agenda, para. 5), which has patents on its queue-ordering system--and is suing Blockbuster for allegedly copying the system.
Small tech companies are taking matters into their own hands, forming patent cooperatives through which they share IPRs. Search company Wink shares in Creative Commons, a group that encourages sharing of copyrights and open source licenses, but there is a line between sharing and protecting intellectual property that creates competitive advantage, says Wink’s Chief Executive Officer (CEO) Michael [54,55] Tanne. “When companies have invested in the development of technologies, they really ought to be able to protect it,” Tanne says (Microsoft Agenda, para. 6). Resolving these issues will influence developing and commercializing tech innovations. Too many lengthy and expensive legal battles will persuade IT departments to stick with familiar technology, and this is something tech vendors should consider as they take one another to court.
The largest and best known pharmaceutical companies in the Pharmaceutical Industry represented by Pharmaceuticals Researchers and Manufacturers of America (PhRMA), Biotechnology Industry Organization (BIO), and the Professional Inventors Alliance disagree with the weakening of patent protection and the long, time frame proposed for patent reexamination. High R&D characterizes these industries and the Pharmaceutical Industry realizes a shortened patent protection because patent protection begins before FDA approval. This shortens patent protection to commercialize the product to the remaining years.
On September 17, 2007, The Professional Inventors Alliance expressed through a letter to President Bush the flaws in the Patent Reform Act of 2007. The Patent Reform Act of 2007 did not pass the United States Senate because of the opposition from PhRMA, small inventors, and small tech firms . The letter from the Professional Inventors Alliance expressed that if the Patent Reform Act of 2007 passed into law it would harm the United States’ innovative character because of the inability to enforce patents and would reduce the royalties associated with a patented technology. In 1980, PHRMA’s members invested $2 billion in R&D for new medicines; although, nearly 30 years later (in 2009), PHRMA’s members invested $50.3 billion in R&D out of the $65.2 billion industry-wide total. Pharmaceutical companies rely on government-granted patents to protect their substantial investments in researching and developing new drugs. It takes 10-15 years and costs $800 million on average to bring a new medicine to market. The Pharmaceutical Research and Manufacturers of America (PhRMA) represents the country’s leading pharmaceutical research and biotechnology companies.
Without patents to protect all the inventions necessary to develop a drug for a limited time, others could simply copy the drugs immediately, offering their versions at a reduced price because they did not incur the high costs to develop the drug. This would seriously affect the pharmaceutical companies’ ability to recoup their costs and reinvest in other research projects. PhRMA stated in 2010 that “a strong patent system is crucial to our economic [56,57] competitiveness, especially in these economically trying times” (PhRMA’s website, 2001, p. 1). The companies in favor and against the Patent Reform Act of 2010 divided into the companies that have favored and opposed the previous patent reform acts, that is, computer software favoring patent reform and pharmaceutical companies and biotechnology companies opposing patent reform. Those opposing and in favor of the patent reform acts through the six years in this study have not changed their needs but, instead, Congress changed trying to create a patent policy agreeable to most patent users.
The large pharmaceutical companies also known as the name brand pharmaceutical companies and the smaller, generic pharmaceutical companies were in general agreement on most issues. Both wanted strong patent protection and both sides were against the Patent Reform Bill [58] of 2005 and 2006 as stated in the congressional hearings on patent reform. The firstinventor- to-file patent system while harmonizing with the large United States trading partners also poses some difficulties and disagreements with United States patentees. The problems lay in the grace period of 1-year and the best mode requirement in the patent application. Harmonizing with other countries’ patent systems as currently written, such as Japan and Europe, would remove the United States grace period of 1 year to file a patent application and would remove the best mode requirement when filing a patent application. The best mode requirement is the descriptive part of the patent application the inventor has to include the inventor’s idea of how best to use or combine the chemicals for complete effectiveness.
The differences between the brand name and generic pharmaceutical companies lay in eliminating the best mode factor of the patent application and the inequitable conduct defense. Brand name pharmaceutical companies say the best mode provision of the patent law is subjective, and therefore should be removed. The generic pharmaceutical companies believe the best mode provision should remain because they cannot copy the patented medication without the recipe or the “best mode” of making the drug. By removing the inequitable conduct defense, brand name pharmaceutical companies will misuse the patent system to the harm of the public and generic pharmaceutical companies. Differences exist between the brand name pharmaceuticals and the generic pharmaceuticals. One example is the issue of patent quality: Best mode. Generic pharmaceuticals want to keep the “best mode” in the patent law language because it lowers cost of medications by allowing generic companies to copy name brand drugs more easily. Ely Lilly [59,60] and PhRMA want to remove the best mode language . The Generic Pharmaceutical Association also has qualms with weakening the inequitable conduct saying that weakening this provision gives brand-name pharmaceutical companies incentive to misrepresent their inventions.
The differences between the brand name and generic pharmaceutical companies lay in eliminating the best mode factor of the patent application and the inequitable conduct defense. Brand name pharmaceutical companies say the best mode provision of the patent law is subjective, and therefore should be removed. The generic pharmaceutical companies believe the best mode provision should remain because they cannot copy the patented medication without the recipe or the “best mode” of making the drug. By removing the inequitable conduct defense, brand name pharmaceutical companies will misuse the patent system to the harm of the public and generic pharmaceutical companies. Differences exist between the brand name pharmaceuticals and the generic pharmaceuticals. One example is the issue of patent quality: Best mode. Generic pharmaceuticals want to keep the “best mode” in the patent law language because it lowers cost of medications by allowing generic companies to copy name brand drugs more easily. Ely Lilly [59,60] and PhRMA want to remove the best mode language . The Generic Pharmaceutical Association also has qualms with weakening the inequitable conduct saying that weakening this provision gives brand-name pharmaceutical companies incentive to misrepresent their inventions.
Together the Case Lawre presented the most comprehensive line of court-led patent reforms, which makes patent reform substantially different in 2010 than 2005. Patent lawyers and the law association, AIPLA [63,64], believe that legislation is not necessary and the court system will eventually find a solution for compromise for the different users of the patent system and will define patent law through successive Case Law. Larger, more market capitalized firms make more noise and are heard more clearly than smaller, less capitalized companies or individual inventors, including companies that specialize in innovation but do not concurrently produce a product, also known as patent trolls. More innovation comes from smaller firms and individual inventors than large entities. The larger software enterprises that often infringe on patents held by companies that do not produce a product (patent trolls) behave similarly to the patent trolls. IBM and Microsoft sit on patents without an accompanying product, when another company discovers something similar the patent surprises the unsuspecting company, and a licensing or royalty agreement can avoid costly litigation. IBM earned over a billion dollars in 2005 solely from license agreements and royalties. Licensing and royalty agreements are another possible direction that companies take to avoid patent infringement suits; however, their use threatens other companies to ransom licensing or royalty agreements but is cheaper and the outcome more certain than litigation.
The Pharmaceutical Industry appreciates the current patent policy and is leery of any changes that would disrupt the current manner in which they use the patent system to optimize patent protection; also the Pharmaceutical Industry like the Software Industry makes the best of the current patent policy . Although pharmaceutical firms have to wait until after drug trials and resulting FDA approval to market the medication, which includes the 20-year patent term and drug approval sometimes lasts as much as 10 years, they too have found ways to evade current patent law to extend the patent length. The Pharmaceutical Industry commonly increases the shortened patent length by adding a known chemical to the patent protected drug therapy, and adds another patent protection term of 20 years by increasing the number of patents on a drug. One specific drug therapy created by a name-brand pharmaceutical firm that a generic company was exploring to copy had patent protection by more than 200 patents spanning 40 years.
Discussion and Conclusions
The specific research questions that framed this qualitative case study were 1. What is the evidence United States Patent Policy adequately protects Intellectual Property Rights [65] (IPRs) for both the Software and Pharmaceutical Industries? 2. How does the United States Patent Policy encourage companies to make research and development (R&D) investment in both the Software Industry and in the Pharmaceutical Industry? Based on the differences on how patent policy should read, issues of effectiveness of the United States Patent Policy to both protect and encourage IPRs and R&D investment should be considered. Patent policy in the United States has remained unchanged for the last 55 years, and has been effective in protecting IPRs and encouraging R&D investment. Pharmaceutical firms have been around many years and have flourished in the current patent policy environment. Only with the creation of the personal computer have software companies entered the scene and have expressed concern for the patent policy changes to reduce the software company’s purposeful infringement. In a few words, the large software companies want to weaken patent protections and reduce their costs to defend against patent infringement lawsuits because big software companies do not care about patents or patent infringement.
Three important findings from this study are
1. The Pharmaceutical and Software Industries use patent policy differently
2. BSA explicitly states they want a strong patent policy, but, in effect, want to weaken the current patent policy, and
3. Differences exist within each industry. Congress has attempted to improve patent law 6 years without success because there is not agreement pleasing all industries, but the principle differences embodied the Software and Pharmaceutical Industries.
Firstly, pharmacy and software use patent policy differently: Pharmacy to protect R&D and Software for defensive purposes. Software Industry (BSA) does not use the patent policy as designed to protect R&D, but to defend against the threat of patent infringement lawsuits. The testimonies to Congress provided evidence to answer my research question of how the patent policy requirements differ between the Software and Pharmaceutical Industries. The testimonies to Congress were clear and straightforward. I did not have to infer the meaning or needs of the witnesses. They clearly stated their position and what they wanted in patent policy. Many people in the Pharmaceutical Industry and smaller software companies specifically stated that larger software and computer companies began calling for patent reform to limit the many patent infringement suits against them. Myhrovold shared his experience working for Microsoft in the late 90s stating that large software companies are not concerned with infringing on another’s patents and the only reason they care at all about patents is to defend against patent infringement lawsuits.
Secondly, the data from congressional testimonies clearly showed that the Software Industry (BSA) verbalized they want a strong patent policy but, instead, they want to weaken the rights of patent holders. This weakening is from: An unlimited post patent review period, placing the burden of proof for infringement on the patent holder (instead of the offender), and limiting the damage awards for infringement to only the infringing part of an innovation. The testimonies clearly stated their position and what they wanted. The previous list clearly communicated to Congress what the Software Industry (BSA) wanted in a patent policy, and refuted by other expert testimonies in the Software Industry.
All BSA representatives stated they wanted strong patent protection, and continued with the above reasons, which amount to weakening a patent holders’ legal rights to their Intellectual Property Rights (IPRs). Many testimonies contrary to BSA stated specifically the reasons BSA wants to limit a patent holders’ IPRs is to stave off patent infringement lawsuits. Myhrovold (2006) shared that patent policy did not enter into Microsoft’s and other BSA members’ culture. Patents are not how software companies protect innovation, but, rather, secrecy, and lead time or economies of scale are more effective to protect innovation in a short product lifecycle industry. Thirdly, the entire Software Industry is not united with BSA, and the entire Pharmaceutical Industry is not united with PhRMA. Differences exist between the two industries and differences exist within each industry, such as difference between larger companies and smaller companies in Software Industry and brand name pharmaceutical versus generic pharmaceutical. Each expert clearly stated what they wanted, why they wanted it, and differences within their respective industries. The witnesses to the congressional hearings succinctly stated that the BSA or PhRMA did not represent the entire industry, and the industry was not united in its desires for patent policy. Siwik [66] said in the exact words that the Pharmaceutical Industry is not united, and based on the non-BSA members’ testimonies with them vehemently disagreeing with BSA’s stance, anyone would reach the same conclusions that BSA is far from united too.
The evidence suggests the two industries use patent policy in different ways. For instance, The Software Industry does not use the patent system to protect intellectual property but rather use the patent system for defensive purposes not so much to protect innovation but to defend against infringement lawsuits. Pharmaceutical industry relies heavily on a patent protection to recover large R&D spending. The evidence was found in examples of how each industry effectively uses the patent system. Based on research of the patent system and the evidence of how each industry uses the patent system, the data would suggest agreement with many of the pharmaceutical, biotechnology, and other industries that use the patent system effectively to protect research and development dollars that the system does not need major change. Research shows the answer to the question of how the United States Patent System encourages R&D and promotes innovation; the patent system performs well according to its design. It protects ideas. The current patent policy is effective in protecting innovation and encouraging research and development spending.
For more Open Access Journals please visit our site: Juniper Publishers For more articles, please click on Journal of Organic Medicinal Chemistry
0 notes